A Photoalkylative Fluorogenic Probe of Guttiferone A for Live Cell Imaging and Proteome Labeling in Plasmodium falciparum
Abstract
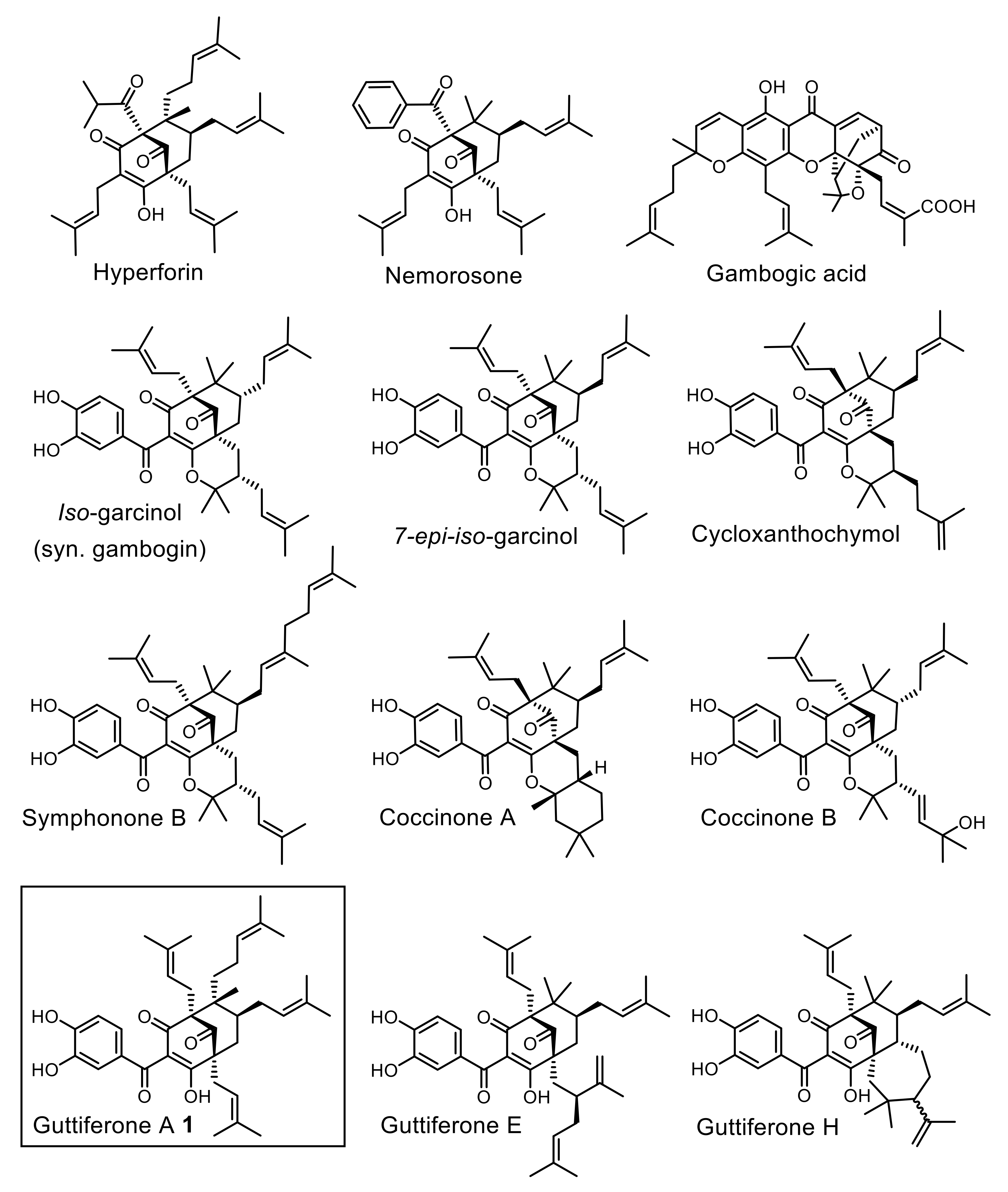
1. Introduction
2. Results and Discussion
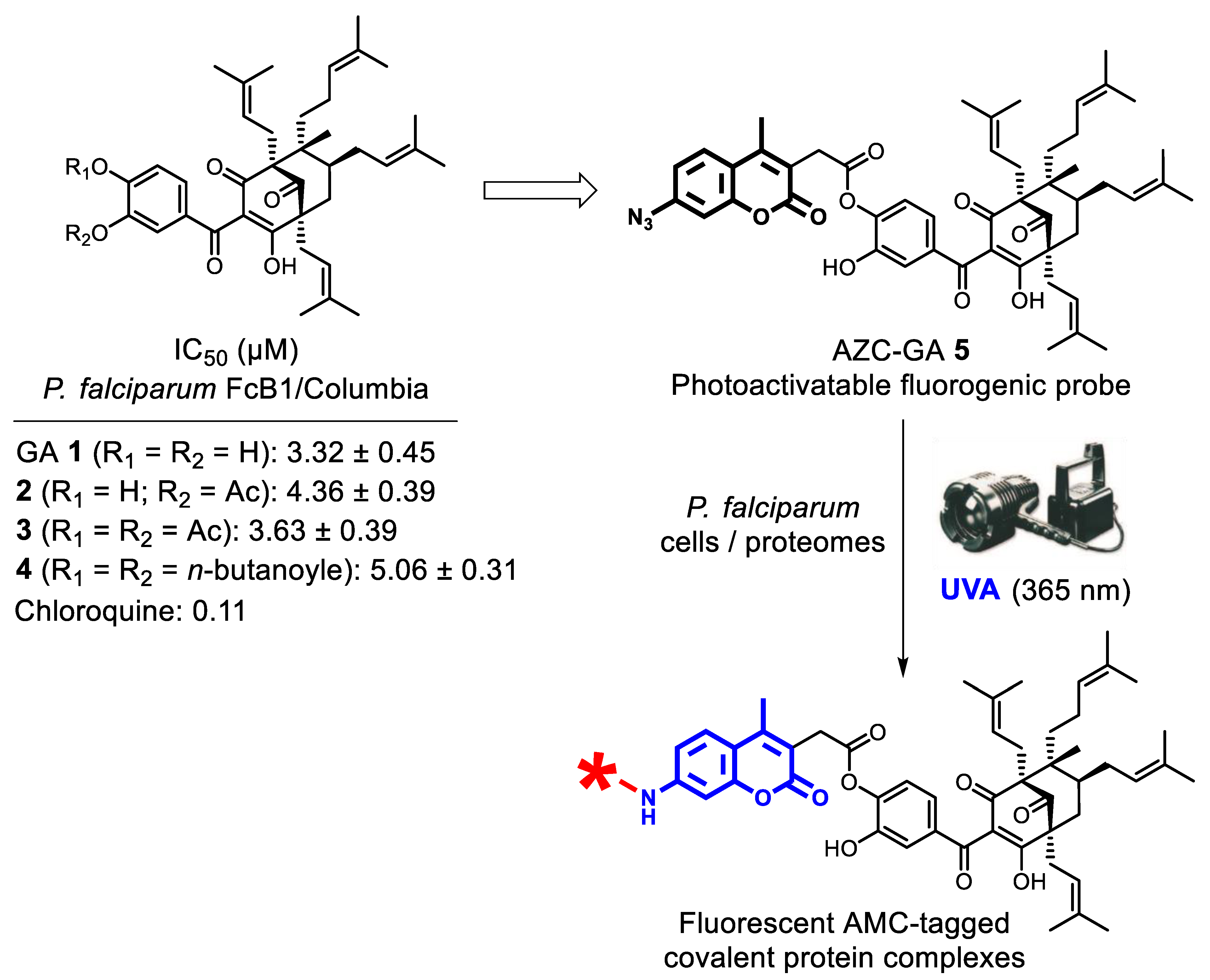
2.1. Probe Principle and Design
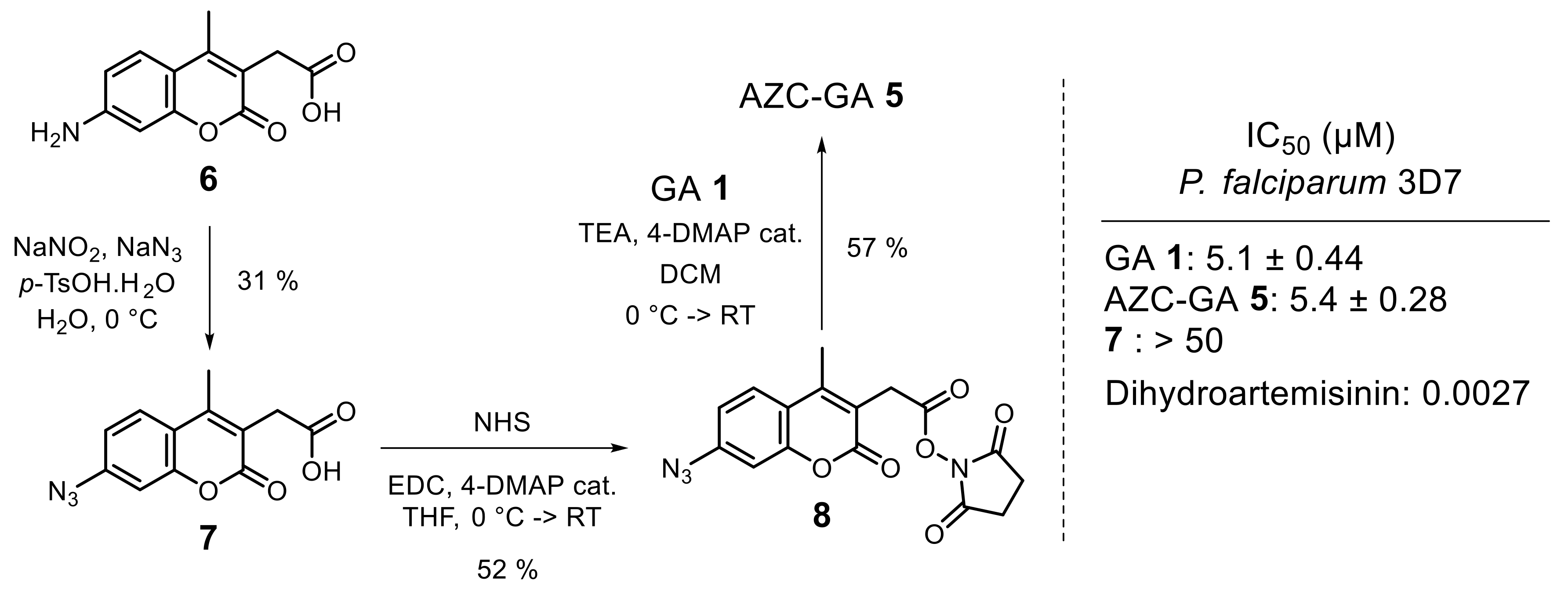
2.2. Probe Synthesis and In Vitro Activity against P. falciparum Blood-Stages
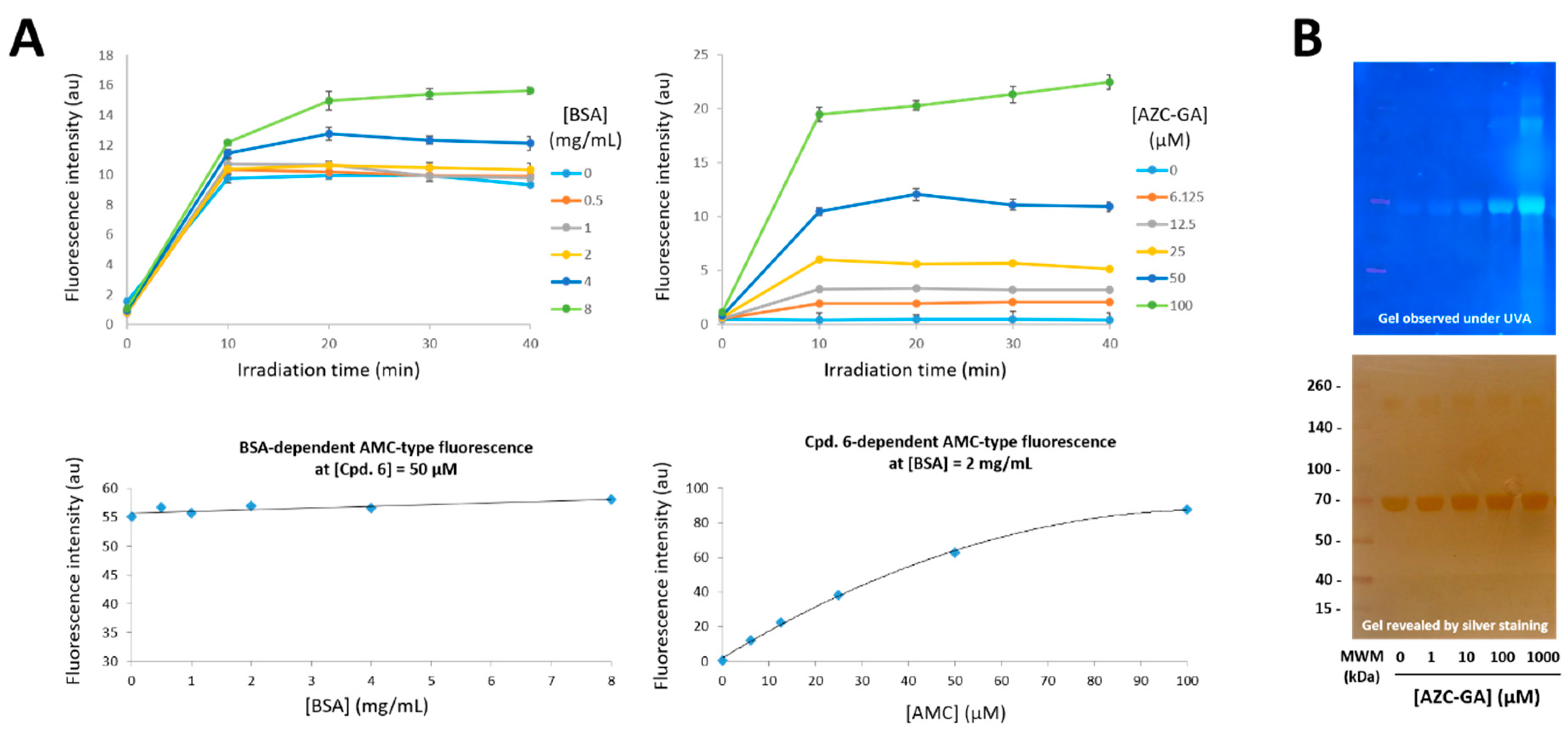
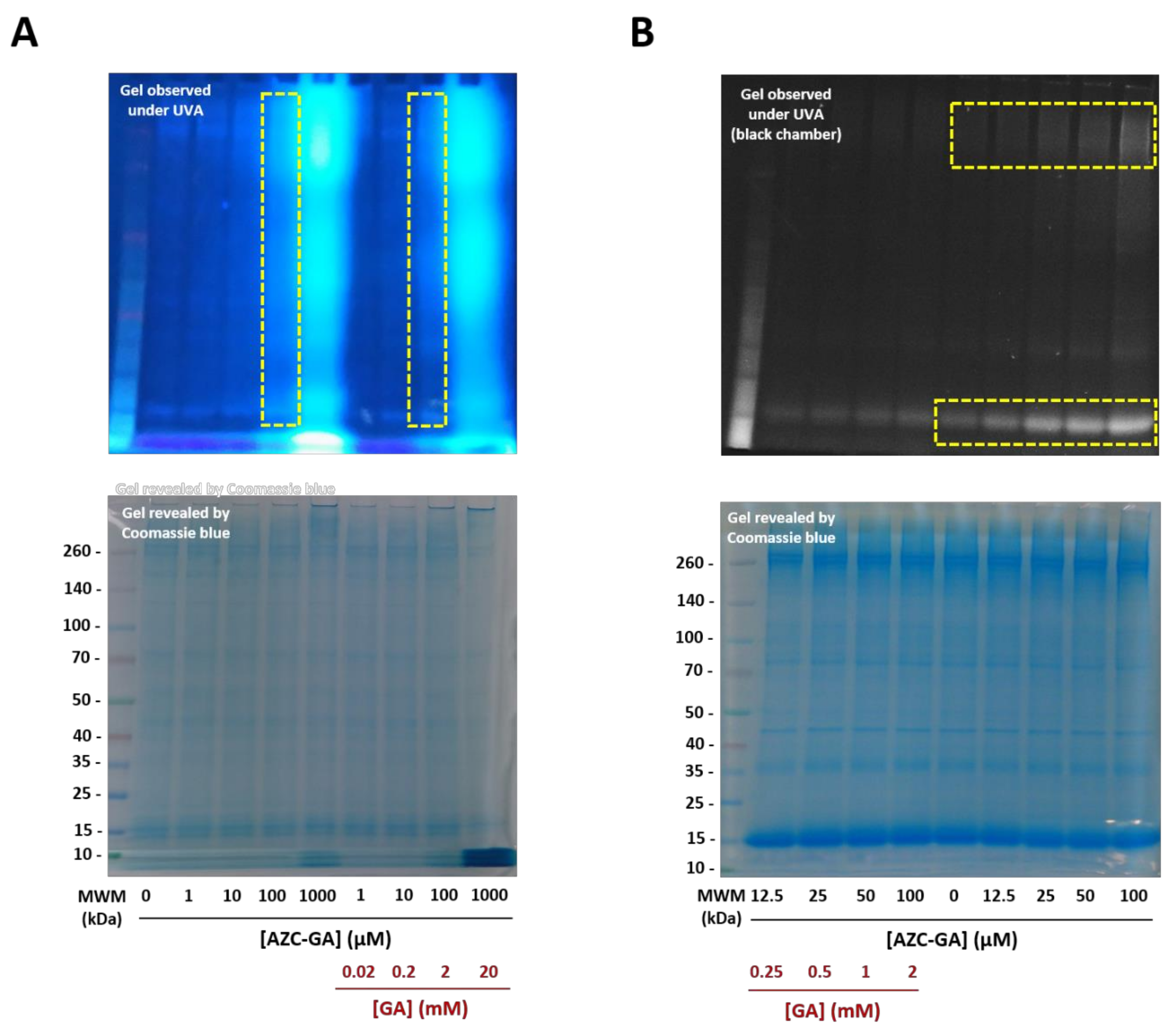
2.3. Proof-of-Principle of the Photoactivated Fluorogenic Covalent Labeling with AZC-GA 5 on BSA
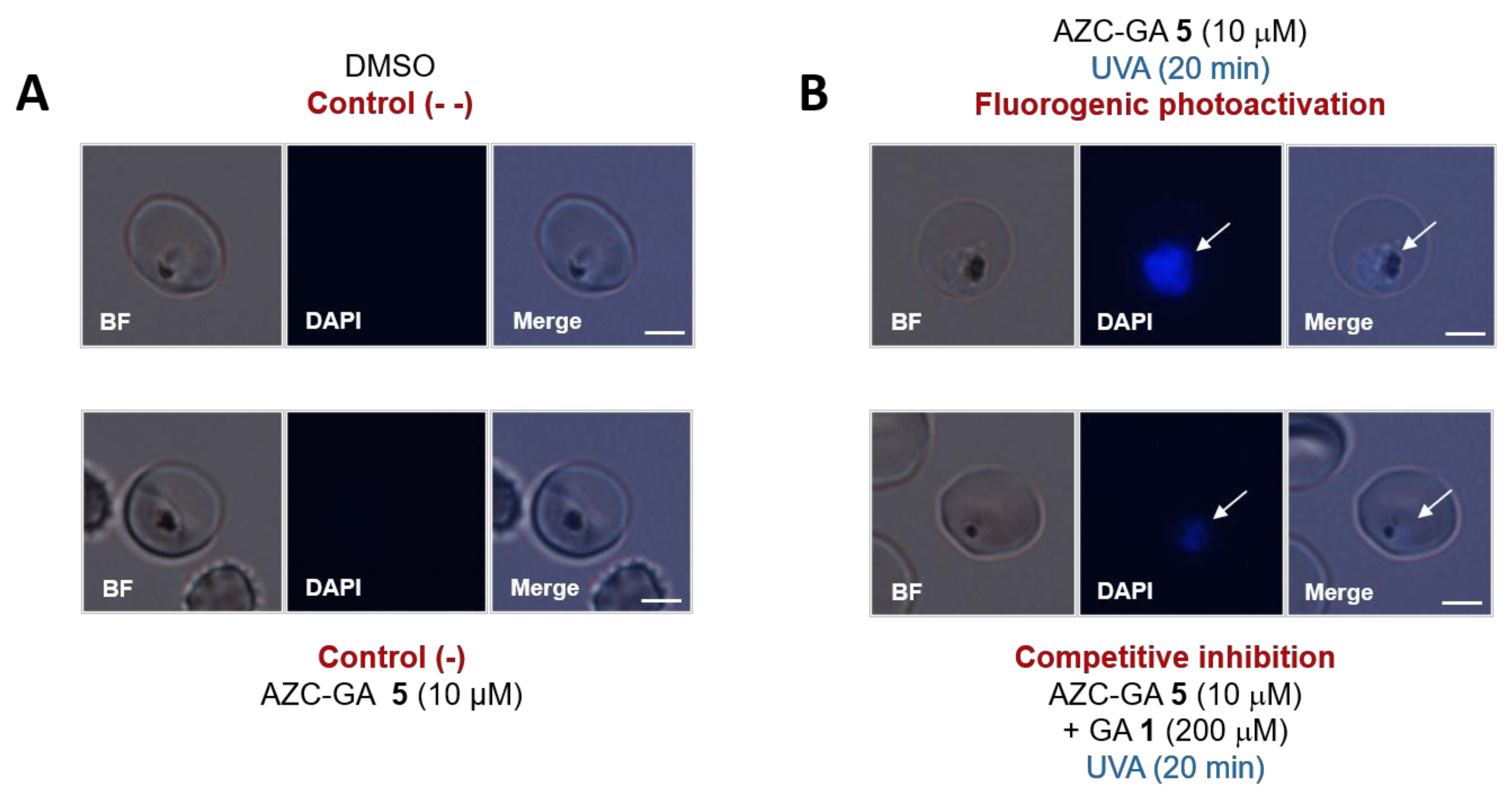
2.4. Fluorogenic Photoactivation Labeling of Live P. falciparum-Infected Erythrocytes with AZC-GA 5
2.5. Fluorogenic Photoactivation Labeling of Fresh P. falciparum Lysate with AZC-GA 5
3. Materials and Methods
3.1. Chemistry
3.1.1. General Information
3.1.2. Compound Synthesis
Cpd. 7: 2-(7-azido-4-methyl-2-oxo-2H-chromen-3-yl)acetic acid
Cpd. 8: 2,5-Dioxopyrrolidin-1-yl 2-(7-azido-4-methyl-2-oxo-2H-chromen-3-yl)acetate
AZC-GA 5: 2-Hydroxy-4-((6R,7S)-2-hydroxy-6-methyl-1,5,7-tris(3-methylbut-2-en-1-yl)-6-(4-methylpent-3-en-1-yl)-4,9-dioxobicyclo [3.3.1]non-2-ene-3-carbonyl)phenyl 2-(7-azido-4-methyl-2-oxo-2H-chromen-3-yl)acetate
3.1.3. Fluorimetric Study
3.2. Biology
3.2.1. General Information
3.2.2. Parasite Culture
3.2.3. IC50 Measurement
3.2.4. Photoactivation Fluorogenic Microscopy
3.2.5. Parasite Lysate Preparation
3.2.6. Covalent Protein Photolabeling in Fresh P. falciparum Lysate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. World Malaria Report 2019; World Health Organization: Geneva, Switzerland, 2019; pp. 1–232. [Google Scholar]
- Bhatt, S.; Weiss, D.J.; Cameron, E.; Bisanzio, D.; Mappin, B.; Dalrymple, U.; Battle, K.E.; Moyes, C.L.; Henry, A.; Eckhoff, P.A.; et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015, 526, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Imwong, M.; Suwannasin, K.; Kunasol, C.; Sutawong, K.; Mayxay, M.; Rekol, H.; Smithuis, F.M.; Hlaing, T.M.; Tun, K.M.; Van Der Pluijm, R.W.; et al. The spread of artemisinin-resistant Plasmodium falciparum in the Greater Mekong subregion: A molecular epidemiology observational study. Lancet Infect. Dis. 2017, 17, 491–497. [Google Scholar] [CrossRef]
- Conrad, M.D.; Rosenthal, P.J. Antimalarial drug resistance in Africa: The calm before the storm? Lancet Infect. Dis. 2019, 19, e338–e351. [Google Scholar] [CrossRef]
- Tajuddeen, N.; Van Heerden, F.R. Antiplasmodial natural products: An update. Malar. J. 2019, 18, 1–62. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Moreno, G.; Cantizani, J.; Sánchez-Carrasco, P.; Ruiz-Pérez, L.M.; Martín, J.; El Aouad, N.; Pérez-Victoria, I.; Tormo, J.R.; González-Menendez, V.; González, I.; et al. Discovery of New Compounds Active against Plasmodium falciparum by High Throughput Screening of Microbial Natural Products. PLoS ONE 2016, 11, e0145812. [Google Scholar] [CrossRef]
- Yoo, E.; Schulze, C.J.; Stokes, B.H.; Onguka, O.; Yeo, T.; Mok, S.; Gnädig, N.F.; Zhou, Y.; Kurita, K.; Foe, I.T.; et al. The Antimalarial Natural Product Salinipostin A Identifies Essential alpha/beta Serine Hydrolases Involved in Lipid Metabolism in P. falciparum Parasites. Cell Chem. Biol. 2020, 27, 143–157.e5. [Google Scholar] [CrossRef]
- Tibon, N.S.; Ng, C.H.; Cheong, S.L. Current progress in antimalarial pharmacotherapy and multi-target drug discovery. Eur. J. Med. Chem. 2020, 188, 111983. [Google Scholar] [CrossRef] [PubMed]
- Cottet, K.; Genta-Jouve, G.; Fromentin, Y.; Odonne, G.; Duplais, C.; Laprévote, O.; Michel, S.; Lallemand, M.-C. Comparative LC–MS-based metabolite profiling of the ancient tropical rainforest tree Symphonia globulifera. Phytochemistry 2014, 108, 102–108. [Google Scholar] [CrossRef]
- Cottet, K.; Fromentin, Y.; Kritsanida, M.; Grougnet, R.; Odonne, G.; Duplais, C.; Michel, S.; Lallemand, M.-C. Isolation of Guttiferones from Renewable Parts of Symphonia globulifera by Centrifugal Partition Chromatography. Planta Medica 2015, 81, 1604–1608. [Google Scholar] [CrossRef]
- Fromentin, Y.; Gaboriaud-Kolar, N.; Lenta, B.N.; Wansi, J.D.; Buisson, D.; Mouray, E.; Grellier, P.; Loiseau, P.M.; Lallemand, M.-C.; Michel, S. Synthesis of novel guttiferone A derivatives: In-vitro evaluation toward Plasmodium falciparum, Trypanosoma brucei and Leishmania donovani. Eur. J. Med. Chem. 2013, 65, 284–294. [Google Scholar] [CrossRef]
- Ngouela, S.; Lenta, B.N.; Noungoué, D.T.; Ngoupayo, J.; Boyom, F.F.; Tsamo, E.; Gut, J.; Rosenthal, P.J.; Connolly, J.D. Anti-plasmodial and antioxidant activities of constituents of the seed shells of Symphonia globulifera Linn f. Phytochemistry 2006, 67, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Monzote, L.; Cuesta-Rubio, O.; Matheeussen, A.; Van Assche, T.; Maes, L.; Cos, P. Antimicrobial Evaluation of the Polyisoprenylated Benzophenones Nemorosone and Guttiferone A. Phytother. Res. 2011, 25, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Phang, Y.; Wang, X.; Lu, Y.; Fu, W.; Zheng, C.; Xu, H. Bicyclic polyprenylated acylphloroglucinols and their derivatives: Structural modification, structure-activity relationship, biological activity and mechanism of action. Eur. J. Med. Chem. 2020, 205, 112646. [Google Scholar] [CrossRef]
- Lyles, J.T.; Negrin, A.; Khan, S.I.; He, K.; Kennelly, E.J. In Vitro Antiplasmodial Activity of Benzophenones and Xanthones from Edible Fruits of Garcinia Species. Planta Medica 2014, 80, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Marti, G.; Eparvier, V.; Moretti, C.; Susplugas, S.; Prado, S.; Grellier, P.; Retailleau, P.; Gueritte, F.; Litaudon, M. Antiplasmodial benzophenones from the trunk latex of Moronobea coccinea (Clusiaceae). Phytochemistry 2009, 70, 75–85. [Google Scholar] [CrossRef]
- Lannang, A.M.; Louh, G.N.; Lontsi, D.; Specht, S.; Sarite, S.R.; Flörke, U.; Hussain, H.; Hoerauf, A.; Krohn, K. Antimalarial Compounds from the Root Bark of Garcinia polyantha Olv. J. Antibiot. 2008, 61, 518–523. [Google Scholar] [CrossRef]
- Marti, G.; Eparvier, V.; Moretti, C.; Prado, S.; Grellier, P.; Hue, N.; Thoison, O.; Delpech, B.; Gueritte, F.; Litaudon, M. Antiplasmodial benzophenone derivatives from the root barks of Symphonia globulifera (Clusiaceae). Phytochemistry 2010, 71, 964–974. [Google Scholar] [CrossRef]
- Ke, H.; Morrisey, J.M.; Qu, S.; Chantarasriwong, O.; Mather, M.W.; Theodorakis, E.A.; Vaidya, A.B. Caged Garcinia Xanthones, a Novel Chemical Scaffold with Potent Antimalarial Activity. Antimicrob. Agents Chemother. 2016, 61. [Google Scholar] [CrossRef]
- Merrick, C.J.; Jiang, R.H.Y.; Skillman, K.M.; Samarakoon, U.; Moore, R.M.; Dzikowski, R.; Ferdig, M.T.; Duraisingh, M.T. Functional Analysis of Sirtuin Genes in Multiple Plasmodium falciparum Strains. PLoS ONE 2015, 10, e0118865. [Google Scholar] [CrossRef]
- Gey, C.; Kyrylenko, S.; Hennig, L.; Nguyen, L.-H.D.; Büttner, A.; Pham, H.D.; Giannis, A. Phloroglucinol Derivatives Guttiferone G, Aristoforin, and Hyperforin: Inhibitors of Human Sirtuins SIRT1 and SIRT2. Angew. Chem. Int. Ed. 2007, 46, 5219–5222. [Google Scholar] [CrossRef]
- Cottet, K.; Xu, B.; Coric, P.; Bouaziz, S.; Michel, S.; Vidal, M.; Lallemand, M.-C.; Broussy, S. Guttiferone A Aggregates Modulate Silent Information Regulator 1 (SIRT1) Activity. J. Med. Chem. 2016, 59, 9560–9566. [Google Scholar] [CrossRef]
- Monzote, L.; Lackova, A.; Staniek, K.; Cuesta-Rubio, O.; Gille, L. Role of mitochondria in the leishmanicidal effects and toxicity of acyl phloroglucinol derivatives: Nemorosone and guttiferone A. Parasitology 2015, 142, 1239–1248. [Google Scholar] [CrossRef]
- Andreu, G.L.P.; Nuñez-Figueredo, Y.; Tudella, V.G.; Cuesta-Rubio, O.; Rodrigues, F.P.; Pestana, C.R.; Uyemura, S.A.; Leopoldino, A.M.; Alberici, L.C.; Curti, C. The anti-cancer agent nemorosone is a new potent protonophoric mitochondrial uncoupler. Mitochondrion 2011, 11, 255–263. [Google Scholar] [CrossRef]
- Guizzunti, G.; Batova, A.; Chantarasriwong, O.; Dakanali, M.; Theodorakis, E.A. Subcellular Localization and Activity of Gambogic Acid. ChemBioChem 2012, 13, 1191–1198. [Google Scholar] [CrossRef]
- Chen, G.; Battaglia, E.; Senay, C.; Falany, C.N.; Radominska-Pandya, A. Photoaffinity labeling probe for the substrate binding site of human phenol sulfotransferase (SULT1A1): 7-azido-4-methylcoumarin. Protein Sci. 1999, 8, 2151–2157. [Google Scholar] [CrossRef]
- Lord, S.J.; Lee, H.-L.D.; Samuel, R.; Weber, R.; Liu, N.; Conley, N.R.; Thompson, M.A.; Twieg, R.J.; Moerner, W.E. Azido Push−Pull Fluorogens Photoactivate to Produce Bright Fluorescent Labels†. J. Phys. Chem. B 2010, 114, 14157–14167. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Chuang, V.T.G.; Maruyama, T.; Otagiri, M. Albumin–drug interaction and its clinical implication. Biochim. Biophys. Acta. Gen. Subj. 2013, 1830, 5435–5443. [Google Scholar] [CrossRef]
- Rimac, H.; Debeljak, Ž.; Bojić, M.; Miller, L. Displacement of Drugs from Human Serum Albumin: From Molecular Interactions to Clinical Significance. Curr. Med. Chem. 2017, 24, 1930–1947. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xue, J.; Shao, J.; Jia, L. Compilation of 222 drugs’ plasma protein binding data and guidance for study designs. Drug Discov. Today 2012, 17, 475–485. [Google Scholar] [CrossRef]
- Colucci-Guyon, E.; Sousa-Batista, A.J.; Oliveira, S.D.S.; Blaud, M.; Bellettini, I.C.; Marteyn, B.; Leblanc, K.; Herbomel, P.; Duval, R. Ultraspecific live imaging of the dynamics of zebrafish neutrophil granules by a histopermeable fluorogenic benzochalcone probe. Chem. Sci. 2019, 10, 3654–3670. [Google Scholar] [CrossRef] [PubMed]
- Merckx, A.; Le Roch, K.; Nivez, M.-P.; Dorin, D.; Alano, P.; Gutierrez, G.J.; Nebreda, A.R.; Goldring, D.; Whittle, C.; Patterson, S.; et al. Identification and Initial Characterization of Three Novel Cyclin-related Proteins of the Human Malaria Parasite Plasmodium falciparum. J. Biol. Chem. 2003, 278, 39839–39850. [Google Scholar] [CrossRef]
- Trager, W.; Jensen, J.B. Human malaria parasites in continuous culture. Science 1976, 193, 673–675. [Google Scholar] [CrossRef]
- Desjardins, R.E.; Canfield, C.J.; Haynes, J.D.; Chulay, J.D. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 1979, 16, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Le Nagard, H.; Vincent, C.; Mentré, F.; Le Bras, J. Online analysis of in vitro resistance to antimalarial drugs through nonlinear regression. Comput. Methods Programs Biomed. 2011, 104, 10–18. [Google Scholar] [CrossRef]
- Kaddouri, H.; Nakache, S.; Houzé, S.; Mentré, F.; Le Bras, J. Assessment of the Drug Susceptibility of Plasmodium falciparum Clinical Isolates from Africa by Using a Plasmodium Lactate Dehydrogenase Immunodetection Assay and an Inhibitory Maximum Effect Model for Precise Measurement of the 50-Percent Inhibitory Concentration. Antimicrob. Agents Chemother. 2006, 50, 3343–3349. [Google Scholar] [CrossRef]
- Elandalloussi, L.M.; Smith, P.J. Preparation of pure and intact Plasmodium falciparum plasma membrane vesicles and partial characterisation of the plasma membrane ATPase. Malar. J. 2002, 1, 6. [Google Scholar] [CrossRef]
- Angelides, K.J. Fluorescent and photoactivatable fluorescent derivatives of tetrodotoxin to probe the sodium channel of excitable membranes. Biochemistry 1981, 20, 4107–4118. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duval, R.; Cottet, K.; Blaud, M.; Merckx, A.; Houzé, S.; Grellier, P.; Lallemand, M.-C.; Michel, S. A Photoalkylative Fluorogenic Probe of Guttiferone A for Live Cell Imaging and Proteome Labeling in Plasmodium falciparum. Molecules 2020, 25, 5139. https://doi.org/10.3390/molecules25215139
Duval R, Cottet K, Blaud M, Merckx A, Houzé S, Grellier P, Lallemand M-C, Michel S. A Photoalkylative Fluorogenic Probe of Guttiferone A for Live Cell Imaging and Proteome Labeling in Plasmodium falciparum. Molecules. 2020; 25(21):5139. https://doi.org/10.3390/molecules25215139
Chicago/Turabian StyleDuval, Romain, Kevin Cottet, Magali Blaud, Anaïs Merckx, Sandrine Houzé, Philippe Grellier, Marie-Christine Lallemand, and Sylvie Michel. 2020. "A Photoalkylative Fluorogenic Probe of Guttiferone A for Live Cell Imaging and Proteome Labeling in Plasmodium falciparum" Molecules 25, no. 21: 5139. https://doi.org/10.3390/molecules25215139
APA StyleDuval, R., Cottet, K., Blaud, M., Merckx, A., Houzé, S., Grellier, P., Lallemand, M.-C., & Michel, S. (2020). A Photoalkylative Fluorogenic Probe of Guttiferone A for Live Cell Imaging and Proteome Labeling in Plasmodium falciparum. Molecules, 25(21), 5139. https://doi.org/10.3390/molecules25215139





