p-Cymene Complexes of Ruthenium(II) as Antitumor Agents
Abstract
1. Introduction
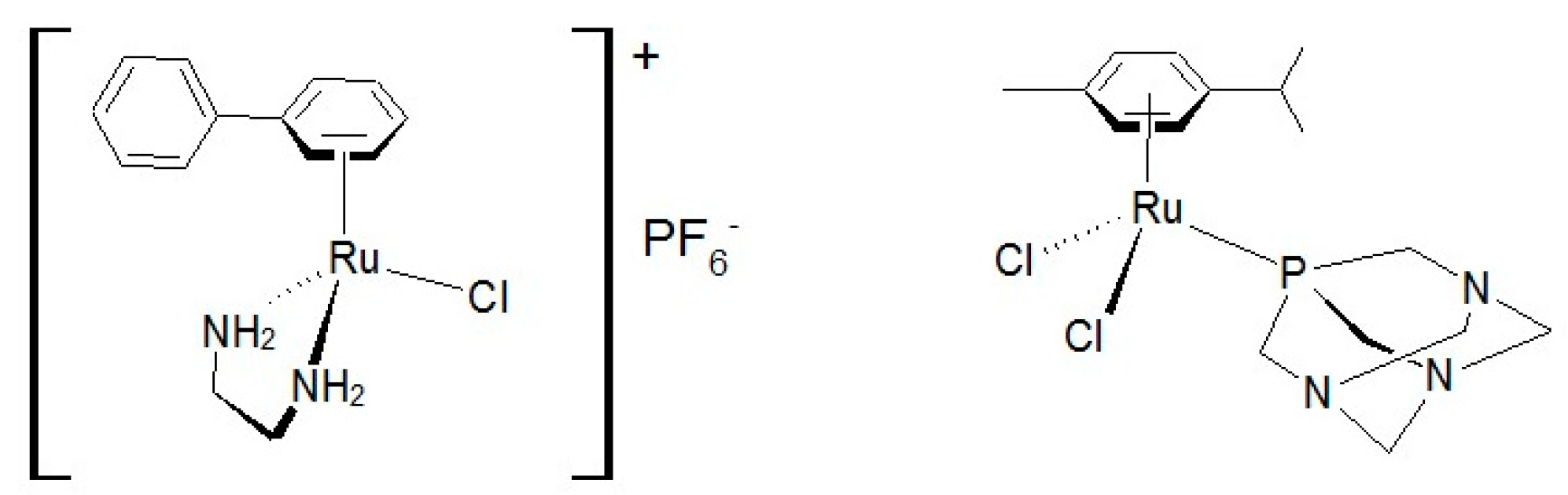
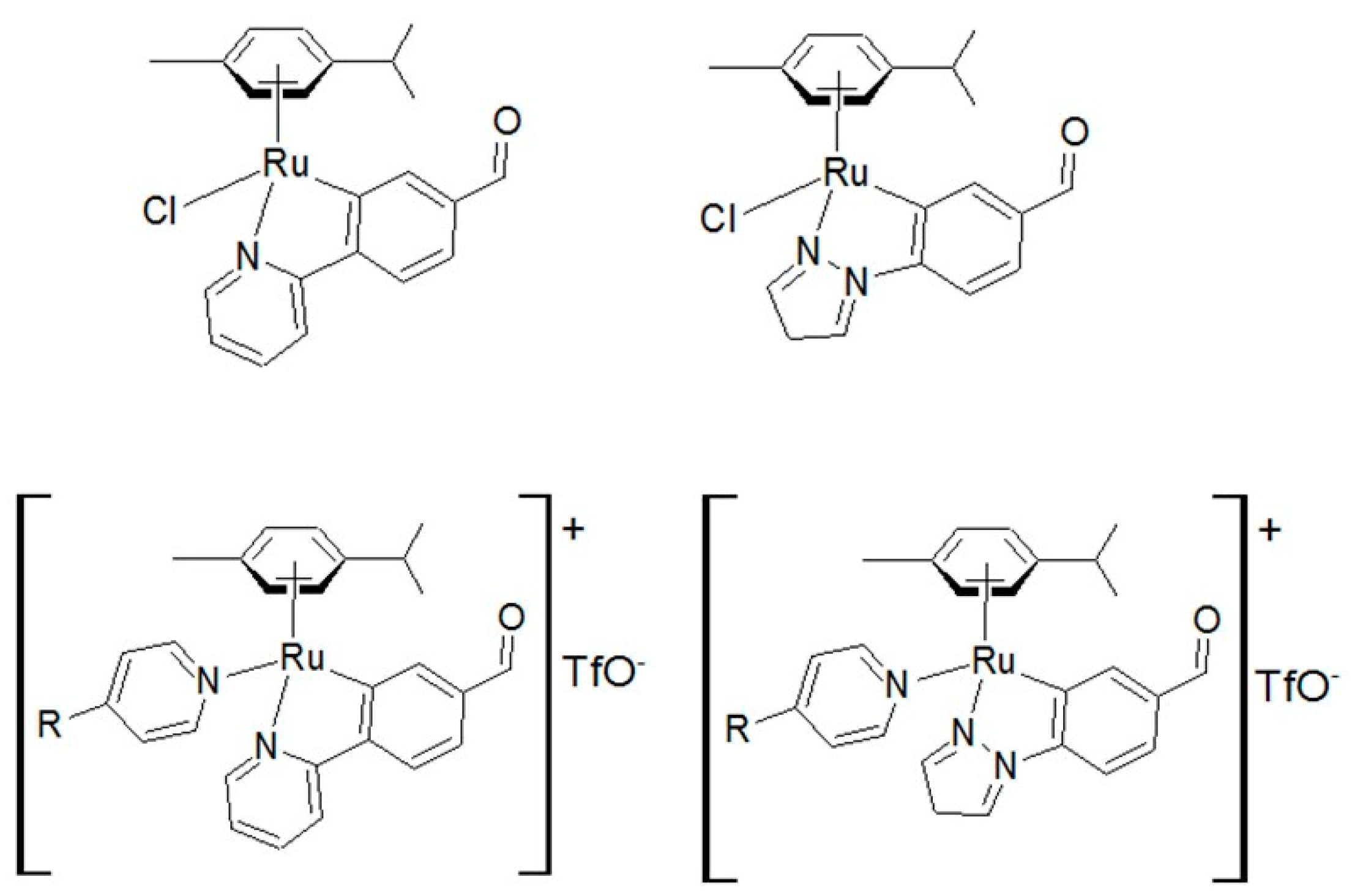
2. Results and Discussion
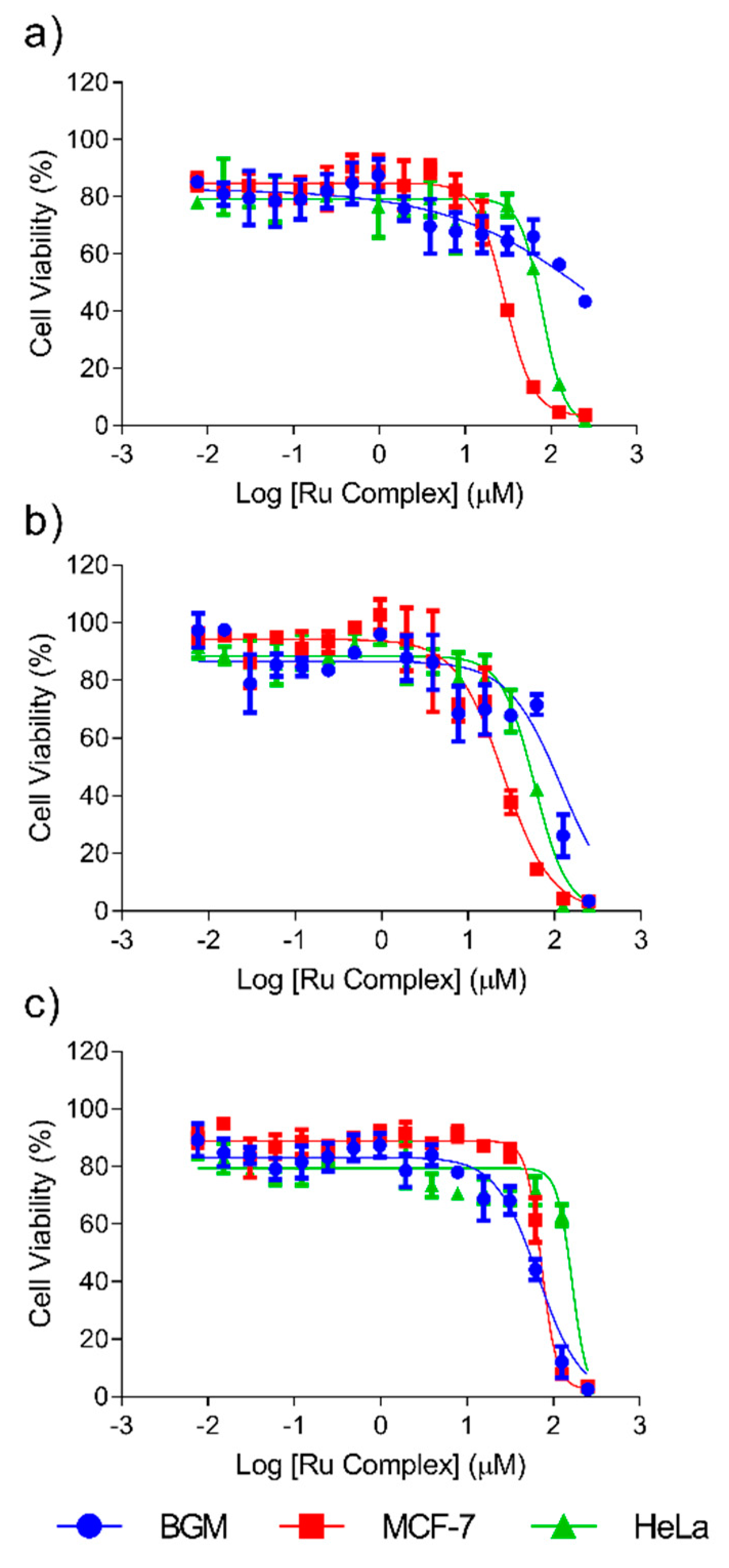
Cytotoxicity of the Complexes and the Ligand
3. Materials and Methods
3.1. Synthesis of the Complexes
3.1.1. Complexes I, II, III and IV
3.1.2. Complexes V and VI
3.2. Cell lines and Culture Media
3.3. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dabrowiak, J.C. Metals in Medicine, 2nd ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; pp. 91–216. [Google Scholar]
- Facchetti, G.; Rimoldi, I. Anticancer platinum(ii) complexes bearing N-heterocycle rings. Bioorg. Med. Chem. Lett. 2019, 29, 1257–1263. [Google Scholar] [CrossRef]
- Zaki, M.; Hairat, S.; Aazam, E.S. Scope of organometallic compounds based on transition metal-arene systems as anticancer agents: Starting from the classical paradigm to targeting multiple strategies. RSC Adv. 2019, 9, 3239–3278. [Google Scholar] [CrossRef]
- Abid, M.; Shamsi, F.; Azam, A. Ruthenium Complexes: An Emerging Ground to the Development of Metallopharmaceuticals for Cancer Therapy. Mini-Rev. Med. Chem. 2016, 16, 772–786. [Google Scholar] [CrossRef] [PubMed]
- Schmid, W.F.; John, R.O.; Arion, V.B.; Jakupec, M.A.; Keppler, B.K. Highly Antiproliferative Ruthenium(II) and Osmium(II) Arene Complexes with Paullone-Derived Ligands. Organometallics 2007, 26, 6643–6652. [Google Scholar] [CrossRef]
- Hearn, J.M.; Romero-Canelón, I.; Qamar, B.; Liu, Z.; Hands-Portman, I.; Sadler, P.J. Organometallic Iridium(III) Anticancer Complexes with New Mechanisms of Action: NCI-60 Screening, Mitochondrial Targeting, and Apoptosis. ACS Chem. Biol. 2013, 8, 1335–1343. [Google Scholar] [CrossRef]
- Romero-Canelón, I.; Salassa, L.; Sadler, P.J. The Contrasting Activity of Iodido versus Chlorido Ruthenium and Osmium Arene Azo- and Imino-pyridine Anticancer Complexes: Control of Cell Selectivity, Cross-Resistance, p53 Dependence, and Apoptosis Pathway. J. Med. Chem. 2013, 56, 1291–1300. [Google Scholar] [CrossRef]
- Namiecińska, E.; Sadowska, B.; Wiȩckowska-Szakiel, M.; Dołȩga, A.; Pasternak, B.; Grazul, M.; Budzisz, E. Anticancer and antimicrobial properties of novel η6-p-cymene ruthenium(ii) complexes containing a N,S-type ligand, their structural and theoretical characterization. RSC Adv. 2019, 9, 38629–38645. [Google Scholar] [CrossRef]
- Sarkar, B.; Mondal, A.; Madaan, Y.; Roy, N.; Moorthy, A.; Kuo, Y.-C.; Paira, P. Luminescent anticancer ruthenium(II)-p-cymene complexes of extended imidazophenanthroline ligands: Synthesis, structure, reactivity, biomolecular interactions and live cell imaging. Dalton Trans. 2019, 48, 12257–12271. [Google Scholar] [CrossRef]
- Zeng, L.; Gupta, P.; Chen, Y.; Wang, E.; Ji, L.; Chao, H.; Chen, Z.-S. The development of anticancer ruthenium(ii) complexes: From single molecule compounds to nanomaterials. Chem. Soc. Rev. 2017, 46, 5771–5804. [Google Scholar] [CrossRef]
- Zhang, C.X.; Lippard, S.J. New metal complexes as potential therapeutics. Curr. Opin. Chem. Biol. 2003, 7, 481–489. [Google Scholar] [CrossRef]
- Kostova, I. Ruthenium Complexes as Anticancer Agents. Curr. Med. Chem. 2006, 13, 1085–1107. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.S.; Therrien, B. Targeted and multifunctional arene ruthenium chemotherapeutics. Dalton Trans. 2011, 40, 10793. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Jia, J.; Zhao, Y.; Wang, K.-Z. Recent advances on dark and light-activated cytotoxity of imidazole-containing ruthenium complexes. Mini-Rev. Med. Chem. 2016, 16, 272–289. [Google Scholar] [CrossRef] [PubMed]
- Levina, A.; Mitra, A.; Lay, P.A. Recent developments in ruthenium anticancer drugs. Metallomics 2009, 1, 458. [Google Scholar] [CrossRef] [PubMed]
- Hartinger, C.G.; Jakupec, M.A.; Zorbas-Seifried, S.; Groessl, M.; Egger, A.; Berger, W.; Zorbas, H.; Dyson, P.J.; Keppler, B.K. KP1019, A New Redox-Active Anticancer Agent - Preclinical Development and Results of a Clinical Phase I Study in Tumor Patients. Chem. Biodivers. 2008, 5, 2140–2155. [Google Scholar] [CrossRef]
- Jakupec, M.A.; Galanski, M.; Arion, V.B.; Hartinger, C.G.; Keppler, B.K. Antitumour metal compounds: More than theme and variations. Dalton Trans. 2008, 183–194. [Google Scholar] [CrossRef]
- Morris, R.E.; Aird, R.E.; del Socorro Murdoch, P.; Chen, H.; Cummings, J.; Hughes, N.D.; Parsons, S.; Parkin, A.; Boyd, G.; Jodrell, D.I.; et al. Inhibition of Cancer Cell Growth by Ruthenium(II) Arene Complexes. J. Med. Chem. 2001, 44, 3616–3621. [Google Scholar] [CrossRef]
- Bruijnincx, P.C.; Sadler, P.J. New trends for metal complexes with anticancer activity. Curr. Opin. Chem. Biol. 2008, 12, 197–206. [Google Scholar] [CrossRef]
- Bugarcic, T.; Habtemariam, A.; Stepankova, J.; Heringova, P.; Kasparkova, J.; Deeth, R.J.; Johnstone, R.D.L.; Prescimone, A.; Parkin, A.; Parsons, S.; et al. The Contrasting Chemistry and Cancer Cell Cytotoxicity of Bipyridine and Bipyridinediol Ruthenium(II) Arene Complexes. Inorg. Chem. 2008, 47, 11470–11486. [Google Scholar] [CrossRef]
- Bugarcic, T.; Nováková, O.; Zerzánková, L.; Vrána, O.; Kašpárková, J.; Habtemariam, A.; Parsons, S.; Sadler, P.J.; Brabec, V. Cytotoxicity, Cellular Uptake, and DNA Interactions of New Monodentate Ruthenium(II) Complexes Containing Terphenyl Arenes. J. Med. Chem. 2008, 51, 5310–5319. [Google Scholar] [CrossRef]
- Scolaro, C.; Chaplin, A.B.; Hartinger, C.G.; Bergamo, A.; Cocchietto, M.; Keppler, B.K.; Sava, G.; Dyson, P.J. Tuning the hydrophobicity of ruthenium(II)–arene (RAPTA) drugs to modify uptake, biomolecular interactions and efficacy. Dalton Trans. 2007, 5065. [Google Scholar] [CrossRef] [PubMed]
- Han Ang, W.; Dyson, P.J. Classical and Non-Classical Ruthenium-Based Anticancer Drugs: Towards Targeted Chemotherapy. Eur. J. Inorg. Chem. 2006, 2006, 4003–4018. [Google Scholar] [CrossRef]
- Ballester, F.J.; Ortega, E.; Porto, V.; Kostrhunova, H.; Davila-Ferreira, N.; Bautista, D.; Brabec, V.; Domínguez, F.; Santana, M.D.; Ruiz, J. New half-sandwich ruthenium(ii) complexes as proteosynthesis inhibitors in cancer cells. Chem. Commun. 2019, 55, 1140–1143. [Google Scholar] [CrossRef] [PubMed]
- García, G.; Solano, I.; Sánchez, G.; Santana, M.D.; López, G.; Casabó, J.; Molins, E.; Miravitlles, C. Reactivity of [{(η6-arene)RuCl(μ-Cl)}2] towards some potentially bidentate ligands. Molecular structure of [(η6-p-cymene)RuCl(taz)]PF6 (p-cymene = p-MeC6H4CH-Me2; taz = 2,6-dimethyl-5-oxo-3-thioxo-2,3,4,5-tetrahydro-1,2,4-triazine). J. Organomet. Chem. 1994, 467, 119–126. [Google Scholar] [CrossRef]
- García, G.; Sánchez, G.; Romero, I.; Solano, I.D.; Santana, M.; López, G. Reactivity of [{η5-C5Me5RhCl(μ-Cl)}2] towards some potentially bidentate ligands. J. Organomet. Chem. 1991, 408, 241–246. [Google Scholar]
- Habtemariam, A.; Sadler, P.J. Ruthenium Compounds. U.S. Patent US7241913B2, 10 July.
- Iida, J.; Bell-Loncella, E.T.; Purazo, M.L.; Lu, Y.; Dorchak, J.; Clancy, R.; Slavik, J.; Lou Cutler, M.; Shriver, C.D. Inhibition of cancer cell growth by ruthenium complexes. J. Transl. Med. 2016, 14, 48. [Google Scholar] [CrossRef]
- Kağıt, R.; Dayan, O.; Özdemir, N. Palladium(II) and Ruthenium(II) complexes bearing arylsulfonate based ligands: Synthesis, structural characterization and catalytic properties. Polyhedron 2016, 117, 504–512. [Google Scholar] [CrossRef]
- Guptar, D.K.; Sahay, A.N.; Pandey, D.S.; Jha, N.K.; Sharma, P.; Espinosa, G.; Cabrera, A.; Puerta, M.C.; Valerga, P. Synthesis, characterization, reactivity and structure of some mono and binuclear (η6-p-cymene)ruthenium(II) complexes. J. Organomet. Chem. 1998, 568, 13–20. [Google Scholar] [CrossRef]
- Singh, S.K.; Joshi, S.; Singh, A.R.; Saxena, J.K.; Pandey, D.S. DNA Binding and Topoisomerase II Inhibitory Activity of Water-Soluble Ruthenium(II) and Rhodium(III) Complexes. Inorg. Chem. 2007, 46, 10869–10876. [Google Scholar] [CrossRef]
- Motswainyana, W.M.; Ajibade, P.A. Anticancer Activities of Mononuclear Ruthenium(II) Coordination Complexes. Adv. Chem. 2015, 2015, 1–21. [Google Scholar] [CrossRef]
- Weiss, A.; Berndsen, R.H.; Dubois, M.; Müller, C.; Schibli, R.; Griffioen, A.W.; Dyson, P.J.; Nowak-Sliwinska, P. In vivo anti-tumor activity of the organometallic ruthenium(ii)-arene complex [Ru(η6-p-cymene)Cl2(pta)] (RAPTA-C) in human ovarian and colorectal carcinomas. Chem. Sci. 2014, 5, 4742–4748. [Google Scholar] [CrossRef]
- Aman, F.; Hanif, M.; Siddiqui, W.A.; Ashraf, A.; Filak, L.K.; Reynisson, J.; Söhnel, T.; Jamieson, S.M.F.; Hartinger, C.G. Anticancer Ruthenium(η6-p-cymene) Complexes of Nonsteroidal Anti-inflammatory Drug Derivatives. Organometallics 2014, 33, 5546–5553. [Google Scholar] [CrossRef]
- Tabrizi, L.; Chiniforoshan, H. Ruthenium(II) p-cymene complexes of naphthoquinone derivatives as antitumor agents: A structure−activity relationship study. J. Organomet. Chem. 2016, 822, 211–220. [Google Scholar] [CrossRef]
- Lenis-Rojas, O.A.; Robalo, M.P.; Tomaz, A.I.; Carvalho, A.; Fernandes, A.R.; Marques, F.; Folgueira, M.; Yánez, J.; Vázquez-García, D.; López Torres, M.; et al. RuII (p-cymene) Compounds as Effective and Selective Anticancer Candidates with No Toxicity in Vivo. Inorg. Chem. 2018, 57, 13150–13166. [Google Scholar] [CrossRef]
- Gill, M.R.; Thomas, J.A. Ruthenium(II) polypyridyl complexes and DNA-from structural probes to cellular imaging and therapeuticsw. Chem. Soc. Rev. 2012, 41, 3179–3192. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, L.; Pei, Q.; He, S.; Liu, S.; Xie, Z. Metal-Organic Framework@Porous Organic Polymer Nanocomposite for Photodynamic Therapy. Chem. Mater. 2017, 29, 2374–2381. [Google Scholar] [CrossRef]
- Zhao, R.; Hammitt, R.; Thummel, R.P.; Liu, Y.; Turro, C.; Snapka, R.M. Nuclear targets of photodynamic tridentate ruthenium complexes. Dalton Trans. 2009, 10926–10931. [Google Scholar] [CrossRef]
- Zeng, L.; Xiao, Y.; Liu, J.; Tan, L. Synthesis, characterization, DNA-binding and cytotoxic properties of Ru(II) complexes: [Ru(MeIm)4L]2+ (MeIm = 1-methylimidazole, L = phen, ip and pip). J. Mol. Struct. 2012, 1019, 183–190. [Google Scholar] [CrossRef]
- Aird, R.E.; Cummings, J.; Ritchie, A.A.; Muir, M.; Morris, R.E.; Chen, H.; Sadler, P.J.; Jodrell, D.I. In vitro and in vivo activity and cross resistance profiles of novel ruthenium (II) organometallic arene complexes in human ovarian cancer. Br. J. Cancer 2002, 86, 1652–1657. [Google Scholar] [CrossRef]
- Wu, K.; Hu, W.; Luo, Q.; Li, X.; Xiong, S.; Sadler, P.J.; Wang, F. Competitive Binding Sites of a Ruthenium Arene Anticancer Complex on Oligonucleotides Studied by Mass Spectrometry: Ladder-Sequencing versus Top-Down. J. Am. Soc. Mass Spectrom. 2013, 24, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.A.; Huang, T.N.; Matheson, T.W.; Smith, A.K. (η6-Hexamethylbenzene) Ruthenium Complexes. Inorg. Synth. 1982, 21, 74–78. [Google Scholar]
- Vieira, N.S.M.; Bastos, J.C.; Rebelo, L.P.N.; Matias, A.; Araújo, J.M.M.; Pereiro, A.B. Human cytotoxicity and octanol/water partition coefficients of fluorinated ionic liquids. Chemosphere 2019, 216, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Wiji Prasetyaningrum, P.; Bahtiar, A.; Hayun, H. Synthesis and Cytotoxicity Evaluation of Novel Asymmetrical Mono-Carbonyl Analogs of Curcumin (AMACs) against Vero, HeLa, and MCF7 Cell Lines. Sci. Pharm. 2018, 86, 25. [Google Scholar] [CrossRef]
- Yellol, J.; Pérez, S.A.; Yellol, G.; Zajac, J.; Donaire, A.; Vigueras, G.; Novohradsky, V.; Janiak, C.; Brabec, V.; Ruiz, J. Highly potent extranuclear-targeted luminescent iridium(iii) antitumor agents containing benzimidazole-based ligands with a handle for functionalization. Chem. Commun. 2016, 52, 14165–14168. [Google Scholar] [CrossRef]
- Chen, T.R. Microscopic demonstration of mycoplasma contamination in cell cultures and cell culture media. Tissue Cult. Assoc. Man. 1976, 1, 229–232. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

| Complex | Color | Yield | Analytical Data a | Mass Data | M.P. b | ||
|---|---|---|---|---|---|---|---|
| (%) | C | H | N | Fragments | |||
| III | Yellow brown | 95 | 45.16 (46.27) | 4.87 (5.10) | 3.33 (3.37) | 281.0131 (281.1013) [M-p-cymene] 344.0595 (344.4141) [M-2Cl] | 220 |
| IV | Orange | 97 | 42.61 (43.53) | 4.35 (4.70) | 7.17 (7.25) | 351.0176 (350.8289) [M-Cl] 315.0444 (315.3762) [M-2Cl] | 195 |
| V | Light brown | 74 | 40.57 (42.35) | 3.46 (3.55) | 8.55 (8.98) | 489.0334 (489.7057) [M-p-cymene] 144.9649 (144.9642) [PF6] | 157 |
| VI | Dark brown | 45 | 40.91 (41.68) | 4.20 (4.45) | 4.33 (4.42) | Decompose into solution | 208 |
| Complex | v(N–H) | v(Ru–Cl) | Others |
|---|---|---|---|
| III | 3210 m | 278 s, 249 sh | 3256 m ν(O–H) |
| IV | 288 s, 278 s | ||
| V | 293 s, | 2246 s ν(C≡N) 849 s,br ν(PF6−) 557 versus ν(PF6−) | |
| VI | 3148 w | 269 w | 846 s, br ν(PF6−) 559 versus ν(PF6−) |
| Complex | 1H δ(SiMe4) | Ligand Structure |
|---|---|---|
| III | 7.29 (ddt, 2H, H3 + H4) 6.80 (m, 2H, H2 + H5) 5.48 (s, 2H, NH2) 5.05–5.11(dd, 4H, C6H4-, J = 6.0) 2.70 (sept, 1H, -CH(CH3)2, J = 7.2) 2.05 (s, 6H, CH3-C6H4-) 1.17 (d, 6H, -CH(CH3)2, J = 6.8) | 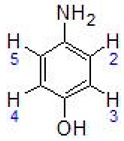 |
| IV | 9.20 (dt, 1H, H6, J = 8.0) 8.96 (m,1H, H3) 7.55 (m, 2H, H4 + H5) 5.37–5.62 (dd, 4H, C6H4-, J = 6.2) 3.09 (sept, 1H, -CH(CH3)2, J = 7.2) 2.28 (s, 3H, CH3-C6H4-) 1.24 (d, 6H, -CH(CH3)2, J = 6.8) | 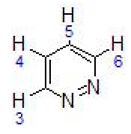 |
| V | 9.22 (dd, 2H, H1 + H4, J = 1.2) 7.73 (d, 2H, H2 + H3, J = 1.6) 5.66–5.95 (dd, 4H, C6H4-, J = 6.2) 2.57 (sept, 1H, -CH(CH3)2, J = 6.8) 1.76 (s, 3H, CH3-C6H4-) 1.50 (d, 6H, -CH(CH3)2, J= 6.8) | 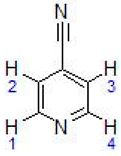 |
| VI | Decompose into solution. |
| Compound | IC50 (μM) | ||
|---|---|---|---|
| HeLa | MCF-7 | BGM | |
| Complex I | >250 | >250 | >250 |
| Complex II | 82.9 ± 0.67 | 28.7 ± 1.8 | >250 |
| Complex III | 171.1 ± 2.1 | 75.8 ± 2.3 | 59.6 ± 1.9 |
| Complex IV | >250 | >250 | >250 |
| Complex V | >250 | >250 | >250 |
| Complex VI | 57.6 ± 4.0 | 24.9 ± 4.3 | 95.0 ± 1.7 |
| cisplatin | 67.6 ± 2.0 [45] | 7.15 ± 0.1 [46] | 5.45 ± 0.2 [46] |
| Ligand | IC50 (μM) | ||
|---|---|---|---|
| HeLa | MCF-7 | BGM | |
| 4-cyanopyridine | >250 | >250 | >250 |
| Pyridazine | >250 | >250 | >250 |
| L = 2-aminophenol | >250 | 131.33 ± 0.15 | >250 |
| L = 4-aminophenol | >250 | >250 | >250 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pujante-Galián, M.A.; Pérez, S.A.; Montalbán, M.G.; Carissimi, G.; Fuster, M.G.; Víllora, G.; García, G. p-Cymene Complexes of Ruthenium(II) as Antitumor Agents. Molecules 2020, 25, 5063. https://doi.org/10.3390/molecules25215063
Pujante-Galián MA, Pérez SA, Montalbán MG, Carissimi G, Fuster MG, Víllora G, García G. p-Cymene Complexes of Ruthenium(II) as Antitumor Agents. Molecules. 2020; 25(21):5063. https://doi.org/10.3390/molecules25215063
Chicago/Turabian StylePujante-Galián, María Angeles, Sergio A. Pérez, Mercedes G. Montalbán, Guzmán Carissimi, Marta G. Fuster, Gloria Víllora, and Gabriel García. 2020. "p-Cymene Complexes of Ruthenium(II) as Antitumor Agents" Molecules 25, no. 21: 5063. https://doi.org/10.3390/molecules25215063
APA StylePujante-Galián, M. A., Pérez, S. A., Montalbán, M. G., Carissimi, G., Fuster, M. G., Víllora, G., & García, G. (2020). p-Cymene Complexes of Ruthenium(II) as Antitumor Agents. Molecules, 25(21), 5063. https://doi.org/10.3390/molecules25215063








