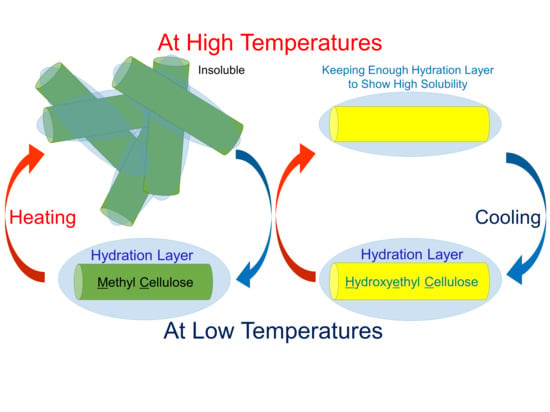
Hydration/Dehydration Behavior of Hydroxyethyl Cellulose Ether in Aqueous Solution
Abstract
1. Introduction
2. Results and Discussion
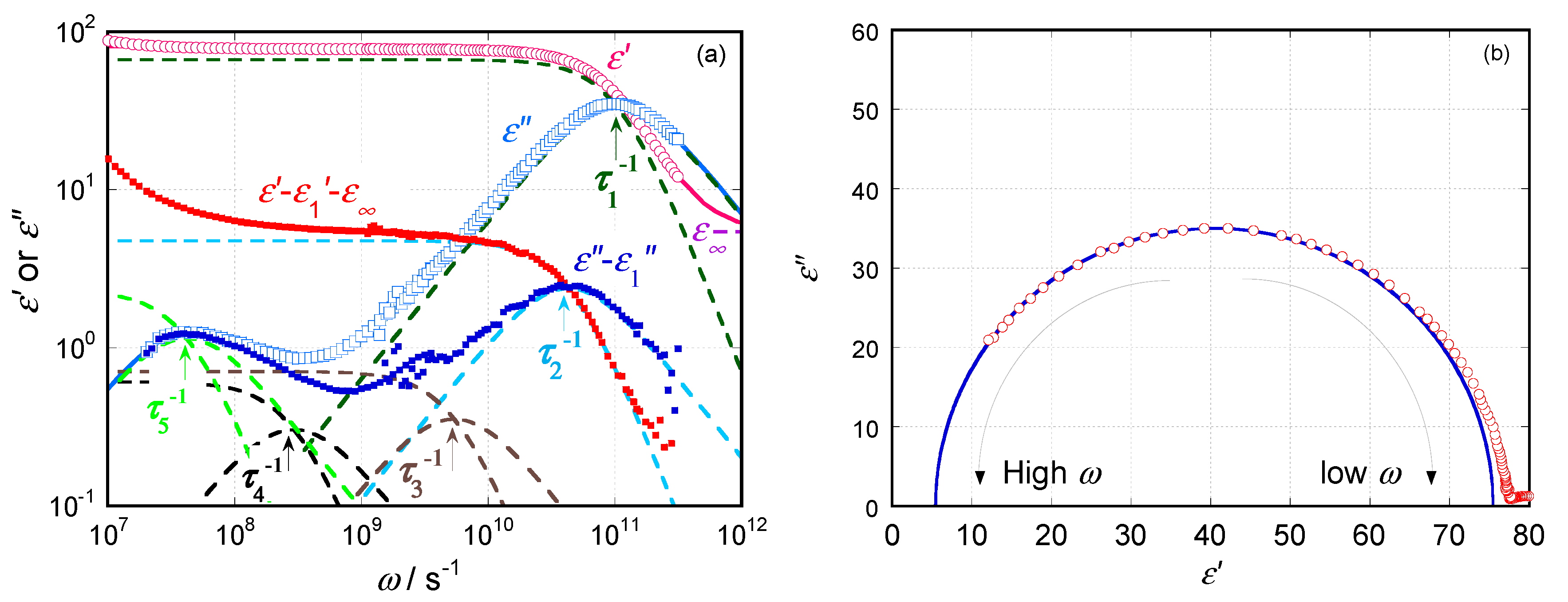
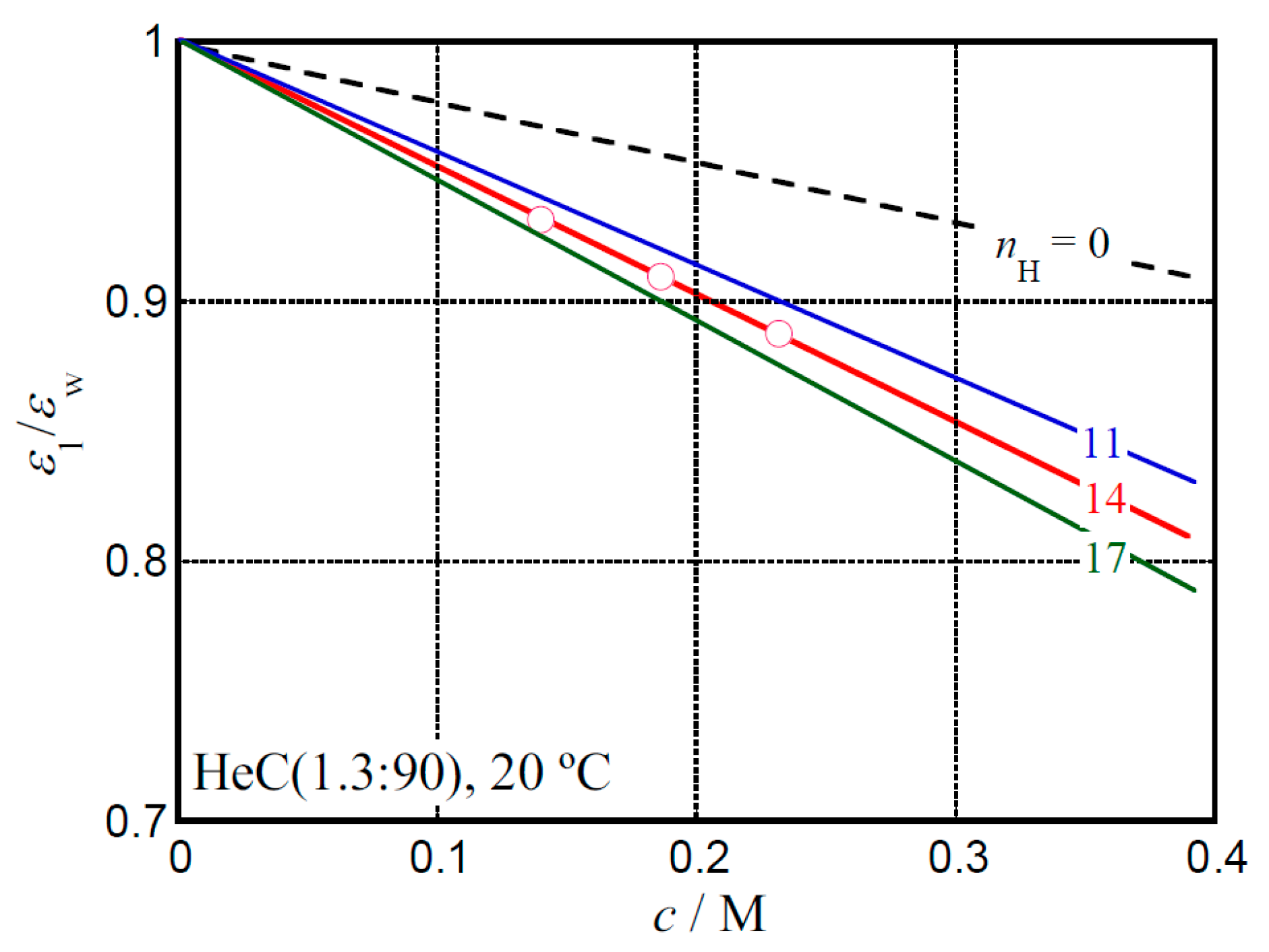
2.1. Dielectric Behavior for Aqueous Solution of Hydroxyethyl Cellulose
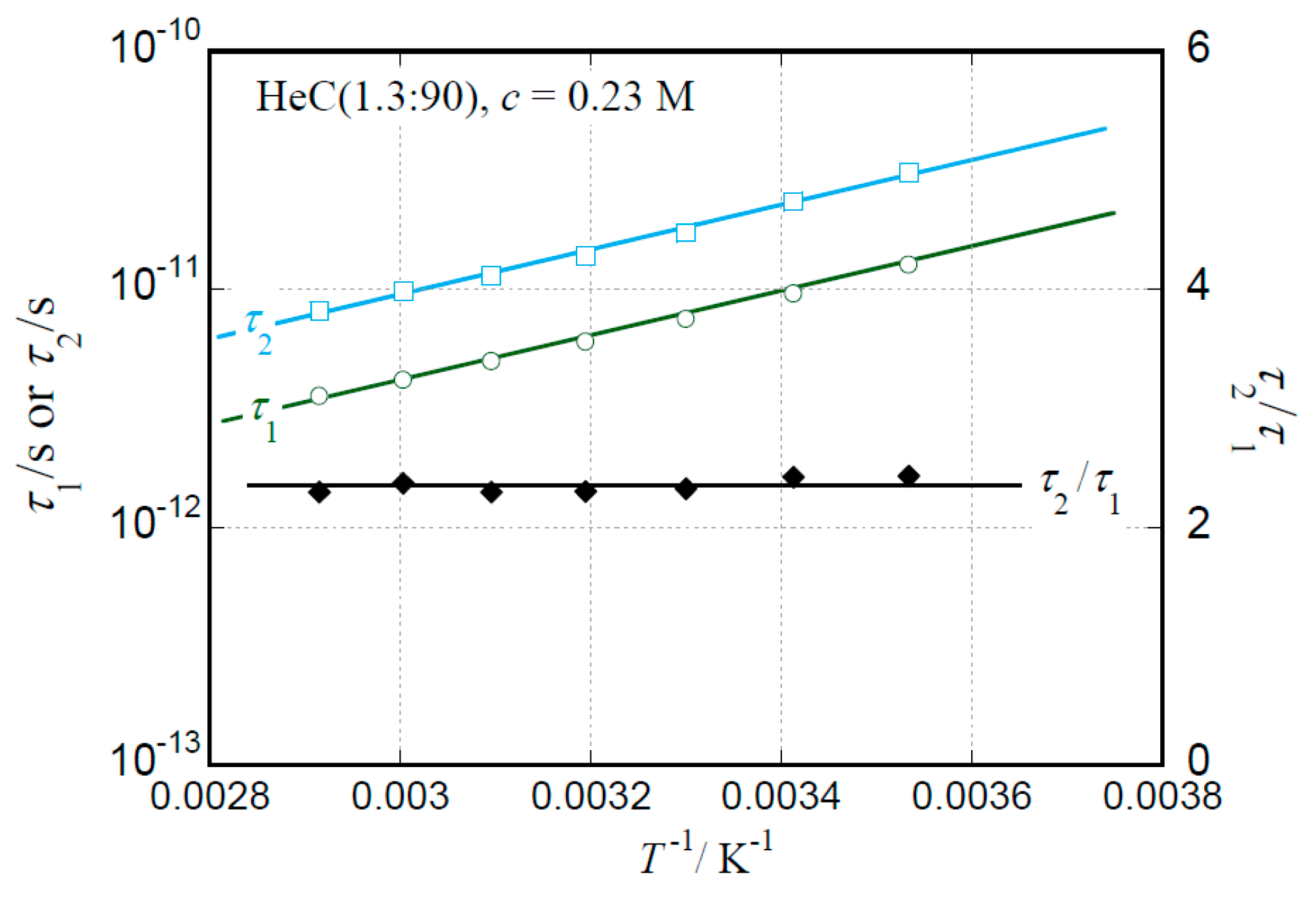
2.2. Temperature Dependence of Relaxation Times
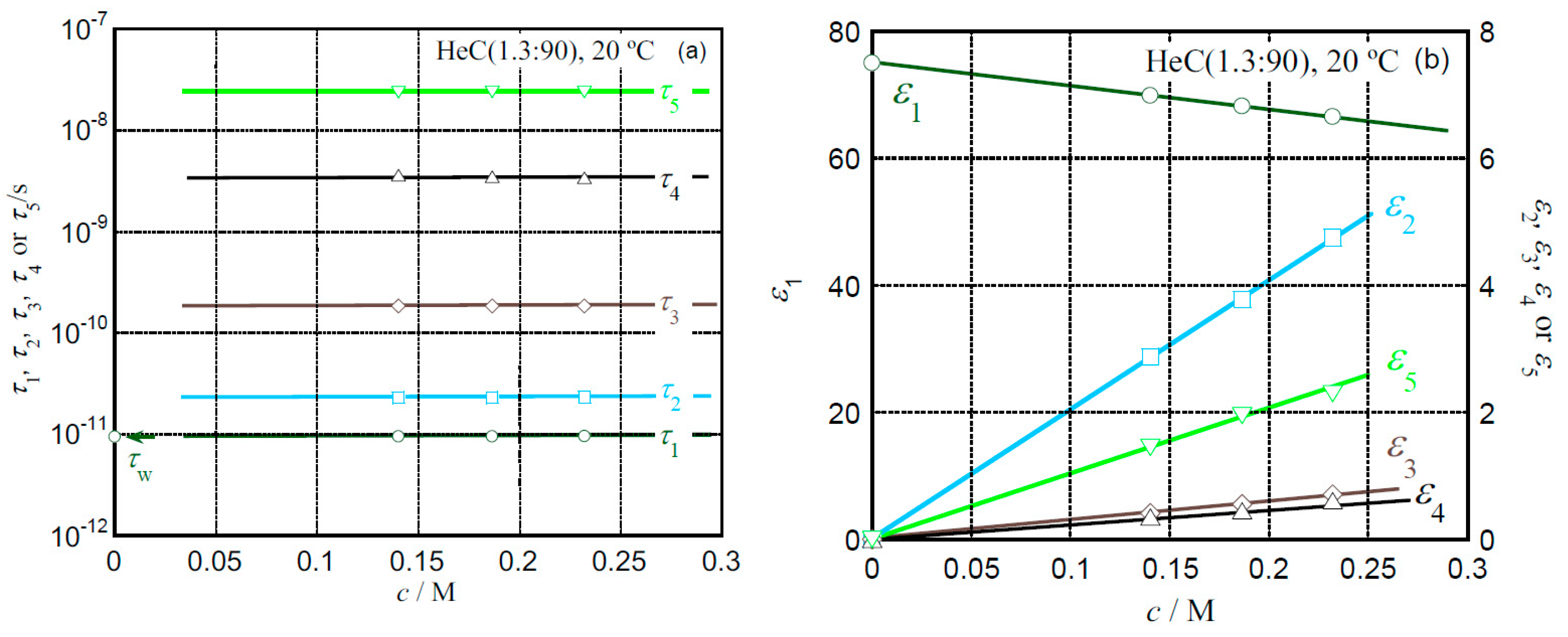
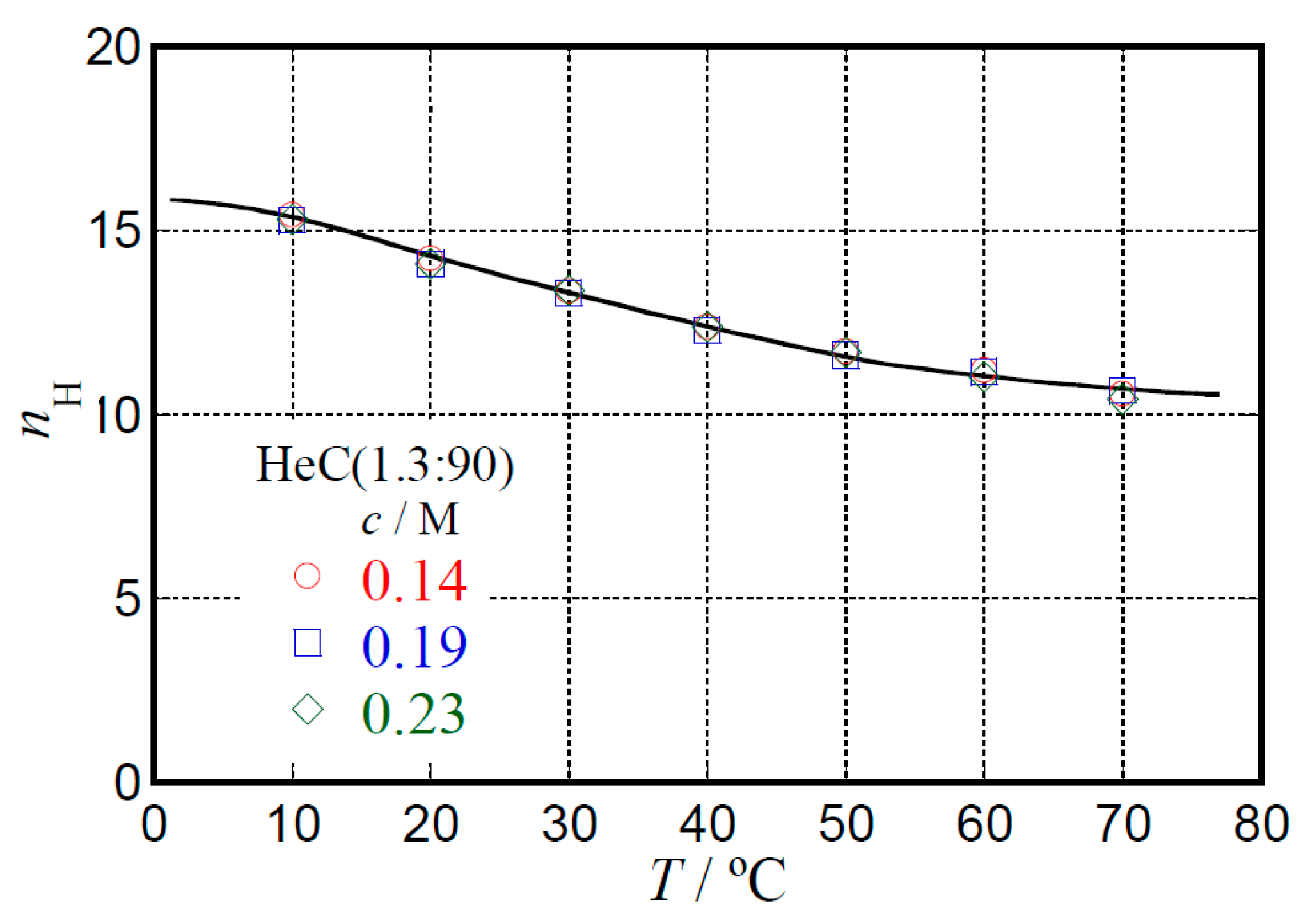
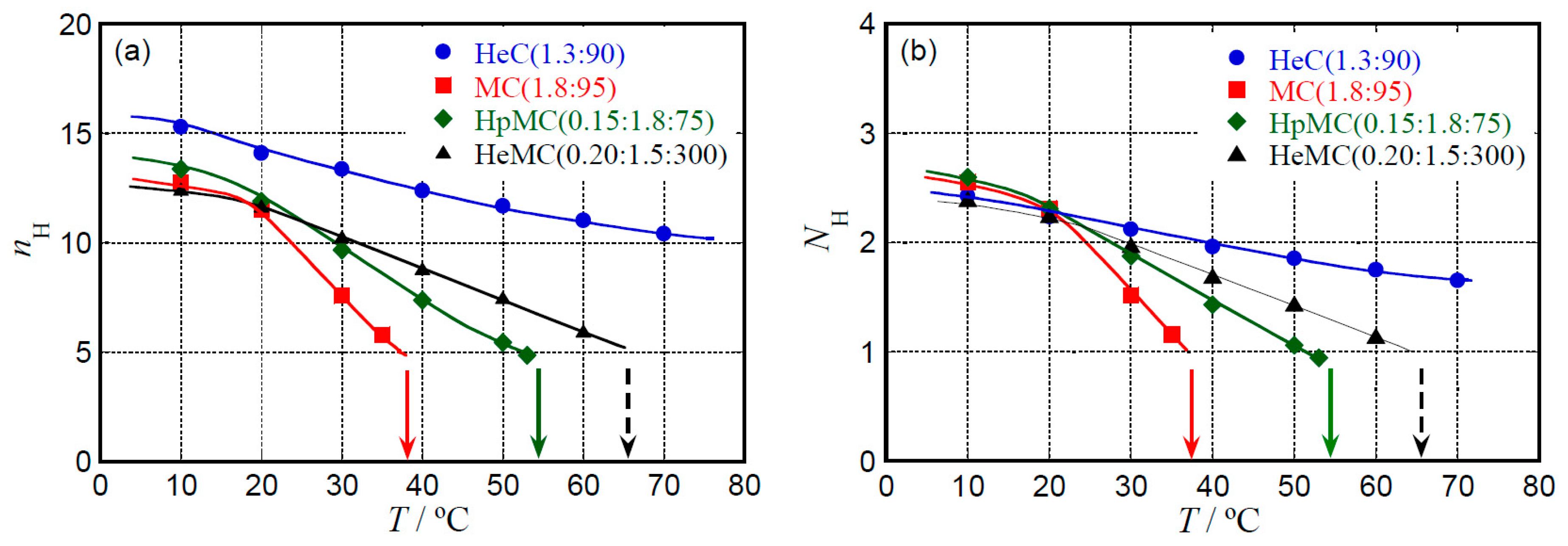
2.3. Hydration Behavior
3. Materials and Methods
3.1. Materials
3.2. Dielectric Spectroscopic Measurements
3.3. Density Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kalia, S.; Kaith, B.S.; Kaur, I. Cellulose Fibers: Bio- and Nano-Polymer Composites; Green Chemistry and Technology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 43–60. [Google Scholar]
- Nishiyama, Y.; Langan, P.; Chanzy, H. Crystal Structure and Hydrogen-Bonding System in Cellulose Iβ from Synchrotron X-ray and Neutron Fiber Diffraction. J. Am. Chem. Soc. 2002, 124, 9074–9082. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Zhang, Y.; Jiang, K.; Wang, J.; Zhang, S. Why Only Ionic Liquids with Unsaturated Heterocyclic Cations Can Dissolve Cellulose: A Simulation Study. ACS Sustain. Chem. Eng. 2017, 5, 3417–3428. [Google Scholar] [CrossRef]
- Clasen, C.; Kulicke, W.M. Determination of viscoelastic and rheo-optical material functions of water-soluble cellulose derivatives. Prog. Polym. Sci. 2001, 26, 1839–1919. [Google Scholar] [CrossRef]
- Xu, A.; Wang, Y.; Gaoa, J.; Wang, J. Facile fabrication of a homogeneous cellulose/polylactic acid composite film with improved biocompatibility, biodegradability and mechanical properties. Green Chem. 2019, 21, 4449–4456. [Google Scholar] [CrossRef]
- Wang, Y.; Duo, T.; Xu, X.; Xiao, Z.; Xu, A.; Liu, R.; Jiang, C.; Lu, J. Eco-Friendly High-Performance Poly (methyl methacrylate) Film Reinforced with Methylcellulose. ACS Omega 2020, 5, 24256–24261. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Wang, F.; Duo, T.; Xiao, Z.; Xu, A.; Liu, R.; Jiang, C. Facile Fabrication of Methylcellulose/PLA Membrane with Improved Properties. Coatings 2020, 10, 499. [Google Scholar] [CrossRef]
- Adden, R.; Müller, R.; Brinkmalm, G.; Ehrler, R.; Mischnick, P. Comprehensive analysis of the substituent distribution in hydroxyethyl celluloses by quantitative MALDI-ToF-MS. Macromol. Biosci. 2006, 6, 435–444. [Google Scholar] [CrossRef]
- Kozlowska, J.; Stachowiak, N.; Sionkowska, A. Collagen/gelatin/hydroxyethyl cellulose composites containing microspheres based on collagen and gelatin: Design and evaluation. Polymers 2018, 10, 456. [Google Scholar] [CrossRef]
- Ou, Z.H.; Ma, B.G.; Jian, S.W. Influence of cellulose ethers molecular parameters on hydration kinetics of Portland cement at early ages. Constr. Build. Mater. 2012, 33, 78–83. [Google Scholar] [CrossRef]
- Lim, D.B.K.; Gong, H. Highly stretchable and transparent films based on cellulose. Carbohydr. Polym. 2018, 201, 446–453. [Google Scholar] [CrossRef]
- Singh, P.; Magalhães, S.; Alves, L.; Antunes, F.; Miguel, M.; Lindman, B.; Medronho, B. Cellulose-based edible films for probiotic entrapment. Food Hydrocoll. 2019, 88, 68–74. [Google Scholar] [CrossRef]
- Chen, Y.C.; Ho, H.O.; Lee, T.Y.; Sheu, M.T. Physical characterizations and sustained release profiling of gastroretentive drug delivery systems with improved floating and swelling capabilities. Int. J. Pharm. 2013, 441, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, C.; Massuelle, D.; Doelker, E. Towards elucidation of the drug release mechanism from compressed hydrophilic matrices made of cellulose ethers. II. Evaluation of a possible swelling-controlled drug release mechanism using dimensionless analysis. J. Control. Release 2010, 141, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Arvidson, S.A.; Lott, J.R.; McAllister, J.W.; Zhang, J.; Bates, F.S.; Lodge, T.P.; Sammler, R.L.; Li, Y.; Brackhagen, M. Interplay of phase separation and thermoreversible gelation in aqueous methylcellulose solutions. Macromolecules 2013, 46, 300–309. [Google Scholar] [CrossRef]
- Lodge, T.P.; Maxwell, A.L.; Lott, J.R.; Schmidt, P.W.; McAllister, J.W.; Morozova, S.; Bates, F.S.; Li, Y.; Sammler, R.L. Gelation, Phase Separation, and Fibril Formation in Aqueous Hydroxypropylmethylcellulose Solutions. Biomacromolecules 2018, 19, 816–824. [Google Scholar] [CrossRef]
- Fettaka, M.; Issaadi, R.; Moulai-Mostefa, N.; Le Cerf, I.; Dez, D.; Picton, L. Thermo sensitive behavior of cellulose derivatives in dilute aqueous solutions: From macroscopic to mesoscopic scale. J. Colloid Interface Sci. 2011, 357, 372–378. [Google Scholar] [CrossRef]
- Hydroxyethyl Cellulose “Cellosize” Thickener, Binder, Stabilizer, Film Former, Protective Colloid. Available online: http://www.dow.com/assets/attachments/industry/building_construction/Cellosize_brochure.pdf (accessed on 7 August 2017).
- Del Giudice, F.; Tassieri, M.; Oelschlaeger, C.; Shen, A.Q. When Microrheology, Bulk Rheology, and Microfluidics Meet: Broadband Rheology of Hydroxyethyl Cellulose Water Solutions. Macromolecules 2017, 50, 2951–2963. [Google Scholar] [CrossRef]
- Koda, S.; Uemura, E.; Nomura, H.; Iwata, M.; Onda, Y. Water retention capacity of cellulose derivatives by the compression method. Macromol. Chem. Phys. 1998, 199, 1489–1493. [Google Scholar] [CrossRef]
- Ouaer, H.; Gareche, M. The rheological behaviour of a water-soluble polymer (HEC) used in drilling fluids. J. Braz. Soc. Mech. Sci. Eng. 2018, 40, 380–387. [Google Scholar] [CrossRef]
- Arfin, N.; Bohidar, H.B. Concentration selective hydration and phase states of hydroxyethyl cellulose (HEC) in aqueous solutions. Int. J. Biol. Macromol. 2012, 50, 759–767. [Google Scholar] [CrossRef]
- Arai, K.; Okuzono, M.; Shikata, T. Reason for the high solubility of chemically modified poly (vinyl alcohol) s in aqueous solution. Macromolecules 2015, 48, 1573–1578. [Google Scholar] [CrossRef]
- Arai, K.; Shikata, T. Hydration/Dehydration Behavior of Cellulose Ethers in Aqueous Solution. Macromolecules 2017, 50, 5920–5928. [Google Scholar] [CrossRef]
- Shikata, T.; Sugimoto, N. Reconsideration of the anomalous dielectric behavior of dimethyl sulfoxide in the pure liquid state. Phys. Chem. Chem. Phys. 2011, 13, 16542–16547. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Shikata, T. Hydration and Dynamic Behavior of Poly (N-isopropylacrylamide) s in Aqueous Solution: A Sharp Phase Transition at the Lower Critical Solution Temperature. J. Am. Chem. Soc. 2006, 128, 10030–10031. [Google Scholar] [CrossRef]
- Lupi, L.; Comez, L.; Paolantoni, M.; Perticaroli, S.; Sassi, P.; Morresi, A.; Ladanyi, B.M.; Fioretto, D. Hydration and aggregation in mono- and disaccharide aqueous solutions by gigahertz-to-terahertz light scattering and molecular dynamics simulations. J. Phys. Chem. B 2012, 116, 14760–14767. [Google Scholar] [CrossRef]
- Lupi, L.; Comez, L.; Paolantoni, M.; Fioretto, D.; Ladanyi, B.M. Dynamics of biological water: Insights from molecular modeling of light scattering in aqueous trehalose solutions. J. Phys. Chem. B 2012, 116, 7499–7508. [Google Scholar] [CrossRef]
- Comez, L.; Paolantoni, M.; Sassi, P.; Corezzi, S.; Morresi, A.; Fioretto, D. Molecular properties of aqueous solutions: A focus on the collective dynamics of hydration water. Soft Matter 2016, 12, 5501–5514. [Google Scholar] [CrossRef]
- Perticaroli, S.; Nakanishi, M.; Pashkovski, E.; Sokolov, A.P. Dynamics of hydration water in sugars and peptides solutions. J. Phys. Chem. B 2013, 117, 7729–7736. [Google Scholar] [CrossRef]
- Denisov, V.P.; Halle, B. Protein hydration dynamics in aqueous solution. Faraday Discuss. 1996, 103, 227–244. [Google Scholar] [CrossRef]
- Steinhoff, H.J.; Kramm, B.; Hess, G.; Owerdieck, C.; Redhardt, A. Rotational and translational water diffusion in the hemoglobin hydration shell: Dielectric and proton nuclear relaxation measurements. Biophys. J. 1993, 65, 1486–1495. [Google Scholar] [CrossRef][Green Version]
- Perticaroli, S.; Ehlers, G.; Stanley, C.B.; Mamontov, E.; O’Neill, H.; Zhang, Q.; Cheng, X.; Myles, D.A.A.; Katsaras, J.; Nickels, J.D. Description of Hydration Water in Protein (Green Fluorescent Protein) Solution. J. Am. Chem. Soc. 2017, 139, 1098–1105. [Google Scholar] [CrossRef]
- Pal, S.K.; Zewail, A.H. Dynamics of Water in Biological Recognition. Chem. Rev. 2004, 104, 2099–2124. [Google Scholar] [CrossRef] [PubMed]
- Shikata, T.; Okuzono, M.; Sugimoto, N. Temperature-Dependent Hydration/Dehydration Behavior of Poly (ethylene oxide) s in Aqueous Solution. Macromolecules 2013, 46, 1956–1961. [Google Scholar] [CrossRef]
- Shiraga, K.; Suzuki, T.; Kondo, N.; Tajima, T.; Nakamura, M.; Togo, H.; Hirata, A.; Ajito, K.; Ogawa, Y. Broadband dielectric spectroscopy of glucose aqueous solution: Analysis of the hydration state and the hydrogen bond network. J. Chem. Phys. 2015, 142, 234504. [Google Scholar] [CrossRef] [PubMed]
- Shiraga, K.; Suzuki, T.; Kondo, N.; De Baerdemaeker, J.; Ogawa, Y. Quantitative characterization of hydration state and destructuring effect of monosaccharides and disaccharides on water hydrogen bond network. Carbohydr. Res. 2015, 406, 46–54. [Google Scholar] [CrossRef]
- Shiraga, K.; Adachi, A.; Nakamura, M.; Tajima, T.; Ajito, K.; Ogawa, Y. Characterization of the hydrogen-bond network of water around sucrose and trehalose: Microwave and terahertz spectroscopic study. J. Chem. Phys. 2017, 146, 105102. [Google Scholar] [CrossRef]
- Deshmukh, S.A.; Sankaranarayanan, S.K.R.S.; Suthar, K.; Mancini, D.C. Role of Solvation Dynamics and Local Ordering of Water in Inducing Conformational Transitions in Poly (N-isopropylacrylamide) Oligomers through the LCST. J. Phys. Chem. B 2012, 116, 2651–2663. [Google Scholar] [CrossRef] [PubMed]
- Marchi, M.; Sterpone, F.; Ceccarelli, M. Water Rotational Relaxation and Diffusion in Hydrated Lysozyme. J. Am. Chem. Soc. 2002, 124, 6787–6791. [Google Scholar] [CrossRef]
- Sterpone, F.; Stirnemann, G.; Laage, D. Magnitude and Molecular Origin of Water Slowdown Next to a Protein. J. Am. Chem. Soc. 2012, 134, 4116–4119. [Google Scholar] [CrossRef]
- Pottel, R. Dielectric properties. In Water, a Comprehensive Treatise, Aqueous Solutions of Simple Electrolytes; Francs, F., Ed.; Plenum: New York, NY, USA, 1973; Volume 3, pp. 401–431. [Google Scholar]
- Cole, K.S.; Cole, R.H. Dispersion and Absorption in Dielectrics 1. Alternating Current Characteristics. J. Chem. Phys. 1941, 9, 341–351. [Google Scholar] [CrossRef]
- Davidson, D.W.; Cole, R.H. Dielectric relaxation in glycerol, propylene glycol, and n-propanol. J. Chem. Phys. 1951, 19, 1484–1490. [Google Scholar] [CrossRef]
- Arai, K.; Shikata, T. Molecular motions, structure and hydration behaviour of glucose oligomers in aqueous solution. Phys. Chem. Chem. Phys. 2019, 21, 25379–25388. [Google Scholar] [CrossRef] [PubMed]
- Shikata, T.; Minakawa, A.; Okuyama, K. Structure, Dynamics, and Hydration of a Collagen Model Polypeptide, (L-Prolyl-L-ProlylGlycyl)10, in Aqueous Media: A Chemical Equilibrium Analysis of Triple Helix-to-Single Coil Transition. J. Phys. Chem. B 2009, 113, 14504–14512. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of all the compounds are available from the authors. |



| Sample Codes | Molar Substitution Number by Hydroxyethyl Groups | Mw/103 |
|---|---|---|
| HeC(1.3:90) | 1.3 | 90 |
| HeC(2.0:220) | 2.0 | 220 |
| HeC(3.6:70) | 3.6 | 70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arai, K.; Shikata, T. Hydration/Dehydration Behavior of Hydroxyethyl Cellulose Ether in Aqueous Solution. Molecules 2020, 25, 4726. https://doi.org/10.3390/molecules25204726
Arai K, Shikata T. Hydration/Dehydration Behavior of Hydroxyethyl Cellulose Ether in Aqueous Solution. Molecules. 2020; 25(20):4726. https://doi.org/10.3390/molecules25204726
Chicago/Turabian StyleArai, Kengo, and Toshiyuki Shikata. 2020. "Hydration/Dehydration Behavior of Hydroxyethyl Cellulose Ether in Aqueous Solution" Molecules 25, no. 20: 4726. https://doi.org/10.3390/molecules25204726
APA StyleArai, K., & Shikata, T. (2020). Hydration/Dehydration Behavior of Hydroxyethyl Cellulose Ether in Aqueous Solution. Molecules, 25(20), 4726. https://doi.org/10.3390/molecules25204726