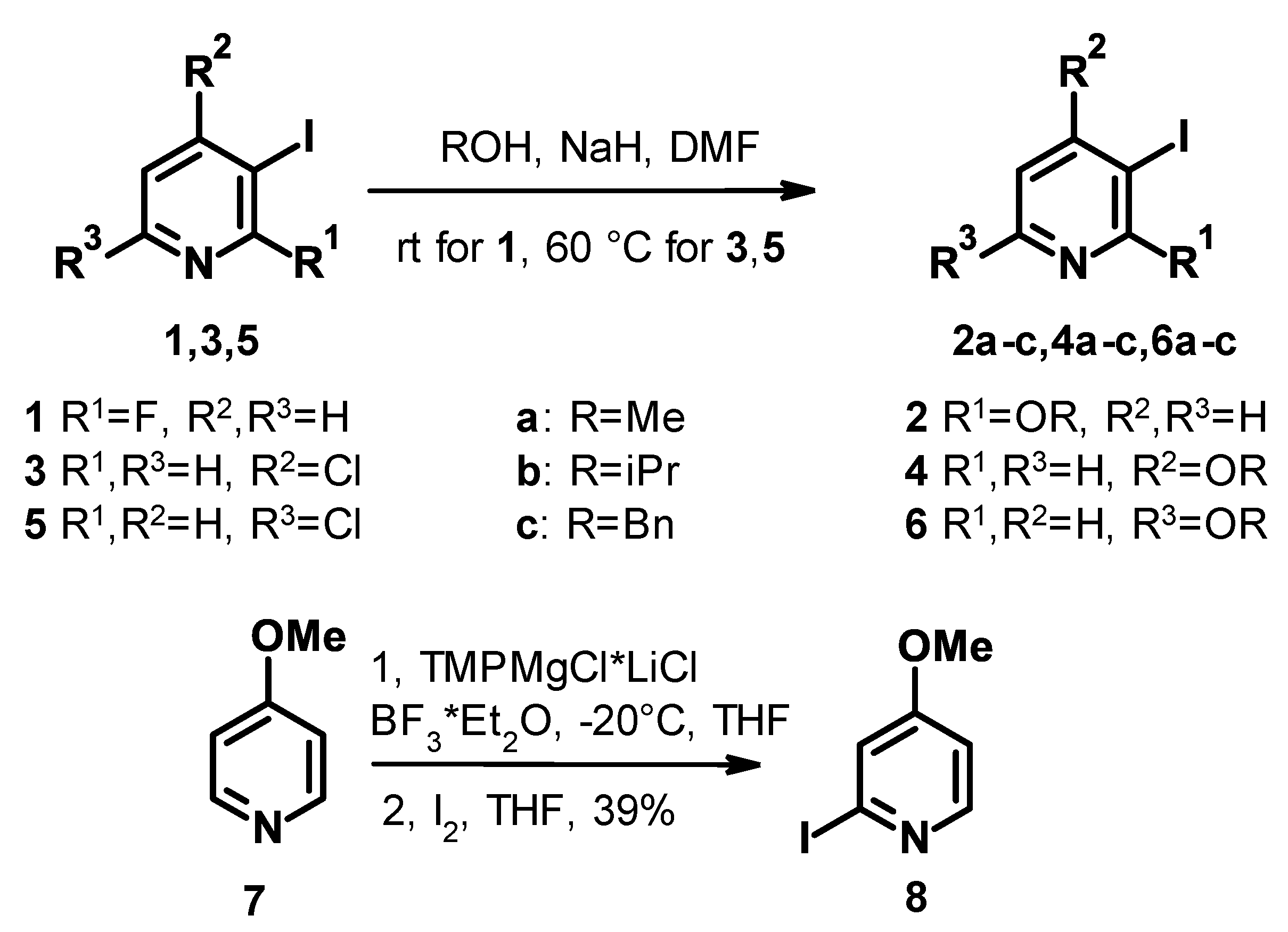
3.1. General Procedure for the Nucleophilic Substitution on 3-Iodopyridine Derivatives
The 0.4 g (10 mmol) of 60% NaH was dissolved in 10 mL dry DMF under an N2 atmosphere. The suspension was cooled to 0 °C, and 10 mmol of the appropriate alcohol was added. The solution was allowed to warm up to room temperature. When the formation of H2 gas stopped, the solution of 5 mmol of the appropriate 3-iodopyridine (1,3,5) in 15 mL DMF was added, and the mixture was stirred at ambient temperature (for 1) or at 60 °C (for 3 and 5) until no further conversion was observed. The mixture was then diluted with water and extracted with EtOAc. The combined organic layers were dried over Na2SO4, filtered, and concentrated under reduced pressure. The crude product was purified via preparative reversed-phase chromatography using 25 mM aqueous NH4HCO3 solution and acetonitrile as eluents or via flash chromatography on silica gel using DCM, MeOH, heptane, and EtOAc as eluents.
2-benzyloxy-3-iodopyridine (2c)
Starting from 1.12 g (5 mmol, 1 equiv.) 2-fluoro-3-iodopyridine (1) using the general nucleophilic substitution procedure and 1.0 mL (10 mmol, 2 equiv.) benzyl-alcohol as appropriate starting material, the mixture was stirred at room temperature for 2 h. Following purification of the crude product via flash chromatography on silica gel using heptane and EtOAc (9:1), 2-benzyloxy-3-iodopyridine (2c) was obtained as a colorless oil (1.08 g, 69% yield).
1H-NMR: (500 MHz, DMSO) δ 8.22 (dd, J = 7.5 Hz, J = 1.6. Hz, 1H), 8.17 (dd, J = 4.8 Hz, J = 1.6 Hz, 1H), 7.47 (d, J = 7.3 Hz, 2H), 7.40 (t, J = 7.5 Hz, 2H), 7.32 (t, J = 7.3 Hz, 1H), 6.82 (dd, J = 7.5 Hz, J = 4.8 Hz, 1H), 5.4 (s, 1H). 13C-NMR: (124 MHz, DMSO) δ 161.3, 148.9, 147.0, 137.5, 128.9, 128.4, 127.8, 119.6, 81.2, 68.4. HR-MS: m/z calculated for C12H11INO ([M + H]+): 311.9880, found: 311.9879.
3-iodo-4-isopropoxypyridine (4b)
Starting from 1.20 g (5 mmol) 4-chloro-3-iodopyridine (3) using the general nucleophilic substitution procedure and 0.8 mL (10 mmol) isopropyl-alcohol as appropriate starting material, following purification by flash chromatography on silica gel with heptane and ethyl-acetate (7:3), 3-iodo-4-isopropoxypyridine (4b) was obtained as a colorless liquid (0.91 g, 69% yield).
1H-NMR: (500 MHz, DMSO) δ 8.68 (s, 1H), 8.33 (d, J = 5.7 Hz, 1H), 7.08 (d, J = 5.7 Hz, 1H), 4.83 (sp, J = 6.0 Hz, 1H), 1.32 (d, J = 6.0 Hz, 6H). 13C-NMR (124 MHz, DMSO) δ 162.6, 157.8, 151.1, 109.9, 87.3, 71.7, 22.0. HR-MS: m/z calculated for C8H11INO ([M + H]+): 263.9880, found: 263.9887.
4-benzyloxy-3-iodopyridine (4c)
Starting from 1.20 g (5 mmol) 4-chloro-3-iodopyridine (3) using the general nucleophilic substitution procedure and 1.0 mL (10 mmol) benzyl-alcohol as appropriate starting material, following purification via preparative HPLC on C18 column with ammonium hydrocarbonate and acetonitrile in 5–95% gradient elution, 4-benzyloxy-3-iodopyridine (4c) was obtained as light brown oil (1.13 g, 72% yield).
1H-NMR: (500 MHz, DMSO) δ 8.72 (s, 1H), 8.38 (d, J = 5.6 Hz, 1H), 7.52–7.35 (m, 5H), 7.16 (d, J = 5.6 Hz, 1H), 5.31 (s, 2H). 13C-NMR (124 MHz, DMSO) δ 163.2, 157.6, 151.3, 136.3, 129.3, 128.6, 127.9, 109.6, 86.5, 70.5. HR-MS: m/z calculated for C12H11INO ([M + H]+): 311.9880, found: 311.9890.
5-iodo-2-isopropoxypyridine (6b)
Starting from 1.20 g (5 mmol) 2-chloro-5-iodopyridine (5) using the general nucleophilic substitution procedure and 0.8 mL (10 mmol) isopropyl-alcohol as appropriate starting material, following purification by flash chromatography on silica gel with heptane and ethyl-acetate (9:1), 5-iodo-2-isopropoxypyridine (6b) was obtained as a pale brown liquid (1.24 g, 95% yield).
1H-NMR (500 MHz, DMSO): δ 8,35 (dd, J = 2,4 Hz, J = 0.7 Hz, 1H), 7,94 (dd, J = 8.6 Hz, J = 2,4 Hz, 1H), 6.64 (dd, J = 8.7 Hz, J = 0.7 Hz, 1H), 5.16 (sp, J = 6.2 Hz, 1H), 1,26 (d, J = 6.2 Hz, 6H). 13C-NMR (124 MHz, DMSO): δ 162.7, 152.8, 147.2, 114.4, 83.3, 68.4, 31.7, 28.9, 22.2, 14.4. HR-MS: m/z calculated for C8H11INO ([M + H]+): 263.9880, found: 263.9880.
2-benzyloxy-5-iodopyridine (6c)
Starting from 1.20 g (5 mmol) 2-chloro-5-iodopyridine (5) using the general nucleophilic substitution procedure and 1.0 mL (10 mmol) benzyl-alcohol as appropriate starting material, following purification by preparative HPLC on C18 with ammonium hydrocarbonate and acetonitrile in 5–95% gradient elution, 2-Benzyloxy-5-iodopyridine (6c) was obtained as a white solid (1.03 g, 66.1% yield).
1H-NMR (500 MHz, DMSO): δ 8.39 (d, J = 2.4 Hz, 1H), 8.00 (dd, J = 8.7 Hz, J = 2.4 Hz, 1H), 7.43–7.30 (m, 5H), 6.79 (d, J = 8.7 Hz, 1H), 5.31 (s, 2H). 13C-NMR (124 MHz, DMSO): δ 162.9, 152.7, 147.4, 137.3, 128.8, 128.4, 128.3, 114.0, 84.1, 67.6. HR-MS: m/z calculated for C12H11INO ([M+H+]): 310.9880, found: 311.9872. Mp: 38.5–39.2 °C
2-iodo-4 methoxypyridine (
8) [
30]
A solution of 4-methoxypyridine (2 g, 18.3 mmol) in 90 mL of dry THF containing BF3·Et2O (2.5 mL, 20 mmol) was treated with TMPMgCl·LiCl (27 mL, 27 mmol, 1M toluene) at −20 °C for 20 h, and the resulting mixture was quenched by adding the solution of iodine (9.14 g, 36 mmol) in 37 mL of THF. Following the warming to room temperature, the resulting mixture was quenched by adding 100 mL of sat. aqueous NH4Cl and NH3 (9 mL) and sat. aqueous Na2S2O3 solution (18 mL), followed by extraction with Et2O (4 × 100 mL). The combined organic layer was dried over MgSO4, and the filtrate was concentrated at reduced pressure. The residue was purified on silica gel using heptane and Et2O (4:1) as eluent, and 8 was isolated as light brown oil (1.65 g, 39% yield).
1H-NMR (500 MHz, DMSO): δ 8.13 (d, J = 5.8 Hz, 1H), 7.42 (d, J = 2.4 Hz, 1H), 7.01 (dd, J = 5.8 Hz, J = 2.4 Hz, 1H), 3.82 (s, 3H). 13C-NMR (124 MHz, DMSO): δ 166.0, 151.9, 120.3, 119.8, 111.4, 56.3. HR-MS: m/z calculated for C6H7INO ([M+H+]): 235.9567, found: 235.9571.
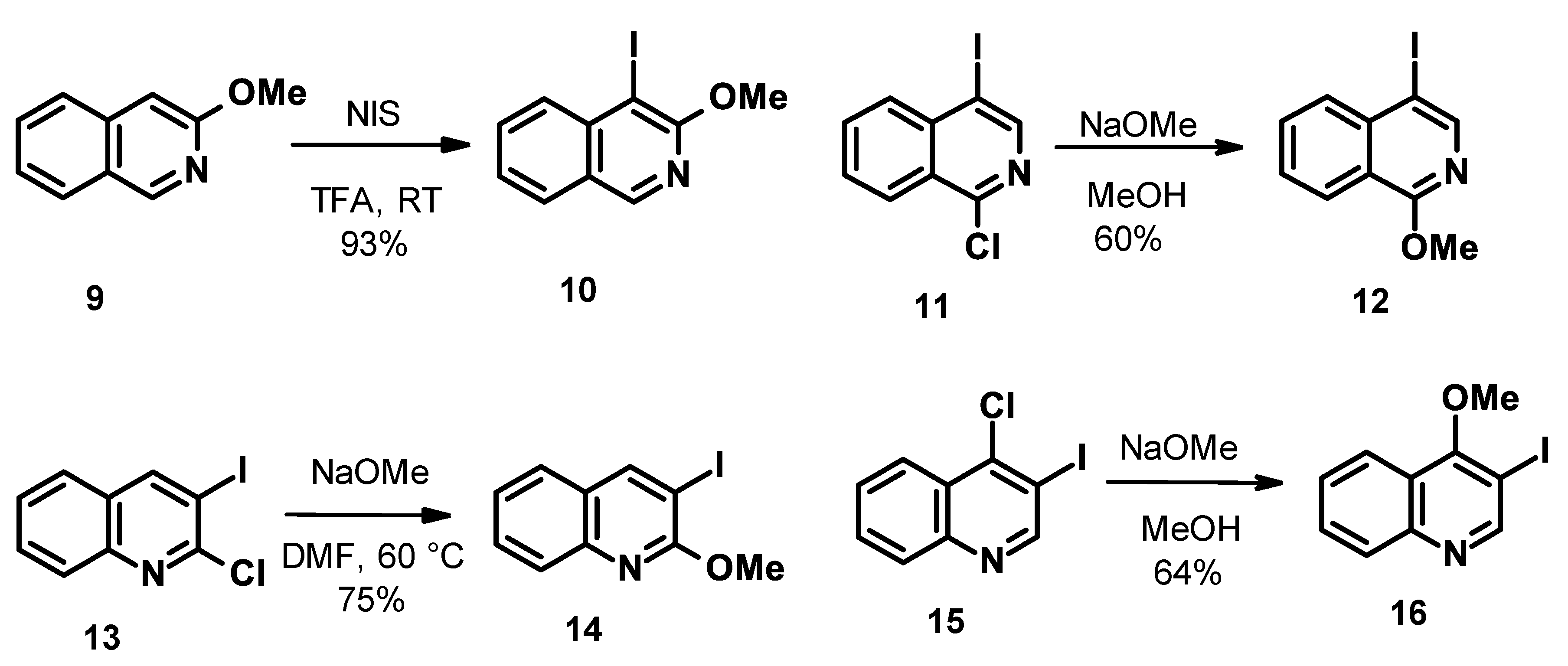
4-iodo-3-methoxyisoquinoline (10)
In an oven-dried two necked round bottom flask, 3-methoxyisoquinoline (9, 508 mg, 3.3 mmol) and N-iodosuccinimide (810 mg, 3.5 mmol) were dissolved in dry acetonitrile under nitrogen. The 77 µL trifluoroacetic acid (1 mmol) was added to the mixture by a syringe. The mixture was stirred at room temperature overnight. The solvent was removed, and the residue was treated with 40 mL of water. The aqueous mixture was extracted with 4 × 25 mL of DCM. The organic layer was washed with 25 mL of 1M Na2S2O3 and 50 mL of brine. The organic layer was dried over MgSO4. The filtrate was concentrated under reduced pressure to give 4-iodo-3-methoxyisoquinoline (10) as off-white solid (870 mg, 93% yield).
1H-NMR (500 MHz, DMSO): δ 9.04 (s, 1H), 8.06 (m, 1H), 7.90 (m, 1H), 7.81 (m, 1H), 7.54 (m, 1H), 4.04 (s, 3H). 13C-NMR (124 MHz, DMSO): δ 159.8, 151.4, 140.4, 133.1, 129.3, 129.0, 126.2, 125.7, 78.5, 55.6. HR-MS: m/z calculated for C10H9INO ([M + H]+): 285.9723, found: 285.9730. Mp: 67.9–69.5 °C.
4-iodo-1-methoxyisoquinoline (12)
NaOMe (112 mg, 2.07 mmol) was dissolved in 5 mL dry MeOH under an N2 atmosphere. 4-iodo-1-chloroisoquinoline (11, 400 mg, 1.38 mmol), dissolved in 5 mL dry dioxane, was added to it, and the mixture was heated to 80 °C for 8 h. The solvents were removed in vacuo, and the residue was diluted with water and extracted with EtOAc. The combined organic layers were washed with brine, dried over MgSO4, filtered, and concentrated under reduced pressure to give 12 as a light brown solid (236 mg, 60% yield).
1H-NMR (500 MHz, DMSO): δ 8.43 (s, 1H), 8.18 (m, J = 8.2 Hz, 1H), 7.91 (m, 1H), 7.89 (m, 1H), 7.73 (m, J = 8.2 Hz, 1H), 4.06 (s, 3H). 13C-NMR (124 MHz, DMSO): δ 161.2, 147.3, 138.0, 133.1, 130.4, 128.9, 124.7, 120.6, 87.6, 54.5. HR-MS: m/z calculated for C10H9INO ([M + H]+): 285.9723, found: 285.9727. Mp: 43.9–44.5 °C.
3-iodo-2-methoxyquinoline (14)
An oven-dried vial was charged with 3-iodo-2-chloroquinoline (13, 200 mg, 0.7 mmol), dissolved in dry DMF (5 mL). The 25 w% NaOMe solution in MeOH (190 µL, 179 mg) was added slowly to the mixture by a syringe under nitrogen. The mixture was stirred for 3 h at 40 °C. The mixture was quenched with 2 mL of water. Purification via preparative HPLC on C18 column with ammonium hydrocarbonate and acetonitrile, in 5–95% gradient elution, with direct injection gave 14 as light-brown solid (148 mg, 75% yield).
1H-NMR (500 MHz, DMSO): δ 8.86 (s, 1H), 7.86 (m, 1H), 7.79 (m, 1H), 7.70 (m, 1H), 7.46 (m, 1H), 4.02 (s, 3H). 13C-NMR (124 MHz, DMSO): δ 159.2, 148.8, 145.6, 130.8, 127.3, 127.0, 126.9, 125.2, 82.4, 55.2. HR-MS: m/z calculated for C10H9INO ([M + H]+): 285.9723, found 285.9717. Mp: 46.8–47.9 °C.
3-iodo-4-methoxyquinoline (16)
NaOMe (169.8 mg, 3.14 mmol) was dissolved in 4 mL of dry CH3OH under an N2 atmosphere. 3-iodo-4-chloroquinoline (15, 700 mg, 2.42 mmol), dissolved in 8 mL of dry dioxane, was added to it, and the mixture was heated to 80 °C for 6 h. The methanol was then removed in vacuo, and the residue was diluted with water and extracted with EtOAc. The combined organic layers were washed with brine, dried over MgSO4, filtered, and concentrated under reduced pressure. The crude product was purified via flash chromatography using heptane and EtOAc as eluents to give 16 as light yellow solid (440 mg, 64% yield).
1H-NMR (500 MHz, DMSO): δ 9.08 (s, 1H), 8.12 (dm, J = 8.4 Hz, 1H), 8.05 (dm, J = 8.4 Hz, 1H), 7.84 (m, J = 8.4 Hz, 1H), 7.69 (m, J = 8.4 Hz, 1H), 4.02 (s, 3H). 13C-NMR (124 MHz, DMSO): δ 163.8, 158.0, 149.4, 130.9, 129.6, 127.9, 124.5, 122.4, 86.3, 62.4. HR-MS: m/z calculated for C10H9INO ([M + H]+): 285.9723, found: 285.9726. Mp: 122.3–124.9 °C.
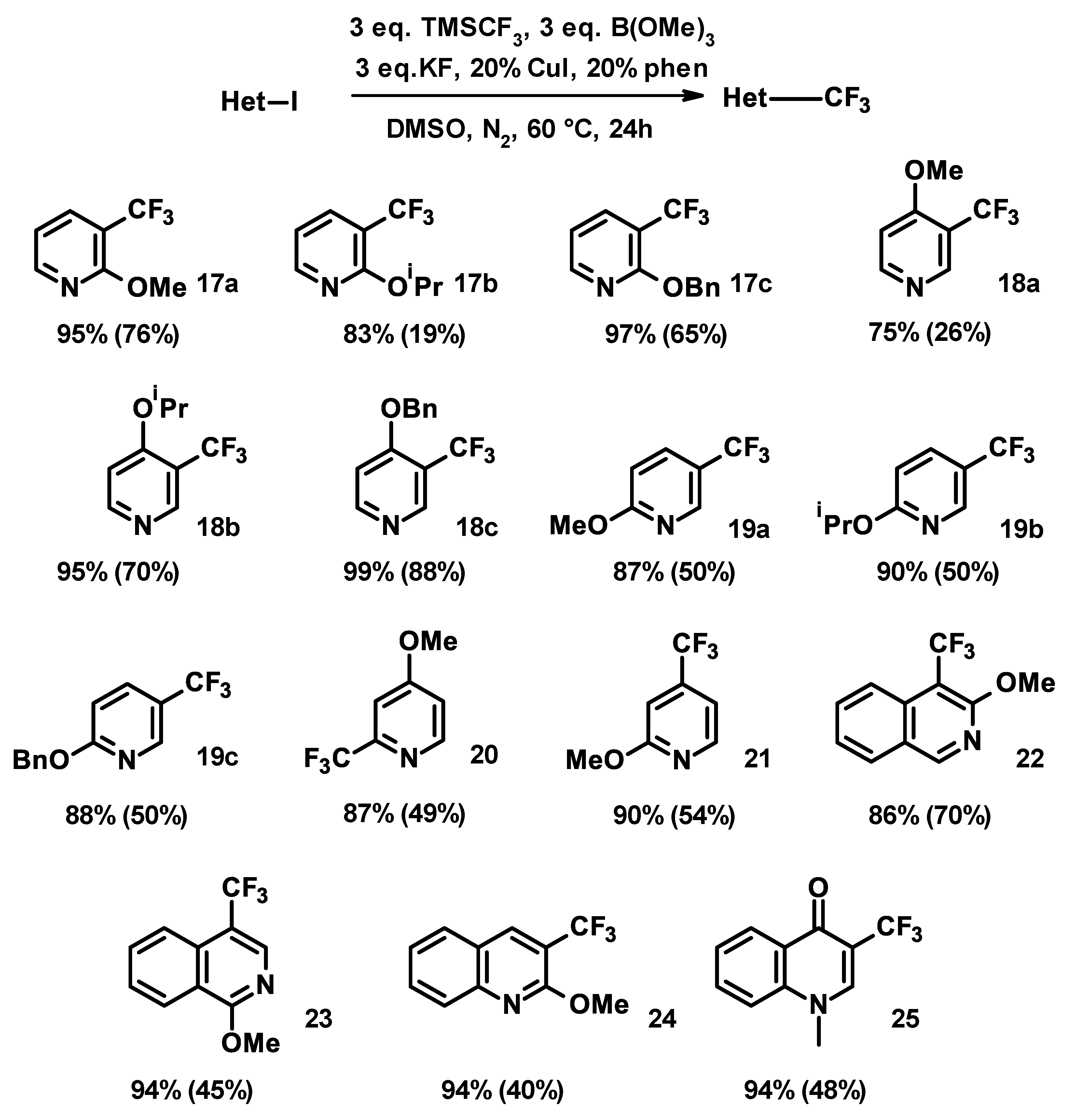
3.2. General Trifluoromethylation Procedure
An oven-dried vial with a septum cap and a stir bar was charged with copper (I) iodide (76 mg, 0.4 mmol), 1,10-phenanthroline (72 mg, 0.4 mmol), KF (348 mg, 6 mmol), and the aryl iodide (2.00 mmol, if solid). The reaction vessel was closed, then evacuated and refilled with argon or nitrogen three times. DMSO (4.0 mL), aryl iodide (2.00 mmol, if liquid), B(OMe)3 (623 mg, 6 mmol), and TMSCF3 (854 mg, 887 μL, 6 mmol) were added via syringe. The resulting orange-brown suspension was stirred for 24 h at 60 °C. After cooling to ambient temperature, the orange solution was diluted with DCM (10 mL) and washed with 1N HCl (25 mL). Acidic washing was omitted for basic products. The washing was re-extracted with DCM (2 × 5 mL), and the combined organic layer was washed with conc. ammonia (25%, 25 mL) to remove traces of copper salts. The washing was re-extracted with DCM (2 × 5 mL), and the combined organic layer was washed with brine (15 mL) and dried over MgSO4 and concentrated. The crude product was purified by flash column chromatography unless otherwise noted.
2-methoxy-3-trifluoromethylpyridine (17a)
Starting from 2a (2.01 g, 8.5 mmol), using the general trifluoromethylation procedure, 17a was obtained as a colorless liquid (1.14 g, 76% yield). No further purification was done after the extraction.
1H-NMR (500 MHz, DMSO): δ 8.46 (dd, J = 4.7 Hz, J = 1.0 Hz, 1H), 8.07 (dd, J = 7.5 Hz, 1H), 7.18 (m, J = 6.9 Hz, J = 5.1 Hz, 1H), 3.98 (s, 3H). 13C-NMR (124 MHz, DMSO): δ 160.5, 151.7, 137.5, 123.6, 117.2, 111.9, 54.4. 19F-NMR (376 MHz, DMSO): δ −62.4. HR-MS: m/z calculated for C7H7F3NO ([M + H]+): 178.0474, found: 178.0473.
2-isopropoxy-3-trifluoromethylpyridine (17b)
Starting from 2b (528 mg, 2 mmol), using the general trifluoromethylation procedure, 17b was obtained following the purification by flash chromatography on silica gel with heptane and ethyl-acetate (1:1) as a colorless liquid (77 mg, 19% yield).
1H-NMR (500 MHz, DMSO): δ 8.42 (m, 1H), 8.07 (m, 1H), 7.13 (m, 1H), 5.39 (sp, J = 6.2 Hz, 1H), 1.31 (d, J = 6.2 Hz, 6H). 13C-NMR (124 MHz, DMSO): δ 160.0, 151.8, 137.6, 123.6, 116.8, 112.3, 69.6, 22.1. 19F-NMR (376 MHz, DMSO): δ -62.4. HR-MS (GC-MS (TOF); [M]+): Calculated for C9H10F3NO([M]+): 205.0714, found: 205.0703.
2-benzyloxy-3-trifluoromethylpyridine (17c)
Starting from 2c (237 mg, 0.75 mmol), using the general trifluoromethylation procedure following the purification via preparative HPLC on C18 column with ammonium hydrocarbonate and acetonitrile in 5–95% gradient elution, 17c was obtained as a colorless liquid (123 mg, 65% yield).
1H-NMR: (500 MHz, DMSO): δ 8.45 (dd, J = 5.0 Hz, J = 1.0 Hz, 1H), 8.14 (dd, J = 7.6 Hz, J = 1.1 Hz, 1H), 7.45–7.30 (m, 5H), 7.20 (m, 1H), 5.51 (s, 2H). 13C-NMR: (124 MHz, DMSO): δ 159.9, 151.8, 137.8, 128.9, 128.3, 127.8, 124.7, 123.6, 117.6, 112.1, 68.0. 19F-NMR (376 MHz, DMSO): δ −62.3. HR-MS: m/z calculated for C13H11F3NO ([M + H]+): 254.0787, found: 254.0795.
4-methoxy-3-trifluoromethylpyridine (18a)
Starting from 4a (473 mg, 2 mmol), using the general trifluoromethylation procedure following the purification by flash chromatography on silica gel with pentane and ethyl-acetate (1:2), 18a was obtained as a colorless liquid (93 mg, 26% yield).
1H-NMR (500 MHz, DMSO): δ 8.72 (d, J = 5.9 Hz, 1H), 8.68 (s, 1H), 7.34 (d, J = 5.9 Hz, 1H), 3.98 (s, 3H). 13C-NMR (500 MHz, DMSO): δ 163.6, 156.2, 147.7, 123.8, 114.1 108.9, 57.1. 19F-NMR (376 MHz, DMSO) δ -61.0. HR-MS: m/z calculated for C7H7F3NO ([M + H]+): 178.0474, found: 178.0475.
4-isopropoxy-3-trifluoromethylpyridine (18b)
Starting from 4b (1.00 g, 3.8 mmol), using the general trifluoromethylation procedure following the purification by flash chromatography on silica gel with pentane and diethyl ether (1:1), 18b was obtained as a colorless liquid (670 mg, 85% yield).
1H-NMR (500 MHz, DMSO): δ 8.66 (s, 1H), 8.65 (s, 1H), 7.35 (d, J = 6.0 Hz, 1H), 4.94 (sp, J = 6.1 Hz, 1H), 1.31 (d, J = 6.1 Hz, 6H). 13C-NMR (124 MHz, DMSO): δ 162.2, 155.8, 148.0, 123.8, 114.7, 110.0, 72.2, 22.5. 19F-NMR (376 MHz, DMSO-d6): δ -61.2. HR-MS: m/z calculated for C9H11F3NO ([M + H]+): 206.0793, found: 206.0788.
4-benzyloxy-3-trifluoromethylpyridine (18c)
Starting from 4c (235 mg, 0.75 mmol), using the general trifluoromethylation procedure following the purification via preparative HPLC on C18 column with ammonium hydrocarbonate and acetonitrile in 5–95% gradient elution, 18c was obtained as an off-white solid (167 mg, 88% yield).
1H-NMR (500 MHz, DMSO): δ 8.72 (m, 1H), 8.71 (m, 1H), 7.43 (m, 5H), 7.36 (m, 1H), 5.39 (s, 2H). 13C-NMR (124 MHz, DMSO): δ 162.6, 156.1, 147.9, 135.9, 129.1, 128.7, 127.9, 123.8, 109.9, 70.6. 19F-NMR (376 MHz, DMSO): δ -61.0. HRMS: m/z calculated for C13H11F3NO ([M + H]+): 254.0793, found: 254.0788. Mp: 66.0–68.6 °C.
2-methoxy-5-trifluoromethylpyridine (19a)
Starting from 6a (1.39 g, 5.9 mmol), using the general trifluoromethylation procedure following the purification by flash chromatography on silica gel with heptane and DCM, 19a was obtained as a colorless liquid (822 mg, 70% yield).
1H-NMR (500 MHz, DMSO): δ 8,60 (m, 1H), 8,07 (dd, J = 8.7 Hz, J = 2,6 Hz, 1H), 7,03 (d, J = 8.7 Hz, 1H), 3,93 (s, 3H). 13C-NMR (124 MHz, DMSO): δ 166.4, 145.4, 136.9, 111.8, 54.4. 19F-NMR (376 MHz, DMSO): δ -59.9. HR-MS: m/z calculated for C7H7F3NO ([M + H]+): 178.0474, found: 178.0474.
2-isopropoxy-5-trifluoromethylpyridine (19b)
Starting from 6b (770 mg, 3.0 mmol), using the general trifluoromethylation procedure following the purification by flash chromatography on silica gel with heptane and ethyl-acetate (9:1), 19b was obtained as a colorless liquid (340 mg, 52% yield).
1H-NMR (500 MHz, DMSO): δ 8.57 (m, 1H), 8.02 (d, J = 8.8 Hz, J = 2.7 Hz, 1H), 6.93 (d, J = 8.8 Hz, 1H), 5.32 (sp, J = 6.2 Hz, 1H), 1.31 (d, J = 6.2 Hz, 6H). 13C-NMR (500 MHz, DMSO): δ 165.7, 145.4, 136.8, 112.2, 69.3, 22.1. 19F-NMR (376 MHz, DMSO): δ -59.8. HR-MS (GC-MS, TOF, EI): calculated for C9H10F3NO ([M]+): 205.0714, found: 205.0705.
2-benzyloxy-5-trifluoromethylpyridine (19c)
Starting from 6c (466 mg, 1.5 mmol), using the general trifluoromethylation procedure following the purification via preparative HPLC on C18 column with ammonium hydrocarbonate and acetonitrile in 5–95% gradient elution, 19c was obtained as a colorless liquid (192 mg, 51% yield).
1H-NMR (500 MHz, DMSO): δ 8.61 (m, 1H), 8.10 (dd, J = 8.8 Hz, J = 2.6 Hz, 1H), 7.47–7.32 (m, 5H), 7.09 (d, J = 8.8 Hz, 1H), 5.43 (s, 2H). 13C-NMR (124 MHz, DMSO): δ 165.8, 145.4, 137.1, 137.0, 128.9, 128.6, 128.5, 124.6, 119.5, 112.1, 68.3. 19F-NMR (376 MHz, DMSO): δ -59.9. HR-MS: m/z calculated for C13H11F3NO ([M + H]+): 254.0793, found: 254.0789.
4-methoxy-2-trifluoromethylpyridine (20)
Starting from 8 (1.18 g, 5 mmol), using the general trifluoromethylation procedure following the purification by flash chromatography on silica gel with pentane and diethyl-ether (3:1), 20 was obtained as a colorless liquid (700 mg, 49% yield).
1H-NMR (500 MHz, DMSO): δ 8.57 (d, J = 5.7 Hz, 1H), 7.44 (d, J = 2.5 Hz, 1H), 7.28 (dd, J = 5.7 Hz, J = 2.6 Hz, 1H), 3.93 (s, 3H). 13C-NMR (124 MHz, DMSO): δ 166.9, 152.0, 148.6, 122.0, 113.3, 108.1, 56.6. 19F-NMR (376 MHz, DMSO): δ -66.6. HR-MS: m/z calculated for C7H7F3NO ([M + H]+): 178.0474, found: 178.0486.
2-methoxy-4-trifluoromethylpyridine (21)
Starting from 4-iodo-2-methoxypyridine (1.17 g, 5.0 mmol), using the general trifluoromethylation procedure following the purification by flash chromatography on silica gel with pentane and diethyl-ether (95:5), 21 was obtained as a colorless liquid (478 mg, 54% yield).
1H-NMR (500 MHz, DMSO): δ 8.45 (d, J = 5.3 Hz, 1H), 7.33 (dd, J = 5.3 Hz, J = 0.8 Hz, 1H), 7.20 (m, 1H), 3.92 (s, 3H). 13C-NMR (124 MHz, DMSO): δ 164.5, 149.5, 140.1, 123.2, 112.8, 107.6, 54.4. 19F-NMR (376 MHz, DMSO): δ -63.5. HR-MS: m/z calculated for C7H7F3NO ([M + H]+): 178.0474, found: 178.0481.
3-methoxy-4-trifluoromethylisoquinoline (22)
Starting from 10 (150 mg, 0.5 mmol), using the general trifluoromethylation procedure following the purification via preparative HPLC on C18 column with ammonium hydrocarbonate and acetonitrile in 5–95% gradient elution, 22 was obtained as a white solid (80 mg, 72% yield).
1H-NMR (500 MHz; DMSO): δ 9.38 (s, 1H), 8.22 (d, J = 8.0 Hz, 1H), 8.07 (d, J = 8.6 Hz, 1H), 7.91–7.87 (m, 1H), 7.63–7.59 (m, 1H), 4.09 (s, 3H). 13C-NMR (124 MHz; DMSO): δ 158.7, 156.7, 135.0, 133.8, 129.9, 125.7, 125.7, 125.0, 122.2, 100.6, 55.2. 19F-NMR (376 MHz): δ -52.7 HR-MS: m/z calculated for C11H9F3NO ([M + H]+): 228.0632, found: 228.0637. Mp: 63.2–65.3 °C.
1-methoxy-4-trifluoromethylisoquinoline (23)
Starting from 12 (300 mg, 1.1 mmol), using the general trifluoromethylation procedure following the purification via preparative HPLC on C18 column with ammonium hydrocarbonate and acetonitrile in 5–95% gradient elution, 23 was obtained as a white solid (115 mg, 46% yield).
1H-NMR (500 MHz, DMSO): δ 8.49 (m, 1H). 8.34 (m, 1H), 7.99 (m, 2H), 7.81 (m, 1H), 4.15 (s, 3H). 13C-NMR (124 MHz, DMSO): δ 163.7, 140.1, 133.4, 132.9, 129.0, 125.1, 123.0, 119.0, 113.9, 55.1. 19F-NMR (376 MHz, DMSO): δ -58.7. HR-MS: m/z calculated for C11H9F3NO ([M+H]+): 228.0631, found: 228.0632. Mp: 56.9–58.4 °C.
2-methoxy-3-trifluoromethylquinoline (24)
Starting from 14 (73 mg, 0.2 mmol), using the general trifluoromethylation procedure following the purification via preparative HPLC on C18 column with ammonium hydrocarbonate and acetonitrile in 5–95% gradient elution, 24 was obtained as a light-brown oil (18 mg, 40% yield).
1H-NMR (500 MHz, DMSO): δ 8.82 (s, 1H), 8.09 (d, 1H), 7.86 (m, 2H), 7.57 (m, 1H), 4.01 (s, 3H). 13C-NMR (124 MHz, DMSO): δ 157.7, 147.5, 139.4, 133.0, 129.7, 127.0, 125.9, 124.1, 54.6. 19F-NMR (376 MHz, DMSO): δ -62.2. HR-MS: m/z calculated for C11H8F3NO (GC-MS (TOF); [M]+): 227.0558, found: 227.0549.
1-methyl-3-trifluoromethyl-4-quinolone (25)
Starting from 16 (285 mg, 1 mmol), using the general trifluoromethylation procedure following the purification via preparative HPLC on PFP column with formic acid and acetonitrile in 56% isocratic elution, 25 was obtained as white crystals (112 mg, 48% yield).
1H-NMR (500 MHz): δ 8.59 (s, 1H), 8.24 (m, J = 8.0 Hz, 1H), 7.89–7.85 (m, J = 8.5 Hz, 1H), 7.77 (m, 1H, J = 8.5 Hz), 7.56–7.52 (m, J = 8.0 Hz, 1H), 3.93 (s, 3H). 13C-NMR (124 MHz): δ 173.0, 145.2, 140.9, 133.7, 127.2, 126.0, 125.5, 117.9, 108.7, 41.1. 19F-NMR (376 MHz): δ -61.4. HR-MS: m/z calculated for C11H9F3NO ([M + H]+): 228.0632, found: 228.0627. Mp: 169.5–179.3 °C.