Drug-Eluting Stents and Balloons—Materials, Structure Designs, and Coating Techniques: A Review
Abstract
1. Introduction
- the mechanisms of releasing the active substances (e.g., by diffusion, ion exchange, or osmosis);
- release kinetics;
- materials used as carriers and delivery routes;
- medicines that can be used for appropriate therapy.
- permanent polymeric coating materials;
- metallic stent platforms;
- optimal drug-releasing condition, and
- the factors that have recently been identified as disadvantages, such as:
- ○
- the degradation of the products of polymers and
- ○
- the presence of metal ions due to the erosion and degradation of metals and their alloys utilized in some stents as a metal base [13].
2. Cardiovascular Stent Design Parameters
3. Drug-Eluting Stents (DES)
- new generations of drug-eluting stents,
- nonpolymeric stents,
- bioresorbable polymer-coated stents, and
- fully bioresorbable scaffolds [1].
3.1. Polymer-Coated Stents (PCS)
- delayed endothelialization caused by the locally delivered drugs,
- inherent thrombogenicity of the stent as a foreign body to the immune system,
- hypersensitivity and inflammatory reactions due to the base framework and/or polymeric coatings,
- insufficient drug amount in addition to a lack of sustained drug release, and
- stent displacement [13].
3.2. Biodegradable Materials for DES
3.2.1. Bioresorbable Materials for Scaffolds
- biocompatibility: before, during, and after degradation;
- adequate radial strength;
- the proper time of degradation: not too fast to increase inflammation and not too long to provoke adverse body reactions—usually 4–6 months;
- no inflammatory process initiative by degradation;
- compatibility with DES technology, eluting drugs at a determined rate without any effect on the radial strength;
- thin struts;
- easy deliverability;
- enhanced visualization under fluoroscopy;
- compatible with currently available equipment for deployment; and
- improved dwell time before implementation.
- lower stiffness and strength of polymer materials that make stent struts be thicker in comparison to conventional metal-based stents;
- increase in diameters of struts may lead to complications within the stent, such as platelet adhesion, and vessel injury;
- at a stress level below the yield and tensile strength of the material under consideration, premature destruction of a polymer may happen; the result is that, long before the polymer is degraded, the device fails in the face of the liquid pressure and the exerted pressure from the vessel wall; and
- mechanical behavior of the polymer and other bioresorbable polymers, due to molecular weight, temperature, molecular orientation, the crystallinity of the polymer, and degradation characteristics, is nonlinear.
3.2.2. Biodegradable Materials as Drug Release Coating Materials
Biodegradable Polymers
Poly(lactic acid)
Poly(glycolic acid)
Poly(lactic-co-glycolic acid)
Poly(caprolactone)
Poly(caprolactone-co-poly(ethylene glycol))
Polyurethanes (PUs)
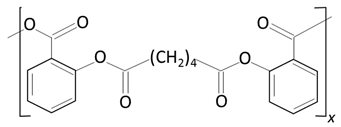
Other Biodegradable Polymers
3.2.3. Biodegradable Metal Scaffolds
4. Drug Release Kinetics
5. DES Coating Techniques
- inhibition of an inflammatory reaction for impeding the thrombosis formation,
- inhibition of excessive SMCs proliferation and preventing intimial hyperplasia,
- fast endothelialization from the early time of implantation to promote the creation of an endothelial layer on the stent surface within one month; a quick endothelialization process is essential to decrease the risk of thrombosis to the least amount, and
- avoiding adverse material-tissue interface interactions; it is necessary for the surface to be biocompatible, especially after complete drug elution [127].
5.1. Dip Coating
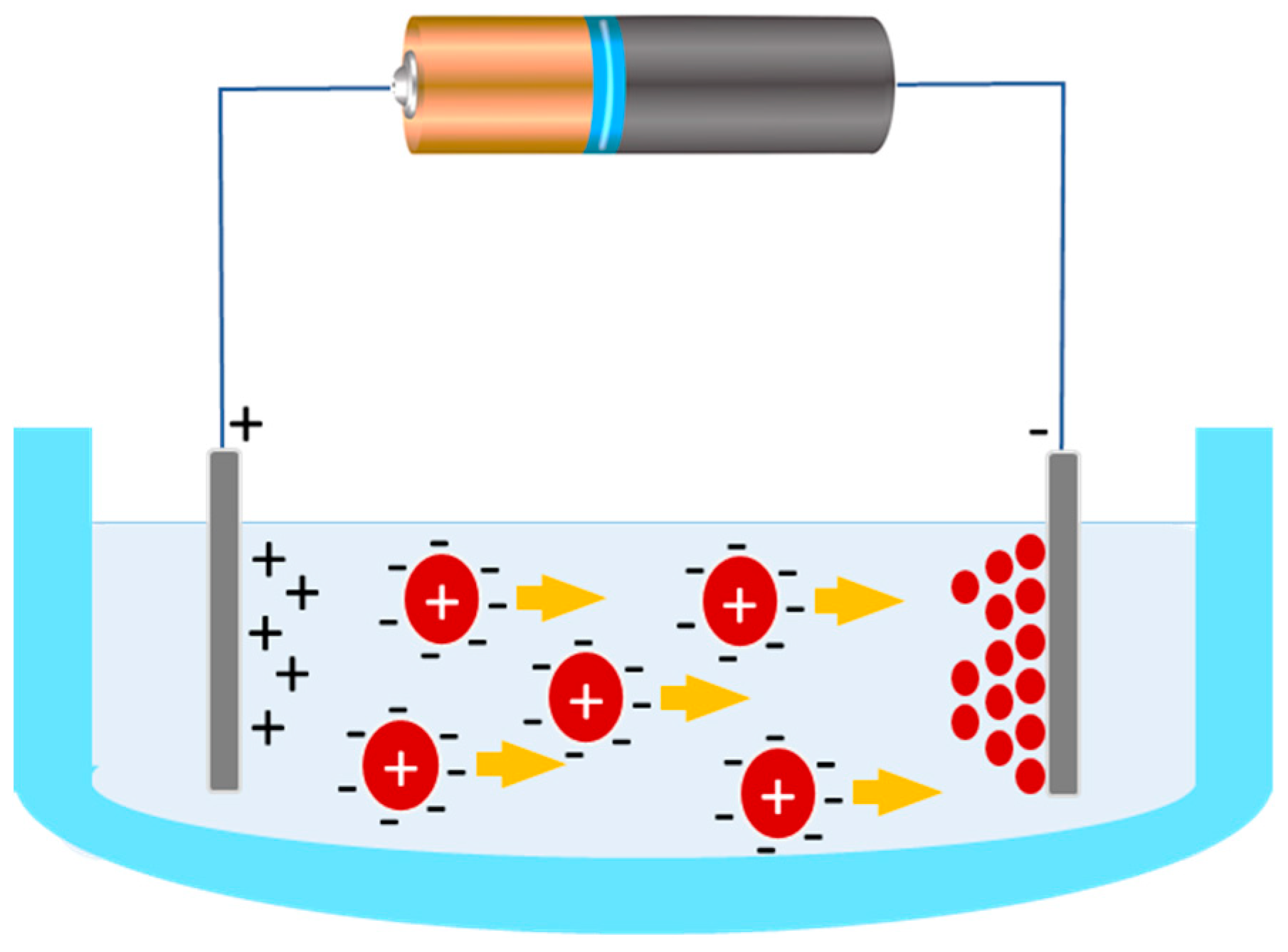
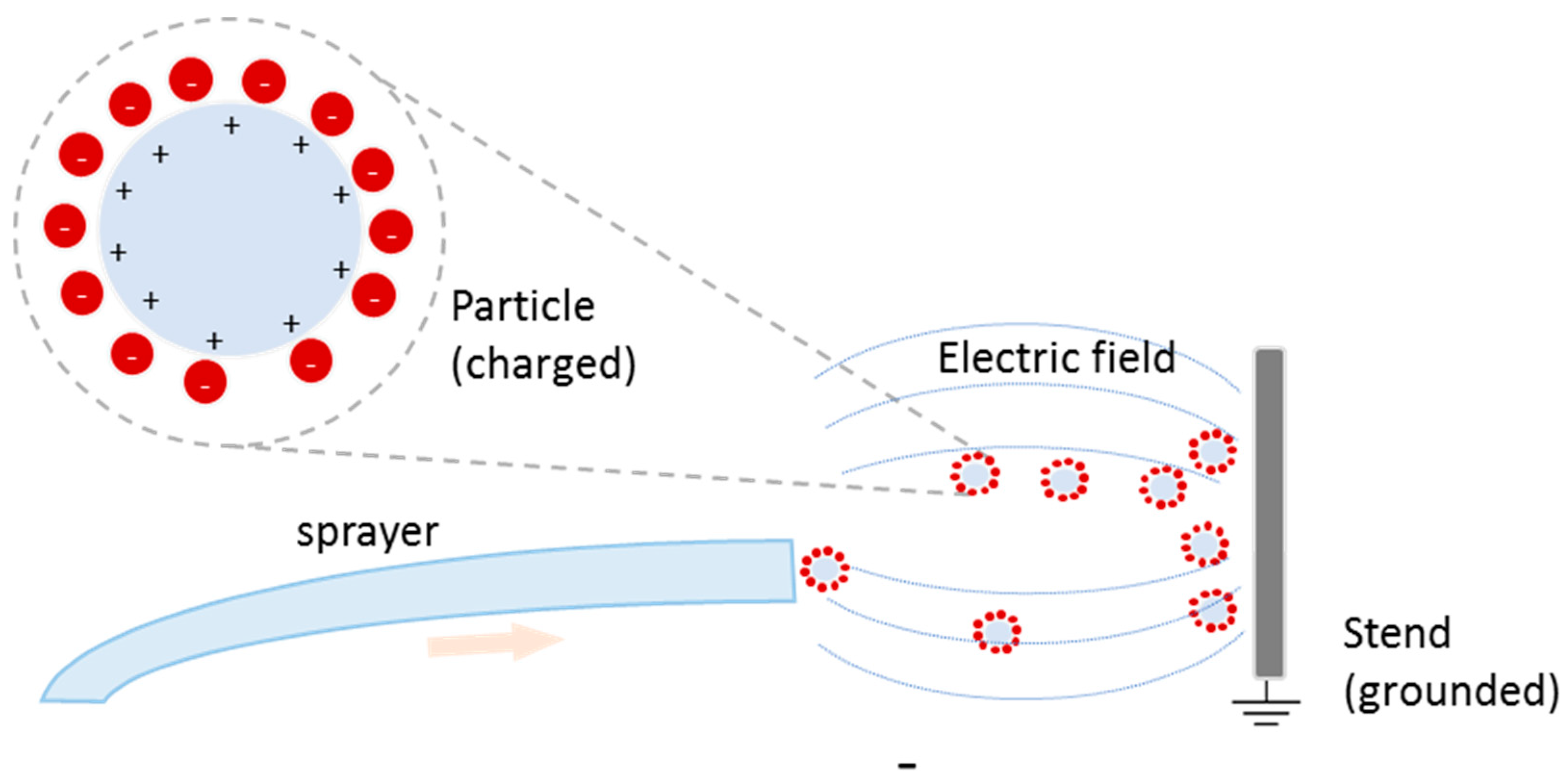
5.2. Electrotreated Coating
5.3. Plasma-Treated Coating
5.4. Spray Coating
5.5. Drug Delivery Mechanism and Effective Parameters
6. New Stent Systems
6.1. Shape-Memory Stents
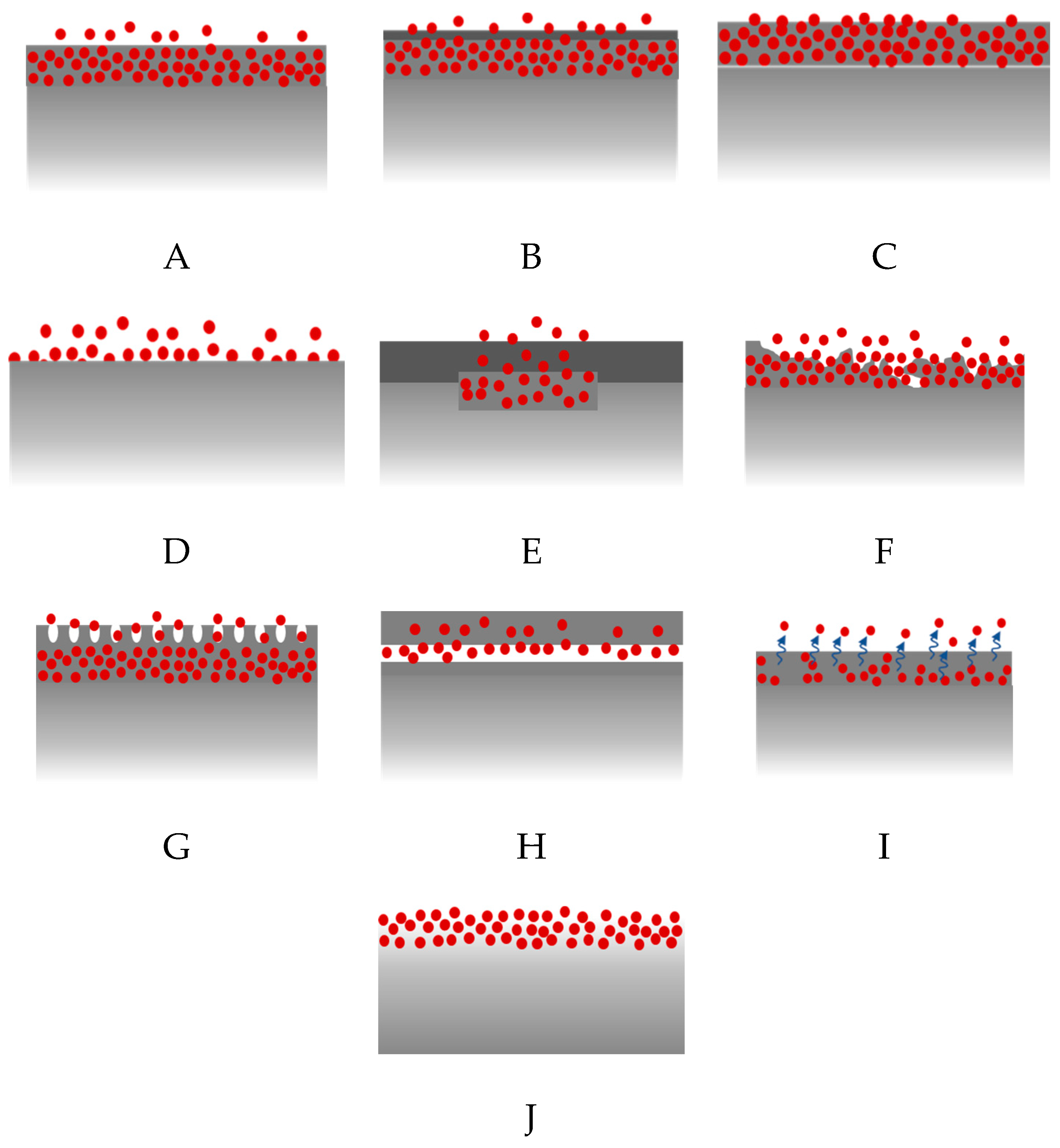
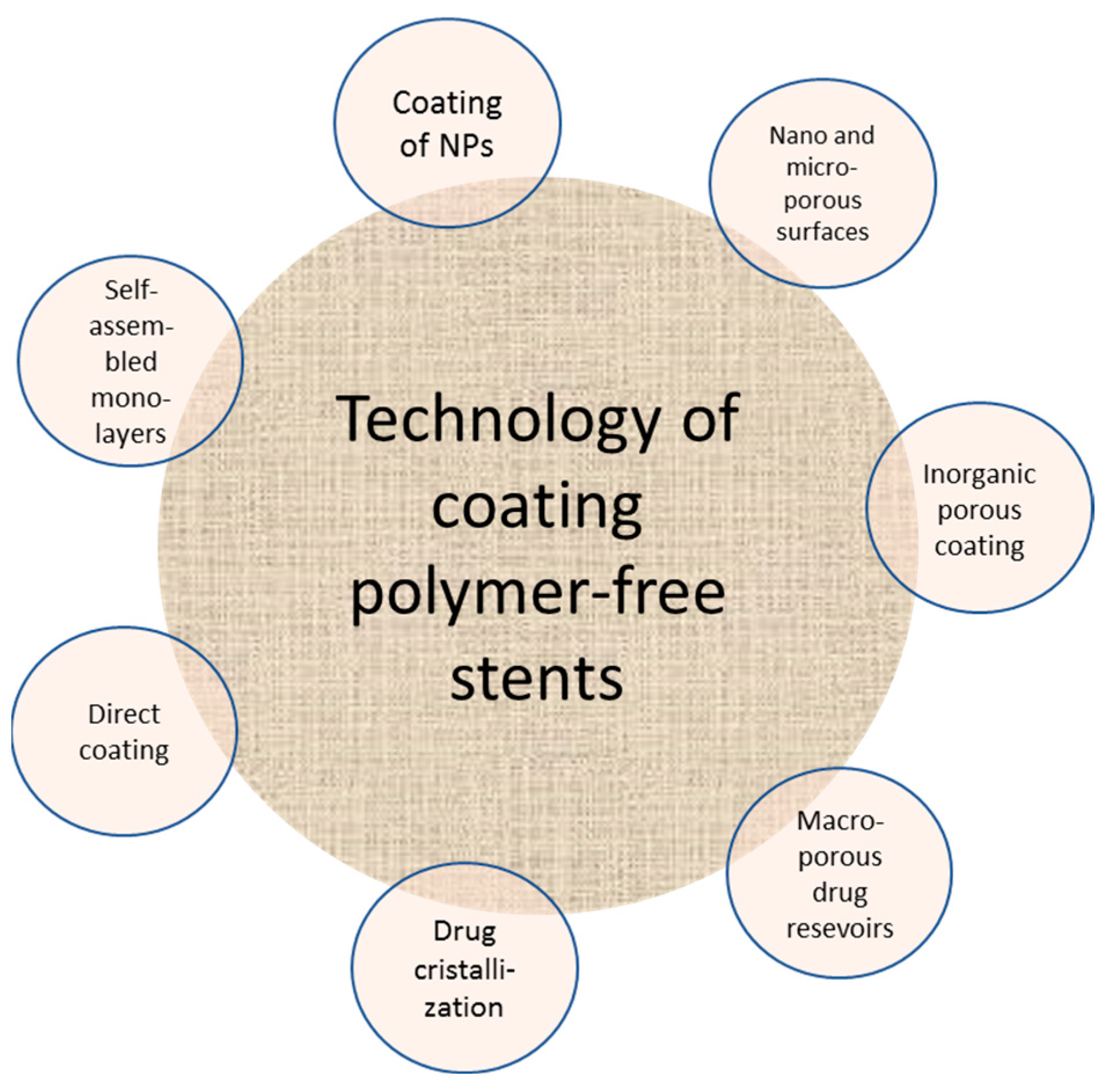
6.2. Polymer-Free DES
6.3. The Future of DES
6.3.1. Solutions for Cardiac Patients
6.3.2. Solutions for Cardiac Patients with Diabetes Mellitus
6.3.3. Stents in the Management of Chronic Rhinosinusitis
6.3.4. Stents in Urological Procedures
6.3.5. Herbal Stent Coatings
- Tripterygium wilfordii [164],
- Atractylodes macrocephala Koidz [165],
- Gastrodia elata Blume [165],
- Citrus unshiu Marcow [165],
- Poria cocos Wolf [165],
- Crataegus pinnatifida Bunge var. typical C. K. Schneider [47],
- Siegesbeckia pubescens Makino [165],
- Coptidis japonica Makino [165], and
- Magnolia Cortex [166].
7. Drug-Eluting Balloons (DEB)
- materials used to make balloons,
- types of medications used for coating, and
- coating techniques.
7.1. Balloon Materials
7.2. Coating Materials for DEB
7.3. Therapeutic Substances
7.4. Excipients
7.5. DEB Coating Techniques
7.5.1. Microcapsule Coating
7.5.2. Hydrogel Coating
7.5.3. Polymer-Free Coating
7.5.4. Immediate Release Coating
7.5.5. Bioadhesive Coating
7.5.6. Multiple-Layer Coating
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BCS | Bioresorbable Cardiovascular Scaffold |
| BMS | Bare Metal Stent |
| CAD | Coronary Artery Disease |
| CDDS | Controlled Drug Delivery System |
| DDS | Controlled Drug Delivery Systems |
| DEB | Drug-Eluting Balloon |
| DCB | Drug-Coated Balloon |
| DES | Drug-Eluting Stent (Drug-Coated Stent) |
| ISR | In-Stent Restenosis |
| PCS | Polymer Coating Stents |
| PDLLA | Poly (D, L-Lactide) |
| PLLA | Poly (L-Lactic Acid) |
| PTCA | Percutaneous Trans Luminal Coronary Angioblasts) |
| ST | Stent Thrombosis |
| SMP | Shape-Memory Polymer |
References
- Chen, D.; Jepson, N. Coronary stent technology: A narrative review. Med. J. Aust. 2016, 205, 277–281. [Google Scholar] [CrossRef]
- Borhani, S.; Hassanajili, S.; Tafti, S.H.A.; Rabbani, S. Cardiovascular stents: Overview, evolution, and next generation. Prog. Biomater. 2018, 7, 175–205. [Google Scholar] [CrossRef] [PubMed]
- Driver, M. Coatings for cardiovascular devices: Coronary stents. In Coatings for Biomedical Applications; Woodhead Publishing: Sawston, UK, 2012; pp. 223–250. [Google Scholar]
- Guildford, A.; Santin, M.; Phillips, G. Cardiovascular stents A2-Gourlay, terence. In Biomaterials and Devices for the Circulatory System; Black, R.A., Ed.; Woodhead Publishing: Sawston, UK, 2010; Volume 7, pp. 173–216. [Google Scholar]
- Grüntzig, A. Transluminal dilatation of coronary artery stenosis experimental report. In Percutaneous Vascular Recanalization; Springer: Berlin/Heidelberg, Germany, 1978; pp. 57–65. [Google Scholar]
- Sigwart, U.; Urban, P.; Golf, S.; Kaufmann, U.; Imbert, C.; Fischer, A.; Kappenberger, L. Emergency stenting for acute occlusion after coronary balloon angioplasty. Circulation 1988, 78, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Palmaz, J.C.; Kopp, D.T.; Hayashi, H.; Schatz, R.A.; Hunter, G.; Tio, F.O.; Garcia, O.; Alvarado, R.; Rees, C.; Thomas, S.C. Normal and stenotic renal arteries: Experimental balloon-expandable intraluminal stenting. Radiology 1987, 164, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Grabow, N.; Martin, D.P.; Schmitz, K.P.; Sternberg, K. Absorbable polymer stent technologies for vascular regeneration. J. Chem. Technol. Biotechnol. 2009, 85, 744–751. [Google Scholar] [CrossRef]
- Buccheri, D.; Piraino, D.; Giuseppe Andolina, G.; Cortese, B. Understanding and managing in-stent restenosis: A review of clinical data, from pathogenesis to treatment. J. Thorac. Dis. 2016, 8, E1150–E1162. [Google Scholar] [CrossRef]
- Kohn, J.; Zeltinger, J. Degradable, drug-eluting stents: A new frontier for the treatment of coronary artery disease. Expert Rev. Med. Devices 2005, 2, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Yang, J.; Cui, K.; Rao, O.; Yin, T.; Tan, L.; Zhang, Y.; Li, Z.; Wang, G. Controlled slow-release drug-eluting stents for the prevention of coronary restenosis: Recent progress and future prospects. ACS Appl. Mater. Interfaces 2015, 7, 11695–11712. [Google Scholar] [CrossRef]
- Ferns, G.A.; Avades, T.Y. The mechanisms of coronary restenosis: Insights from experimental models. Int. J. Exp. Pathol. 2000, 81, 63–88. [Google Scholar] [CrossRef] [PubMed]
- Saleh, Y.E.; Gepreel, M.A.; Allam, N.K. Functional nanoarchitectures for enhanced drug eluting stents. Sci. Rep. 2017, 7, 40291. [Google Scholar] [CrossRef]
- Bukka, M.; Rednam, P.J.; Sinha, M. Drug-eluting balloon: Design, technology and clinical aspects. Biomed. Mater. 2018, 13, 032001. [Google Scholar] [CrossRef] [PubMed]
- Farah, S. Protective layer development for enhancing stability and drug-delivery capabilities of des surface-crystallized coatings. ACS Appl. Mater. Interfaces. 2018, 10, 9010–9022. [Google Scholar] [CrossRef] [PubMed]
- Levy, Y.; Tal, N.; Tzemach, G.; Weinberger, J.; Domb, A.J.; Mandler, D. Drug-eluting stent with improved durability and controllability properties, obtained via electrocoated adhesive promotion layer. J. Biomed. Mater. Res. Part B 2009, 91, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Otsuka, F.; Gupta, A.; Jinnouchi, H.; Torii, S.; Harari, E.; Virmani, R. Revisiting the role of durable polymers in cardiovascular devices. Expert Rev. Cardiovasc. Ther. 2017, 15, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, P.; Lally, C.; Daly, S.; Reid, A.; Lee, T.; Quinn, D.; Dolan, F. Analysis of 18 in cardiovascular stents: A constitutive equation for vascular tissue and finite-element modelling. J. Biomech. Eng. 2003, 125, 692–699. [Google Scholar] [CrossRef]
- Alexander, G.C.; Hwang, P.T.; Chen, J.; Kim, J.; Brott, B.C.; Yoon, Y.S.; Jun, H.W. Nanomatrix coated stent enhances endothelialization but reduces platelet, smooth muscle cell, and monocyte adhesion under physiologic conditions. ACS Biomater. Sci. Eng. 2017, 4, 107–115. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Dooley, J.F.; Kopia, G.; Edelman, E.R. Intravascular drug release kinetics dictate arterial drug deposition, retention, and distribution. J. Control. Release 2007, 123, 100–108. [Google Scholar] [CrossRef]
- Htay, T.; Liu, M.W. Drug-Eluting Stent: A Review and Update. Vasc. Health Risk Manag. 2005, 1, 263–276. [Google Scholar] [CrossRef]
- Burt, H.M.; Hunter, W.L. Drug-eluting stents: A multidisciplinary success story. Adv. Drug Deliv. Rev. 2006, 3, 350–357. [Google Scholar] [CrossRef]
- Martin, D.M.; Boyle, F.J. Drug-eluting stents for coronary artery disease: A review. Med. Eng. Phys. 2011, 33, 148–163. [Google Scholar] [CrossRef]
- Doostzadeh, J.; Clark, L.N.; Bezenek, S.; Pierson, W.; Sood, P.R.; Sudhir, K. Recent progress in percutaneous coronary intervention: Evolution of the drug-eluting stents, focus on the XIENCE V drug-eluting stent. Coron. Artery Dis. 2010, 21, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Silber, S.; Herdeg, C. Drug-eluting stents for diabetic patients. A critical appraisal of the currently available data from randomized trials. Herz Kardiovask. Erkrank. 2008, 33, 196–205. [Google Scholar]
- Li, Y.; Bhindi, R.; Khachigian, L.M. Recent developments in drug-eluting stents. J. Mol. Med. 2011, 89, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, K.; Steinvil, A.; Waksman, R. Does the new generation of drug-eluting stents render bare metal stents obsolete? Cardiovasc. Revascularization Med. 2017, 18, 456–461. [Google Scholar] [CrossRef]
- Fusaro, M.; Cassese, S.; Ndrepepa, G.; Tepe, G.; King, L.; Ott, I.; Nerad, M.; Schunkert, H.; Kastrati, A. Drug-eluting stents for revascularization of infrapopliteal arteries: Updated meta-analysis of randomized trials. JACC Cardiovasc. Interv. 2013, 6, 1284–1293. [Google Scholar] [CrossRef]
- Shlofmitz, E.; Iantorno, M.; Waksman, R. Restenosis of Drug-Eluting Stents: A New Classification System Based on Disease Mechanism to Guide Treatment and State-of-the-Art Review. Circ. Cardiovasc. Interv. 2019, 12, e007023. [Google Scholar] [CrossRef]
- Wiesinger, C.G.; Lee, J.; Herrera-Caceres, J.O. Future developments in ureteral stents. Curr. Opin. Urol. 2019, 29, 124–128. [Google Scholar] [CrossRef]
- Lukman, S.K.; Al-Ashwal, R.H.; Khudzari, A.Z.M.; Saidin, S. Emerging of cardiovascular metal stent: A review on drug-eluting stent towards the utilisation of herbal coating. Malays. J. Fundam. Appl. Sci. 2019, 15, 225–231. [Google Scholar] [CrossRef]
- Wu, J.J.; Way, J.A.H.; Kritharides, L.; Brieger, D. Polymer-free versus durable polymer drug-eluting stents in patients with coronary artery disease: A meta-analysis. Ann. Med. Surg. 2019, 38, 13–21. [Google Scholar] [CrossRef]
- Kommineni, N.; Saka, R.; Khan, W.; Domb, A. Non-polymer drug-eluting coronary stents. Drug Deliv. Transl. Res. 2018, 8, 903–917. [Google Scholar] [CrossRef]
- Livingston, M.; Tan, A. Coating Techniques and Release Kinetics of Drug-Eluting Stents. Med. Devices 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Tousoulis, D.; Oikonomou, E.; Economou, E.K.; Crea, F.; Kaski, J.C. Inflammatory cytokines in atherosclerosis: Current therapeutic approaches. Eur. Heart J. 2016, 37, 1723–1732. [Google Scholar] [CrossRef]
- Simard, T.; Hibbert, B.; Ramirez, F.D.; Froeschl, M.; Chen, Y.X.; O’Brien, E.R. The evolution of coronary stents: A brief review. Can. J. Cardiol. 2014, 30, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Habraken, T.C.; Hennink, W.E.; Kok, R.J. Polymer-free drug-eluting stents: An overview of coating strategies and comparison with polymer-coated drug-eluting stents. Bioconjug. Chem. 2015, 26, 1277–1288. [Google Scholar] [CrossRef] [PubMed]
- Tugtekin, S.M.; Kappert, U.; Jung, F.; Park, J.W.; Knaut, M. Do drugeluting stents influence the spectrum of coronary artery bypass surgery? Herz 2004, 29, 201–207. [Google Scholar]
- Levy, Y.; Mandler, D.; Weinberger, J.; Domb, A.J. Evaluation of drug-eluting stents’ coating durability-clinical and regulatory implications. J. Biomed. Mater. Res. Part B 2009, 91, 441–451. [Google Scholar] [CrossRef]
- Parker, T.; Dave, V.; Falotico, R. Polymers for drug eluting stents. Curr. Pharm. Des. 2010, 16, 3978–3988. [Google Scholar] [CrossRef] [PubMed]
- Naseem, R.; Zhao, L.; Liu, Y.; Silberschmidt, V.V. Experimental and computational studies of poly-l-lactic acid for cardiovascular applications: Recent progress. Mech. Adv. Mater. Modern Process 2017, 3, 13–21. [Google Scholar] [CrossRef]
- Celermajer, D.S. Endothelial dysfunction: Does it matter? Is it reversible? J. Am. Coll. Cardiol. 1997, 30, 325–333. [Google Scholar] [CrossRef]
- Oberhauser, J.P.; Hossainy, S.; Rapoza, R.J. Design principles and performance of bioresorbable polymeric vascular scaffolds. EuroIntervention 2009, 5, F15–F22. [Google Scholar] [CrossRef]
- Wayangankar, S.A.; Ellis, S.G. Bioresorbable stents: Is this where we are headed? Prog. Cardiovasc. Dis. 2015, 58, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, J.; Nef, H.M.; Hamm, C.W. Current status of bioresorbable scaffolds in the treatment of coronary artery disease. J. Am. Coll. Cardiol. 2014, 64, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Mariano, E.; Sangiorgi, G.M.; Fioranelli, M. Coronary stents. In Imaging Coronary Arteries; Springer: New York, NY, USA, 2013; pp. 101–113. [Google Scholar]
- Bourantas, C.V.; Onuma, Y.; Farooq, V.; Zhang, Y.; Serruys, H.M.; Garcia-Garcia, P.W. Bioresorbable scaffolds: Current knowledge, potentialities and limitations experienced during their first clinical applications. Int. J. Cardiol. 2013, 167, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Kereiakes, D.J.; Onuma, Y.; Serruys, P.W.; Stone, G.W. Bioresorbable vascular scaffolds for coronary revascularization. Circulation 2016, 134, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Onuma, Y.; Serruys, P.W. Bioresorbable scaffold: The advent of a new era in percutaneous coronary and peripheral revascularization? Circulation 2011, 123, 779–797. [Google Scholar] [CrossRef] [PubMed]
- Sharkawi, T.; Cornhill, F.; Lafont, A.; Sabaria, P.; Vert, M. Intravascular bioresorbable polymeric stents: A potential alternative to current drug eluting metal stents. J. Pharm. Sci. 2007, 96, 2829–2837. [Google Scholar] [CrossRef]
- Bergström, J.S.; Hayman, D. An overview of mechanical properties and material modeling of polylactide (PLA) for medical applications. Ann. Biomed. Eng. 2016, 44, 330–340. [Google Scholar]
- Rizas, K.D.; Mehilli, J. Stent Polymers Do They Make a Difference? Circ. Cardiovasc. Interv. 2016, 9, e002943. [Google Scholar] [CrossRef]
- Stewart, S.A.; Domínguez-Robles, J.; Donnelly, R.F.; Larrañeta, E. Implantable Polymeric Drug Delivery Devices: Classification, Manufacture, Materials, and Clinical Applications. Polymers 2018, 10, 1379. [Google Scholar] [CrossRef]
- Strohbach, A.; Busch, R. Polymers for Cardiovascular Stent Coatings. Review. Int. J. Polym. Sci. 2015. [Google Scholar] [CrossRef]
- Joseph, J.; Patel, R.M.; Wenham, A.; Smith, J.R. Biomedical applications of polyurethane materials and coatings. Trans. Inst. Met. Finish. 2018, 96, 121–129. [Google Scholar] [CrossRef]
- Englert, C.; Brendel, J.C.; Majdanski, T.C.; Yildirim, T.; Schubert, S.; Gottschaldt, M.; Windhab, N.; Schubert, U.S. Pharmapolymers in the 21st century: Synthetic polymers in drug delivery applications Pharmapolymers in the 21st century: Synthetic polymers in drug delivery applications. Prog. Polym. Sci. 2018, 87, 107–164. [Google Scholar] [CrossRef]
- Miao, T.; Wang, J.; Zeng, Y.; Liu, G.; Chen, X. Polysaccharide-based controlledrelease systems for therapeutics delivery and tissue engineering: Frombench to bedside. Adv. Sci. 2018, 5, 1700513. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Shavi, G.V.; Averineni, R.K.; Bhat, M.; Udupa, N.; Upadhya, P.N. Poly(-hydroxy acid)based polymers: A review on material and degradation aspects. Polym. Degrad. Stab. 2017, 144, 520–535. [Google Scholar]
- Grayson, A.C.R.; Voskerician, G.; Lynn, A.; Anderson, J.M.; Cima, M.J.; Langer, R. Differential degradationrates in vivo and in vitro of biocompatible poly(lactic acid) and poly(glycolic acid) homo- and co-polymers for a polymeric drug-delivery microchip. J. Biomater. Sci. Polym. Ed. 2004, 15, 1281–1304. [Google Scholar] [CrossRef]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
- Crimi, G.; Gritti, V.; Galiffa, V.A.; Scotti, V.; Leonardi, S.; Ferrario, M.; Ferlini, M.; De Ferrari, G.M.; Visconti, L.O.; Klersy, C. Drugeluting stents are superior to bare metal stents to reduce clinical outcomeand stent-related complications in CKD patients, a systematic review, meta-analysis and network meta-analysis. J. Interven. Cardiol. 2018, 31, 319–329. [Google Scholar] [CrossRef]
- Palmerini, T.; Biondi-Zoccai, G.; Della Riva, D.; Mariani, A.; Genereux, P.; Branzi, A.; Stone, G.W. Stent thrombosis with drug-eluting stents: Is the paradigm shifting? J. Coll. Cardiol. 2013, 62, 1915–1921. [Google Scholar] [CrossRef]
- Chin-Quee, S.L.; Hsu, S.H.; Nguyen-Ehrenreich, K.L.; Tai, J.T.; Abraham, G.M.; Pacetti, S.D.; Chan, Y.F.; Nakazawa, G.; Kolodgie, F.D.; Virmani, R.; et al. Endothelial cell recovery, acute thrombogenicity, and monocyteadhesion and activation on fluorinated copolymer and phosphorylcholinepolymer stent coatings. Biomaterials 2010, 31, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ng, H.C.A.; Ng, X.W.; Subbu, V. Drug-eluting biostable and erodiblestents. J. Control. Release 2014, 193, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.J.; Gunn, P.W. Serruys, Coronary stents: Historical development, currentstatus and future directions. Br. Med. Bull. 2013, 106, 193–211. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Byrne, R.A.; Cassese, S.; King, L.; Schulz, S.; Mehilli, J.; Schömig, A.; Kastrati, A. Comparativeefficacy of 2 zotarolimus-eluting stent generations: Resolute versus endeavor stents in patients with coronary artery disease. Am. Heart J. 2013, 165, 80–86. [Google Scholar] [CrossRef]
- Gogas, B.D.; McDaniel, M.; Samady, H.; King, S.B.I. Novel drug-eluting stents forcoronary revascularization. Trends Cardiovasc. Med. 2014, 24, 305–313. [Google Scholar] [CrossRef]
- Sammel, A.M.; Chen, D.; Jepson, N. New generation coronary stenttechnology—Is the future biodegradable? Heart Lung. Circ. 2013, 22, 495–506. [Google Scholar] [CrossRef]
- Bundhun, P.K.; Pursun, M.; Huang, F. Biodegradable polymer drug-eluting stentsversus first-generation durable polymer drug-eluting stents: A systematicreview and meta-analysis of 12 randomized controlled trials. Medicine 2017, 96, e8878. [Google Scholar] [CrossRef]
- Nogic, J.; Mc Cormick, L.M.; Francis, R.; Nerlekar, N.; Jaworski, C.; West, N.E.J.; Brown, A.J. Novel bioabsorbable polymer and polymer-free metallic drug-elutingstents. Int. J. Cardiol. 2018, 71, 435–443. [Google Scholar]
- Stefanini, G.G.; Kalesan, B.; Serruys, P.W.; Heg, D.; Buszman, P.; Linke, A.; Ischinger, T.; Klauss, V.; Eberli, F.; Wijns, W.; et al. Long-term clinical outcomes of biodegradable polymer biolimus-elutingstents versus durable polymer sirolimus-eluting stents in patients withcoronary artery disease (LEADERS): 4-year follow-up of a randomisednon-inferiority trial. Lancet 2011, 378, 1940–1948. [Google Scholar] [CrossRef]
- Upendra, K.; Sanjeev, B. Advantages of novel BioMimeTM sirolimus elutingcoronary stent system. Mov. Towards Biomimicry. Minerva Cardioangiol. 2012, 60, 23–31. [Google Scholar]
- Lemos, P.A.; Bienert, I. The supralimus®sirolimus-eluting stent. Exp. Rev. Med. Devices 2013, 10, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Kereiakes, D.J.; Meredith, I.T.; Windecker, S.; Jobe, R.L.; Mehta, S.R.; Sarembock, I.J.; Feldman, R.L.; Stein, B.; Dubois, C.; Grady, T.; et al. Efficacy and safety of a novel bioabsorbable polymer-coated, everolimus-eluting coronary stent: The EVOLVE II randomized trial. Circ. Cardiovasc. Interv. 2015, 8, e002372. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.O.; Thayssen, P.; Maeng, M.; Ravkilde, J.; Hansen, H.S.; Jensen, S.E.; Krusell, L.R.; Raungaard, B.; Junker, A.; Terkelsen, C.J.; et al. Randomized comparison of a sirolimus-eluting orsiro stent with abiolimus-eluting nobori stent in patients treated with percutaneouscoronary intervention: Rationale and study design of the scandinavianorganization for randomized trials with clinical outcome VII trial. Am. Heart J. 2015, 170, 210–215. [Google Scholar] [PubMed]
- Turner, E.; Megan, E.; Atigh, M.; Christians, U.; Saul, J.M.; Yazdani, S.K. In vitro and in vivo Assessment of Keratose as a Novel Excipient of Paclitaxel Coated Balloons. Front Pharmacol. 2018, 9, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.A. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials 2000, 21, 2475–2490. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles-based drug delivery systems. Coll. Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Anderson, J.M.; Shive, M.S. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 1997, 28, 5–24. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications-A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Mukherjee, T.; Kao, N. PLA Based Biopolymer Reinforced with Natural Fibre: A Review. J. Polym. Environ. 2011, 19, 714–725. [Google Scholar] [CrossRef]
- Da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Wang, B.; Yang, G.; Gauthier, M. Poly(Lactic Acid)-Based Biomaterials: Synthesis, Modification and Applications. In Biomedical Science; Engineering and Technology InTech: London, UK, 2012. [Google Scholar]
- Avérous, L.; Pollet, E. Environmental Silicate Nano-Biocomposites. In Green Energy and Technology; Avérous, L., Pollet, E., Eds.; Springer: London, UK, 2012; ISBN 978-1-4471-4101-3. [Google Scholar]
- Bao, L.; Dorgan, J.R.; Knauss, D.; Hait, S.; Oliveira, N.S.; Maruccho, I.M. Gas permeation properties of poly(lactic acid) revisited. J. Membr. Sci. 2006, 285, 166–172. [Google Scholar] [CrossRef]
- Vieira, A.C.; Vieira, J.C.; Ferra, J.M.; Magalhăes, F.D.; Guedes, R.; Marques, A.T. Mechanical study of PLA–PCL fibers during in vitro degradation. J. Mech. Behav. Biomed. Mater. 2011, 4, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Maurus, P.B.; Kaeding, C.C. Bioabsorbable implant material review. Oper. Tech. Sports Med. 2004, 12, 158–160. [Google Scholar] [CrossRef]
- Xinteng, Z.; Weisan, P.; Ruhua, Z.; Feng, Z. Preparation and evaluation of poly (D, L-lactic acid) (PLA) or D,L-lactide/glycolide copolymer (PLGA) microspheres with estradiol. Pharmazie 2002, 57, 695–697. [Google Scholar] [PubMed]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacquot, M.; Desobry, S. Poly-Lactic Acid: Production, Applications, Nanocomposites, and Release Studies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 552–571. [Google Scholar] [CrossRef]
- Fukushima, K.; Hirata, M.; Kimura, Y. Synthesis and Characterization of Stereoblock Poly(lactic acid)s with Nonequivalent D/L Sequence Ratios. Macromolecules 2007, 40, 3049–3055. [Google Scholar] [CrossRef]
- Mooney, D.J.; Baldwin, D.F.; Suh, N.P.; Vacanti, J.P.; Langer, R. Novel approach to fabricate porous sponges of poly(D,L-lactic-co-glycolic acid) without the use of organic solvents. Biomaterials 1996, 17, 1417–1422. [Google Scholar] [CrossRef]
- Grizzi, I.; Garreau, H.; Li, S.; Vert, M. Hydrolytic degradation of devices based on poly(DL-lactic acid) size-dependence. Biomaterials 1995, 16, 305–311. [Google Scholar] [CrossRef]
- Pitt, C.G.; Gratzl, M.M.; Kimmel, G.L.; Surles, J.; Schindler, A. Aliphatic polyesters II. The degradation of poly (DL-lactide), poly (epsilon-caprolactone), and their copolymers in vivo. Biomaterials 1981, 2, 215–220. [Google Scholar] [CrossRef]
- Bini, R.A.; Silva, M.F.; Varanda, L.C.; da Silva, M.A.; Dreiss, C.A. Soft nanocomposites of gelatin and poly(3-hydroxybutyrate) nanoparticles for dual drug release. Coll. Surf. B Biointerfaces 2017, 157, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Goldbart, R.; Traitel, T.; Lapidot, S.A.; Kost, J. Enzymatically controlled responsive drug delivery systems. Polym. Adv. Technol. 2002, 13, 1006–1018. [Google Scholar] [CrossRef]
- Athanasiou, K.; Agrawal, C.; Barber, F.; Burkhart, S. Orthopaedic applications for PLA-PGA biodegradable polymers. Arthrosc. J. Arthrosc. Relat. Surg. 1998, 14, 726–737. [Google Scholar] [CrossRef]
- Rajgor, N.; Bhaskar, V.; Patel, M. Implantable drug delivery systems: An overview. Syst. Rev. Pharm. 2011, 2, 91–95. [Google Scholar] [CrossRef]
- Sun, H.; Mei, L.; Song, C.; Cui, X.; Wang, P. The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials 2006, 27, 1735–1740. [Google Scholar] [CrossRef]
- Castilla-Cortázar, I.; Más-Estellés, J.; Meseguer-Dueñas, J.M.; Escobar Ivirico, J.L.; Marí, B.; Vidaurre, A. Hydrolytic and enzymatic degradation of a poly(ε-caprolactone) network. Polym. Degrad. Stab. 2012, 97, 1241–1248. [Google Scholar] [CrossRef]
- Mondal, D.; Griffith, M.; Venkatraman, S.S. Polycaprolactone-based biomaterials for tissue engineering and drug delivery: Current scenario and challenges. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 255–265. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer-Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Jenkins, M.J.; Harrison, K.L. The effect of molecular weight on the crystallization kinetics of polycaprolactone. Polym. Adv. Technol. 2006, 17, 474–478. [Google Scholar] [CrossRef]
- Escobar Ivirico, J.L.; Salmerón Sánchez, M.; Sabater i Serra, R.; Meseguer Dueñas, J.M.; Gómez Ribelles, J.L.; Monleón Pradas, M. Structure and Properties of Poly(“-caprolactone) Networks with Modulated Water Uptake. Macromol. Chem. Phys. 2006, 207, 2195–2205. [Google Scholar] [CrossRef]
- Serra, R.S.; Escobar Ivirico, J.L.; Meseguer Dueñas, J.M. Andrio Balado, A.; Gómez Ribelles, J.L.; Salmerón Sánchez, M. Dielectric relaxation spectrum of poly (Epsilon-caprolactone) networks hydrophilized by copolymerization with 2-hydroxyethyl acrylate. Eur. Phys. J. E 2007, 22, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.W.; Gong, C.Y.; Gou, M.L.; Fu, S.Z.; Guo, Q.F.; Shi, S.; Luo, F.; Guo, G.; Qiu, L.Y.; Qian, Z.Y. Biodegradable poly(ε-caprolactone)-poly(ethylene glycol) copolymers as drug delivery system. Int. J. Pharm. 2009, 381, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Uhrich, K.E.; Cannizzaro, S.M.; Langer, R.S.; Shakesheff, K.M. Polymeric Systems for Controlled Drug Release. Chem. Rev. 1999, 99, 3181–3198. [Google Scholar] [CrossRef] [PubMed]
- Doppalapudi, S.; Jain, A.; Khan, W.; Domb, A.J. Biodegradable polymers-an overview. Polym. Adv. Technol. 2014, 25, 427–435. [Google Scholar] [CrossRef]
- Goonoo, N.; Jeetah, R.; Bhaw-Luximon, A.; Jhurry, D. Polydioxanone-based biomaterials for tissue engineering and drug/gene delivery applications. Eur. J. Pharm. Biopharm. 2015, 97, 371–391. [Google Scholar] [CrossRef]
- Witte, F. Reprint of: The history of biodegradable magnesium implants: A review. Acta Biomater. 2015, 23, S28–S40. [Google Scholar] [CrossRef]
- Nguyen, T.Y.; Liew, C.G.; Liu, H. An in vitro mechanism study on the proliferation and pluripotency of human embryonic stems cells in response to magnesium degradation. PLoS ONE 2013, 8, e76547. [Google Scholar] [CrossRef][Green Version]
- Heublein, B.; Rohde, R.; Kaese, V.; Niemeyer, M.; Hartung, W.; Haverich, A. Biocorrosion of magnesium alloys: A new principle in cardiovascular implant technology? Heart 2003, 89, 651–656. [Google Scholar] [CrossRef]
- Waksman, R.; Pakala, R.; Baffour, R.; Seabron, R.; Hellinga, D.; Tio, F.O. Short-term effects of biocorrodible iron stents in porcine coronary arteries. J. Interv. Cardiol. 2008, 21, 15–20. [Google Scholar] [CrossRef]
- Haude, M.; Erbel, R.; Erne, P.; Verheye, S.; Degen, H.; Vermeersch, P.; Weissman, N.; Prati, F.; Bruining, N.; Waksman, R.; et al. Safety and performance of the DRug-Eluting Absorbable Metal Scaffold (DREAMS) in patients with de novo coronary lesions: 3-year results of the prospective, multicentre, first-in-man BIOSOLVE-I trial. EuroIntervention 2016, 12, 160–166. [Google Scholar] [CrossRef]
- Haude, M.; Ince, H.; Abizaid, A.; Toelg, R.; Lemos, P.A.; von Birgelen, C.; Christiansen, E.H.; Wijns, W.; Neumann, F.J.; Kaiser, C.; et al. Safety and performance of the second-generation drug-eluting absorbable metal scaffold in patients with de-novo coronary artery lesions (BIOSOLVE-II): 6 month results of a prospective, multicentre, non-randomised, first-in-man trial. Lancet 2016, 387, 31–39. [Google Scholar] [CrossRef]
- Jiang, W.; Tian, Q.; Vuong, T.; Shashaty, M.; Gopez, C.; Sanders, T.; Liu, H. Comparison study on four biodegradable polymer coatings for controlling magnesium degradation and human endothelial cell adhesion and spreading. ACS Biomater. Sci. Eng. 2017, 3, 936–950. [Google Scholar] [CrossRef]
- Johnson, I.; Akari, K.; Liu, H. Nanostructured hydroxyapatite/poly (lactic-co-glycolic acid) composite coating for controlling magnesium degradation in simulated body fluid. Nanotechnology 2013, 24, 375103. [Google Scholar] [CrossRef]
- Li, J.; Cao, P.; Zhang, X.; Zhang, S.; He, Y. In vitro degradation and cell attachment of a PLGA coated biodegradable Mg–6Zn based alloy. J. Mater. Sci. 2010, 45, 6038–6045. [Google Scholar] [CrossRef]
- Liu, J.; Wang, P.; Chu, C.C.; Xi, T. A novel biodegradable and biologically functional arginine-based poly (ester urea urethane) coating for Mg–Zn–Y–Nd alloy: Enhancement in corrosion resistance and biocompatibility. J. Mater. Chem. B 2017, 5, 1787–1802. [Google Scholar] [CrossRef] [PubMed]
- Hornberger, H.; Virtanen, S.; Boccaccini, A. Biomedical coatings on magnesium alloys—A review. Acta Biomater. 2012, 8, 2442–2455. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Fan, H.; Liu, Y.; Cao, L.; Wu, X.; Xu, X. Controllable biodegradability, drug release behavior and hemocompatibility of PTXeluting magnesium stents. Coll. Surf. B 2011, 83, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Li, F.Q.; Gu, Y.Q.; Hua, R.X.; Ni, Z.H.; Zhao, G.T. In vitro release study of sirolimus from a PDLLA matrix on a bioresorbable drug-eluting stent. J. Drug Deliv. Sci. Technol. 2018, 48, 88–95. [Google Scholar] [CrossRef]
- Chakravarty, K.; Dalal, D.C. An analytical study of drug release kinetics from a degradable polymeric matrix. Int. J. Biomath. 2018, 11, 1850011. [Google Scholar] [CrossRef]
- Zhang, H.J.; Li, X.D.; Deng, W.; Wang, X.F.; Wang, S.G.; Ge, J.B.; Toft, E. Drug release kinetics from a drug-eluting stent with asymmetrical coat HPLC. Front. Biosci. Landmark 2017, 22, 407–415. [Google Scholar]
- Li, J.; Wu, F.; Zhang, K.; He, Z.; Zou, D.; Luo, X.; Fan, Y.; Yang, P.; Zhao, A.; Huang, N. Controlling molecular weight of hyaluronic acid conjugated on amine-rich surface: Toward better multifunctional biomaterials for cardiovascular implants. ACS Appl. Mater. Interfaces 2017, 9, 30343–30358. [Google Scholar] [CrossRef]
- Qi, P.; Maitz, M.F.; Huang, N. Surface modification of cardiovascular materials and implants. Surf. Coat. Technol. 2013, 233, 80–90. [Google Scholar] [CrossRef]
- Luo, L.; Wang, G.; Li, Y.; Yin, T.; Jiang, T.; Ruan, C. Layer-by-layer assembly of chitosan and platelet monoclonal antibody to improve biocompatibility and release character of PLLA coated stent. J. Biomed. Mater. Res. Part A 2011, 97, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Menning, M. 2000, “Wet Coating Technologies for Glass,” SolGel.com. Available online: http://www.solgel.com/articles/nov00/mennig.htm (accessed on 28 September 2020).
- Ammam, M. Electrophoretic Deposition Under Modulated Electric Fields: A Review. RSC Adv. 2012, 2, 7633–7646. [Google Scholar] [CrossRef]
- Leong, K.; Langer, R. Polymeric controlled drug delivery. Adv. Drug Deliv. Rev. 1988, 1, 199–233. [Google Scholar] [CrossRef]
- Nukala, R.K.; Boyapally, H.; Slipper, I.J.; Mendham, A.P.; Douroumis, D. The Application of Electrostatic Dry Powder Deposition Technology to Coat Drug-Eluting Stents. Pharm. Res. 2010, 27, 72–81. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Acharya, G.; Shim, Y.B.; Choe, E.S.; Lee, C.H. Advanced Stent Coating for Drug Delivery and In Vivo Biocompatibility. J. Nanopar. Res. 2013, 15, 1962–1978. [Google Scholar] [CrossRef]
- Hagiwara, K.; Hasebe, T.; Hotta, A. Effects of Plasma Treatments on the Controlled Drug Release From Poly(Ethylene-co-Vinyl Acetate). Surf. Coat. Technol. 2013, 216, 318–323. [Google Scholar] [CrossRef]
- Shanshan, C.; Lili, T.; Yingxue, T.; Bingchun, Z.; Ke, Y. Study of Drug-Eluting Coating on Metal Coronary Stent, Mater. Sci. Eng. C 2013, 33, 1476–1480. [Google Scholar] [CrossRef]
- Martín del Valle, E.M.; Galan, M.A.; Carbonell, R.G. Drug delivery technologies: The way forward in the new decade. Ind. Eng. Chem. Res. 2009, 48, 2475–2486. [Google Scholar] [CrossRef]
- Chow, A.H.; Tong, H.H.; Chattopadhyay, P.; Shekunov, B.Y. Particle engineering for pulmonary drug delivery. Pharm. Res. 2007, 24, 411–437. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Peppas, N.A. Advances in biomaterials, drug delivery, and bionanotechnology. AIChE J 2003, 49, 2990–3006. [Google Scholar] [CrossRef]
- Nam, K.; Watanabe, J.; Ishihara, K. Modeling of swelling and drug release behavior of spontaneously forming hydrogels composed of phospholipid polymers. Int. J. Pharm. 2004, 275, 259–269. [Google Scholar] [CrossRef]
- Davis, S.S. Drug delivery systems. Interdiscip. Sci. Rev. 2000, 25, 175–183. [Google Scholar] [CrossRef]
- Ajili, S.H.; Ebrahimi, N.G.; Soleimani, M. Polyurethane/polycaprolactane blend with shape memory effect as a proposed material for cardiovascular implants. Acta Biomater. 2009, 5, 1519–1530. [Google Scholar] [CrossRef]
- Navarese, E.P.; Kowalewski, M.; Cortese, B.; Kandzari, D.; Dias, S.; Wojakowski, W.; Buffon, A.; Lansky, A.; Angelini, P.; Torguson, R.; et al. Short and long-term safety and efficacy of polymer-free vs. durable polymer drug-eluting stents. A comprehensive meta-analysis of randomized trials including 6178 patients. Atherosclerosis 2014, 233, 224–231. [Google Scholar] [CrossRef]
- Acharya, G.; Park, K. Mechanisms of controlled drug release from drug-eluting stents. Adv Drug. Deliv. Rev. 2006, 58, 387–401. [Google Scholar] [CrossRef]
- Thiruppathi, E.; Mani, G. Vitamin-c delivery from CoCr alloy surfacesusing polymer-free and polymer-based platforms for cardiovascular stent applications. Langmuir 2014, 30, 6237–6249. [Google Scholar] [CrossRef]
- Gallo, A.; Mani, G. A stent for co-delivering paclitaxel and nitric oxide from abluminal and luminal surfaces: Preparation, Surface characterization, and in vitro drug release studies. Appl Surf. Sci. 2013, 279, 216–232. [Google Scholar] [CrossRef]
- Mani, G.; Torres, N.; Oh, S. Paclitaxel delivery from cobalt-chromium alloy surfaces using self-assembled monolayers. Biointerphases 2011, 6, 33–42. [Google Scholar] [CrossRef]
- Lancaster, S.; Kakade, S.; Mani, G. Microrough cobalt–chromium alloy surfaces for paclitaxel delivery: Preparation, characterization, and in vitro drug release studies. Langmuir 2012, 28, 11511–11526. [Google Scholar] [CrossRef] [PubMed]
- Tsujino, I.; Ako, J.; Honda, Y.; Fitzgerald, P.J. Drug delivery via nano-, micro and macroporous coronary stent surfaces. Expert Opin. Drug Deliv. 2007, 4, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Wessely, R.; Hausleiter, J.; Michaelis, C.; Jaschke, B.; Vogeser, M.; Milz, S.; Behnisch, B.; Schratzenstaller, T.; Gluszko-Renke, M.; Stöver, M.; et al. Inhibition of neointima formation by a novel drug-eluting stent system that allows for dose-adjustable, multiple, and on-site stent coating. Arterioscler Thromb. Vasc. Biol. 2005, 25, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Morice, M.C.; Bestehorn, H.P.; Carrié, D.; Macaya, C.; Aengevaeren, W.; Wijns, W.; Dubois, C.; de Winter, R.; Verheye, S.; Hoffmann, S.; et al. Direct stenting of de novo coronary stenoses with tacrolimus-eluting versus carbon-coated carbostents. The randomized JUPITER II Trial. EuroIntervention 2006, 2, 45–52. [Google Scholar] [PubMed]
- Kakade, S.; Mani, G. A comparative study of the effects of vitamin C, sirolimus, and paclitaxel on the growth of endothelial and smooth muscle cells for cardiovascular medical device applications. Drug Des. Dev. Ther. 2013, 7, 529–543. [Google Scholar]
- Popat, A.; Hartono, S.B.; Stahr, F.; Liu, J.; Qiao, S.Z.; Lu, G.Q.M. Mesoporous silica nanoparticles for bioadsorption, enzyme immobilisation, and delivery carriers. Nanoscale 2011, 3, 2801–2818. [Google Scholar] [CrossRef]
- Vivero-Escoto, J.L.; Slowing, I.I.; Trewyn, B.G.; Lin, V.S.Y. Mesoporous silica nanoparticles for intracellular controlled drug delivery. Small 2010, 6, 1952–1967. [Google Scholar] [CrossRef]
- Zhang, L.; Shizhang, Q.; Yonggang, J.; Yang, H.; Budihartono, S.; Stahr, F.; Yan, Z.; Wang, X.; Hao, Z.; Lu, G.Q. Fabrication and size-selective bioseparation of magnetic silica nanospheres with highly ordered periodic mesostructure. Adv. Func. Mater. 2008, 18, 3203–3212. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Zhang, J.; Sun, W.; Zhang, R.; Gu, H. Fabrication of a novel polymer-free nanostructured drug-eluting coating for cardiovascular stents. ACS Appl. Mater. Interfaces 2013, 5, 10337–10345. [Google Scholar] [CrossRef]
- Alviar, C.L.; Tellez, A.; Wang, M.; Potts, P.; Smith, D.; Tsui, M.; Budzynski, W.; Raizner, A.E.; Kleiman, N.S.; Lev, E.I.; et al. Low-dose sirolimus-eluting hydroxyapatite coating on stents does not increase platelet activation and adhesion ex vivo. J. Thromb. Thrombolysis 2012, 34, 91–98. [Google Scholar] [CrossRef]
- Costa, J.R.; Abizaid, A.; Costa, R.; Feres, F.; Tanajura, L.F.; Abizaid, A.; Maldonado, G.; Staico, R.; Siqueira, D.; Sousa, A.G.M.R.; et al. 1-year results of the hydroxyapatite polymer-free sirolimus-eluting stent for the treatment of single de novo coronary lesions: The VESTASYNC I trial. JACC 2009, 2, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, H.M.; Chiu, Y.H.; Wu, T.Y.; Shen, J.K.; Lee, T.Y. Effects of through-hole drug reservoirs on key clinical attributes for drugeluting depot stent. Med. Eng. Phys. 2013, 35, 884–897. [Google Scholar] [CrossRef]
- Lee, S.J.; Jo, H.H.; Lim, K.S.; Lim, D.; Lee, S.; Lee, J.H.; Kim, W.D.; Jeong, M.H.; Lim, J.Y.; Kwon, I.K.; et al. Heparin coating on 3D printed poly (l-lactic acid) biodegradable cardiovascular stent via mild surface modification approach for coronary artery implantation. Chem. Eng. J. 2019, 378, 122116. [Google Scholar] [CrossRef]
- Lee, C.H.; Hsieh, M.J.; Chang, S.H.; Hung, K.C.; Wang, C.J.; Hsu, M.Y.; Juang, J.H.; Hsieh, I.C.; Wen, M.S.; Liu, S.J. Nanofibrous vildagliptin-eluting stents enhance re-endothelialization and reduce neointimal formation in diabetes: In vitro and in vivo. Int. J. Nanomed. 2019, 14, 7503–7513. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.G.; Kennedy, D.W. What is new and promising with drug-eluting stents in sinus surgery? Curr. Opin. Otolaryngol. Head Neck Surg. 2014, 22, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Parikh, A.; Anand, U.; Ugwu, M.C.; Feridooni, T.; Massoud, E.; Agu, R.U. Drug-eluting Nasal Implants: Formulation, Characterization, Clinical Applications and Challenges. Pharmaceutics 2014, 6, 249–267. [Google Scholar] [CrossRef]
- Tao, R.; Lu, L.; Zhang, R.; Hu, J.; Ni, J.; Shen, W. Triptolide inhibits rat vascular smooth muscle cell proliferation and cell cycle progression via attenuation of ERK1/2 and Rb phosphorylation. Exp. Mol. Pathol. 2011, 90, 137–142. [Google Scholar] [CrossRef]
- Lee, J.W.Y.; Lee, B.S.; Lee, J.Y.; Ku, H.J.; Jeon, S.R.; Kim, J.Y.; Ban, J.M.; Sung, S.H.; Shin, H.M.; Park, J.E. The herbal extract HMC05 inhibits neointima formation in balloon-injured rat carotid arteries: Possible therapeutic implications of HMC05. J. Ethnopharmacol. 2011, 133, 168–176. [Google Scholar] [CrossRef]
- Karki, R.; Jeon, E.R.; Kim, D.W. Magnoliae cortex inhibits intimal thickening of carotid artery through modulation of proliferation and migration of vascular smooth muscle cells. Food Chem. Toxicol. 2012, 50, 634–640. [Google Scholar] [CrossRef]
- Trzeciak, P. Drug eluting balloons-new weapon in the treatment of coronary artery disease? Choroby Serca Naczyń 2011, 8, 12–16. [Google Scholar]
- Serruys, P.W.; Kutryk, M.J.B.; Ong, A.T.L. Coronary stents. N. Engl. J. Med. 2006, 354, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Ellis, S.G.; Cox, D.A.; Hermiller, J.; O’Shaughnessy, C.; Mann, J.T.; Turco, M.; Caputo, R.; Bergin, P.; Greenberg, J.; et al. TAXUS-IV Investigators, A polymer-based, paclitaxel-eluting stentin patients with coronary artery disease. N. Engl. J. Med. 2004, 350, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.E.; Costa, M.A.; Abizaid, A.; Feres, F.; Seixas, A.C.; Tanajura, L.F.; Mattos, L.A.; Falotico, R.; Jaeger, J.; Popma, J.J.; et al. Four-year angiographic and intrava-scular ultrasound follow-up of patients treated with sirolimus-eluting stents. Circulation 2005, 111, 2326–2329. [Google Scholar] [CrossRef] [PubMed]
- Mauri, L.; Hsieh, W.H.; Massaro, J.M.; Ho, K.K.L.; D’Agostino, R.; Cutlip, D.E. Stent thrombosis in randomized clini-cal trials of drug-eluting-stents. N. Engl. J. Med. 2007, 356, 1010–1029. [Google Scholar] [CrossRef]
- Sanchis-Gomar, F.; Perez-Quilis, C.; Leischik, R.; Lucia, A. Epidemiology of coronary heart disease and acute coronary syndrome. Ann. Transl. Med. 2016, 4, 1–12. [Google Scholar] [CrossRef]
- Naghi, J.; Yalvac, E.; Pourdjabbar, A.; Ang, L.; Bahadorani, J.; Reeves, R.; Mahmud, E.; Patel, M. New developments in the clinical use of drug-coated balloon catheters in peripheral arterial disease Review. Med. Devices Evid. Res. 2016, 9, 161–174. [Google Scholar]
- Byrne, R.A.; Joner, M.; Alfonso, F.; Kastrati, A. Drug-coated balloon therapy in coronary and peripheral artery disease Review. Nat. Rev. 2014, 11, 13–23. [Google Scholar]
- Schorn, I.; Malinoff, H.; Anderson, S.; Lecy, C.; Wang, J.; Giorgianni, J.; Papandreou, G. The LUTONIX® drug-coated balloon: A novel drug delivery technology forthe treatment of vascular disease. Adv. Drug Deliv. Rev. 2017, 112, 78–87. [Google Scholar] [CrossRef]
- Cortesea, B.; Bertoletti, A. Paclitaxel coated balloons for coronary artery interventions: A comprehensive review of preclinical and clinical data. Int. J. Cardiol. 2012, 161, 4–12. [Google Scholar] [CrossRef]
- Loh, J.P.; Waksman, R. Paclitaxel Drug-Coated Balloons, A Review of Current Status and Emerging Applicationsin Native Coronary Artery De Novo Lesions. J. Am. Coll. Cardiol. Intv. 2012, 5, 1002–1012. [Google Scholar]
- Katsanos, K.; Kitrou, P.; Spiliopoulos, S.; Diamantopoulos, A.; Karnabatidis, D. Comparative Effectiveness of Plain Balloon Angioplasty, Bare Metal Stents, Drug-Coated Balloons, and Drug-Eluting Stents for the Treatment of Infrapopliteal Artery Disease: Systematic Review and Bayesian Network Meta-analysis of Randomized Controlled Trials. J. Endovasc. Ther. 2016, 6, 851–863. [Google Scholar]
- Zhang, J.; Xu, X.; Kong, J.; Xu, R.; Fan, X.; Chen, J.; Zheng, X.; Ma, B.; Sun, M.; Ye, Z.; et al. Systematic Review and Meta-Analysis of Drug-Eluting Balloon and Stent for Infrapopliteal Artery Revascularization. Vasc. Endovasc. Surg. 2017, 51, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, K.; Spiliopoulos, S.; Karunanithy, N.; Krokidis, M.; Sabharwal, T.; Taylor, P. Bayesian network meta-analysis of nitinol stents, covered stents, drug-eluting stents, and drug-coated balloons in the femoropopliteal artery. J. Vasc. Surg. 2014, 59, 1123–1133.e8. [Google Scholar] [CrossRef] [PubMed]
- Spiliopoulos, S.; Kamarinos, N.V.; Brountzos, E. Current evidence of drug-elution therapy for infrapopliteal arterial disease. Current evidence of drug-elution therapy for infrapopliteal arterial disease. World J. Cardiol. 2019, 11, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, J.; Zheng, C.; He, Y.; Jia, J.; Wang, X.; Li, D.; Shang, T.; Li, M. Drug-delivering endovascular treatment versus angioplasty in artery occlusion diseases: A systematic review and meta-analysis. Curr. Med. Res. Opin. 2018, 34, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Caradu, C.; Lakhlifi, E.; Colacchio, E.C.; Midy, D.; Bérard, X.; Poirier, M.; Ducasse, E. Systematic review and updated meta-analysis of the use of drug-coated balloon angioplasty versus plain old balloon angioplasty for femoropopliteal arterial disease. J. Vasc. Surg. 2019, 70, 981–995. [Google Scholar] [CrossRef]
- Yang, J.Q.; Peng, J.H.; Xu, T.; Liu, L.Y.; Tu, J.H.; Li, S.H.; Chen, H. Meta-analysis of the effects of drug-coated balloons among patients with small-vessel coronary artery disease. Medicine 2019, 98, e15797. [Google Scholar] [CrossRef]
- Li, M.; Guo, C.; Lv, Y.H.; Zhang, M.B.; Wang, Z.L. Drug-coated balloon versus drug-eluting stent in de novo small coronary vessel disease: A systematic review and meta-analysis. Medicine 2019, 98, e15622. [Google Scholar] [CrossRef]
- Kayssi, A.; Al-Jundi, W.; Papia, G.; Kucey, D.S.; Forbes, T.; Rajan, D.K.; Neville, R.; Dueck, A.D. Drug-eluting balloon angioplasty versus uncoated balloon angioplasty for the treatment of in-stent restenosis of the femoropopliteal arteries. Cochrane Database Syst. Rev. 2019, 1, CD012510. [Google Scholar] [CrossRef]
- Lindquist, J.; Schramm, K. Drug-Eluting Balloons and Drug-Eluting Stents in the Treatment of Peripheral Vascular Disease. Semin. Int. Radiol. 2018, 35, 443–452. [Google Scholar] [CrossRef]
- Mohiaddin, H.; Wong, T.D.F.K.; Burke-Gaffney, A.; Bogle, R.G. Drug-Coated Balloon-Only Percutaneous Coronary Intervention for the Treatment of De Novo Coronary Artery Disease: A Systematic Review. Cardiol. Ther. 2018, 7, 127–149. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.B.; Worme, M.; Yanagawa, B.; Kumar, N.; Buller, C.E.; Cheema, A.N.; Bagai, A. Treatment of Drug-Eluting Stent In-Stent Restenosis with Drug-Eluting Balloons: A Systematic Review and Meta-Analysis. J. Invasive Cardiol. 2018, 30, 360–366. [Google Scholar] [PubMed]
- Meneguz-Moreno, R.A.; Costa, J.R.; Abizaid, A. Drug-Coated Balloons: Hope or Hot Air: Update on the Role of Coronary DCB. Curr. Cardiol. Rep. 2018, 20, 100. [Google Scholar] [CrossRef] [PubMed]
- Kokkinidis, D.G.; Prouse, A.F.; Avner, S.J.; Lee, J.M.; Waldo, S.W.; Armstrong, E.J. Second-generation drug-eluting stents versus drug-coated balloons for the treatment of coronary in-stent restenosis: A systematic review and meta-analysis. Catheter. Cardiovasc. Interv. 2018, 92, 285–299. [Google Scholar] [CrossRef]
- Merinopoulos, I.; Gunawardena, T.; Wickramarachchi, U.; Ryding, A.; Eccleshall, S.; Vassiliou, V.S. Percutaneous Coronary Intervention in the Elderly: Are Drug-coated Balloons the Future? Curr. Cardiol. Rev. 2018, 14, 45–52. [Google Scholar] [CrossRef]
- Berg, M.C.; Kolodziej, H.; Cremers, B.; Gershony, G.; Speck, U. Drug-coated angioplasty balloon catheters: Coating compositions and methods. Adv. Eng. Mater. 2012, 14, B45–B50. [Google Scholar] [CrossRef]
- Levy, S.B. Balloon and Manufacture Thereof. US Patent Application No. 07/287,234, 25 December 1984. [Google Scholar]
- Lixiao, W.; Jianhua, C.N.L. Laminate Catheter Balloons with Additive Burst Strength and Methods for Preparation of Same. US Patent Specification US 6124007 A, 26 September 2000. [Google Scholar]
- Roorda, W.E. Biocompatible Carrier Containing a Bioadhesive Material. US Patent Specification US 8,728,510, 20 May 2014. [Google Scholar]
- Zou, W.; Cao, G.; Xi, Y.; Zhang, N. New approach for local delivery of rapamycin by bioadhesive PLGA-carbopol nanoparticles. Drug Deliv. 2009, 16, 15–23. [Google Scholar] [CrossRef]
- Pacetti, S.; Stankus, J.D. Coating on a Balloon Comprising a Polymer and a Drug. US Patent Specification US 8367090 B2, 5 February 2013. [Google Scholar]
- Radhakrishnan, R.; Larsen, S.; Schewe, S.; Feng, J.; Warner, R.; Folan, M.; Flanagan, A.; Clarke, J.; O’connor, T.; Malone, A. Drug Eluting Medical Device Utilizing Bioadhesives. US Patent Specification US 20120095396 A1, 19 April 2012. [Google Scholar]
- Gertz, Z.M.; Wilensky, R.L. Local Drug Delivery for Treatment of Coronary and Peripheral Artery Disease. Cardiovasc. Ther. 2011, 29, e54–e66. [Google Scholar] [CrossRef]
- Chang, H.G.; Azar, D.A.; Lyle, C.; Chitalia, V.C.; Shazly, T.; Kolachalama, V.B. Intrinsic coating morphology. Sci. Rep. 2019, 9, 6839. [Google Scholar] [CrossRef]
- Tepe, G.; Schnorr, B.; Albrecht, T.; Brechtel, K.; Claussen, C.D.; Scheller, B.; Speck, U.; Zeller, T. Angioplasty of Femoral-Popliteal Arteries with Drug-Coated Balloons: 5-Year Follow-Up of the THUNDER Trial. JACC Cardiovasc. Interv. 2015, 8, 102–108. [Google Scholar] [CrossRef]
- Turner, E.A.; Atigh, M.K.; Erwin, M.M.; Christians, U.; Yazdani, S.K. Coating and pharmacokinetic evaluation of air spray coated drug coated balloons. Cardiovasc. Eng. Technol. 2018, 9, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Cortese, B.; Micheli, A.; Picchi, A.; Coppolaro, A.; Bandinelli, L.; Severi, S.; Limbruno, U. Paclitaxel-coated balloon versus drug-eluting stent during PCI of small coronary vessels, a prospective randomized clinical trial. Heart 2010, 96, 1291–1296. [Google Scholar] [CrossRef] [PubMed]
- Scheinert, D.; Duda, S.; Zeller, T.; Krankenberg, H.; Ricke, J.; Bosiers, M.; Tepe, G.; Naisbitt, S.; Rosenfield, K. The LEVANT I (Lutonix paclitaxel-coated balloon for the prevention of femoropopliteal restenosis) trial for femoropopliteal revascularization: First-in-human randomized trial of low-dose drug-coated balloon versus uncoated balloon angioplasty. JACC Cardiovasc. Interv. 2014, 7, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Pósa, A.; Nyolczas, N.; Hemetsberger, R.; Pavo, N.; Petnehazy, O.; Petrasi, Z.; Sangiorgi, G.; Gyongyosi, M. Optimization of drug-eluting balloon use for safety and efficacy: Evaluation of the 2nd generation paclitaxel-eluting DIOR-balloon in porcine coronary arteries, Catheter. Cardiovasc. Interv. 2010, 76, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Scheller, B.; Speck, U.; Romeike, B.; Schmitt, A.; Sovak, M.; Böhm, M.; Stoll, H.P. Contrast media as carriers for local drug delivery Successful inhibition of neointimal proliferation in the porcine coronary stent model. Eur. Heart J. 2003, 24, 1462–1467. [Google Scholar] [CrossRef]
- Hehrlein, C.; Richardt, G.; Wiemer, M.; Schneider, H.; Naber, C.; Hoffmann, E.; Dietz, U. Description of Pantera Lux paclitaxel-releasing balloon and preliminary quantitative coronary angiography (QCA) results at six months in patients with coronary in-stent restenosis. EuroIntervention 2011, 7 (Suppl. K), K119–K124. [Google Scholar] [CrossRef]
- Schroeder, H.; Meyer, D.R.; Lux, B.; Ruecker, F.; Martorana, M.; Duda, S. Two-year results of a low-dose drug-coated balloon for revascularization of the femoropopliteal artery: Outcomes from the ILLUMENATE first-inhuman study. Catheter Cardiovasc. Interv. 2015, 86, 278–286. [Google Scholar] [CrossRef]
- Raval, A.; Parikh, J.; Engineer, C. Mechanism of Controlled Release Kinetics from Medical Devices. Braz. J. Chem. Eng. 2010, 27, 211–225. [Google Scholar] [CrossRef]
- Sternberg, K.; Kramer, S.; Nischan, C.; Grabow, N.; Langer, T.; Hennighausen, G.; Schmitz, K.P. In vitro study of drug-eluting stent coatings based on poly(L-lactide) incorporating cyclosporine A—Drug release, polymer degradation and mechanical integrity. J. Mater. Sci. Mater. Med. 2007, 18, 1423–1432. [Google Scholar] [CrossRef]
- Wang, L. Drug Releasing Coatings for Medical Devices. US Patent US9289539B22016, 22 March 2016. [Google Scholar]
- Ruebben, A.; Boeing, J.; Weiss, N. Usage of different vessel models in a flow-through cell: In vitro study of a novel coated balloon catheter. Int. Cardiol. Rev. 2011, 6, 56–57. [Google Scholar]
- Byrne, R.A.; Neumann, F.J.; Mehilli, J.; Pinieck, S.; Wolff, B.; Tiroch, K.; Schulz, S.; Fusaro, M.; Ott, I.; Ibrahim, T.; et al. Paclitaxel-eluting balloons, paclitaxel-eluting stents, and balloon angioplasty in patients with restenosis after implantation of a drug-eluting stent (ISAR-DESIRE 3): A randomised, open-label trial. Lancet 2013, 381, 461–467. [Google Scholar] [CrossRef]
- Berg, M.C.; Speck, T. Drug-Coated Medical Devices. US Patent Specification US 9,233,191, 12 January 2016. [Google Scholar]
- Stankus, J.; Trollsas, M.; Hossainy, S. Coatings with Tunable Solubility Profile for Drug-Coated Balloon. US Patent Application 8480620 B2, 9 July 2013. [Google Scholar]
- Paclitaxel-Eluting Balloon Essential Catheter by i Vascula. Available online: http://ivascular.global/coronary/essential/ (accessed on 28 September 2020).
- Speck, U. Formulations for Drug-Coated Medical Devices. US Patent Application 14/350,483, 16 August 2016. [Google Scholar]
- Borck, A. Balloon Catheter Having Coating. US Patent Specification 8,486,013, 16 July 2013. [Google Scholar]
- Gandhi, P.J.; Murthy, Z.V.P. Investigation of Different Drug Deposition Techniques on Drug Releasing Properties of Cardiovascular Drug Coated Balloons. Ind. Eng. Chem. Res. 2012, 51, 10800–10823. [Google Scholar] [CrossRef]
- Petersen, S.; Kaule, S.; Stein, F.; Minrath, I.; Schmitz, K.P.; Kragl, U.; Sternberg, K. Novel Paclitaxel-Coated Angioplasty Balloon Catheter Based on Cetylpyridinium Salicylate: Preparation, Characterization and Simulated Use in an in Vitro Vessel Model Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 4244–4250. [Google Scholar] [CrossRef] [PubMed]
- Amundson, R.R.; Hull, V.W.; Dror, M.; Schwartz, R.S. Method for Making a Drug Delivery Balloon Catheter. US Patent Specification 5,370,614, 6 December 1994. [Google Scholar]
- Hull, V.W.; Schwartz, R.S.; Dror, M. Releasable Microcapsules on Balloon Catheters. US Patent Specification 5,893,840, 13 April 1999. [Google Scholar]
- Palasis, M. Multi-Balloon Catheter with Hydrogel Coating. US Patent Specification 7,060,051, 13 June 2006. [Google Scholar]
- Christiansen, F.K. Drug Eluting Balloon. US Patent No. 8211055 B2, 3 July 2012. [Google Scholar]
- Speck, U. Lmus-Coated Medical Devices. US Patent Application 13/641,490, 25 April 2017. [Google Scholar]
- Orlowski, M. Shellac and Paclitaxel Coated Catheter Balloons. US Patent Application 13/266,059, 21 May 2019. [Google Scholar]
- Von Strandmann, R.P. Catheter Balloon Coated with Rapamycin and Shella. US Patent Application 14/113,953, 17 April 2014. [Google Scholar]
- Rowe, S.J. Ethod and Apparatus for Delivery Stokes of Therapeuticagent. US Patent Specification 6,616,650, 9 September 2003. [Google Scholar]
- Clarke, J.T.; Weber, J.; Flanagan, A. Drug-Delivery Balloons. US Patent Application 12/961,927, 30 June 2011. [Google Scholar]
- Weber, J.; Clarke, J.T.; Flanagan, A. Alloon Catheters with Fibers for Delivery of Therapeuticagent and Methods of Making the Same. US Patent Specification 9,227,041, 5 January 2016. [Google Scholar]
- Nordmann, A.J.; Briel, M.; Bucher, H.C. Mortality in randomizedcontrolled trials comparing drug-eluting vs. bare metal stents incoronary artery disease: A meta-analysis. Eur. Heart J. 2006, 27, 2784–2814. [Google Scholar] [CrossRef]
- Camenzind, E.; Steg, P.G.; Wijns, W. Stent thrombosis late afterimplantation of first-generation drug-eluting stents: A cause forconcern. Circulation 2007, 115, 1440–1455. [Google Scholar] [CrossRef]
- Lagerqvist, B.; James, S.K.; Stenestrand, U.; Lindbäck, J.; Nilsson, T.; Wallentin, L.; SCAAR Study Group. Long-term outcomeswith drug-eluting stents versus bare-metal stents in Sweden. N. Engl. J. Med. 2007, 356, 1009–1019. [Google Scholar] [CrossRef]
- Garg, S.; Serruys, P.W. Coronary stents: Looking forward. J. Am. Coll Cardiol. 2010, 56, S43–S78. [Google Scholar] [CrossRef]
- Silber, S.; Borggrefe, M.; Böhm, M.; Hoffmeister, H.M.; Dietz, R.; Ertl, G.; Heusch, G. Drug-eluting coronary stentsand drug eluting balloon catheters: Summary of the positionpapers of the DGK. Clin. Res. Cardiol. 2008, 97, 548–563. [Google Scholar]





| Authors | Year | Title | Abstract | Ref. |
|---|---|---|---|---|
| Chen et al. | 2016 | Coronary stent technology: a narrative review | A description of the evolution of coronary stent technology, the efficacy and safety of currently available devices, and the rationales for new-generation techniques in this domain. | [1] |
| Htay et al. | 2005 | Drug-Eluting Stent: A Review and Update | A summary of the recent development and progress of drug-eluting stents, followed by the results of their clinical trials. | [21] |
| Burt et al. | 2006 | Drug-eluting stents: A multidisciplinary success story | A comprehensive view of the disciplines related to the design and the development of drug-eluting stents, followed by a discussion on future directions in this domain. | [22] |
| Martin et al. | 2011 | Drug-eluting stents for coronary artery disease: A review | A review of both approved and most-promising proposals of drug-eluting stents. The study is a starting point for an indicator of the ways of the evolution of drug-eluting stents. | [23] |
| Doostzadeh et al. | 2010 | Recent progress in percutaneous coronary intervention: evolution of the drug-eluting stents focuses on the XIENCE V drug-eluting stent. | A discussion of clinical outcomes of drug-elution stents: clinical trials and development problems, design methods, and critical features, followed by an analysis of the future of this domain | [24] |
| Silber et al. | 2008 | Drug-eluting stents for diabetic patients. A critical appraisal of the currently available data from randomized trials | A review is summarizing the results of clinical trials and analysis for patients with coronary artery disease and parallel diabetosis. | [25] |
| Li et al. | 2011 | Recent developments in drug-eluting stents | A summary of recent developments of drug-eluting stents as a base for novel methods in the management of symptomatic coronary artery disease, followed by a discussion of problems associated with the usage of this technology. | [26] |
| Buchanan et al. | 2017 | Does the new generation of drug-eluting stents render bare-metal stents obsolete? | A review of the literature devoted to the safety and efficacy of drug-eluting stents and a comparison of this technique with bare-metal stents. | [27] |
| Fusaro et al. | 2013 | Drug-eluting stents for revascularization of infrapopliteal arteries: an updated meta-analysis of randomized trials | An updated meta-analysis of randomized trials investigating the outcomes of percutaneous revascularization with primary drug-eluting stenting in patients with atherosclerotic disease of infrapopliteal arteries. | [28] |
| Shlofmitz et al. | 2019 | Restenosis of Drug-Eluting Stents: A New Classification System Based on Disease Mechanism to Guide Treatment and State-of-the-Art Review | A new classification of in-stent restenosis by different mechanical, biological, and mixed etiologies, to enable individual treating of patients with drug-eluting stents to improve clinical outcome. | [29] |
| Wiesinger et al. | 2019 | Future developments in ureteral stents | A review of recent literature to summarize the most recent evidence on the use of ureteral stents, including the use of different materials and treatment of stent-related symptoms. | [30] |
| Lukman et al. | 2019 | Emerging of cardiovascular metal stent: A review on drug-eluting stent towards the utilization of herbal coating | A review of the utilization of various drugs as coating materials in identifying a possible alternative to overcome the current complications of DES. The discussion was divided into three sections: Stent; Commercial drug coating on DES; Herb coating on DES for cardiovascular application. | [31] |
| Wu et al. | 2019 | Polymer-free versus durable polymer drug-eluting stents in patients with coronary artery disease: A meta-analysis | A meta-analysis of randomized controlled trials to evaluate the safety and efficacy profiles of polymer-free drug-eluting stents compared with durable polymer drug-eluting stents. | [32] |
| Kommineni et al. | 2018 | Nonpolymer drug-eluting coronary stents | A review of nonpolymer drug-eluting stents loaded with different drugs like anti-inflammatory agents, antithrombotic, antiplatelet agents, immune suppressants, and others, followed by a description of surface modification techniques on stents like crystalline coating; microporous, macroporous, and nanoporous coatings; and chemically modified self-assembled monolayers. | [33] |
| Livingston et al. | 2019 | Coating Techniques and Release Kinetics of Drug-Eluting Stents | A review paper discusses recent drug-eluting stents designs utilizing individual or a combination of several coating techniques and their resulting drug-release profiles. | [34] |
| Authors | Year | Title | Abstract | Ref. |
|---|---|---|---|---|
| Mori et al. | 2017 | Revisiting the role of durable polymers in cardiovascular devices | Presentation and discussion of the problems related to the 1st generation DP-DES, areas of success and failure of the 2nd generation DP-DES, as well as a summary of the advantages and disadvantages of BP-DES. | [17] |
| Rizas et al. | 2016 | Stent Polymers: Do They Make a Difference? | A review of various permanent (biostable) and biodegradable polymers (BPs) that are used on DES platforms, followed by a discussion of needed features: biocompatibility, lack of interaction with the active drug, appropriate drug-eluting kinetics, biological inertion after the drug has been wholly eluted, and mechanical stability. | [52] |
| Stewart et al. | 2018 | Implantable Polymeric Drug Delivery Devices: Classification, Manufacture, Materials, and Clinical Applications Implantable Polymeric Drug Delivery Devices: Classification, Manufacture, Materials, and Clinical Applications | A classification of the implantable drug delivery devices, as well as a description of the drug-release mechanisms, followed by a discussion on materials and manufacture methods, and finally, examples of clinical applications. | [53] |
| Strohbach et al. | 2015 | Polymers for Cardiovascular Stent Coatings. Review | Discussion on the parameters of tissue and blood cell functions to be considered to evaluate the biocompatibility of stent polymers, especially towards biodegradable polymers; additionally, a summary of the methods to assess these parameters in certain physiological conditions. | [54] |
| Joseph et al. | 2018 | Biomedical applications of polyurethane materials and coatings | A review summarizes state-of-the-art from 2014 to 2018 in the domain of polyurethane materials and coatings and their biomedical applications, taking into account the biocompatibility, biodegradability, and tailorable chemical and physical forms. | [55] |
| Englert et al. | 2018 | Pharmapolymers in the 21st century: Synthetic polymers in drug delivery applications Pharmapolymers in the 21st century: Synthetic polymers in drug delivery applications | A summary of the classes of synthetic polymers and their applications in polymer-drug conjugates, excipients, and in nano- and macroscopic drug carriers as coatings and as drugs. | [56] |
| Trade Name. | Stent Platform | Polymer System | Drug | Drug Release (Days) | Manufacturer | Approval |
|---|---|---|---|---|---|---|
| Cypher® | SS | PEVA, PBMA, PCh | Sirolimus | 40% (5) 85% (30) 100% (90) | Cordis Corporation (Hialeah, FL) | FDA, CE |
| Taxus® | SS | Poly(styrene-b-isobutylene-b-styrene) | Paclitaxel | <10% (28) | Boston Scientific (Marlborough, MA) | FDA, CE |
| Promus PREMIERTM | Pt-Cr | PBMA, poly(vinylidene-co-hexafluoropropylene) | Everolimus | 71% (28) 100% (120) | Boston Scientific (Marlborough, MA) | FDA, CE |
| Xience V® | Co-Cr | PBMA, poly(vinylidene-co-hexafluoropropylene) | Everolimus | 80% (28) 100% (120) | Abbot Vascular (Chicago, IL) | FDA, CE |
| Endeavor® | Co-Cr | Phosphorylcholine polymer | Zotarolimus | 75% (2) 95% (15) 100% (28) | Metronic (Fridley, MN) | FDA, CE |
| Endeavor® Resolute | Co-Cr | Blend of PVP, poly(hexyl methacrylate)-co-PVP-co-PVAc, and PBMA-co-PVAc (BioLinx) | Zotarolimus | 50% (7) 70% (28) 100% (31) | Metronic (Fridley, MN) | FDA, CE |
| Firebird 2® | Co-Cr | Poly(styrene-butylene styrene) | Sirolimus | 50% (7) 90% (30) | Essen Technology (Beijing, China) | Phase IV NCT01257373 |
| Trade Name | Stent Platform | Polymer System | Drug | Drug Release (Days) | Manufacturer | Approval |
|---|---|---|---|---|---|---|
| SynergyTM | Pt-Cr | PLGA | Everolimus | (60) 50% (90) 100% | Boston Scientific (Marlborough, MA) | FDA, CE |
| AxxessTM | Nitinol | PLA | Biolimus A9 | (30) 45% | Biosensors (Irvine, CA) | CE |
| BioMatrix FlexTM | SS | PLA | Biolimus A9 | (30) 45% | Biosensors (Irvine, CA) | CE |
| Nobori® | SS | PLA | Biolimus A9 | (30) 45% | Terumo (Somerset, NJ) | CE |
| Supralimus® | SS | PLLA-PLGA-PCL-PVP | Sirolimus | (48) 100% | SMT (Mumbai, India) | CE |
| Orsiro | Co-Cr | PLLA + silicon carbide | Sirolimus | (30) 50% (90) 80% | Biotronik (Poznań, Poland) | CE |
| BioMimeTM | Co-Cr | PLLA + PLGA PLLA + PLGA | Sirolimus | (30) 100% | Meril (Gujarat, India) | CE |
| Inspiron® | Co-Cr | PLLA, PDLLGA PLLA, PDLLGA | Sirolimus | (10) 60% (45) 100% | SciTech Medical (Aparecida de Goiânia, Brasil) | Phase IV NCT01856088 |
| Firehawk® | Co-Cr | PDLLA | Sirolimus | (90) 90% | MicroPort Medica (Shanghai, China) | CE |
| DESyne® BD | Co-Cr | PLA | Novolimus M | (90) 90% | Elixir® (Milpitas, CA) | CE |
| MiStent SES® | Co-Cr | PLGA | Sirolimus | (270) 100% | Micell Technologies (Durham, SC) | CE |
| Tivoli® | Co-Cr | PLGA | Sirolimus | (7) 50% (28) 80% | Essen Technology (Beijing, China) | Phase III NCT02448524 |
| Structure | Products of Degradation | |
|---|---|---|
 |  | |
| polylactic acid (PLA) | lactic acid (LA) | |
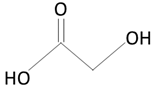
 | 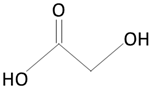 | |
| polyglycolic acid (PGA) | glycolic acid (GA) | |
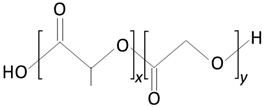 |  |  |
| poly(lactic-co-glycolic acid) (PLGA) | lactic acid (LA) | glycolic acid (GA) |
 | 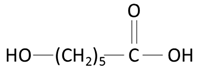 | |
| poly(caprolactone) (PCL) | caproic acid | |
 |  |  |
| poly(anhydride ester) | salicylic acid (SA) | sebacic acid |
| Material | E | σ | ε | Tg | Tmelt | Loss of Mech. Prop. | Total Degradation |
|---|---|---|---|---|---|---|---|
| (GPa) | (MPa) | (%) | (°C) | (°C) | (Months) | (Months) | |
| PLLA | 3.4–4.8 | 10–100 | 2–6 | 60–65 | 170–180 | 6 | 24–67 |
| PGA | 6.8–12.5 | 70–647 | min | 35–40 | 180–230 | 1–2 | 6–12 |
| PLGA (D/L/PLG) 85/15-50/50 | 2 | 20–50 | 3–10 | 45–55 | - | 1–4 | 2–6 |
| PCL | 0.3–0.4 | 16–23 | 300–700 | −60 | 59–64 | 0.8 | >34 |
| Trade Name | Stent Platform | Polymer System | Drug | Drug Release (DAYS) | Manufacturer | APPROVAL |
|---|---|---|---|---|---|---|
| AbsorbTM | PLLA | PDLLA | Evorolimus | (28) 80% | Abbot Vascular (Northbrook, IL) | FDA approval |
| DESolve® | PLLA | PLLA | NovolimusTM | (180–270) 100% | Elixir® (Milpitas, CA) | CE approval |
| Dreams I | Mg | PLGA | Paclitaxel | (90) 100% | Biotronik (Poznań, Poland) | Phase 0 NCT01168830 |
| Dreams II | Mg | PLLA | Sirolimus | n.a. | Biotronik (Poznań, Poland) | Phase 0 NCT01960504 |
| ReZolve2 | PTD-PC | n.a. | Sirolimus | (90)~100% | REVA (San Diego, CA) | Clinical study NCT01845311 |
| Antineoplastics and Anti-Inflammatory Immunomodulators | Antiproliferative | Migration Inhibitors and ECM Modulators | Enhanced Healing and Re-Endothelialization Factors |
|---|---|---|---|
| Sirolimus | QP-2, Taxol (Paclitaxel) | Batimastat | BCP671 |
| Tacrolimus | Actinomycin | Prolyl hydroxylase inhibitors | VEGF |
| Everolimus | Methotrexate | Halofuginone | Estradiols |
| Leflunomide | Angiopeptin | C-proteinase inhibitors | NO donor compounds |
| M-Prednisolone | Vincristine | Probucol | EPC antibodies |
| Dexamethasone | Mitomycin | Biorest | |
| Interferon r-1b | Statins | ||
| Mycophenolic acid | C-myc antisense | ||
| Mizoribine | Abbott ABT-578 | ||
| Cyclosporine | Resten ASE | ||
| Tranilast | 2-chloro-deoxyadenosine PCNA ribozyme |
| Tacrolimus | Present I–III | Preliminary Safety Evaluation of Nanoporous Tacrolimus-Eluting Stents |
|---|---|---|
| EVIDENT | The endovascular investigation determining the safety of new tacrolimus-eluting stent grafts. | |
| Everolimus | FUTURE I–IV | First used to underscore the reduction in restenosis with everolimus. |
| SPIRITS-FIRST | ||
| M-Prednisolone | IMPRESS | Immunosuppressive therapy for the prevention of restenosis after coronary artery stent implantation. |
| Dexamethasone | STRIDE | The study of antirestenosis with a BiodivYsio dexamethasone-eluting stent. |
| EMPEROR | Evaluation of the 9α-F-16 methylprednisolone (dexamethasone)-eluting stent on the reduction of restenosis. | |
| DESIRE | Dexamethasone-eluting stent, Italian registry. | |
| SAFE | Sorin and aspirin following elective stenting. | |
| Mycophenolic acid | IMPACT | Inhibition with MPA of a coronary restenosis trial. |
| Batimastat | BATMAN | BiodivYsio batimastat SV stent versus balloon angioplasty for the reduction of restenosis in small coronary arteries. |
| BRILLIANT | Batimastat (BB-94) antirestenosis trial utilizing the BiodivYsio local drug delivery PC stent. | |
| Actinomycin | ACTION | Recruitment in the actinomycin-eluting stent improves outcomes by reducing neointimal hyperplasia. |
| Angiopeptin | SWAN | Stent with angiopeptin. |
| Medtronic ABT-578 | ENDEAVOR I–III | A randomized controlled trial to evaluate the safety and efficacy of the Medtronic AVE ABT-578- eluting driverTM coronary stent in de novo native coronary artery lesions |
| Abbott ABT-578 | Zomaxx 1 | Zomaxx coronary drug-eluting stent for de novo lesion in coronary arteries. |
| Estradiols | EASTER | Estrogen and stent to eliminate restenosis. |
| NO donor compounds | NOBLESSE | Nitric oxide through a biodegradable layer elective study for safety and efficacy. |
| EPC antibodies | HEALING I–II | Healthy endothelial accelerated lining inhibits neointimal growth. |
| Authors | Year | Title | Abstract | Ref |
|---|---|---|---|---|
| Borhani et al. | 2018 | Cardiovascular stents: overview, evolution, and next generation. Review | A discussion on different techniques for stent design, mainly based on recent advances in drug-eluting stents. | [2] |
| Bukka et al. | 2018 | Drug-eluting balloon: design, technology and clinical aspects. Topical review | A review and discussion of the evolution, rationale, and comparison of the drug-eluting balloons currently available on the market, with a comparison of different coating techniques. | [14] |
| Naghi et al. | 2016 | New developments in the clinical use of drug-coated balloon catheters in peripheral arterial disease | A review summarizes currently available clinical data on the application of drug-coated balloons, followed by a presentation of new paclitaxel drug-coated balloons. | [173] |
| Byrne et al. | 2013 | Drug-coated balloon therapy in coronary and peripheral artery disease Review | A review of the clinical applications of balloons coated with drugs in the treatment of coronary and peripheral artery disease. | [174] |
| Schorn et al. | 2017 | The LUTONIX® drug-coated balloon: A novel drug delivery technology for the treatment of vascular disease | A review summarizes the development of the LUTONIX® drug-coated balloon catheter. | [175] |
| Cortesea et al. | 2012 | A comprehensive review of preclinical and clinical data | A review of specific parameters of the paclitaxel-coated balloons for the treatment of coronary artery disease. | [176] |
| Loh et al. | 2012 | Paclitaxel Drug-Coated Balloons A Review of Current Status and Emerging Applications Native Coronary Artery De Novo Lesions | A review of the role of drug-coated balloon DCB in de novo coronary lesions based on clinical evidence. | [177] |
| Katsanos et al. | 2016 | Comparative Effectiveness of Plain Balloon Angioplasty, Bare Metal Stents, Drug-Coated Balloons, and Drug-Eluting Stents for the Treatment of Infrapopliteal Artery Disease: Systematic Review and Bayesian Network Meta-analysis of Randomized Controlled Trials. | A meta-analysis of randomized controlled trials comparing bare-metal stents, paclitaxel-coated balloons, and drug-eluting stents with balloon angioplasty or with each other in the infrapopliteal arteries. | [178] |
| Zhang et al. | 2017 | Systematic Review and Meta-Analysis of Drug-Eluting Balloon and Stent for Infrapopliteal Artery Revascularization. | A review and meta-analysis of the current available studies investigating outcomes of drug-eluting balloons and drug-eluting stents in the treatment of infrapopliteal artery disease. | [179] |
| Katsanos et al. | 2014 | Bayesian network meta-analysis of nitinol stents, covered stents, drug-eluting stents, and drug-coated balloons in the femoropopliteal artery. | A meta-analysis of randomized controlled trials comparing bare nitinol stents, covered nitinol stents, paclitaxel- or sirolimus-eluting stents, and paclitaxel-coated balloons with plain balloon angioplasty or with each other. | [180] |
| Spiliopoulos et al. | 2019 | Current evidence of drug-elution therapy for infrapopliteal arterial disease. | A review summarizes and discussing data related to the application of infrapopliteal drug-elution devices and their future perspectives. | [181] |
| Chen et al. | 2018 | Drug-delivering endovascular treatment versus angioplasty in artery occlusion diseases: a systematic review and meta-analysis. | A comparison of the efficacy of drug-coated balloons and drug-eluting stents with percutaneous transluminal angioplasty in patients with femoropopliteal or infrapopliteal arterial occlusive disease. | [182] |
| Wua et al. | 2019 | Is There a Safety Concern for Drug-Coated Balloons in Peripheral Arterial Disease? | A description of the evolution of endovascular therapy for peripheral arterial disease, with highlights regarding the recent debates on the long-term safety of the drug-coated devices for the treatment of this disease. | [32] |
| Caradu et al. | 2019 | Systematic Review and updated meta-analysis of the use of drug-coated balloon angioplasty versus plain old balloon angioplasty for femoropopliteal arterial disease | A review of the use of drug-coated balloons in the management of femoropopliteal disease and a comparison of this technique with plain old balloon angioplasty. | [183] |
| Yang et al. | 2019 | A meta-analysis of the effects of drug-coated balloons among patients with small-vessel coronary artery disease | Clinical evaluation of drug-coated balloons for patients with small-vessel coronary artery disease. | [184] |
| Li et al. | 2019 | Drug-coated balloon versus drug-eluting stent in de novo small coronary vessel disease A systematic review and meta-analysis | A discussion on the safety and efficacy of the drug-coated balloons and the drug-eluting stents. | [185] |
| Kayssi et al. | 2019 | Drug-eluting balloon angioplasty versus uncoated balloon angioplasty for the treatment of in-stent restenosis of the femoropopliteal arteries | A description of the role of drug-eluting technologies, such as drug-eluting balloons, in the management of in-stent restenosis, summarized by the analysis of the efficacy of drug-eluting balloons compared with conventional uncoated balloon angioplasty in people with in-stent restenosis of the femoropopliteal arteries. | [186] |
| Lindquist et al. | 2018 | Drug-Eluting Balloons and Drug-Eluting Stents in the Treatment of Peripheral Vascular Disease | A review of the usage of drug-eluting technologies in the applications addressing the peripheral arterial system. | [187] |
| Mohiaddin et al. | 2018 | Drug-Coated Balloon-Only Percutaneous Coronary Intervention for the Treatment of De Novo Coronary Artery Disease: A Systematic Review | A review of stentless a drug-coated-balloon-only angioplasty in de novo coronary artery disease, comparing more than 40 studies examining the effects of drug-coated-balloon-only percutaneous coronary intervention in a variety of clinical scenarios, including small vessels, bifurcations, calcified lesions, and primary percutaneous coronary intervention. | [188] |
| Liuet al. | 2018 | Treatment of Drug-Eluting Stent In-Stent Restenosis With Drug-Eluting Balloons: A Systematic Review and Meta-Analysis | A description of the application of drug-eluting stents in the treatment of in-stent restenosis, with the outcome of investigating the death, myocardial infarction, and target-lesion revascularization at longest available follow-up (up to 3 years). | [189] |
| Meneguz-Moreno et al. | 2018 | Drug-Coated Balloons: Hope or Hot Air: Update on the Role of Coronary DCB | A review of applications of drug-coated balloons with the clinical evidence, to enumerate profits, especially in the implementation of bare-metal stents and drug-eluting stents in-stent restenosis. | [190] |
| Kokkinidis et al. | 2018 | Second-generation drug-eluting stents versus drug-coated balloons for the treatment of coronary in-stent restenosis: A systematic review and meta-analysis | A comparison of second-generation drug-eluting stents and drug-coated balloons for the treatment of coronary artery in-stent restenosis. | [191] |
| Merinopouloset al. | 2018 | Percutaneous Coronary Intervention in the Elderly: Are Drug-coated Balloons the Future? | A review and history of percutaneous coronary intervention and the application of drug-coated balloons, especially within the elderly population. | [192] |
| Material | Tensile Stress | Compliance | Maximum Sustainable Pressure |
|---|---|---|---|
| (104 psi) | (%) | (atm) | |
| Polyethylene terephthalate (PET) | 3–6 | Low compliance (0–10) | 18–27 |
| Nylon | 2–4 | Semi-compliant (5–15) | 5–18 |
| Polyethylene with additives | 1 | Compliant (>15) | 10 |
| Polyvinyl chloride (PVC) | <1 | Compliant (>15) | 6–8 |
| Polyurethane used along with nylon | 1–2 | Semi-compliant (5–15) | 10 |
| Class of Therapeutic Agent | Examples | Mechanism of Action |
|---|---|---|
| Antiplatelet | Aspirin, clopidogrel | Reduces blood clotting |
| Anti-inflammatory | Glucocorticoids, betamethasone, dexamethasone prednisolone | Inhibits monocyte and macrophage function and influences smooth muscle cell proliferation |
| Antihyperlipidemic | Statins (simvastatin, pravastatin), probucol | Decreases blood cholesterol level |
| Antiproliferative | Taxanes (paclitaxel docetaxel) limus (sirolimus, everolimus, tacrolimus) | Inhibits the G1 or G2 phase and the proliferation of cells |
| Cytotoxic antibiotics | Actinomycin-D | Inhibits the G1 phase and the proliferation of cells |
| Antithrombogenic agents | Heparin, urokinase | Prevents the formation of thrombin |
| Excipient | Role | Ref. | |
|---|---|---|---|
| Bioadhesives | Amino acids (DOPA) | To deliver the therapeutic agent from the vehicle and gain adhesion to the vessel | [142,146] |
| Adhesive surface proteins (microbial surface components recognizing adhesive matrix molecules) | To adhere to the lesion site, produced by pathogens | ||
| Polymer materials (polysaccharides, alginic acid, PVA, etc.) | To deliver the therapeutic agent from the vehicle and gain adhesion to the vessel | ||
| Minigel particles (poly(N-iso propyl acrylamide)) | To gain adhesion to the vessel at body temperature and behave as a liquid | ||
| Endothelial cell stimulant (mono- and disaccharides and polymers) | Promotes the uptake of a therapeutic agent when it comes into contact with endothelial cells lining the vasculature | ||
| Hydrophilic carriers | Shellac | To increase the adhesion of paclitaxel, considered as a superior excipient in binding paclitaxel | [146,218] |
| Urea | To increase homogenous delivery of a drug | [146] | |
| Iopramide | To monitor balloon movements, a radio spacer agent used in angiography | [146,148] | |
| Butyryl-trihexyl citrate | Hydrophilic carrier in binding a lipophilic drug to the vessel wall | [146] | |
| Lipophilic lubricant | C6-C30 magnesium, zinc, calcium, or ammonium Monocarboxylic acid salt, talc, or magnesium stearate | To decrease the frictional force between the polymer layer and balloon and to increase drug binding to the balloon | [150] |
| Antioxidants | Butylated hydroxyl toluene | To prevent the decomposition of the drug through oxidation and promote adherence of medicine to the balloon | [219] |
| Balloon | Application | Clinical trials | Groups | CE Approval | FDA Approval |
|---|---|---|---|---|---|
| PACCOCATH® COTAVANCETM MEDRADIN, USA | Infrainguinal and iliac arteries | ISR I | PACCOCATH® DEB versus uncoated DEB | Yes, 2011 | No |
| ISR II (12 months) | |||||
| FEMPAC | Uncoated balloon, DEB | ||||
| THUNDER | Plain balloon, DEB, a drug with contrast media | ||||
| DIOR®, Eurocor GmbH, Germany | Coronary arteries | DIOR Registry | DEB after BMS-ISR, DES-ISR | No | No |
| Small coronary arteries | DEBUIT | DEB after BMS, DEB | YES, 2007 for coating | ||
| PICCOLETO | DES (versus) DEB | ||||
| VALENTINES II | Lesions with DEB | ||||
| DEB-AMI | DEB+BMS, BMS, Taxus® DES | ||||
| IN.PACT™Admiral™, Medtronic, USA | Peripheral artery diseases (upper leg) | IN.PACT™ SFA I | DEB (versus) plain balloon | Yes, 2009 | PMA 2015 |
| SeQuent® Please, B. Braun Vascular Systems, Germany | Coronary artery and small vessels | PEPCAD I | Sequent™Please (versus) Sequent™Please+BMS | Yes, 2009 | No |
| PEPCAD II | Sequent™Please (versus) Taxus® | ||||
| PEPCAD III | Sequent™Please + Coroflex™ DEBlue (versus) Cypher® | ||||
| PEPCAD IV | Sequent™Please (versus) Coroflex™DEBlue and Taxus® | ||||
| PEPCAD V | Sequent™Please (versus) Coroflex™DEBlue and Taxus | ||||
| PEPCAD DES | Sequent™Please (versus) plain balloon | ||||
| PEPCADCTO | Sequent™Please+BMS (versus) Taxus® | ||||
| INDICOR | BMS after Sequent™Please (versus) Sequent™Please after BMS | ||||
| PERFECT | Sequent™Please + EPC stent (versus) EPC stent | ||||
| ISAR DESIRE | Sequent™Please, DES, uncoated balloon |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rykowska, I.; Nowak, I.; Nowak, R. Drug-Eluting Stents and Balloons—Materials, Structure Designs, and Coating Techniques: A Review. Molecules 2020, 25, 4624. https://doi.org/10.3390/molecules25204624
Rykowska I, Nowak I, Nowak R. Drug-Eluting Stents and Balloons—Materials, Structure Designs, and Coating Techniques: A Review. Molecules. 2020; 25(20):4624. https://doi.org/10.3390/molecules25204624
Chicago/Turabian StyleRykowska, I., I. Nowak, and R. Nowak. 2020. "Drug-Eluting Stents and Balloons—Materials, Structure Designs, and Coating Techniques: A Review" Molecules 25, no. 20: 4624. https://doi.org/10.3390/molecules25204624
APA StyleRykowska, I., Nowak, I., & Nowak, R. (2020). Drug-Eluting Stents and Balloons—Materials, Structure Designs, and Coating Techniques: A Review. Molecules, 25(20), 4624. https://doi.org/10.3390/molecules25204624





