Tert-butyl-(4-hydroxy-3-((3-(2-methylpiperidin-yl)propyl)carbamoyl)phenyl)carbamate Has Moderated Protective Activity in Astrocytes Stimulated with Amyloid Beta 1-42 and in a Scopolamine Model
Abstract
1. Introduction
2. Results
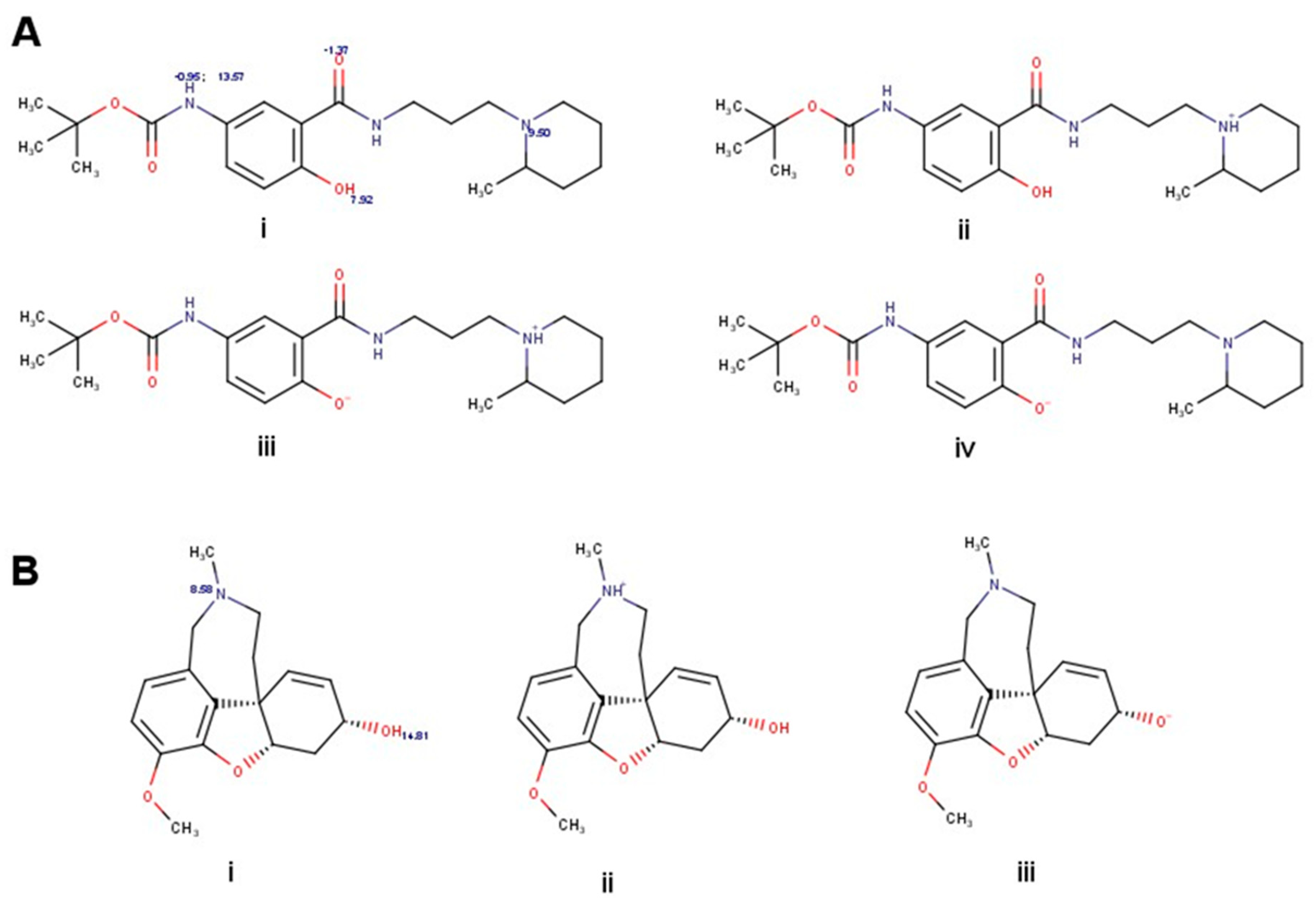
2.1. Synthesis and Purification of the M4 Compound
2.2. Identification of Fibrilar Aβ1-42 by Atomic Force Microscopy
2.3. Astrocyte Characterization
2.4. M4 Compound Increases the Cell Viability of Astrocytes in the Presence of Aβ1-42
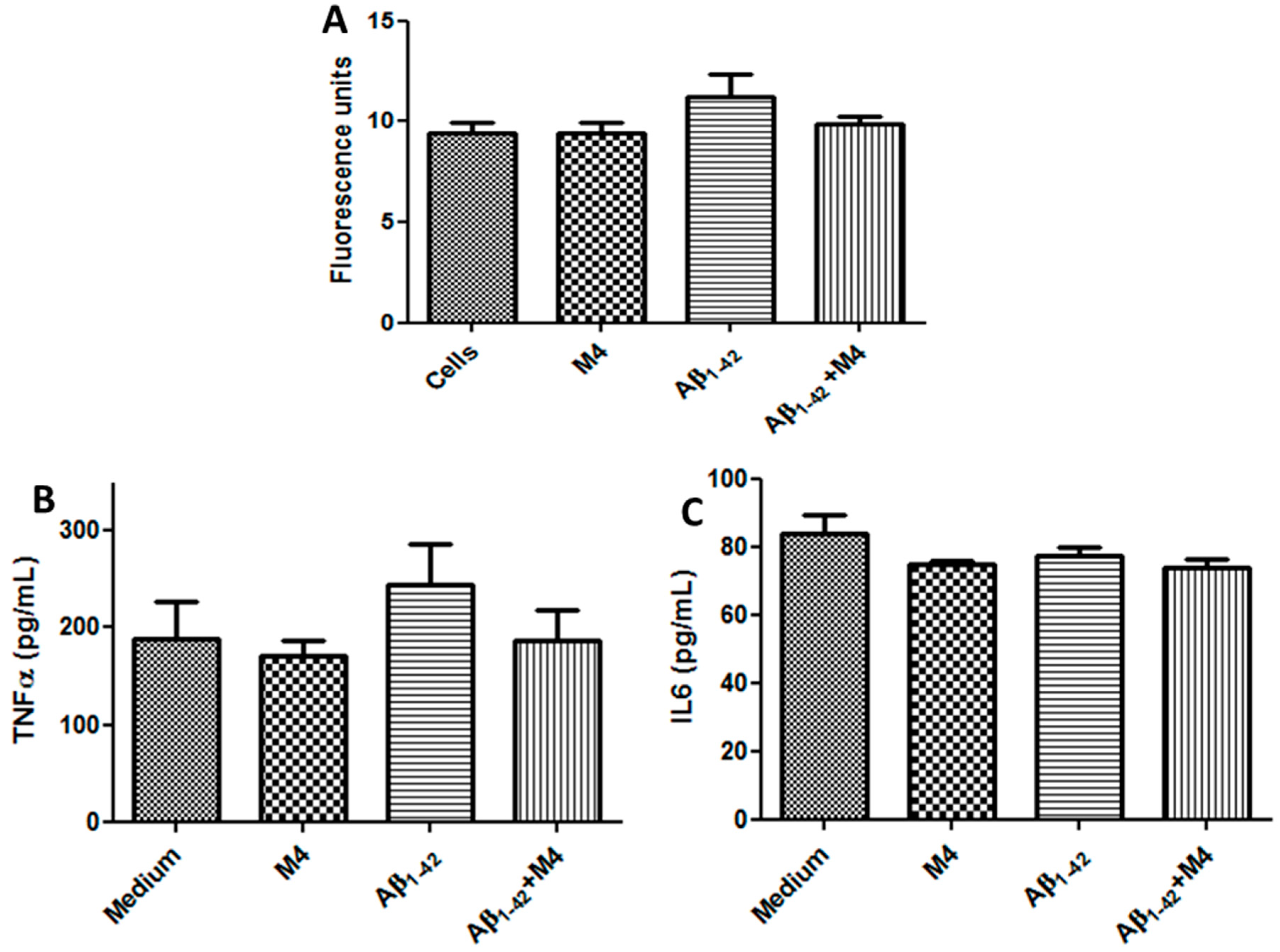
2.5. M4 Compound Protect against Reactive Oxigen Species (ROS) Produced by Aβ1-42 in Astrocyte Culture Cells
2.6. Effect of M4 Compound on IL-6 and TNF-α Astrocytes Activated with Aβ1-42
2.7. Moderated Activity of M4 on Aβ1-42 Deposition
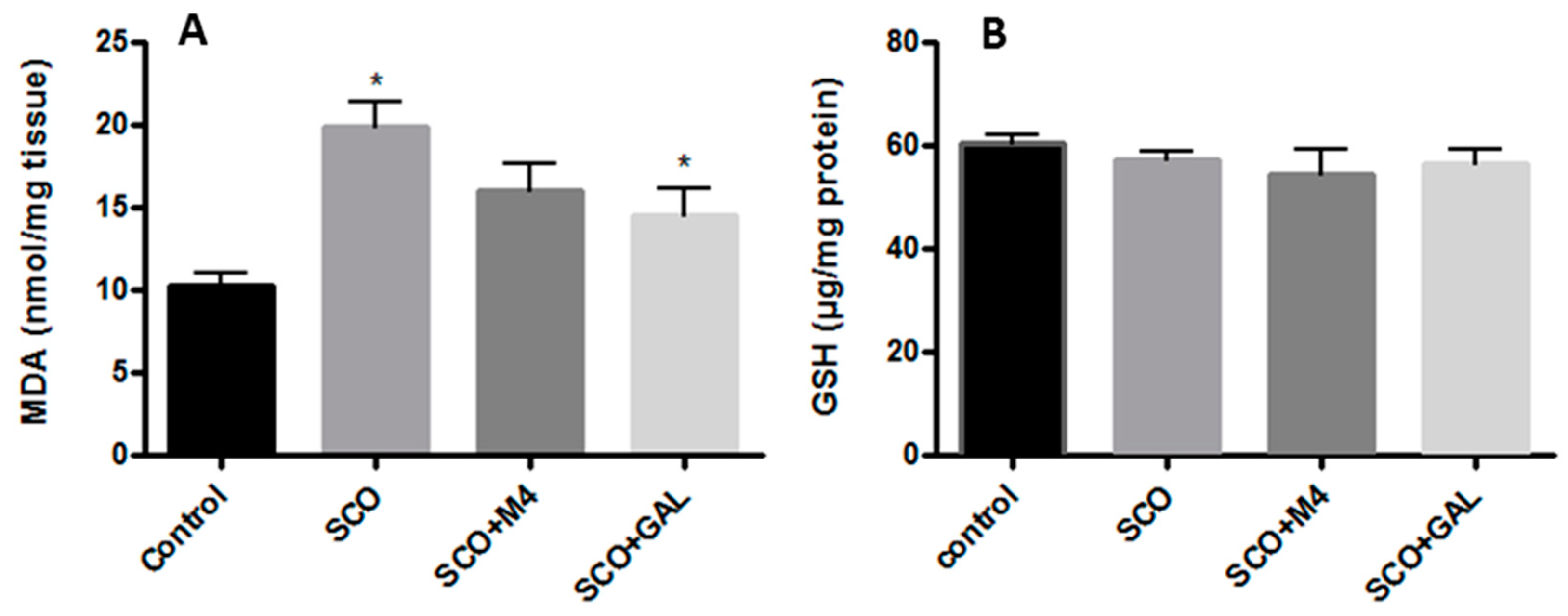
2.8. Moderated Activity of M4 on Scopolamine-Induced Oxidative Stress
3. Discussion
4. Materials and Methods
4.1. Chemicals and General Characterization Methods
Synthesis and Compound Characterization
4.2. In Vitro Evaluation of Compound M4
Formation and Determination of Fibrilar Aβ1-42
4.3. Cortical Astrocyte Primary Culture
4.4. Cell Viability in the Presence of Compounds M4 Was Determined by (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) MTT Assay with and without Aβ1-42
4.5. Reactive Oxygen Species Quantification by 2,7-Dichlorodihydrofluorescein Diacetate (DCFH-DA) Assay
4.6. IL-6 and TNF-α Determination
4.7. Effect of M4 Compound on the Scopolamine-Induced Aβ1-42 Aggregation Model
4.8. Determination of Amyloid Deposits in Hippocampal Histological Sections
4.9. β-Secretase Activity
4.10. Quantification of Aβ1-42 by ELISA Assay
4.11. Malondialdehyde (MDA) Content Determination
4.12. Quantification of Reduced Form of Glutathione (GSH)
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Querfurth, H.W.; LaFerla, F.M. Alzheimer′s disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Novick, P.A.; Lopes, D.H.; Branson, K.M.; Chopo, A.E.; Graef, I.A.; Bitan, G.; Pande, V.S. Design of β-amyloid aggregation inhibitors from a predicted structural motif. J. Med. Chem. 2012, 55, 3002–3010. [Google Scholar] [CrossRef] [PubMed]
- Taneja, V.; Verma, M.; Vats, A. Toxic species in amyloid disorders: Oligomers or mature fibrils. Ann. Indian Acad. Neurol. 2015, 18, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Ji, X.; Han, W.; Han, H. Oxymatrine exhibits anti-neuroinflammatory effects on Aβ1-42-induced primary microglia cells by inhibiting NF-κB and MAPK signaling pathways. Int. Immunopharmacol. 2019, 74, 105686. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, J.; Yao, X.C.; Xue, X.; Zhang, Q.C.; Wang, X.X.; Ding, L.L.; Wu, C. Oligomeric Aβ-Induced Microglial Activation is Possibly Mediated by NADPH Oxidase. Neurochem. Res. 2012, 38, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Heurtaux, T.; Michelucci, A.; Losciuto, S.; Gallotti, C.; Felten, P.; Dorban, G.; Grandbarbe, L.; Morga, E.; Heuschling, P. Microglial activation depends on beta-amyloid conformation: Role of the formylpeptide receptor 2. J. Neurochem. 2010, 114, 576–586. [Google Scholar] [CrossRef]
- Frost, G.R.; Li, Y.-M. The role of astrocytes in amyloid production and Alzheimer’s disease. Open Biol. 2017, 7, 170228. [Google Scholar] [CrossRef]
- Streit, W.J.; Braak, H.; Del Tredici, K.; Leyh, J.; Lier, J.; Khoshbouei, H.; Eisenlöffel, C.; Müller, W.; Bechmann, I. Microglial activation occurs late during preclinical Alzheimer’s disease. Glia 2018, 66, 2550–2562. [Google Scholar] [CrossRef]
- Hughes, C.; Choi, M.L.; Yi, J.-H.; Kim, S.-C.; Drews, A.; George-Hyslop, P.S.; Bryant, C.; Gandhi, S.; Cho, K.; Klenerman, D. Beta amyloid aggregates induce sensitised TLR4 signalling causing long-term potentiation deficit and rat neuronal cell death. Commun. Biol. 2020, 3, 1–7. [Google Scholar] [CrossRef]
- Farfara, D.; Lifshitz, V.; Frenkel, D. Neuroprotective and neurotoxic properties of glial cells in the pathogenesis of Alzheimer’s disease. J. Cell. Mol. Med. 2008, 12, 762–780. [Google Scholar] [CrossRef]
- Yang, X.; Sheng, W.; Ridgley, D.M.; Haidekker, M.A.; Sun, G.Y.; Lee, J.C. Astrocytes regulate α-secretase-cleaved soluble amyloid precursor protein secretion in neuronal cells: Involvement of group IIA secretory phospholipase A2. Neuroscientist 2015, 300, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Hussain, M.D.; Yan, L.-J. Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer’s disease. Int. J. Neurosci. 2013, 124, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Cherbuin, N.; Walsh, E.I.; Baune, B.T.; Anstey, K.J. Oxidative stress, inflammation and risk of neurodegeneration in a population sample. Eur. J. Neurol. 2019, 26, 1347–1354. [Google Scholar] [CrossRef]
- Lanni, C.; Fagiani, F.; Racchi, M.; Preda, S.; Pascale, A.; Grilli, M.; Allegri, N.; Govoni, S. Beta-amyloid short- and long-term synaptic entanglement. Pharmacol. Res. 2019, 139, 243–260. [Google Scholar] [CrossRef]
- Ali, M.M.; Ghouri, R.G.; Ans, A.H.; Akbar, A.; Toheed, A. Recommendations for Anti-inflammatory Treatments in Alzheimer’s Disease: A Comprehensive Review of the Literature. Cureus 2019, 11, e4620. [Google Scholar] [CrossRef]
- Giorgetti, S.; Greco, C.; Tortora, P.; Aprile, F.A. Targeting Amyloid Aggregation: An Overview of Strategies and Mechanisms. Int. J. Mol. Sci. 2018, 19, 2677. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, M.L.; Cavalli, A.; Melchiorre, C. Memoquin: A multi-target-directed ligand as an innovative therapeutic opportunity for Alzheimer’s disease. Neurotherapeutics 2009, 6, 152–162. [Google Scholar] [CrossRef]
- Oxford, A.E.; Stewart, E.S.; Rohn, T.T. Clinical Trials in Alzheimer’s Disease: A Hurdle in the Path of Remedy. Int. J. Alzheimer’s Dis. 2020, 2020, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kabir, T.; Uddin, S.; Al Mamun, A.; Jeandet, P.; Aleya, L.; Mansouri, R.A.; Ashraf, G.M.; Mathew, B.; Bin-Jumah, M.N.; Abdel-Daim, M.M. Combination Drug Therapy for the Management of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 3272. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Rodríguez, M.; Correa-Basurto, J.; Martínez-Ramos, F.; Padilla-Martínez, I.I.; Benítez-Cardoza, C.G.; Mera-Jiménez, E.; Rosales-Hernández, M.C. Design of Multi-Target Compounds as AChE, BACE1, and Amyloid-β1-42 Oligomerization Inhibitors: In Silico and In Vitro Studies. J. Alzheimer Dis. 2014, 41, 1073–1085. [Google Scholar] [CrossRef] [PubMed]
- Valeur, E.; Bradley, M. Amide bond formation: Beyond the myth of coupling reagents. Chem. Soc. Rev. 2009, 38, 606–631. [Google Scholar] [CrossRef] [PubMed]
- Malmsten, L.; Vijayaraghavan, S.; Hovatta, O.; Marutle, A.; Darreh-Shori, T. Fibrillar β-amyloid 1-42 alters cytokine secretion, cholinergic signalling and neuronal differentiation. J. Cell. Mol. Med. 2014, 18, 1874–1888. [Google Scholar] [CrossRef] [PubMed]
- Konno, H.; Watanabe-Nakayama, T.; Uchihashi, T.; Okuda, M.; Zhu, L.; Kodera, N.; Kikuchi, Y.; Ando, T.; Taguchi, H. Dynamics of oligomer and amyloid fibril formation by yeast prion Sup35 observed by high-speed atomic force microscopy. Proc. Natl. Acad. Sci. USA 2020, 117, 7831–7836. [Google Scholar] [CrossRef]
- Azouz, M.; Cullin, C.; LeComte, S.; LaFleur, M. Membrane domain modulation of Aβ1–42 oligomer interactions with supported lipid bilayers: An atomic force microscopy investigation. Nanoscale 2019, 11, 20857–20867. [Google Scholar] [CrossRef] [PubMed]
- Bihaqi, S.W.; Singh, A.P.; Tiwari, M. Supplementation of Convolvulus pluricaulis attenuates scopolamine-induced increased tau and Amyloid precursor protein (AβPP) expression in rat brain. Indian J. Pharmacol. 2012, 44, 593–598. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, M.; Arciniega-Martínez, I.M.; García-Marín, I.D.; Correa-Basurto, J.; Rosales-Hernández, M.C. Chronic Administration of Scopolamine Increased GSK3βP9, Beta Secretase, Amyloid Beta, and Oxidative Stress in the Hippocampus of Wistar Rats. Mol. Neurobiol. 2020, 57, 3979–3988. [Google Scholar] [CrossRef]
- Tang, K.S. The cellular and molecular processes associated with scopolamine-induced memory deficit: A model of Alzheimer’s biomarkers. Life Sci. 2019, 233, 116695. [Google Scholar] [CrossRef]
- Rao, C.V.; Asch, A.S.; Carr, D.J.J.; Yamada, H.Y. “Amyloid-beta accumulation cycle” as a prevention and/or therapy target for Alzheimer’s disease. Aging Cell 2020, 19, e13109. [Google Scholar] [CrossRef]
- Chen, G.-F.; Xu, T.-H.; Yan, Y.; Zhou, Y.-R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef]
- González-Reyes, R.E.; Nava-Mesa, M.O.; Vargas-Sánchez, K.; Ariza-Salamanca, D.; Mora-Muñoz, L. Involvement of Astrocytes in Alzheimer’s Disease from a Neuroinflammatory and Oxidative Stress Perspective. Front. Mol. Neurosci. 2017, 10, 427. [Google Scholar] [CrossRef]
- Batarseh, Y.S.; Duong, Q.-V.; Mousa, Y.; Al Rihani, S.B.; Elfakhri, K.; Kaddoumi, A. Amyloid-β and Astrocytes Interplay in Amyloid-β Related Disorders. Int. J. Mol. Sci. 2016, 17, 338. [Google Scholar] [CrossRef] [PubMed]
- Khondker, A.; Alsop, R.J.; Rheinstädter, M.C. Membrane-Accelerated Amyloid-β Aggregation and Formation of Cross-β Sheets. Membranes 2017, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kumar, A. Microglial Inhibitory Mechanism of Coenzyme Q10 against Aβ (1-42) Induced Cognitive Dysfunctions: Possible Behavioral, Biochemical, Cellular, and Histopathological Alterations. Front. Pharmacol. 2015, 6, 268. [Google Scholar] [CrossRef] [PubMed]
- Fakhoury, M. Microglia and Astrocytes in Alzheimer’s Disease: Implications for Therapy. Curr. Neuropharmacol. 2018, 16, 508–518. [Google Scholar] [CrossRef]
- Cunningham, C.; Dunne, A.; Lopez-Rodriguez, A.B. Astrocytes: Heterogeneous and Dynamic Phenotypes in Neurodegeneration and Innate Immunity. Neuroscientist 2018, 25, 455–474. [Google Scholar] [CrossRef]
- Tsvetkova, D.; Obreshkova, D.; Zheleva-Dimitrova, D.; Saso, L. Antioxidant Activity of Galantamine and Some of its Derivatives. Curr. Med. Chem. 2013, 20, 4595–4608. [Google Scholar] [CrossRef]
- Li, Q.; Wu, D.; Zhang, L.; Zhang, Y. Effects of galantamine on β-amyloid release and beta-site cleaving enzyme 1 expression in differentiated human neuroblastoma SH-SY5Y cells. Exp. Gerontol. 2010, 45, 842–847. [Google Scholar] [CrossRef]
- Castillo, W.O.; Aristizabal-Pachon, A.F. Galantamine protects against beta amyloid peptide-induced DNA damage in a model for Alzheimer’s disease. Neural. Regen. Res. 2017, 12, 916–917. [Google Scholar] [CrossRef]
- Joseph, E.; Villalobos-Acosta, D.M.Á.; Torres-Ramos, M.A.; Farfán-García, E.D.; Gómez-López, M.; Miliar-García, Á.; Fragoso-Vázquez, M.J.; García-Marín, I.D.; Correa-Basurto, J.; Rosales-Hernández, M.C. Neuroprotective Effects of Apocynin and Galantamine During the Chronic Administration of Scopolamine in an Alzheimer’s Disease Model. J. Mol. Neurosci. 2019, 70, 180–193. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Bilcer, G.; Harwood, C.; Kawahama, R.; Shin, N.; Hussain, K.A.; Hong, L.; Loy, J.A.; Nguyen, C.; Koelsch, G.; et al. Structure-Based Design: Potent Inhibitors of Human Brain Memapsin 2 (β-Secretase). J. Med. Chem. 2001, 44, 2865–2868. [Google Scholar] [CrossRef]
- Perluigi, M.; Di Domenico, F.; Giorgi, A.; Schininà, M.; Coccia, R.; Cini, C.; Bellia, F.; Cambria, M.; Cornelius, C.; Butterfield, D.; et al. Redox proteomics in aging rat brain: Involvement of mitochondrial reduced glutathione status and mitochondrial protein oxidation in the aging process. J. Neurosci. Res. 2010, 88, 3498–3507. [Google Scholar] [CrossRef] [PubMed]





Sample Availability: Sample of the compound M4 is available with the first author and the correspondence author. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camarillo-López, R.H.; Hernández Rodríguez, M.; Torres-Ramos, M.A.; Arciniega-Martínez, I.M.; García-Marín, I.D.; Correa Basurto, J.; Méndez Méndez, J.V.; Rosales-Hernández, M.C. Tert-butyl-(4-hydroxy-3-((3-(2-methylpiperidin-yl)propyl)carbamoyl)phenyl)carbamate Has Moderated Protective Activity in Astrocytes Stimulated with Amyloid Beta 1-42 and in a Scopolamine Model. Molecules 2020, 25, 5009. https://doi.org/10.3390/molecules25215009
Camarillo-López RH, Hernández Rodríguez M, Torres-Ramos MA, Arciniega-Martínez IM, García-Marín ID, Correa Basurto J, Méndez Méndez JV, Rosales-Hernández MC. Tert-butyl-(4-hydroxy-3-((3-(2-methylpiperidin-yl)propyl)carbamoyl)phenyl)carbamate Has Moderated Protective Activity in Astrocytes Stimulated with Amyloid Beta 1-42 and in a Scopolamine Model. Molecules. 2020; 25(21):5009. https://doi.org/10.3390/molecules25215009
Chicago/Turabian StyleCamarillo-López, Raúl Horacio, Maricarmen Hernández Rodríguez, Mónica Adriana Torres-Ramos, Ivonne Maciel Arciniega-Martínez, Iohanan Daniel García-Marín, José Correa Basurto, Juan Vicente Méndez Méndez, and Martha Cecilia Rosales-Hernández. 2020. "Tert-butyl-(4-hydroxy-3-((3-(2-methylpiperidin-yl)propyl)carbamoyl)phenyl)carbamate Has Moderated Protective Activity in Astrocytes Stimulated with Amyloid Beta 1-42 and in a Scopolamine Model" Molecules 25, no. 21: 5009. https://doi.org/10.3390/molecules25215009
APA StyleCamarillo-López, R. H., Hernández Rodríguez, M., Torres-Ramos, M. A., Arciniega-Martínez, I. M., García-Marín, I. D., Correa Basurto, J., Méndez Méndez, J. V., & Rosales-Hernández, M. C. (2020). Tert-butyl-(4-hydroxy-3-((3-(2-methylpiperidin-yl)propyl)carbamoyl)phenyl)carbamate Has Moderated Protective Activity in Astrocytes Stimulated with Amyloid Beta 1-42 and in a Scopolamine Model. Molecules, 25(21), 5009. https://doi.org/10.3390/molecules25215009






