Anti-Angiogenic Properties of Ginsenoside Rg3
Abstract
1. Introduction
2. The Controversies on the Effects of Rg3 on Angiogenesis
3. Pharmacodynamic Aspects of the Effect of Rg3 on Angiogenesis
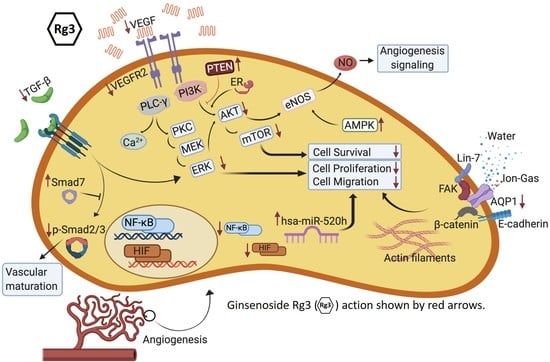
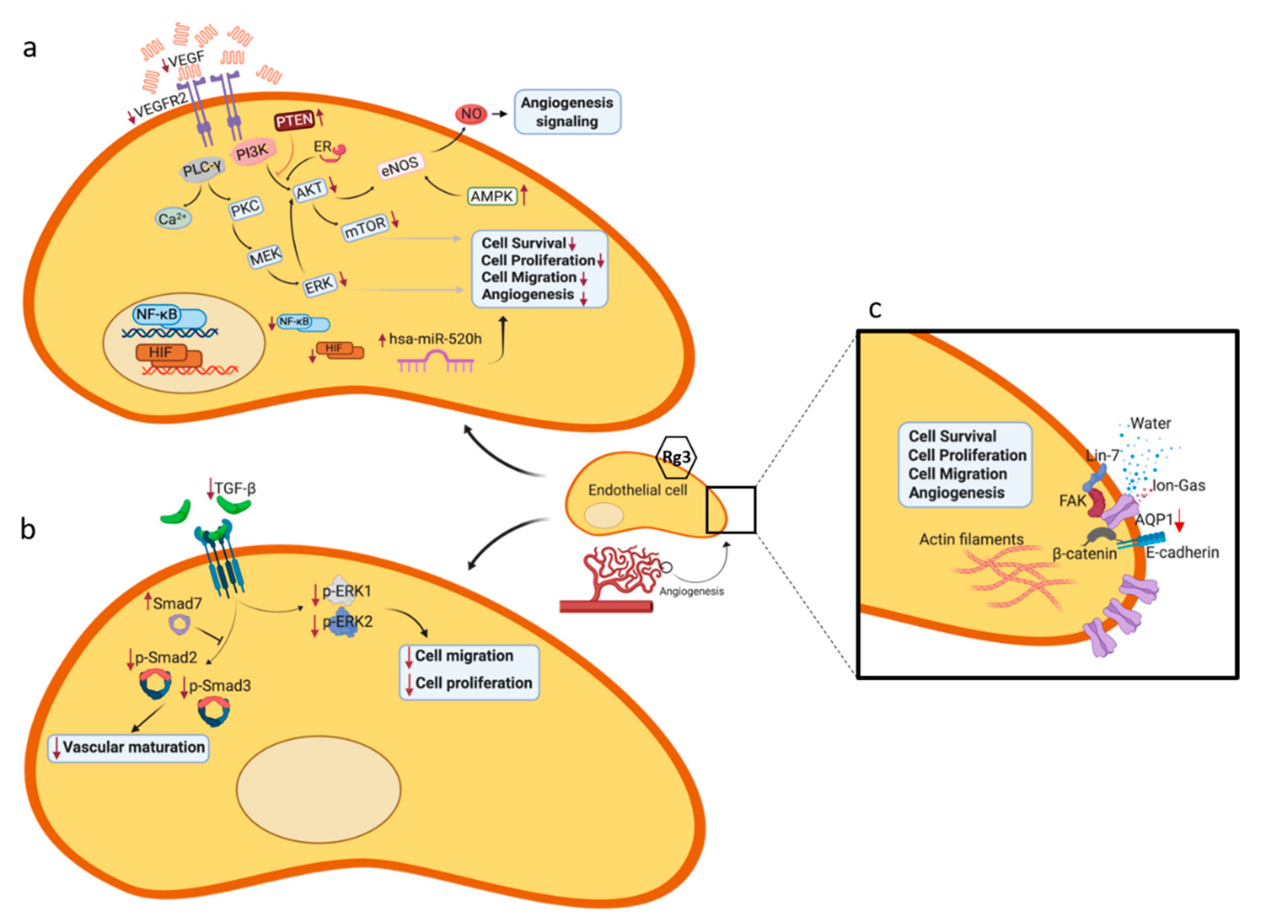
4. Molecular Mechanisms of Rg3 in Targeting Angiogenesis
4.1. VEGF and its Receptor, VEGFR2
4.2. Signaling Pathways Leading to Activation of eNOS
4.3. Role of Mammalian Target of Rapamycin (TOR), Angiogenesis and Autophagy
4.4. Signal Transducer and Activator of Transcription 3 (STAT3)
4.5. TGF-β1
4.6. Aquaporin 1 (AQP1)
4.7. MicroRNAs (miRs)
4.8. CD31 and CD34
5. Pharmacokinetic Aspects of Administering Rg3
6. Safety of Rg3
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miao, X.S.; Metcalfe, C.D.; Hao, C.; March, R.E. Electrospray ionization mass spectrometry of ginsenosides. J. Mass Spectrom. 2002, 37, 495–506. [Google Scholar] [CrossRef]
- Jo, S.K.; Kim, I.S.; Yoon, K.S.; Yoon, H.H.; Yoo, H.H. Preparation of ginsenosides Rg3, Rk1, and Rg5-selectively enriched ginsengs by a simple steaming process. Eur. Food Res. Technol. 2015, 240, 251–256. [Google Scholar] [CrossRef]
- Chen, C.-f.; Chiou, W.-f.; Zhang, J.-t. Comparison of the pharmacological effects of Panax ginseng and Panax quinquefolium. Acta Pharmacol. Sin. 2008, 29, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.-Q.; Na, J.R.; Bang, M.H.; Kim, M.K.; Yang, D.-C. Conversion of major ginsenoside Rb1 to 20 (S)-ginsenoside Rg3 by Microbacterium sp. GS514. Phytochemistry 2008, 69, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Zhou, W.; Li, X.; Feng, M.; Zhou, P. Purification method improvement and characterization of a novel ginsenoside-hydrolyzing β-glucosidase from Paecilomyces Bainier sp. 229. Biosci. Biotechnol. Biochem. 2008, 72, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, J.; Ma, S.; Liu, K. Isolation and elucidation of alkaline degradation Product from total saponins in leaves and stems of Panax quinquefolium L. J. Guangzhou Univ. Tradit. Chin. Med. 2006, 19, 142–147. [Google Scholar]
- Sun, C.; Gao, W.; Zhao, B.; Cheng, L. Optimization of the selective preparation of 20 (R)-ginsenoside Rg3 catalyzed by d, l-tartaric acid using response surface methodology. Fitoterapia 2013, 84, 213–221. [Google Scholar] [CrossRef]
- Bae, S.H.; Lee, H.-S.; Kim, M.-R.; Kim, S.Y.; Kim, J.-M.; Suh, H.J. Changes of ginsenoside content by mushroom mycelial fermentation in red ginseng extract. J. Ginseng Res. 2011, 35, 235–242. [Google Scholar] [CrossRef]
- Lee, H.S.; Lee, H.J.; Yu, H.J.; Ju, D.W.; Kim, Y.; Kim, C.T.; Kim, C.J.; Cho, Y.J.; Kim, N.; Choi, S.Y. A comparison between high hydrostatic pressure extraction and heat extraction of ginsenosides from ginseng (Panax ginseng CA Meyer). J. Sci. Food Agric. 2011, 91, 1466–1473. [Google Scholar] [CrossRef]
- Lee, S.A.; Jo, H.K.; Im, B.O.; Kim, S.; Whang, W.K.; Ko, S.K. Changes in the contents of prosapogenin in the red ginseng (Panax ginseng) depending on steaming batches. J. Ginseng Res. 2012, 36, 102–106. [Google Scholar] [CrossRef]
- Nakhjavani, M.; Hardingham, J.E.; Palethorpe, H.M.; Tomita, Y.; Smith, E.; Price, T.J.; Townsend, A.R. Ginsenoside Rg3: Potential molecular targets and therapeutic indication in metastatic breast cancer. Medicines 2019, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Nakhjavani, M.; Palethorpe, H.M.; Tomita, Y.; Smith, E.; Price, T.J.; Yool, A.J.; Pei, J.V.; Townsend, A.R.; Hardingham, J.E. Stereoselective Anti-Cancer Activities of Ginsenoside Rg3 on Triple Negative Breast Cancer Cell Models. Pharmaceuticals 2019, 12, 117. [Google Scholar] [CrossRef]
- Kang, D.-I.; Lee, J.-Y.; Yang, J.-Y.; Jeong, S.M.; Lee, J.-H.; Nah, S.-Y.; Kim, Y. Evidence that the tertiary structure of 20 (S)-ginsenoside Rg3 with tight hydrophobic packing near the chiral center is important for Na+ channel regulation. Biochem. Biophys. Res. Commun. 2005, 333, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, M.; Yoo, Y.C.; Matsuzawa, K.; Sato, K.; Saiki, I.; Tono-oka, S.; Samukawa, K.; Azuma, I. Inhibitory effect of tumor metastasis in mice by saponins, ginsenoside-Rb2, 20(R)- and 20(S)-ginsenoside-Rg3, of red ginseng. Biol. Pharm. Bull. 1995, 18, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.Y.; Wong, D.Y.; Wu, P.K.; Leung, P.Y.; Mak, N.K.; Yeung, H.W.; Liu, L.; Cai, Z.; Jiang, Z.H.; Fan, T.P.; et al. The angiosuppressive effects of 20(R)- ginsenoside Rg3. Biochem. Pharmacol. 2006, 72, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Kwon, H.K.; Jung, I.H.; Cho, Y.B.; Kim, K.J.; Kim, J.L. Anti-cancer Activities of Ginseng Extract Fermented with Phellinus linteus. Mycobiology 2009, 37, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Jung, S.Y.; Kwon, Y.H.; Lee, J.H.; Lee, Y.M.; Lee, B.Y.; Kwon, S.M. Ginsenoside Rg3 attenuates tumor angiogenesis via inhibiting bioactivities of endothelial progenitor cells. Cancer Biol. Ther. 2012, 13, 504–515. [Google Scholar] [CrossRef]
- Kim, J.W.; Jung, S.Y.; Kwon, Y.H.; Lee, S.H.; Lee, J.H.; Lee, B.Y.; Kwon, S.M. Ginsenoside Rg3 inhibits endothelial progenitor cell differentiation through attenuation of VEGF-dependent Akt/eNOS signaling. Phytother. Res. 2012, 26, 1286–1293. [Google Scholar] [CrossRef]
- Keung, M.H.; Chan, L.S.; Kwok, H.H.; Wong, R.N.; Yue, P.Y. Role of microRNA-520h in 20(R)-ginsenoside-Rg3-mediated angiosuppression. J. Ginseng Res. 2016, 40, 151–159. [Google Scholar] [CrossRef]
- Kwok, H.H.; Guo, G.L.; Lau, J.K.; Cheng, Y.K.; Wang, J.R.; Jiang, Z.H.; Keung, M.H.; Mak, N.K.; Yue, P.Y.; Wong, R.N. Stereoisomers ginsenosides-20(S)-Rg(3) and -20(R)-Rg(3) differentially induce angiogenesis through peroxisome proliferator-activated receptor-gamma. Biochem. Pharmacol. 2012, 83, 893–902. [Google Scholar] [CrossRef]
- Hien, T.T.; Kim, N.D.; Pokharel, Y.R.; Oh, S.J.; Lee, M.Y.; Kang, K.W. Ginsenoside Rg3 increases nitric oxide production via increases in phosphorylation and expression of endothelial nitric oxide synthase: Essential roles of estrogen receptor-dependent PI3-kinase and AMP-activated protein kinase. Toxicol. Appl. Pharmacol. 2010, 246, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Li, J.P.; Zhao, F.L.; Yuan, Y.; Sun, T.T.; Zhu, L.; Zhang, W.Y.; Liu, M.X. Studies on anti-angiogenesis of ginsenoside structure modification HRG in vitro. Biochem. Biophys. Res. Commun. 2017, 492, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Yu, Y.; Wang, L.; Wu, B.; Xia, L.; Feng, F.; Ling, Z.; Wang, S. Additive antiangiogenesis effect of ginsenoside Rg3 with low-dose metronomic temozolomide on rat glioma cells both in vivo and in vitro. J. Exp. Clin. Cancer Res. 2016, 35, 32. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.Z.; Usher, M.G.; Mortensen, R.M. PPARs: The vasculature, inflammation and hypertension. Curr. Opin. Nephrol. Hypertens. 2009, 18, 128–133. [Google Scholar] [CrossRef]
- Calabrese, E.J. Estrogen and related compounds: Biphasic dose responses. Crit. Rev. Toxicol. 2001, 31, 503–515. [Google Scholar] [CrossRef]
- Calabrese, E.J. Nitric oxide: Biphasic dose responses. Crit. Rev. Toxicol. 2001, 31, 489–501. [Google Scholar] [CrossRef]
- Hao, C.; Hao, W.; Wei, X.; Xing, L.; Jiang, J.; Shang, L. The role of MAPK in the biphasic dose-response phenomenon induced by cadmium and mercury in HEK293 cells. Toxicol. In Vitro 2009, 23, 660–666. [Google Scholar] [CrossRef]
- Calabrese, E.J. Opiates: Biphasic dose responses. Crit. Rev. Toxicol. 2001, 31, 585–604. [Google Scholar] [CrossRef]
- Calabrese, E.J. Dopamine: Biphasic dose responses. Crit. Rev. Toxicol. 2001, 31, 563–583. [Google Scholar] [CrossRef]
- Celik, I.; Sürücü, O.; Dietz, C.; Heymach, J.V.; Force, J.; Höschele, I.; Becker, C.M.; Folkman, J.; Kisker, O. Therapeutic efficacy of endostatin exhibits a biphasic dose-response curve. Cancer Res. 2005, 65, 11044–11050. [Google Scholar] [CrossRef]
- Weis, M.; Heeschen, C.; Glassford, A.J.; Cooke, J.P. Statins have biphasic effects on angiogenesis. Circulation 2002, 105, 739–745. [Google Scholar] [CrossRef]
- Volpert, O.V.; Ward, W.F.; Lingen, M.W.; Chesler, L.; Solt, D.B.; Johnson, M.D.; Molteni, A.; Polverini, P.J.; Bouck, N.P. Captopril inhibits angiogenesis and slows the growth of experimental tumors in rats. J. Clin. Investig. 1996, 98, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Slaton, J.W.; Perrotte, P.; Inoue, K.; Dinney, C.P.; Fidler, I.J. Interferon-α-mediated down-regulation of angiogenesis-related genes and therapy of bladder cancer are dependent on optimization of biological dose and schedule. Clin. Cancer Res. 1999, 5, 2726–2734. [Google Scholar]
- Takayasu, M.; Kajita, Y.; Suzuki, Y.; Shibuya, M.; Sugita, K.; Ishikawa, T.; Hidaka, H. Triphasic response of rat intracerebral arterioles to increasing concentrations of vasopressin in vitro. J. Cereb. Blood Flow Metab. 1993, 13, 304–309. [Google Scholar] [CrossRef]
- Oishi, M.; Inagaki, C.; Fujiwara, M.; Takaori, S.; Yajima, H.; Akazawa, Y. Possible mechanisms of the triphasic effects of neurotensin on the rat blood pressure. Jpn. J. Pharmacol. 1981, 31, 1043–1049. [Google Scholar] [CrossRef]
- James, R.C.; Franklin, M.R. The triphasic amphetamine lethal dose curve in mice and its possible relationship to drug metabolism. Toxicol. Appl. Pharmacol. 1978, 44, 63–73. [Google Scholar] [CrossRef]
- Marech, I.; Leporini, C.; Ammendola, M.; Porcelli, M.; Gadaleta, C.D.; Russo, E.; De Sarro, G.; Ranieri, G. Classical and non-classical proangiogenic factors as a target of antiangiogenic therapy in tumor microenvironment. Cancer Lett. 2016, 380, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, C.; Liu, L.; Li, X. Hepatic arterial administration of ginsenoside Rg3 and transcatheter arterial embolization for the treatment of VX2 liver carcinomas. Exp. Ther. Med. 2013, 5, 761–766. [Google Scholar] [CrossRef]
- Chen, Q.J.; Zhang, M.Z.; Wang, L.X. Gensenoside Rg3 inhibits hypoxia-induced VEGF expression in human cancer cells. Cell. Physiol. Biochem. 2010, 26, 849–858. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, Q.; Zhao, W.; Gong, Y.; Su, A.; Liu, F.; Liu, Y.; Li, Z.; Zhu, J. Ginsenoside Rg3 Inhibition of Thyroid Cancer Metastasis Is Associated with Alternation of Actin Skeleton. J. Med. Food 2018, 21, 849–857. [Google Scholar] [CrossRef]
- Tang, M.; Wang, W.; Cheng, L.; Jin, R.; Zhang, L.; Bian, W.; Zhang, Y. The inhibitory effects of 20(R)-ginsenoside Rg3 on the proliferation, angiogenesis, and collagen synthesis of hypertrophic scar derived fibroblasts in vitro. Iran. J. Basic Med. Sci. 2018, 21, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Breen, E.; Tang, K.; Olfert, M.; Knapp, A.; Wagner, P. Skeletal muscle capillarity during hypoxia: VEGF and its activation. High. Alt. Med. Biol. 2008, 9, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.S.; Tsujii, M.; Reese, J.; Dey, S.K.; DuBois, R.N. Host cyclooxygenase-2 modulates carcinoma growth. J. Clin. Investig. 2000, 105, 1589–1594. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Gordon, E.; Claesson-Welsh, L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 2016, 17, 611. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. VEGFR and type-V RTK activation and signaling. Cold Spring Harb. Perspect. Biol. 2013, 5, a009092. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; Heymach, J.V. Vascular endothelial growth factor (VEGF) pathway. J. Thorac. Oncol. 2006, 1, 768–770. [Google Scholar] [CrossRef]
- Zhuang, G.; Yu, K.; Jiang, Z.; Chung, A.; Yao, J.; Ha, C.; Toy, K.; Soriano, R.; Haley, B.; Blackwood, E. Phosphoproteomic analysis implicates the mTORC2-FoxO1 axis in VEGF signaling and feedback activation of receptor tyrosine kinases. Sci. Signal. 2013, 6, ra25. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.F.; Granger, D.N. Blood cells and endothelial barrier function. Tissue Barriers 2015, 3, e978720. [Google Scholar] [CrossRef]
- Zhang, Q.; Kang, X.; Yang, B.; Wang, J.; Yang, F. Antiangiogenic effect of capecitabine combined with ginsenoside Rg3 on breast cancer in mice. Cancer Biother. Radiopharm. 2008, 23, 647–653. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.Z.; Xing, S.P.; Zhang, J.L. Inhibiting effect of Endostar combined with ginsenoside Rg3 on breast cancer tumor growth in tumor-bearing mice. Asian Pac. J. Trop. Med. 2016, 9, 180–183. [Google Scholar] [CrossRef]
- Xu, T.M.; Xin, Y.; Cui, M.H.; Jiang, X.; Gu, L.P. Inhibitory effect of ginsenoside Rg3 combined with cyclophosphamide on growth and angiogenesis of ovarian cancer. Chin. Med. J. (Engl.) 2007, 120, 584–588. [Google Scholar] [CrossRef]
- Xu, T.M.; Cui, M.H.; Xin, Y.; Gu, L.P.; Jiang, X.; Su, M.M.; Wang, D.D.; Wang, W.J. Inhibitory effect of ginsenoside Rg3 on ovarian cancer metastasis. Chin. Med. J. (Engl.) 2008, 121, 1394–1397. [Google Scholar] [CrossRef]
- Cao, Y.; Ye, Q.; Zhuang, M.; Xie, S.; Zhong, R.; Cui, J.; Zhou, J.; Zhu, Y.; Zhang, T.; Cao, L. Ginsenoside Rg3 inhibits angiogenesis in a rat model of endometriosis through the VEGFR-2-mediated PI3K/Akt/mTOR signaling pathway. PLoS ONE 2017, 12, e0186520. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.C.; Zhang, Y.; Zhou, J.; Zhi, Q.; Wu, M.Y.; Gong, F.R.; Shen, M.; Liu, L.; Tao, M.; Shen, B.; et al. Ginsenoside Rg3 targets cancer stem cells and tumor angiogenesis to inhibit colorectal cancer progression in vivo. Int. J. Oncol. 2018, 52, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Duo, L.; Duan, P. Ginsenoside Rg3 Sensitizes Colorectal Cancer to Radiotherapy through Downregulation of Proliferative and Angiogenic Biomarkers. Evid. Based Complement. Alternat. Med. 2018, 2018, 1580427. [Google Scholar] [CrossRef]
- Liu, T.G.; Huang, Y.; Cui, D.D.; Huang, X.B.; Mao, S.H.; Ji, L.L.; Song, H.B.; Yi, C. Inhibitory effect of ginsenoside Rg3 combined with gemcitabine on angiogenesis and growth of lung cancer in mice. BMC Cancer 2009, 9, 250. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Teng, L.; Meng, Q.; Li, Y.; Sun, X.; Lu, J.; R, J.L.; Teng, L. Development of liposomal Ginsenoside Rg3: Formulation optimization and evaluation of its anticancer effects. Int. J. Pharm. 2013, 450, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Peng, H.; Ou-Yang, X.; He, X. Research on the antitumor effect of ginsenoside Rg3 in B16 melanoma cells. Melanoma Res. 2008, 18, 322–329. [Google Scholar] [CrossRef]
- Meng, L.; Ji, R.; Dong, X.; Xu, X.; Xin, Y.; Jiang, X. Antitumor activity of ginsenoside Rg3 in melanoma through downregulation of the ERK and Akt pathways. Int. J. Oncol. 2019, 54, 2069–2079. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, J.; Yan, Z. Ginsenoside Rg3 attenuates hepatoma VEGF overexpression after hepatic artery embolization in an orthotopic transplantation hepatocellular carcinoma rat model. Onco Targets Ther. 2014, 7, 1945–1954. [Google Scholar] [CrossRef][Green Version]
- Hu, S.; Zhu, Y.; Xia, X.; Xu, X.; Chen, F.; Miao, X.; Chen, X. Ginsenoside Rg3 Prolongs Survival of the Orthotopic Hepatocellular Carcinoma Model by Inducing Apoptosis and Inhibiting Angiogenesis. Anal. Cell. Pathol. (Amst.) 2019, 2019, 3815786. [Google Scholar] [CrossRef] [PubMed]
- Murohara, T.; Asahara, T.; Silver, M.; Bauters, C.; Masuda, H.; Kalka, C.; Kearney, M.; Chen, D.; Symes, J.; Fishman, M. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J. Clin. Investig. 1998, 101, 2567–2578. [Google Scholar] [CrossRef] [PubMed]
- Papapetropoulos, A.; García-Cardeña, G.; Madri, J.A.; Sessa, W.C. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J. Clin. Investig. 1997, 100, 3131–3139. [Google Scholar] [CrossRef] [PubMed]
- Gerber, H.-P.; McMurtrey, A.; Kowalski, J.; Yan, M.; Keyt, B.A.; Dixit, V.; Ferrara, N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway requirement for Flk-1/KDR activation. J. Biol. Chem. 1998, 273, 30336–30343. [Google Scholar] [CrossRef]
- Michell, B.; Griffiths, J.; Mitchelhill, K.; Rodriguez-Crespo, I.; Tiganis, T.; Bozinovski, S.; de Montellano, P.O.; Kemp, B.; Pearson, R. The Akt kinase signals directly to endothelial nitric oxide synthase. Curr. Biol. 1999, 9, 845–848. [Google Scholar] [CrossRef]
- Kim, N.D.; Kang, S.Y.; Schini, V.B. Ginsenosides evoke endothelium-dependent vascular relaxation in rat aorta. Gen. Pharmacol. 1994, 25, 1071–1077. [Google Scholar] [CrossRef]
- Kang, Y.J.; Sohn, J.-T.; Chang, K.C. Relaxation of canine corporal smooth muscle relaxation by ginsenoside saponin Rg3 is independent from eNOS activation. Life Sci. 2005, 77, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Fei, Z.; Zhang, G. Synergistic anticancer activity of 20 (S)-Ginsenoside Rg3 and Sorafenib in hepatocellular carcinoma by modulating PTEN/Akt signaling pathway. Biomed. Pharmacother. 2018, 97, 1282–1288. [Google Scholar] [CrossRef]
- Hisamoto, K.; Ohmichi, M.; Kurachi, H.; Hayakawa, J.; Kanda, Y.; Nishio, Y.; Adachi, K.; Tasaka, K.; Miyoshi, E.; Fujiwara, N. Estrogen induces the Akt-dependent activation of endothelial nitric-oxide synthase in vascular endothelial cells. J. Biol. Chem. 2001, 276, 3459–3467. [Google Scholar] [CrossRef]
- Applanat, M.P.; Buteau-Lozano, H.; Herve, M.A.; Corpet, A. Vascular endothelial growth factor is a target gene for estrogen receptor and contributes to breast cancer progression. In Hormonal Carcinogenesis V; Springer: Berlin/Heidelberg, Germany, 2008; pp. 437–444. [Google Scholar]
- Mueller, M.D.; Vigne, J.-L.; Minchenko, A.; Lebovic, D.I.; Leitman, D.C.; Taylor, R.N. Regulation of vascular endothelial growth factor (VEGF) gene transcription by estrogen receptors α and β. Proc. Natl. Acad. Sci. USA 2000, 97, 10972–10977. [Google Scholar] [CrossRef]
- Zarubin, T.; Jiahuai, H. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005, 15, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Ji, L.; Lu, B.; Wang, Z. c-Jun N-terminal kinase mediated VEGFR2 sustained phosphorylation is critical for VEGFA-induced angiogenesis in vitro and in vivo. Cell Biochem. Biophys. 2012, 64, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Park, M.; Byun, C.J.; Jo, I. c-Jun N-terminal kinase 2 phosphorylates endothelial nitric oxide synthase at serine 116 and regulates nitric oxide production. Biochem. Biophys. Res. Commun. 2012, 417, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Gee, E.; Milkiewicz, M.; Haas, T.L. p38 MAPK activity is stimulated by vascular endothelial growth factor receptor 2 activation and is essential for shear stress-induced angiogenesis. J. Cell. Physiol. 2010, 222, 120–126. [Google Scholar] [CrossRef]
- Chrestensen, C.A.; McMurry, J.L.; Salerno, J.C. MAP kinases bind endothelial nitric oxide synthase. FEBS Open Bio. 2012, 2, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Nagata, D.; Mogi, M.; Walsh, K. AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J. Biol. Chem. 2003, 278, 31000–31006. [Google Scholar] [CrossRef]
- Morrow, V.A.; Foufelle, F.; Connell, J.M.; Petrie, J.R.; Gould, G.W.; Salt, I.P. Direct activation of AMP-activated protein kinase stimulates nitric-oxide synthesis in human aortic endothelial cells. J. Biol. Chem. 2003, 278, 31629–31639. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR signaling at a glance. J. Cell Sci. 2009, 122, 3589–3594. [Google Scholar] [CrossRef]
- Karar, J.; Maity, A. PI3K/AKT/mTOR pathway in angiogenesis. Front. Mol. Neurosci. 2011, 4, 51. [Google Scholar] [CrossRef]
- Chen, Z.; Han, Z.C. STAT3: A critical transcription activator in angiogenesis. Med. Res. Rev. 2008, 28, 185–200. [Google Scholar] [CrossRef]
- Tang, M.; Bian, W.; Cheng, L.; Zhang, L.; Jin, R.; Wang, W.; Zhang, Y. Ginsenoside Rg3 inhibits keloid fibroblast proliferation, angiogenesis and collagen synthesis in vitro via the TGFbeta/Smad and ERK signaling pathways. Int. J. Mol. Med. 2018, 41, 1487–1499. [Google Scholar] [CrossRef]
- Tomita, Y.; Dorward, H.; Yool, A.J.; Smith, E.; Townsend, A.R.; Price, T.J.; Hardingham, J.E. Role of aquaporin 1 signalling in cancer development and progression. Int. J. Mol. Sci. 2017, 18, 299. [Google Scholar] [CrossRef]
- De Ieso, M.L.; Yool, A.J. Mechanisms of aquaporin-facilitated cancer invasion and metastasis. Front. Chem. 2018, 6, 135. [Google Scholar] [CrossRef]
- Perego, C.; Vanoni, C.; Massari, S.; Longhi, R.; Pietrini, G. Mammalian LIN-7 PDZ proteins associate with β-catenin at the cell–cell junctions of epithelia and neurons. EMBO J. 2000, 19, 3978–3989. [Google Scholar] [CrossRef]
- Monzani, E.; Bazzotti, R.; Perego, C.; La Porta, C.A. AQP1 is not only a water channel: It contributes to cell migration through Lin7/beta-catenin. PLoS ONE 2009, 4. [Google Scholar] [CrossRef]
- Meng, F.; Rui, Y.; Xu, L.; Wan, C.; Jiang, X.; Li, G. Aqp1 enhances migration of bone marrow mesenchymal stem cells through regulation of FAK and β-catenin. Stem Cells Dev. 2014, 23, 66–75. [Google Scholar] [CrossRef]
- Wei, X.; Dong, J. Aquaporin 1 promotes the proliferation and migration of lung cancer cell in vitro. Oncol. Rep. 2015, 34, 1440–1448. [Google Scholar] [CrossRef]
- Galán-Cobo, A.; Ramírez-Lorca, R.; Toledo-Aral, J.J.; Echevarría, M. Aquaporin-1 plays important role in proliferation by affecting cell cycle progression. J. Cell. Physiol. 2016, 231, 243–256. [Google Scholar] [CrossRef]
- Almasalmeh, A.; Krenc, D.; Wu, B.; Beitz, E. Structural determinants of the hydrogen peroxide permeability of aquaporins. FEBS J. 2014, 281, 647–656. [Google Scholar] [CrossRef]
- Tafani, M.; Sansone, L.; Limana, F.; Arcangeli, T.; De Santis, E.; Polese, M.; Fini, M.; Russo, M.A. The interplay of reactive oxygen species, hypoxia, inflammation, and sirtuins in cancer initiation and progression. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef]
- Schenk, B.; Fulda, S. Reactive oxygen species regulate Smac mimetic/TNFα-induced necroptotic signaling and cell death. Oncogene 2015, 34, 5796–5806. [Google Scholar] [CrossRef]
- Clapp, C.; de la Escalera, G.M. Aquaporin-1: A novel promoter of tumor angiogenesis. Trends Endocrinol. Metab. 2006, 17, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Saadoun, S.; Papadopoulos, M.C.; Hara-Chikuma, M.; Verkman, A. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature 2005, 434, 786–792. [Google Scholar] [CrossRef]
- Abreu-Rodríguez, I.; Silva, R.S.; Martins, A.P.; Soveral, G.; Toledo-Aral, J.J.; López-Barneo, J.; Echevarría, M. Functional and transcriptional induction of aquaporin-1 gene by hypoxia; analysis of promoter and role of Hif-1α. PLoS ONE 2011, 6, e28385. [Google Scholar] [CrossRef]
- Dorward, H.S.; Du, A.; Bruhn, M.A.; Wrin, J.; Pei, J.V.; Evdokiou, A.; Price, T.J.; Yool, A.J.; Hardingham, J.E. Pharmacological blockade of aquaporin-1 water channel by AqB013 restricts migration and invasiveness of colon cancer cells and prevents endothelial tube formation in vitro. J. Exp. Clin. Cancer Res. 2016, 35, 36. [Google Scholar] [CrossRef]
- Tomita, Y.; Palethorpe, H.M.; Smith, E.; Nakhjavani, M.; Townsend, A.R.; Price, T.J.; Yool, A.J.; Hardingham, J.E. Bumetanide-derived aquaporin 1 inhibitors, aqb013 and aqb050 inhibit tube formation of endothelial cells through induction of apoptosis and impaired migration in vitro. Int. J. Mol. Sci. 2019, 20, 1818. [Google Scholar] [CrossRef]
- Palethorpe, H.M.; Tomita, Y.; Smith, E.; Pei, J.V.; Townsend, A.R.; Price, T.J.; Young, J.P.; Yool, A.J.; Hardingham, J.E. The aquaporin 1 inhibitor bacopaside II reduces endothelial cell migration and tubulogenesis and induces apoptosis. Int. J. Mol. Sci. 2018, 19, 653. [Google Scholar] [CrossRef]
- Pan, X.-Y.; Guo, H.; Han, J.; Hao, F.; An, Y.; Xu, Y.; Xiaokaiti, Y.; Pan, Y.; Li, X.-J. Ginsenoside Rg3 attenuates cell migration via inhibition of aquaporin 1 expression in PC-3M prostate cancer cells. Eur. J. Pharmacol. 2012, 683, 27–34. [Google Scholar] [CrossRef]
- Peng, M.; Li, X.; Zhang, T.; Ding, Y.; Yi, Y.; Le, J.; Yang, Y.; Chen, X. Stereoselective pharmacokinetic and metabolism studies of 20 (S)-and 20 (R)-ginsenoside Rg3 epimers in rat plasma by liquid chromatography-electrospray ionization mass spectrometry. J. Pharm. Biomed. Anal. 2016, 121, 215–224. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, X.-y.; Li, K.; Li, A.-p.; Yang, W.-d.; Yang, R.; Wang, P.; Zhao, Z.-h.; Cui, F.; Qin, Y. Protopanaxadiol inhibits epithelial–mesenchymal transition of hepatocellular carcinoma by targeting STAT3 pathway. Cell Death Dis. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Song, W.; Zhang, Y.; Dong, X.; Tan, M. Ginsenoside Rh2 Inhibits Migration of Lung Cancer Cells under Hypoxia via mir-491. Anti-Cancer Agents Med. Chem. 2019, 19, 1633–1641. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.-T.; Wang, G.-J.; Sun, J.-G.; Tucker, I.; Zhao, X.-C.; Xie, Y.-Y.; Li, H.; Jiang, X.-l.; Wang, R.; Xu, M.-J. High performance liquid chromatographic–mass spectrometric determination of ginsenoside Rg3 and its metabolites in rat plasma using solid-phase extraction for pharmacokinetic studies. J. Chromatogr. B 2005, 818, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Qian, T.; Wong, R.N.; Jiang, Z.-H. Liquid chromatography–electrospray ionization mass spectrometry for metabolism and pharmacokinetic studies of ginsenoside Rg3. Anal. Chim. Acta 2003, 492, 283–293. [Google Scholar] [CrossRef]
- Qian, T.; Cai, Z.; Wong, R.N.; Mak, N.K.; Jiang, Z.-H. In vivo rat metabolism and pharmacokinetic studies of ginsenoside Rg3. J. Chromatogr. B 2005, 816, 223–232. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Li, G.; Wang, Z.; Sun, Y.; Liu, K.; Wang, Z. Ginsenoside 20 (S)-protopanaxadiol inhibits the proliferation and invasion of human fibrosarcoma HT1080 cells. Basic Clin. Pharmacol. Toxicol. 2006, 98, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-J.; Zhang, X.-J.; Shui, Y.-M.; Wan, J.-B.; Gao, J.-L. Anticancer activities of protopanaxadiol-and protopanaxatriol-type ginsenosides and their metabolites. Evid. Based Complement. Alternat. Med. 2016, 2016. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, H.; Han, Z.; Li, W.; Mai, Z.; Yuan, R. Ginsenoside Rh2 inhibits angiogenesis in prostate cancer by targeting CNNM1. J. Nanosci. Nanotechnol. 2019, 19, 1942–1950. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, P.; Jiang, J.; Hu, P. Pharmacokinetics of single ascending doses and multiple doses of 20 (S)-ginsenoside Rg3 in Chinese healthy volunteers. Eur. J. Drug Metab. Pharmacokinet. 2016, 41, 845–853. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.; Li, G.; Wang, Z.; Yang, J.; Li, Y.; Wang, H.; Jin, H.; Qiao, J.; Wang, H. Acute and repeated dose 26-week oral toxicity study of 20 (S)-ginsenoside Rg3 in Kunming mice and Sprague–Dawley rats. J. Ginseng Res. 2020, 44, 222–228. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, G.; Wang, T.; Li, G.; Lin, J.; Sun, L.; Wu, X.; Sun, X.; Wang, H.; Li, C. A 26-week 20 (S)-ginsenoside Rg3 oral toxicity study in Beagle dogs. Regul. Toxicol. Pharmacol. 2020, 110, 104522. [Google Scholar] [CrossRef]
- Lu, P.; Su, W.; Miao, Z.-h.; Niu, H.-r.; Liu, J.; Hua, Q.-l. Effect and mechanism of ginsenoside Rg3 on postoperative life span of patients with non-small cell lung cancer. Chin. J. Integr. Med. 2008, 14, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Niu, K.; Chen, X.; Xia, L.; Lu, D.; Kong, R.; Chen, Z.; Duan, Y.; Sun, J. Clinical benefit from EGFR-TKI plus ginsenoside Rg3 in patients with advanced non-small cell lung cancer harboring EGFR active mutation. Oncotarget 2016, 7, 70535. [Google Scholar] [CrossRef]
- Zhou, B.; Yan, Z.; Liu, R.; Shi, P.; Qian, S.; Qu, X.; Zhu, L.; Zhang, W.; Wang, J. Prospective study of transcatheter arterial chemoembolization (TACE) with ginsenoside Rg3 versus TACE alone for the treatment of patients with advanced hepatocellular carcinoma. Radiology 2016, 280, 630–639. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
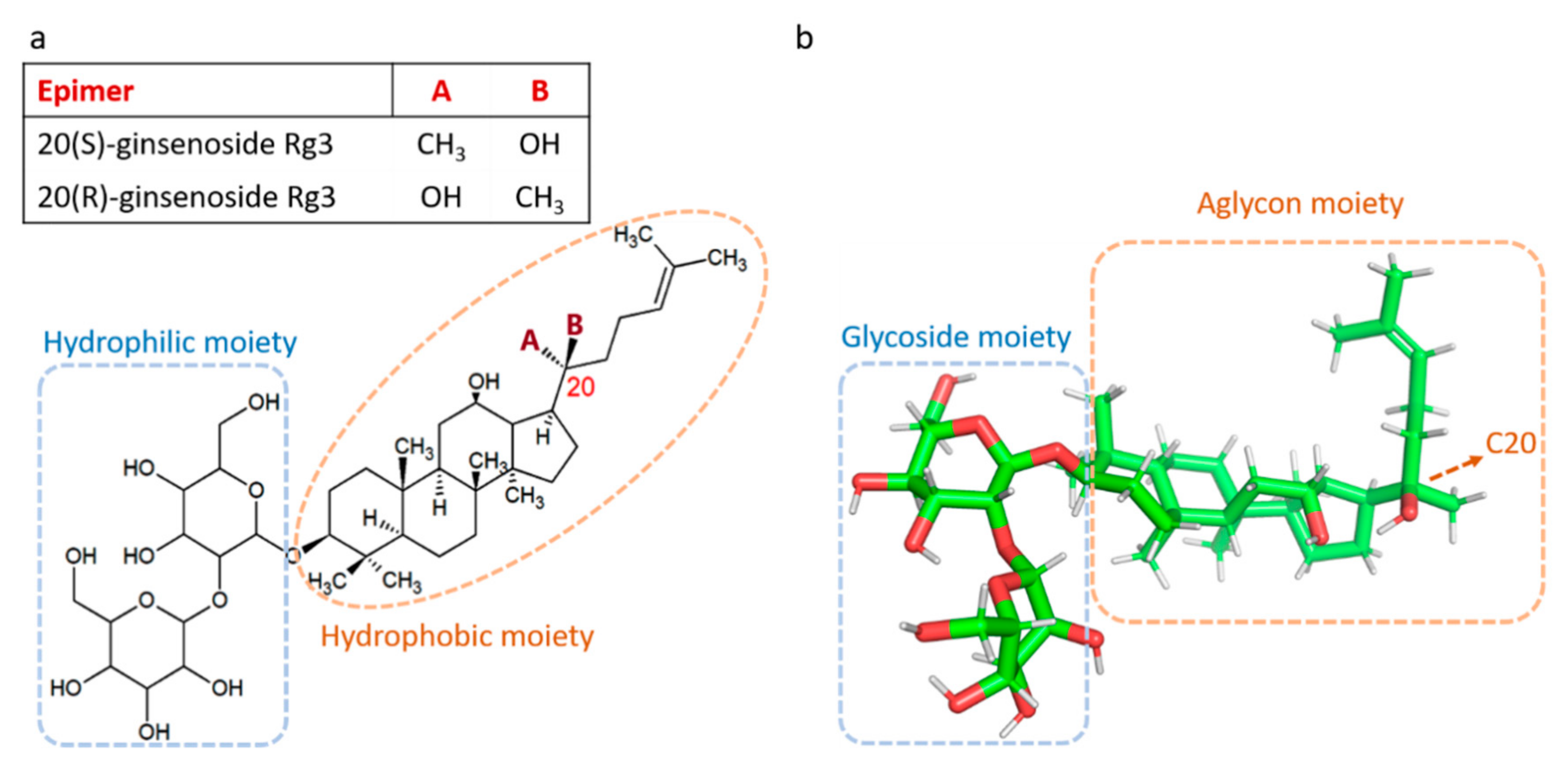
| Epimer | Concentration | Tested Cell | Effect | Ref | |
|---|---|---|---|---|---|
| Anti-angiogenic | RRg3 | 1–1000 nM | HUVEC | ↓ tube-formation ↓ chemotactic migration ↓ microvascular sprouting ↓ hemoglobin content of tumors | [15] |
| Rg3 | 1.3 µM | HUVEC | ↓ tube-forming capacity ↓ hemoglobin content of Matrigel plugs | [16] | |
| Rg3 | 60–600 nm/mL | EPC | ↓ expression of VEGF and VEGFR2 ↓ proliferation, migration and tube formation | [17] | |
| Rg3 | 60, 300 ng/mL | EPC | inhibition of differentiation | [18] | |
| RRg3 | 100 nM | HUVEC | ↑ miR-520h ↓ EphB2 and EphB4 ↓ proliferation and loop formation | [19] | |
| Pro-angiogenic | Rg3 | 1–10 µg/mL | ECV 304 | ↑ expression and phosphorylation of eNOS ↑ expression of PI3K, JNK, p38 MAPK ↑ gene transcription mediated by ER and GR ↑ CaMK-II and AMPK | [21] |
| SRg3 | 15 µM | HUVEC | ↑ proliferation (50%) ↑ DNA synthesis ↑ migration ↑ loop formation ↑ activation of ERK/Akt/eNOS ↑activation of PPARγ | [20] | |
| RRg3 | 15 µM | HUVEC | ↑ proliferation (10%) ↑ loop formation | ||
| Anti-angiogenic | RRg3 | 65 µM | HUVEC | ↓ tube formation and migration ↓ protein and transcript expression of VEGF, b-FGF, MMP-2, MMP-9 | [22] |
| Rg3 | 180 µg/mL | HUVEC | ↓ proliferation ↓ expression of VEGF and Bcl-2 S-phase cell cycle arrest | [23] |
| Cancer | Animal Model | Rg3, Dose and Route of Administration | Other Drugs in Study | Results | Ref |
|---|---|---|---|---|---|
| Breast | BALB/c mouse | 10 mg/kg/day, p.o. | Low dose capecitabine, 200 mg/kg/day, p.o. | ↓ MVD a and VEGF expression (especially in the combination group) | [49] |
| Nude mouse | 5 mg/kg q.a.d., s.c. | Recombinant human endostatin, 10 mg/kg, q.a.d. | ↓ VEGF-A, -B, -C (especially in the combination group), proteins involved in autophagy pathway, mTOR, PI3K, Akt, JNK and Beclin-1 | [50] | |
| Ovary | Nude mouse | i.p. | Cyclophosphamide | ↓ MVD and VEGF expression (combination) | [51] |
| Nude mouse | 0.3, 1 and 3 mg/kg/d for 20 days, i.p. | ↓ number of vessels oriented toward the tumor mass | [52] | ||
| Uterus | Rats | 5 or 10 mg/kg/d for 21 days | Gestrinone | Rg3 (10 mg/kg/d) + gestrinone significantly decreased the expression of VEGF, VEGFR2, p-Akt and p-mTOR, suggesting Rg3 blocks the effect of VEGFR2 via PI3K/Akt/mTOR signaling pathway | [53] |
| Colorectal cancer | Nude mouse | 25 mg/kg/d for 12 days, gastric perfusion | Inhibited the expression of angiogenesis-related genes, MVD and decreased neo-vessel formation | [54] | |
| Nude mouse | 10 mg/kg/d for 30 days, p.o. | Radiotherapy twice weekly (2 Gy) for 2 weeks | ↑ effects of radiation on the expression of CD31 | [55] | |
| Thyroid | Nude mouse | 10 mg/kg/d, intragastric | ↓ CD31 in the tumors | [40] | |
| Lung | Mouse | 20 mg/kg/day for 18 days, (gastric perfusion) | Gemcitabine, 10 mg/kg, i.p. every 3rd day | ↓ VEGF expression, MVD and signals of blood flow and peak systolic velocity of the tumor | [56] |
| Mouse | 600 µg/kg/day (p.o.) for 23 days | ↓ arterial and capillary density, decreased number of CD34+/VEGFR2+ EPCs | [17] | ||
| Wistar rats | 1 mg/kg | ↓ tumor volume and MVD | [57] | ||
| Melanoma | C57BL/6 mouse | 1.5 mg/kg every other day for 20 days (i.v.) | ↓ MVD | [58] | |
| C57BL/6 mouse | 0.3, 1.0 or 3.0 mg/kg Rg3 (i.p.) for 10 days | 5-Fluorouracil, 20 mg/kg | ↓ vessel numbers, MVD and VEGF and proliferating cell nuclear antigen (PCNA) | [59] | |
| Liver | A rabbit model of liver VX2 carcinoma | 6 mg/kg (i.v.) | TAE b | ↓ CD31 and VEGF and ↑ Bcl-2 and caspase-3 | [38] |
| Buffalo rat | 1 mg/kg (i.p.) | TAE b | ↓ MVD, CD31 expression, VEGF overexpression, and VEGFR2 expression and phosphorylation | [60] | |
| C57BL/6 mouse | 10 mg/kg for 10 days | ↓ MVD | [61] | ||
| Glioma | Rat | 10 mg/kg/d for 8 days (p.o.) | LDT c 5 mg/kg/d for 8 days MDT d 30 mg/kg/d for 3 days | ↑ rCBV e; Untreated: 90% Rg3: 65% MDT: 64% LDT: 51% LDT + Rg3: 15%. ↓ MVD | [23] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakhjavani, M.; Smith, E.; Townsend, A.R.; Price, T.J.; Hardingham, J.E. Anti-Angiogenic Properties of Ginsenoside Rg3. Molecules 2020, 25, 4905. https://doi.org/10.3390/molecules25214905
Nakhjavani M, Smith E, Townsend AR, Price TJ, Hardingham JE. Anti-Angiogenic Properties of Ginsenoside Rg3. Molecules. 2020; 25(21):4905. https://doi.org/10.3390/molecules25214905
Chicago/Turabian StyleNakhjavani, Maryam, Eric Smith, Amanda R. Townsend, Timothy J. Price, and Jennifer E. Hardingham. 2020. "Anti-Angiogenic Properties of Ginsenoside Rg3" Molecules 25, no. 21: 4905. https://doi.org/10.3390/molecules25214905
APA StyleNakhjavani, M., Smith, E., Townsend, A. R., Price, T. J., & Hardingham, J. E. (2020). Anti-Angiogenic Properties of Ginsenoside Rg3. Molecules, 25(21), 4905. https://doi.org/10.3390/molecules25214905