Antiviral and Immunomodulatory Effects of Phytochemicals from Honey against COVID-19: Potential Mechanisms of Action and Future Directions
Abstract
1. Introduction
2. The Medicinal Properties of Honey
2.1. Honey as an Immune System Booster
2.2. The Antiviral Activity of Honey
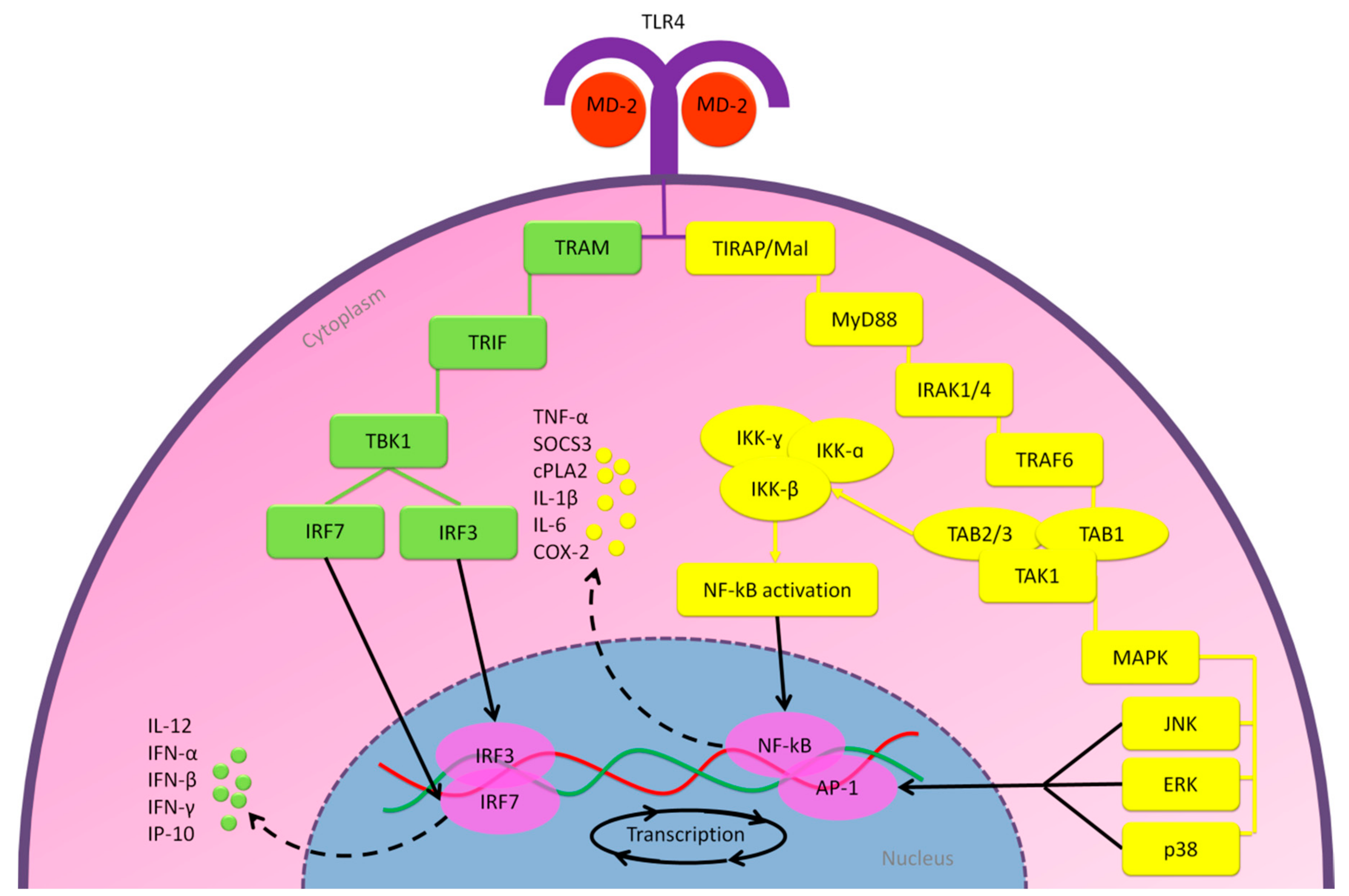
2.2.1. MD-2/TLR4 Pathway
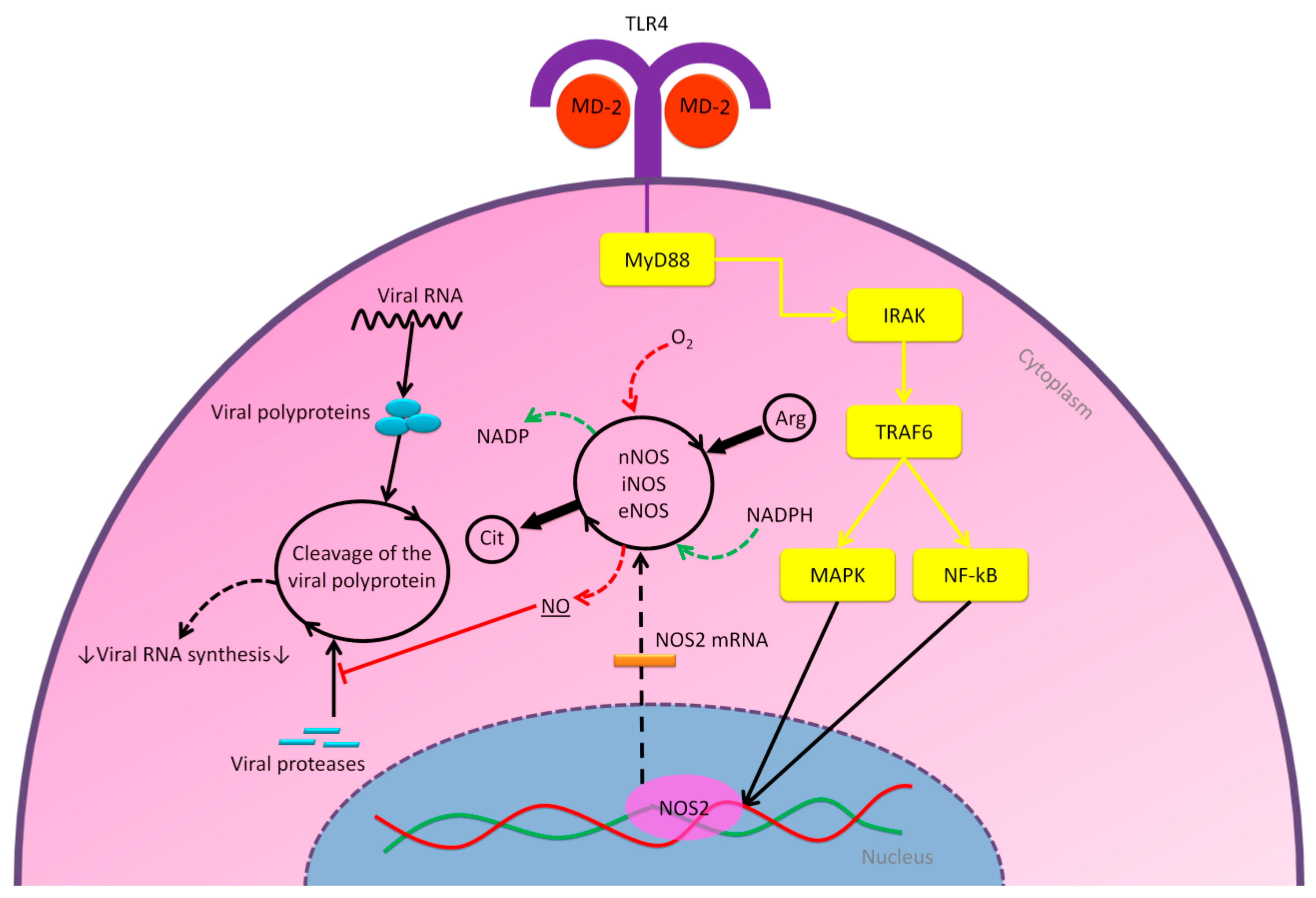
2.2.2. Nitric Oxide Pathway
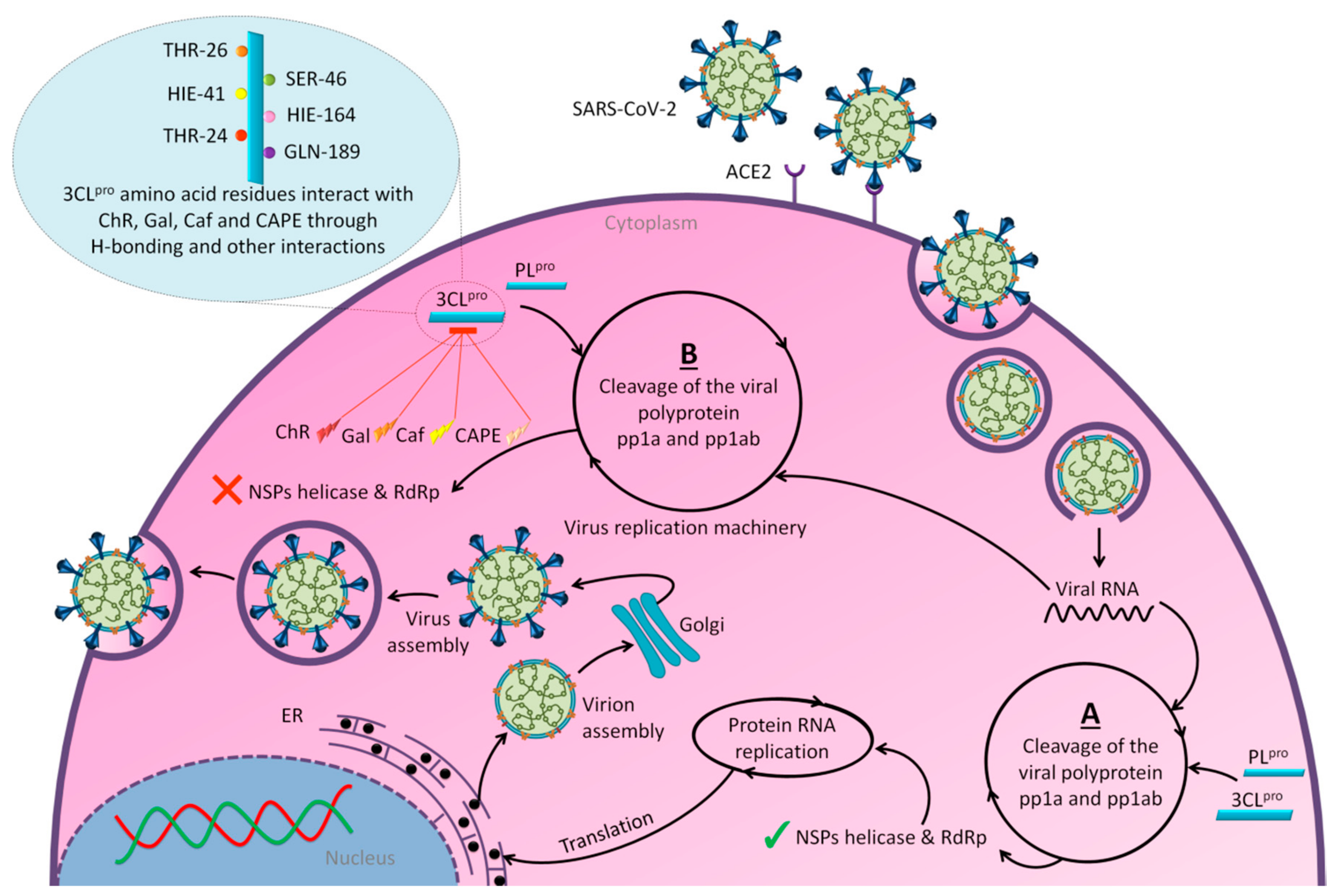
3. Promising Insights for Honey Research amid COVID-19 Outbreak
4. Future Directions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO Timeline—COVID-19. Available online: https://www.who.int/news-room/detail/27-04-2020-who-timeline---covid-19 (accessed on 7 June 2020).
- Coronavirus Disease (COVID-2019) Situation Reports. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed on 14 September 2020).
- Wertheim, J.O.; Chu, D.K.; Peiris, J.S.; Kosakovsky Pond, S.L.; Poon, L.L. A case for the ancient origin of coronaviruses. J. Virol. 2013, 87, 7039–7045. [Google Scholar] [CrossRef] [PubMed]
- Estola, T. Coronaviruses, a new group of animal RNA viruses. Avian Dis. 1970, 14, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Kahn, J.S.; McIntosh, K. History and recent advances in coronavirus discovery. Pediatr. Infect. Dis. J. 2005, 24, S223–227, discussion S226. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, C.S.; Tatti, K.M.; Ksiazek, T.G.; Rollin, P.E.; Comer, J.A.; Lee, W.W.; Rota, P.A.; Bankamp, B.; Bellini, W.J.; Zaki, S.R. Ultrastructural characterization of SARS coronavirus. Emerg. Infect. Dis. 2004, 10, 320–326. [Google Scholar] [CrossRef] [PubMed]
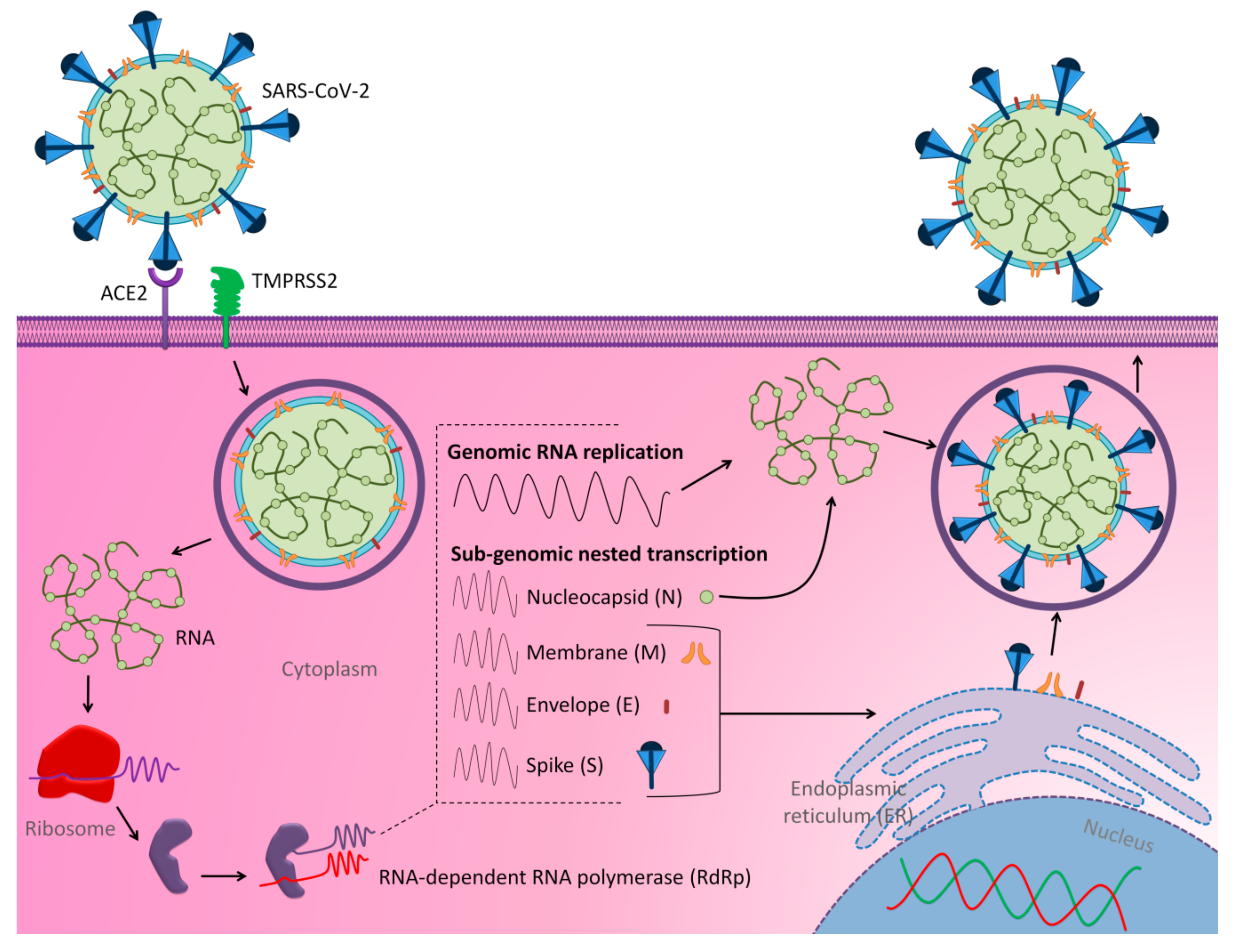
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. In Coronaviruses; Maier, H.J.B., Erica Britton, P., Eds.; Springer: New York, NY, USA, 2015; Volume 1282, pp. 1–23. [Google Scholar]
- Masters, P.S. The molecular biology of coronaviruses. Adv. Virus Res. 2006, 66, 193–292. [Google Scholar] [CrossRef] [PubMed]
- Coronavirus Disease (COVID-19): What Parents Should Know. Available online: https://www.unicef.org/northmacedonia/coronavirus-disease-covid-19-what-parents-should-know (accessed on 3 May 2020).
- Astuti, I.; Ysrafil. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): An overview of viral structure and host response. Diabetes Metab. Syndr. 2020, 14, 407–412. [Google Scholar] [CrossRef]
- Muus, C.; Luecken, M.D.; Eraslan, G.; Waghray, A.; Heimberg, G.; Sikkema, L.; Kobayashi, Y.; Vaishnav, E.D.; Subramanian, A.; Smilie, C.; et al. Integrated analyses of single-cell atlases reveal age, gender, and smoking status associations with cell type-specific expression of mediators of SARS-CoV-2 viral entry and highlights inflammatory programs in putative target cells. bioRxiv 2020. [Google Scholar] [CrossRef]
- Snijder, E.J.; Decroly, E.; Ziebuhr, J. The Nonstructural Proteins Directing Coronavirus RNA Synthesis and Processing. Adv. Virus Res. 2016, 96, 59–126. [Google Scholar] [CrossRef]
- Sola, I.; Almazan, F.; Zuniga, S.; Enjuanes, L. Continuous and discontinuous RNA synthesis in coronaviruses. Ann. Rev. Virol. 2015, 2, 265–288. [Google Scholar] [CrossRef]
- Xia, S.; Liu, M.; Wang, C.; Xu, W.; Lan, Q.; Feng, S.; Qi, F.; Bao, L.; Du, L.; Liu, S. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020, 30, 343–355. [Google Scholar] [CrossRef]
- Coutard, B.; Valle, C.; de Lamballerie, X.; Canard, B.; Seidah, N.; Decroly, E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antivir. Res. 2020, 176, 104742. [Google Scholar] [CrossRef] [PubMed]
- Malik, Y.A. Properties of Coronavirus and SARS-CoV-2. Malays. J. Pathol. 2020, 42, 3–11. [Google Scholar] [PubMed]
- Wu, J.; Liu, J.; Zhao, X.; Liu, C.; Wang, W.; Wang, D.; Xu, W.; Zhang, C.; Yu, J.; Jiang, B.; et al. Clinical Characteristics of Imported Cases of COVID-19 in Jiangsu Province: A Multicenter Descriptive Study. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Chen, T.; Dai, Z.; Mo, P.; Li, X.; Ma, Z.; Song, S.; Chen, X.; Luo, M.; Liang, K.; Gao, S.; et al. Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID-19) in Wuhan, China (2019): A single-centered, retrospective study. J. Gerontol. A Biol. Sci. Med. Sci. 2020. [Google Scholar] [CrossRef]
- Q&A on Coronaviruses (COVID-19). Available online: https://www.who.int/news-room/q-a-detail/q-a-coronaviruses (accessed on 3 May 2020).
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- The, L.I.D. Challenges of coronavirus disease 2019. Lancet Infect. Dis. 2020, 20, 261. [Google Scholar]
- Lu, H. Drug treatment options for the 2019-new coronavirus (2019-nCoV). Biosci. Trends 2020, 14, 69–71. [Google Scholar] [CrossRef]
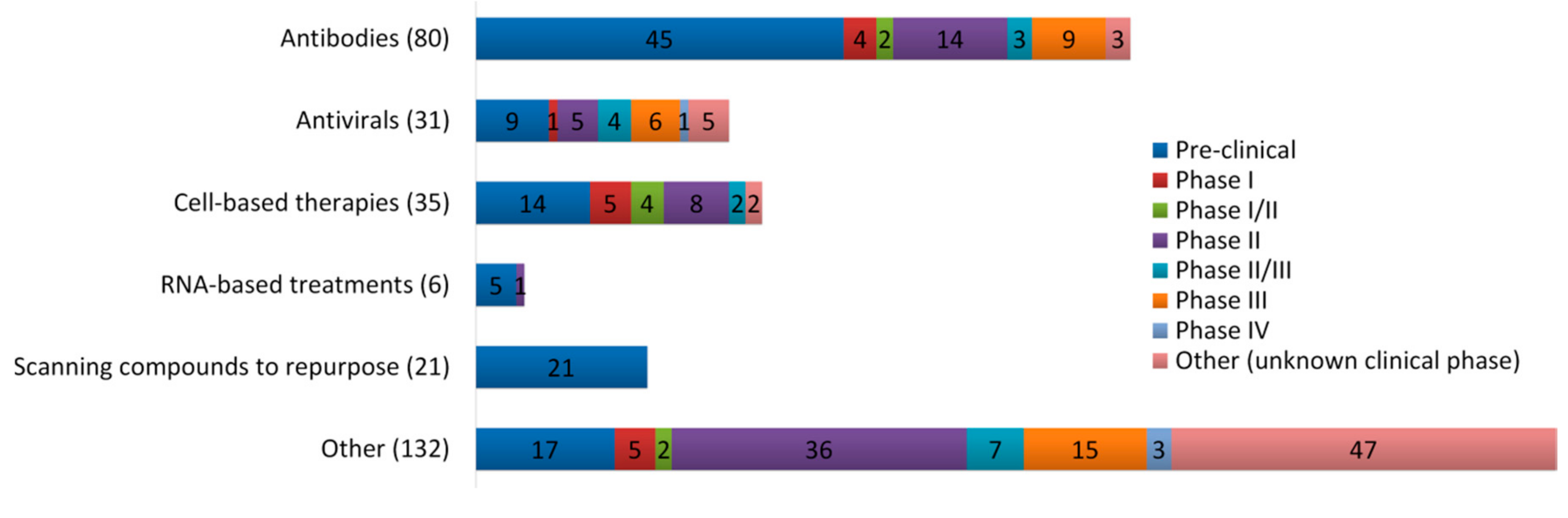
- COVID-19 Treatment and Vaccine Tracker. Available online: https://covid-19tracker.milkeninstitute.org/#vaccines_intro (accessed on 7 September 2020).
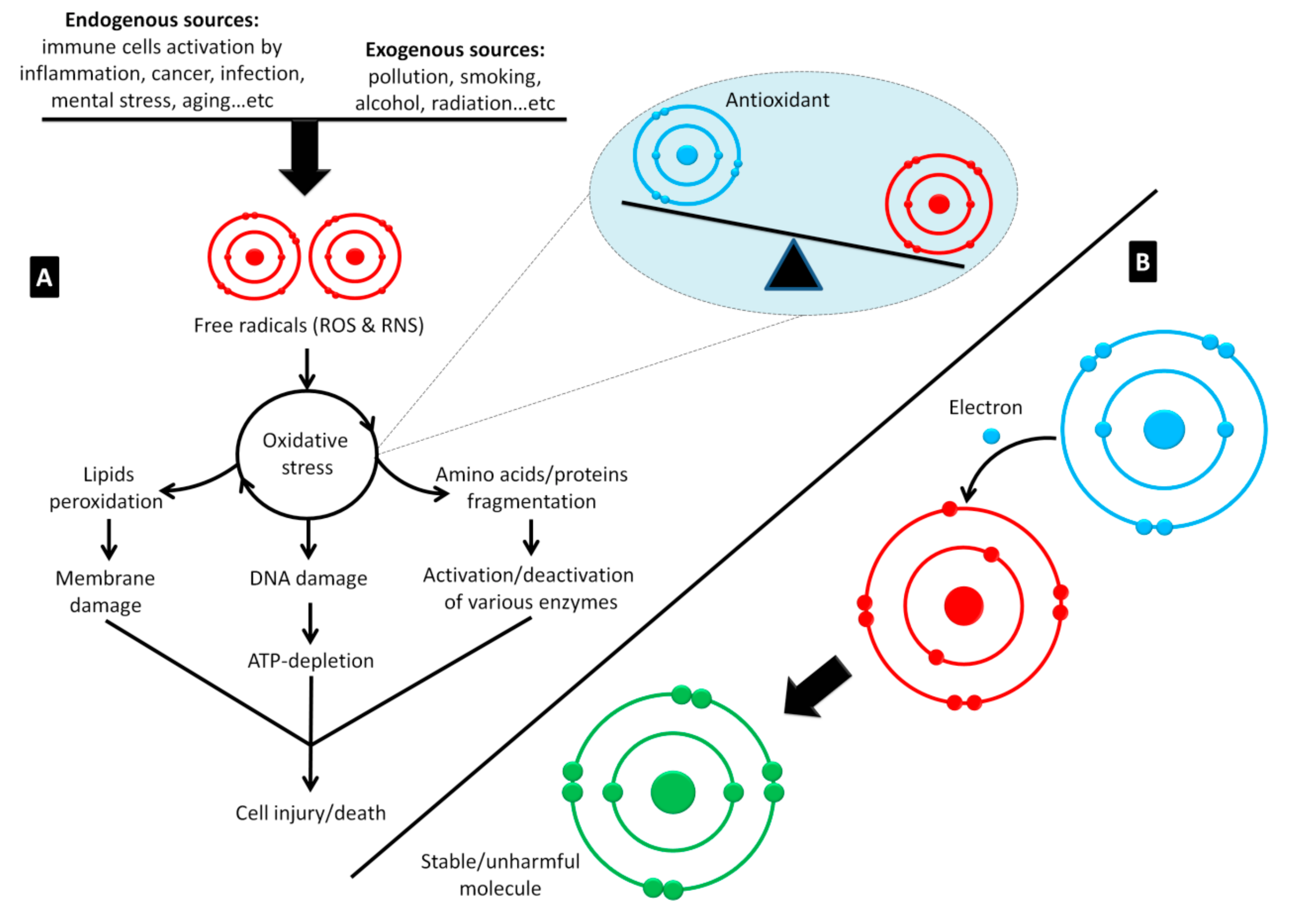
- Ahmed, S.; Sulaiman, S.A.; Baig, A.A.; Ibrahim, M.; Liaqat, S.; Fatima, S.; Jabeen, S.; Shamim, N.; Othman, N.H. Honey as a Potential Natural Antioxidant Medicine: An Insight into Its Molecular Mechanisms of Action. Oxid. Med. Cell. Longev. 2018, 2018, 8367846. [Google Scholar] [CrossRef]
- Khan, S.U.; Anjum, S.I.; Rahman, K.; Ansari, M.J.; Khan, W.U.; Kamal, S.; Khattak, B.; Muhammad, A.; Khan, H.U. Honey: Single food stuff comprises many drugs. Saudi J. Biol. Sci. 2018, 25, 320–325. [Google Scholar] [CrossRef]
- Adeleye, I.A.; Opiah, L. Antimicrobial activity of extracts of local cough mixtures on upper respiratory tract bacterial pathogens. West Indian Med. J. 2003, 52, 188–190. [Google Scholar]
- Hashemipour, M.A.; Tavakolineghad, Z.; Arabzadeh, S.A.; Iranmanesh, Z.; Nassab, S.A. Antiviral Activities of Honey, Royal Jelly, and Acyclovir Against HSV-1. Wounds 2014, 26, 47–54. [Google Scholar] [PubMed]
- Shahzad, A.; Cohrs, R.J. In vitro antiviral activity of honey against varicella zoster virus (VZV): A translational medicine study for potential remedy for shingles. Transl. Biomed. 2012, 3. [Google Scholar] [CrossRef]
- Watanabe, K.; Rahmasari, R.; Matsunaga, A.; Haruyama, T.; Kobayashi, N. Anti-influenza viral effects of honey in vitro: Potent high activity of manuka honey. Arch. Med. Res. 2014, 45, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Esawy, M.A.; Ahmed, E.F.; Helmy, W.A.; Mansour, N.M.; El-Senousy, W.M.; El-Safty, M.M. Production of levansucrase from novel honey Bacillus subtilis isolates capable of producing antiviral levans. Carbohydr. Polym. 2011, 86, 823–830. [Google Scholar] [CrossRef]
- Zareie, P.P. Honey as an Antiviral Agent against Respiratory Syncytial Virus. Master’s Thesis, University of Waikato, Hamilton, New Zealand, 2011. [Google Scholar]
- Abuharfeil, N.; Al-Oran, R.; Abo-Shehada, M. The Effect of Bee Honey on the Proliferative Activity of Human B-and T-Lymphocytes and the Activity of Phagocytes. Food Agric. Immunol. 1999, 11, 169–177. [Google Scholar] [CrossRef]
- Tonks, A.J.; Cooper, R.A.; Jones, K.P.; Blair, S.; Parton, J.; Tonks, A. Honey stimulates inflammatory cytokine production from monocytes. Cytokine 2003, 21, 242–247. [Google Scholar] [CrossRef]
- Miguel, M.G.; Antunes, M.D.; Faleiro, M.L. Honey as a Complementary Medicine. Integr. Med. Insights 2017, 12. [Google Scholar] [CrossRef]
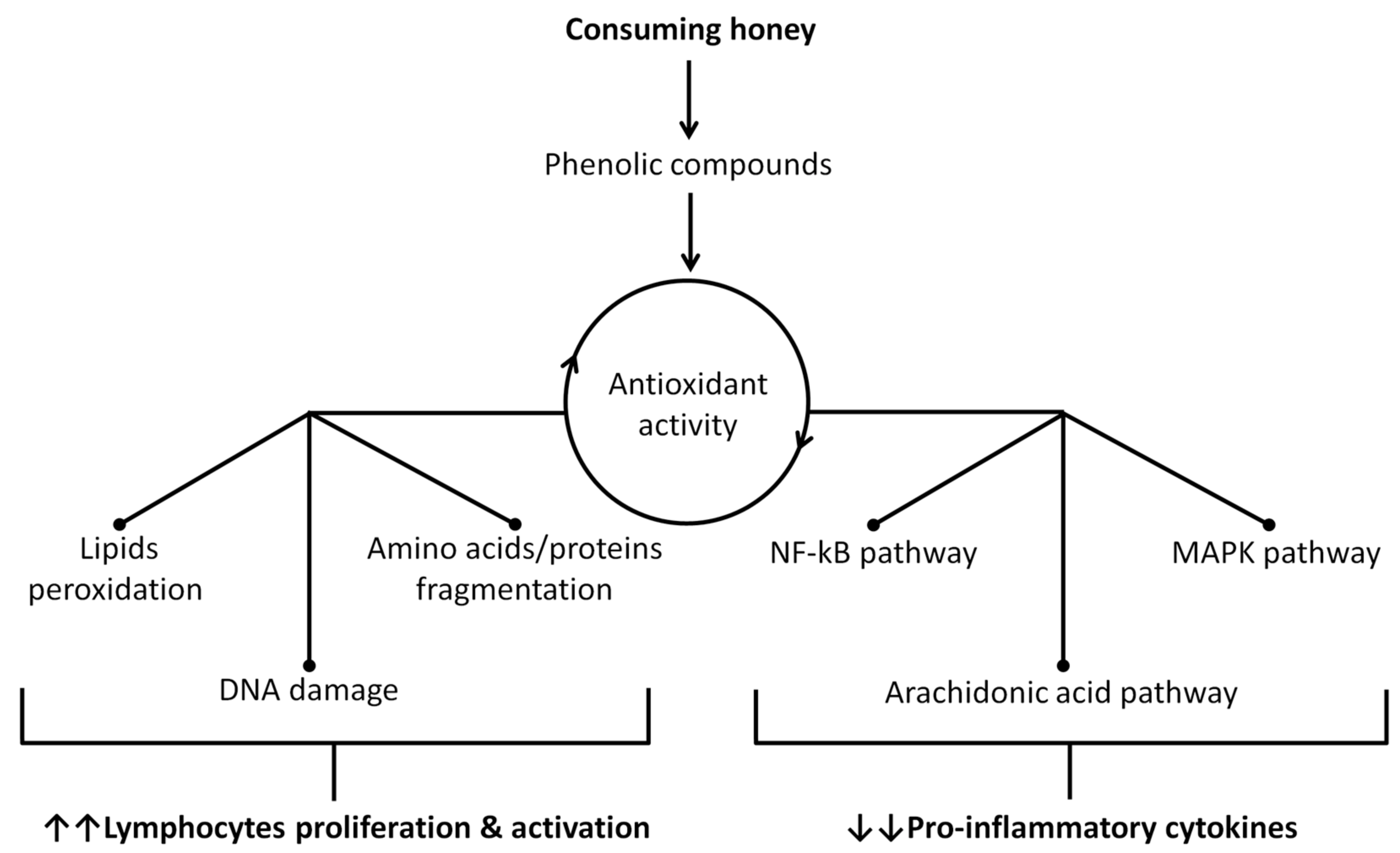
- Cianciosi, D.; Forbes-Hernandez, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Bravo Lamas, L.; Martinez Florez, S.; Agudo Toyos, P.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef]
- Al-Hatamleh, M.A.I.; Boer, J.C.; Wilson, K.L.; Plebanski, M.; Mohamud, R.; Mustafa, M.Z. Antioxidant-Based Medicinal Properties of Stingless Bee Products: Recent Progress and Future Directions. Biomolecules 2020, 10, 923. [Google Scholar] [CrossRef]
- Jibril, F.I.; Hilmi, A.B.M.; Manivannan, L. Isolation and characterization of polyphenols in natural honey for the treatment of human diseases. Bull. Natl. Res. Cent. 2019, 43, 4. [Google Scholar] [CrossRef]
- Wilczynska, A. Phenolic content and antioxidant activity of different types of polish honey-a short report. Polish J. Food Nutr. Sci. 2010, 60, 309–313. [Google Scholar]
- Walle, T. Absorption and metabolism of flavonoids. Free Radic. Biol. Med. 2004, 36, 829–837. [Google Scholar] [CrossRef]
- D’Archivio, M.; Filesi, C.; Vari, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef] [PubMed]
- Schramm, D.D.; Karim, M.; Schrader, H.R.; Holt, R.R.; Cardetti, M.; Keen, C.L. Honey with high levels of antioxidants can provide protection to healthy human subjects. J. Agric. Food Chem. 2003, 51, 1732–1735. [Google Scholar] [CrossRef]
- Spencer, J.P.; Chowrimootoo, G.; Choudhury, R.; Debnam, E.S.; Srai, S.K.; Rice-Evans, C. The small intestine can both absorb and glucuronidate luminal flavonoids. FEBS Lett. 1999, 458, 224–230. [Google Scholar] [CrossRef]
- Marin, L.; Miguelez, E.M.; Villar, C.J.; Lombo, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. Biomed Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef]
- Ranneh, Y.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A.; Mahmoud, A.M. Stingless bee honey protects against lipopolysaccharide induced-chronic subclinical systemic inflammation and oxidative stress by modulating Nrf2, NF-kappaB and p38 MAPK. Nutr. Metab. 2019, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- Kek, S.P.; Chin, N.L.; Yusof, Y.A.; Tan, S.W.; Chua, L.S. Total phenolic contents and colour intensity of Malaysian honeys from the Apis spp. and Trigona spp. bees. Agric. Agric. Sci. Procedia 2014, 2, 150–155. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Correction to: Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020. [Google Scholar] [CrossRef]
- Conti, P.; Ronconi, G.; Caraffa, A.; Gallenga, C.E.; Ross, R.; Frydas, I.; Kritas, S.K. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): Anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents 2020, 34. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Dzialo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The Potential of Plant Phenolics in Prevention and Therapy of Skin Disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef] [PubMed]
- Dharmaraja, A.T. Role of Reactive Oxygen Species (ROS) in Therapeutics and Drug Resistance in Cancer and Bacteria. J. Med. Chem. 2017, 60, 3221–3240. [Google Scholar] [CrossRef]
- Reinisalo, M.; Karlund, A.; Koskela, A.; Kaarniranta, K.; Karjalainen, R.O. Polyphenol Stilbenes: Molecular Mechanisms of Defence against Oxidative Stress and Aging-Related Diseases. Oxid. Med. Cell. Longev. 2015, 2015, 340520. [Google Scholar] [CrossRef]
- Albaridi, N.A. Antibacterial Potency of Honey. Int. J. Microbiol. 2019, 2019, 2464507. [Google Scholar] [CrossRef]
- Israili, Z.H. Antimicrobial properties of honey. Am. J. Ther. 2014, 21, 304–323. [Google Scholar] [CrossRef]
- Zeina, B.; Othman, O.; al-Assad, S. Effect of honey versus thyme on Rubella virus survival in vitro. J. Altern. Complement. Med. 1996, 2, 345–348. [Google Scholar] [CrossRef]
- Al-Waili, N.S. Topical honey application vs. acyclovir for the treatment of recurrent herpes simplex lesions. Med. Sci. Monit. 2004, 10, MT94–MT98. [Google Scholar]
- Vahed, H.; Jafri, S.; Jamil, N. Propagation of influenza virus in lymphocytes determine by antiviral effects of honey, ginger and garlic decoction. J. Antivir. Antiretrovir. 2016, 8, 12–19. [Google Scholar] [CrossRef]
- Al-Waili, N.S.; Al-Waili, T.N.; Al-Waili, A.N.; Saloom, K.S. Influence of natural honey on biochemical and hematological variables in AIDS: A case study. Sci. World J. 2006, 6, 1985–1989. [Google Scholar] [CrossRef]
- Heidari, A.; Zia, H.; Amiri, G.; Afsahi, S.; Sarahroodi, S. Has the natural raw honey any effect on HIV infection. Int. J. Pharm. Res. Biosci. 2012, 1, 205–210. [Google Scholar]
- Mahomoodally, M.F.; Ragoo, L.; Sreekeesoon, P.D.; Suroowan, S.; Khedoo, Z.M. The potential of complementary and alternative medicines in the management of HIV infection and related complications. Spatula DD 2013, 3, 127–140. [Google Scholar] [CrossRef]
- Wan Yusuf, W.N.; Wan Mohammad, W.M.Z.; Gan, S.H.; Mustafa, M.; Abd Aziz, C.B.; Sulaiman, S.A. Tualang honey ameliorates viral load, CD4 counts and improves quality of life in asymptomatic human immunodeficiency virus infected patients. J. Tradit. Complement. Med. 2019, 9, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; McGrath, M.S.; Xu, H. Inhibition of HIV Expression and Integration in Macrophages by Methylglyoxal-Bis-Guanylhydrazone. J. Virol. 2015, 89, 11176–11189. [Google Scholar] [CrossRef]
- Behbahani, M. Anti-HIV-1 activity of eight monofloral Iranian honey types. PLoS ONE 2014, 9, e108195. [Google Scholar] [CrossRef]
- Vasdev, S.; Stuckless, J. Role of methylglyoxal in essential hypertension. Int. J. Angiol. 2010, 19, e58–65. [Google Scholar] [CrossRef]
- Allaman, I.; Belanger, M.; Magistretti, P.J. Methylglyoxal, the dark side of glycolysis. Front. Neurosci. 2015, 9, 23. [Google Scholar] [CrossRef]
- Rabie, E.; Serem, J.C.; Oberholzer, H.M.; Gaspar, A.R.M.; Bester, M.J. How methylglyoxal kills bacteria: An ultrastructural study. Ultrastruct. Pathol. 2016, 40, 107–111. [Google Scholar] [CrossRef]
- Mukherjee, S.; Karmakar, S.; Babu, S.P. TLR2 and TLR4 mediated host immune responses in major infectious diseases: A review. Braz. J. Infect. Dis. 2016, 20, 193–204. [Google Scholar] [CrossRef]
- Vaure, C.; Liu, Y. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front. Immunol. 2014, 5, 316. [Google Scholar] [CrossRef] [PubMed]
- Mazgaeen, L.; Gurung, P. Recent Advances in Lipopolysaccharide Recognition Systems. Int. J. Mol. Sci. 2020, 21, 379. [Google Scholar] [CrossRef] [PubMed]
- Olejnik, J.; Hume, A.J.; Muhlberger, E. Toll-like receptor 4 in acute viral infection: Too much of a good thing. PLoS Pathog. 2018, 14, e1007390. [Google Scholar] [CrossRef]
- Okumura, A.; Pitha, P.M.; Yoshimura, A.; Harty, R.N. Interaction between Ebola virus glycoprotein and host toll-like receptor 4 leads to induction of proinflammatory cytokines and SOCS1. J. Virol. 2010, 84, 27–33. [Google Scholar] [CrossRef]
- Escudero-Perez, B.; Volchkova, V.A.; Dolnik, O.; Lawrence, P.; Volchkov, V.E. Shed GP of Ebola virus triggers immune activation and increased vascular permeability. PLoS Pathog. 2014, 10, e1004509. [Google Scholar] [CrossRef] [PubMed]
- Iampietro, M.; Younan, P.; Nishida, A.; Dutta, M.; Lubaki, N.M.; Santos, R.I.; Koup, R.A.; Katze, M.G.; Bukreyev, A. Ebola virus glycoprotein directly triggers T lymphocyte death despite of the lack of infection. PLoS Pathog. 2017, 13, e1006397. [Google Scholar] [CrossRef]
- Rallabhandi, P.; Phillips, R.L.; Boukhvalova, M.S.; Pletneva, L.M.; Shirey, K.A.; Gioannini, T.L.; Weiss, J.P.; Chow, J.C.; Hawkins, L.D.; Vogel, S.N.; et al. Respiratory syncytial virus fusion protein-induced toll-like receptor 4 (TLR4) signaling is inhibited by the TLR4 antagonists Rhodobacter sphaeroides lipopolysaccharide and eritoran (E5564) and requires direct interaction with MD-2. mBio 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Modhiran, N.; Watterson, D.; Muller, D.A.; Panetta, A.K.; Sester, D.P.; Liu, L.; Hume, D.A.; Stacey, K.J.; Young, P.R. Dengue virus NS1 protein activates cells via Toll-like receptor 4 and disrupts endothelial cell monolayer integrity. Sci. Transl. Med. 2015, 7, 304ra142. [Google Scholar] [CrossRef]
- Tay, M.Z.; Poh, C.M.; Renia, L.; MacAry, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, W.; Hu, G.; Xia, S.; Sun, Z.; Liu, Z.; Xie, Y.; Zhang, R.; Jiang, S.; Lu, L. SARS-CoV-2 infects T lymphocytes through its spike protein-mediated membrane fusion. Cell. Mol. Immunol. 2020. [Google Scholar] [CrossRef]
- Zheng, M.; Gao, Y.; Wang, G.; Song, G.; Liu, S.; Sun, D.; Xu, Y.; Tian, Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, Y.-Z.; Huang, G.; Wu, W.; Dong, S.-Y.; Xu, Y. Mortality of COVID-19 is Associated with Cellular Immune Function Compared to Immune Function in Chinese Han Population. medRxiv. 2020. [Google Scholar] [CrossRef]
- Miyake, K. Innate recognition of lipopolysaccharide by Toll-like receptor 4-MD-2. Trends Microbiol 2004, 12, 186–192. [Google Scholar] [CrossRef]
- Feng, Y.; Chao, W. Toll-like receptors and myocardial inflammation. Int. J. Inflam. 2011, 2011, 170352. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S. Toll-like receptor-4 modulation for cancer immunotherapy. Front. Immunol. 2014, 5, 328. [Google Scholar] [CrossRef] [PubMed]
- Shirey, K.A.; Lai, W.; Scott, A.J.; Lipsky, M.; Mistry, P.; Pletneva, L.M.; Karp, C.L.; McAlees, J.; Gioannini, T.L.; Weiss, J.; et al. The TLR4 antagonist Eritoran protects mice from lethal influenza infection. Nature 2013, 497, 498–502. [Google Scholar] [CrossRef]
- Perrin-Cocon, L.; Aublin-Gex, A.; Sestito, S.E.; Shirey, K.A.; Patel, M.C.; Andre, P.; Blanco, J.C.; Vogel, S.N.; Peri, F.; Lotteau, V. TLR4 antagonist FP7 inhibits LPS-induced cytokine production and glycolytic reprogramming in dendritic cells, and protects mice from lethal influenza infection. Sci. Rep. 2017, 7, 40791. [Google Scholar] [CrossRef]
- Shirey, K.A.; Lai, W.; Patel, M.C.; Pletneva, L.M.; Pang, C.; Kurt-Jones, E.; Lipsky, M.; Roger, T.; Calandra, T.; Tracey, K.J.; et al. Novel strategies for targeting innate immune responses to influenza. Mucosal Immunol. 2016, 9, 1173–1182. [Google Scholar] [CrossRef]
- Younan, P.; Ramanathan, P.; Graber, J.; Gusovsky, F.; Bukreyev, A. The Toll-Like Receptor 4 Antagonist Eritoran Protects Mice from Lethal Filovirus Challenge. mBio 2017, 8. [Google Scholar] [CrossRef]
- Diehl, N.; Schaal, H. Make yourself at home: Viral hijacking of the PI3K/Akt signaling pathway. Viruses 2013, 5, 3192–3212. [Google Scholar] [CrossRef]
- Laird, M.H.; Rhee, S.H.; Perkins, D.J.; Medvedev, A.E.; Piao, W.; Fenton, M.J.; Vogel, S.N. TLR4/MyD88/PI3K interactions regulate TLR4 signaling. J. Leukoc. Biol. 2009, 85, 966–977. [Google Scholar] [CrossRef]
- Xu, Q.; Yajima, T.; Li, W.; Saito, K.; Ohshima, Y.; Yoshikai, Y. Levan (beta-2, 6-fructan), a major fraction of fermented soybean mucilage, displays immunostimulating properties via Toll-like receptor 4 signalling: Induction of interleukin-12 production and suppression of T-helper type 2 response and immunoglobulin E production. Clin. Exp. Allergy 2006, 36, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Peiris, M. Pathogenesis of avian flu H5N1 and SARS. Novartis Found. Symp. 2006, 279, 56–60. [Google Scholar] [PubMed]
- Chu, C.; Tsang, K. Newly emerged respiratory infections-SARS and H5NI. J. Royal Coll. Physicians Edinb. 2006, 36, 245. [Google Scholar]
- Rairakhwada, D.; Pal, A.K.; Bhathena, Z.P.; Sahu, N.P.; Jha, A.; Mukherjee, S.C. Dietary microbial levan enhances cellular non-specific immunity and survival of common carp (Cyprinus carpio) juveniles. Fish Shellfish Immunol. 2007, 22, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Al-Waili, N.S.; Boni, N.S. Honey increased saliva, plasma, and urine content of total nitrite concentrations in normal individuals. J. Med. Food 2004, 7, 377–380. [Google Scholar] [CrossRef]
- Mehta, D.R.; Ashkar, A.A.; Mossman, K.L. The nitric oxide pathway provides innate antiviral protection in conjunction with the type I interferon pathway in fibroblasts. PLoS ONE 2012, 7, e31688. [Google Scholar] [CrossRef]
- Reiss, C.S.; Komatsu, T. Does nitric oxide play a critical role in viral infections? J. Virol. 1998, 72, 4547–4551. [Google Scholar] [CrossRef]
- Lane, T.E.; Paoletti, A.D.; Buchmeier, M.J. Disassociation between the in vitro and in vivo effects of nitric oxide on a neurotropic murine coronavirus. J. Virol. 1997, 71, 2202–2210. [Google Scholar] [CrossRef]
- Lin, Y.L.; Huang, Y.L.; Ma, S.H.; Yeh, C.T.; Chiou, S.Y.; Chen, L.K.; Liao, C.L. Inhibition of Japanese encephalitis virus infection by nitric oxide: Antiviral effect of nitric oxide on RNA virus replication. J. Virol. 1997, 71, 5227–5235. [Google Scholar] [CrossRef]
- Rimmelzwaan, G.F.; Baars, M.M.; de Lijster, P.; Fouchier, R.A.; Osterhaus, A.D. Inhibition of influenza virus replication by nitric oxide. J. Virol. 1999, 73, 8880–8883. [Google Scholar] [CrossRef]
- Saura, M.; Zaragoza, C.; McMillan, A.; Quick, R.A.; Hohenadl, C.; Lowenstein, J.M.; Lowenstein, C.J. An antiviral mechanism of nitric oxide: Inhibition of a viral protease. Immunity 1999, 10, 21–28. [Google Scholar] [CrossRef]
- Colasanti, M.; Persichini, T.; Venturini, G.; Ascenzi, P. S-nitrosylation of viral proteins: Molecular bases for antiviral effect of nitric oxide. IUBMB Life 1999, 48, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Åkerström, S.; Mousavi-Jazi, M.; Klingström, J.; Leijon, M.; Lundkvist, Å.; Mirazimi, A. Nitric Oxide Inhibits the Replication Cycle of Severe Acute Respiratory Syndrome Coronavirus. J. Virol. 2005, 79, 1966–1969. [Google Scholar] [CrossRef] [PubMed]
- Zamanian, R.T.; Pollack, C.V., Jr.; Gentile, M.A.; Rashid, M.; Fox, J.C.; Mahaffey, K.W.; de Jesus Perez, V. Outpatient Inhaled Nitric Oxide in a Patient with Vasoreactive Idiopathic Pulmonary Arterial Hypertension and COVID-19 Infection. Am. J. Respir. Crit. Care Med. 2020, 202, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.A.; Berra, L.; Gladwin, M.T. Home Nitric Oxide Therapy for COVID-19. Am. J. Respir. Crit. Care Med. 2020, 202, 16–20. [Google Scholar] [CrossRef]
- NCT04323345. Efficacy of Natural Honey Treatment in Patients with Novel Coronavirus. ClinicalTrials.gov. 2020. Available online: https://clinicaltrials.gov/ct2/show/study/NCT04323345 (accessed on 14 September 2020).
- Hashem, H. IN Silico Approach of Some Selected Honey Constituents as SARS-CoV-2 Main Protease (COVID-19) Inhibitors. ChemRxiv. 2020. Available online: https://chemrxiv.org/articles/preprint/IN_Silico_Approach_of_Some_Selected_Honey_Constituents_as_SARS-CoV-2_Main_Protease_COVID-19_Inhibitors/12115359/2 (accessed on 14 September 2020).
- Utsunomiya, H.; Ichinose, M.; Ikeda, K.; Uozaki, M.; Morishita, J.; Kuwahara, T.; Koyama, A.H.; Yamasaki, H. Inhibition by caffeic acid of the influenza A virus multiplication in vitro. Int. J. Mol. Med. 2014, 34, 1020–1024. [Google Scholar] [CrossRef]
- Ikeda, K.; Tsujimoto, K.; Uozaki, M.; Nishide, M.; Suzuki, Y.; Koyama, A.H.; Yamasaki, H. Inhibition of multiplication of herpes simplex virus by caffeic acid. Int. J. Mol. Med. 2011, 28, 595–598. [Google Scholar] [CrossRef]
- Erdemli, H.K.; Akyol, S.; Armutcu, F.; Akyol, O. Antiviral properties of caffeic acid phenethyl ester and its potential application. J. Intercult. Ethnopharmacol. 2015, 4, 344–347. [Google Scholar] [CrossRef]
- Meyer, J.J.; Afolayan, A.J.; Taylor, M.B.; Erasmus, D. Antiviral activity of galangin isolated from the aerial parts of Helichrysum aureonitens. J. Ethnopharmacol. 1997, 56, 165–169. [Google Scholar] [CrossRef]
- Song, J.H.; Kwon, B.E.; Jang, H.; Kang, H.; Cho, S.; Park, K.; Ko, H.J.; Kim, H. Antiviral Activity of Chrysin Derivatives against Coxsackievirus B3 in vitro and in vivo. Biomol. Ther. 2015, 23, 465–470. [Google Scholar] [CrossRef]
- Lyu, S.Y.; Rhim, J.Y.; Park, W.B. Antiherpetic activities of flavonoids against herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) in vitro. Arch. Pharm. Res. 2005, 28, 1293–1301. [Google Scholar] [CrossRef]
- Schnitzler, P.; Neuner, A.; Nolkemper, S.; Zundel, C.; Nowack, H.; Sensch, K.H.; Reichling, J. Antiviral activity and mode of action of propolis extracts and selected compounds. Phytother. Res. 2010, 24 (Suppl. 1), S20–28. [Google Scholar] [CrossRef]
- Ul Qamar, M.T.; Alqahtani, S.M.; Alamri, M.A.; Chen, L.L. Structural basis of SARS-CoV-2 3CL(pro) and anti-COVID-19 drug discovery from medicinal plants. J. Pharm. Anal. 2020. [Google Scholar] [CrossRef]
- Wu, C.; Liu, Y.; Yang, Y.; Zhang, P.; Zhong, W.; Wang, Y.; Wang, Q.; Xu, Y.; Li, M.; Li, X. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B 2020. [Google Scholar] [CrossRef] [PubMed]
- Baez-Santos, Y.M.; St John, S.E.; Mesecar, A.D. The SARS-coronavirus papain-like protease: Structure, function and inhibition by designed antiviral compounds. Antivir. Res. 2015, 115, 21–38. [Google Scholar] [CrossRef]
- Cheung, Y.; Meenu, M.; Yu, X.; Xu, B. Phenolic acids and flavonoids profiles of commercial honey from different floral sources and geographic sources. Int. J. Food Prop. 2019, 22, 290–308. [Google Scholar] [CrossRef]
- Brudzynski, K.; Abubaker, K.; St-Martin, L.; Castle, A. Re-examining the role of hydrogen peroxide in bacteriostatic and bactericidal activities of honey. Front. Microbiol. 2011, 2, 213. [Google Scholar] [CrossRef] [PubMed]
- Mentel, R.; Shirrmakher, R.; Kevich, A.; Dreizin, R.S.; Shmidt, I. Virus inactivation by hydrogen peroxide. Vopr. Virusol. 1977, 6, 731–733. [Google Scholar]
- Neighbor, N.K.; Newberry, L.A.; Bayyari, G.R.; Skeeles, J.K.; Beasley, J.N.; McNew, R.W. The effect of microaerosolized hydrogen peroxide on bacterial and viral poultry pathogens. Poult. Sci. 1994, 73, 1511–1516. [Google Scholar] [CrossRef]
- Montazeri, N.; Manuel, C.; Moorman, E.; Khatiwada, J.R.; Williams, L.L.; Jaykus, L.A. Virucidal Activity of Fogged Chlorine Dioxide- and Hydrogen Peroxide-Based Disinfectants against Human Norovirus and Its Surrogate, Feline Calicivirus, on Hard-to-Reach Surfaces. Front. Microbiol. 2017, 8, 1031. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, H.; Bae, S.; Choi, J.; Lim, S.Y.; Lee, N.; Kong, J.M.; Hwang, Y.I.; Kang, J.S.; Lee, W.J. Vitamin C Is an Essential Factor on the Anti-viral Immune Responses through the Production of Interferon-alpha/beta at the Initial Stage of Influenza A Virus (H3N2) Infection. Immune Netw. 2013, 13, 70–74. [Google Scholar] [CrossRef]
- da Silva, V.L.; Cerqueira, M.R.F.; Lowinsohn, D.; Matos, M.A.C.; Matos, R.C. Amperometric detection of ascorbic acid in honey using ascorbate oxidase immobilised on amberlite IRA-743. Food Chem. 2012, 133, 1050–1054. [Google Scholar] [CrossRef]
- COVID-19 Information. Available online: https://www.drugbank.ca/covid-19#drugs (accessed on 7 June 2020).
- Lianda, R.L.; Sant’Ana, L.D.O.; Echevarria, A.; Castro, R.N. Antioxidant activity and phenolic composition of Brazilian honeys and their extracts. J. Braz. Chem. Soc. 2012, 23, 618–627. [Google Scholar] [CrossRef]
- Ibrahim, A.K.; Youssef, A.I.; Arafa, A.S.; Ahmed, S.A. Anti-H5N1 virus flavonoids from Capparis sinaica Veill. Nat. Prod. Res. 2013, 27, 2149–2153. [Google Scholar] [CrossRef]
- Dzopalic, T.; Vucevic, D.; Tomic, S.; Djokic, J.; Chinou, I.; Colic, M. 3,10-Dihydroxy-decanoic acid, isolated from royal jelly, stimulates Th1 polarising capability of human monocyte-derived dendritic cells. Food Chem. 2011, 126, 1211–1217. [Google Scholar] [CrossRef]
- Kolayli, S.; Sahin, H.; Can, Z.; Yildiz, O.; Malkoc, M.; Asadov, A. A Member of Complementary Medicinal Food: Anatolian Royal Jellies, Their Chemical Compositions, and Antioxidant Properties. J. Evid. Based Complement. Altern. Med. 2016, 21, NP4–48. [Google Scholar] [CrossRef] [PubMed]
- Hegazi, A.G.; Abd El-Hady, F.K. Influence of Honey on the Suppression of Human Low Density Lipoprotein (LDL) Peroxidation (In vitro). Evid. Based Complement. Alternat. Med. 2009, 6, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Biluca, F.C.; da Silva, B.; Caon, T.; Mohr, E.T.B.; Vieira, G.N.; Gonzaga, L.V.; Vitali, L.; Micke, G.; Fett, R.; Dalmarco, E.M.; et al. Investigation of phenolic compounds, antioxidant and anti-inflammatory activities in stingless bee honey (Meliponinae). Food Res. Int. 2020, 129, 108756. [Google Scholar] [CrossRef]
- Niphade, S.R.; Asad, M.; Chandrakala, G.K.; Toppo, E.; Deshmukh, P. Immunomodulatory activity of Cinnamomum zeylanicum bark. Pharm. Biol. 2009, 47, 1168–1173. [Google Scholar] [CrossRef]
- Jaafar, M.H.M.; Hamid, K.A.; Anuar, N.; Zohdi, R.M.; Effendi, T.J.B. Physicochemical properties and pharmacokinetic profiles of selected Malaysian honey. In Proceedings of the 2012 IEEE Symposium on Business, Engineering and Industrial Applications, Bandung, Indonesia, 23–26 September 2012; pp. 140–145. [Google Scholar]
- Balasooriya, E.R.; Jayasinghe, C.D.; Jayawardena, U.A.; Ruwanthika, R.W.D.; Mendis de Silva, R.; Udagama, P.V. Honey mediated green synthesis of nanoparticles: New era of safe nanotechnology. J. Nanomater. 2017, 2017. [Google Scholar] [CrossRef]
- Al-Hatamleh, M.A.; Ahmad, S.; Boer, J.C.; Lim, J.; Chen, X.; Plebanski, M.; Mohamud, R. A Perspective Review on the Role of Nanomedicine in the Modulation of TNF-TNFR2 Axis in Breast Cancer Immunotherapy. J. Oncol. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Mohamud, R.; Xiang, S.D.; Selomulya, C.; Rolland, J.M.; O’Hehir, R.E.; Hardy, C.L.; Plebanski, M. The effects of engineered nanoparticles on pulmonary immune homeostasis. Drug Metab. Rev. 2014, 46, 176–190. [Google Scholar] [CrossRef]
- Al-Hatamleh, M.A.I.; Engku Nur Syafirah, A.R.E.; Boer, J.C.; Ferji, K.; Six, J.L.; Chen, X.; Elkord, E.; Plebanski, M.; Mohamud, R. Synergistic Effects of Nanomedicine Targeting TNFR2 and DNA Demethylation Inhibitor-An Opportunity for Cancer Treatment. Cells 2019, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.C.W. Nano Research for COVID-19. ACS Nano 2020, 14, 3719–3720. [Google Scholar] [CrossRef]
- Fuenmayor, C.A.; Díaz-Moreno, A.C.; Zuluaga-Domínguez, C.M.; Quicazán, M.C. Honey of Colombian stingless bees: Nutritional characteristics and physicochemical quality indicators. In Pot-Honey; Vit, P., Pedro, S.R., Roubik, D., Eds.; Springer: New York, NY, USA, 2013; pp. 383–394. [Google Scholar]
- Brown, E.; O’Brien, M.; Georges, K.; Suepaul, S. Physical characteristics and antimicrobial properties of Apis mellifera, Frieseomelitta nigra and Melipona favosa bee honeys from apiaries in Trinidad and Tobago. BMC Complement. Med. Ther. 2020, 20, 85. [Google Scholar] [CrossRef]
- Siddiqi, H.K.; Mehra, M.R. COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J. Heart Lung Transplant. 2020, 39, 405–407. [Google Scholar] [CrossRef]
- Mangalmurti, N.; Hunter, C.A. Cytokine Storms: Understanding COVID-19. Immunity 2020, 53, 19–25. [Google Scholar] [CrossRef]
| Compound | Discretion | Mechanisms of Antiviral Activities | Stage of Research | Reference |
|---|---|---|---|---|
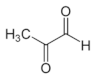 Methylglyoxal | Dicarbonyl resulted from the conversion of DHA during the ripening of honey | Blocks formation of virion assembly and maturation | In-vitro | [63] |
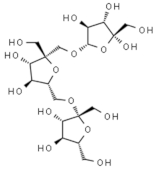 Levan | Polysaccharide produced by fermentation of Bacillus subtilis | Activation of antiviral immune responses | In-vitro | [30] |
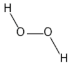 Hydrogen peroxide | Produced mainly during glucose oxidation | Viral inactivation | - | [118] |
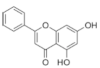 Chrysin | Flavonoid | Inhibition of viral protease enzymes | In-silico | [105] |
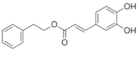 CAPE | Polyphenolic ester | Inhibition of viral protease enzymes | In-silico | [105] |
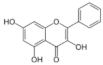 Galangin | Flavonoid | Inhibition of viral protease enzymes | In-silico | [105] |
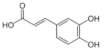 Caffeic acid | Flavonoid | Inhibition of viral protease enzymes | In-silico | [105] |
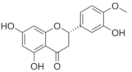 Hesperidin | Flavonoid | - Inhibition of viral protease enzymes - Binding to S-RBD and then blocking the interaction with ACE2 | In-silico | [114] |
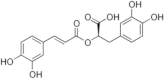 Rosmarinic acid | Polyphenolic hydroxycinnamic acid | Inhibition of viral protease enzymes | In-silico | [114] |
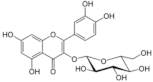 Isoquercetin | Flavonoid | Reduction of viral load | - | [125] |
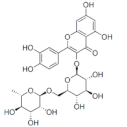 Rutin | Flavonoid | Reduction of viral load | - | [125] |
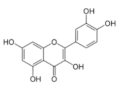 Quercetin | Flavonoid | Reduction of viral load | - | [125] |
 3-hydroxy-sebacic acid | Fatty acid | Unknown | - | [128] |
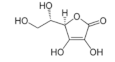 Ascorbic acid | Sugar acid | Activation of antiviral immune responses | - | [121] |
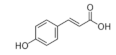 p-Coumaric acid | Phenolic acid | Unknown | - | [112] |
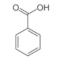 Benzoic acid | Aromatic carboxylic acid | Unknown | - | [112] |
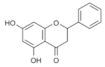 Pinocembrin | Flavonoid | Unknown | - | [112] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Hatamleh, M.A.I.; Hatmal, M.M.; Sattar, K.; Ahmad, S.; Mustafa, M.Z.; Bittencourt, M.D.C.; Mohamud, R. Antiviral and Immunomodulatory Effects of Phytochemicals from Honey against COVID-19: Potential Mechanisms of Action and Future Directions. Molecules 2020, 25, 5017. https://doi.org/10.3390/molecules25215017
Al-Hatamleh MAI, Hatmal MM, Sattar K, Ahmad S, Mustafa MZ, Bittencourt MDC, Mohamud R. Antiviral and Immunomodulatory Effects of Phytochemicals from Honey against COVID-19: Potential Mechanisms of Action and Future Directions. Molecules. 2020; 25(21):5017. https://doi.org/10.3390/molecules25215017
Chicago/Turabian StyleAl-Hatamleh, Mohammad A. I., Ma’mon M. Hatmal, Kamran Sattar, Suhana Ahmad, Mohd Zulkifli Mustafa, Marcelo De Carvalho Bittencourt, and Rohimah Mohamud. 2020. "Antiviral and Immunomodulatory Effects of Phytochemicals from Honey against COVID-19: Potential Mechanisms of Action and Future Directions" Molecules 25, no. 21: 5017. https://doi.org/10.3390/molecules25215017
APA StyleAl-Hatamleh, M. A. I., Hatmal, M. M., Sattar, K., Ahmad, S., Mustafa, M. Z., Bittencourt, M. D. C., & Mohamud, R. (2020). Antiviral and Immunomodulatory Effects of Phytochemicals from Honey against COVID-19: Potential Mechanisms of Action and Future Directions. Molecules, 25(21), 5017. https://doi.org/10.3390/molecules25215017







