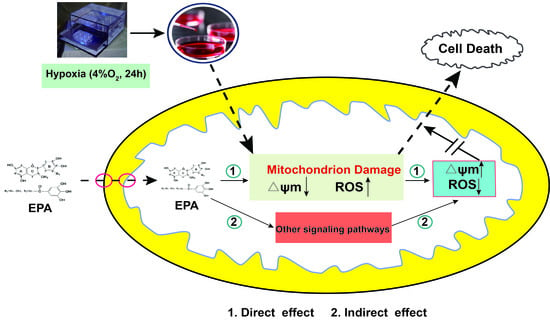
Phenolic Acids-Rich Fractions from Agaricus bitorguis (Quél.) Sacc. Chaidam ZJU-CDMA-12 Mycelia Modulate Hypoxic Stress on Hypoxia-Damaged PC12 Cells
Abstract
:1. Introduction
2. Results
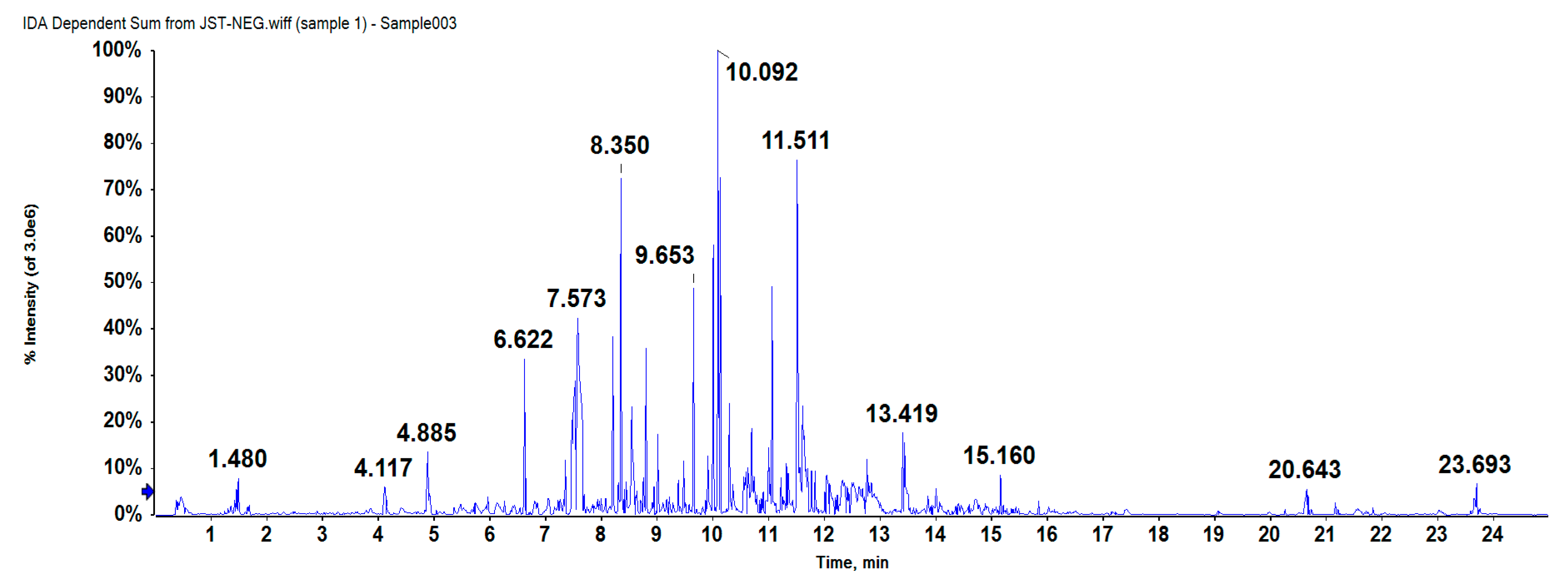
2.1. Compound Separation and Identification
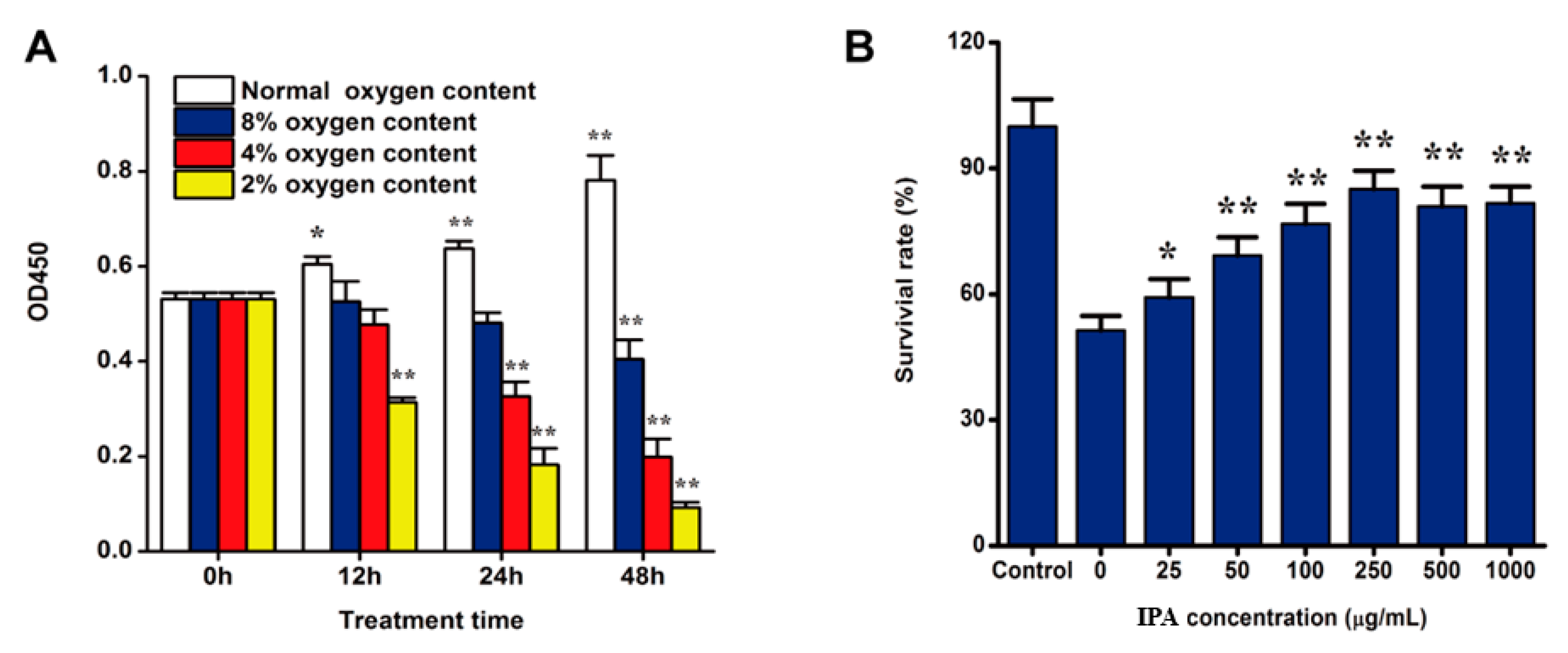
2.2. Hypoxia Induced PC12 Cell Injury
2.3. IPA Promotes the Survival of Hypoxia-Damaged PC12 Cells
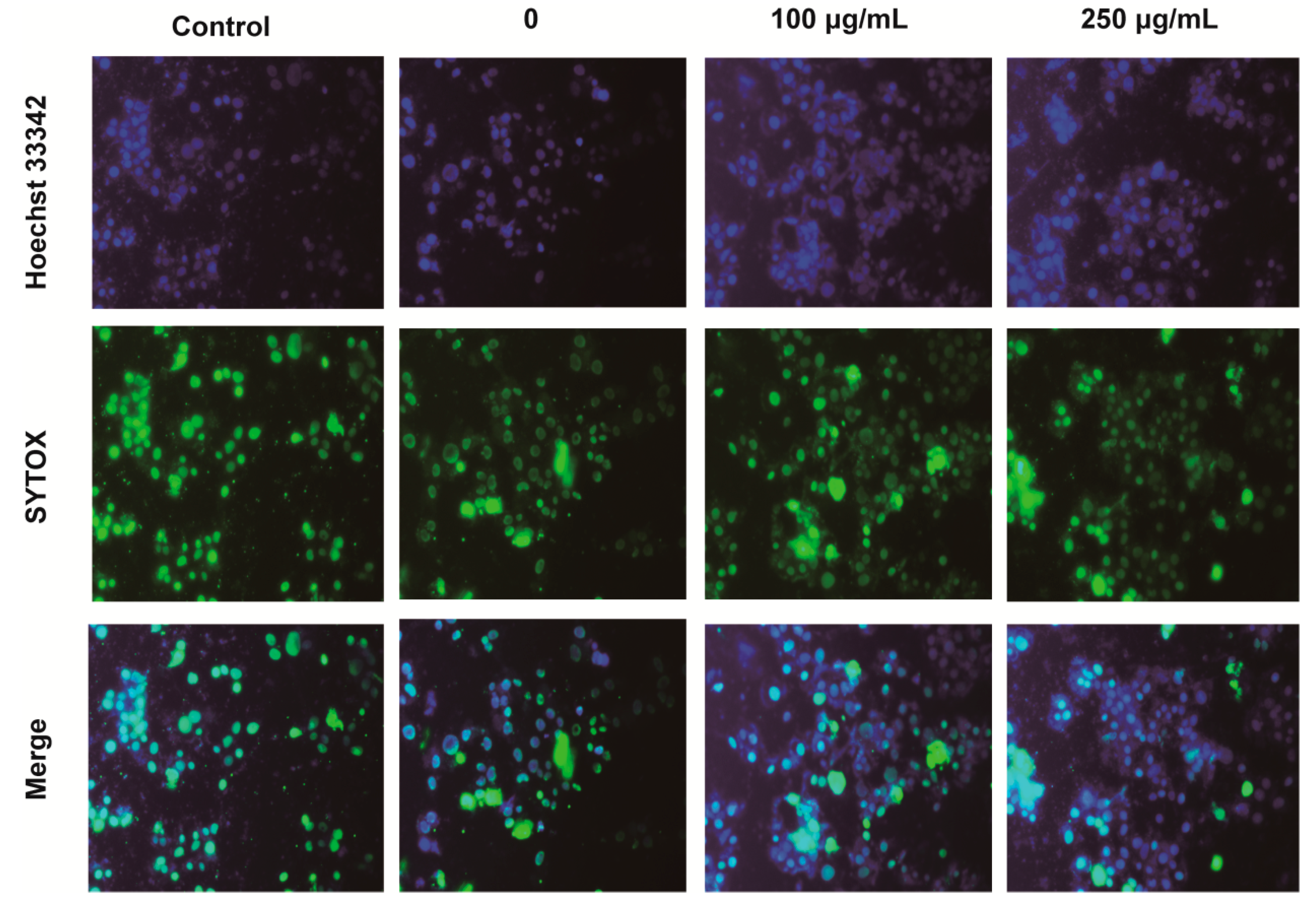
2.4. IPA Decreases the Number of Apoptotic and Necrotic Hypoxia-Damaged Cells
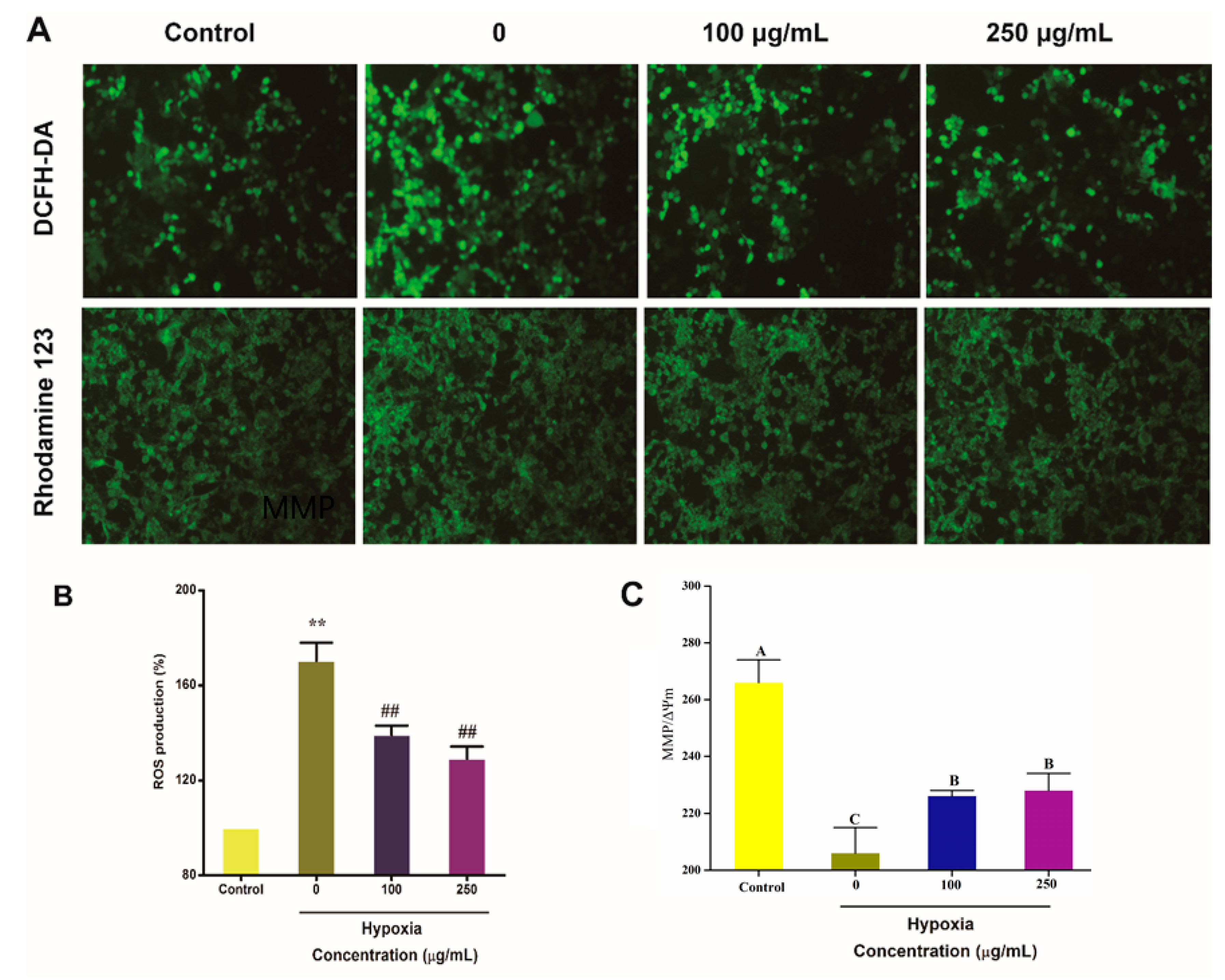
2.5. IPA Protects Hypoxia-Damaged PC12 Cells from Overproduced ROS Damage and Mitochondrial Damage
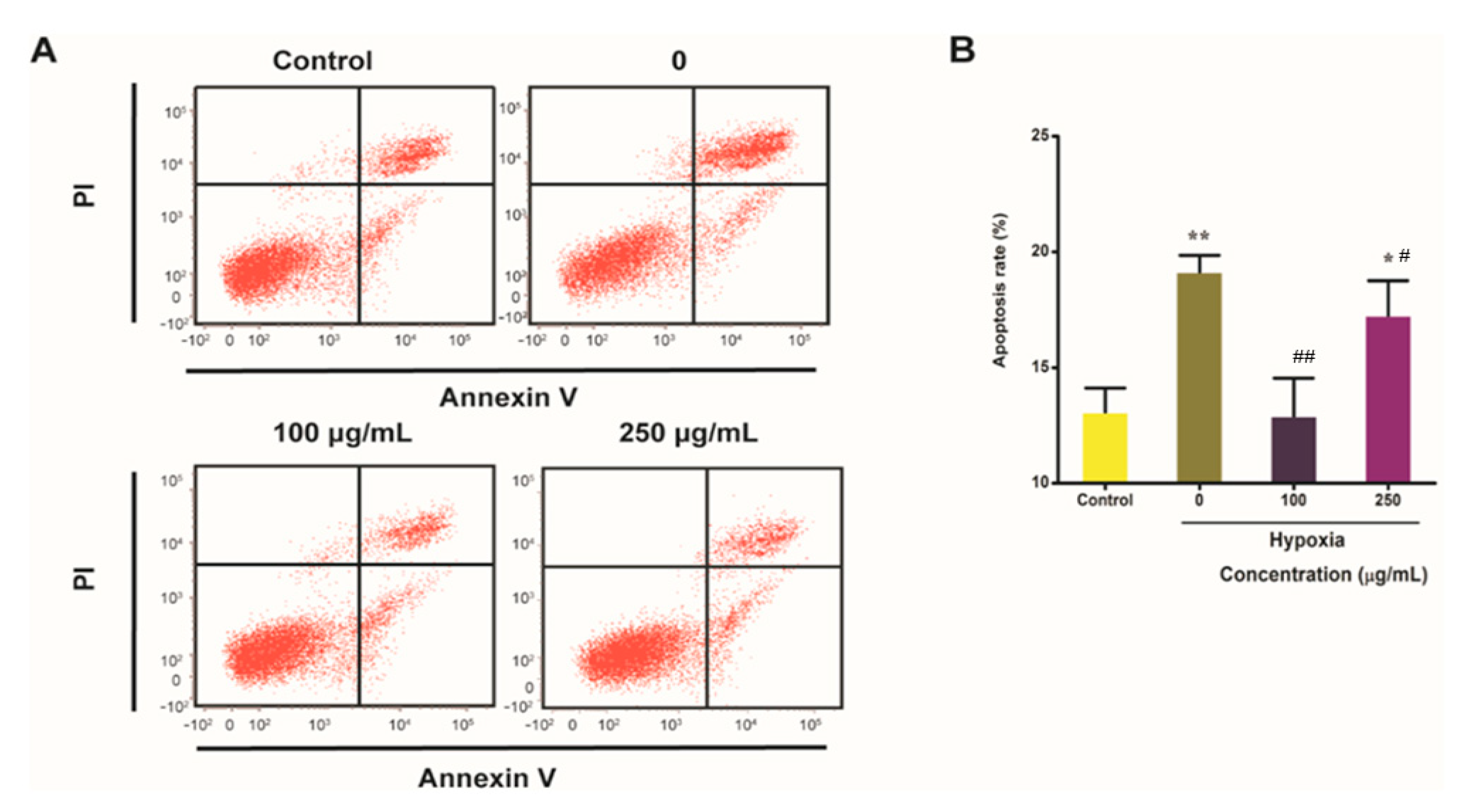
2.6. IPA Reduces Hypoxia-Induced Apoptosis Rate of PC12 Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Determination of IPA Content
4.3. Isolation and Characterization of IPA
4.3.1. Isolation Procedure
4.3.2. HPLC-Q-TOF-MS Analysis
4.4. Anti-Hypoxia Activity
4.4.1. Cell Culture and Cytotoxicity Assay
4.4.2. Treatment Condition
4.4.3. CCK-8 Assay
4.4.4. Hoechst 33342 and SYTOX Staining
4.4.5. Intracellular ROS Measurement and Mitochondrial Membrane Potential (∆ψm)
4.4.6. Detection of Cell Apoptosis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- de Mejia, E.G.; Ramirez-Mares, M.V.; Puangpraphant, S. Bioactive components of tea: Cancer, inflammation and behavior. Brain Behav. Immun. 2009, 23, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, A.; Viane, J.; Mahjoub, M.A.; Majouli, K.; Gad, M.H.H.; Kharbach, M.; Demeyer, K.; Marzouk, Z.; Heyden, Y.V. Polyphenolic contents, antioxidant activities and UPLC-ESI-MS analysis of Haplophyllum tuberculatum A. Juss leaves extracts. Int. J. Biol. Macromol. 2018, 106, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Holt, P.R. Dairy foods and prevention of colon cancer: Human studies. J. Am. Coll. Nutr. 1999, 18, 379S–391S. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Wang, Y.; Yang, M.; Cao, J.; Khan, A.; Cheng, G. UHPLC-ESI-HRMS/MS analysis on phenolic compositions of different E Se tea extracts and their antioxidant and cytoprotective activities. Food Chem. 2020, 318, 126512. [Google Scholar] [CrossRef]
- Rahman, I.; Biswas, S.K.; Kirkham, P.A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol. 2006, 72, 1439–1452. [Google Scholar] [CrossRef]
- Ahmad, N.; Mahmood, F.; Khalil, S.A.; Zamir, R.; Fazal, H.; Abbasi, B.H. Antioxidant activity via DPPH, gram-positive and gram-negative antimicrobial potential in edible mushrooms. Toxicol. Ind. Health 2014, 30, 826–834. [Google Scholar] [CrossRef]
- Carneiro, A.A.J.; Ferreira, I.C.F.R.; Duenas, M.; Barros, L.; da Silva, R.; Gomes, E.; Santos-Buelga, C. Chemical composition and antioxidant activity of dried powder formulations of Agaricus blazei and Lentinus edodes. Food Chem. 2013, 138, 2168–2173. [Google Scholar] [CrossRef]
- Gasecka, M.; Siwulski, M.; Mleczek, M. Evaluation of bioactive compounds content and antioxidant properties of soil-growing and wood-growing edible mushrooms. J. Food Process. Preserv. 2018, 42, e13386. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.; Liu, H.-W.; Zhao, W.-M.; Wei, D.-Z.; Zhong, J.-J. Ganoderic acid T from Ganoderma lucidum mycelia induces mitochondria mediated apoptosis in lung cancer cells. Life Sci. 2006, 80, 205–211. [Google Scholar] [CrossRef]
- Bach, F.; Zielinski, A.A.F.; Helm, C.V.; Maciel, G.M.; Pedro, A.C.; Stafussa, A.P.; Avila, S.; Haminiuk, C.W.I. Bio compounds of edible mushrooms: In vitro antioxidant and antimicrobial activities. LWT-Food Sci. Technol. 2019, 107, 214–220. [Google Scholar] [CrossRef]
- Lin, J.-T.; Liu, C.-W.; Chen, Y.-C.; Hu, C.-C.; Juang, L.-D.; Shiesh, C.-C.; Yang, D.-J. Chemical composition, antioxidant and anti-inflammatory properties for ethanolic extracts from Pleurotus eryngii fruiting bodies harvested at different time. LWT-Food Sci. Technol. 2014, 55, 374–382. [Google Scholar] [CrossRef]
- Jha, N.K.; Jha, S.K.; Sharma, R.; Kumar, D.; Ambasta, R.K.; Kumar, P. Hypoxia-Induced Signaling Activation in Neurodegenerative Diseases: Targets for New Therapeutic Strategies. J. Alzheimers Dis. 2018, 62, 15–38. [Google Scholar] [CrossRef]
- Clanton, T.L. Hypoxia-induced reactive oxygen species formation in skeletal muscle. J. Appl. Physiol. 2007, 102, 2379–2388. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Qiu, H.; Xiao, Q.; Le, W. Chronic Hypoxia-Induced Autophagy Aggravates the Neuropathology of Alzheimer’s Disease through AMPK-mTOR Signaling in the APP(Swe)/PS1(dE9) Mouse Model. J. Alzheimers Dis. 2015, 48, 1019–1032. [Google Scholar] [CrossRef]
- Hao, S.; Li, X.; Han, A.; Yang, Y.; Luo, X.; Fang, G.; Wang, H.; Liu, J.; Wang, S. Hydroxycinnamic Acid from Corncob and Its Structural Analogues Inhibit Aβ40 Fibrillation and Attenuate Aβ40-Induced Cytotoxicity. J. Agric. Food Chem. 2020, 68, 8788–8796. [Google Scholar] [CrossRef]
- Wu, H.; Luo, D.; Li, C.X.; Zhang, H.A.S.X.; Zhang, Y.X.; Sun, C. Chicoric Acid Improves Heart and Blood Responses to Hypobaric Hypoxia in Tibetan Yaks. Am. J. Chin. Med. 2018, 46, 339–355. [Google Scholar] [CrossRef]
- Mishra, J.; Joshi, A.; Rajput, R.; Singh, K.; Bansal, A.; Misra, K. Phenolic Rich Fractions from Mycelium and Fruiting Body of Ganoderma lucidum Inhibit Bacterial Pathogens Mediated by Generation of Reactive Oxygen Species and Protein Leakage and Modulate Hypoxic Stress in HEK 293 Cell Line. Adv. Pharmacol. Sci. 2018, 2018, 10. [Google Scholar] [CrossRef]
- Jiao, Y.C.; Kuang, H.; Hu, J.J.; Chen, Q.H. Structural characterization and anti-hypoxia activities of polysaccharides from the sporocarp, fermentation broth and cultured mycelium of Agaricus bitorquis (Quel.) Sacc. Chaidam in mice. J. Funct. Foods 2018, 51, 75–85. [Google Scholar] [CrossRef]
- Jiao, Y.; Kuang, H.; Wu, J.; Chen, Q. Polysaccharides from the Edible Mushroom Agaricus bitorquis (Quel.) Sacc. Chaidam Show Anti-hypoxia Activities in Pulmonary Artery Smooth Muscle Cells. Int. J. Mol. Sci. 2019, 20, 637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ying-chun, J.; Hui, K.; Jia-nan, W.; Cheng-ri, H.; Qi-he, C. The anti-fatigue activities of polysaccharides extracted from the Agaricus bitorquis (QueL.) Sacc.Chaidam. Mod. Food Sci. Technol. 2018, 34, 24–31. [Google Scholar] [CrossRef]
- Jeon, S.Y.; Bae, K.H.; Seong, Y.H.; Song, K.S. Green tea catechins as a BACE1 (beta-secretase) inhibitor. Bioorg. Med. Chem. Lett. 2003, 13, 3905–3908. [Google Scholar] [CrossRef]
- Sridhar, C.; Subbaraju, G.V.; Venkateswarlu, Y.; Venugopal, R.T. New acylated iridoid glucosides from Vitex altissima. J. Nat. Prod. 2004, 67, 2012–2016. [Google Scholar] [CrossRef]
- Griffith, D.R.; Botta, L.; St Denis, T.G.; Snyder, S.A. Explorations of Caffeic Acid Derivatives: Total Syntheses of Rufescenolide, Yunnaneic Acids C and D, and Studies toward Yunnaneic Acids A and B. J. Org. Chem. 2014, 79, 88–105. [Google Scholar] [CrossRef]
- Tanaka, T.; Nishimura, A.; Kouno, I.; Nonaka, G.; Young, T.J. Isolation and characterization of Yunnaneic acids A-D, four novel caffeic acid metabolites from Salvia yunnanensis. J. Nat. Prod. 1996, 59, 843–849. [Google Scholar] [CrossRef]
- Schmidt, C.A.; Murillo, R.; Bruhn, T.; Bringmann, G.; Goettert, M.; Heinzmann, B.; Brecht, V.; Laufer, S.A.; Merfort, I. Catechin Derivatives from Parapiptadenia rigida with in Vitro Wound-Healing Properties. J. Nat. Prod. 2010, 73, 2035–2041. [Google Scholar] [CrossRef]
- Alonso, D.; Serrano, E.; Bermejo, F.J.; Corral, R.S. HIF-1 alpha-regulated MIF activation and Nox2-dependent ROS generation promote Leishmania amazonensis killing by macrophages under hypoxia. Cell. Immunol. 2019, 335, 15–21. [Google Scholar] [CrossRef]
- Carneiro, P.P.; Conceicao, J.; Macedo, M.; Magalhaes, V.; Carvalho, E.M.; Bacellar, O. The Role of Nitric Oxide and Reactive Oxygen Species in the Killing of Leishmania braziliensis by Monocytes from Patients with Cutaneous Leishmaniasis. PLoS ONE 2016, 11, e0148084. [Google Scholar] [CrossRef] [Green Version]
- Fuhrmann, D.C.; Bruene, B. Mitochondrial composition and function under the control of hypoxia. Redox Biol. 2017, 12, 208–215. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar]
- Dhar, P.; Das, S.K.; Barhwal, K.; Hota, S.K.; Mishra, K.P.; Singh, S.B. Trans-Himalayan Phytococktail Confers Protection Against Hypobaric Hypoxia-Induced Hippocampal Neurodegeneration and Memory Impairment in Male Sprague Dawley Rats. High Alt. Med. Biol. 2019, 20, 279–292. [Google Scholar] [CrossRef]
- Webster, K.A. Mitochondrial membrane permeabilization and cell death during myocardial infarction: Roles of calcium and reactive oxygen species. Future Cardiol. 2012, 8, 863–884. [Google Scholar] [CrossRef] [Green Version]
- Tait, S.W.G.; Green, D.R. Mitochondria and cell death: Outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 2010, 11, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Silachev, D.N.; Plotnikov, E.Y.; Pevzner, I.B.; Zorova, L.D.; Balakireva, A.V.; Gulyaev, M.V.; Pirogov, Y.A.; Skulachev, V.P.; Zorov, D.B. Neuroprotective Effects of Mitochondria-Targeted Plastoquinone in a Rat Model of Neonatal Hypoxic-Ischemic Brain Injury. Molecules 2018, 23, 1871. [Google Scholar] [CrossRef] [Green Version]
- Ten, V.S.; Starkov, A. Hypoxic-ischemic injury in the developing brain: The role of reactive oxygen species originating in mitochondria. Neurol. Res. Int. 2012, 2012, 542976. [Google Scholar] [CrossRef] [Green Version]
- Arteaga, O.; Revuelta, M.; Urigueen, L.; Alvarez, A.; Montalvo, H.; Hilario, E. Pretreatment with Resveratrol Prevents Neuronal Injury and Cognitive Deficits Induced by Perinatal Hypoxia-Ischemia in Rats. PLoS ONE 2015, 10, e0142424. [Google Scholar] [CrossRef] [Green Version]
- Luo, C.; Li, Q.; Gao, Y.; Shen, X.; Ma, L.; Wu, Q.; Wang, Z.; Zhang, M.; Zhao, Z.; Chen, X.; et al. Poloxamer 188 Attenuates Cerebral Hypoxia/Ischemia Injury in Parallel with Preventing Mitochondrial Membrane Permeabilization and Autophagic Activation. J. Mol. Neurosci. 2015, 56, 988–998. [Google Scholar] [CrossRef]
- Digicaylioglu, M.; Bichet, S.; Marti, H.H.; Wenger, R.H.; Rivas, L.A.; Bauer, C.; Gassmann, M. Localization of Specific Erythropoietin Binding-Sites in Defined Areas of the Mouse-Brain. Proc. Natl. Acad. Sci. USA 1995, 92, 3717–3720. [Google Scholar] [CrossRef] [Green Version]
- Duncan, O.F.; Bateman, J.M. Mitochondrial retrograde signaling in the Drosophila nervous system and beyond. Fly 2016, 10, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, A.J.; Trad, L.A.; Cymerman, A. Alterations in human upper extremity motor function during acute exposure to simulated altitude. Aviat. Space Environ. Med. 1991, 62, 759–764. [Google Scholar]
- Shukitt-Hale, B.; Banderet, L.E.; Lieberman, H.R. Elevation-dependent symptom, mood, and performance changes produced by exposure to hypobaric hypoxia. Int. J. Aviat. Psychol. 1998, 8, 319–334. [Google Scholar] [CrossRef]
- Sharma, V.K.; Das, S.K.; Dhar, P.; Hota, K.B.; Mahapatra, B.B.; Vashishtha, V.; Kumar, A.; Hota, S.K.; Norboo, T.; Srivastava, R.B. Domain Specific Changes in Cognition at High Altitude and Its Correlation with Hyperhomocysteinemia. PLoS ONE 2014, 9, e101448. [Google Scholar] [CrossRef] [Green Version]
- Frisancho, A.R. Developmental Functional Adaptation to High Altitude: Review. Am. J. Human Biol. 2013, 25, 151–168. [Google Scholar] [CrossRef] [Green Version]
- Gogoi, P.; Chutia, P.; Singh, P.; Mahanta, C.L. Effect of optimized ultrasound-assisted aqueous and ethanolic extraction of Pleurotus citrinopileatus mushroom on total phenol, flavonoids and antioxidant properties. J. Food Process Eng. 2019, 42, 12. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In Oxidants and Antioxidants, Pt A; Packer, L., Ed.; Elsevier Academic Press Inc: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader11Mention of a trade name, proprietary product, or specific equipment does not constitute a guarantee by the United States Department of Agriculture and does not imply its approval to the exclusion of other products that may be suitable. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| [M-H]−(m/z) | |||||||
|---|---|---|---|---|---|---|---|
| Peak no. | Rt (min) | Detected | Expected | Error (ppm) | Formula | Identification | Fragments (m/z) |
| 1 | 6.629 | 673.1795 | 674.612 | 2.3 | C32H34O16 | arabelline | 321.0422/381.0646/441.0862 |
| 2 | 6.637 | 441.0827 | 442.379 | −1.9 | C22H18O10 | (−)-epicatechin gallate | 251.0347/295.0243/381.0627 |
| 3 | 8.827 | 657.1778 | 656.597 | 1.8 | C32H32O15 | 2′-O-p-hydroxybenzoyl-6′-O-trans-caffeoylgardoside | 455.0980/561.1363/579.1468 |
| 4 | 9.659 | 539.1201 | 540.480 | 0.4 | C27H24O12 | yunnaneic acid D | 169.0291/197.0237/311.0569 |
| 5 | 10.012 | 637.1575 | 638.582 | 2.4 | C32H30O14 | 4′-O-methylgallocatechin-(4->8)-4′-O-methylepigallocatechin | 331.0625/375.0513/577.1390 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Jiao, Z.; Jiao, Y.; Wang, W.; Chen, Q. Phenolic Acids-Rich Fractions from Agaricus bitorguis (Quél.) Sacc. Chaidam ZJU-CDMA-12 Mycelia Modulate Hypoxic Stress on Hypoxia-Damaged PC12 Cells. Molecules 2020, 25, 4845. https://doi.org/10.3390/molecules25204845
Lu H, Jiao Z, Jiao Y, Wang W, Chen Q. Phenolic Acids-Rich Fractions from Agaricus bitorguis (Quél.) Sacc. Chaidam ZJU-CDMA-12 Mycelia Modulate Hypoxic Stress on Hypoxia-Damaged PC12 Cells. Molecules. 2020; 25(20):4845. https://doi.org/10.3390/molecules25204845
Chicago/Turabian StyleLu, Hongyun, Zhihua Jiao, Yingchun Jiao, Wei Wang, and Qihe Chen. 2020. "Phenolic Acids-Rich Fractions from Agaricus bitorguis (Quél.) Sacc. Chaidam ZJU-CDMA-12 Mycelia Modulate Hypoxic Stress on Hypoxia-Damaged PC12 Cells" Molecules 25, no. 20: 4845. https://doi.org/10.3390/molecules25204845
APA StyleLu, H., Jiao, Z., Jiao, Y., Wang, W., & Chen, Q. (2020). Phenolic Acids-Rich Fractions from Agaricus bitorguis (Quél.) Sacc. Chaidam ZJU-CDMA-12 Mycelia Modulate Hypoxic Stress on Hypoxia-Damaged PC12 Cells. Molecules, 25(20), 4845. https://doi.org/10.3390/molecules25204845