Drying Techniques and Storage: Do They Affect the Nutritional Value of Bee-Collected Pollen?
Abstract
1. Introduction
2. Results
2.1. Influence of Plant Species, Drying Treatment, and Shelf Life on Phenolic Compounds, Flavonoids, and Rutin
2.2. Influence of Plant Species, Drying Treatment, and Shelf Life on the Content of Total and Free Amino Acids and Proline
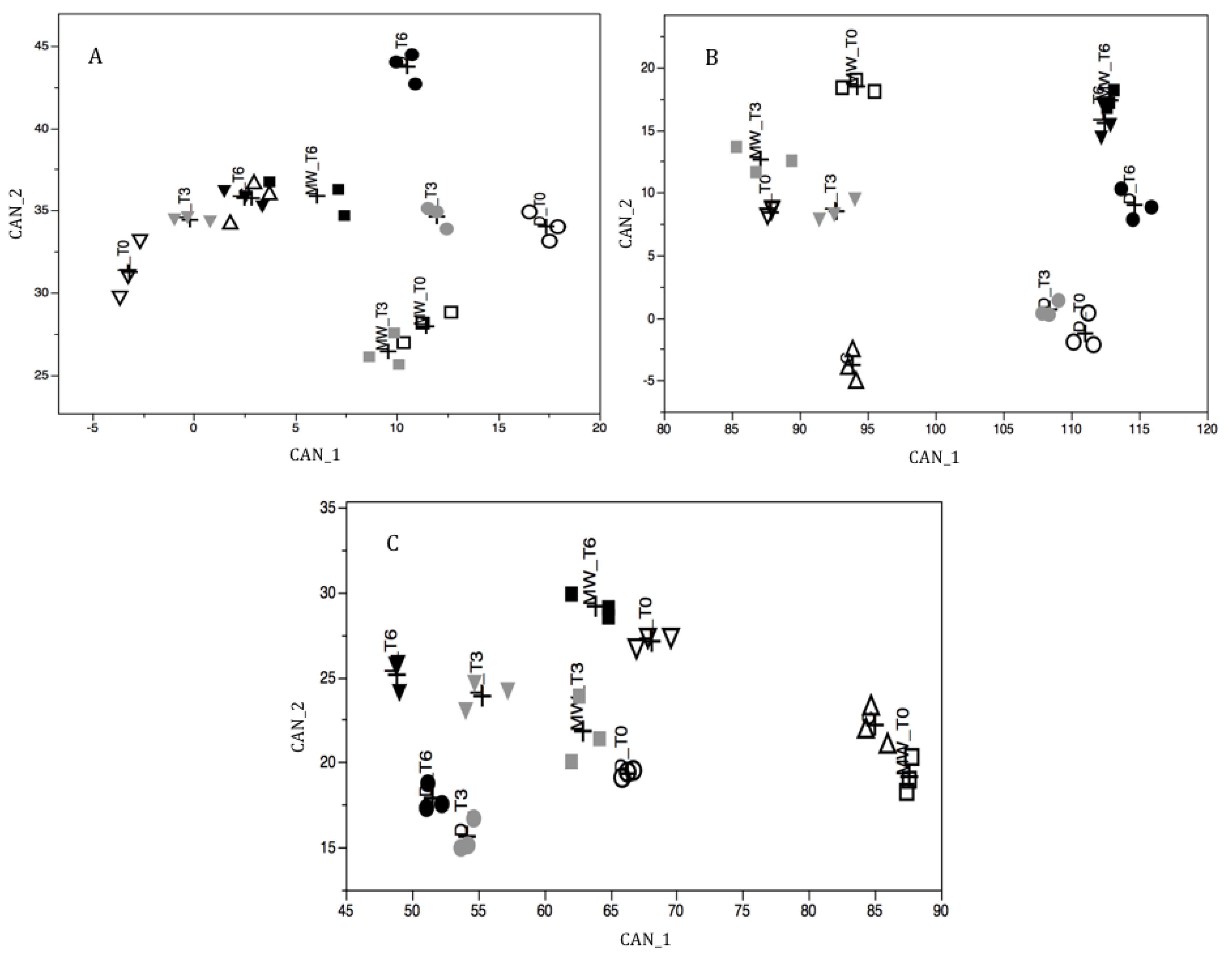
2.3. Multivariate Analysis of Pollen Components
3. Discussion
4. Materials and Methods
4.1. Bee-Collected Pollen
4.2. Conventional Drying
4.3. Freeze-Drying
4.4. Microwave-Assisted Drying
4.5. Extraction and Quantification of Phenolics and Flavonoids
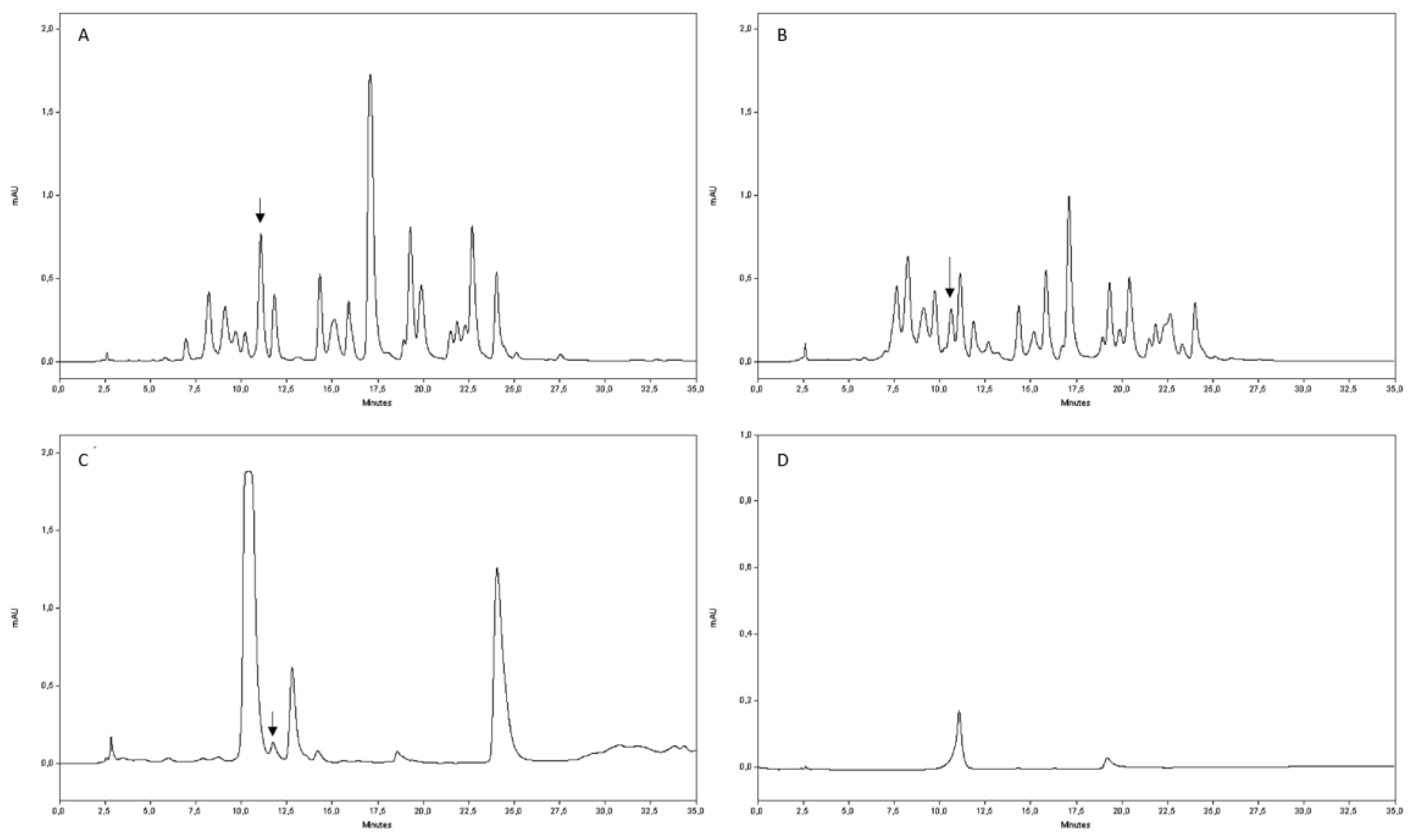
4.6. Rutin Quantification by HPLC Analysis
4.7. Extraction and Quantification of Free and Total Amino Acids
4.8. Quantification of Free and Total Proline
4.9. Data Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hasegawa, T.; Havlík, P.; Frank, S.; Palazzo, A.; Valin, H. Tackling food consumption inequality to fight hunger without pressuring the environment. Nat. Sustain. 2019, 2, 826–833. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, L.; Zeng, X.; Han, Z.; Brennan, C.S. Non-thermal technologies and its current and future application in the food industry: A review. Int. J. Food Sci. Technol. 2019, 54, 1–13. [Google Scholar] [CrossRef]
- Amit, S.K.; Uddin, M.M.; Rahman, R.; Islam, S.M.R.; Khan, M.S. A review on mechanisms and commercial aspects of food preservation and processing. Agric. Food Secur. 2017, 6, 51. [Google Scholar] [CrossRef]
- Ekezie, F.-G.C.; Sun, D.-W.; Han, Z.; Cheng, J.-H. Microwave-assisted food processing technologies for enhancing product quality and process efficiency: A review of recent developments. Trends Food Sci. Technol. 2017, 67, 58–69. [Google Scholar] [CrossRef]
- Kasote, D.M. Propolis: A neglected product of value in the Indian beekeeping sector. Bee World 2017, 94, 80–83. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Ansary, J.; Gil, E.; Amici, A.; Bompadre, S.; Simal-Gandara, J.; Giampieri, F.; Battino, M. Phenolic compounds from Mediterranean foods as nutraceutical tools for the prevention of cancer: The effect of honey polyphenols on colorectal cancer stem-like cells from spheroids. Food Chem. 2020, 325, 126881. [Google Scholar] [CrossRef]
- Canale, A.; Benelli, G.; Castagna, A.; Sgherri, C.; Poli, P.; Serra, A.; Mele, M.; Ranieri, A.; Signorini, F.; Bientinesi, M. Microwave-assisted drying for the conservation of honeybee pollen. Materials 2016, 9, 363. [Google Scholar] [CrossRef]
- Tomás, A.; Falcão, S.I.; Russo-Almeida, P.; Vilas-Boas, M. Potentialities of beebread as a food supplement and source of nutraceuticals: Botanical origin, nutritional composition and antioxidant activity. J. Apic. Res. 2017, 56, 219–230. [Google Scholar] [CrossRef]
- Almaraz-Abarca, N.; da Graça Campos, M.; Avila-Reyes, J.A.; Naranjo-Jimenez, N.; Corral, J.H.; Gonzalez-Valdez, L.S. Antioxidant activity of polyphenolic extract of monofloral honeybee-collected pollen from mesquite (Prosopis juliflora, Leguminosae). J. Food Compos. Anal. 2007, 20, 119–124. [Google Scholar] [CrossRef]
- Šarić, A.; Balog, T.; Sobočanec, S.; Kušić, B.; Šverko, V.; Rusak, G.; Likić, S.; Bubalo, D.; Pinto, B.; Reali, D. Antioxidant effects of flavonoid from Croatian Cystus incanus L. rich bee pollen. Food Chem. Toxicol. 2009, 47, 547–554. [Google Scholar] [CrossRef]
- Ranieri, A.; Benelli, G.; Castagna, A.; Sgherri, C.; Signorini, F.; Bientinesi, M.; Nicolella, C.; Canale, A. Freeze-drying duration influences the amino acid and rutin content in honeybee-collected chestnut pollen. Saudi J. Biol. Sci. 2019, 26, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Mărghitaş, L.A.; Stanciu, O.G.; Dezmirean, D.S.; Bobiş, O.; Popescu, O.; Bogdanov, S.; Campos, M.G. In vitro antioxidant capacity of honeybee-collected pollen of selected floral origin harvested from Romania. Food Chem. 2009, 115, 878–883. [Google Scholar] [CrossRef]
- Fanali, C.; Dugo, L.; Rocco, A. Nano-liquid chromatography in nutraceutical analysis: Determination of polyphenols in bee pollen. J. Chromatogr. A 2013, 1313, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wu, D.; Ye, X.; Liu, D.; Chen, J.; Sun, P. Characterization of chemical composition of bee pollen in China. J. Agric. Food Chem. 2013, 61, 708–718. [Google Scholar] [CrossRef]
- Margaoan, R.; Mărghitaş, L.A.; Dezmirean, D.S.; Dulf, F.V.; Bunea, A.; Socaci, S.A.; Bobiş, O. Predominant and secondary pollen botanical origins influence the carotenoid and fatty acid profile in fresh honeybee-collected pollen. J. Agric. Food Chem. 2014, 62, 6306–6316. [Google Scholar] [CrossRef]
- Conte, G.; Benelli, G.; Serra, A.; Signorini, F.; Bientinesi, M.; Nicolella, C.; Mele, M.; Canale, A. Lipid characterization of chestnut and willow honeybee-collected pollen: Impact of freeze-drying and microwave-assisted drying. J. Food Compos. Anal. 2017, 55, 12–19. [Google Scholar] [CrossRef]
- de Arruda, V.A.S.; Vieria dos Santos, A.; Figueiredo Sampaio, D.; da Silva Araújo, E.; de Castro Peixoto, A.L.; Estevinho, M.L.F.; Bicudo de Almeida-Muradian, L. Microbiological quality and physicochemical characterization of Brazilian bee pollen. J. Apic. Res. 2017, 56, 231–238. [Google Scholar] [CrossRef]
- De-Melo, A.A.M.; Estevinho, M.L.M.F.; Sattler, J.A.G.; Souza, B.R.; da Silva Freitas, A.; Barth, O.M.; Almeida-Muradian, L.B. Effect of processing conditions on characteristics of dehydrated bee-pollen and correlation between quality parameters. LWT Food Sci. Technol. 2016, 65, 808–815. [Google Scholar] [CrossRef]
- Kayacan, S.; Sagdic, O.; Doymaz, I. Effects of hot-air and vacuum drying on drying kinetics, bioactive compounds and color of bee pollen. J. Food Meas. Charact. 2018, 12, 1274–1283. [Google Scholar] [CrossRef]
- Kanar, Y.; Mazı, B.G. Effect of different drying methods on antioxidant characteristics of bee-pollen. J. Food Meas. Charact. 2019, 13, 3376–3386. [Google Scholar] [CrossRef]
- Isik, A.; Ozdemir, M.; Doymaz, I. Effect of hot air drying on quality characteristics and physicochemical properties of bee pollen. Food Sci. Technol. 2019, 39, 224–231. [Google Scholar] [CrossRef]
- Loper, G.M.; Standifer, L.N.; Thompson, M.J.; Gilliam, M. Biochemistry and microbiology of bee-collected almond (Prunus dulcis) pollen and bee bread. I-Fatty Acids, Sterols, Vitamins and Minerals. Apidologie 1980, 11, 63–73. [Google Scholar] [CrossRef]
- Human, H.; Nicolson, S.W. Nutritional content of fresh, bee-collected and stored pollen of Aloe greatheadii var. davyana (Asphodelaceae). Phytochemistry 2006, 67, 1486–1492. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, J.; Zhang, Z.; Pan, Y. Proteome analysis of tea pollen (Camellia sinensis) under different storage conditions. J. Agric. Food Chem. 2008, 56, 7535–7544. [Google Scholar] [CrossRef] [PubMed]
- de Melo, I.L.P.; de Almeida-Muradian, L.B. Stability of antioxidants vitamins in bee pollen samples. Quim. Nova 2010, 33, 514–518. [Google Scholar] [CrossRef]
- Siuda, M.; Wilde, J.; Bąk, T. The effect of various storage methods on organoleptic quality of bee pollen loads. J. Apic. Sci. 2012, 56, 71–79. [Google Scholar] [CrossRef]
- de Arruda, V.A.S.; Pereira, A.A.S.; Estevinho, L.M.; de Almeida-Muradian, L.B. Presence and stability of B complex vitamins in bee pollen using different storage conditions. Food Chem. Toxicol. 2013, 51, 143–148. [Google Scholar] [CrossRef]
- Almeida-Muradian, L.B.; Pamplona, L.C.; Coimbra, S.; Barth, O.M. Chemical composition and botanical evaluation of dried bee pollen pellets. J. Food Compos. Anal. 2005, 18, 105–111. [Google Scholar] [CrossRef]
- Palla, M.; Turrini, A.; Sbrana, C.; Signorini, F.; Nicolella, C.; Benelli, G.; Canale, A.; Giovannetti, M.; Agnolucci, M. Honeybee-collected pollen for human consumption: Impact of post-harvest conditioning on the microbiota. Agrochimica 2018. [Google Scholar] [CrossRef]
- Serra Bonvehí, J.; Soliva Torrentó, M.; Centelles Lorente, E. Evaluation of polyphenolic and flavonoid compounds in honeybee-collected pollen produced in Spain. J. Agric. Food Chem. 2001, 49, 1848–1853. [Google Scholar] [CrossRef]
- Di Cagno, R.; Filannino, P.; Cantatore, V.; Gobbetti, M. Novel solid-state fermentation of bee-collected pollen emulating the natural fermentation process of bee bread. Food Microbiol. 2019, 82, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Komosinska-Vassev, K.; Olczyk, P.; Kaźmierczak, J.; Mencner, L.; Olczyk, K. Bee pollen: Chemical composition and therapeutic application. Evid. Based Complement. Altern. Med. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Barberán, F.A.; Tomás-Lorente, F.; Ferreres, F.; Garcia-Viguera, C. Flavonoids as biochemical markers of the plant origin of bee pollen. J. Sci. Food Agric. 1989, 47, 337–340. [Google Scholar] [CrossRef]
- Dias, L.G.; Tolentino, G.; Pascoal, A.; Estevinho, L.M. Efecto de las condiciones de procesado sobre los compuestos bioactivos y las propiedades biológicas del polen de abeja. J. Apic. Res. 2016, 55, 357–365. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant. Signal. Behav. 2012, 7. [Google Scholar] [CrossRef]
- Benelli, G.; Benvenuti, S.; Desneux, N.; Canale, A. Cephalaria transsylvanica-based flower strips as potential food source for bees during dry periods in European Mediterranean basin countries. PLoS ONE 2014, 9, e93153. [Google Scholar] [CrossRef]
- Di Pasquale, G.; Alaux, C.; Le Conte, Y.; Odoux, J.F.; Pioz, M.; Vaissière, B.E.; Belzunces, L.P.; Decourtye, A. Variations in the availability of pollen resources affect honey bee health. PLoS ONE 2016, 11, e0162818. [Google Scholar] [CrossRef]
- Domínguez-Valhondo, D.; Bohoyo Gil, D.; Hernández, M.T.; González-Gómez, D. Influence of the commercial processing and floral origin on bioactive and nutritional properties of honeybee-collected pollen. Int. J. Food Sci. Technol. 2011, 46, 2204–2211. [Google Scholar] [CrossRef]
- Serra Bonvehi, J.; Escolà Jordà, R. Nutrient composition and microbiological quality of honeybee-collected pollen in Spain. J. Agric. Food Chem. 1997, 45, 725–732. [Google Scholar] [CrossRef]
- Kanar, Y.; Mazı, B.G. HMF formation, diastase activity and proline content changes in bee pollen dried by different drying methods. LWT 2019, 113. [Google Scholar] [CrossRef]
- Erdtman, G. Handbook of Palynolgy: Morphology, Taxonomy, Ecology. An Introduction to the Study of Pollen Grains and Spores; Munksgaard: Copenhagen, Danmark, 1969; pp. 1–486. [Google Scholar]
- Ricciardelli, D.; Albore, G. Mediterranean Melissopalynology; Institute of Agricultural Entomology, University of Perugia: Perugia, Italy, 1998. [Google Scholar]
- Borbalan, A.M.A.; Zorro, L.; Guillen, D.A.; Barroso, C.G. Study of the polyphenol content of red and white grape varieties by liquid chromatography–mass spectrometry and its relationship to antioxidant power. J. Chromatogr. A 2003, 1012, 31–38. [Google Scholar]
- Kim, D.O.; Chun, O.K.; Kim, Y.J.; Moon, H.Y.; Lee, C.Y. Quantification of Polyphenolics and Their Antioxidant Capacity in Fresh Plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef]
- De Maesschalck, R.; Jouan-Rimbaud, D.; Massart, D.L. The Mahalanobis distance. Chemom. Intell. Lab. Syst. 2000, 50, 1–18. [Google Scholar] [CrossRef]
- Mardia, K.V.; Bookstein, F.L.; Moreton, I.J. Statistical assessment of bilateral symmetry of shapes. Biometrika 2000, 87, 285–300. [Google Scholar] [CrossRef]
| Time | Treatment | Species | Factors † | p-Value | Standard Error (SE) | ||
|---|---|---|---|---|---|---|---|
| Chestnut | Ivy | Willow | |||||
| Phenolics (mg GAE g−1 DW) | |||||||
| t0 | Fresh | 24.77 AY1 | 6.37 CY1 | 21.10 BX1 | P | *** | 0.22 |
| HAD | 23.27 AZ1 | 6.24 CY1 | 17.29 BZ1 | T | *** | ||
| FD | 24.37 AY1 | 6.83 CX1 | 18.26 BY1 | S | *** | ||
| MWD | 30.50 AX1 | 6.20 CY1 | 19.44 BY1 | P × T | ** | ||
| P × S | *** | ||||||
| t3 | HAD | 21.68 AZ2 | 5.27 CZ1 | 17.60 BX2 | S × T | ns | |
| FD | 22.87 AY2 | 6.89 CY1 | 16.65 BY2 | P × T × S | *** | ||
| MWD | 25.10 AX2 | 7.68 CX1 | 16.78 BY2 | ||||
| t6 | HAD | 20.61 AY3 | 6.20 CZ1 | 14.28 BY3 | |||
| FD | 23.05 AX3 | 7.75 CY1 | 16.11 BX3 | ||||
| MWD | 23.28 AX3 | 9.78 CX1 | 16.41 BX3 | ||||
| Flavonoids (mg of catechin equivalents g−1 DW) | |||||||
| t0 | Fresh | 10.13 Ab1 | 1.54 Cb1 | 8.20 Ba1 | P | *** | 0.10 |
| HAD | 7.25 Ac1 | 1.87 Cb1 | 5.86 Bc1 | T | *** | ||
| FD | 8.53 Ac1 | 2.00 Ca1 | 7.22 Bb1 | S | *** | ||
| MWD | 11.66 Aa1 | 1.68 Cb1 | 6.93 Bb1 | P × T | *** | ||
| P × S | *** | ||||||
| t3 | HAD | 8.13 Ab2 | 1.77 Cb1 | 6.10 Bb2 | S × T | ns | |
| FD | 9.19 Aa2 | 2.12 Ca1 | 6.53 Ba2 | P × T × S | *** | ||
| MWD | 9.72 Aa2 | 1.84 Ca1 | 6.62 Ba2 | ||||
| t6 | HAD | 7.05 Ac2 | 1.87 Cb1 | 4.57 Bc3 | |||
| FD | 7.91 Ab2 | 2.28 Ca1 | 5.95 Bb3 | ||||
| MWD | 8.47 Aa2 | 2.29 Ca1 | 6.31 Ba3 | ||||
| Rutin (µg g−1 DW) | |||||||
| t0 | Fresh | 326.35 Ab1 | 9.04 Ca1 | 78.37 Bc1 | P | *** | 3.51 |
| HAD | 242.42 Ac1 | 3.81 Cb1 | 138.04 Ba1 | T | *** | ||
| FD | 316.46 Ab1 | 10.99 Ca1 | 94.14 Bb1 | S | * | ||
| MWD | 369.29 Aa1 | 5.81 Cb1 | 121.67 Ba1 | P × T | *** | ||
| P × S | *** | ||||||
| t3 | HAD | 253.69 Ab1 | 4.21 Ca2 | 87.68 Bb2 | S × T | * | |
| FD | 331.09 Aa1 | 6.53 Ca2 | 104.00 Ba2 | P × T × S | *** | ||
| MWD | 324.93 Aa1 | 5.87 Ca2 | 56.67 Bc2 | ||||
| t6 | HAD | 287.30 Ac1 | 4.83 Ca2 | 54.89 Bc2 | |||
| FD | 363.09 Aa1 | 7.27 Ca2 | 108.68 Ba2 | ||||
| MWD | 344.20 Ab1 | 6.17 Ca2 | 86.59 Bb2 | ||||
| Time | Treatment | Species | Factors † | p-Value | Standard Error (SE) | |||
|---|---|---|---|---|---|---|---|---|
| Chestnut | Ivy | Willow | ||||||
| Total Amino Acid (mg g−1 DW) | ||||||||
| t0 | Fresh | 183.50 Cc2 | 363.15 Aa2 | 259.72 Ba2 | P | *** | 3.83 | |
| HAD | 261.14 Bb2 | 287.85 Ab2 | 261.22 Ba2 | T | *** | |||
| FD | 223.60 Bc2 | 273.92 Ac2 | 214.93 Bc2 | S | *** | |||
| MWD | 287.89 Aa2 | 287.12 Ab2 | 247.43 Bc2 | P × T | ** | |||
| P × S | *** | |||||||
| t3 | HAD | 233.15 Cb2 | 347.75 Ab1 | 247.84 Ba2 | S × T | ns | ||
| FD | 233.15 Bb2 | 327.47 Ac1 | 214.66 Cb2 | P × T × S | *** | |||
| MWD | 242.71 Ba2 | 402.28 Aa1 | 215.16 Cb2 | |||||
| t6 | HAD | 286.18 CY1 | 358.16 Ab1 | 299.59 Ba1 | ||||
| FD | 300.37 Ba1 | 345.66 Ab1 | 264.71 Cb1 | |||||
| MWD | 310.98 Ba1 | 393.61 Aa1 | 276.33 Cb1 | |||||
| Total Proline (mg g−1 DW) | ||||||||
| t0 | Fresh | 28.99 Bc1 | 95.07 Aa2 | 29.03 Bb2 | P | *** | 0.79 | |
| HAD | 57.02 Ba1 | 77.05 Ac2 | 52.13 Ca2 | T | *** | |||
| FD | 39.31 Bc1 | 71.24 Ac2 | 23.80 Cc2 | S | * | |||
| MWD | 61.06 Ba1 | 84.60 Ab2 | 25.58 Cc2 | P × T | *** | |||
| P × S | *** | |||||||
| t3 | HAD | 36.23 Cb1 | 84.64 Aa1 | 41.08 Ba1 | S × T | ** | ||
| FD | 36.23 Bb1 | 78.66 Ab1 | 22.91 Cc1 | P × T × S | *** | |||
| MWD | 45.40 Ba1 | 85.72 Aa1 | 27.63 Cb1 | |||||
| t6 | HAD | 36.75 Cb2 | 76.75 Ac1 | 44.01 Ba1 | ||||
| FD | 37.79 Bb2 | 80.78 Ab1 | 26.21 Cc1 | |||||
| MWD | 40.74 Ba2 | 95.82 Aa1 | 29.43 Cc1 | |||||
| Free Amino Acid (mg g−1 DW) | ||||||||
| t0 | Fresh | 25.01 Cb2 | 87.44 Aa1 | 27.91 Ba2 | P | *** | 0.55 | |
| HAD | 29.31 Ba2 | 69.74 Ac1 | 23.17 Cb2 | Tr | ns | |||
| FD | 21.93 Cc2 | 71.85 Ac1 | 28.40 Ba2 | Ti | *** | |||
| MWD | 22.97 Bc2 | 79.26 Ab1 | 19.90 Cc2 | P × Tr | *** | |||
| P × Ti | *** | |||||||
| t3 | HAD | 29.31 Ba2 | 58.00 Ac2 | 22.33 Ca2 | Ti × Tr | ns | ||
| FD | 23.44 Bb2 | 60.45 Ab2 | 22.37 Ba2 | P × Tr × Ti | *** | |||
| MWD | 21.63 Bb2 | 67.10 Aa2 | 17.60 Cb2 | |||||
| t6 | HAD | 28.87 Ba1 | 55.16 Aa2 | 27.40 Ba1 | ||||
| FD | 27.42 Bb1 | 53.41 Aa2 | 25.52 Cb1 | |||||
| MWD | 27.57 Bb1 | 64.86 Aa2 | 26.47 Ba1 | |||||
| Free Proline (mg g−1 DW) | ||||||||
| t0 | Fresh | 16.24 Bb1 | 63.47 Aa1 | 10.75 Ca2 | P | *** | 0.38 | |
| HAD | 17.95 Ba1 | 50.88 Ac1 | 11.04 Ca2 | T | * | |||
| FD | 13.88 Bc1 | 48.37 Ac1 | 9.17 Cb2 | S | *** | |||
| MWD | 14.97 Bc1 | 55.24 Ab1 | 7.15 Cc2 | P × T | *** | |||
| P × S | *** | |||||||
| t3 | HAD | 11.58 Bb2 | 40.43 Ab2 | 11.73 Bb2 | S × T | ns | ||
| FD | 14.04Ba2 | 36.96Ac2 | 13.56Ba2 | P × T × S | *** | |||
| MWD | 13.42 Ba2 | 42.39 Aa2 | 7.35 Cc2 | |||||
| t6 | HAD | 15.13 Ba2 | 37.37 Ab2 | 14.56 Ba1 | ||||
| FD | 14.91 Bb2 | 33.61 Ac2 | 11.42 Cb1 | |||||
| MWD | 14.31 Bb2 | 41.87 Aa2 | 9.72 Cc1 | |||||
| CAN_1 | CAN_2 | |
|---|---|---|
| Willow | ||
| Polyphenol | −0.17 | −0.34 |
| Flavonoids | −0.45 | −0.38 |
| Rutin | 0.06 | −0.07 |
| Total Amino Acids | 0.40 | 0.71 |
| Total Proline | 0.78 | 0.41 |
| Free Amino Acids | 0.46 | 0.65 |
| Free Proline | −0.06 | 0.87 |
| Variance Explained (%) | 49.38 | 31.99 |
| Cumulative Variance (%) | 49.38 | 81.37 |
| Chestnut | ||
| Polyphenol | −0.47 | 0.32 |
| Flavonoids | −0.63 | 0.24 |
| Rutin | −0.40 | 0.69 |
| Total Amino Acids | 0.63 | 0.74 |
| Total Proline | −0.03 | 0.31 |
| Free Amino Acids | 0.94 | −0.31 |
| Free Proline | 0.18 | −0.23 |
| Variance Explained (%) | 61.07 | 29.53 |
| Cumulative Variance (%) | 61.07 | 90.60 |
| Ivy | ||
| Polyphenol | −0.14 | 0.82 |
| Flavonoids | −0.57 | 0.62 |
| Rutin | 0.25 | 0.67 |
| Total Amino Acids | 0.05 | −0.04 |
| Total Proline | 0.47 | 0.07 |
| Free Amino Acids | 0.99 | −0.01 |
| Free Proline | 0.97 | −0.09 |
| Variance Explained (%) | 84.46 | 8.83 |
| Cumulative Variance (%) | 84.46 | 93.29 |
Sample Availability: Samples of the bee-collected willow, chestnut and ivy pollens are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castagna, A.; Benelli, G.; Conte, G.; Sgherri, C.; Signorini, F.; Nicolella, C.; Ranieri, A.; Canale, A. Drying Techniques and Storage: Do They Affect the Nutritional Value of Bee-Collected Pollen? Molecules 2020, 25, 4925. https://doi.org/10.3390/molecules25214925
Castagna A, Benelli G, Conte G, Sgherri C, Signorini F, Nicolella C, Ranieri A, Canale A. Drying Techniques and Storage: Do They Affect the Nutritional Value of Bee-Collected Pollen? Molecules. 2020; 25(21):4925. https://doi.org/10.3390/molecules25214925
Chicago/Turabian StyleCastagna, Antonella, Giovanni Benelli, Giuseppe Conte, Cristina Sgherri, Francesca Signorini, Cristiano Nicolella, Annamaria Ranieri, and Angelo Canale. 2020. "Drying Techniques and Storage: Do They Affect the Nutritional Value of Bee-Collected Pollen?" Molecules 25, no. 21: 4925. https://doi.org/10.3390/molecules25214925
APA StyleCastagna, A., Benelli, G., Conte, G., Sgherri, C., Signorini, F., Nicolella, C., Ranieri, A., & Canale, A. (2020). Drying Techniques and Storage: Do They Affect the Nutritional Value of Bee-Collected Pollen? Molecules, 25(21), 4925. https://doi.org/10.3390/molecules25214925









