Predicted Reversal in N-Methylazepine/N-Methyl-7-azanorcaradiene Equilibrium upon Formation of Their N-Oxides
Abstract
1. Introduction
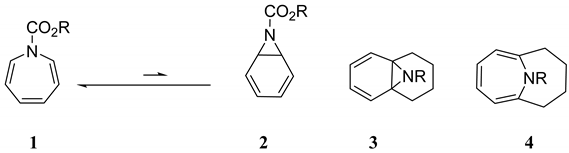

2. Results and Discussion
2.1. N-Methylazepine, N-Methyl-7-Azanorcaradiene, and Their N-Oxides
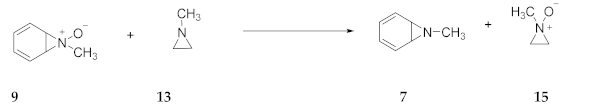

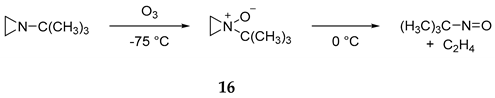

2.2. Aromaticity and Antiaromaticity of Pyrroles, Azepines and Their N-Oxides
2.3. Enthalpies of Hydrogenation and Resonance Stabilization
2.4. Aromatic Delocalization from Equalization of Double and Single Bond Lengths
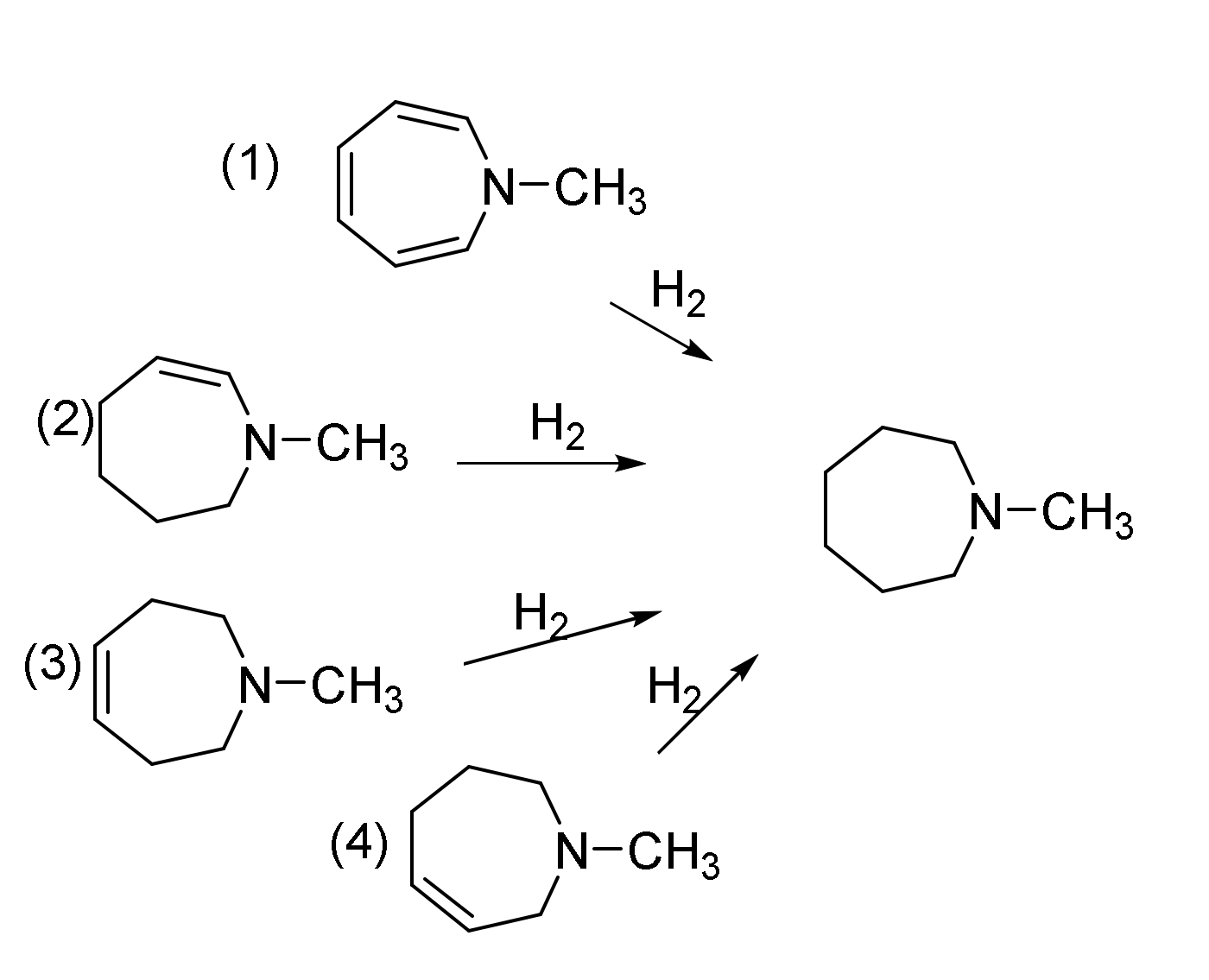
2.5. Enthalpies of Hydrogenation and Resonance Stabilization of N-Methylazepine
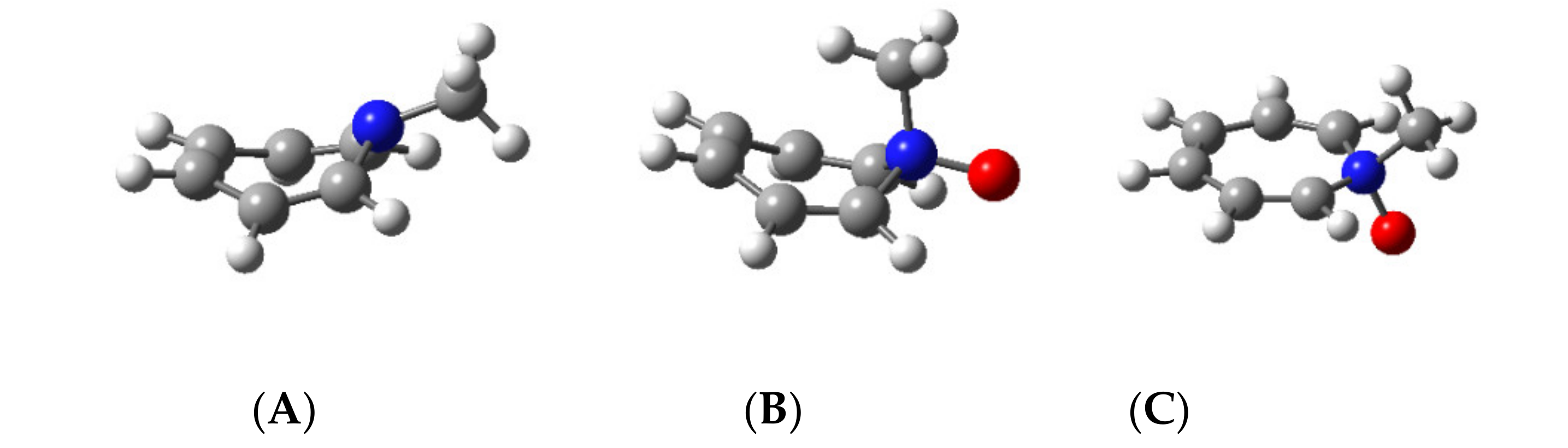
2.6. Computed Structures for N-Methylazepine and Its N-Oxide
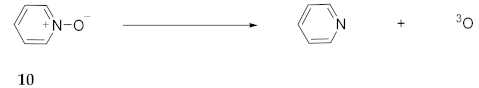
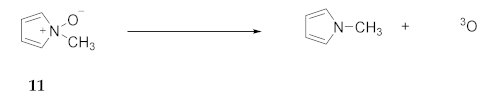
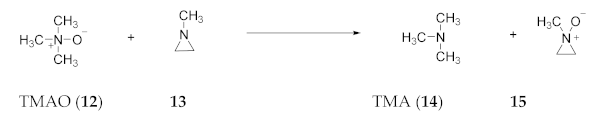
2.7. Experimental Evidence for Azepine N-Oxides
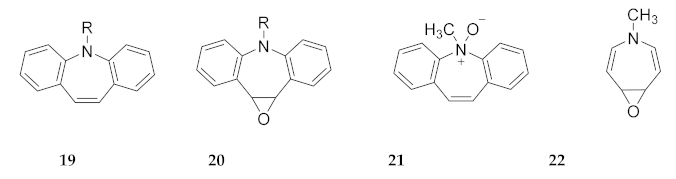
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hafner, K.; Konig, C. N-Ethoxycarbonylazepine. Angew. Chem. Int. Ed. 1963, 2, 96. [Google Scholar] [CrossRef]
- Vogel, E.; Günther, H. Benzene Oxide-Oxepin Valence Tautomerism. Angew. Chem. Int. Ed. 1967, 6, 385–401. [Google Scholar] [CrossRef]
- Paquette, L.A.; Kuhla, D.E.; Barrett, J.H.; Haluska, R.J. Unsaturated Heterocyclic Systems. LII. A General Synthetic Entry to Derivatives of 1H-Azepine. J. Org. Chem. 1969, 34, 2866–2878. [Google Scholar] [CrossRef]
- Paquette, L.A.; Barrett, J.H.; Kuhla, D.E. Thermochemical Reactions of 1H-Azepine Derivatives. I. Dimerization. J. Am. Chem. Soc. 1969, 91, 3616–3624. [Google Scholar] [CrossRef]
- Paquette, L.A.; Kuhla, D.E.; Barrett, J.H. Unsaturated heterocyclic systems. LIII. Thermochemical reactions of 1H-azepine derivatives. 2. Aromatization and sigmatropic migrations involving nitrogen. J. Org. Chem. 1969, 34, 2879–2884. [Google Scholar] [CrossRef]
- Meigh, J.-P.K. Benzazepines and Their Group 15 Analogues. Chemin 2005, 36, 825–927. [Google Scholar] [CrossRef]
- Dardonville, C.; Alkorta, I. Homoheteroaromaticity: The case study of azepine and dibenzazepineElectronic supplementary information (ESI) available: Absolute chemical shieldings calculated at the B3LYP/6-311++G**//B3LYP/6-311++G** computational level for compounds 1b, 2b, 4b and 5b. Org. Biomol. Chem. 2004, 2, 1587–1591. Available online: http://www.rsc.org/suppdata/ob/b3/b314742h/ (accessed on 30 August 2020). [CrossRef]
- Greenberg, A.; Green, A.R.; Liebman, J.F. Computational Study of Selected Amine and Lactam N-Oxides Including Comparisons of N-O Bond Dissociation Enthalpies with Those of Pyridine N-Oxides. Molecules 2020, 25, 3703. [Google Scholar] [CrossRef]
- Drakenberg, T.; Lehn, J.M. Nuclear magnetic resonance studies of rate processes and conformations. Part XX. Nitrogen inversion in the gas phase. J. Chem. Soc. Perkin Trans. 2 1972, 532. [Google Scholar] [CrossRef]
- Jansen, H.; Slootweg, J.C.; Lammertsma, K. Valence isomerization of cyclohepta-1,3,5-triene and its heteroelement analogues. Beilstein J. Org. Chem. 2011, 7, 1713–1721. [Google Scholar] [CrossRef]
- Schleyer, P.v.R.; Maerker, C.; Dransfeld, A.; Jiao, H.; van Eikema Hommes, N.J. R Nucleus-Independent Chemical Shifts: A Simple and Efficient Aromaticity Probe. J. Am. Chem. Soc. 1996, 118, 6317–6318. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wannere, C.S.; Cominboeuf, C.; Puchta, R.; Schleyer, P.v.R. Nuclear-Independent Chemical Shifts (NICS) as an Aromatic Criterion. Chem. Rev. 2005, 105, 3842–3888. [Google Scholar]
- Stanger, A. NICS—Past and Present. Eur. J. Org. Chem. 2020, 2020, 3120–3127. [Google Scholar] [CrossRef]
- Acree, W.E.; Pilcher, G.; Da Silva, M.D.M.C.R. The Dissociation Enthalpies of Terminal (N–O) Bonds in Organic Compounds. J. Phys. Chem. Ref. Data 2005, 34, 553–572. [Google Scholar] [CrossRef][Green Version]
- Adam, W.; Bottke, N.; Engels, B.; Krebs, O. An experimental and computational study on the reactivity and regioselectivity for the nitrosoarene ene reaction: Comparison with triazolinedione and singlet oxygen. J. Am. Chem. Soc. 2001, 123, 5542–5548. [Google Scholar] [CrossRef]
- Leach, A.G.; Houk, K.N. The Ene Reactions of Nitroso Compounds Involve Polarized Diradical Intermediates. J. Am. Chem. Soc. 2002, 124, 14820–14821. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, J.E.; Bhatnagar, A.K.; Choi, S.C.; Shortridge, T.J. Rearrangement of strained dipolar species, aziridine N-oxides. II. J. Am. Chem. Soc. 1971, 93, 4082–4084. [Google Scholar] [CrossRef]
- Dardonville, C. The behavior of 5H-dibenz[b,f]azepine in sulfuric acid solution. ArkNOC 2004, 2004, 206. [Google Scholar] [CrossRef]
- Hückel, E. Quantentheoretische Beiträge zum Benzolproblem. Eur. Phys. J. A 1931, 70, 204–286. [Google Scholar]
- Breslow, R.; Groves, J.T.; Ryan, G. Cyclopropenyl cation. J. Am. Chem. Soc. 1967, 89, 5048. [Google Scholar] [CrossRef]
- Hasson, B.F.; Ma, C.; Wang, L.; Wazer, D.E.; Reiff, J.E.; Fisher, B.J.; Speer, T.W.; Brashears, J.H.; Christodouleas, J.P.; Dragun, A.E.; et al. Encyclopedia of Radiation Oncology; Springer: Berlin, Germany, 2013; Volume 6, p. 1. [Google Scholar] [CrossRef]
- Liu, C.; Ni, Y.; Lu, X.; Li, G.; Wu, J. Global Aromaticity in Macrocyclic Polyradicaloids: Hückel’s Rule or Baird’s Rule? Accounts Chem. Res. 2019, 52, 2309–2321. [Google Scholar] [CrossRef] [PubMed]
- Popov, I.A.; Starikov, A.G.; Steglenko, D.V.; Boldyrev, A.I. Frontispiece: Usefulness of the σ-Aromaticity and σ-Antiaromaticity Concepts for Clusters and Solid-State Compounds. Chem. A Eur. J. 2018, 24, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Slayden, S.W.; Liebman, J.F. The Energetics of Aromatic Hydrocarbons: An Experimental Thermochemical Perspective. Chem. Rev. 2001, 101, 1541–1566. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-F.; Liu, Z.-Z.; Liu, H.; Zhang, H.-Q. Bond Length Equalization with molecular aromaticity—A new measurement of aromaticity. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2018, 201, 392–398. [Google Scholar] [CrossRef]
- Masui, H. Metalloaromaticity. Coord. Chem. Rev. 2001, 219, 957–992. [Google Scholar] [CrossRef]
- Klarner, F. About the Antiaromaticity of Planar Cyclooctatetraene. Angew. Chem. 2001, 40, 3977–3981. [Google Scholar]
- Saito, T.; Nishihara, S.; Yamanaka, S.; Kitigawa, Y.; Kawakami, T.; Yamada, S.; Isobe, H.; Okumura, M.; Yamaguchi, K. Symmetry and broken symmetry in molecular orbital description of unstable molecules IV: comparison between single- and multi-reference computational results for antiaromtic molecules. Theor. Chim. Acc. 2011, 130, 749–763. [Google Scholar] [CrossRef]
- Franklin, J.L. Calculation of Resonance Energies. J. Am. Chem. Soc. 1950, 72, 4278–4280. [Google Scholar] [CrossRef]
- Takeuchi, H.; Inoue, K.; Enmi, J.; Hamada, T.; Shibuya, T.; Konaka, S. Molecular structure of N-methylpyrrole studied by gas electron diffraction using rotational constants and liquid–crystal NMR spectroscopy. J. Mol. Struct. 2001, 107–111. [Google Scholar] [CrossRef]
- Császár, A.G.; Demaison, J.; Rudolph, H.D. Equilibrium Structures of Three-, Four-, Five-, Six-, and SeveN-Membered Unsaturated N-Containing Heterocycles. J. Phys. Chem. A 2014, 119, 1731–1746. [Google Scholar] [CrossRef] [PubMed]
- Itoh, S.; Yokoyama, Y.; Okumoto, T.; Saito, K.; Takahashi, K.; Satake, K.; Takamuku, H.; Kimura, M.; Morosawa, S. Hindered Internal Rotation Around the C–N Bond in 1H-Azepine Derivatives as Studied by the NMR Techniques. Bull. Chem. Soc. Jpn. 1990, 63, 3162–3166. [Google Scholar] [CrossRef]
- Frigerio, A.; Cavo-Briones, M.; Belvedere, G. Formation of Stable Epoxides in the Metabolism of Tricyclic Drugs. Drug Metab. Rev. 1976, 5, 197–218. [Google Scholar] [CrossRef] [PubMed]
- Ohta, T.; Miyata, N.; Hirube, M. Studies on the Oxidation of 5H-N-Substituted Dibenzo[b,f]-azepines. III. Oxidative Metabolism by Rat Liver Microsomes. Chem. Pharm. Bull. 1986, 34, 2494–2500. [Google Scholar] [CrossRef]
- Zbaida, S.; Kariv, R. Simulation of the hepatic metabolism of stilbene and its tricyclic derivatives by fenton and ruff reagents: Models for cytochrome P-450 activation of chemical carcinogens. Biopharm. Drug Dispos. 1990, 11, 39–51. [Google Scholar] [CrossRef]
- Ohta, T.; Miyata, N.; Hirobe, M. Studies on the oxidation of N-substituted-dibenz(b,f)azepines. I. Oxidation with m-chloroperbenzoic acid. Chem. Pharm. Bull. 1981, 29, 1221–1230. [Google Scholar] [CrossRef][Green Version]
- Pedley, J.B. Thermochemical Data and Structures of Organic Compounds; Thermodynamics Research Center: College Station, TX, USA, 1994; Volume 1.
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Austin, A.; Petersson, G.A.; Frisch, M.J.; Dobek, F.J.; Scalmani, G.; Throssell, K. A Density Functional with Spherical Atom Dispersion Terms. J. Chem. Theory Comput. 2012, 8, 4989–5007. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Accounts 2007, 120, 215–241. [Google Scholar] [CrossRef]
- Petersson, G.A.; Bennett, A.; Tensfeldt, T.G.; Al-Laham, M.A.; Shirley, W.A.; Mantzaris, J. A complete basis set model chemistry. I. The total energies of closed-shell atoms and hydrides of the first-row elements. J. Chem. Phys. 1988, 89, 2193–2218. [Google Scholar] [CrossRef]
- Dunning, T.H., Jr. Gaussian Basis Sets for Use in Correlated Molecular Calculations. I. The Atoms Boron Through Neon and Hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Kendall, R.A.; Dunning, T.H., Jr.; Harrison, R.J. Electron Affinities of the First-Row Atoms Revisited. Systematic Basis Sets and Wave Functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef]
- Gauss, J. Accurate Calculation of NMR Chemical Shifts. Berichte der Bunsengesellschaft für physikalische Chemie 1995, 99, 1001–1008. [Google Scholar] [CrossRef]
- Cheeseman, J.R.; Trucks, G.W.; Keith, T.A.; Frisch, M.J. A comparison of models for calculating nuclear magnetic resonance shielding tensors. J. Chem. Phys. 1996, 104, 5497–5509. [Google Scholar] [CrossRef]
- Wolinski, K.; Hinton, J.F.; Pulay, P. Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations. J. Am. Chem. Soc. 1990, 112, 8251–8260. [Google Scholar] [CrossRef]
- Dill, J.D.; Greenberg, A.; Liebman, J.F. Substituent effects on strain energies. J. Am. Chem. Soc. 1979, 101, 6814–6826. [Google Scholar] [CrossRef]
- Greenberg, A.; Stevenson, T. Application of Linear Free Energy Relationships to Calculated Stabilization Energies of Strained and Unsaturated Molecules. J. Am. Chem. Soc. 1985, 107, 3488–3494. [Google Scholar] [CrossRef]
- Kassaee, M.Z.; Arshadi, S.; Haerizade, B.N.; Vessally, E. Electronic Effects on 1H-Azepines Valence Tautomerization: An Ab Initio Comparative Study. J. Molec. Struct. THEOCHEM 2005, 731, 29–37. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
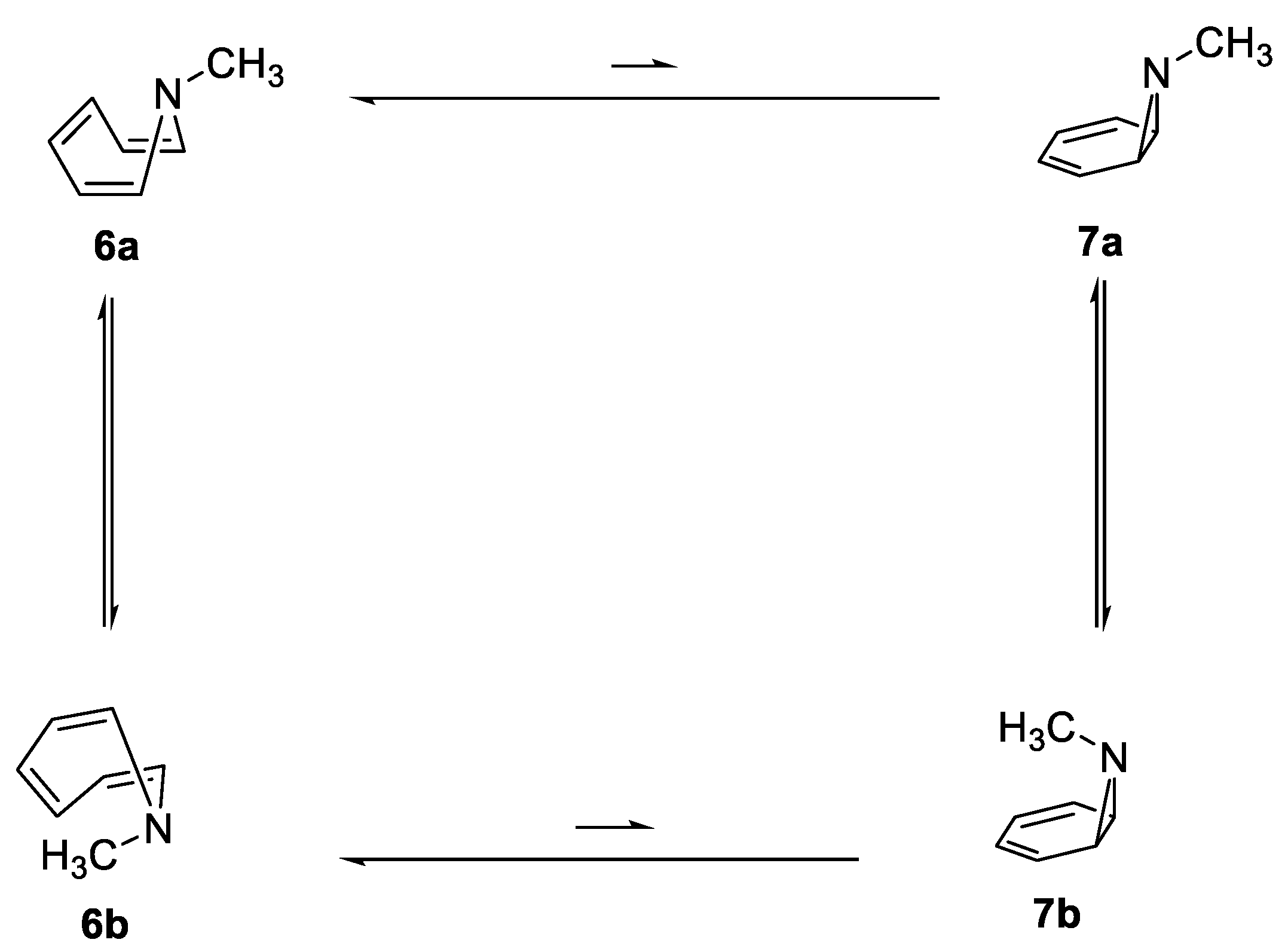
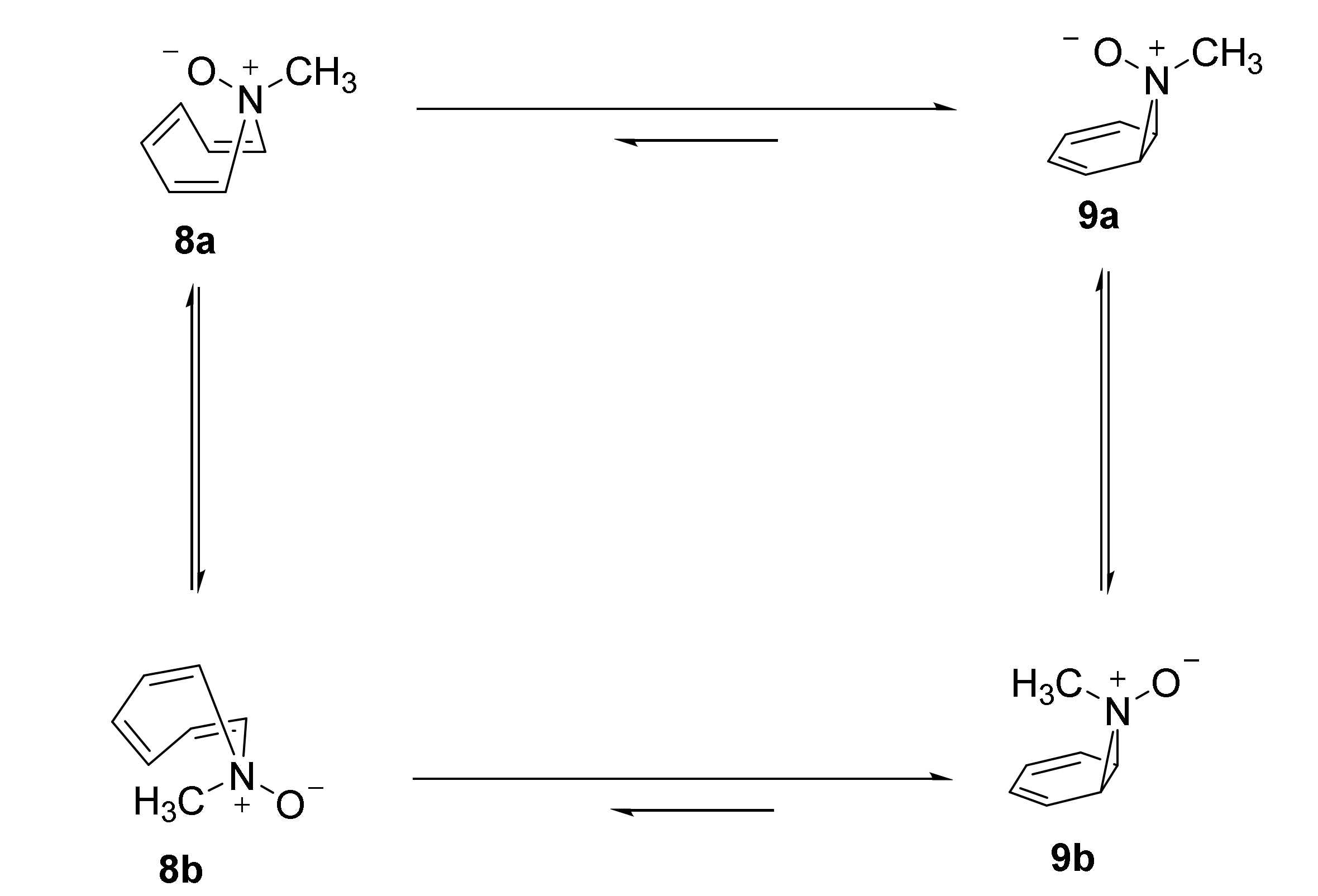
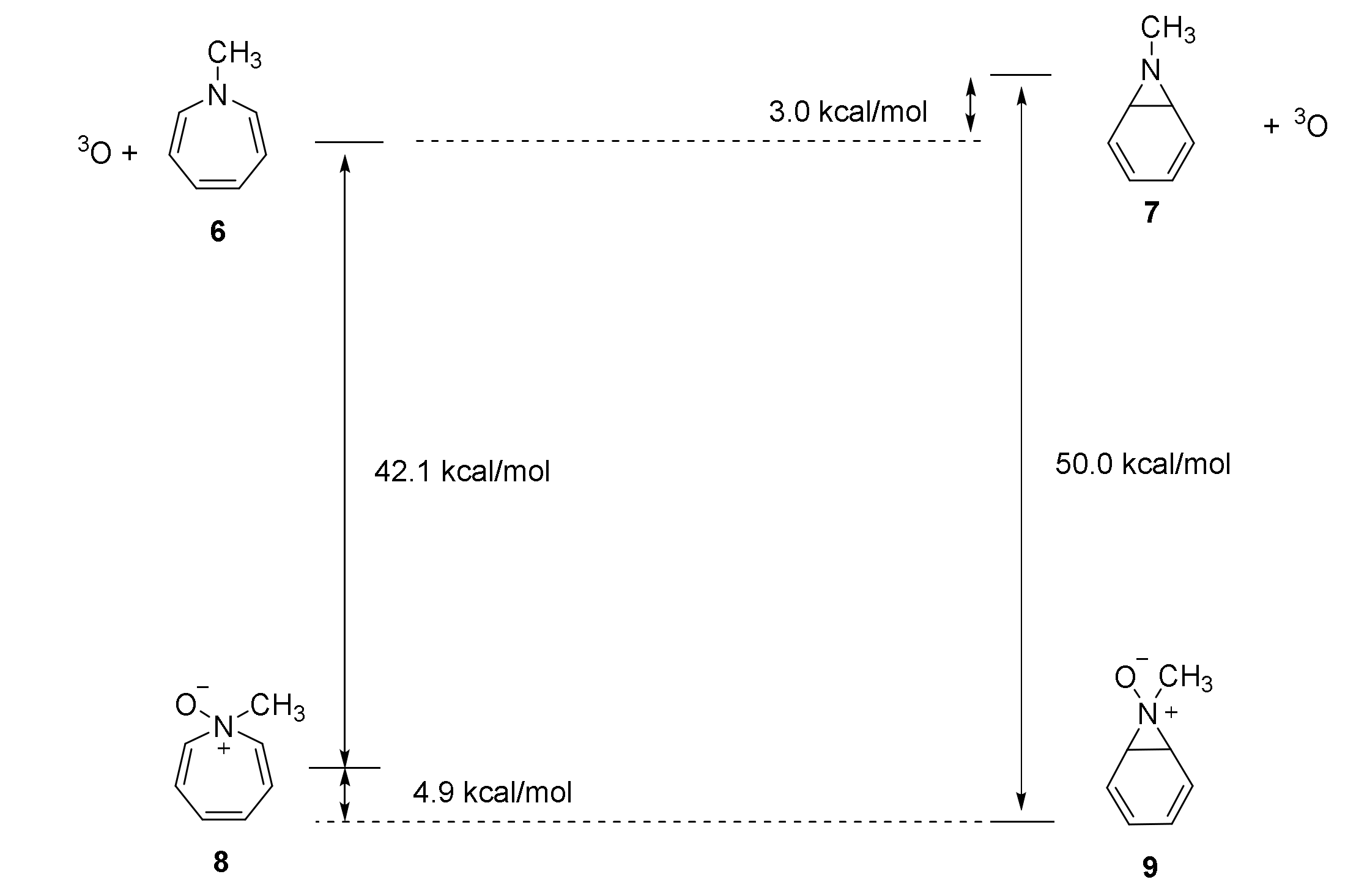
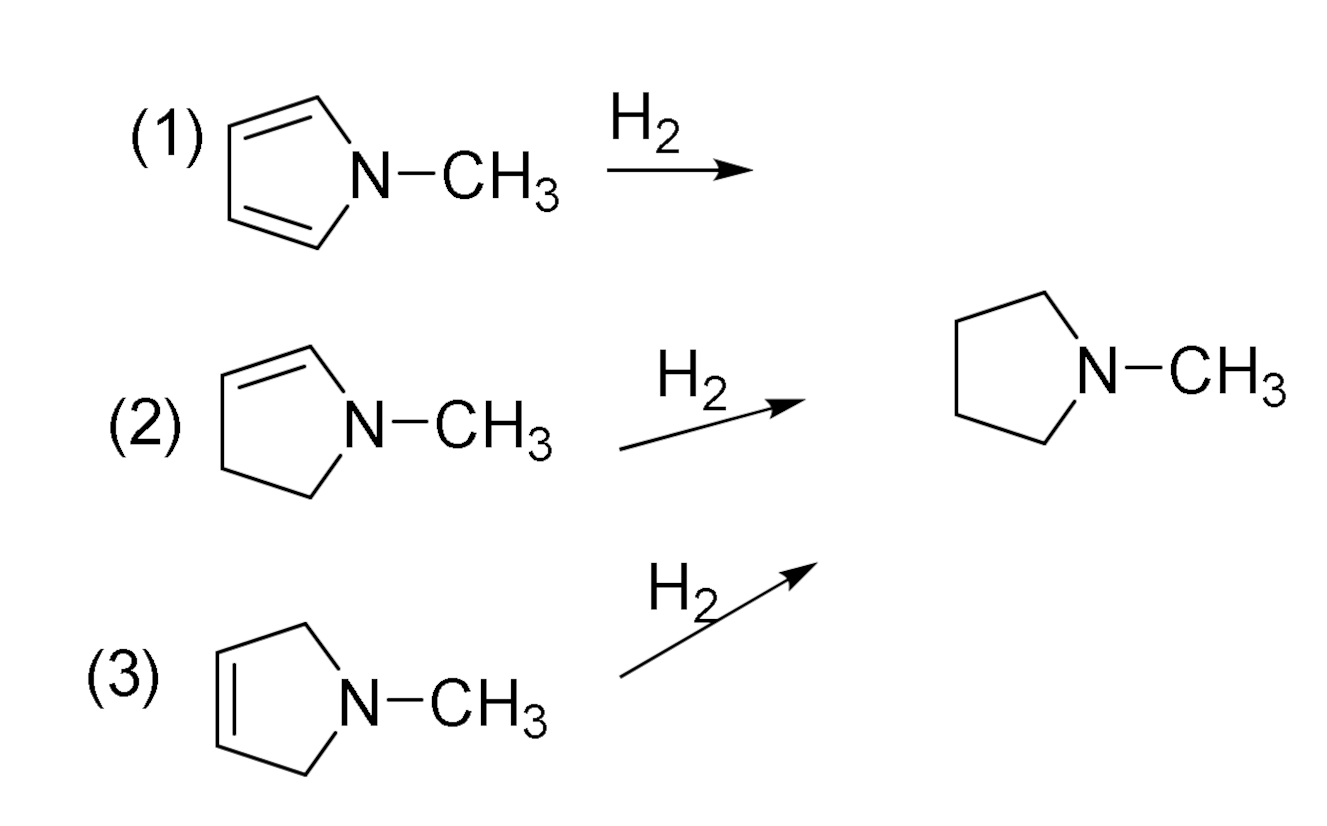
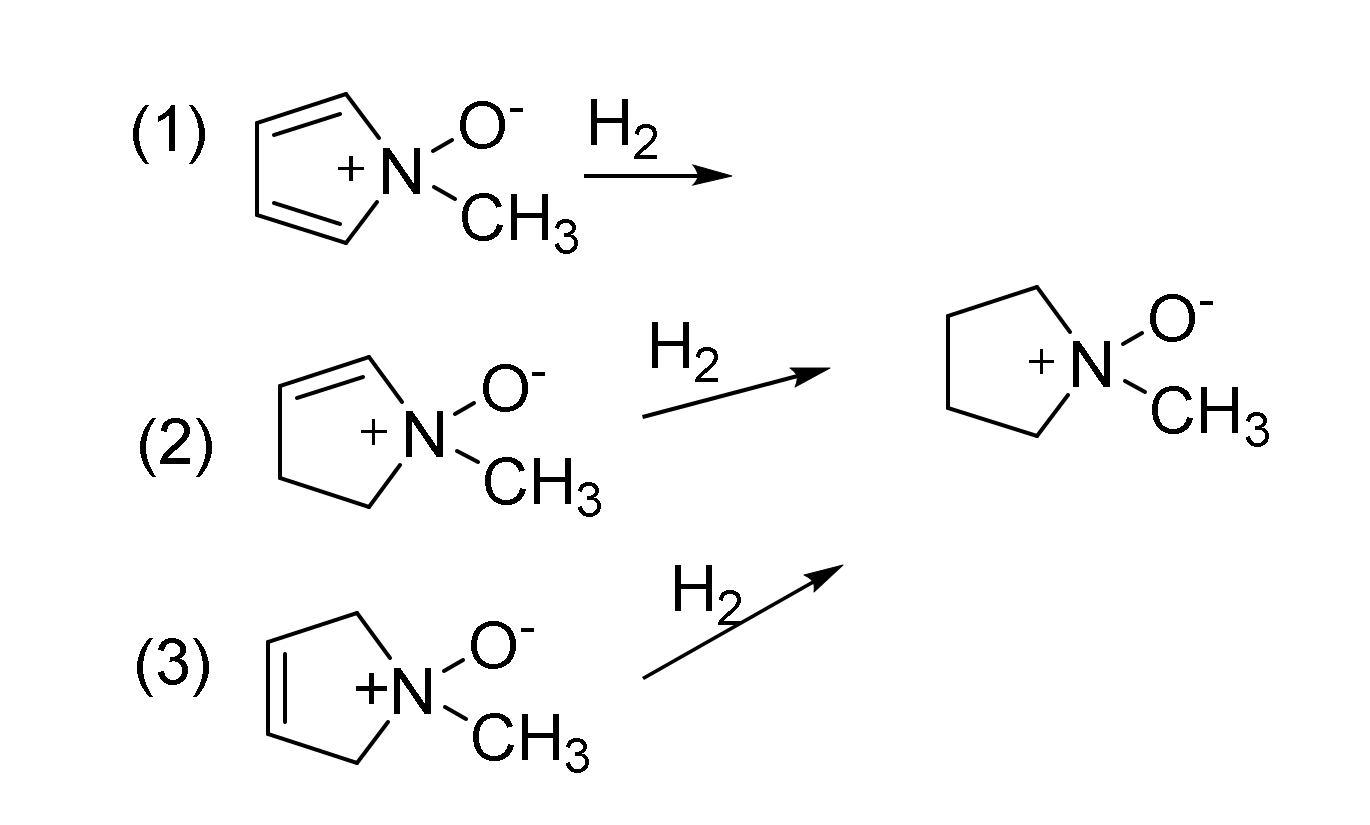
| A. Relative Enthalpy (kcal/mol) | |||||
| Molecule | B3LYP/6-31G(d) | B3LYP/6-31G(d,p) | M06/6-311+G(d,p) | APFD/aug-cc-pVDZ | APFD/aug-cc-pVTZ |
| 6a | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 6b | |||||
| 7a | 5.2 | 7.5 | 3.9 | 4.7 | 5.7 |
| 7b | 6.2 | 6.3 | 3.0 | ||
| B. Relative Free Energy (kcal/mol) | |||||
| Molecule | B3LYP/6-31G(d) | B3LYP/6-31G(d,p) | M06/6-311+G(d,p) | APFD/aug-cc-pVDZ | APFD/aug-cc-pVTZ |
| 6a | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 6b | |||||
| 7a | 8.2 | 8.5 | 4.8 | 5.6 | 6.6 |
| 7b | 7.1 | 7.4 | 3.9 | ||
| A. Relative Enthalpy (kcal/mol) | |||||
| Molecule | B3LYP/6-31G(d) | B3LYP/6-31G(d,p) | M06/6-311+G(d,p) | APFD/aug-cc-pVDZ | APFD/aug-cc-pVTZ |
| 8a | 1.5 | 1.3 | 4.9 | ||
| 8b | 5.1 | 4.3 | |||
| 9a | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 |
| 9b | 0.0 | 0.1 | 0.0 | ||
| B. Relative Free Energy (kcal/mol) | |||||
| Molecule | B3LYP/6-31G(d) | B3LYP/6-31G(d,p) | M06/6-311+G(d,p) | APFD/aug-cc-pVDZ | APFD/aug-cc-pVTZ |
| 8a | 1.4 | 1.4 | 5.0 | ||
| 8b | 5.2 | 4.3 | |||
| 9a | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 |
| 9b | 0.0 | 0.1 | 0.0 | ||
| Molecule | B3LYP/6-31G(d) | B3LYP/6-31G(d,p) | M06/6-311+G(d,p) | APFD/aug-cc-pVDZ | APFD/aug-cc-pVTZ |
|---|---|---|---|---|---|
| PNO (10) | 62.1 | 62.3 | 61.5 | ||
| TMAO (12) | 48.6 | 48.7 | 51.5 | ||
| 8 | 40.7 | 40.8 | 42.1 | 41.6 | 44.4 |
| 9 | 47.3 | 49.6 | 50.9 | 51.4 | 54.0 |
| 15 | 47.4 | 47.6 | 50.0 | ||
| 11 | 14.7 | 14.6 | 17.6 | 13.1 | 16.9 |
| Equation | B3LYP/6-31G(d) | B3LYP/6-31G(d,p) | M06/6-311+G(d,p) |
|---|---|---|---|
| (3) | +1.2 | +1.1 | +1.5 |
| (4) | −0.1 | +2.0 | +0.9 |
| Hydrogenation | (1) | (2) | (3) |
|---|---|---|---|
| B3LYP/6-31G(d,p) | −27.1 | −24.7 | −29.3 |
| APFD/aug-cc-pVDZ | −34.6 | −28.9 | −33.7 |
| APFD/aug-cc-pVTZ | −28.2 | −23.9 | −28.8 |
| Reaction | (1) | (2) | (3) |
|---|---|---|---|
| B3LYP/6-31G(d,p) | −60.9 | −29.3 | −28.9 |
| APFD/aug-cc-pVDZ | −70.7 | −34.2 | −33.3 |
| APFD/aug-cc-pVTZ | −67.2 | −32.4 | −31.8 |
| N-Methylpyrrole | N-Methylpyrrole N-Oxide | |
|---|---|---|
| B3LYP/6-31G(d,p) | ||
| Shielding (ppm) | 15.15 | 2.34 |
| APFD/aug-cc-pVTZ | ||
| Shielding (ppm) | 13.38 | 1.68 |
| A. Experimental Bond Lengths (Angstroms) | |||
| Bond | GED | MW | |
| 1 | 1.372 | 1.361 | |
| 2 | 1.383 | 1.393 | |
| 3 | 1.425 | 1.422 | |
| B. Computed Bond Lengths (Angstroms) | |||
| Bond | B3LYP/6-31G(d,p) | APFD/aug-cc-pVDZ | APFD/aug-cc-pVTZ |
| 1 | 1.376 | 1.369 | 1.364 |
| 2 | 1.378 | 1.379 | 1.372 |
| 3 | 1.423 | 1.421 | 1.415 |
| Bond | B3LYP/6-31G(d,p) | APFD/aug-cc-pVDZ | APFD/aug-cc-pVTZ |
|---|---|---|---|
| 1 | 1.479 | 1.470 | 1.467 |
| 2 | 1.335 | 1.336 | 1.327 |
| 3 | 1.475 | 1.472 | 1.468 |
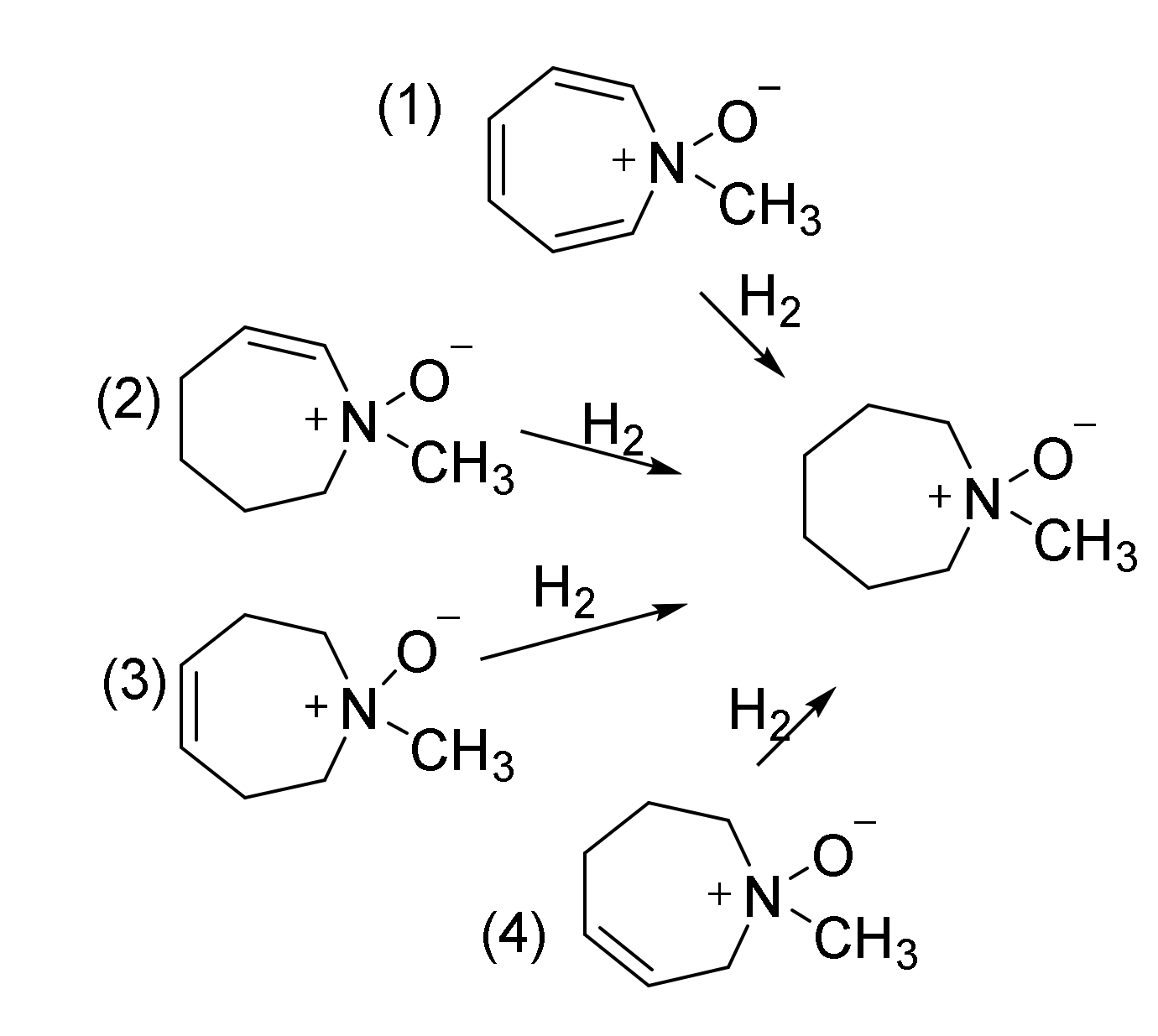
| Hydrogenation | (1) | (2) | (3) | (4) |
|---|---|---|---|---|
| B3LYP/6-31G(d,p) | −67.3 | −22.4 | −29.8 | −25.8 |
| APFD/aug-cc-pVDZ | −82.8 | −27.8 | −34.7 | −31.0 |
| APFD/aug-cc-pVTZ | −77.1 | −25.5 | −32.6 | −29.0 |
| Hydrogenation | (1) | (2) | (3) | (4) |
|---|---|---|---|---|
| B3LYP/6-31G(d,p) | −74.3 | −28.7 | −32.1 | −25.4 |
| APFD/aug-cc-pVDZ | −90.6 | −34.3 | −37.4 | −30.5 |
| APFD/aug-cc-pVTZ | −84.7 | −31.9 | −34.1 | −28.0 |
| N-Methylazepine | N-Methylazepine N-Oxide | |
|---|---|---|
| B3LYP/6-31G(d,p) | ||
| Shielding (ppm) | −5.13 | +0.26 |
| APFD/aug-cc-pVTZ | ||
| Shielding (ppm) | −5.38 | +0.73 |
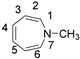
| Bond | B3LYP/6-31G(d,p) | APFD/aug-cc-pVDZ | APFD/aug-cc-pVTZ |
|---|---|---|---|
| 1 | 1.420 | 1.414 | 1.409 |
| 2 | 1.344 | 1.345 | 1.336 |
| 3 | 1.459 | 1.456 | 1.452 |
| 4 | 1.352 | 1.354 | 1.345 |
| 5 | 1.459 | 1.456 | 1.452 |
| 6 | 1.344 | 1.345 | 1.336 |
| 7 | 1.420 | 1.414 | 1.409 |
| C2−C7 distance 2.387 | C2−C7 distance 2.362 | C2−C7 distance 2.363 |
| Bond | B3LYP/6-31G(d,p) | APFD/aug-cc-pVDZ | APFD/aug-cc-pVTZ |
|---|---|---|---|
| 1 | 1.469 | 1.461 | 1.456 |
| 2 | 1.345 | 1.347 | 1.337 |
| 3 | 1.440 | 1.437 | 1.431 |
| 4 | 1.355 | 1.356 | 1.347 |
| 5 | 1.440 | 1.437 | 1.431 |
| 6 | 1.345 | 1.347 | 1.337 |
| 7 | 1.469 | 1.461 | 1.456 |
| C2−C7 distance 2.550 | C2−C7 distance 2.537 | C2−C7 distance 2.531 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fournier, R.; Green, A.R.; Greenberg, A.; Lee-Ruff, E.; Liebman, J.F.; Rágyanszki, A. Predicted Reversal in N-Methylazepine/N-Methyl-7-azanorcaradiene Equilibrium upon Formation of Their N-Oxides. Molecules 2020, 25, 4767. https://doi.org/10.3390/molecules25204767
Fournier R, Green AR, Greenberg A, Lee-Ruff E, Liebman JF, Rágyanszki A. Predicted Reversal in N-Methylazepine/N-Methyl-7-azanorcaradiene Equilibrium upon Formation of Their N-Oxides. Molecules. 2020; 25(20):4767. https://doi.org/10.3390/molecules25204767
Chicago/Turabian StyleFournier, René, Alexa R. Green, Arthur Greenberg, Edward Lee-Ruff, Joel F. Liebman, and Anita Rágyanszki. 2020. "Predicted Reversal in N-Methylazepine/N-Methyl-7-azanorcaradiene Equilibrium upon Formation of Their N-Oxides" Molecules 25, no. 20: 4767. https://doi.org/10.3390/molecules25204767
APA StyleFournier, R., Green, A. R., Greenberg, A., Lee-Ruff, E., Liebman, J. F., & Rágyanszki, A. (2020). Predicted Reversal in N-Methylazepine/N-Methyl-7-azanorcaradiene Equilibrium upon Formation of Their N-Oxides. Molecules, 25(20), 4767. https://doi.org/10.3390/molecules25204767







