Modeling of Chemoperfusion vs. Intravenous Administration of Cisplatin in Wistar Rats: Adsorption and Tissue Distribution
Abstract
1. Introduction
2. Results
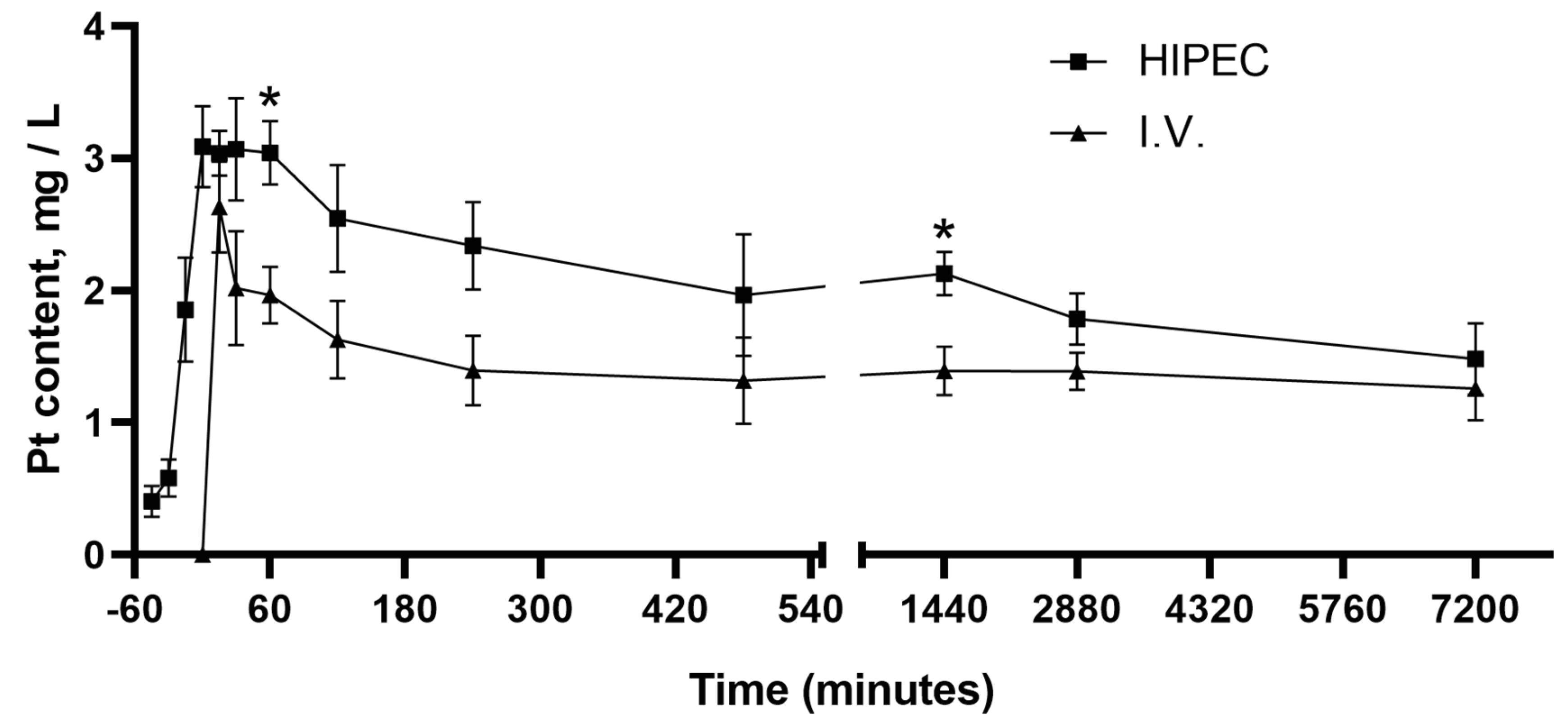
2.1. Pharmacokinetics of Cisplatin in HIPEC Compared to i.v. Administration
2.1.1. Pharmacokinetics Curves for HIPEC vs. i.v. Administration
2.1.2. Recovery of Perfusate
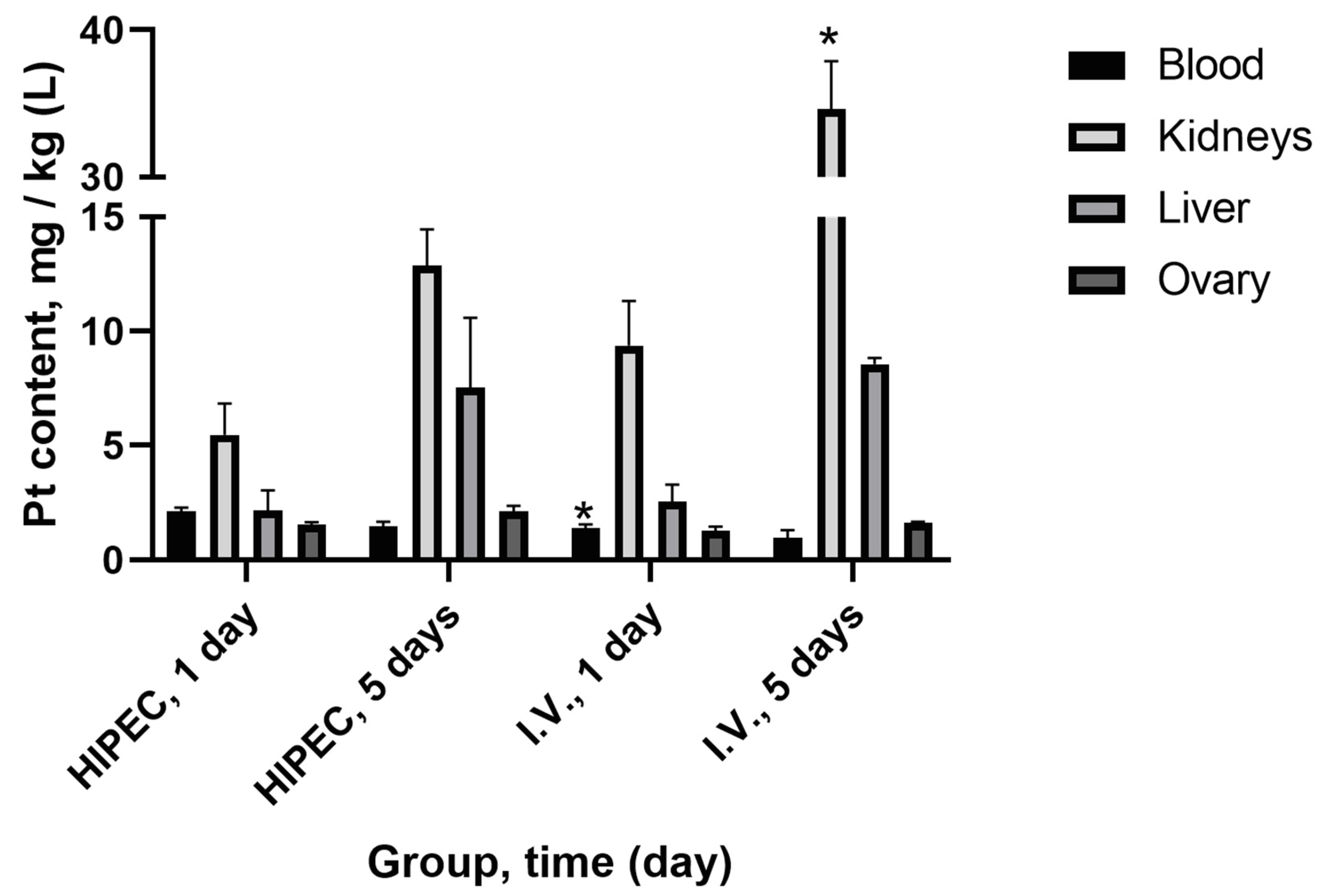
2.1.3. Platinum Distribution in Organs and Tissue
2.2. Ultrafiltration
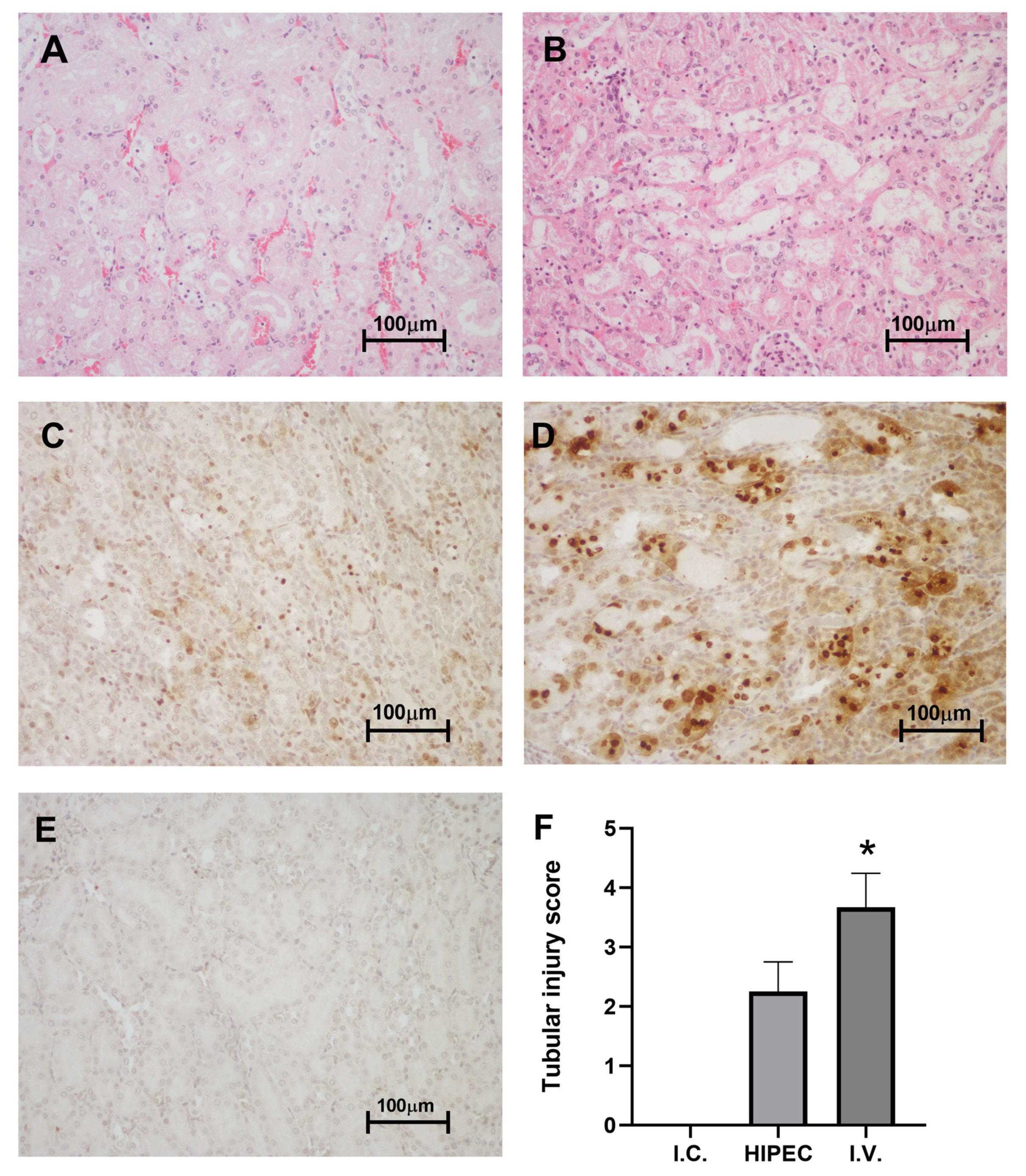
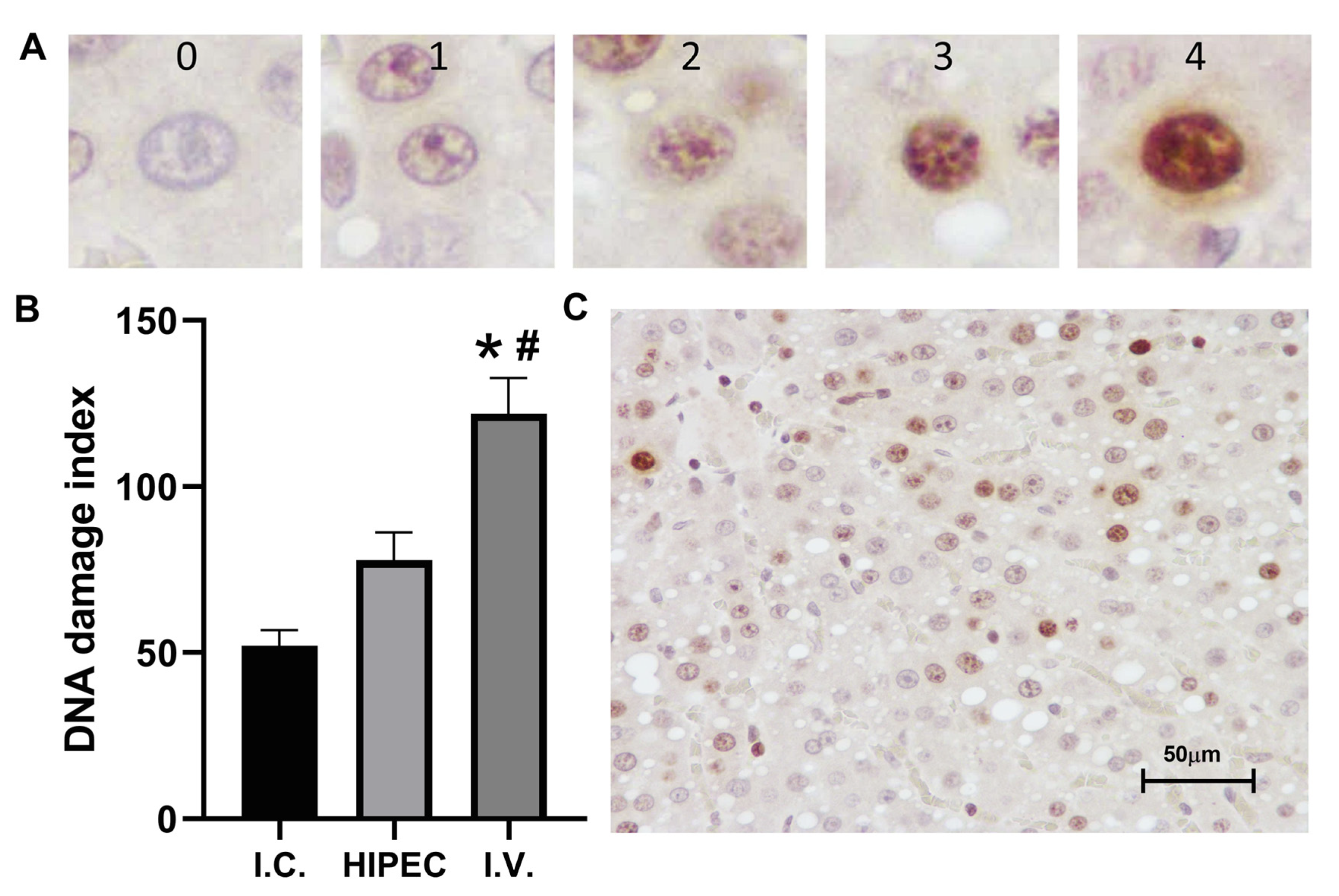
2.3. Histology and Immunohistochemistry
2.3.1. Kidney
2.3.2. Liver
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Study Design
4.3. Cisplatin Administration
4.3.1. Chemoperfusion
4.3.2. Intravenous Injection
4.4. Samples and Sample Handling
4.4.1. Blood Sampling
4.4.2. Blood Ultrafiltrate for ‘Free’ Platinum Assay
4.4.3. Platinum-Containing Perfusate
4.4.4. Organs for Platinum Quantification
4.4.5. Samples for Pathological Examination
4.5. Quantification of Platinum
4.5.1. Total Platinum Quantification in Blood and Tissue
4.5.2. Ultrafiltrable Platinum Quantification
4.6. Histology and Immunohistochemistry
4.6.1. Pathological Examination
4.6.2. Immunohistochemical Anti-H2AX Staining
4.7. Statistics
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Parameter | WBC 109/L | Lymph 109/L | Mon 109/L | Gran 109/L | RBC 1012/L | HGB g/L | PLT 109/L |
|---|---|---|---|---|---|---|---|
| I.C. | 16.8 ± 0.7 | 12.6 ± 1.1 | 0.50 ± 0.06 | 3.70 ± 0.78 | 9.36 ± 0.05 | 157.3 ± 4.3 | 905.3 ± 324.1 |
| HIPEC | 12.2 ± 2.0 | 8.3 ± 1.0 * | 0.37 ± 0.09 | 3.57 ± 0.92 | 7.44 ± 0.16 * | 129.7 ± 3.9 | 591.7 ± 257.3 |
| I.V. | 11.5 ± 1.5 | 8.2 ± 1.1 * | 0.27 ± 0.03* | 3.00 ± 0.38 | 7.07 ± 0.34 * | 124.7 ± 10.8 | 524.7 ± 300.2 |
References
- Takahara, P.M.; Frederick, C.A.; Lippard, S.J. Crystal structure of the anticancer drug cisplatin bound to duplex DNA. J. Am. Chem. Soc. 1996, 118, 12309–12321. [Google Scholar] [CrossRef]
- Zayed, A.; Jones, G.D.D.; Reid, H.J.; Shoeib, T.; Taylor, S.E.; Thomas, A.L.; Wood, J.P.; Sharp, B.L. Speciation of oxaliplatin adducts with DNA nucleotides. Metallomics 2011, 3, 991–1000. [Google Scholar] [CrossRef]
- Rosenberg, B.; Vancamp, L.; Trosko, J.E.; Mansour, V.H. Platinum compounds—A new class of potent antitumour agents. Nature 1969, 222, 385–386. [Google Scholar] [CrossRef]
- Dilruba, S.; Kalayda, G.V. Platinum-based drugs: Past, present and future. Cancer Chemother Pharm. 2016, 77, 1103–1124. [Google Scholar] [CrossRef]
- Michalke, B. Platinum speciation used for elucidating activation or inhibition of Pt-containing anti-cancer drugs. J. Trace Elem. Med. Biol. 2010, 24, 69–77. [Google Scholar] [CrossRef]
- Todd, R.C.; Lippard, S.J. Inhibition of transcription by platinum antitumor compounds. Metallomics 2009, 1, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Itamochi, H.; Kigawa, J. Nedaplatin: A cisplatin derivative in cancer chemotherapy. Cancer Manag. Res. 2013, 5, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Greaves, E.D.; Angeli-Greaves, M.; Jaehde, U.; Drescher, A.; von Bohlen, A. Rapid determination of platinum plasma concentrations of chemotherapy patients using total reflection X-ray fluorescence. Spectrochim Acta B. 2006, 61, 1194–1200. [Google Scholar] [CrossRef]
- Piccolini, V.M.; Bottone, M.G.; Bottiroli, G.; De Pascali, S.A.; Fanizzi, F.P.; Bernocchi, G. Platinum drugs and neurotoxicity: Effects on intracellular calcium homeostasis. Cell Biol. Toxicol. 2013, 29, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Marsili, S.; Vitale, I.; Senovilla, L.; Michels, J.; Garcia, P.; Vacchelli, E.; Chatelut, E.; Castedo, M.; Kroemer, G. Vitamin B6 metabolism influences the intracellular accumulation of cisplatin. Cell Cycle 2013, 12, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Marrache, S.; Pathak, R.K.; Dhar, S. Detouring of cisplatin to access mitochondrial genome for overcoming resistance. Proc. Natl. Acad. Sci. USA 2014, 111, 10444–10449. [Google Scholar] [CrossRef] [PubMed]
- Auer, R.C.; Sivajohanathan, D.; Biagi, J.; Conner, J.; Kennedy, E.; May, T. Indications for hyperthermic intraperitoneal chemotherapy with cytoreductive surgery: A systematic review. Eur. J. Cancer 2020, 127, 76–95. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.L.; Morris, D.L.; Rao, A.; Chua, T.C. Intraperitoneal chemotherapy in ovarian cancer: A review of tolerance and efficacy. Cancer Manag. Res. 2012, 4, 413–422. [Google Scholar] [CrossRef]
- Cristea, M.; Han, E.; Salmon, L.; Morgan, R.J., Jr. Review: Practical considerations in ovarian cancer chemotherapy. Ther. Adv. Med Oncol. 2010, 2, 175–187. [Google Scholar] [CrossRef] [PubMed]
- van Rhoon, G.C.; Franckena, M.; ten Hagen, T.L.M. A moderate thermal dose is sufficient for effective free and TSL based thermochemotherapy. Adv. Drug Deliv. Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Helm, C.W. The role of hyperthermic intraperitoneal chemotherapy (HIPEC) in ovarian cancer. Oncologist 2009, 14, 683–694. [Google Scholar] [CrossRef]
- Badgwell, B.; Blum, M.; Das, P.; Estrella, J.; Wang, X.; Ho, L.; Fournier, K.; Royal, R.; Mansfield, P.; Ajani, J. Phase II trial of laparoscopic hyperthermic intraperitoneal chemoperfusion for peritoneal carcinomatosis or positive peritoneal cytology in patients with gastric adenocarcinoma. Ann. Surg. Oncol. 2017, 24, 3338–3344. [Google Scholar] [CrossRef]
- Rizvi, S.A.; Syed, W.; Shergill, R. Approach to pseudomyxoma peritonei. World J. Gastrointest Surg. 2018, 10, 49–56. [Google Scholar] [CrossRef]
- Enomoto, L.M.; Shen, P.; Levine, E.A.; Votanopoulos, K.I. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal mesothelioma: Patient selection and special considerations. Cancer Manag. Res. 2019, 11, 4231–4241. [Google Scholar] [CrossRef]
- van Driel, W.J.; Koole, S.N.; Sikorska, K.; Schagen van Leeuwen, J.H.; Schreuder, H.W.R.; Hermans, R.H.M.; de Hingh, I.H.J.T.; van der Velden, J.; Arts, H.J.; Massuger, L.F.A.G.; et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef]
- Desiderio, J.; Chao, J.; Melstrom, L.; Warner, S.; Tozzi, F.; Fong, Y.; Parisi, A.; Woo, Y. The 30-year experience—A meta-analysis of randomised and high-quality non-randomised studies of hyperthermic intraperitoneal chemotherapy in the treatment of gastric cancer. Eur. J. Cancer 2017, 79, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Vermorken, J.B.; van Dam, P.; Brand, A. HIPEC in advanced epithelial ovarian cancer: Why is there controversy? Curr. Opin. Oncol. 2020, 32, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Bespalov, V.G.; Kireeva, G.S.; Belyaeva, O.A.; Kalinin, O.E.; Senchik, K.Y.; Stukov, A.N.; Gafton, G.I.; Guseynov, K.D.; Belyaev, A.M. Both heat and new chemotherapeutic drug dioxadet in hyperthermic intraperitoneal chemoperfusion improved survival in rat ovarian cancer model. J. Surg. Oncol. 2016, 113, 438–442. [Google Scholar] [CrossRef] [PubMed]
- González-Moreno, S.; González-Bayón, L.A.; Ortega-Pérez, G. Hyperthermic intraperitoneal chemotherapy: Rationale and technique. World J. Gastrointest Oncol. 2010, 2, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Fagotti, A.; Petrillo, M.; Costantini, B.; Fanfani, F.; Gallotta, V.; Chiantera, V.; Turco, L.C.; Bottoni, C.; Scambia, G. Minimally invasive secondary cytoreduction plus HIPEC for recurrent ovarian cancer: A case series. Gynecol. Oncol. 2014, 132, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Ansaloni, L.; Coccolini, F.; Morosi, L.; Ballerini, A.; Ceresoli, M.; Grosso, G.; Bertoli, P.; Busci, L.M.; Lotti, M.; Cambria, F.; et al. Pharmacokinetics of concomitant cisplatin and paclitaxel administered by hyperthermic intraperitoneal chemotherapy to patients with peritoneal carcinomatosis from epithelial ovarian cancer. Br. J. Cancer 2015, 112, 306–312. [Google Scholar] [CrossRef]
- Deraco, M.; Baratti, D.; Laterza, B.; Balestra, M.R.; Mingrone, E.; Macrì, A.; Virzì, S.; Puccio, F.; Ravenda, P.S.; Kusamura, S. Advanced cytoreduction as surgical standard of care and hyperthermic intraperitoneal chemotherapy as promising treatment in epithelial ovarian cancer. Eur. J. Surg. Oncol. 2011, 37, 4–9. [Google Scholar] [CrossRef]
- Fujiwara, K.; Nagao, S.; Aotani, E.; Hasegawa, K. Principle and evolving role of intraperitoneal chemotherapy in ovarian cancer. Expert Opin. Pharmacother. 2013, 14, 1797–1806. [Google Scholar] [CrossRef]
- Markman, M.; Walker, J.L. Intraperitoneal Chemotherapy of Ovarian Cancer: A Review, with a Focus on Practical Aspects of Treatment. J. Clin. Oncol. 2006, 24, 988–994. [Google Scholar] [CrossRef]
- Lemoine, L.; Sugarbaker, P.; Van der Speeten, K. Drugs, doses, and durations of intraperitoneal chemotherapy: Standardising HIPEC and EPIC for colorectal, appendiceal, gastric, ovarian peritoneal surface malignancies and peritoneal mesothelioma. Int. J. Hyperth. 2017, 33, 582–592. [Google Scholar] [CrossRef]
- Di Giorgio, A.; Naticchioni, E.; Biacchi, D.; Sibio, S.; Accarpio, F.; Rocco, M.; Tarquini, S.; Di Seri, M.; Ciardi, A.; Montruccoli, D.; et al. Cytoreductive surgery (peritonectomy procedures) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in the treatment of diffuse peritoneal carcinomatosis from ovarian cancer. Cancer 2008, 113, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Goodman, M.D.; McPartland, S.; Detelich, D.; Saif, M.W. Chemotherapy for intraperitoneal use: A review of hyperthermic intraperitoneal chemotherapy and early post-operative intraperitoneal chemotherapy. J. Gastrointest Oncol. 2016, 7, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Urano, M.; Ling, C.C. Thermal enhancement of melphalan and oxaliplatin cytotoxicity in vitro. Int. J. Hyperth. 2002, 18, 307–315. [Google Scholar] [CrossRef]
- Helderman, R.; Löke, D.R.; Verhoeff, J.; Rodermond, H.M.; van Bochove, G.G.W.; Boon, M.; van Kesteren, S.; Garcia Vallejo, J.J.; Kok, H.P.; Tanis, P.J.; et al. The temperature-dependent effectiveness of platinum-based drugs mitomycin-c and 5-fu during hyperthermic intraperitoneal chemotherapy (HIPEC) in colorectal cancer cell lines. Cells 2020, 9, 1775. [Google Scholar] [CrossRef]
- Xu, M.J.; Alberts, D.S. Potentiation of platinum analogue cytotoxicity by hyperthermia. Cancer Chemother Pharm. 1988, 21, 191–196. [Google Scholar] [CrossRef]
- Muller, M.; Chérel, M.; Dupré, P.F.; Gouard, S.; Collet, M.; Classe, J.M. Cytotoxic effect of hyperthermia and chemotherapy with platinum salt on ovarian cancer cells: Results of an in vitro study. Eur. Surg. Res. 2011, 46, 139–147. [Google Scholar] [CrossRef]
- Piché, N.; Leblond, F.A.; Sidéris, L.; Pichette, V.; Drolet, P.; Fortier, L.P.; Mitchell, A.; Dubé, P. Rationale for heating oxaliplatin for the intraperitoneal treatment of peritoneal carcinomatosis: A study of the effect of heat on intraperitoneal oxaliplatin using a murine model. Ann. Surg. 2011, 254, 138–144. [Google Scholar] [CrossRef]
- Zeamari, S.; Floot, B.; van der Vange, N.; Stewart, F.A. Pharmacokinetics and pharmacodynamics of cisplatin after intraoperative hyperthermic intraperitoneal chemoperfusion (HIPEC). Anticancer Res. 2003, 23, 1643–1648. [Google Scholar]
- Ausmus, P.L.; Wilke, A.V.; Frazier, D.L. Effects of hyperthermia on blood flow and cis-diamminedichloroplatinum(II) pharmacokinetics in murine mammary adenocarcinomas. Cancer Res. 1992, 52, 4965–4968. [Google Scholar] [PubMed]
- Facy, O.; Radais, F.; Ladoire, S.; Delroeux, D.; Tixier, H.; Ghiringhelli, F.; Rat, P.; Chauffert, B.; Ortega-Deballon, P. Comparison of hyperthermia and adrenaline to enhance the intratumoral accumulation of cisplatin in a murin model of peritoneal carcinomatosis. J. Exp. Clin. Cancer Res. 2011, 30, 4. [Google Scholar] [CrossRef]
- Johnsson, A.; Olsson, C.; Nygren, O.; Nilsson, M.; Seiving, B.; Cavallin-Stahl, E. Pharmacokinetics and tissue distribution of cisplatin in nude mice: Platinum levels and cisplatin-DNA adducts. Cancer Chemother. Pharmacol. 1995, 37, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Whitlam, J.B.; Brown, K.F. Ultrafiltration in serum protein binding determinations. J. Pharm. Sci. 1981, 70, 146–150. [Google Scholar] [CrossRef] [PubMed]
- van der Vijgh, W.J.F.; Klein, I. Protein binding of five platinum compounds. Cancer Chemother. Pharmacol. 1986, 18, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Gamelin, E.; Boisdron-Celle, M.; Lebouil, A.; Turcant, A.; Cailleux, A.; Krikorian, A.; Brienza, S.; Cvitkovic, E.; Larra, F.; Robert, J.; et al. Determination of unbound platinum after oxaliplatin administration: Comparison of currently available methods and influence of various parameters. Anti-Cancer Drugs 1998, 9, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Solovyev, N.; Ala, A.; Schilsky, M.; Mills, C.; Willis, K.; Harrington, C.F. Biomedical copper speciation in relation to Wilson’s disease using strong anion exchange chromatography coupled to triple quadrupole inductively coupled plasma mass spectrometry. Anal. Chim. Acta 2020, 1098, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Gu, J. Analytical Morphology. Theory, Applications and Protocols; Birkhäuser Basel: Basel, Switzerland, 1997. [Google Scholar]
- Navolotskii, D.V.; Ivanenko, N.B.; Solovyev, N.D.; Fedoros, E.I.; Panchenko, A.V. Pharmacokinetics and tissue distribution of novel platinum containing anticancer agent BP-C1 studied in rabbits using sector field inductively coupled plasma mass spectrometry. Drug Test. Anal. 2015, 7, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Solovyev, N.D.; Fedoros, E.I.; Drobyshev, E.J.; Ivanenko, N.B.; Pigarev, S.E.; Tyndyk, M.L.; Anisimov, V.N.; Vilpan, Y.A.; Panchenko, A.V. Anticancer activity and tissue distribution of platinum (II) complex with lignin-derived polymer of benzene-poly-carboxylic acids. J. Trace Elem. Med. Biol. 2017, 43, 72–79. [Google Scholar] [CrossRef]
- Marnett, L.J.; Plastaras, J.P. Endogenous DNA damage and mutation. Trends Genet. 2001, 17, 214–221. [Google Scholar] [CrossRef]
| Body Weight, g | Volume of Perfusate, mL | Initial Pt in Perfusate, mg/L | Final Pt in Perfusate, mg/L | Retained Pt in Rats, mg/kg | |
|---|---|---|---|---|---|
| Average | 250.00 | 93.67 | 34.75 | 21.28 | 5.01 |
| SD | 17.00 | 7.78 | 2.16 | 1.23 | 0.44 |
| Injection Route/Dose | HIPEC, Cisplatin 20 mg/kg b.w. | i.v., Cisplatin 4 mg/kg b.w. |
|---|---|---|
| Blood Pt conc., mg/L ± SEM | 2666 ± 266 | 1051 ± 80 |
| Plasma Pt conc., mg/L ± SEM | 2653 ± 277 | 972.9 ± 45.9 |
| Filtrate Pt conc., mg/L ± SEM | 1310 ± 117 | 682.1 ± 21.3 |
| Permeability, % ± SEM | 53.08 ± 0.92 | 73.76 ± 1.32 * |
| Binding, % ± SEM | 46.92 ± 0.92 | 26.24 ± 1.32 * |
| Model Solution Used | Cisplatin, 40 mg/L | Cisplatin, 7.5 mg/L |
|---|---|---|
| Initial solution Pt conc., mg/L ± SEM | 25.49 ± 0.33 | 4.89 ± 0.07 |
| Filtrate Pt conc., mg/L ± SEM | 24.90 ± 0.09 | 4.70 ± 0.05 |
| Recovery, % ± SEM | 97.70 ± 0.92 | 96.46 ± 0.29 |
Sample Availability: In the current study, commercially available drugs were tested, which are freely available on the market at the time of publication. The lot number is provided. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kireeva, G.; Kruglov, S.; Maydin, M.; Gubareva, E.; Fedoros, E.; Zubakina, E.; Ivanenko, N.; Bezruchko, M.; Solovyev, N. Modeling of Chemoperfusion vs. Intravenous Administration of Cisplatin in Wistar Rats: Adsorption and Tissue Distribution. Molecules 2020, 25, 4733. https://doi.org/10.3390/molecules25204733
Kireeva G, Kruglov S, Maydin M, Gubareva E, Fedoros E, Zubakina E, Ivanenko N, Bezruchko M, Solovyev N. Modeling of Chemoperfusion vs. Intravenous Administration of Cisplatin in Wistar Rats: Adsorption and Tissue Distribution. Molecules. 2020; 25(20):4733. https://doi.org/10.3390/molecules25204733
Chicago/Turabian StyleKireeva, Galina, Stepan Kruglov, Mikhail Maydin, Ekaterina Gubareva, Elena Fedoros, Ekaterina Zubakina, Natalya Ivanenko, Marina Bezruchko, and Nikolay Solovyev. 2020. "Modeling of Chemoperfusion vs. Intravenous Administration of Cisplatin in Wistar Rats: Adsorption and Tissue Distribution" Molecules 25, no. 20: 4733. https://doi.org/10.3390/molecules25204733
APA StyleKireeva, G., Kruglov, S., Maydin, M., Gubareva, E., Fedoros, E., Zubakina, E., Ivanenko, N., Bezruchko, M., & Solovyev, N. (2020). Modeling of Chemoperfusion vs. Intravenous Administration of Cisplatin in Wistar Rats: Adsorption and Tissue Distribution. Molecules, 25(20), 4733. https://doi.org/10.3390/molecules25204733







