Diels-Alder Additions as Mechanistic Probes–Interception of Silyl-Isoindenes: Organometallic Derivatives of Polyphenylated Cycloheptatrienes and Related Seven-Membered Rings
Abstract
1. Introduction
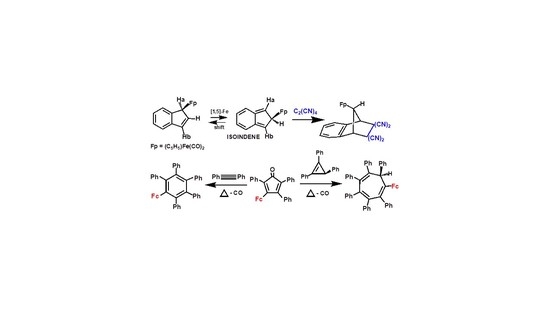
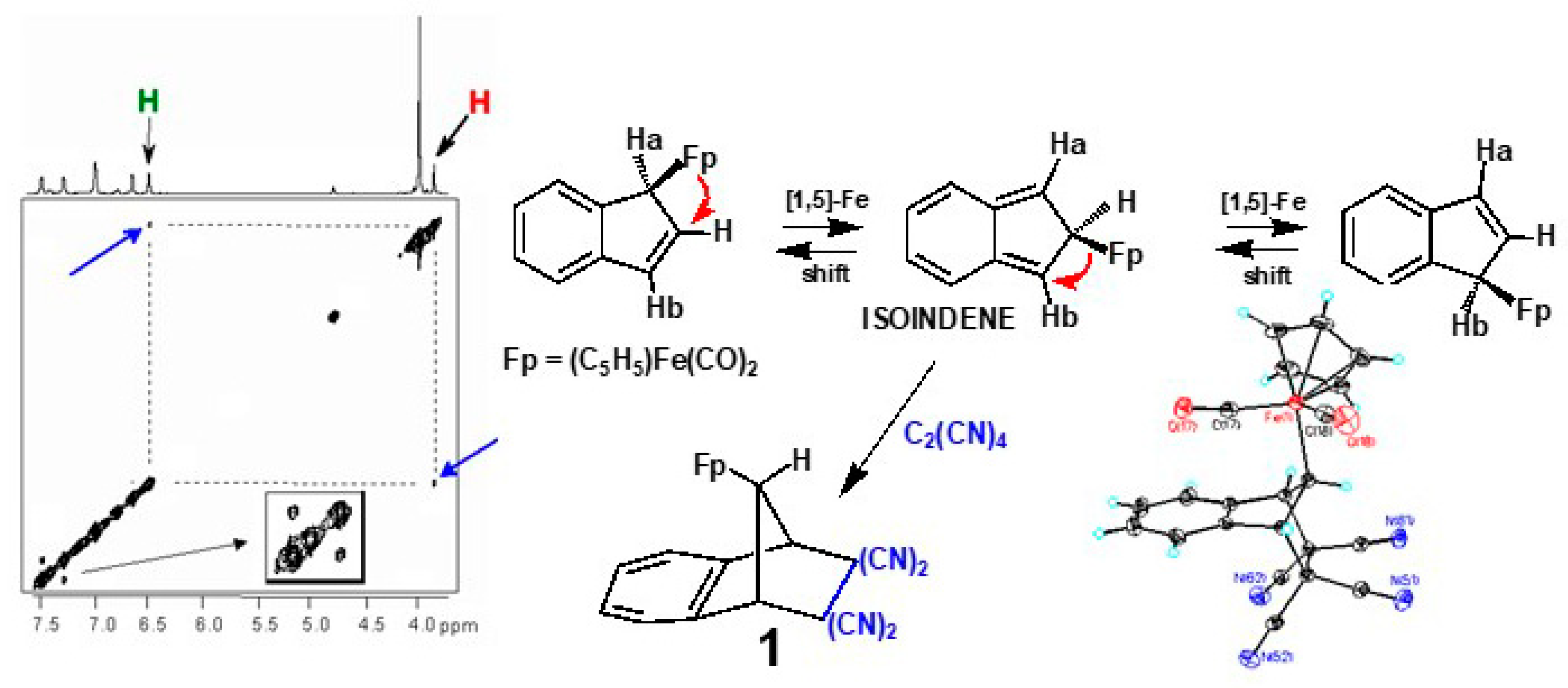
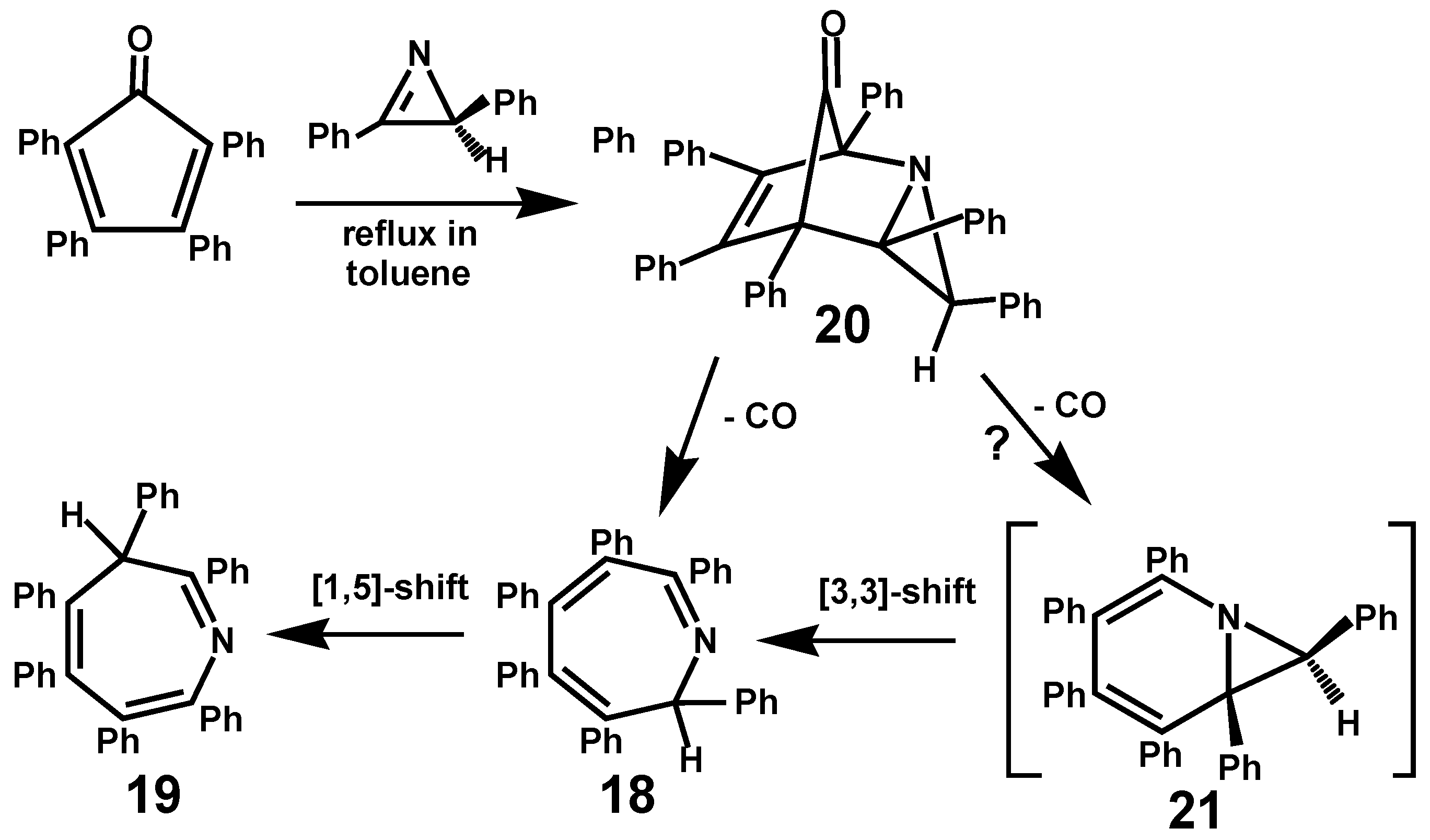
2. Diels-Alder Trapping of Isoindenes
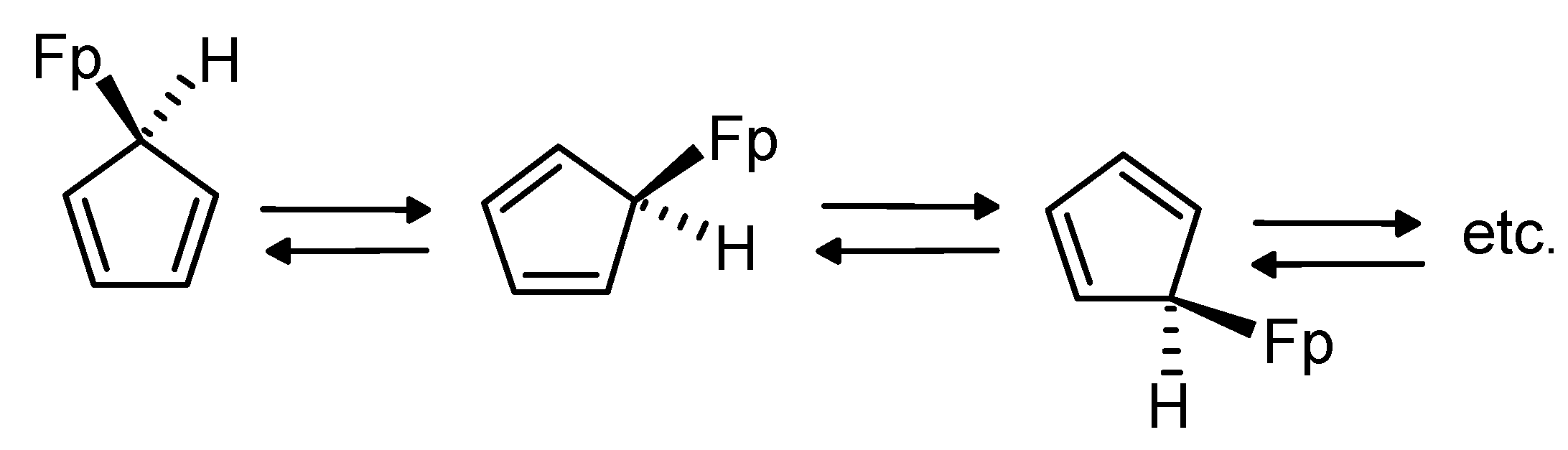
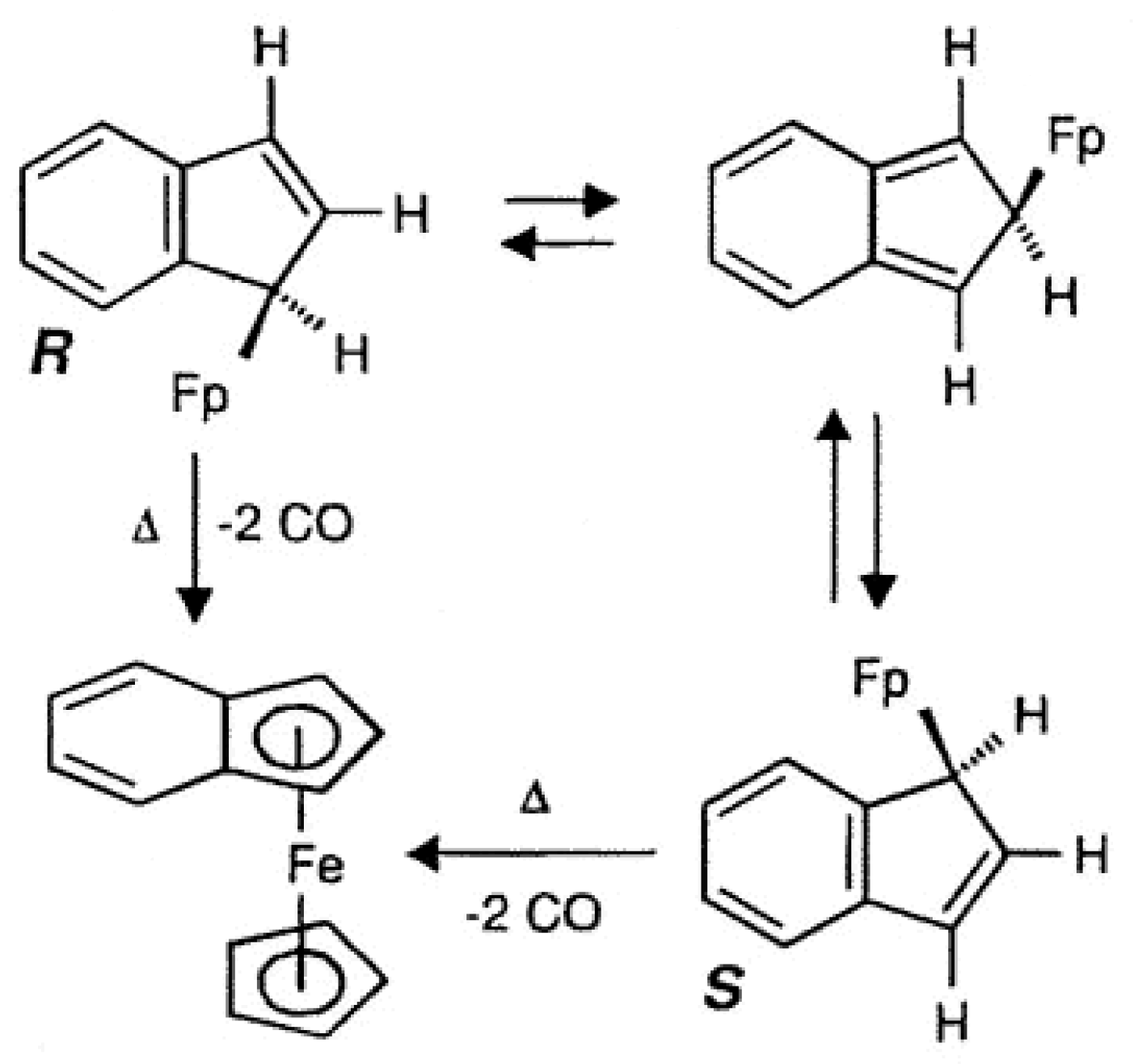
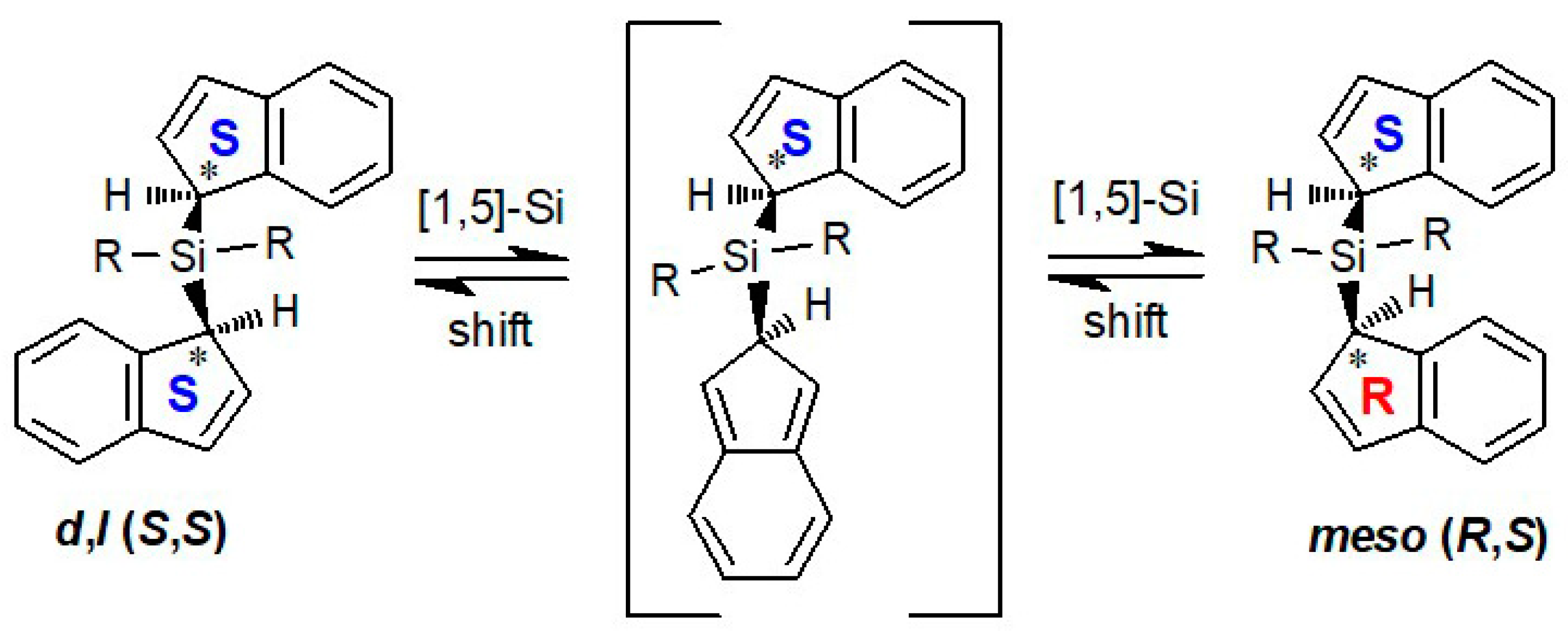
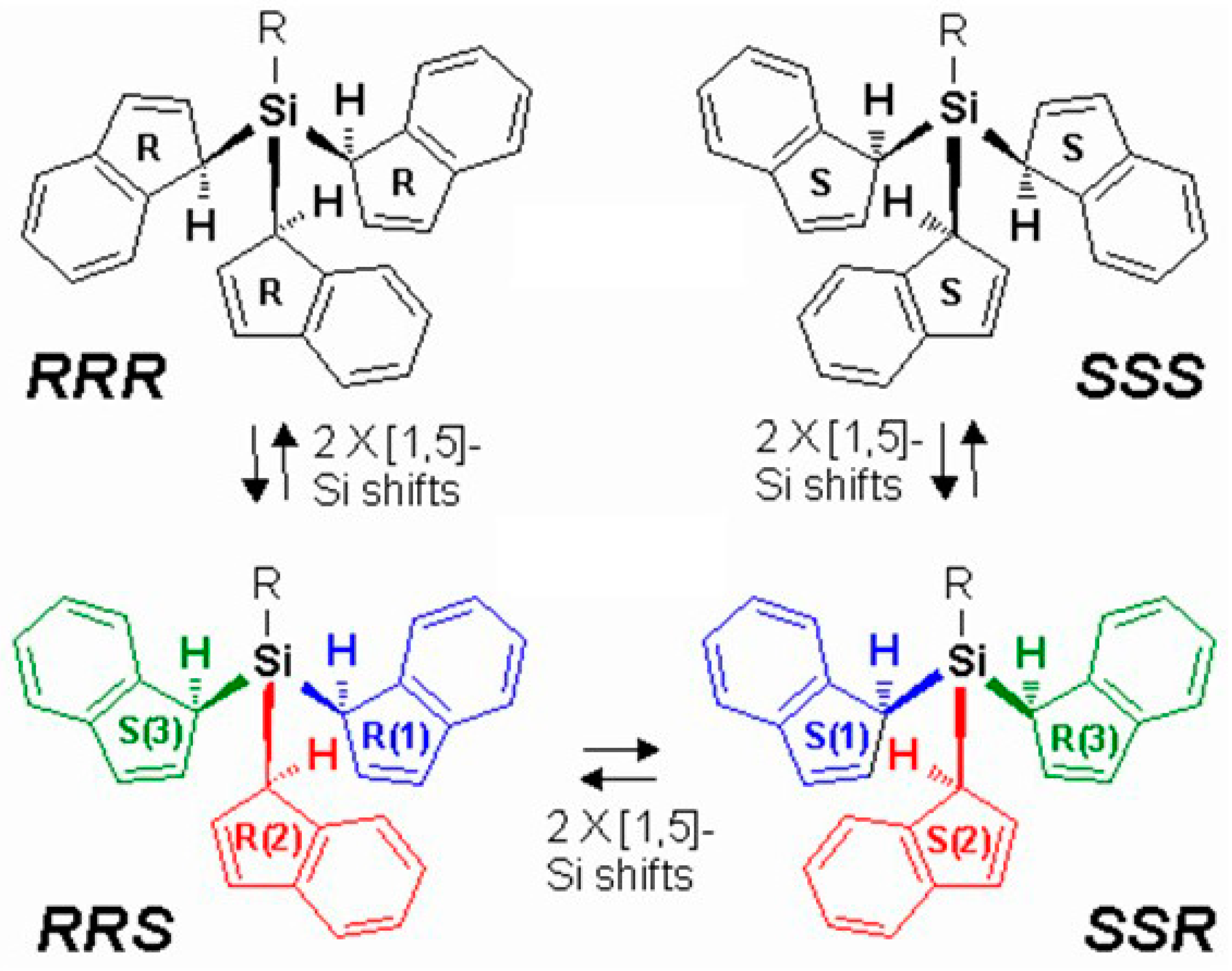
2.1. Metallotropic Shifts
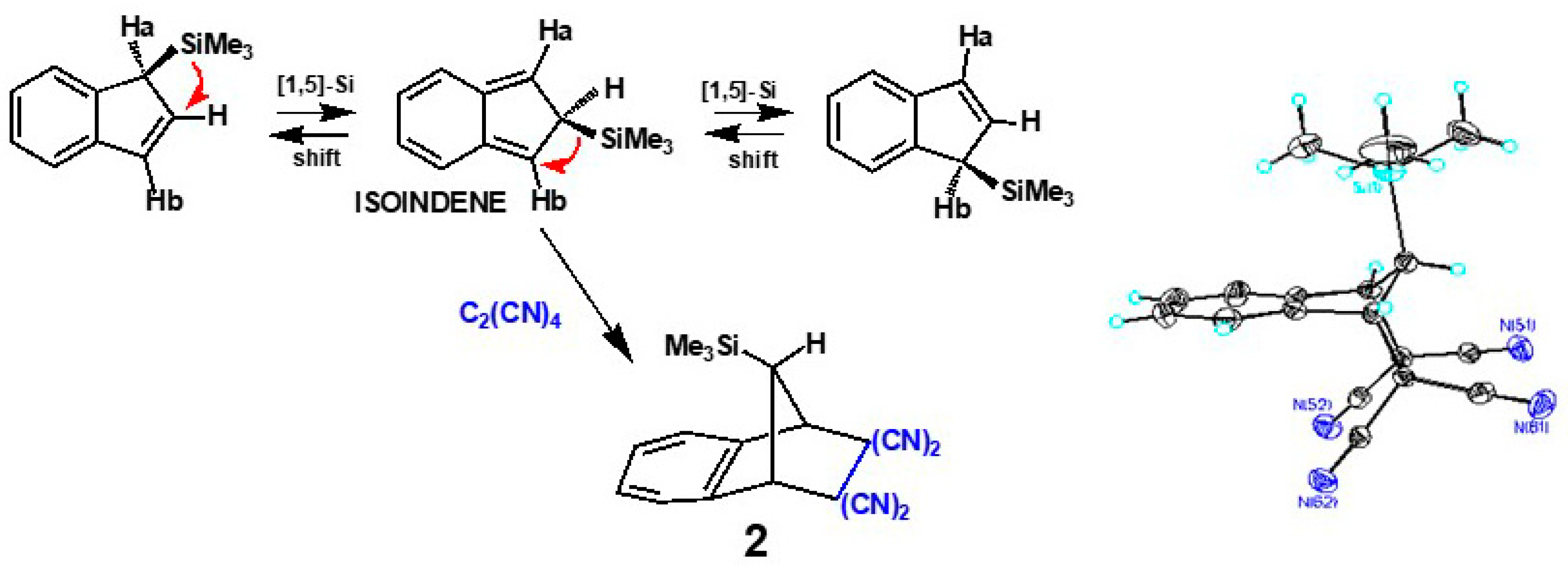
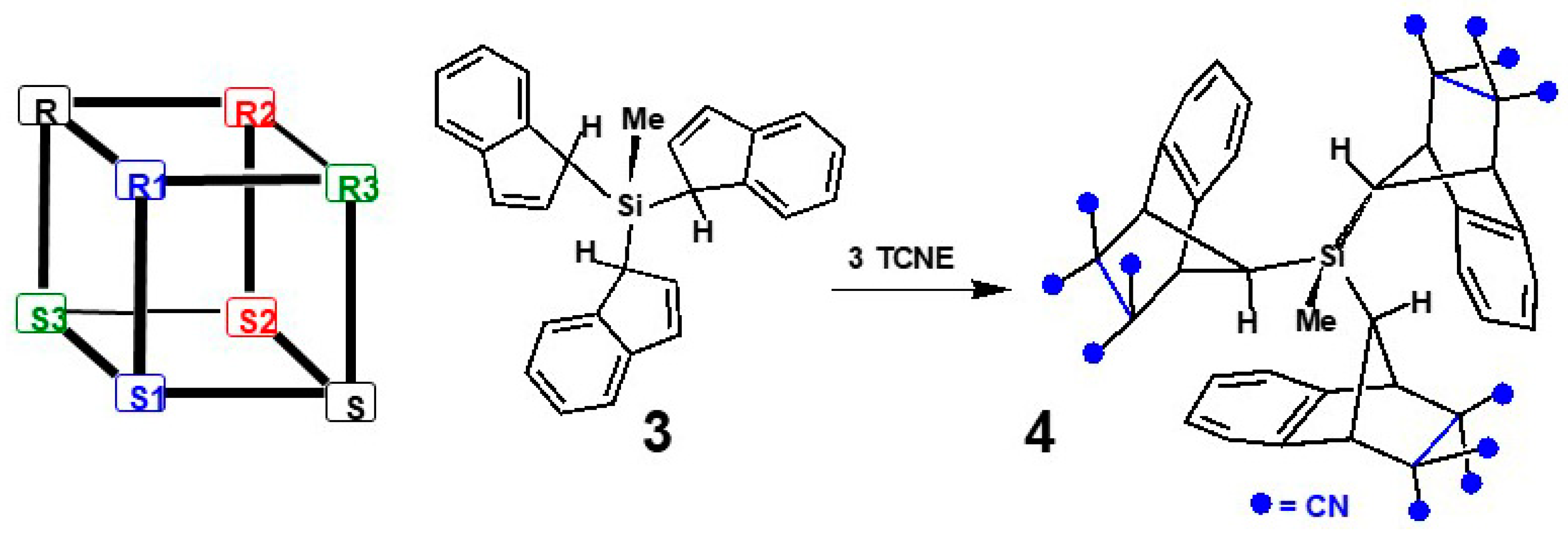
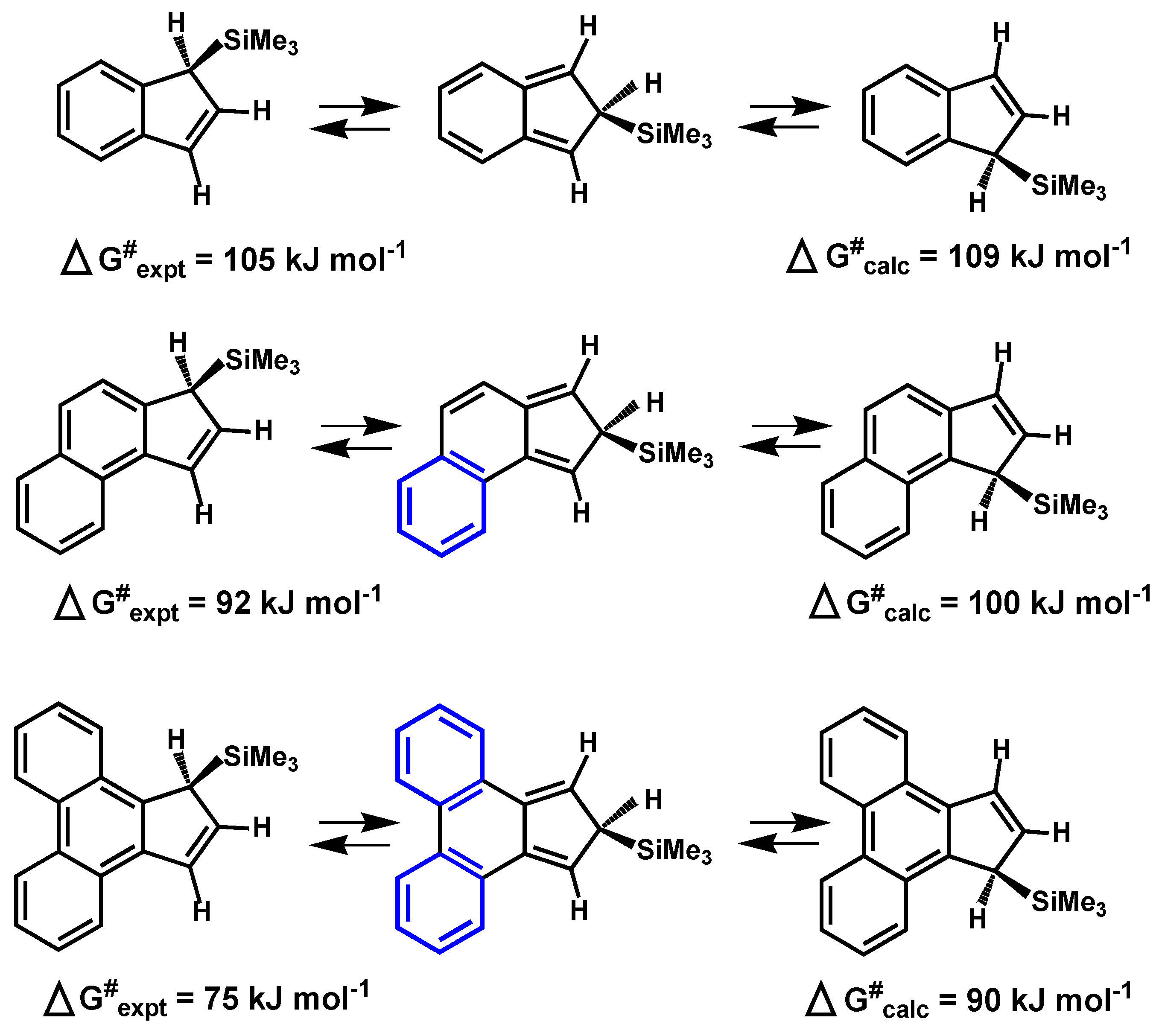
2.2. Silatropic Shifts
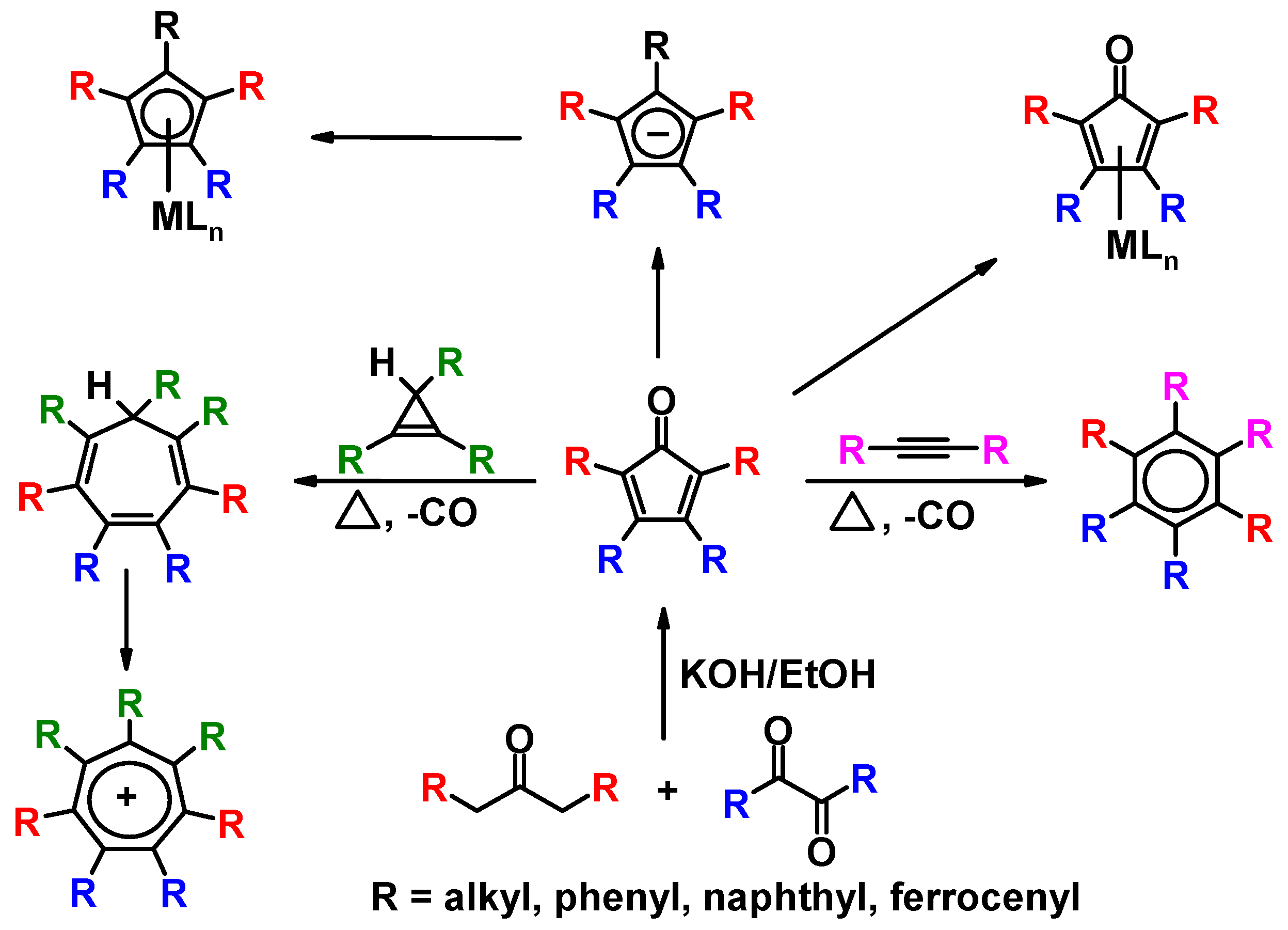
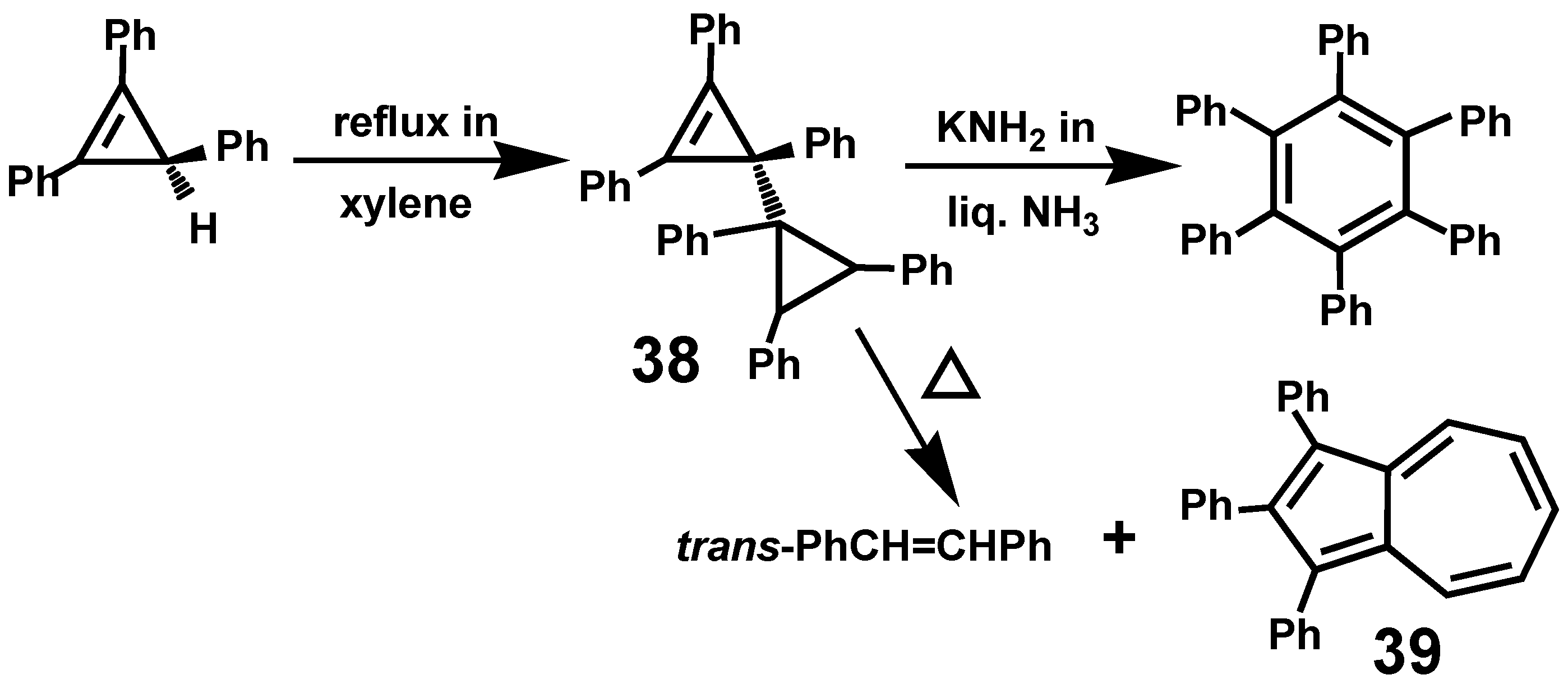
3. Diels-Alder Cycloadditions of Alkynes or Triphenylcyclopropene to Cyclopentadienones
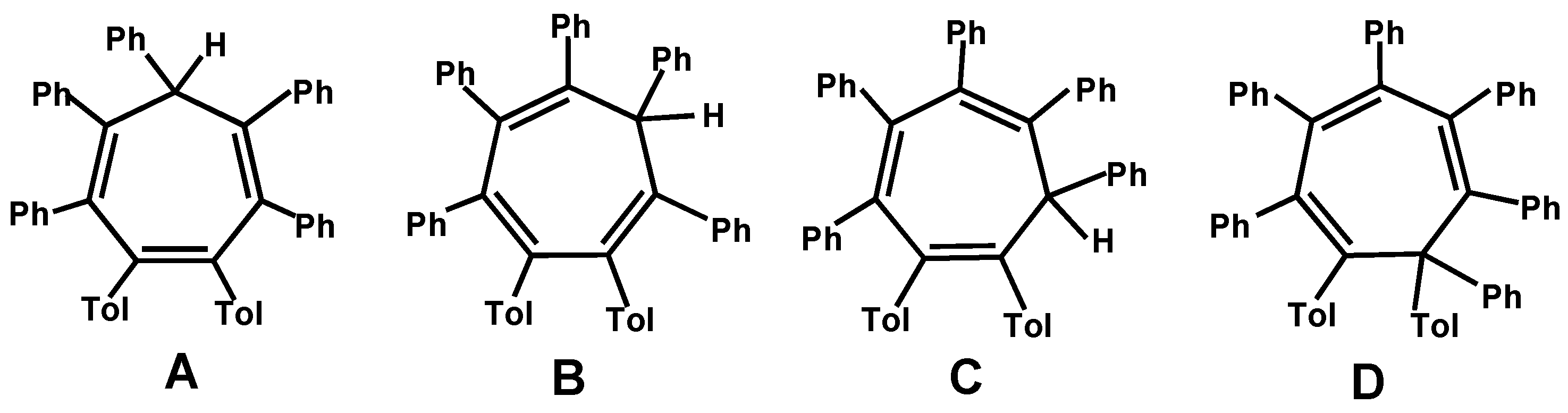
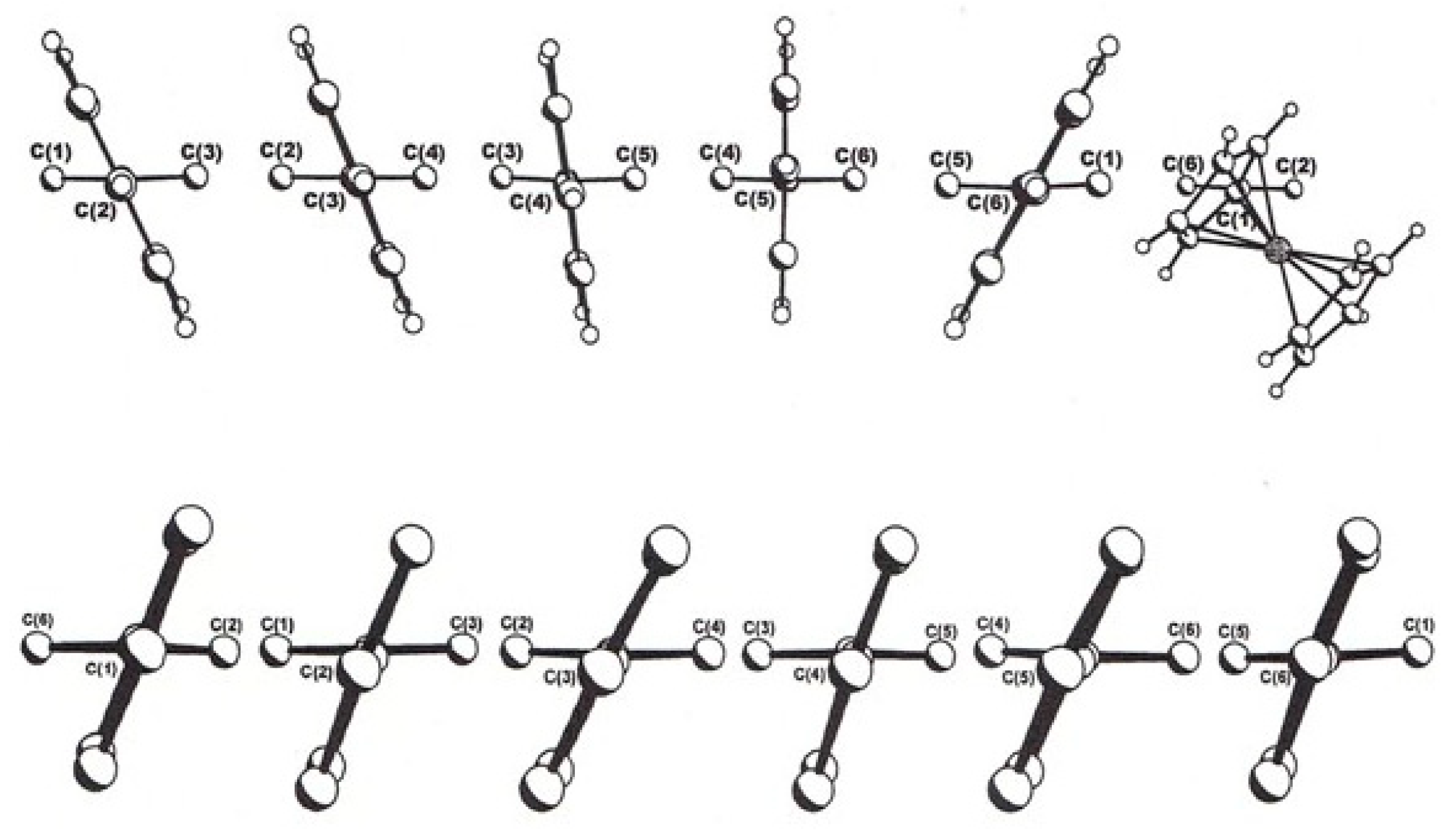
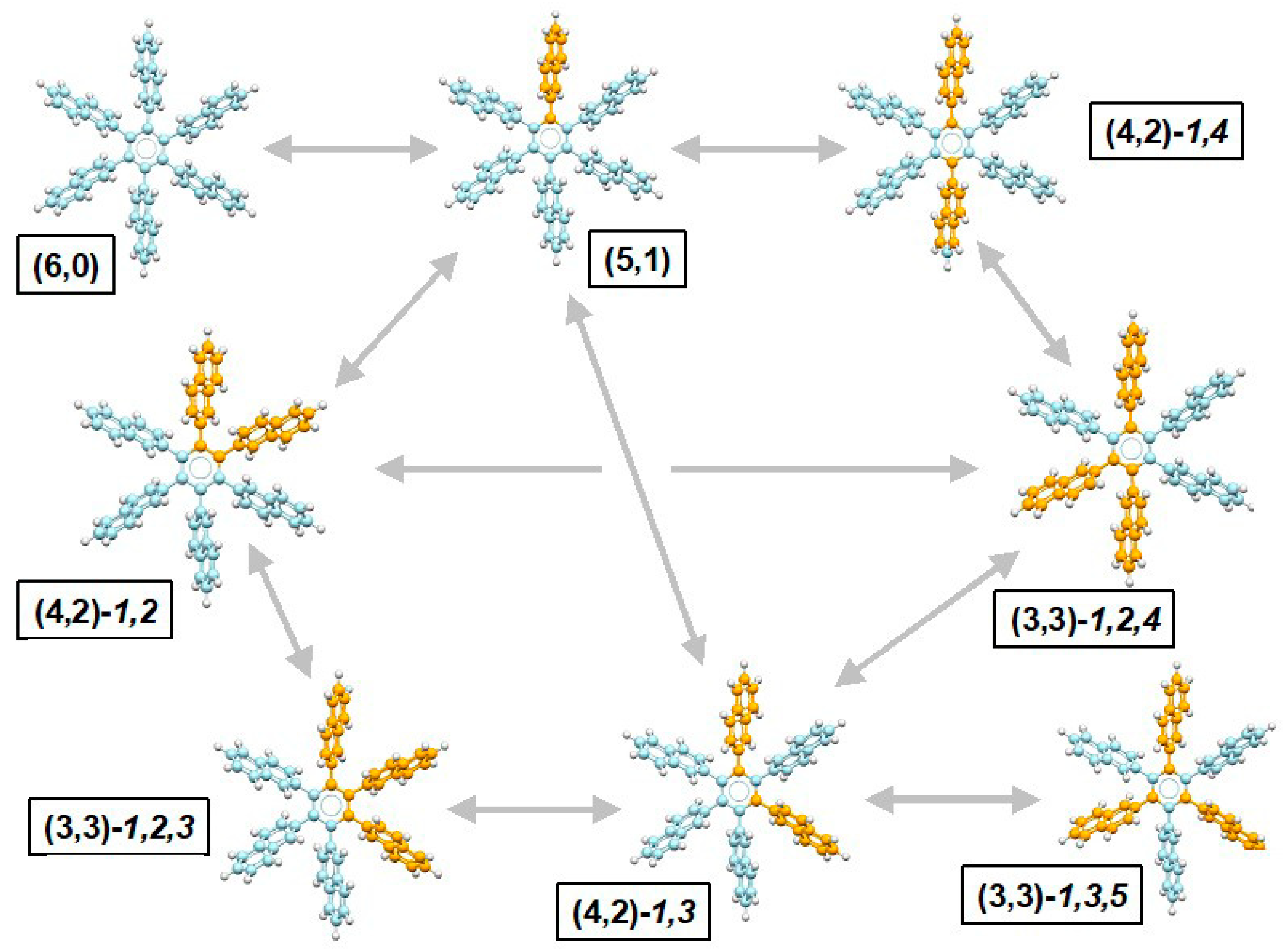
3.1. Geometric Factors in CnPhn Ring Systems
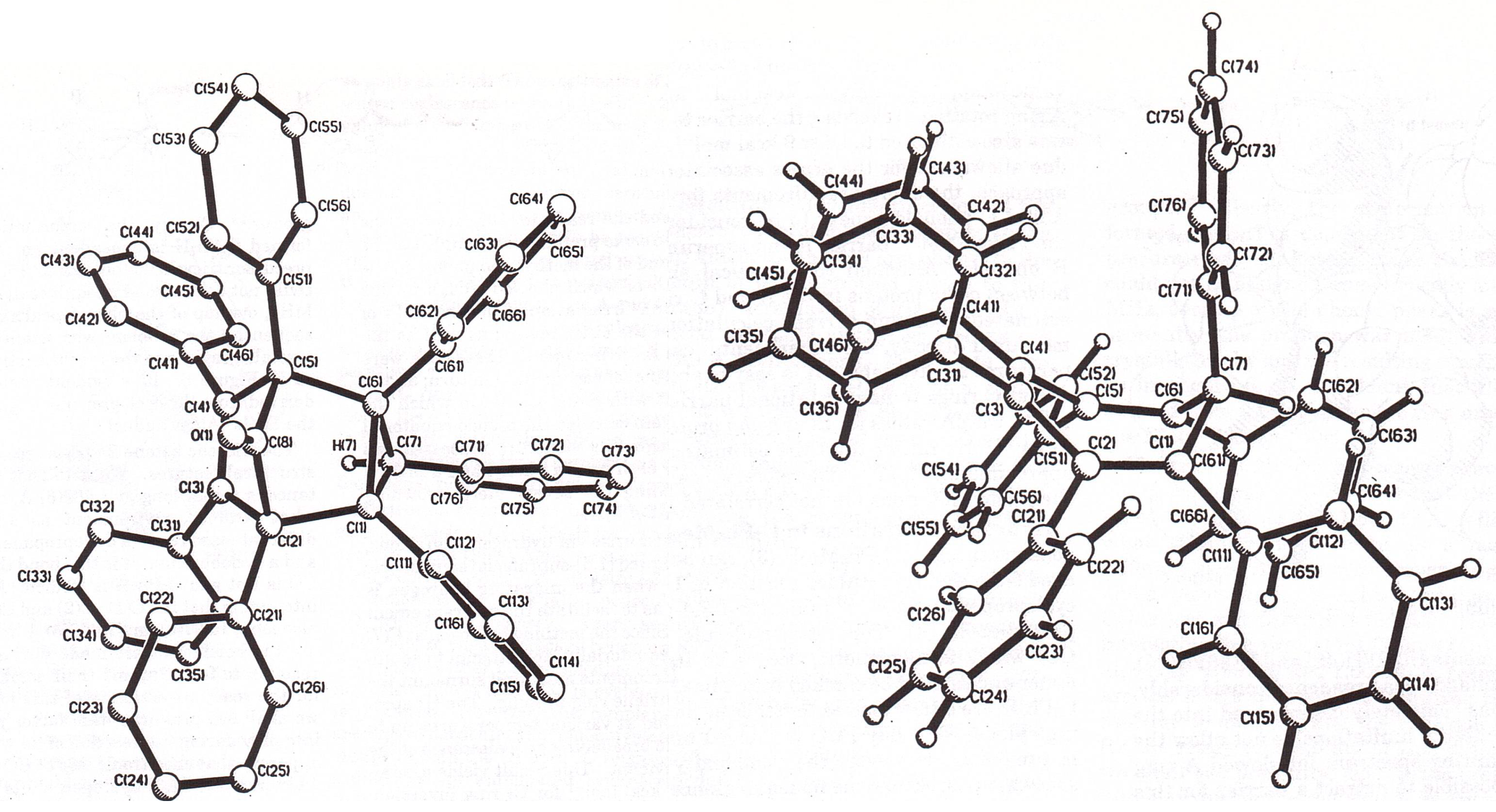
3.2. Syntheses and Structures of Heptaarylcycloheptatrienes
3.3. The Heptaphenyltropylium Cation
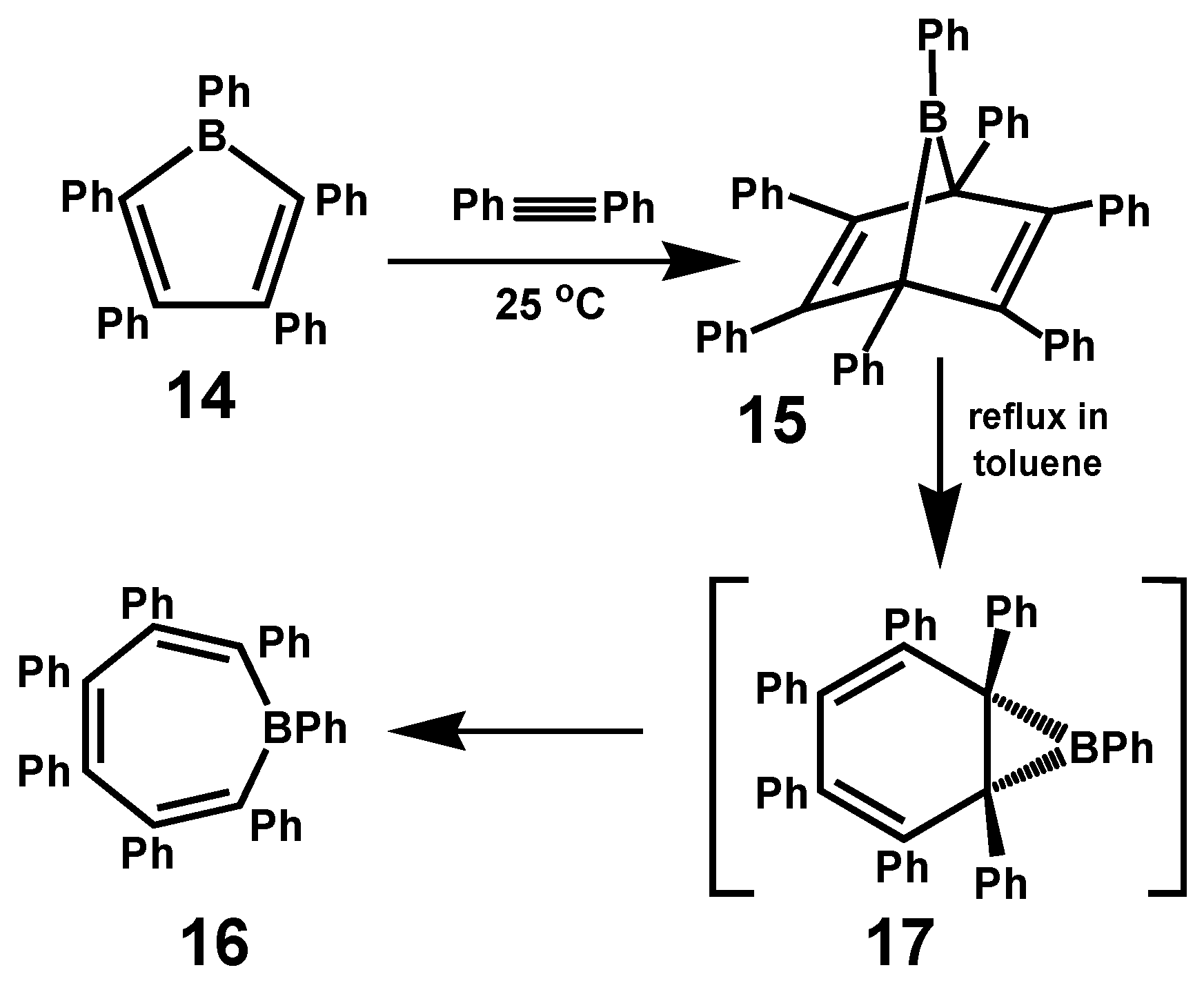
3.4. Boron and Nitrogen Analogues of Heptaphenylcycloheptatrienes
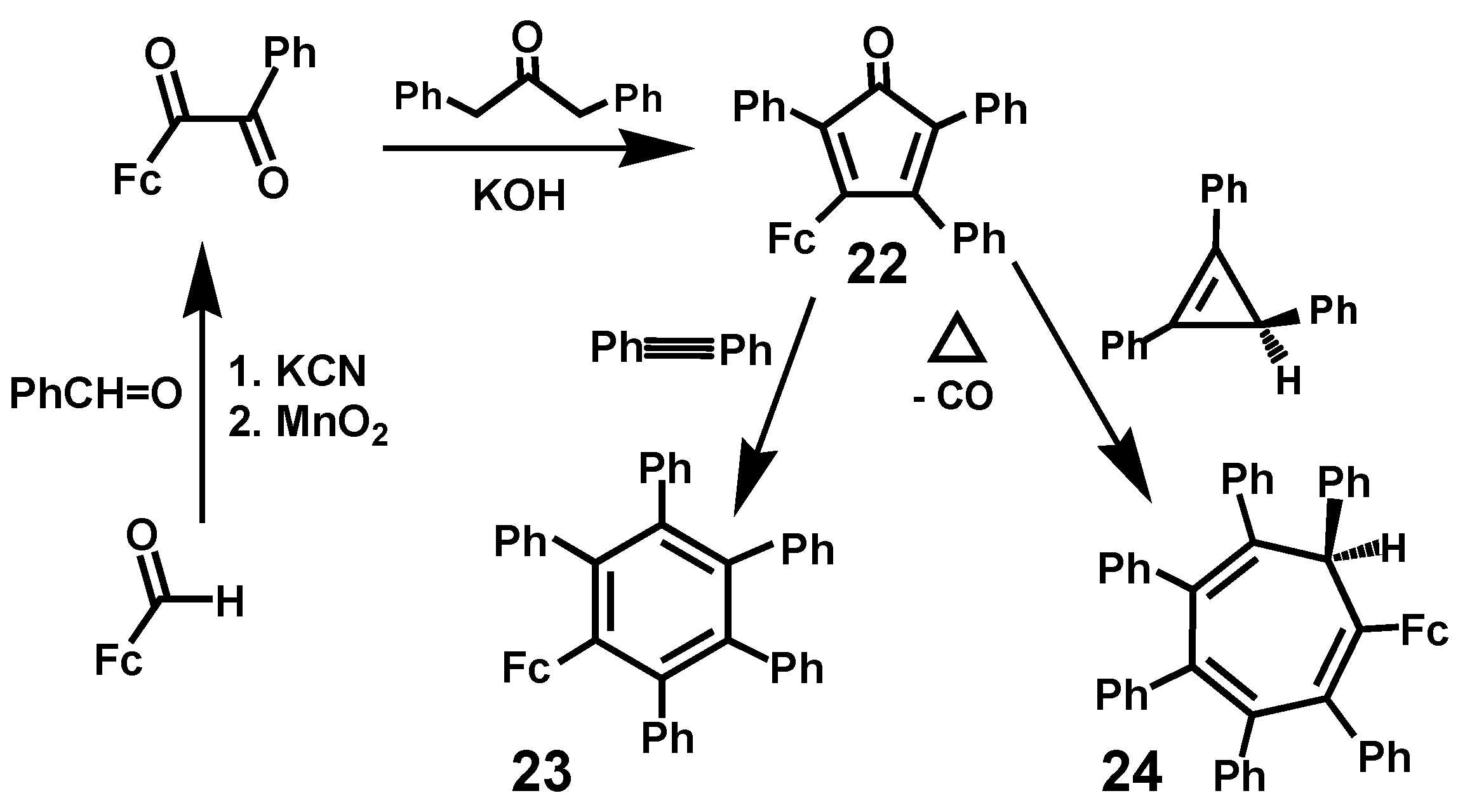
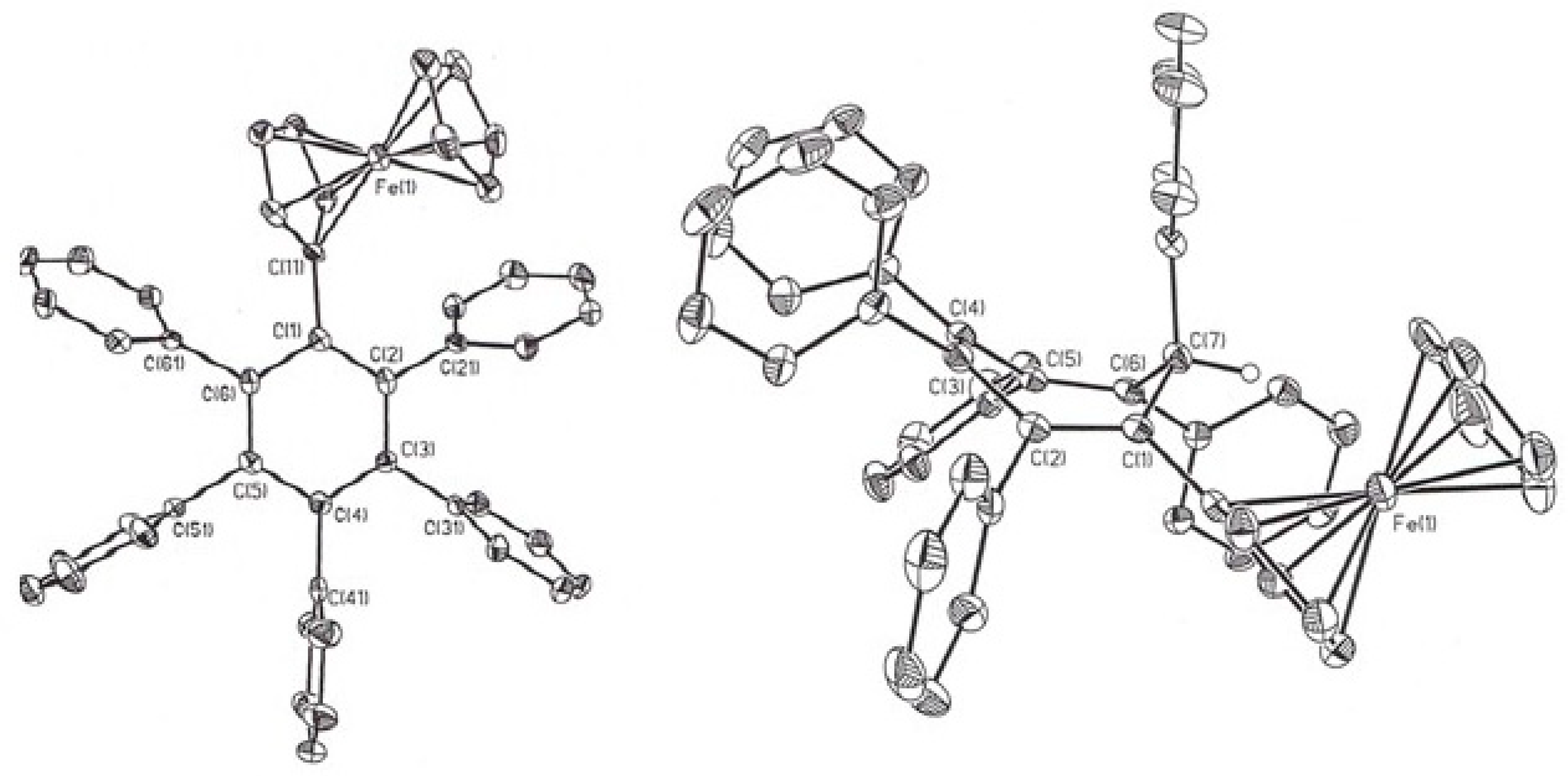
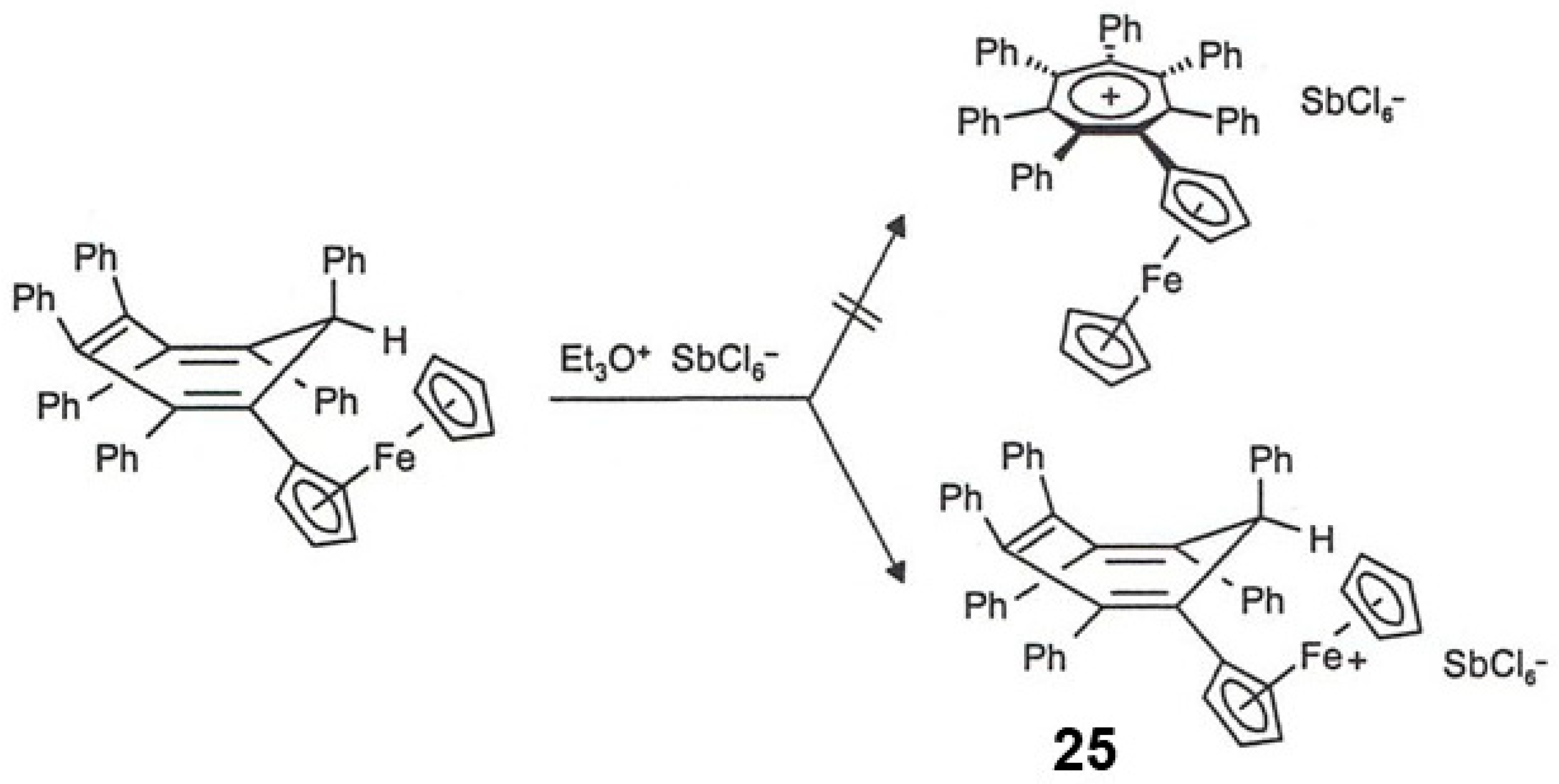
3.5. Ferrocenyl-Substituted Polyaryl-Benzenes and -Cycloheptatrienes
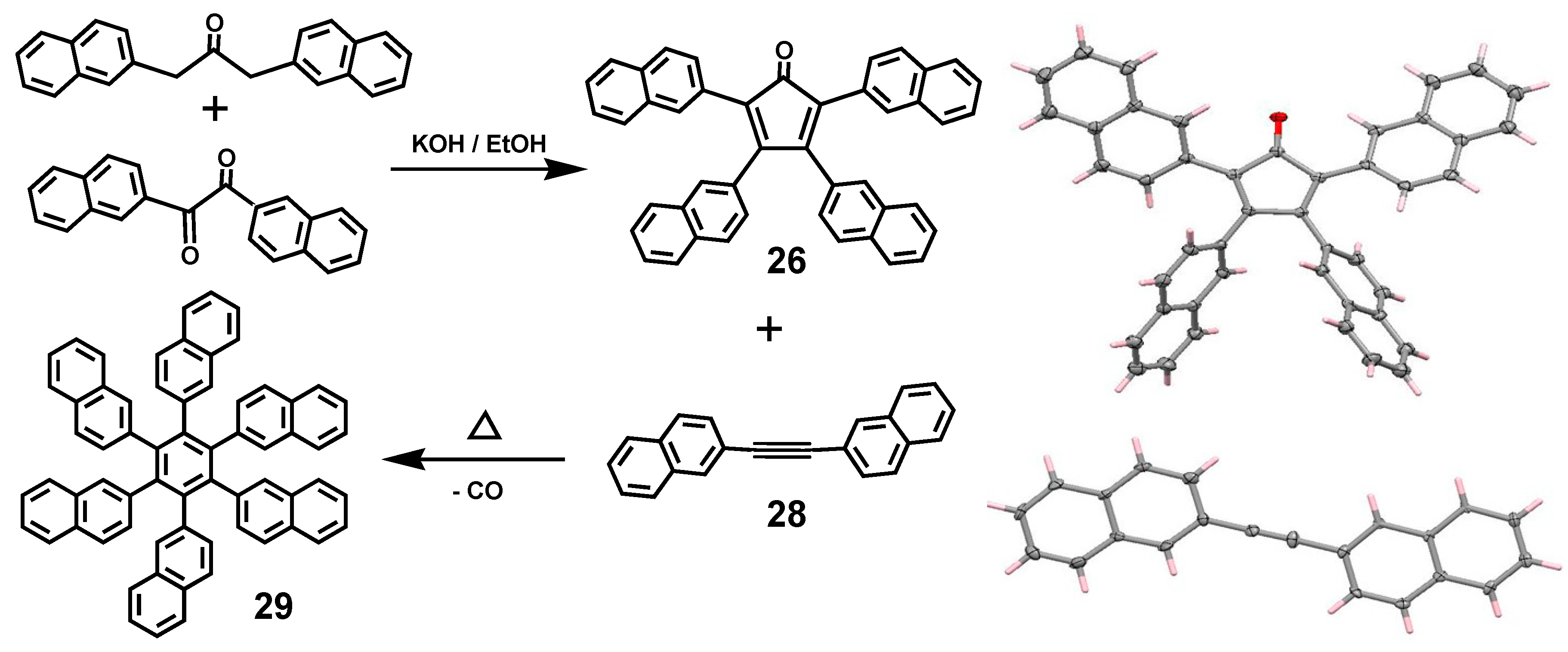
3.6. Hexa(β-naphthyl)benzene and Ferrocenyl-penta(β-naphthyl)benzene
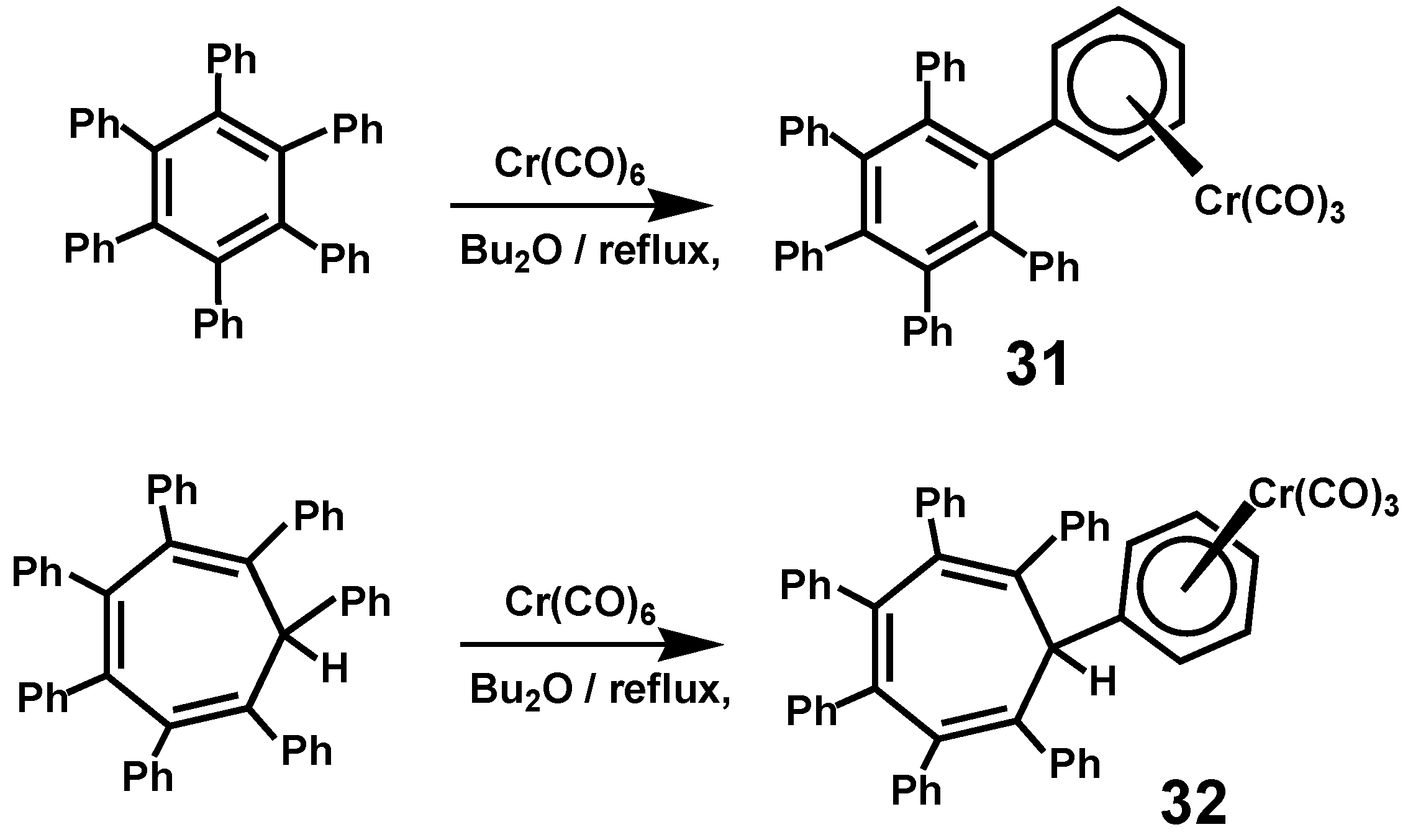
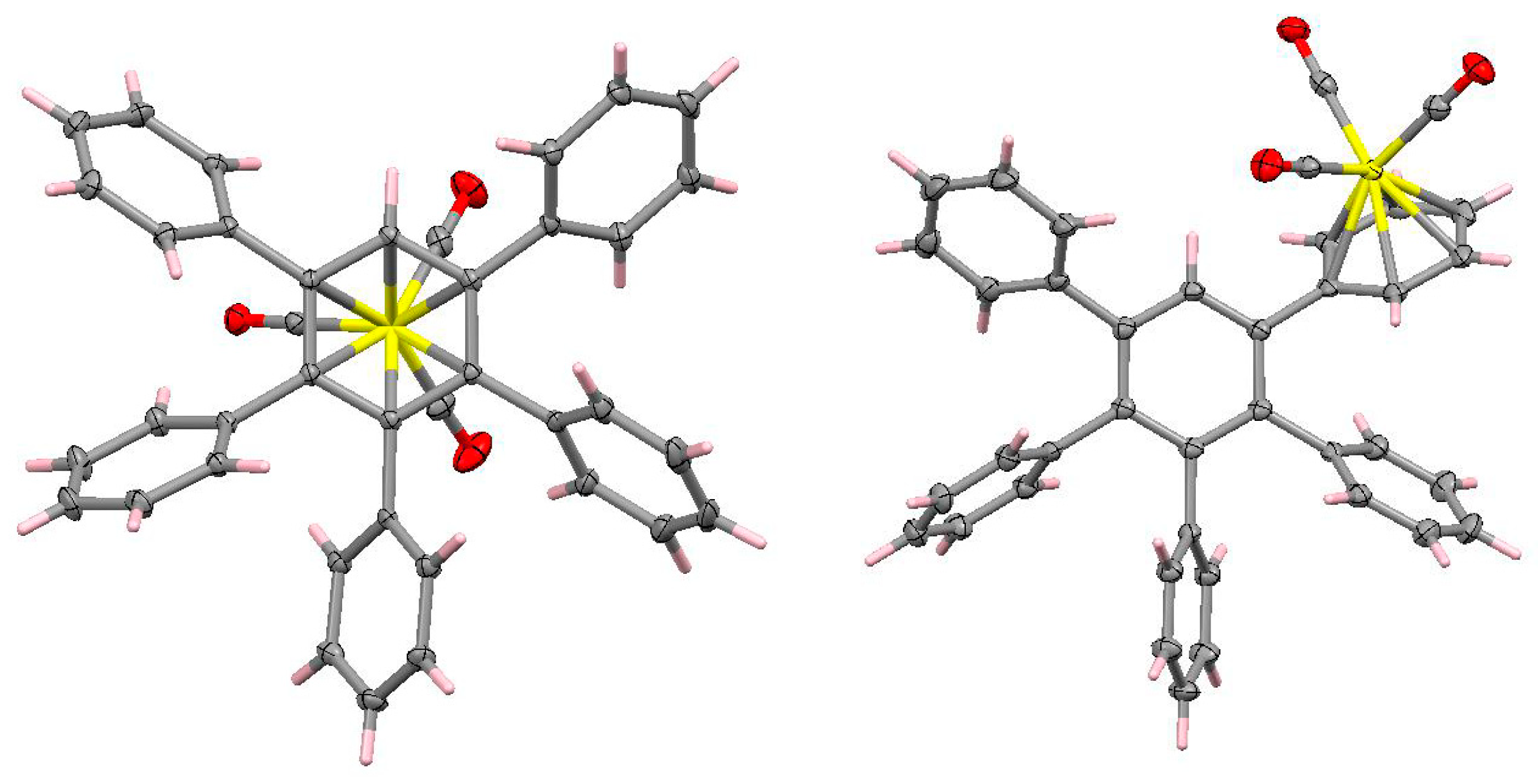
3.7. Other Organometallic Derivatives of C6Ph6 and C7Ph7H
3.8. Organometallic Derivatives of Persubstituted Cycloheptatrienes
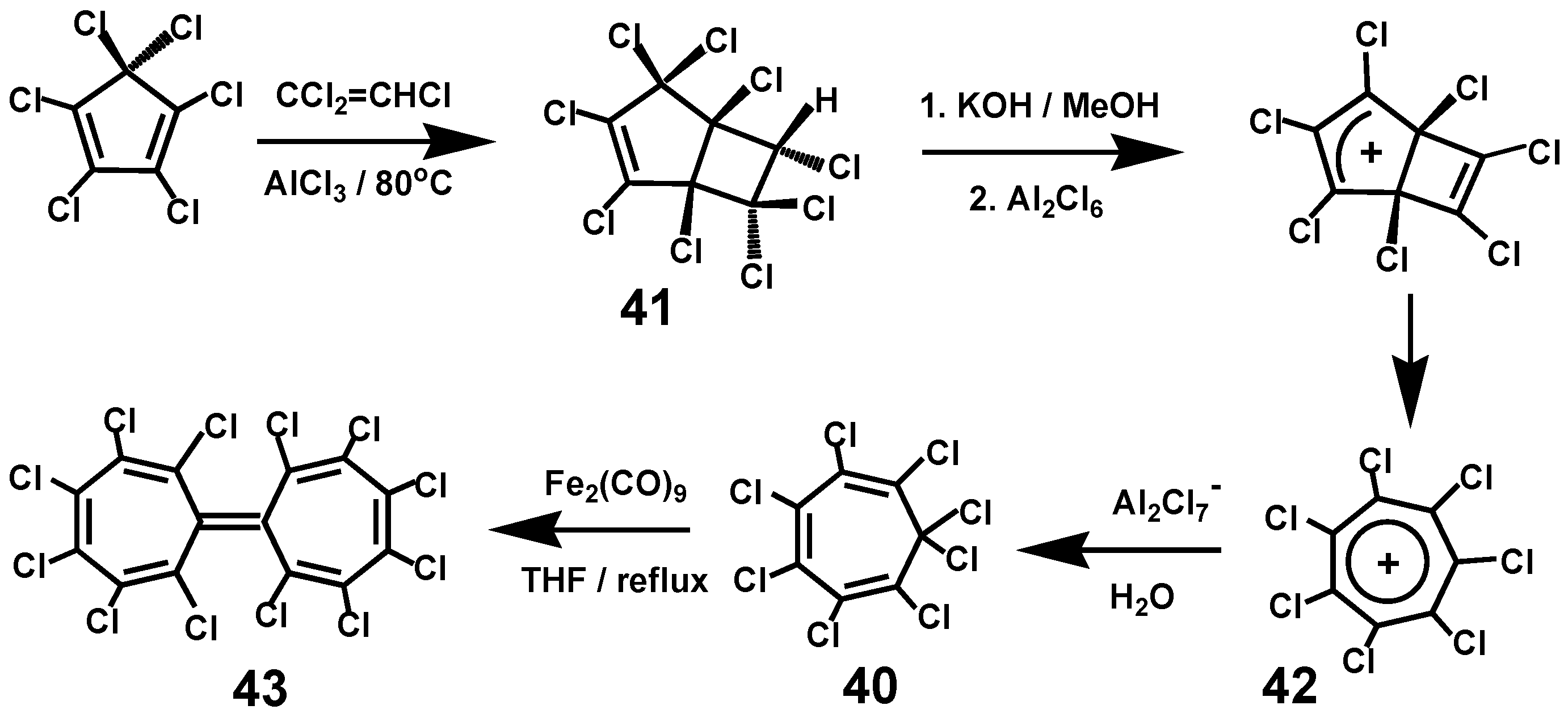
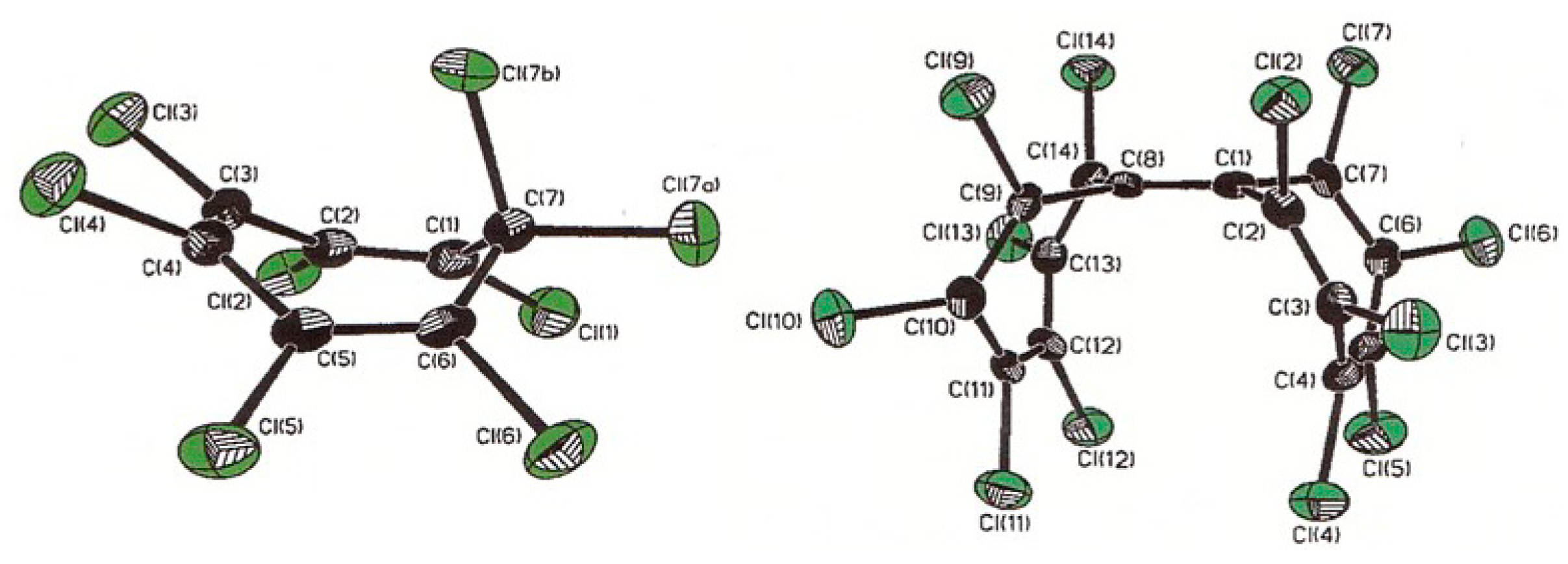
3.8.1. Octachlorocycloheptatriene, C7Cl8
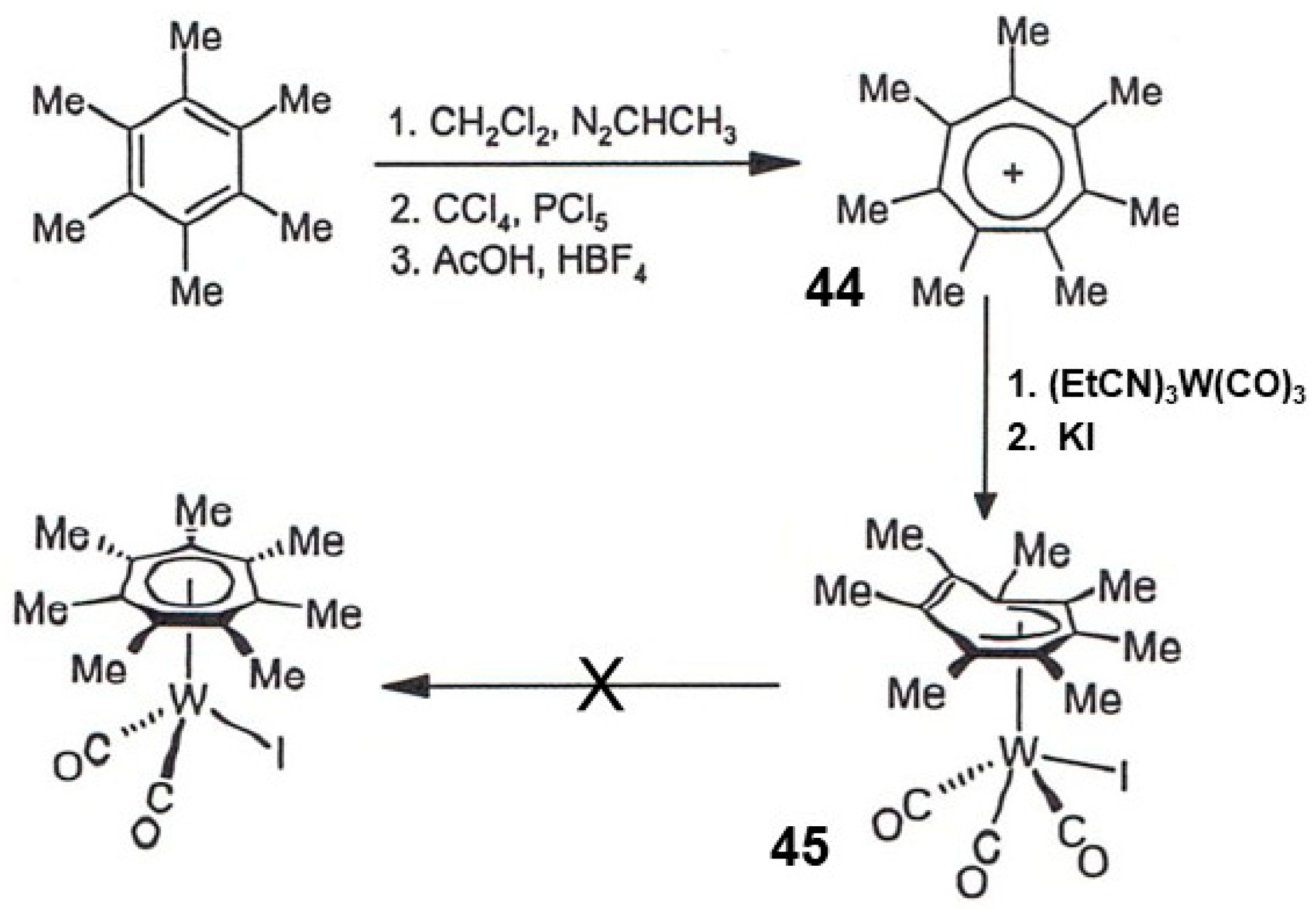
3.8.2. The heptamethyltropylium Cation, [C7Me7+]
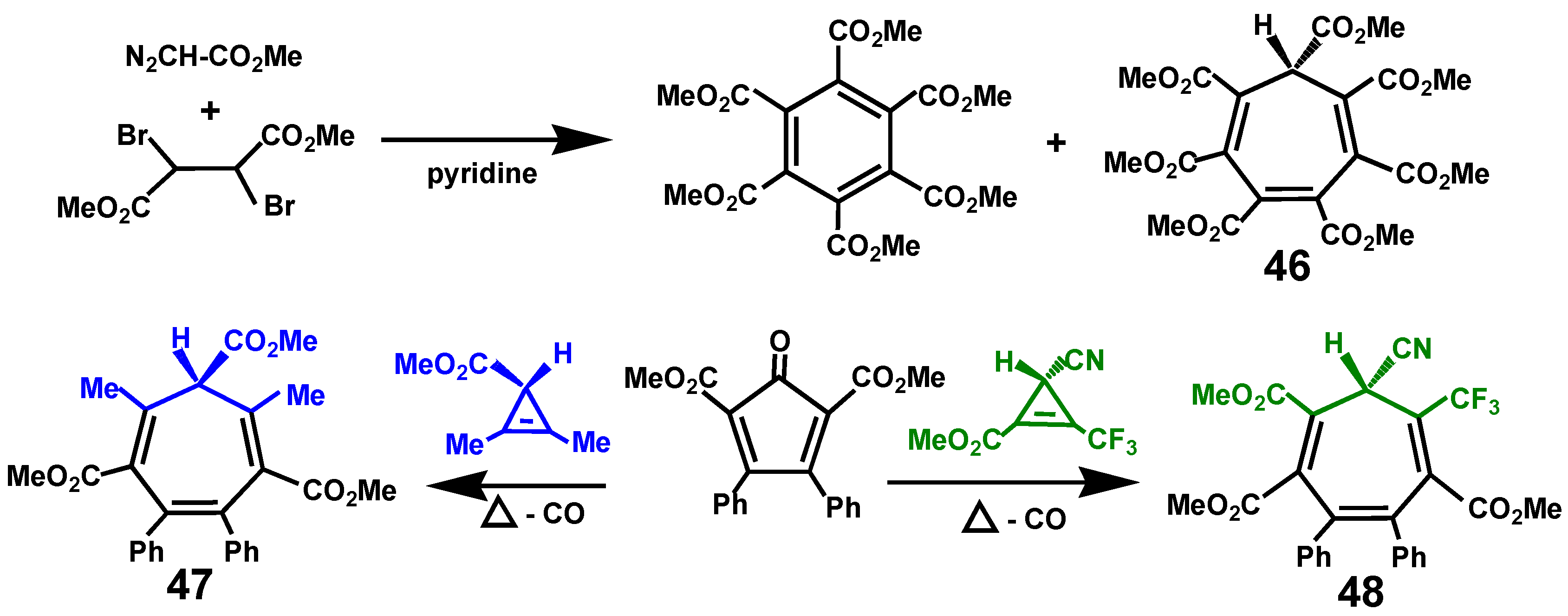
3.8.3. Heptacarbomethoxycycloheptatriene, C7(CO2Me)7H
4. Diels-Alder Cycloadditions en Route to Organometallic Molecular Brakes
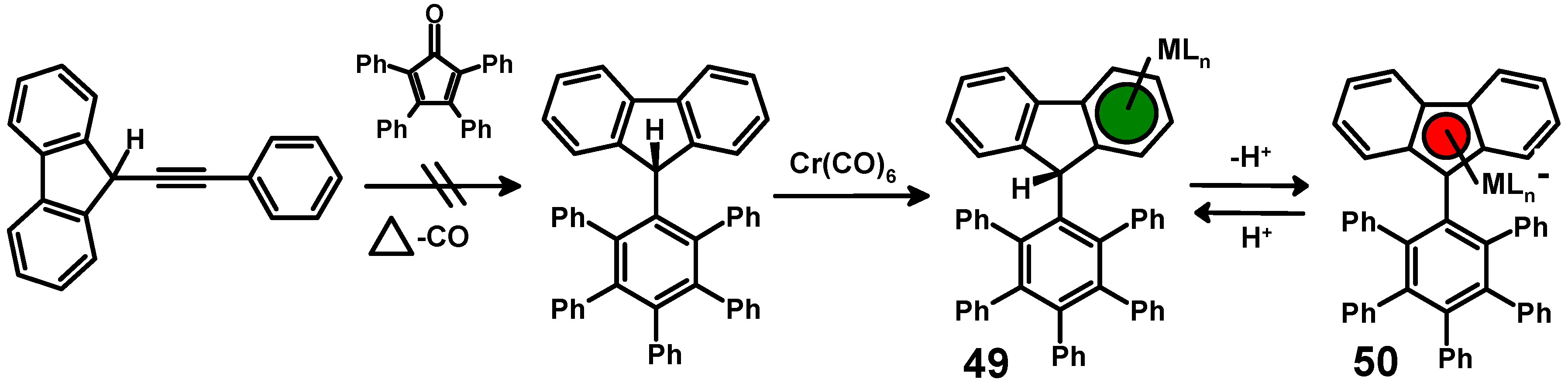
4.1. Attempted Preparation of Fluorenyl-Pentaphenylbenzene
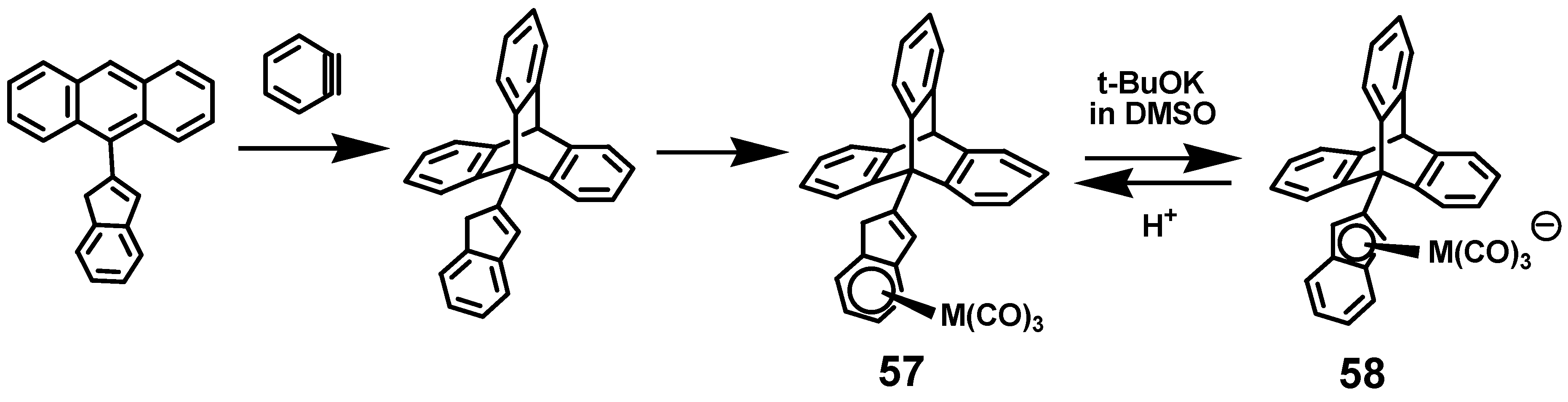
4.2. Successful Route to a Molecular Brake via Addition of Benzyne to 9-(2-Indenyl)anthracene
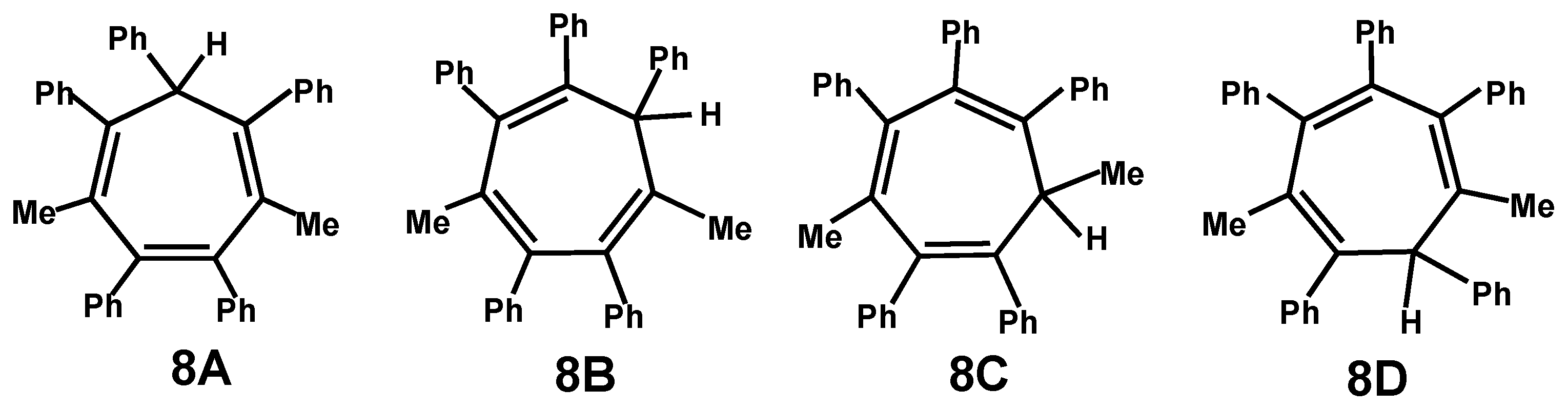
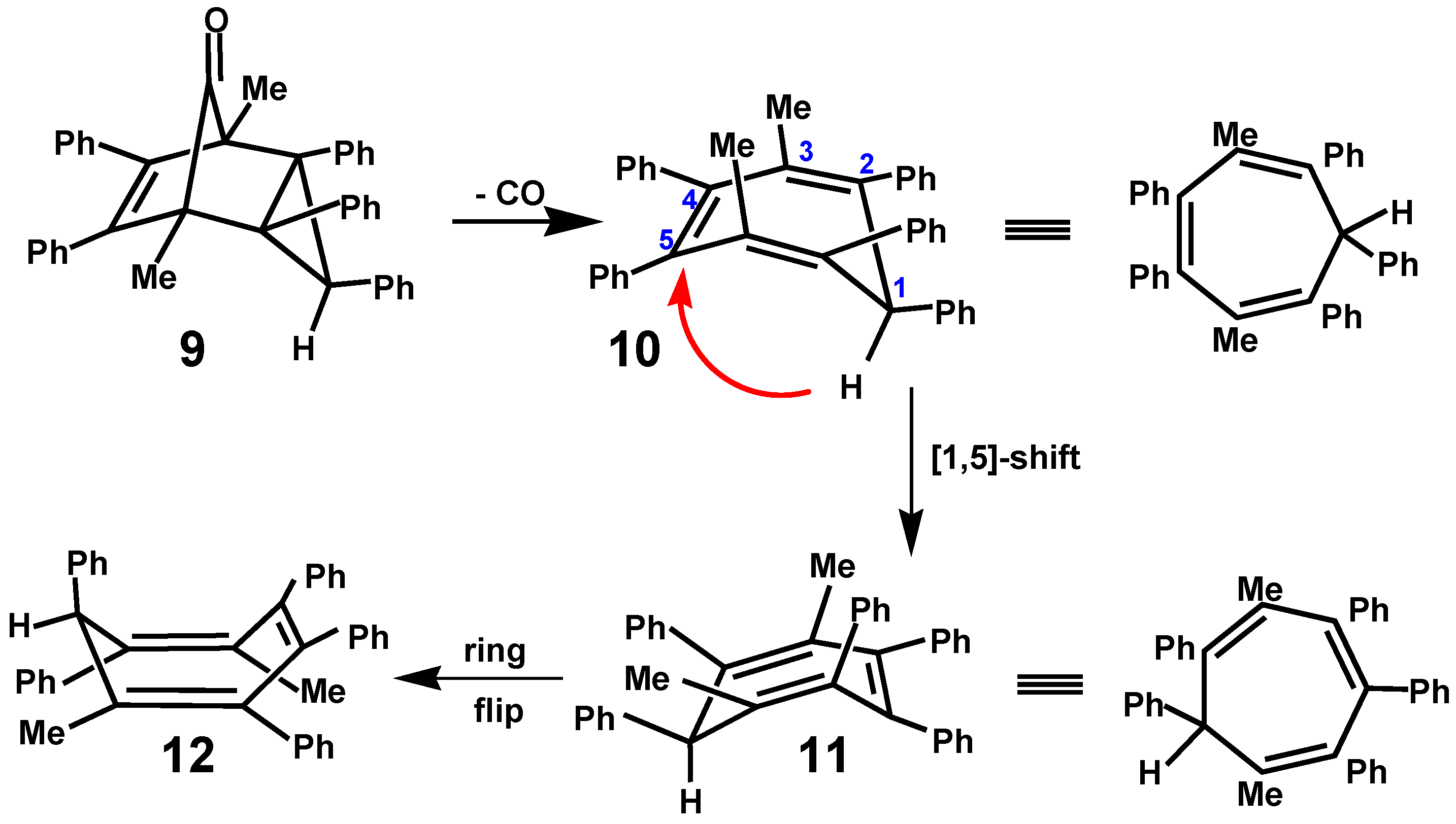
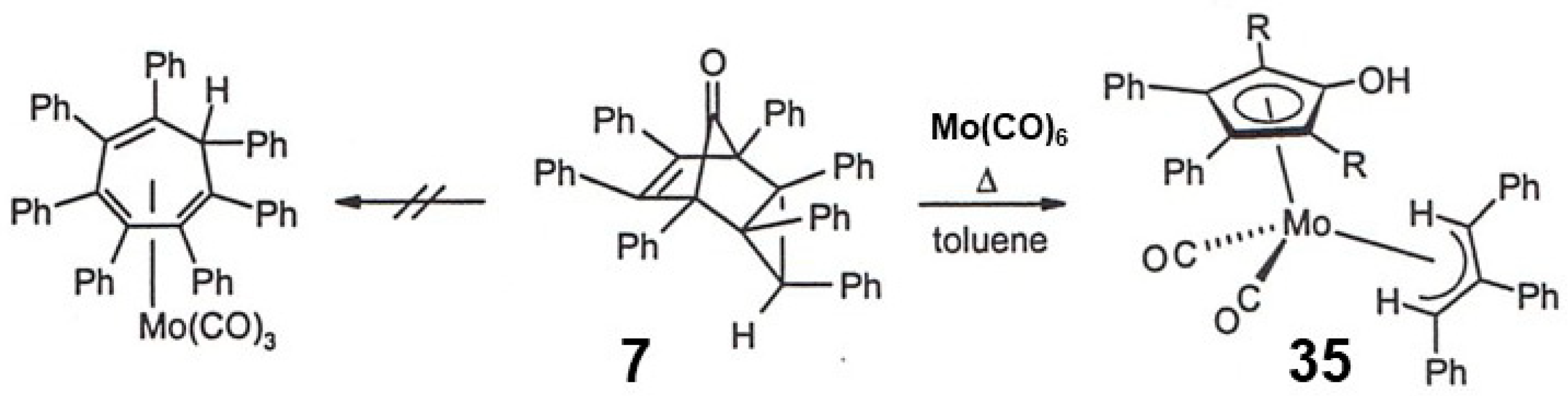
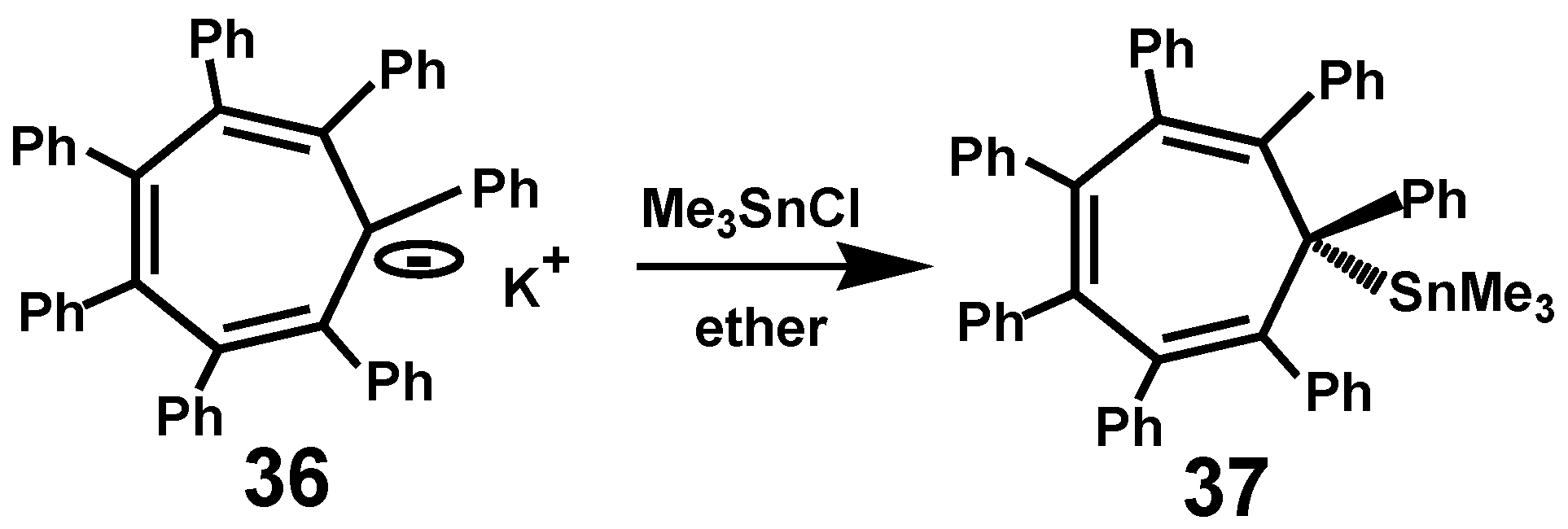
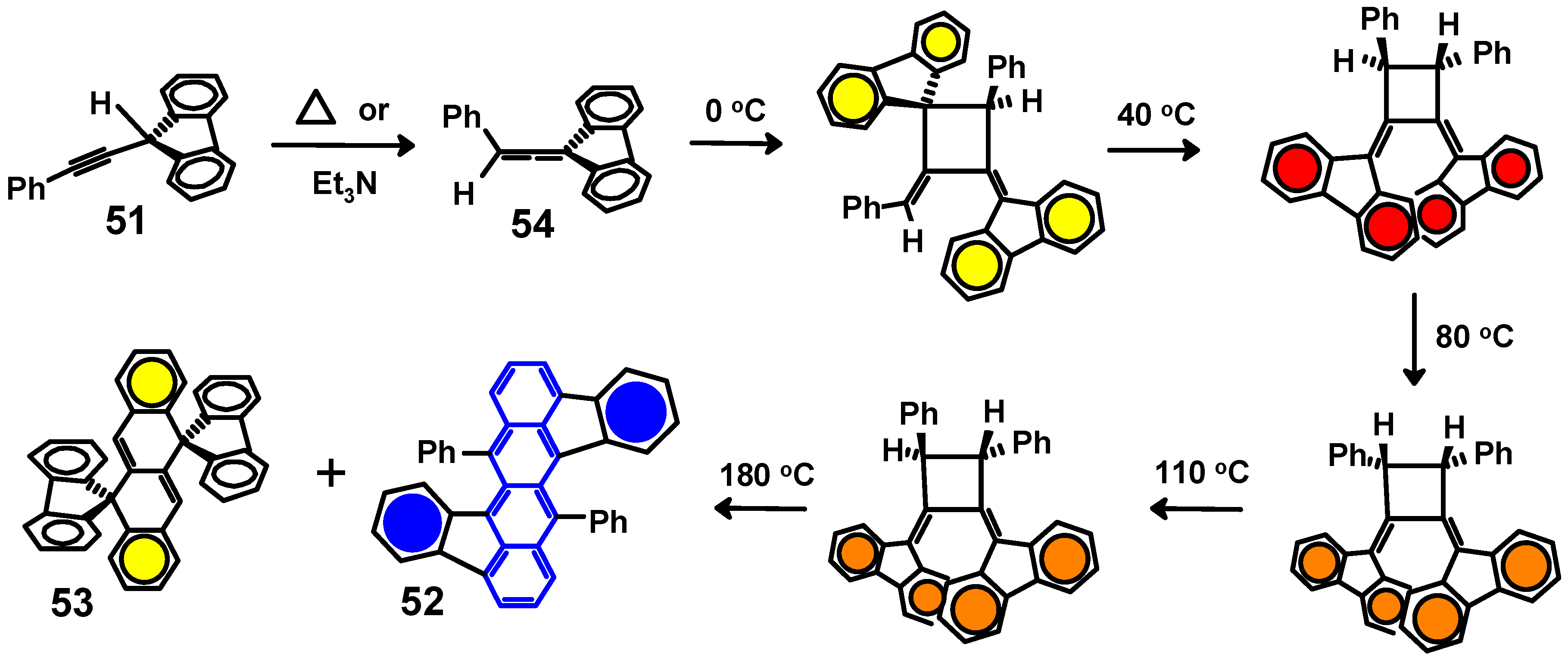
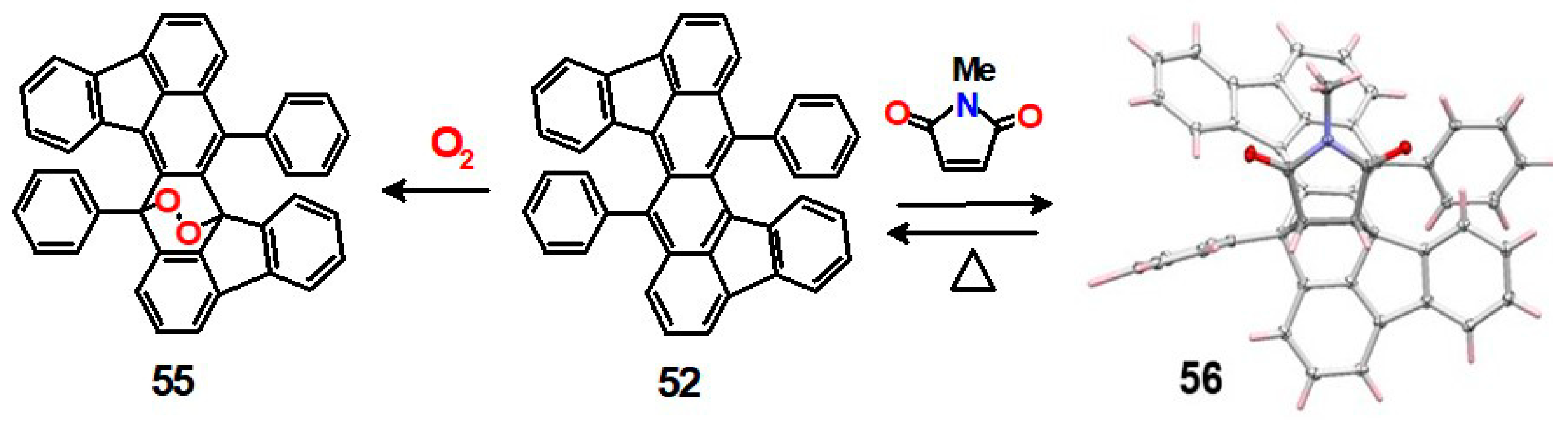
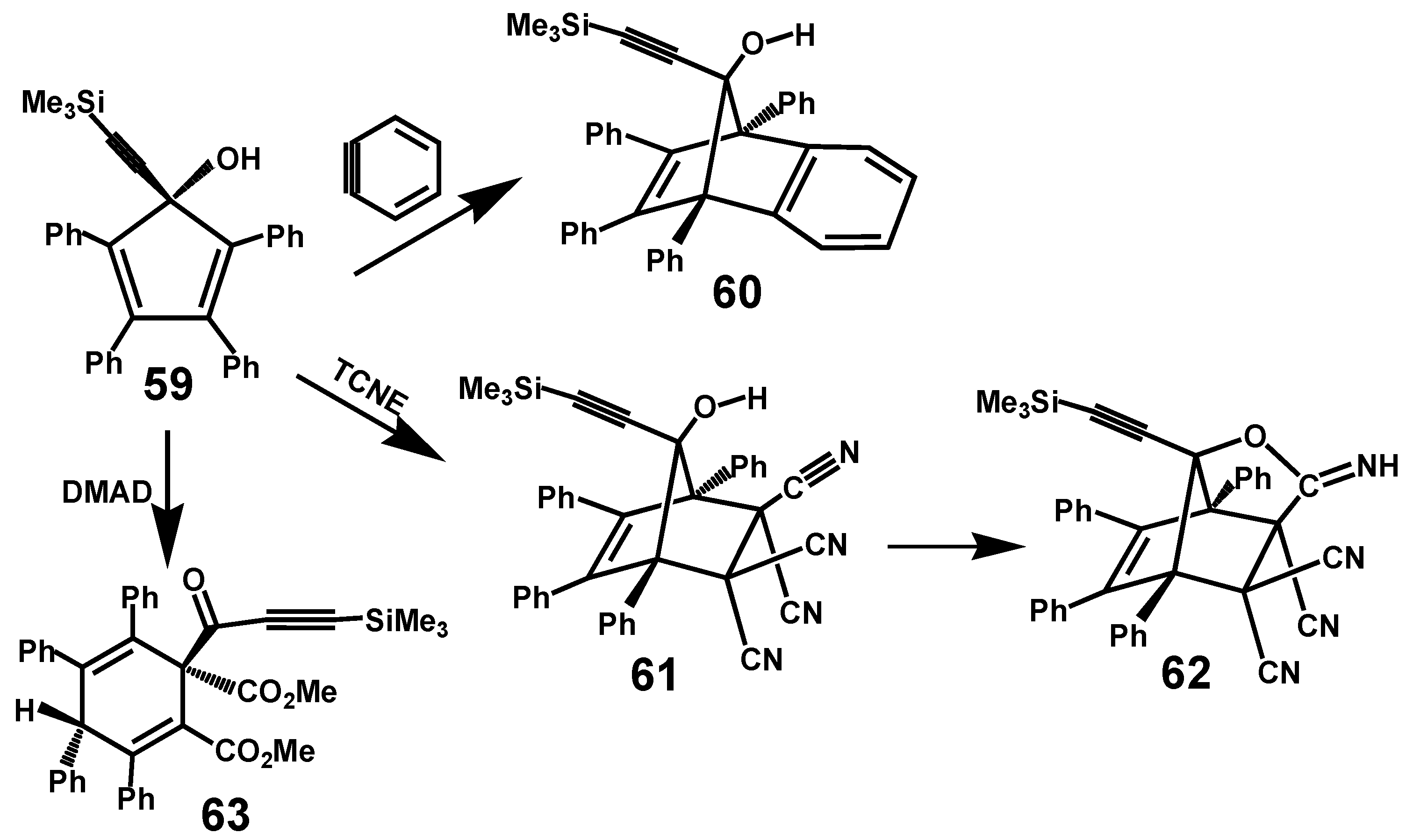
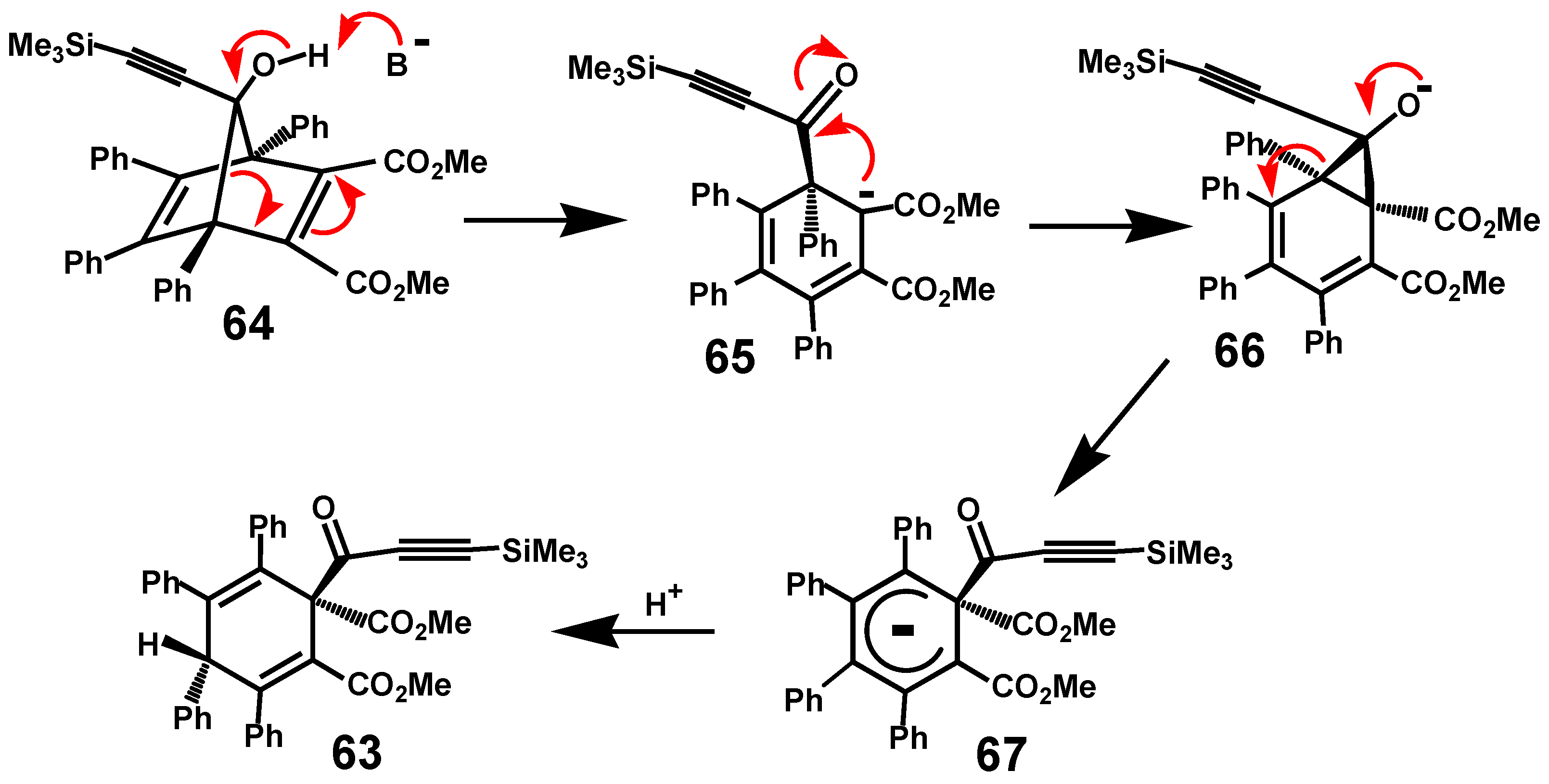
5. Diels-Alder Cycloadditions with Subsequent Molecular Rearrangement
6. Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- Woodward, R.B.; Sondheimer, F.; Taub, D. The total synthesis of cortisone. J. Am. Chem. Soc. 1951, 73, 4057. [Google Scholar] [CrossRef]
- Eaton, P.E.; Cole, T.W. The cubane system. J. Am. Chem. Soc. 1964, 86, 962–964. [Google Scholar] [CrossRef]
- Wyvratt, M.J.; Paquette, L.A. Domino Diels-Alder reactions. II A four-step conversion of cyclopentadienide to triquinacene. Tetrahedron Lett. 1974, 15, 2433–2436. [Google Scholar] [CrossRef]
- Shen, X.F.; Ho, D.M.; Pascal, R.A. Synthesis of polyphenylene dendrimers related to cubic graphite. J. Am. Chem. Soc. 2004, 126, 5798–5805. [Google Scholar] [CrossRef] [PubMed]
- Iyer, V.; Wehmeier, M.; Brand, J.D.; Keegstra, M.A.; Müllen, K. From Hexa-peri-hexabenzocoronene to “Superacenes”. Angew. Chem. Int. Ed. 1997, 36, 1604–1607. [Google Scholar] [CrossRef]
- Bennett, M.J.; Cotton, F.A.; Davison, A.; Faller, J.W.; Lippard, S.J.; Morehouse, S.M. Stereochemically nonrigid organometallic compounds. I. π-Cyclopentadienyliron dicarbonyl σ-cyclopentadiene. J. Am. Chem. Soc. 1966, 88, 4371–4376. [Google Scholar] [CrossRef]
- Cotton, F.A.; Musco, A.; Yagupsky, G. Stereochemically nonrigid organometallic compounds. VIII. Further Studies of σ-cyclopentadienylmetal compounds. J. Am. Chem. Soc. 1967, 89, 6136–6139. [Google Scholar] [CrossRef]
- Cotton, F.A.; Marks, T.J. Stereochemically nonrigid organometallic compounds. XXII. A fluxional indenylmercury compound. J. Am. Chem. Soc. 1969, 91, 3178–3182. [Google Scholar] [CrossRef]
- Stradiotto, M.; Hughes, D.W.; Bain, A.D.; Brook, M.A.; McGlinchey, M.J. The fluxional character of (η5-C5H5)FeCO)2(η1-C9H7): Evidence for the [4+2] cycloaddition of a metal-substituted isoindene with tetracyanoethylene. Organometallics 1997, 16, 5563–5568. [Google Scholar] [CrossRef]
- Kerber, R.C.; Garcia, R.; Nobre, A.L. Cycloaddition reactions of dicarbonyl(η5-cyclopentadienyl)(η1-indenyl)iron. Organometallics 1996, 15, 5756–5758. [Google Scholar] [CrossRef]
- Larrabee, R.B.; Dowben, B.F. Organometallic rearrangements. 1-Trimethylsilyl-and 1-trimethylgermylindene. Tetrahedron Lett. 1970, 11, 915–918. [Google Scholar] [CrossRef]
- Ashe, A.J. III 1,5-Trimethylsilyl migrations in 1-trimethylsilylindene. Tetrahedron Lett. 1970, 11, 2105–2108. [Google Scholar] [CrossRef]
- Luzikov, Y.N.; Sergeyev, N.M.; Ustynyuk, Y.A. Relative migratory abilities of metals for cyclopentadienyl and indenyl ligands. J. Organometal. Chem. 1974, 65, 303–310. [Google Scholar] [CrossRef]
- McMaster, A.D.; Stobart, S.R. Stereochemically nonrigid silanes, germanes, and stannanes. 10. Diastereoisomerism and metallotropic behavior in polyindenyl derivatives of germanium and tin. Facile stereomutation. J. Am. Chem. Soc. 1982, 104, 2109–2112. [Google Scholar] [CrossRef]
- Rigby, S.S.; Girard, L.; Bain, A.D.; McGlinchey, M.J. Molecular dynamics of dl-bis(indenyl)dimethylsilane and meso-bis(indenyl)dimethylsilane—reexamination of the mechanism of interconversion by using single selective inversion NMR. Organometallics 1995, 14, 3798–3801. [Google Scholar] [CrossRef]
- Atwood, J.L.; McMaster, A.D.; Rogers, R.D.; Stobart, S.R. Stereochemically nonrigid silanes, germanes, and stannanes. 12. Crystal and molecular structures of tetrakis(η1-indenyl) derivatives of germanium and tin: Meso diastereomers with S4 symmetry. Organometallics 1984, 3, 1500–1504. [Google Scholar] [CrossRef]
- Stradiotto, M.; Rigby, S.S.; Hughes, D.W.; Brook, M.A.; Bain, A.D.; McGlinchey, M.J. Multidimensional NMR Study of tris(indenyl)methylsilane: Molecular dynamics mapped onto a hypercube. Organometallics 1996, 15, 5645–5652. [Google Scholar] [CrossRef]
- Stradiotto, M.; Brook, M.A.; McGlinchey, M.J. The molecular dynamics and cycloaddition chemistry of tris(1-indenyl)allylsilane Part 1-Generation of the first crystallographically characterized tris(benzonorbornyl)silane. New J. Chem. 1999, 23, 317–321. [Google Scholar] [CrossRef]
- Stradiotto, M.; Brook, M.A.; McGlinchey, M.J. The molecular dynamics and reactivity of tris(inden-1-yl)silane: An NMR spectroscopic, and X-ray crystallographic study. J. Chem. Soc. Perkin Trans. 2 2000, 611–618. [Google Scholar] [CrossRef]
- Rigby, S.S.; Gupta, H.K.; Werstiuk, N.H.; Bain, A.D.; McGlinchey, M.J. The barriers to trimethylsilyl migrations in indenes and benzindenes: Silatropic shifts via aromatic transition states. Polyhedron 1995, 14, 2787–2796. [Google Scholar] [CrossRef]
- Rigby, S.S.; Gupta, H.K.; Werstiuk, N.H.; Bain, A.D.; McGlinchey, M.J. Do aromatic transition states lower barriers to silatropic shifts? A synthetic, NMR spectroscopic, and computational study. Inorg. Chim. Acta 1996, 251, 355–364. [Google Scholar] [CrossRef]
- Stradiotto, M.; McGlinchey, M.J. η1-Indenyl derivatives of transition metal and main group elements: Synthesis, characterization and molecular dynamics. Coord. Chem. Rev. 2001, 219, 311–378. [Google Scholar] [CrossRef]
- Rigby, S.S.; Stradiotto, M.; Brydges, S.; Pole, D.L.; Top, S.; Bain, A.D.; McGlinchey, M.J. Diels-Alder dimerization of cyclopenta[l]phenanthrene (dibenz[e,g]indene) with isodibenzindene: A computational, NMR spectroscopic, and X-ray crystallographic study. J. Org. Chem. 1998, 63, 3735–3740. [Google Scholar] [CrossRef]
- Brydges, S.; Harrington, L.E.; McGlinchey, M.J. Sterically hindered organometallics: Multi-n-rotor (n = 5, 6 and 7) molecular propellers and the search for correlated rotations. Coord. Chem. Res. 2002, 233, 75–105. [Google Scholar] [CrossRef]
- Battiste, M.A. Heptaphenyltropilidene–product of a Diels-Alder reaction of triphenylcyclopropene. Chem. Ind. 1961, 550–551. [Google Scholar]
- Battiste, M.A. Salts of the heptaphenyltropylium ion and their stability. J. Am. Chem. Soc. 1961, 83, 4101–4102. [Google Scholar] [CrossRef]
- Battiste, M.A. The heptaphenyltropylium (heptaphenylcycloheptatrienyl) radical in solution. J. Am. Chem. Soc. 1962, 84, 3780–3782. [Google Scholar] [CrossRef]
- Breslow, R.; Chang, H.W. Heptaphenylcycloheptatrienyl anion. J. Am. Chem. Soc. 1965, 87, 2200–2203. [Google Scholar] [CrossRef]
- Chao, L.C.F.; Gupta, H.K.; Hughes, D.W.; Britten, J.F.; Rigby, S.S.; Bain, A.D.; McGlinchey, M.J. Chromium and molybdenum carbonyl complexes of C7Ph7H and C7Ph5Me2H and of C7Ph7H(CO), the Diels-Alder adduct of tetracyclone and triphenylcyclopropene: Variable-temperature NMR and X-ray crystallography study. Organometallics 1995, 14, 1139–1151. [Google Scholar] [CrossRef]
- Battiste, M.A. Cyclopropenes as Dienophiles. The stereochemistry and nuclear magnetic resonance spectra of some diene adducts of triphenylcyclopropene and diphenylcyclopropene carboxylic acid. Tetrahedron Lett. 1964, 5, 3795–3802. [Google Scholar] [CrossRef]
- Traetteberg, M. The molecular structure of 1,3,5-cycloheptatriene in the vapor phase as determined by the sector electron diffraction method. J. Am. Chem. Soc. 1964, 86, 4265–4270. [Google Scholar] [CrossRef]
- Brydges, S.; Britten, J.F.; Chao, L.C.F.; Gupta, H.K.; McGlinchey, M.J.; Pole, D.L. The structure of a seven-bladed propeller: C7Ph7+ is not planar. Chem. Eur. J. 1998, 4, 1201–1205. [Google Scholar] [CrossRef]
- Larson, E.M.; Von Dreele, R.B.; Hanson, P.; Gust, J.D. The structure of hexaphenylbenzene anisole clathrate, 2 C6(C6Ph5)6.C6H5OCH3. Acta Crystallogr. 1990, C46, 784–788. [Google Scholar] [CrossRef]
- Bart, J.C.J. Crystal structure of a modification of hexaphenylbenzene. Acta Crystallogr. 1968, B24, 1277–1287. [Google Scholar] [CrossRef]
- Schumann, H.; Lentz, A.; Weimann, R.; Pickardt, J. Decaphenylferrocene and decaphenylferrocenium tetrafluoroborate. Angew. Chem. Int. Ed. 1994, 33, 1731–1733. [Google Scholar] [CrossRef]
- Li, L.; Decken, A.; Sayer, B.G.; McGlinchey, M.J.; Brégaint, P.; Thépot, J.Y.; Toupet, L.; Hamon, J.R.; Lapinte, C. Multiple fluxional processes in chiral (pentaphenylcyclopentadienyl)iron complexes: X-ray crystal structure of (C5Ph5)Fe(CO)(PMe3)(HC=O) and the barriers to aryl, phosphine, and tripodal rotation in (C5Ph5)Fe(CO)(PMe2Ph)C(O)Et. Organometallics 1994, 13, 682–689. [Google Scholar] [CrossRef]
- Adams, H.; Bailey, N.A.; Browning, A.F.; Ramsden, J.A.; White, C. The stereochemical and kinetic consequences of binding a pentaphenylcyclopentadienyl ligand–crystal structure of Ru(C5Ph5)(CO)(PPh3)Br. J. Organometal. Chem. 1990, 387, 305–314. [Google Scholar] [CrossRef]
- Iyoda, M.; Sultana, F.; Sasaki, S.; Butenschön, H. Synthesis of novel fullerene complex–the [4+2]-cycloadduct of (bicyclo[3.2.0]hepta-1,3-dienyl)cobalt(I) complex with C-60. Tetrahedron Lett. 1995, 36, 579–582. [Google Scholar] [CrossRef]
- Bailey, P.J.; Blake, A.J.; Dyson, P.J.; Ingham, S.L.; Johnson, B.F.G. The preparation and solid-state structure of Ru5C(CO)13(η4-C4Ph4)–the first cluster to carry a cyclobutadiene ring. J. Chem. Soc. Chem. Commun. 1994, 2233–2234. [Google Scholar] [CrossRef]
- Borgias, B.A.; Scarrow, R.C.; Seidler, M.D.; Weiner, W.P. Sym-triphenylcyclopropenium hexabromotellurate(IV), (C21H15)2[TeBr6]. Acta Crystallogr. 1985, C41, 476–479. [Google Scholar] [CrossRef]
- Sundarlingam, M.; Jensen, L.H. Structure of a carbonium ion. Refinement of crystal and molecular structure of sym-triphenylcyclopropenium perchlorate. J. Am. Chem. Soc. 1966, 88, 198–204. [Google Scholar] [CrossRef]
- Eisch, J.J.; Galle, J.E. The synthesis of heptaphenylborepin via the thermal rearrangement of heptaphenyl-7-borabicyclo[2.2.1]heptadiene. J. Am. Chem. Soc. 1975, 97, 4436–4437. [Google Scholar] [CrossRef]
- Hassner, A.; Anderson, D.J. Cycloaddition of 1-azirines with cyclopentadienones. Formation of 2H-and 3H-azepines, and mechanistic interpretation. J. Org. Chem. 1974, 39, 3070–3076. [Google Scholar] [CrossRef]
- Rybinskaya, M.I.; Kreindlin, A.Z.; Fadeeva, S.S. On the problem of stabilization of alpha-carbocationic centers in metallocene series-related interconversions of permethylated alpha-metallocenylcarbocations and metallicenium cation-radicals of the iron sub-group. J. Organometal. Chem. 1988, 358, 363–374. [Google Scholar] [CrossRef]
- Gupta, H.K.; Brydges, S.; McGlinchey, M.J. Diels-Alder reactions of 3-ferrocenyl-2,4,5-triphenylcyclopentadienone: Syntheses and structures of the sterically crowded systems C6Ph5Fc, C7Ph6FcH, and [C7Ph6FcH][SbCl6]. Organometallics 1999, 18, 115–122. [Google Scholar] [CrossRef]
- Harrington, L.E.; Britten, J.F.; Nikitin, K.; McGlinchey, M.J. A synthetic, X-ray, NMR spectroscopy and DFT study of di(β-naphthyl)acetylene, tetra(β-naphthyl)cyclopentadienone, and hexa(β-naphthyl)benzene: C6(C10H7)6 is a disordered molecular propeller. ChemPlusChem 2017, 82, 433–441. [Google Scholar] [CrossRef]
- Harrington, L.E.; Britten, J.F.; McGlinchey, M.J. Ferrocenyl-penta-(β-naphthyl)benzene–Synthesis, structure and dynamic behaviour. Can. J. Chem. 2003, 81, 1180–1186. [Google Scholar] [CrossRef]
- Mailvaganam, B.; Sayer, B.G.; McGlinchey, M.J. The fluxional behavior of hexaphenylbenzene chromium tricarbonyl–a variable-temperature C-13 NMR spectroscopic study. J. Organometal. Chem. 1990, 395, 177–185. [Google Scholar] [CrossRef]
- Pauson, P.L.; Smith, G.H.; Valentin, J.H. Cycloheptatriene and tropylium metal complexes. 4. Preparation of 7-exosubstituted tricarbonylcycloheptatrienechromiums. J. Chem. Soc. C 1967, 1057–1061. [Google Scholar] [CrossRef]
- Brydges, S.; Gildea, B.; Grealis, J.P.; Müller-Bunz, H.; Stradiotto, M.; Casey, M.; McGlinchey, M.J. Organic and organometallic derivatives of pentaphenylbenzene C6Ph5X: Correlation of peripheral phenyl ring orientations with the steric bilk of “X”. Can. J. Chem. 2013, 91, 1098–1111. [Google Scholar] [CrossRef]
- Breslow, R.; Dowd, P. The dimerization of triphenylcyclopropene. J. Am. Chem. Soc. 1963, 85, 2729–2735. [Google Scholar] [CrossRef]
- Kusada, K.; West, R.; Rao, V.N.M. Octachloroheptatriene and related compounds. J. Am. Chem. Soc. 1971, 93, 3627–3632. [Google Scholar] [CrossRef]
- Tomilov, Y.V.; Platonov, D.N.; Salikov, R.F.; Okonnishnikova, G.P. Synthesis and properties of stable 1,2,3,4,5,6,7-heptamethoxycarbonylcyclohepta-2,4,6-trienyl potassium and its reactions with electrophilic reagents. Tetrahedron 2008, 64, 10201–10206. [Google Scholar] [CrossRef]
- Tamm, M.; Dressel, B.; Fröhlich, R. Molecular structure of a heptadentate cogwheel: C7Me7+ is not planar. J. Org. Chem. 2000, 65, 6795–6797. [Google Scholar] [CrossRef]
- Harvey, J.A.; Ogliaruso, M.A. Synthesis, electronic spectra, and thermal behavior of bis(heptaphenylcycloheptatrienes). J. Org. Chem. 1976, 41, 3374–3377. [Google Scholar] [CrossRef]
- Dunn, J.A.; Gupta, H.K.; Bain, A.D.; McGlinchey, M.J. The metal-mediated conversion of octachlorocycloheptatriene into dodecachloroheptafulvalene: A synthetic, structural and EHMO study. Can. J. Chem. 1996, 74, 2258–2267. [Google Scholar] [CrossRef]
- Tamm, M.; Dressel, B.; Fröhlich, R.; Bergander, K. Complexes containing the heptadentate cogwheel C7Me7: Synthesis and structural characterisation of heptamethylcycloheptatrienyl (CHT*) tungsten complexes. Chem. Commun. 2000, 1731–1732. [Google Scholar] [CrossRef]
- Platonov, D.N.; Belyy, A.Y.; Ananyev, I.V.; Tomilov, Y.V. Syntheses of 1,2,3,4,5,6,7-heptasubstituted cycloheptatrienes through cycloaddition reactions of substituted cyclopentadienones. Eur. J. Org. Chem. 2016, 2016, 4105–4110. [Google Scholar] [CrossRef]
- Harrington, L.E.; Britten, J.F.; McGlinchey, M.J. Dimerization of 9-phenylethynylfluorene to di-indeno-naphthacene and dispiro[fluorene-dihydronaphthacene-fluorene]: An X-ray crystallographic and NMR study. Org. Lett. 2004, 6, 787–790. [Google Scholar] [CrossRef]
- Banide, E.V.; Ortin, Y.; Seward, C.M.; Harrington, L.E.; Müller-Bunz, H.; McGlinchey, M.J. Sequential formation of yellow, red and orange 1-phenyl-3,3-biphenylene-allene dimers prior to blue tetracene formation: Helicity reversal in trans-3,4-diphenyl-1,2-bis(fluorenylidene)cyclobutene. Chem. Eur. J. 2006, 12, 3275–3286. [Google Scholar] [CrossRef]
- Banide, E.V.; Oulié, P.; McGlinchey, M.J. From allenes to tetracenes: Syntheses, structures and reactivity of the intermediates. Pure Appl. Chem. 2009, 81, 1–17. [Google Scholar] [CrossRef]
- Banide, E.V.; O’Connor, C.; Fortune, N.; Ortin, Y.; Milosevic, S.; Müller-Bunz, H.; McGlinchey, M.J. Syntheses, X-ray structures and reactivity of fluorenylidene- and dibenzosuberylidene-allenes: Convenient precursors to dispirotetracenes, di-indenotetracenes and 2-phenyl-11bH-dibenz[cd,h]azulene. Org. Biomol. Chem. 2010, 8, 3997–4010. [Google Scholar] [CrossRef] [PubMed]
- Nikitin, K.; Müller-Bunz, H.; Ortin, Y.; McGlinchey, M.J. A molecular paddlewheel with a sliding organometallic latch: X-ray crystal structures and dynamic behaviour of [Cr(CO)3{η6-2-(9-triptycyl)indene}], and of [M(CO)3{η5-2-(9-triptycyl)indenyl}] (M = Mn, Re). Chem. Eur. J. 2009, 15, 1836–1843. [Google Scholar] [CrossRef]
- McGlinchey, M.J.; Nikitin, K. Palladium-catalysed coupling reactions en route to molecular machines: Sterically hindered indenyl and ferrocenyl anthracenes and triptycenes. Molecules 2020, 25, 1950. [Google Scholar] [CrossRef]
- Dunn, J.A.; Stradiotto, M.; McGlinchey, M.J. Diels-Alder adducts of 5-alkynylcyclopentadienols with tetracyanoethylene and dimethyl acetylenedicarboxylate: An X-ray crystallographic study of unexpected rearrangement products. J. Org. Chem. 2000, 65, 4861–4863. [Google Scholar] [CrossRef] [PubMed]
- Werstiuk, N.H.; Ma, J. An AM1 calculational study of the Diels-Alder addition of maleic anhydride to C5-substituted pentamethylcyclopentadienes and 2,5-dimethylthiophene oxide. An attempt to ascertain the factors controlling the π-facial selectivities and relative reactivities. Can. J. Chem. 1994, 72, 2493–2505. [Google Scholar] [CrossRef]
- Diels, O.; Alder, K. Synthesen in der hydroaromatischen Reihe VII. Justus Liebigs Ann. Chem. 1931, 486, 191–202. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGlinchey, M.J. Diels-Alder Additions as Mechanistic Probes–Interception of Silyl-Isoindenes: Organometallic Derivatives of Polyphenylated Cycloheptatrienes and Related Seven-Membered Rings. Molecules 2020, 25, 4730. https://doi.org/10.3390/molecules25204730
McGlinchey MJ. Diels-Alder Additions as Mechanistic Probes–Interception of Silyl-Isoindenes: Organometallic Derivatives of Polyphenylated Cycloheptatrienes and Related Seven-Membered Rings. Molecules. 2020; 25(20):4730. https://doi.org/10.3390/molecules25204730
Chicago/Turabian StyleMcGlinchey, Michael J. 2020. "Diels-Alder Additions as Mechanistic Probes–Interception of Silyl-Isoindenes: Organometallic Derivatives of Polyphenylated Cycloheptatrienes and Related Seven-Membered Rings" Molecules 25, no. 20: 4730. https://doi.org/10.3390/molecules25204730
APA StyleMcGlinchey, M. J. (2020). Diels-Alder Additions as Mechanistic Probes–Interception of Silyl-Isoindenes: Organometallic Derivatives of Polyphenylated Cycloheptatrienes and Related Seven-Membered Rings. Molecules, 25(20), 4730. https://doi.org/10.3390/molecules25204730