Naturally Occurring Isocoumarins Derivatives from Endophytic Fungi: Sources, Isolation, Structural Characterization, Biosynthesis, and Biological Activities
Abstract
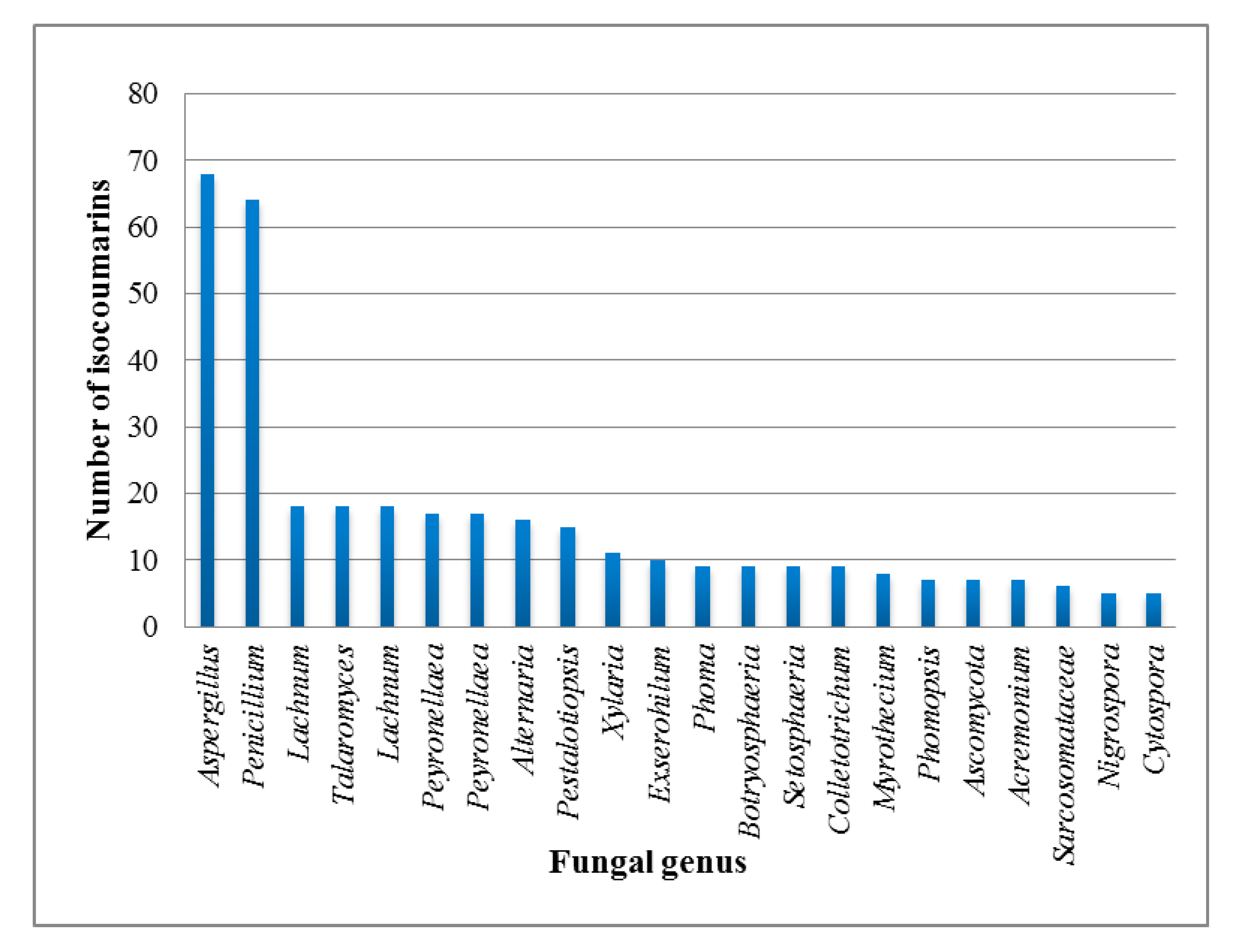
1. Introduction
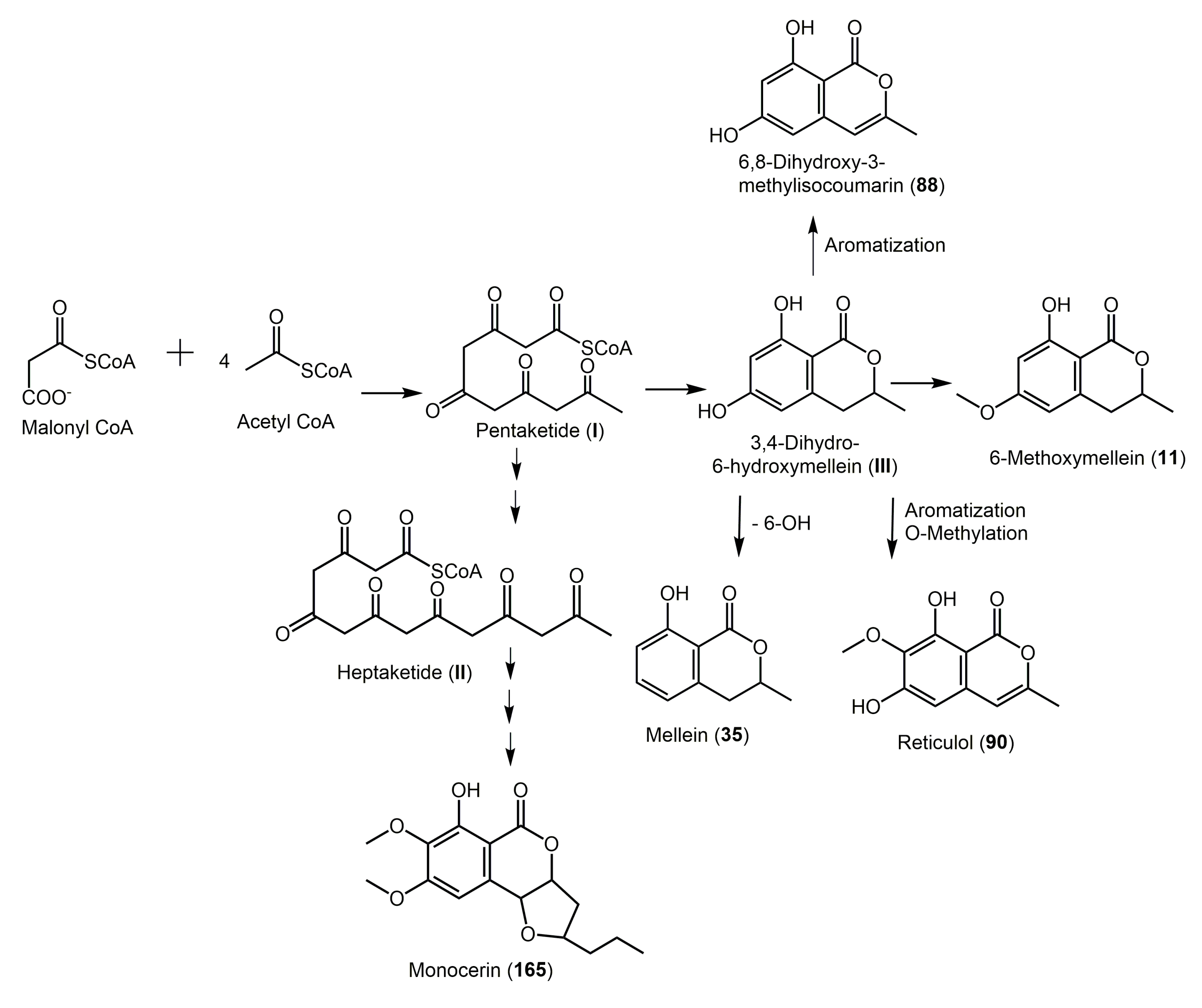
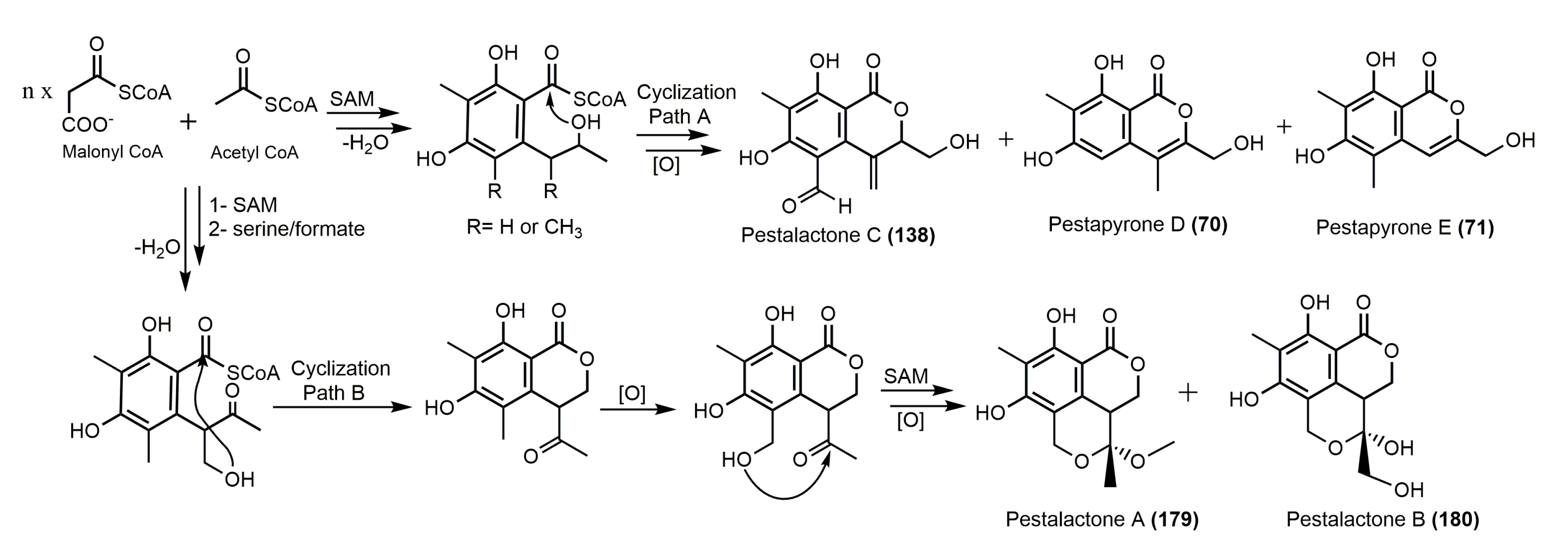
2. Biosynthesis
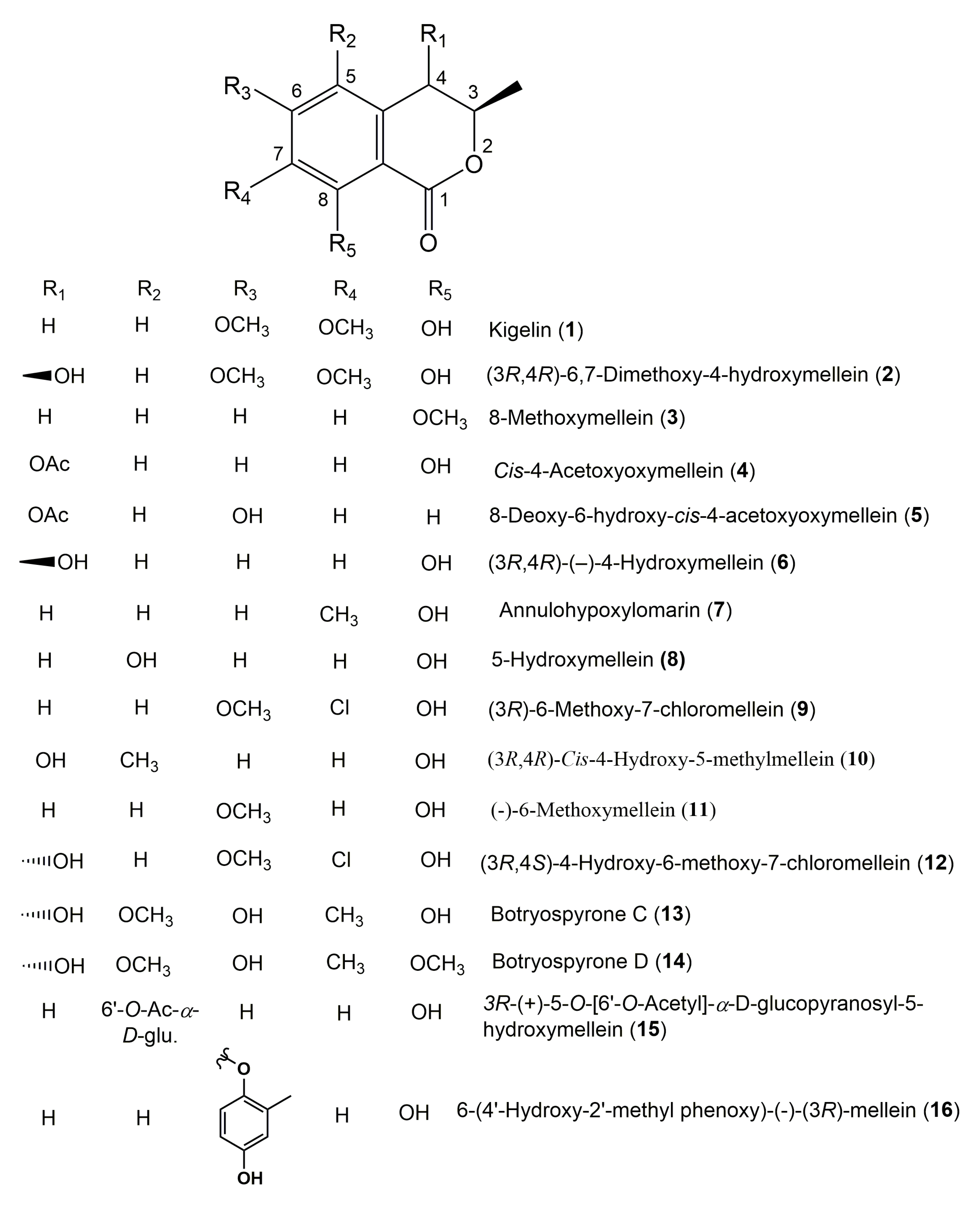
3. Structural Characterization of Isocoumarins Derivatives
4. Methods of Extraction and Purification of Isocoumarins Derivatives
5. Biological Activities
5.1. Antimicrobial Activity
5.2. Cytotoxic Activity
5.3. Antioxidant Activity
5.4. α-Glucosidase, Acetylcholinesterase (AChE), and Protein Kinase Inhibitory Activities
5.5. Anti-Inflammatory Activity
5.6. Anti-Mycobacterial, Antiplasmodial, Antiviral, and Insecticidal Activities
5.7. Other Biological Activities
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Ibrahim, S.R.; Abdallah, H.M.; Mohamed, G.A.; Ross, S.A. Integracides HJ: New tetracyclic triterpenoids from the endophytic fungus Fusarium sp. Fitoterapia 2016, 112, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.; Mohamed, G.A.; Khedr, A.I. γ-Butyrolactones from Aspergillus species: Structures, biosynthesis, and biological activities. Nat. Prod. Commun. 2017, 12, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.; Elkhayat, E.S.; Mohamed, G.A.; Khedr, A.I.; Fouad, M.A.; Kotb, M.H.; Ross, S.A. Aspernolides F and G, new butyrolactones from the endophytic fungus Aspergillus terreus. Phytochem. Lett. 2015, 14, 84–90. [Google Scholar] [CrossRef]
- Ibrahim, S.R.; Mohamed, G.A.; Moharram, A.M.; Youssef, D.T. Aegyptolidines A and B: New pyrrolidine alkaloids from the fungus Aspergillus aegyptiacus. Phytochem. Lett. 2015, 12, 90–93. [Google Scholar] [CrossRef]
- Ibrahim, S.R.; Mohamed, G.A.; Al Haidari, R.A.; El-Kholy, A.A.; Zayed, M.F. Potential anti-malarial agents from endophytic fungi: A review. Mini Rev. Med. Chem. 2018, 18, 1110–1132. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.; Mohamed, G.A.; Al Haidari, R.A.; El-Kholy, A.A.; Zayed, M.F.; Khayat, M.T. Biologically active fungal depsidones: Chemistry, biosynthesis, structural characterization, and bioactivities. Fitoterapia 2018, 129, 317–365. [Google Scholar] [CrossRef]
- Tan, R.X.; Zou, W.X. Endophytes: A rich source of functional metabolites. Nat. Prod. Rep. 2001, 18, 448–459. [Google Scholar] [CrossRef]
- Gunatilaka, A.L. Natural products from plant-associated microorganisms: Distribution, structural diversity, bioactivity, and implications of their occurrence. J. Nat. Prod. 2006, 69, 509–526. [Google Scholar] [CrossRef]
- Ibrahim, S.R.; Mohamed, G.A.; Ross, S.A. Integracides F and G: New tetracyclic triterpenoids from the endophytic fungus Fusarium sp. Phytochem. Lett. 2016, 15, 125–130. [Google Scholar] [CrossRef]
- Elkhayat, E.S.; Ibrahim, S.R.; Mohamed, G.A.; Ross, S.A. Terrenolide S, a new antileishmanial butenolide from the endophytic fungus Aspergillus terreus. Nat. Prod. Res. 2016, 30, 814–820. [Google Scholar] [CrossRef]
- Saeed, A.; Qasim, M. Total synthesis of cytotoxic metabolite (±)-desmethyldiaportinol from Ampelomyces sp. Nat. Prod. Res. 2014, 28, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Chatare, V.; Pal, M. Isocoumarin and its derivatives: An overview on their synthesis and applications. Curr. Org. Chem. 2011, 15, 782–800. [Google Scholar] [CrossRef]
- Pal, S.; Pal, M. Isocoumarin, Thiaisocoumarin and Phosphaisocoumarin: Natural Occurrences, Synthetic Approaches and Pharmaceutical Applications; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Lutz-Kutschera, G.; Engelmeier, D.; Hadacek, F.; Werner, A.; Greger, H.; Hofer, O. Synthesis of side chain substituted 3-butylisocoumarins and absolute configurations of natural isocoumarins from Artemisia dracunculus. Monatsh. Chem. 2003, 134, 1195–1206. [Google Scholar] [CrossRef]
- Engelmeier, D.; Hadacek, F.; Hofer, O.; Lutz-Kutschera, G.; Nagl, M.; Wurz, G.; Greger, H. Antifungal 3-butylisocoumarins from asteraceae-anthemideae. J. Nat. Prod. 2004, 67, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Barry, R. Isocoumarins. Development since 1950. Chem. Rev. 1964, 64, 229–260. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Regasini, L.O.; Silva, G.H.; Pfenning, L.H.; Young, M.C.; Berlinck, R.G.; Bolzani, V.S.; Araujo, A.R. Dihydroisocoumarins produced by Xylaria sp. and Penicillium sp., endophytic fungi associated with Piper aduncum and Alibertia macrophylla. Phytochem. Lett. 2011, 4, 93–96. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Silva, G.H.; Regasini, L.O.; Zanardi, L.M.; Evangelista, A.H.; Young, M.C.; Bolzani, V.S.; Araujo, A.R. Bioactive metabolites produced by Penicillium sp. 1 and sp. 2, two endophytes associated with Alibertia macrophylla (Rubiaceae). Z. Naturforsch. C 2009, 64, 824–830. [Google Scholar] [CrossRef]
- Kuramata, M.; Fujioka, S.; Shimada, A.; Kawano, T.; Kimura, Y. Citrinolactones A, B and C, and sclerotinin C, plant growth regulators from Penicillium citrinum. Biosci. Biotechnol. Biochem. 2007. [Google Scholar] [CrossRef]
- Krohn, K.; Flörke, U.; Rao, M.S.; Steingröver, K.; Aust, H.-J.; Draeger, S.; Schulz, B. Metabolites from fungi 15. New isocoumarins from an endophytic fungus isolated from the Canadian thistle Cirsium arvense. Nat. Prod. Lett. 2001, 15, 353–361. [Google Scholar] [CrossRef]
- Wu, C.; Zhu, H.; van Wezel, G.P.; Choi, Y.H. Metabolomics-guided analysis of isocoumarin production by Streptomyces species MBT76 and biotransformation of flavonoids and phenylpropanoids. Metabolomics 2016, 12, 90. [Google Scholar] [CrossRef][Green Version]
- Song, R.-Y.; Wang, X.-B.; Yin, G.-P.; Liu, R.-H.; Kong, L.-Y.; Yang, M.-H. Isocoumarin derivatives from the endophytic fungus, Pestalotiopsis sp. Fitoterapia 2017, 122, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Kurosaki, F.; Kizawa, Y.; Nishi, A. Biosynthesis of dihydroisocoumarin by extracts of elicitor-treated carrot root. Phytochemistry 1989, 28, 1843–1845. [Google Scholar] [CrossRef]
- Turner, W.B. Fungal Metabolites; Academic Press: London, UK; New York, NY, USA, 1971. [Google Scholar]
- Scott, F.E.; Simpson, T.J.; Trimble, L.A.; Vederas, J.C. Biosynthesis of monocerin. Incorporation of 2 H-, 13 C-, and 18 O-labelled acetates by Drechslera ravenelii. J. Chem. Soc. Chem. Commun. 1984, 756–758. [Google Scholar] [CrossRef]
- Liu, S.-S.; Jiang, J.-X.; Huang, R.; Wang, Y.-T.; Jiang, B.-G.; Zheng, K.-X.; Wu, S.-H. A new antiviral 14-nordrimane sesquiterpenoid from an endophytic fungus Phoma sp. Phytochem. Lett. 2019, 29, 75–78. [Google Scholar] [CrossRef]
- Krohn, K.; Sohrab, M.H.; Aust, H.-J.; Draeger, S.; Schulz, B. Biologically active metabolites from fungi, 19: New isocoumarins and highly substituted benzoic acids from the endophytic fungus, Scytalidium sp. Nat. Prod. Res. 2004, 18, 277–285. [Google Scholar] [CrossRef]
- Prompanya, C.; Fernandes, C.; Cravo, S.; Pinto, M.; Dethoup, T.; Silva, A.; Kijjoa, A. A new cyclic hexapeptide and a new isocoumarin derivative from the marine sponge-associated fungus Aspergillus similanensis KUFA 0013. Mar. Drugs 2015, 13, 1432–1450. [Google Scholar] [CrossRef]
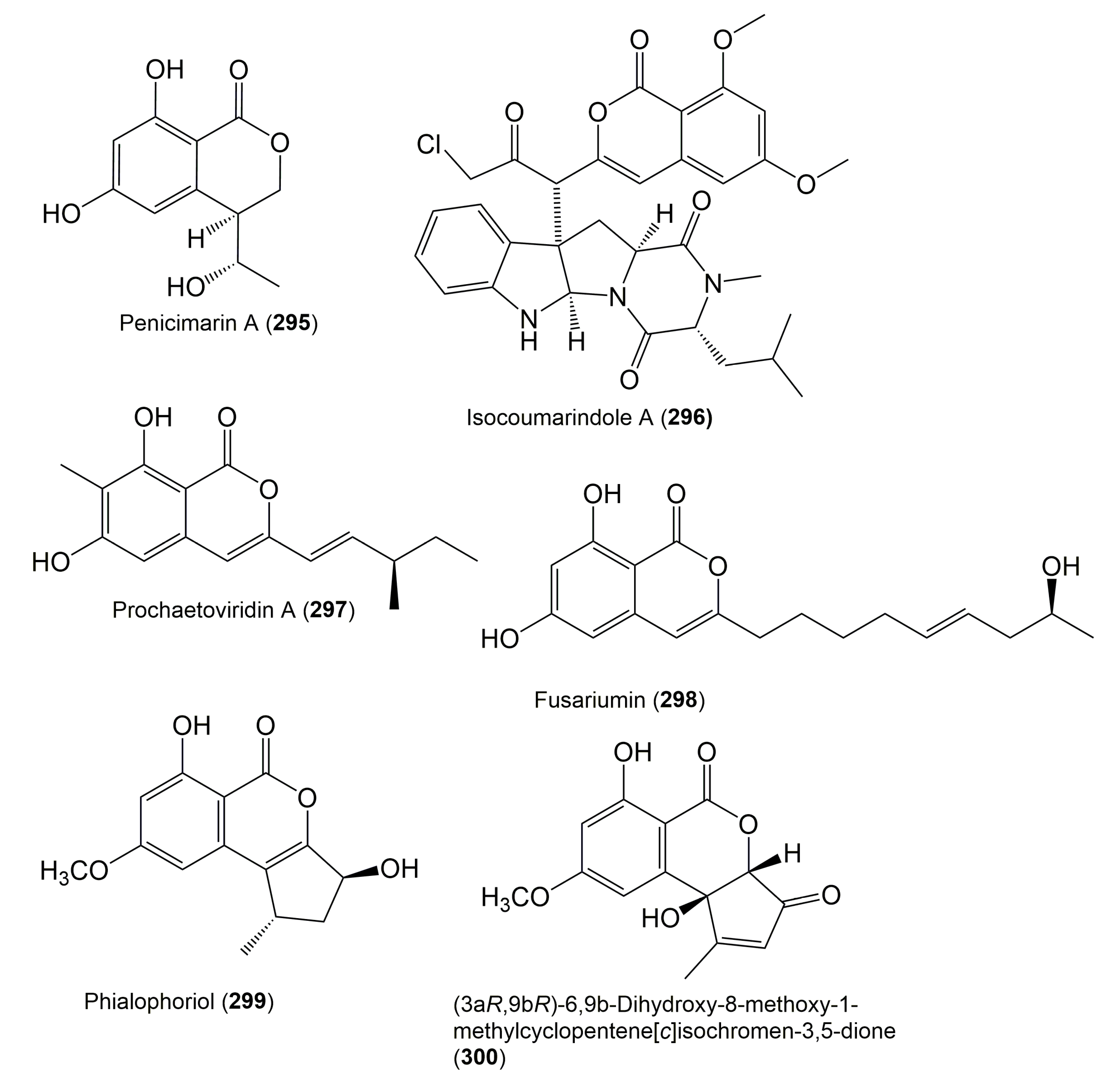
- Chen, M.; Wang, R.; Zhao, W.; Yu, L.; Zhang, C.; Chang, S.; Li, Y.; Zhang, T.; Xing, J.; Gan, M. Isocoumarindole A, a chlorinated isocoumarin and indole alkaloid hybrid metabolite from an endolichenic fungus Aspergillus sp. Org. Lett. 2019, 21, 1530–1533. [Google Scholar] [CrossRef]
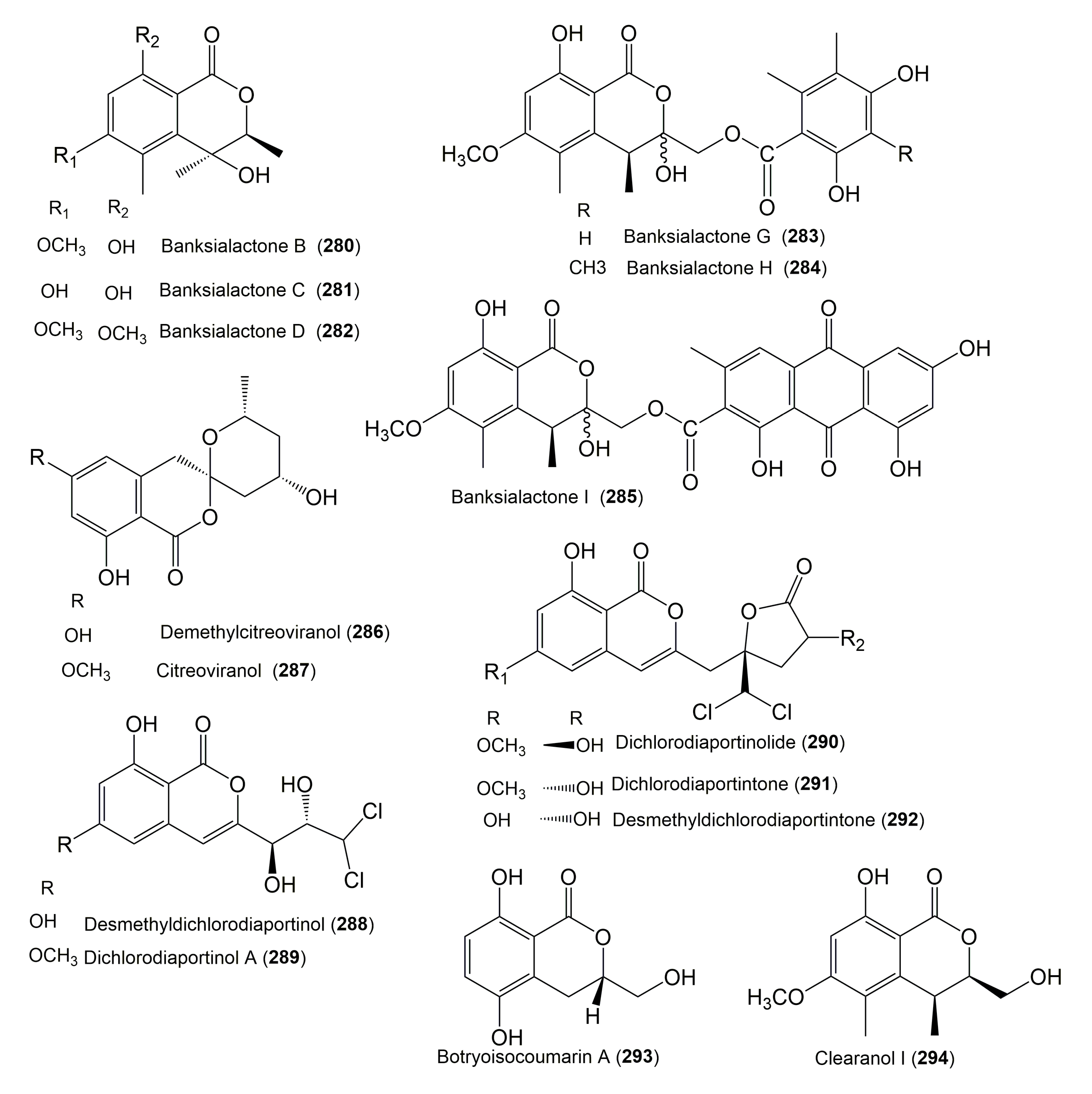
- Chaudhary, N.K.; Pitt, J.I.; Lacey, E.; Crombie, A.; Vuong, D.; Piggott, A.M.; Karuso, P. Banksialactones and Banksiamarins: Isochromanones and Isocoumarins from an Australian Fungus, Aspergillus banksianus. J. Nat. Prod. 2018, 81, 1517–1526. [Google Scholar] [CrossRef]
- Cai, R.; Wu, Y.; Chen, S.; Cui, H.; Liu, Z.; Li, C.; She, Z. Peniisocoumarins A–J: Isocoumarins from Penicillium commune QQF-3, an endophytic fungus of the mangrove plant Kandelia candel. J. Nat. Prod. 2018, 81, 1376–1383. [Google Scholar] [CrossRef]
- Elsebai, M.F.; Ghabbour, H.A. Isocoumarin derivatives from the marine-derived fungus Phoma sp. 135. Tetrahedron Lett. 2016, 57, 354–356. [Google Scholar] [CrossRef]
- Cui, H.; Liu, Y.; Nie, Y.; Liu, Z.; Chen, S.; Zhang, Z.; Lu, Y.; He, L.; Huang, X.; She, Z. Polyketides from the mangrove-derived endophytic fungus Nectria sp. HN001 and their α-glucosidase inhibitory activity. Mar. Drugs 2016, 14, 86. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Ishigami, K.; Watanabe, H. Synthesis of (−)-mellein,(+)-ramulosin, and related natural products. Tetrahedron 2007, 63, 1074–1079. [Google Scholar] [CrossRef]
- Kimura, Y.; Nakadoi, M.; Shimada, A.; Nakajima, H.; Hamasaki, T. Biosyntheses of sescandelin and sescandelin B: New isocoumarin compounds produced by the fungus, Sesquicilium candelabrum. Biosci. Biotechnol. Biochem. 1994, 58, 1525–1526. [Google Scholar] [CrossRef]
- Ju, Z.; Lin, X.; Lu, X.; Tu, Z.; Wang, J.; Kaliyaperumal, K.; Liu, J.; Tian, Y.; Xu, S.; Liu, Y. Botryoisocoumarin A, a new COX-2 inhibitor from the mangrove Kandelia candel endophytic fungus Botryosphaeria sp. KcF6. J. Antibiot. 2015, 68, 653. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Z.; Liu, H.; Pan, Y.; Li, J.; Liu, L.; She, Z. Dichloroisocoumarins with potential anti-Inflammatory activity from the mangrove endophytic fungus Ascomycota sp. CYSK-4. Mar. Drugs 2018, 16, 54. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, S.; Liu, Z.; Lu, Y.; Xia, G.; Liu, H.; He, L.; She, Z. Bioactive metabolites from mangrove endophytic fungus Aspergillus sp. 16-5B. Mar. Drugs 2015, 13, 3091–3102. [Google Scholar] [CrossRef]
- Aly, A.H.; Edrada-Ebel, R.; Wray, V.; Müller, W.E.; Kozytska, S.; Hentschel, U.; Proksch, P.; Ebel, R. Bioactive metabolites from the endophytic fungus Ampelomyces sp. isolated from the medicinal plant Urospermum picroides. Phytochemistry 2008, 69, 1716–1725. [Google Scholar] [CrossRef]
- Aly, A.H.; Edrada-Ebel, R.; Indriani, I.D.; Wray, V.; Müller, W.E.; Totzke, F.; Zirrgiebel, U.; Schächtele, C.; Kubbutat, M.H.; Lin, W. Cytotoxic metabolites from the fungal endophyte Alternaria sp. and their subsequent detection in its host plant Polygonum senegalense. J. Nat. Prod. 2008, 71, 972–980. [Google Scholar] [CrossRef]
- Arunpanichlert, J.; Rukachaisirikul, V.; Phongpaichit, S.; Supaphon, O.; Sakayaroj, J. Meroterpenoid, isocoumarin, and phenol derivatives from the seagrass-derived fungus Pestalotiopsis sp. PSU-ES194. Tetrahedron 2015, 71, 882–888. [Google Scholar] [CrossRef]
- Hussain, H.; Akhtar, N.; Draeger, S.; Schulz, B.; Pescitelli, G.; Salvadori, P.; Antus, S.; Kurtán, T.; Krohn, K. New bioactive 2, 3-epoxycyclohexenes and isocoumarins from the endophytic fungus Phomopsis sp. from Laurus azorica. Eur. J. Org. Chem. 2009, 2009, 749–756. [Google Scholar] [CrossRef]
- Hussain, H.; Krohn, K.; Draeger, S.; Meier, K.; Schulz, B. Bioactive chemical constituents of a sterile endophytic fungus from Meliotus dentatus. Rec. Nat. Prod. 2009, 3, 114–117. [Google Scholar]
- Li, Q.-Q.; Dang, L.-Z.; Zhang, Y.-P.; Jiang, J.-X.; Zhang, C.-M.; Xiang, N.-J.; Yang, H.-Y.; Du, G.; Duan, Y.-Q. Isocoumarins from the fermentation products of a plant entophytic fungus Penicillium oxalicum. J. Asian Nat. Prod. Res. 2015, 17, 876–881. [Google Scholar] [CrossRef]
- Li, W.; Lee, C.; Bang, S.H.; Ma, J.Y.; Kim, S.; Koh, Y.-S.; Shim, S.H. Isochromans and related constituents from the endophytic fungus Annulohypoxylon truncatum of Zizania caduciflora and their anti-inflammatory effects. J. Nat. Prod. 2016, 80, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Sappapan, R.; Sommit, D.; Ngamrojanavanich, N.; Pengpreecha, S.; Wiyakrutta, S.; Sriubolmas, N.; Pudhom, K. 11-Hydroxymonocerin from the plant endophytic fungus Exserohilum rostratum. J. Nat. Prod. 2008, 71, 1657–1659. [Google Scholar] [CrossRef]
- Cao, J.; Li, X.-M.; Li, X.; Li, H.-L.; Meng, L.-H.; Wang, B.-G. New lactone and isocoumarin derivatives from the marine mangrove-derived endophytic fungus Penicillium coffeae MA-314. Phytochem. Lett. 2019, 32, 1–5. [Google Scholar] [CrossRef]
- Prompanya, C.; Dethoup, T.; Bessa, L.; Pinto, M.; Gales, L.; Costa, P.; Silva, A.; Kijjoa, A. New isocoumarin derivatives and meroterpenoids from the marine sponge-associated fungus Aspergillus similanensis sp. nov. KUFA 0013. Mar. Drugs 2014, 12, 5160–5173. [Google Scholar] [CrossRef]
- Zhang, W.; Krohn, K.; Draeger, S.; Schulz, B. Bioactive isocoumarins isolated from the endophytic fungus Microdochium bolleyi. J. Nat. Prod. 2008, 71, 1078–1081. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Jabeen, F.; Krohn, K.; Al-Harrasi, A.; Ahmad, M.; Mabood, F.; Shah, A.; Badshah, A.; Rehman, N.U.; Green, I.R. Antimicrobial activity of two mellein derivatives isolated from an endophytic fungus. Med. Chem. Res. 2015, 24, 2111–2114. [Google Scholar] [CrossRef]
- Zhao, M.; Yuan, L.-Y.; Guo, D.-L.; Ye, Y.; Da-Wa, Z.-M.; Wang, X.-L.; Ma, F.-W.; Chen, L.; Gu, Y.-C.; Ding, L.-S. Bioactive halogenated dihydroisocoumarins produced by the endophytic fungus Lachnum palmae isolated from Przewalskia tangutica. Phytochemistry 2018, 148, 97–103. [Google Scholar] [CrossRef]
- Qi, J.; Shao, C.-L.; Li, Z.-Y.; Gan, L.-S.; Fu, X.-M.; Bian, W.-T.; Zhao, H.-Y.; Wang, C.-Y. Isocoumarin derivatives and benzofurans from a sponge-derived Penicillium sp. fungus. J. Nat. Prod. 2013, 76, 571–579. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Y.; Wang, L.; Wang, J.; Zhang, C. Bioactive secondary metabolites from Nigrospora sp. LLGLM003, an endophytic fungus of the medicinal plant Moringa oleifera Lam. World J. Microbiol. Biotechnol. 2012, 28, 2107–2112. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.-X.; Xue, Y.-B.; Bi, X.-B.; Zhang, J.-W.; Luo, Z.-W.; Li, X.-N.; Yao, G.-M.; Wang, J.-P.; Zhang, Y.-H. Five new secondary metabolites produced by a marine-associated fungus, Daldinia eschscholzii. Mar. Drugs 2014, 12, 5563–5575. [Google Scholar] [CrossRef] [PubMed]
- Kokubun, T.; Veitch, N.C.; Bridge, P.D.; Simmonds, M.S. Dihydroisocoumarins and a tetralone from Cytospora eucalypticola. Phytochemistry 2003, 62, 779–782. [Google Scholar] [CrossRef]
- Huang, G.-L.; Zhou, X.-M.; Bai, M.; Liu, Y.-X.; Zhao, Y.-L.; Luo, Y.-P.; Niu, Y.-Y.; Zheng, C.-J.; Chen, G.-Y. Dihydroisocoumarins from the mangrove-derived fungus Penicillium citrinum. Mar. Drugs 2016, 14, 177. [Google Scholar] [CrossRef]
- Wang, X.-Z.; Luo, X.-H.; Xiao, J.; Zhai, M.-M.; Yuan, Y.; Zhu, Y.; Crews, P.; Yuan, C.-S.; Wu, Q.-X. Pyrone derivatives from the endophytic fungus Alternaria tenuissima SP-07 of Chinese herbal medicine Salvia przewalskii. Fitoterapia 2014, 99, 184–190. [Google Scholar] [CrossRef]
- Xu, R.; Li, X.-M.; Wang, B.-G. Penicisimpins A-C, three new dihydroisocoumarins from Penicillium simplicissimum MA-332, a marine fungus derived from the rhizosphere of the mangrove plant Bruguiera sexangula var. rhynchopetala. Phytochem. Lett. 2016, 17, 114–118. [Google Scholar] [CrossRef]
- Pinheiro, E.A.; Pina, J.R.; Feitosa, A.O.; Carvalho, J.M.; Borges, F.C.; Marinho, P.S.; Marinho, A.M. Bioprospecting of antimicrobial activity of extracts of endophytic fungi from Bauhinia guianensis. Rev. Argent. Microbiol. 2017, 49, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Chen, S.; Niu, S.; Guo, L.; Yin, J.; Che, Y. Exserolides A–F, new isocoumarin derivatives from the plant endophytic fungus Exserohilum sp. Fitoterapia 2014, 96, 88–94. [Google Scholar] [CrossRef]
- Li, S.; Wei, M.; Chen, G.; Lin, Y. Two new dihydroisocoumarins from the endophytic fungus Aspergillus sp. collected from the South China Sea. Chem. Nat. Compd. 2012, 48, 371–373. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Y.; Liu, Z.; Cai, R.; Lu, Y.; Huang, X.; She, Z. Isocoumarins and benzofurans from the mangrove endophytic fungus Talaromyces amestolkiae possess α-glucosidase inhibitory and antibacterial activities. RSC Adv. 2016, 6, 26412–26420. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, M.-H.; Wang, X.-B.; Li, T.-X.; Kong, L.-Y. Bioactive metabolites from the endophytic fungus Alternaria alternata. Fitoterapia 2014, 99, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Lin, X.; Yang, J.; Zhou, X.; Yang, B.; Wang, J.; Liu, Y. Spiro-Phthalides and Isocoumarins isolated from the marine-sponge-derived fungus Setosphaeria sp. SCSIO41009. J. Nat. Prod. 2018, 81, 1860–1868. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, D.; Proksch, P.; Yu, S.; Lin, W. Isocoumarin derivatives from the sponge-associated fungus Peyronellaea glomerata with antioxidant activities. Chem. Biodivers. 2016, 13, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xu, J.; Li, F.; Xu, L.; Li, C. A new antifungal isocoumarin from the endophytic fungus Trichoderma sp. 09 of Myoporum bontioides A. gray. Pharmacogn. Mag. 2016, 12, 259–261. [Google Scholar] [PubMed]
- Bai, M.; Zheng, C.-J.; Huang, G.-L.; Mei, R.-Q.; Wang, B.; Luo, Y.-P.; Zheng, C.; Niu, Z.-G.; Chen, G.-Y. Bioactive Meroterpenoids and Isocoumarins from the Mangrove-Derived Fungus Penicillium sp. TGM112. J. Nat. Prod. 2019, 82, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, M.I.; Musharraf, S.G.; Mukhmoor, T.; Shaheen, F.; Ali, S.; Rahman, A.-U. Isolation of bioactive compounds from Aspergillus terreus. Z. Naturforsch. B 2004, 59, 324–328. [Google Scholar] [CrossRef]
- Ding, Z.; Tao, T.; Wang, L.; Zhao, Y.; Huang, H.; Zhang, D.; Liu, M.; Wang, Z.; Han, J. Bioprospecting of novel and bioactive metabolites from endophytic fungi Isolated from rubber tree Ficus elastica leaves. J. Microbiol. Biotechnol. 2019, 29, 731–738. [Google Scholar] [CrossRef]
- Ma, X.; Liang, X.; Huang, Z.-H.; Qi, S.-H. New alkaloids and isocoumarins from the marine gorgonian-derived fungus Aspergillus sp. SCSIO 41501. Nat. Prod. Res. 2019. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, J.; Zhang, X.; Chen, Z.; Li, T.; She, Z.; Ding, W.; Li, C. Four New isocoumarins and a new natural tryptamine with antifungal activities from a mangrove endophytic fungus Botryosphaeria ramosa L29. Mar. Drugs 2019, 17, 88. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, X.; Li, G.; Feng, Z.; Xu, J. Pestalotiopisorin B, a new isocoumarin derivative from the mangrove endophytic fungus Pestalotiopsis sp. HHL101. Nat. Prod. Res. 2018. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, S.; Liu, H.; Huang, X.; Liu, Y.; Tao, Y.; She, Z. Cytotoxic isocoumarin derivatives from the mangrove endophytic fungus Aspergillus sp. HN15-5D. Arch. Pharm. Res. 2019, 42, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Kongsaeree, P.; Prabpai, S.; Sriubolmas, N.; Vongvein, C.; Wiyakrutta, S. Antimalarial dihydroisocoumarins produced by Geotrichum sp., an endophytic fungus of Crassocephalum crepidioides. J. Nat. Prod. 2003, 66, 709–711. [Google Scholar] [CrossRef]
- Lei, H.; Lin, X.; Han, L.; Ma, J.; Ma, Q.; Zhong, J.; Liu, Y.; Sun, T.; Wang, J.; Huang, X. New metabolites and bioactive chlorinated benzophenone derivatives produced by a marine-derived fungus Pestalotiopsis heterocornis. Mar. Drugs 2017, 15, 69. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Cai, R.; Hong, K.; She, Z. New furoisocoumarins and isocoumarins from the mangrove endophytic fungus Aspergillus sp. 085242. Beilstein J. Org. Chem. 2016, 12, 2077–2085. [Google Scholar]
- Yan, W.; Cao, L.-L.; Zhang, Y.-Y.; Zhao, R.; Zhao, S.-S.; Khan, B.; Ye, Y.-H. New metabolites from endophytic fungus Chaetomium globosum CDW7. Molecules 2018, 23, 2873. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.-Y.; Wu, Y.-Y.; Zhang, M.-Y.; Cheng, J.; Dube, B.; Yu, H.-J.; Zhang, Y.-X. New antimicrobial compounds produced by Seltsamia galinsogisoli sp. nov., isolated from Galinsoga parviflora as potential inhibitors of FtsZ. Sci. Rep. 2019, 9, 8319. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Gong, B.; Cox, D.G.; Li, C.; Wang, J.; Ding, W. Dichlorodiaportinol A–A new chlorine-containing isocoumarin from an endophytic fungus Trichoderma sp. 09 from Myoporum bontioides A. Gray and its cytotoxic activity. Pharmacogn. Mag. 2014, 10 (Suppl. 1), S153–S158. [Google Scholar] [CrossRef]
- Tianpanich, K.; Prachya, S.; Wiyakrutta, S.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Radical scavenging and antioxidant activities of isocoumarins and a phthalide from the endophytic fungus Colletotrichum sp. J. Nat. Prod. 2010, 74, 79–81. [Google Scholar] [CrossRef]
- Ebada, S.S.; El-Neketi, M.; Ebrahim, W.; Mándi, A.; Kurtán, T.; Kalscheuer, R.; Müller, W.E.; Proksch, P. Cytotoxic secondary metabolites from the endophytic fungus Aspergillus versicolor KU258497. Phytochem. Lett. 2018, 24, 88–93. [Google Scholar] [CrossRef]
- Kamdem, R.S.; Wang, H.; Wafo, P.; Ebrahim, W.; Özkaya, F.C.; Makhloufi, G.; Janiak, C.; Sureechatchaiyan, P.; Kassack, M.U.; Lin, W. Induction of new metabolites from the endophytic fungus Bionectria sp. through bacterial co-culture. Fitoterapia 2018, 124, 132–136. [Google Scholar] [CrossRef]
- Kumar, M.; Qadri, M.; Sharma, P.R.; Kumar, A.; Andotra, S.S.; Kaur, T.; Kapoor, K.; Gupta, V.K.; Kant, R.; Hamid, A. Tubulin inhibitors from an endophytic fungus isolated from Cedrus deodara. J. Nat. Prod. 2013, 76, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Sang, X.-N.; Chen, S.-F.; An, X.; Chen, G.; Wang, H.-F.; Pei, Y.-H. A novel 3, 4-dihydronaphthalen-1 (2 H)-one with spiro-butyrolactone and a new isocoumarin isolated from the endophytic fungus Phoma sp. YN02-P-3. J. Asian Nat. Prod. Res. 2017, 19, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-F.; Li, L.-H.; Tian, L.; Qiao, L.; Hua, H.-M.; Pei, Y.-H. Sg17-1-4, a novel isocoumarin from a marine fungus Alternaria tenuis Sg17-1. J. Antibiot. 2006, 59, 355. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-X.; Gao, J.-M.; Zhang, Q.; Laatsch, H. Toxic polyketides produced by Fusarium sp., an endophytic fungus isolated from Melia azedarach. Biorg. Med. Chem. Lett. 2011, 21, 1887–1889. [Google Scholar] [CrossRef]
- Han, Z.; Mei, W.; Zhao, Y.; Deng, Y.; Dai, H. A new cytotoxic isocoumarin from endophytic fungus Penicillium sp. 091402 of the mangrove plant Bruguiera sexangula. Chem. Nat. Compd. 2009, 45, 805–807. [Google Scholar] [CrossRef]
- Huang, Z.; Shao, C.; Chen, Y.; She, Z.; Lin, Y.; Zhou, S. A new isocoumarin from mangrove endophytic fungus (No. dz17) on the South China Sea coast. Chem. Nat. Compd. 2007, 43, 655–658. [Google Scholar] [CrossRef]
- Ji, B.-K.; Dong, W.; Wang, Y.-D.; Zhou, K.; Li, Y.-K.; Zhou, M.; Du, G.; Hu, Q.-F.; Ye, Y.-Q.; Yang, H.-Y. A new isocoumarin from fermentation products of endophytic fungus of Aspergillus versicolor. Asian J. Chem. 2015, 27, 3915–3916. [Google Scholar] [CrossRef]
- Tsukada, M.; Fukai, M.; Miki, K.; Shiraishi, T.; Suzuki, T.; Nishio, K.; Sugita, T.; Ishino, M.; Kinoshita, K.; Takahashi, K. Chemical constituents of a marine fungus, Arthrinium sacchari. J. Nat. Prod. 2011, 74, 1645–1649. [Google Scholar] [CrossRef]
- Schmeda-Hirschmann, G.; Hormazabal, E.; Astudillo, L.; Rodriguez, J.; Theoduloz, C. Secondary metabolites from endophytic fungi isolated from the Chilean gymnosperm Prumnopitys andina (Lleuque). World J. Microbiol. Biotechnol. 2005, 21, 27–32. [Google Scholar] [CrossRef]
- Pinheiro, Â.; Dethoup, T.; Bessa, J.; Silva, A.M.; Kijjoa, A. A new bicyclic sesquiterpene from the marine sponge associated fungus Emericellopsis minima. Phytochem. Lett. 2012, 5, 68–70. [Google Scholar] [CrossRef]
- Ye, Y.-Q.; Xia, C.-F.; Yang, J.-X.; Qin, Y.; Zhou, M.; Gao, X.-M.; Du, G.; Yang, H.-Y.; Li, X.-M.; Hu, Q.-F. Isocoumarins from the fermentation products of an endophytic fungus of Aspergillus versicolor. Phytochem. Lett. 2014, 10, 215–218. [Google Scholar] [CrossRef]
- Zhou, M.; Zhou, K.; He, P.; Wang, K.-M.; Zhu, R.-Z.; Wang, Y.-D.; Dong, W.; Li, G.-P.; Yang, H.-Y.; Ye, Y.-Q. Antiviral and cytotoxic isocoumarin derivatives from an endophytic fungus Aspergillus oryzae. Planta Med. 2016, 82, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Rukachaisirikul, V.; Buadam, S.; Sukpondma, Y.; Phongpaichit, S.; Sakayaroj, J.; Hutadilok-Towatana, N. Indanone and mellein derivatives from the Garcinia-derived fungus Xylaria sp. PSU-G12. Phytochem. Lett. 2013, 6, 135–138. [Google Scholar] [CrossRef]
- Sritharan, T.; Savitri Kumar, N.; Jayasinghe, L.; Araya, H.; Fujimoto, Y. Isocoumarins and dihydroisocoumarins from the endophytic fungus Biscogniauxia capnodes isolated from the fruits of Averrhoa carambola. Nat. Prod. Commun. 2019, 14, 1–3. [Google Scholar] [CrossRef]
- Kimura, A.; Lee, J.-H.; Lee, I.-S.; Lee, H.-S.; Park, K.-H.; Chiba, S.; Kim, D. Two potent competitive inhibitors discriminating α-glucosidase family I from family II. Carbohydr. Res. 2004, 339, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.; Mohamed, G.A.; Abdel-Latif, M.M.; El-Messery, S.M.; Al Musayeib, N.M.; Shehata, I.A. Minutaside A, new α-amylase inhibitor flavonol glucoside from Tagetes minuta: Antidiabetic, antioxidant, and molecular modeling studies. Starch-Stärke 2015, 67, 976–984. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A.; Khayat, M.T.A.; Ahmed, S.; Abo-Haded, H. α-Amylase inhibition of xanthones from Garcinia mangostana pericarps and their possible use for the treatment of diabetes with molecular docking studies. J. Food Biochem. 2019, 43, e12844. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A.; Khayat, M.T.A.; Ahmed, S.; Abo-Haded, H. Garcixanthone D, a new xanthone, and other xanthone derivatives from Garcinia mangostana pericarps: Their α-amylase inhibitory potential and molecular docking studies. Starch-Stärke 2019, 71, 1800354. [Google Scholar] [CrossRef]
- Lebovitz, H.E. Alpha-glucosidase inhibitors. Endocrinol. Metab. Clin. N. Am. 1997, 26, 539–551. [Google Scholar] [CrossRef]
- Indrianingsih, A.W.; Tachibana, S. α-Glucosidase inhibitor produced by an endophytic fungus, Xylariaceae sp. QGS 01 from Quercus gilva Blume. Food Sci. Hum. Wellness 2017, 6, 88–95. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, C.; Liu, H.; Zhu, G.; Fu, P.; Wang, L.; Zhu, W. Meroterpenoids and Isocoumarinoids from a Myrothecium Fungus Associated with Apocynum venetum. Mar. Drugs 2018, 16, 363. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.-F.; Wang, K.; Ren, J.-W.; Liu, L.; Cai, L.; Han, J.-J.; Liu, H.-W. 2 H-Pyranone and isocoumarin derivatives isolated from the plant pathogenic fungus Leptosphaena maculans. J. Asian Nat. Prod. Res. 2019, 21, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.P.; Faraoni, M.B.; Castro, M.J.; Alza, N.P.; Cavallaro, V. Natural AChE inhibitors from plants and their contribution to Alzheimer’s disease therapy. Curr. Neuropharmacol. 2013, 11, 388–413. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.S.; Raghuwanshi, R.; Masood, M.; Acharya, A.; Jain, S.K. Medicinal plants with acetylcholinesterase inhibitory activity. Rev. Neurosci. 2018, 29, 491–529. [Google Scholar] [CrossRef]
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The protein kinase complement of the human genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, G.; Cao, S.; Zhang, Y.; Li, X.; Lin, X. Development of certain protein kinase inhibitors with the components from traditional Chinese medicine. Front. Pharmacol. 2017, 7, 523. [Google Scholar] [CrossRef]
- Fan, N.W.; Chang, H.S.; Cheng, M.J.; Hsieh, S.Y.; Liu, T.W.; Yuan, G.F.; Chen, I.S. Secondary metabolites from the endophytic fungus Xylaria cubensis. Helv. Chim. Acta 2014, 97, 1689–1699. [Google Scholar] [CrossRef]
- Sommart, U.; Rukachaisirikul, V.; Sukpondma, Y.; Phongpaichit, S.; Sakayaroj, J.; Kirtikara, K. Hydronaphthalenones and a dihydroramulosin from the endophytic fungus PSU-N24. Chem. Pharm. Bull. 2008, 56, 1687–1690. [Google Scholar] [CrossRef]
- Tansuwan, S.; Pornpakakul, S.; Roengsumran, S.; Petsom, A.; Muangsin, N.; Sihanonta, P.; Chaichit, N. Antimalarial benzoquinones from an endophytic fungus, Xylaria sp. J. Nat. Prod. 2007, 70, 1620–1623. [Google Scholar] [CrossRef]
- Duan, Y.-Q.; Dang, L.-Z.; Jiang, J.-X.; Zhang, Y.-P.; Xiang, N.-J.; Yang, H.-M.; Du, G.; Yang, H.-Y.; Li, Q.-Q. Anti-tobacco Mosaic virus isocoumarins from the fermentation products of the endophytic fungus Aspergillus versicolor. Chem. Nat. Compd. 2018, 54, 249–252. [Google Scholar] [CrossRef]
- Ramos, H.P.; Simão, M.R.; de Souza, J.M.; Magalhães, L.G.; Rodrigues, V.; Ambrósio, S.R.; Said, S. Evaluation of dihydroisocoumarins produced by the endophytic fungus Arthrinium state of Apiospora montagnei against Schistosoma mansoni. Nat. Prod. Res. 2013, 27, 2240–2243. [Google Scholar] [CrossRef]
- Evidente, A.; Punzo, B.; Andolfi, A.; Cimmino, A.; Melck, D.; Luque, J. Lipophilic phytotoxins produced by Neofusicoccum parvum, a grapevine canker agent. Phytopathol. Mediterr. 2010, 49, 74–79. [Google Scholar]
- Nakashima, K.-i.; Tomida, J.; Hirai, T.; Morita, Y.; Kawamura, Y.; Inoue, M. A new isocoumarin derivative from an endophytic fungus Thielavia sp. isolated from Crassula ovata. Heterocycles Int. J. Rev. Commun. Heterocycl. Chem. 2017, 94, 117–121. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhang, X.; Wang, L.; Li, G.; Zhou, J.-C.; Lou, H.-X. Metabolites from Penicillium sp., an endophytic fungus from the liverwort Riccardia multifida (L.) S. Gray. Phytochem. Lett. 2013, 6, 14–17. [Google Scholar] [CrossRef]
- Krohn, K.; Kock, I.; Elsässer, B.; Flörke, U.; Schulz, B.; Draeger, S.; Pescitelli, G.; Antus, S.; Kurtán, T. Bioactive natural products from the endophytic fungus Ascochyta sp. from Meliotus dentatus–configurational assignment by solid-State CD and TDDFT calculations. Eur. J. Org. Chem. 2007, 2007, 1123–1129. [Google Scholar] [CrossRef]
- Wang, F.; Han, S.; Hu, S.; Xue, Y.; Wang, J.; Xu, H.; Chen, L.; Zhang, G.; Zhang, Y. Two new secondary metabolites from Xylaria sp. cfcc 87468. Molecules 2014, 19, 1250–1257. [Google Scholar] [CrossRef]
- Sumarah, M.W.; Puniani, E.; Blackwell, B.A.; Miller, J.D. Characterization of polyketide metabolites from foliar endophytes of Picea glauca. J. Nat. Prod. 2008, 71, 1393–1398. [Google Scholar] [CrossRef]
- Qian, C.-D.; Fu, Y.-H.; Jiang, F.-S.; Xu, Z.-H.; Cheng, D.-Q.; Ding, B.; Gao, C.-X.; Ding, Z.-S. Lasiodiplodia sp. ME4-2, an endophytic fungus from the floral parts of Viscum coloratum, produces indole-3-carboxylic acid and other aromatic metabolites. BMC Microbial. 2014, 14, 297. [Google Scholar] [CrossRef]
- Tian, J.-F.; Yu, R.-J.; Li, X.-X.; Gao, H.; Hu, D.; Guo, L.-D.; Tang, J.-S.; Yao, X.-S. Cyclohexenones and isocoumarins from an endophytic fungus of Sarcosomataceae sp. J. Asian Nat. Prod. Res. 2015, 17, 550–558. [Google Scholar] [CrossRef]
- Hu, Q.-F.; Xing, H.-H.; Wang, Y.-D.; Yu, Z.-H.; Yan, K.-L.; Zhou, K.; Dong, W.; Zhou, M.; Yang, H.-Y.; Zhu, D.-L. Prenylated isocoumarins from the fermentation products of the endophytic fungus Aspergillus versicolor and their anti-tobacco mosaic virus activities. Chem. Nat. Compd. 2017, 53, 436–439. [Google Scholar] [CrossRef]
- Luo, J.; Liu, X.; Li, E.; Guo, L.; Che, Y. Arundinols A–C and arundinones A and B from the plant endophytic fungus Microsphaeropsis arundinis. J. Nat. Prod. 2013, 76, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Arunpanichlert, J.; Rukachaisirikul, V.; Sukpondma, Y.; Phongpaichit, S.; Tewtrakul, S.; Rungjindamai, N.; Sakayaroj, J. Azaphilone and isocoumarin derivatives from the endophytic fungus Penicillium sclerotiorum PSU-A13. Chem. Pharm. Bull. 2010, 58, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Rukachaisirikul, V.; Rodglin, A.; Sukpondma, Y.; Phongpaichit, S.; Buatong, J.; Sakayaroj, J. Phthalide and isocoumarin derivatives produced by an Acremonium sp. isolated from a mangrove Rhizophora apiculata. J. Nat. Prod. 2012, 75, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Lou, J.; Li, Y.-K.; Wang, Y.-D.; Zhou, K.; Ji, B.-K.; Dong, W.; Gao, X.-M.; Du, G.; Hu, Q.-F. Versicolols A and B, two new prenylated isocoumarins from endophytic fungus Aspergillus versicolor and their cytotoxic activity. Arch. Pharm. Res. 2017, 40, 32–36. [Google Scholar] [CrossRef]
- Huang, H.; Li, Q.; Feng, X.; Chen, B.; Wang, J.; Liu, L.; She, Z.; Lin, Y. Structural elucidation and NMR assignments of four aromatic lactones from a mangrove endophytic fungus (No. GX4-1B). Magn. Reson. Chem. 2010, 48, 496–499. [Google Scholar] [CrossRef]
- Ariefta, N.R.; Kristiana, P.; Aboshi, T.; Murayama, T.; Tawaraya, K.; Koseki, T.; Kurisawa, N.; Kimura, K.-i.; Shiono, Y. New isocoumarins, naphthoquinones, and a cleistanthane-type diterpene from Nectria pseudotrichia 120-1NP. Fitoterapia 2018, 127, 356–361. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, P.; Xue, T.-D.; Meng, L.-L.; Gao, W.-B.; Zhang, J.; Zhao, Y.-x.; Luo, D.-Q. Two new isocoumarin derivatives from an endophytic fungi Pestalotiopsis coffeae isolated from a mangrove Fishtail Palm. Nat. Prod. Commun. 2018, 13, 57–59. [Google Scholar] [CrossRef]
- Fang, Z.F.; Yu, S.S.; Zhou, W.Q.; Chen, X.G.; Ma, S.G.; Li, Y.; Qu, J. A new isocoumarin from metabolites of the endophytic fungus Alternaria tenuissima (Nees & T. Nees: Fr.) Wiltshire. Chin. Chem. Lett. 2012, 23, 317–320. [Google Scholar]
- Shi, T.; Qi, J.; Shao, C.-L.; Zhao, D.-L.; Hou, X.-M.; Wang, C.-Y. Bioactive diphenyl ethers and isocoumarin derivatives from a gorgonian-derived fungus Phoma sp.(TA07-1). Mar. Drugs 2017, 15, 146. [Google Scholar] [CrossRef]
- Hsiao, Y.; Cheng, M.-J.; Chang, H.-S.; Wu, M.-D.; Hsieh, S.-Y.; Liu, T.-W.; Lin, C.-H.; Yuan, G.-F.; Chen, I.-S. Six new metabolites produced by Colletotrichum aotearoa 09F0161, an endophytic fungus isolated from Bredia oldhamii. Nat. Prod. Res. 2016, 30, 251–258. [Google Scholar] [CrossRef]
- Wijeratne, E.K.; Paranagama, P.A.; Gunatilaka, A.L. Five new isocoumarins from Sonoran desert plant-associated fungal strains Paraphaeosphaeria quadriseptata and Chaetomium chiversii. Tetrahedron 2006, 62, 8439–8446. [Google Scholar] [CrossRef]

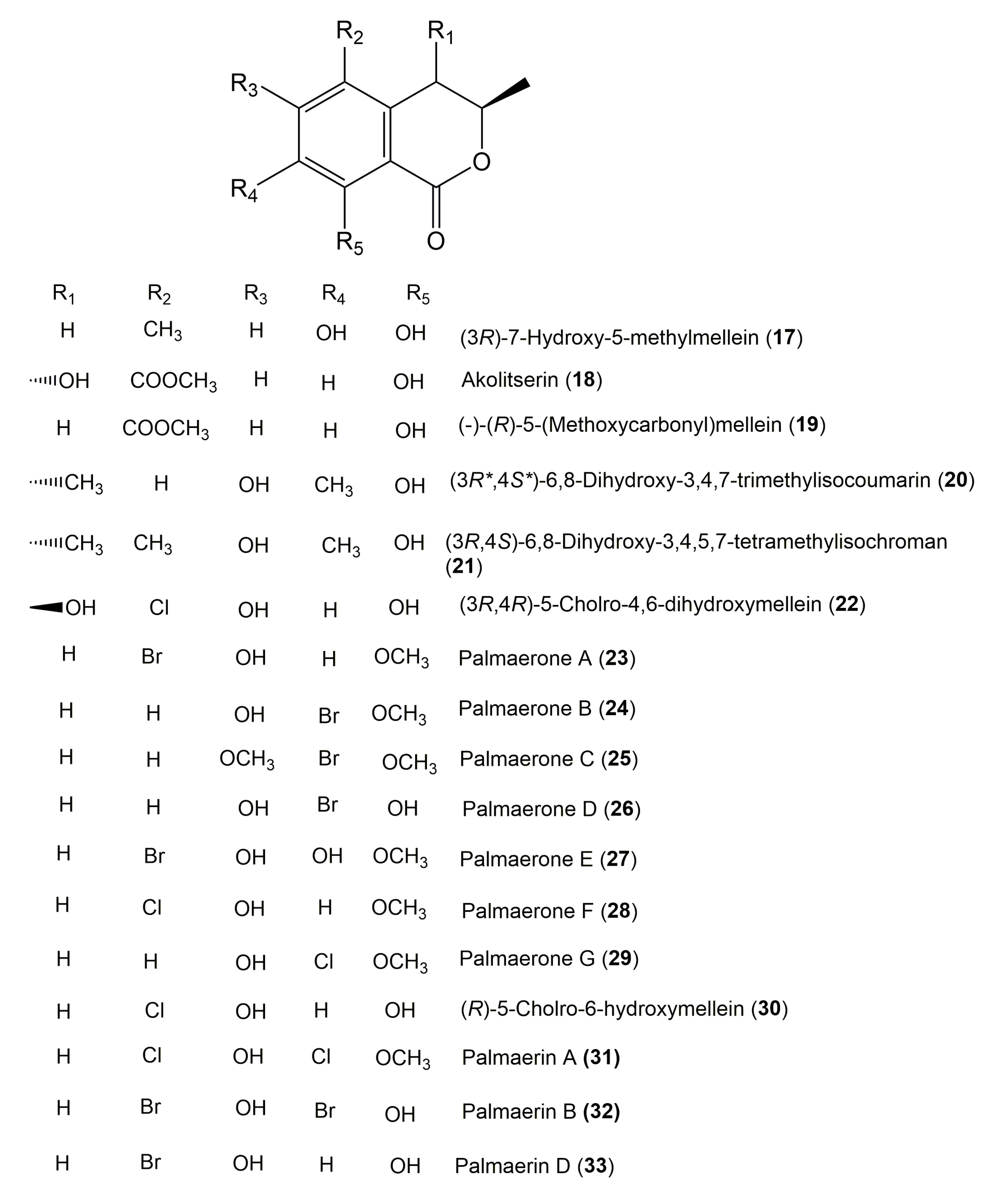
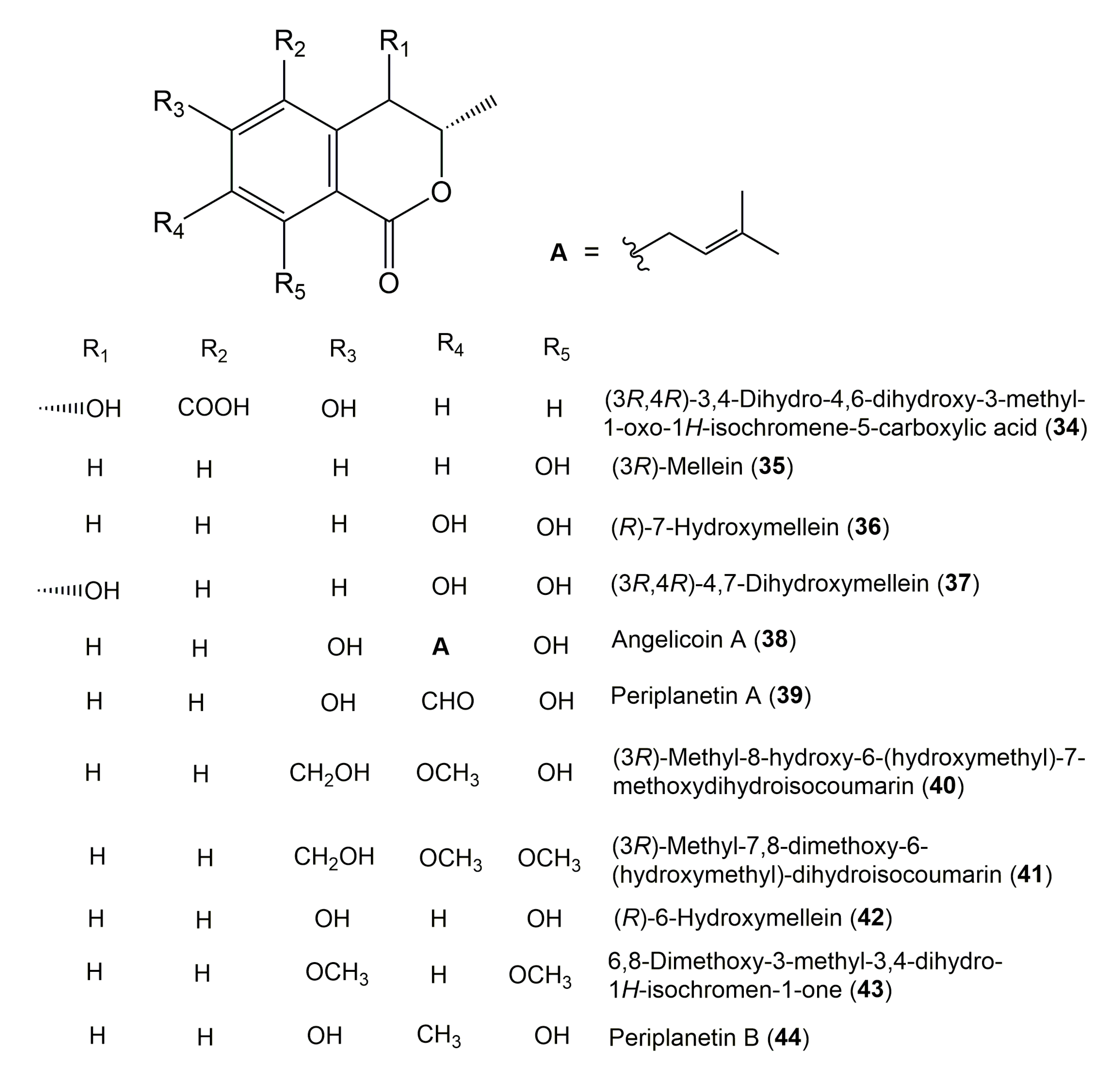

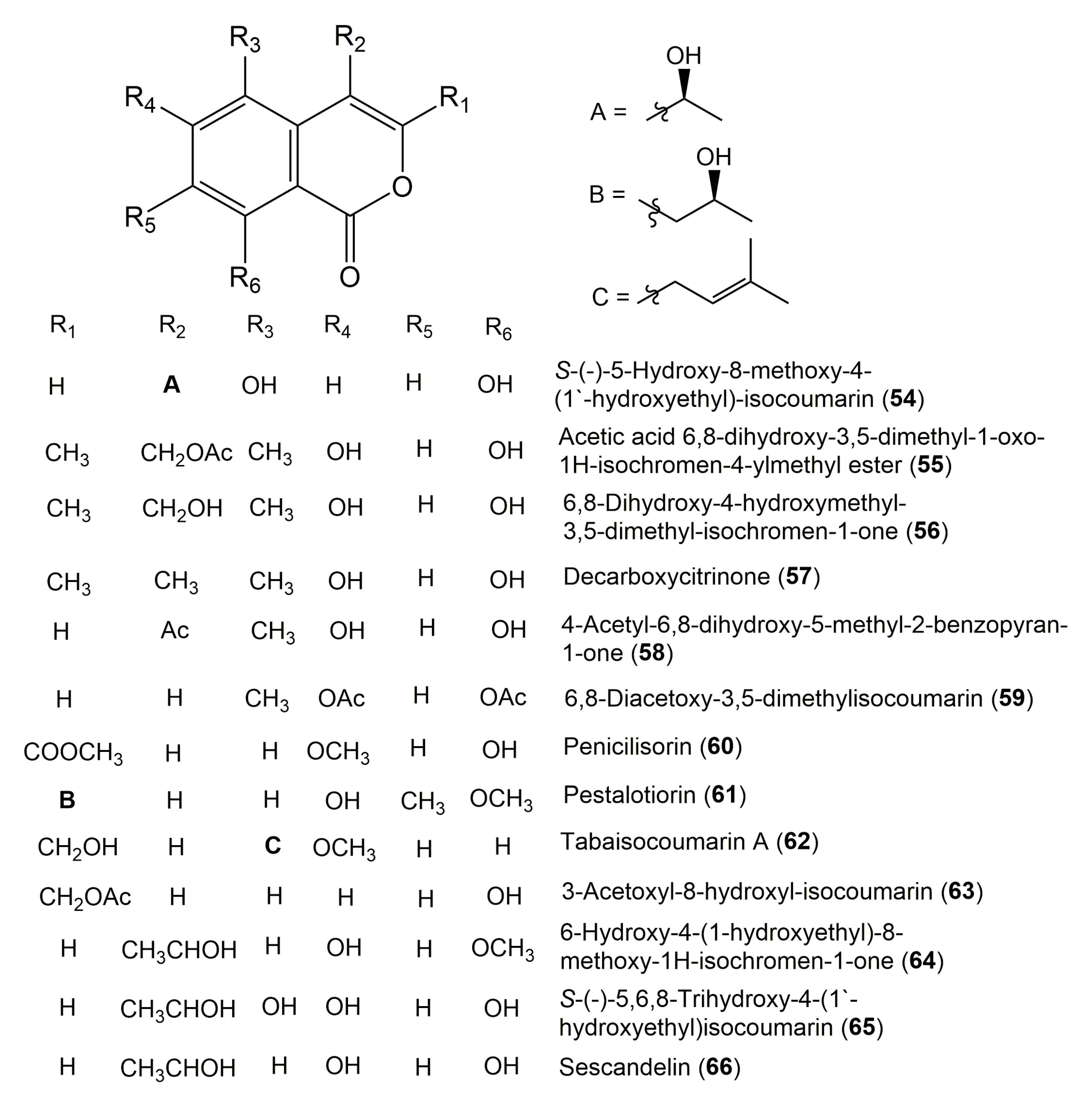


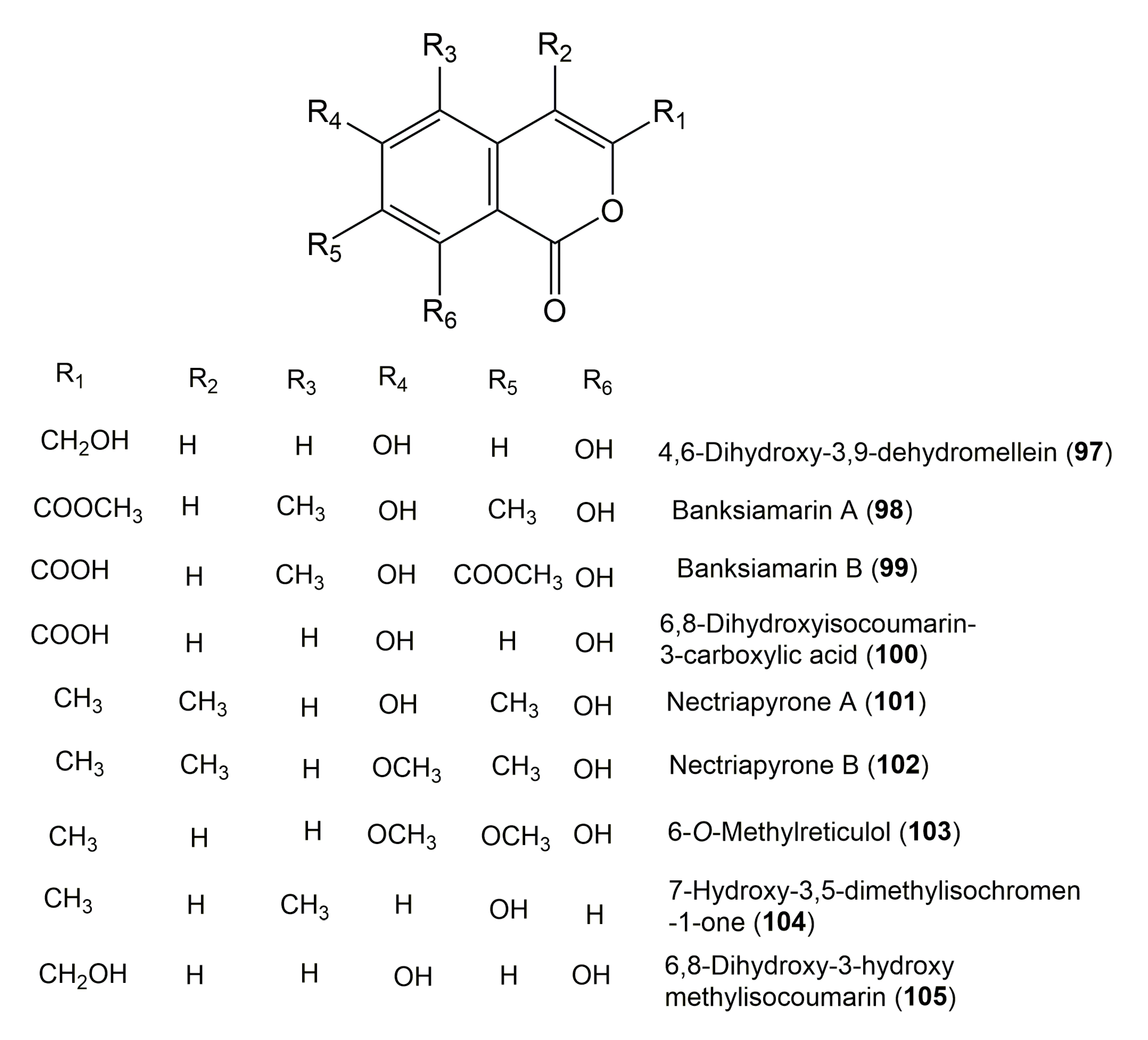

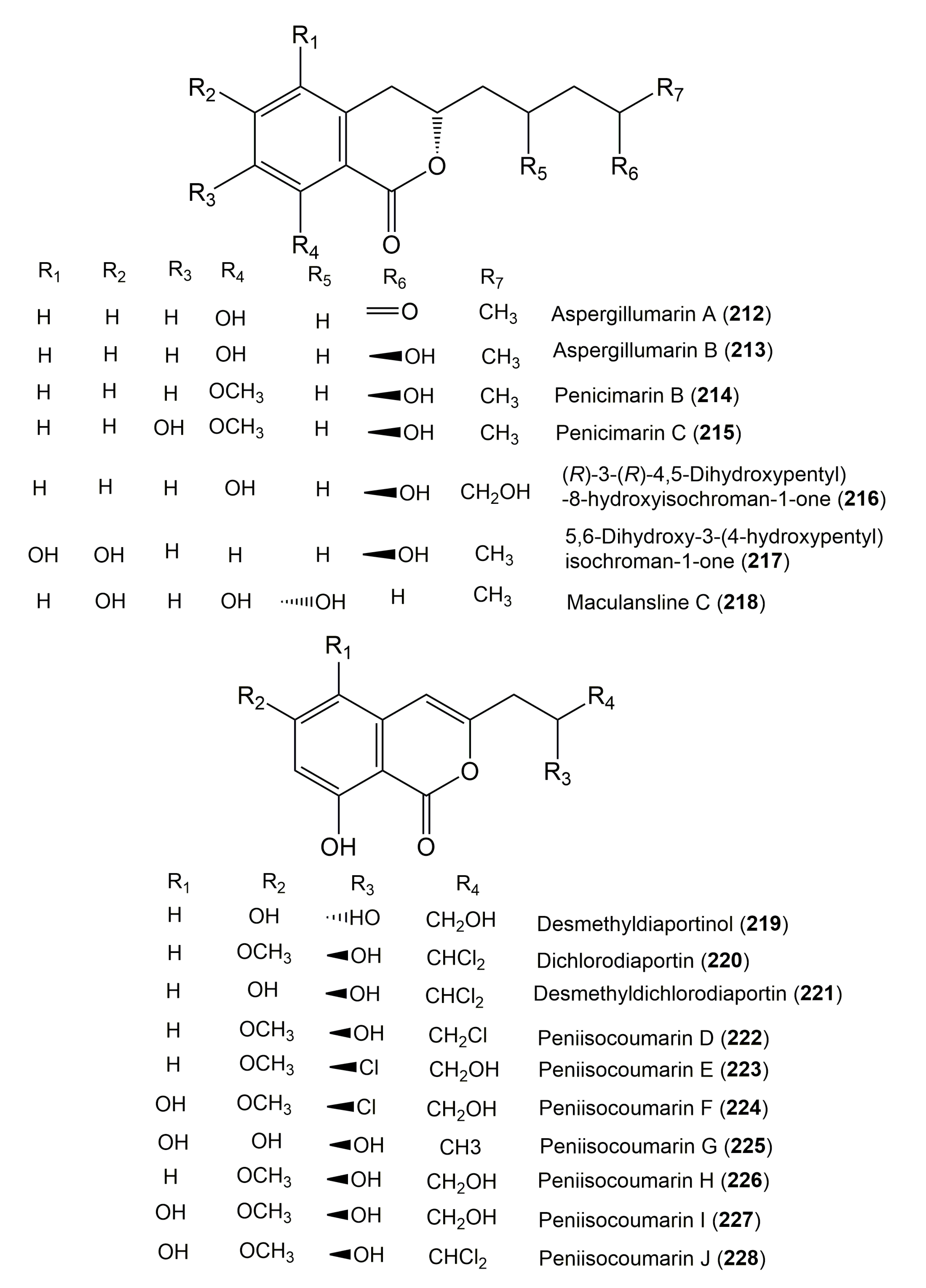
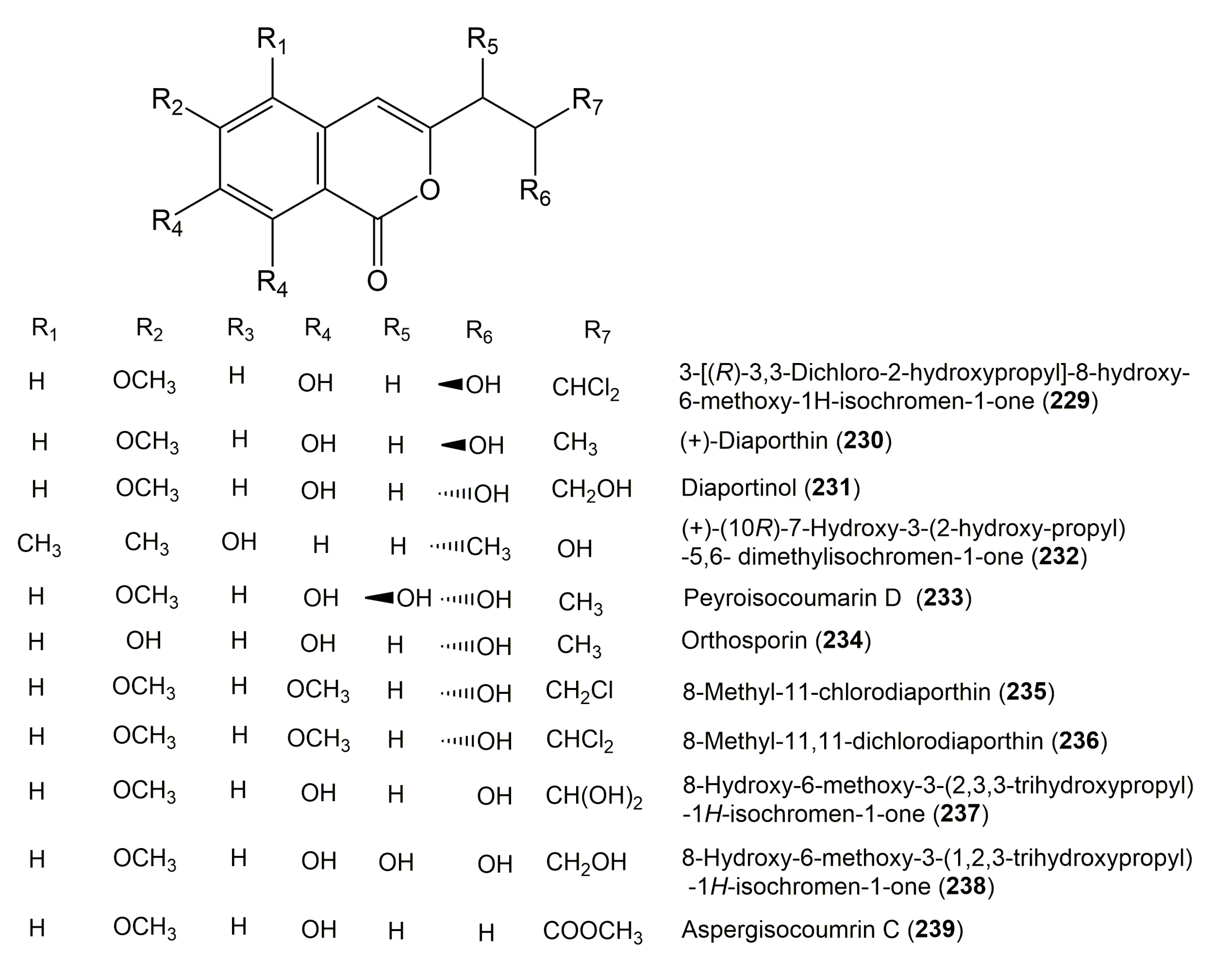
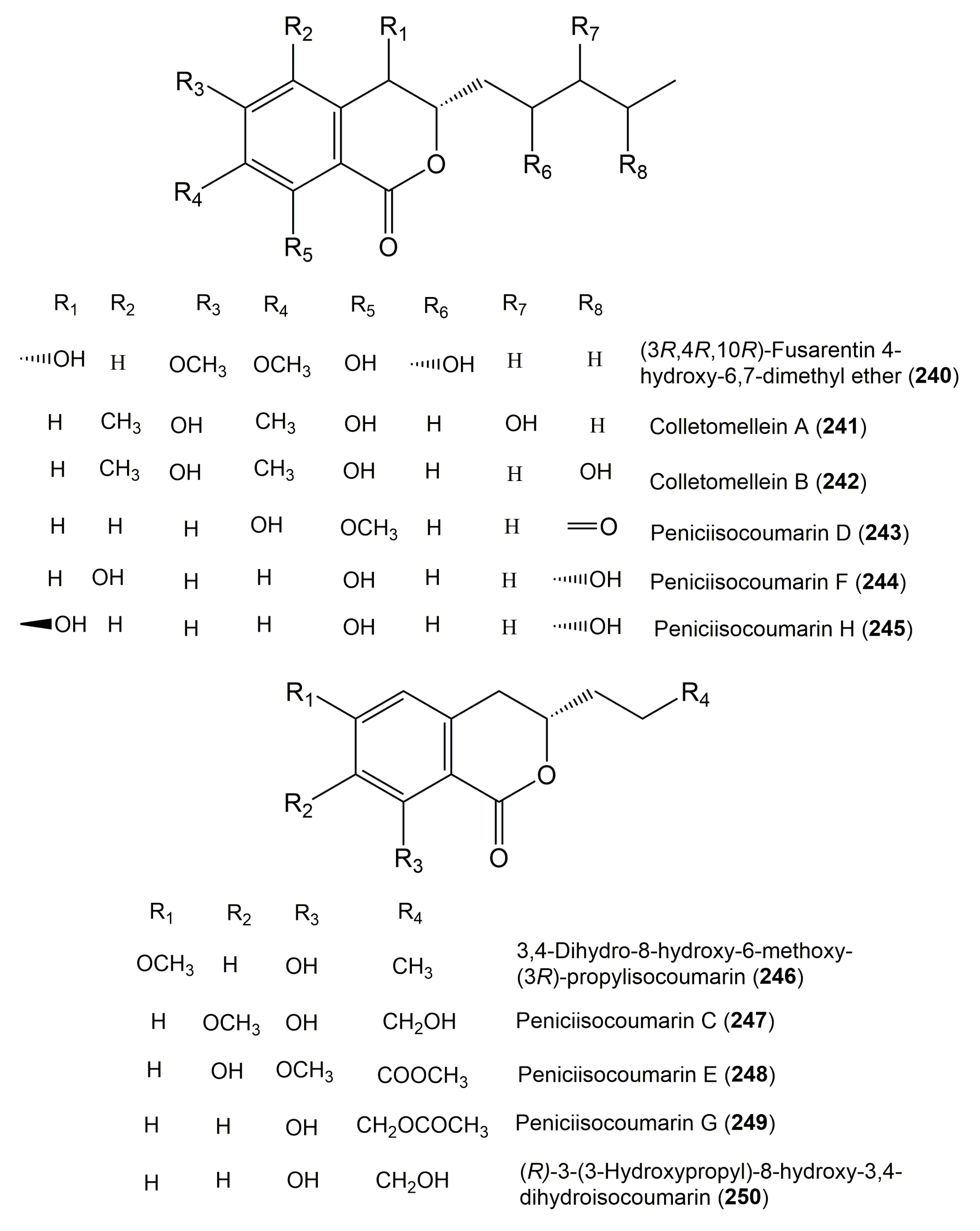
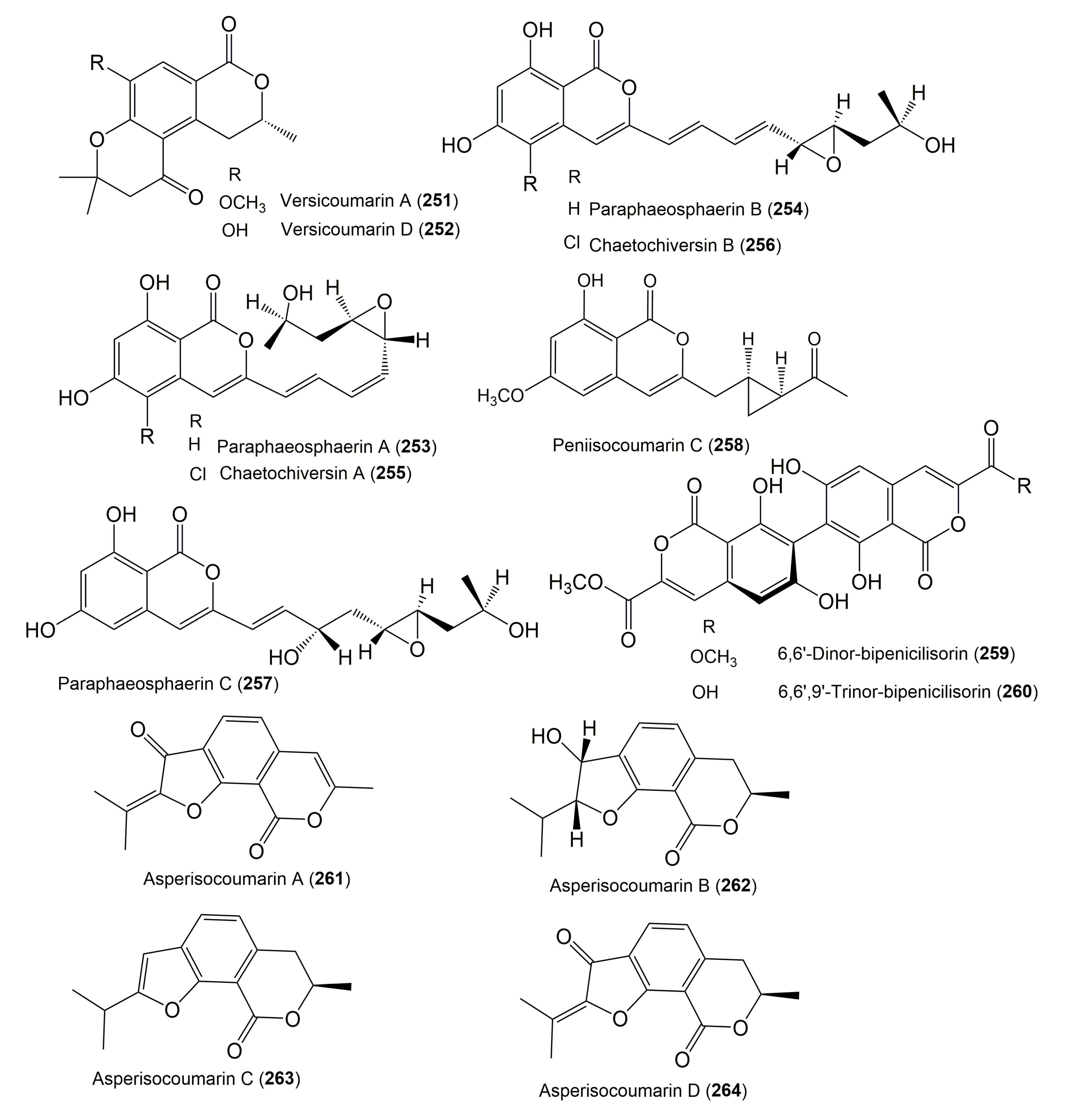
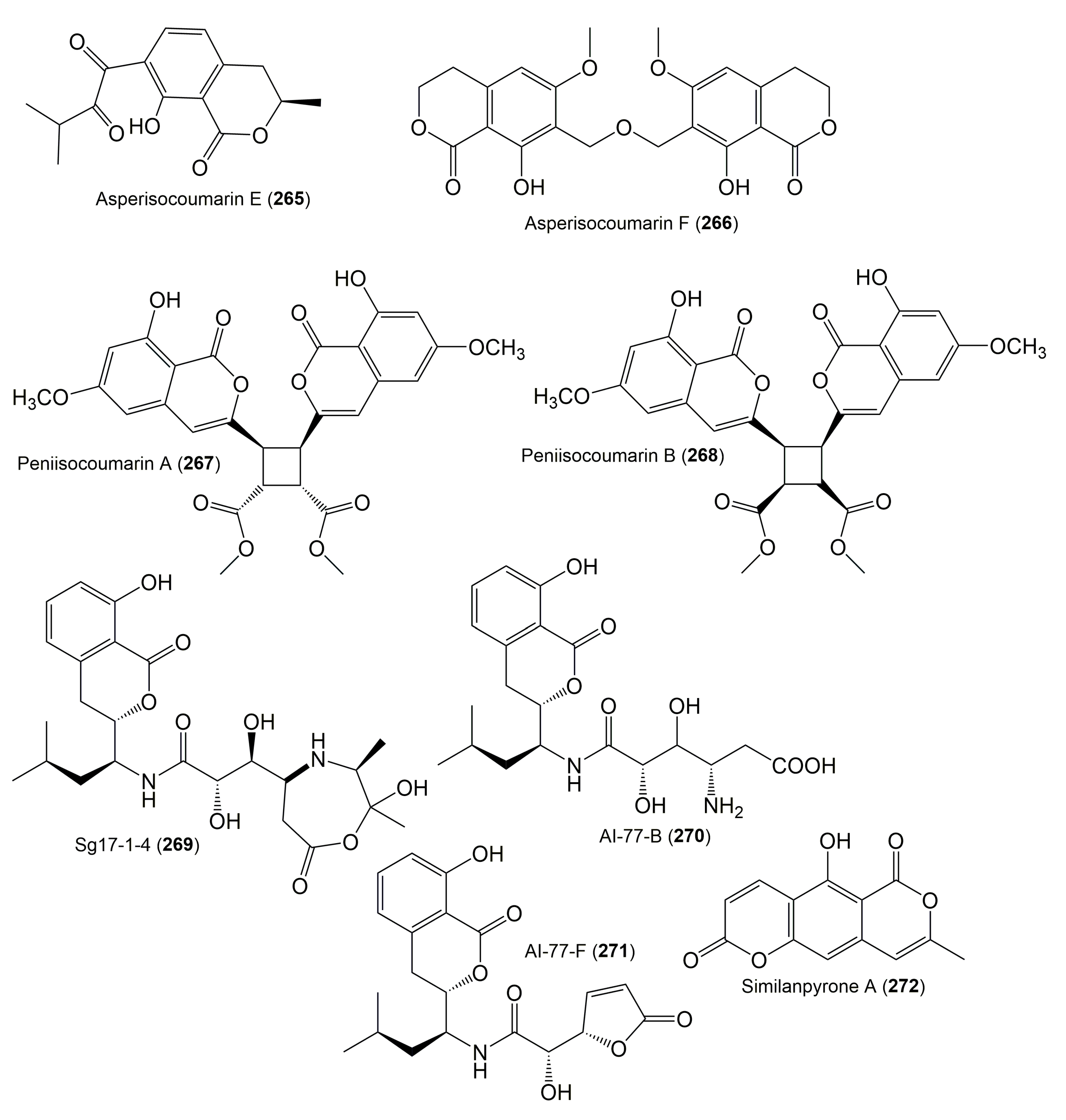
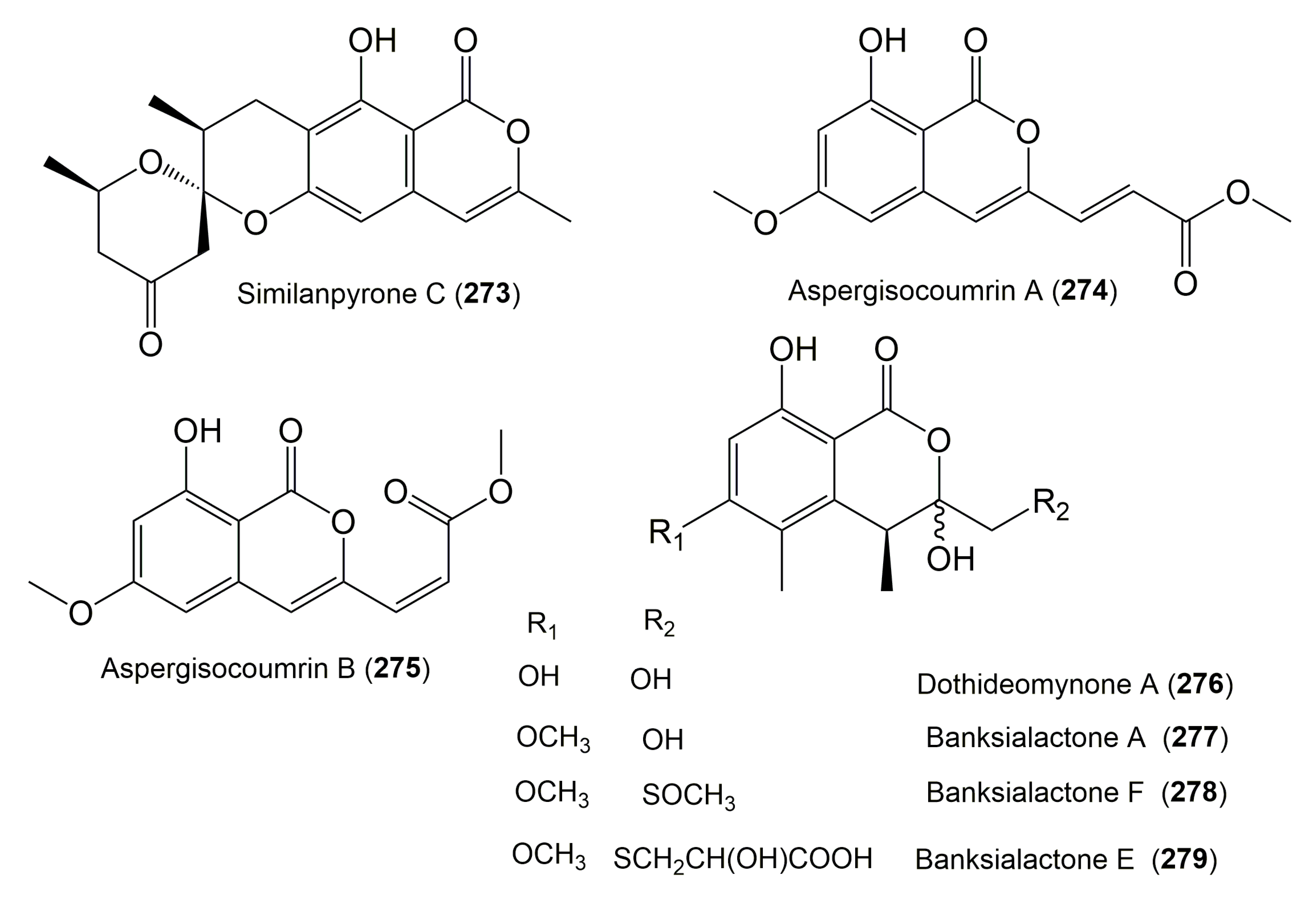


| Compound Name | Fungus | Host (Part, Family) | Source, Place | References |
|---|---|---|---|---|
| Kigelin (1) (−)-(3R)-6,7-Dimethoxymellein | Aspergillus terrus BDKU 1164 | Marine alga | Mubarak village beach, Karachi, Pakistan | [68] |
| (3R,4R)-6,7-Dimethoxy-4-hydroxymellein (2) | Aspergillus terrus BDKU 1164 | Marine alga | Mubarak village beach, Karachi, Pakistan | [68] |
| 8-Methoxymellein (3) | Penicillium sp.1 and sp.2 | Alibertia macrophylla (Leaves, Rubiaceae) | Mogi-Guaçu, São Paulo, Brazil | [18] |
| Botryosphaeria sp. KcF6 | Kandelia candel (Fruits, Rhizophoraceae) | Daya Bay, Shenzhen, China | [36] | |
| Xylaria cubensis BCRC 09F 0035 | Litsea akoensis Hayata (Leaves, Lauraceae) | Kaohsiung, Taiwan | [109] | |
| Cis-4-Acetoxyoxymellein (4) | Ascomycete 6650 | Meliotus dentatus (Leaves, Fabaceae) | Baltic Sea, Ahrenshoop, Germany | [50] |
| 8-Deoxy-6-hydroxy-cis-4-acetoxyoxymellein (5) | Ascomycete 6650 | Meliotus dentatus (Leaves, Fabaceae) | Baltic Sea, Ahrenshoop, Germany | [50] |
| (3R,4R)-(−)-4-Hydroxymellein (3R,4R)-Cis-4-Hydroxymellein (6) | Aspergillus terrus (BDKU 1164) | Marine alga | Mubarak village beach, Karachi, Pakistan | [68] |
| Xylaria sp. PBR-30 | Sandoricum koetjape (Leaves, Meliaceae) | Prachinburi Province, Thailand | [111] | |
| Ascochyta sp. | Meliotus dentatus (Whole plant, Fabaceae) | Shores of the Baltic Sea, near Ahrenshoop, Germany | [117] | |
| Nigrospora sp. PSU-N24 | Garcinia nigrolineata (Branches, Clusiaceae) | Ton Nga Chang wildlife sanctuary, Songkhla province, Southern Thailand | [110] | |
| Neofusicoccum parvum | Vitis vinifera L. (Cankered branchs, Vitaceae) | Catalonia, NE Spain | [114] | |
| Emericellopsis minima | Hyrtios erecta (Marine sponge) | Similan islands, Phag Nga Province, Thailand | [92] | |
| Apiospora montagnei Sacc. | Smallanthus sonchifolius (Roots, Asteraceae) | Ribeirão Preto city, S. P. State, Brazil | [113] | |
| Lachnum palmae | Przewalskia tangutica Maxim. (Leaves, Solanaceae) | Linzhou Country of the Tibet Autonomous Region, China | [51] | |
| Annulohypoxylomarin (7) | Annulohypoxylon truncatum | Zizania caduciflora (Leaves, Poaceae) | Suncheon, South Korea | [45] |
| 5-Hydroxymellein (8) | Penicillium sp.1 and sp.2 | Alibertia macrophylla (Leaves, Rubiaceae) | Mogi-Guaçu, São Paulo, Brazil | [18] |
| Lachnum palmae | Przewalskia tangutica Maxim. (Leaves, Solanaceae) | Linzhou Country of the Tibet Autonomous Region, China | [51] | |
| (3R)-6-Methoxy-7-chloromellein (9) | Phoma sp. 135 | Ectyplasia perox | Lauro Club Reef, Dominica | [32] |
| (3R,4R)-Cis-4-Hydroxy-5-methylmellein (10) | Unidentified Ascomycete 6650 | Meliotus dentatus (Leaves, Fabaceae) | Baltic Sea, Ahrenshoop, Germany | [43] |
| (−)-6-Methoxymellein (11) | Lachnum palmae | Przewalskia tangutica Maxim. (Leaves, Solanaceae) | Linzhou Country of the Tibet Autonomous Region, China | [51] |
| Phoma sp. YE3135 | Aconitum vilmorinianum (Roots, Ranunculaceae) | Yunnan University, China | [26] | |
| (3R,4S)-4-Hydroxy-6-methoxy-7-chloromellein (12) | Phoma sp. 135 | Ectyplasia perox | Lauro Club Reef, Dominica | [32] |
| Botryospyrone C (13) | Botryosphaeria ramosa L29 | Myoporum bontioides (Leaves, Scrophulariaceae) | Leizhou Peninsula, China | [71] |
| Botryospyrone D (14) | Botryosphaeria ramosa L29 | Myoporum bontioides (Leaves, Scrophulariaceae) | Leizhou Peninsula, China | [71] |
| 3R-(+)-5-O-[6′-O-Acetyl]-α-D-glucopyranosyl-5-hydroxymellein (15) | Xylaria sp. cfcc 87468 | Pinus tabuliformis (Leaves, Pinaceae) | China Forestry Culture Collection Center, Beijing, China | [118] |
| 6-(4′-Hydroxy-2′-methyl phenoxy)-(−)-(3R)-mellein (16) | Aspergillus terrus BDKU 1164 | Marine alga | Mubarak village beach, Karachi, Pakistan | [68] |
| (3R)-7-Hydroxy-5-methylmellein (17) | Phomopsis sp. 7233 | Laurus azorica (Leaves, Lauraceae) | Gomera, Spain | [42] |
| Biscogniauxia capnodes | Averrhoa carambola L. (Fruits, Oxalidaceae) | Home garden in Kandy, Central Province, Sri Lanka | [96] | |
| Akolitserin (18) (+)-(3R,4S)-5-Carbomethoxy-3-hydroxymellein Methyl (3R,4S)-3,4-Dihydro-4,8-dihydroxy-3-methyl-1-oxo-1H-isochromene-5-carboxylate | Xylaria cubensis BCRC 09F 0035 | Litsea akoensis Hayata (Leaves, Lauraceae) | Kaohsiung, Taiwan | [109] |
| (−)-(R)-5-(Methoxycarbonyl)mellein (19) | Xylaria cubensis BCRC 09F 0035 | Litsea akoensis Hayata (Leaves, Lauraceae) | Kaohsiung, Taiwan | [109] |
| (3R*,4S*)-6,8-Dihydroxy-3,4,7-trimethylisocoumarin (20) | Penicillium sp. 091402 | Bruguiera sexangula (Roots, Rhizophoraceae) | Qinglan Port, Hainan, China | [87] |
| (3R,4S)-6,8-Dihydroxy-3,4,5,7-tetramethylisochroman (21) | Penicillium sp. 091402 | Bruguiera sexangula (Roots, Rhizophoraceae) | Qinglan Port, Hainan, China | [87] |
| Aspergillus versicolor | Paris marmorata Stearn (Rhizomes, Melanthiaceae) | Dali, Yunnan, China | [93] | |
| (3R,4R)-5-Cholro-4,6-dihydroxymellein (22) | Lachnum palmae | Przewalskia tangutica Maxim. (Leaves, Solanaceae) | Linzhou Country of the Tibet Autonomous Region, China | [51] |
| Palmaerone A (23) (R)-5-Bromo-6-hydroxy-8-methoxy-mellein | Lachnum palmae | Przewalskia tangutica Maxim. (Leaves, Solanaceae) | Linzhou Country of the Tibet Autonomous Region, China | [51] |
| Palmaerone B (24) (R)-7-Bromo-6-hydroxy-8-methoxy-mellein | Lachnum palmae | Przewalskia tangutica Maxim. (Leaves, Solanaceae) | Linzhou Country of the Tibet Autonomous Region, China | [51] |
| Palmaerone C (25) (R)-7-Bromo-6,8-dimethoxy-mellein | Lachnum palmae | Przewalskia tangutica Maxim. (Leaves, Solanaceae) | Linzhou Country of the Tibet Autonomous Region, China | [51] |
| Palmaerone D (26) (R)-7-Bromo-6-hydroxy-mellein | Lachnum palmae | Przewalskia tangutica Maxim. (Leaves, Solanaceae) | Linzhou Country of the Tibet Autonomous Region, China | [51] |
| Palmaerone E (27) (R)-5-Bromo-6,7-dihydroxy-8-methoxy-mellein | Lachnum palmae | Przewalskia tangutica Maxim. (Leaves, Solanaceae) | Linzhou Country of the Tibet Autonomous Region, China | [51] |
| Palmaerone F (28) (R)-5-Cholro-6-hydroxy-8-methoxy-mellein | Lachnum palmae | Przewalskia tangutica Maxim. (Leaves, Solanaceae) | Linzhou Country of the Tibet Autonomous Region, China | [51] |
| Palmaerone G (29) (R)-7-Cholro-6-hydroxy-8-methoxy-mellein | Lachnum palmae | Przewalskia tangutica Maxim. (Leaves, Solanaceae) | Linzhou Country of the Tibet Autonomous Region, China | [51] |
| (R)-5-Cholro-6-hydroxymellein (30) | Lachnum palmae | Przewalskia tangutica Maxim. (Leaves, Solanaceae) | Linzhou Country of the Tibet Autonomous Region, China | [51] |
| Palmaerin A (31) | Lachnum palmae | Przewalskia tangutica Maxim. (Leaves, Solanaceae) | Linzhou Country of the Tibet Autonomous Region, China | [51] |
| Palmaerin B (32) | Lachnum palmae | Przewalskia tangutica Maxim. (Leaves, Solanaceae) | Linzhou Country of the Tibet Autonomous Region, China | [51] |
| Palmaerin D (33) | Lachnum palmae | Przewalskia tangutica Maxim. (Leaves, Solanaceae) | Linzhou Country of the Tibet Autonomous Region, China | [51] |
| (3R,4R)-3,4-Dihydro-4,6-dihydroxy-3-methyl-1-oxo-1H-isochromene-5-carboxylic acid (34) | Xylaria sp. PA-01 | Piper aduncum (Leaves, Piperaceae) | Mogi-Guaçu, Săo Paulo, Brazil | [17] |
| (3R)-Mellein (35) 3,4-Dihydro-(3R)-methyl-8-hydroxyisocoumarin | Centraalbureau voor Schimmel 120379 | Picea glauca (Leaves, Pinaceae) | Sussex, New Brunswick, Canada | [119] |
| Nigrospora sp. PSU-N24 | Garcinia nigrolineata (Branches, Clusiaceae) | Ton Nga Chang wildlife sanctuary, Songkhla province, Southern Thailand | [119] | |
| Nigrospora sp. LLGLM003 | Moringa oleifera (Roots, Moringaceae) | Xiamen municipality, Fujian Province, China | [53] | |
| Apiospora montagnei Sacc. | Smallanthus sonchifolius (Roots, Asteraceae) | Ribeirão Preto city, S. P. State, Brazil | [113] | |
| Lasiodiplodia sp. ME4-2 | Viscum coloratum (Flowers, Santalaceae) | Hangzhou City, Zhejiang Province, China | [120] | |
| Sarcosomataceae sp. NO.49-14-2-1 | Everniastrum nepalense (Taylor) Hale ex Sipman (Lichen, Parmeliaceae) | Panzhihua, Sichuan province, China | [121] | |
| Penicillium janczewskii | Prumnopitys andina (Phloem, Podocarpaceae) | Western Andean slopes near Las Trancas, Chillan | [91] | |
| Lachnum palmae | Przewalskia tangutica Maxim. (Leaves, Solanaceae) | Linzhou Country of the Tibet Autonomous Region, China | [51] | |
| (R)-7-Hydroxymellein (36) | Penicillium sp. 05070032-C | Alibertia macrophylla (Leaves, Rubiaceae) | Mogi-Guaçu, Săo Paulo, Brazil | [17] |
| Xylaria cubensis BCRC 09F 0035 | Litsea akoensis Hayata (Leaves, Lauraceae) | Kaohsiung, Taiwan | [109] | |
| (3R,4R)-4,7-Dihydroxymellein (37) | Penicillium sp. 05070032-C | Alibertia macrophylla (Leaves, Rubiaceae) | Mogi-Guaçu, Săo Paulo, Brazil | [17] |
| Angelicoin A (38) | Aspergillus versicolor 0456 | Nicotiana sanderae (Leaves, Solanaceae) | Shilin, Yunnan Province, China | [122] |
| Aspergillus versicolor | Paris marmorata Stearn (Rhizomes, Melanthiaceae) | Dali, Yunnan, China | [93] | |
| Periplanetin A (39) | Penicillium oxalicum 0403 | Nicotiana sanderae (Leaves, Solanaceae) | Shilin, Yunnan Province, China | [44] |
| (3R)-Methyl-8-hydroxy-6-(hydroxymethyl)-7-methoxydihydroisocoumarin (40) | Aspergillus versicolor | Nicotiana tabacum (Rhizomes, Solanaceae) | Chuxiong, Yunnan, China | [112] |
| (3R)-Methyl-7,8-dimethoxy-6-(hydroxymethyl) dihydro-isocoumarin (41) | Aspergillus versicolor | Nicotiana tabacum (Rhizomes, Solanaceae) | Chuxiong, Yunnan, China | [112] |
| (R)-6-Hydroxymellein (42) | Aspergillus versicolor | Nicotiana tabacum (Rhizomes, Solanaceae) | Chuxiong, Yunnan, China | [112] |
| Lachnum palmae | Przewalskia tangutica Maxim. (Leaves, Solanaceae) | Linzhou Country of the Tibet Autonomous Region, | [51] | |
| Seltsamia galinsogisoli sp. nov. SYPF 7336 | Galinsoga parviflora (Whole plant, Asteraceae) | Huludao, China | [78] | |
| 6,8-Dimethoxy-3-methyl-3,4-dihydro-1H-isochromen-1-one (43) | Aspergillus versicolor | Nicotiana tabacum (Rhizomes, Solanaceae) | Chuxiong, Yunnan, China | [112] |
| Periplanetin B (44) | Aspergillus versicolor | Nicotiana tabacum (Rhizomes, Solanaceae) | Chuxiong, Yunnan, China | [112] |
| Arundinone A (45) | Microsphaeropsis arundinis | Ulmus macrocarpa (Stems, Ulmaceae) | Dongling Mountain, Beijing, China | [123] |
| Aspergillspin F (46) | Aspergillus sp. SCSIO 41501 | Melitodes squamata (Gorgonian, Plexauridae) | South China Sea, Sanya Hainan Province, China | [70] |
| (3R)-5-Carbomethoxymellein (47) 5-Carbomethyoxy-3,4-dihydro-8-hydroxy-(3R)-methylisocoumarin | Centra albureau voor Schimmel cultures 120379 | Picea glauca (Leaves, Pinaceae) | Sussex, New Brunswick, Canada | [119] |
| Xylaria sp. PSU-G12 | Garcinia hombroniana (Branch, Clusiaceae) | Songkhla province, Thailand | [95] | |
| (3R)-5-Formylmellein (48) 3,4-Dihydro-5-formyl-8-hydroxy-(3R)-methylisocoumarin | Centraalbureau voor Schimmel 120379 | Picea glauca (Leaves, Pinaceae) | Sussex, New Brunswick, Canada | [119] |
| Xylarellein (49) | Xylaria sp. PSU-G12 | Garcinia hombroniana (Branch, Clusiaceae) | Songkhla province, Thailand | [95] |
| (3R)-5-Carboxylmellein (50) | Xylaria sp. PSU-G12 | Garcinia hombroniana (Branches, Clusiaceae) | Songkhla province, Thailand | [95] |
| Gamahorin (51) | Pestalotiopsis heterocornis | Phakellia fusca (Sponge, Axinellidae | Xisha Islands, China | [75] |
| Versicoumarin B (52) | Aspergillus versicolor | Paris marmorata Stearn (Rhizomes, Melanthiaceae) | Dali, Yunnan, China | [93] |
| Versicoumarin C (53) | Aspergillus versicolor | Paris marmorata Stearn (Rhizomes, Melanthiaceae) | Dali, Yunnan, China | [93] |
| S-(−)-6-Hydroxy-8-methoxy-4-(1′-hydroxyethyl)-isocoumarin (54) | Talaromyces Amestolkiae YX1 | Kandelia obovata (Leaves, Rhizophoraceae) | Zhanjiang, Guangdong Province, China | [62] |
| Acetic acid 6,8-dihydroxy-3,5-dimethyl-1-oxo-1H-isochromen-4-ylmethyl ester (55) | Scytalidium sp. 5681 | Salix sp. (Leaves, Salicaceae) | Harz Mountains, Lower Saxony, Germany | [27] |
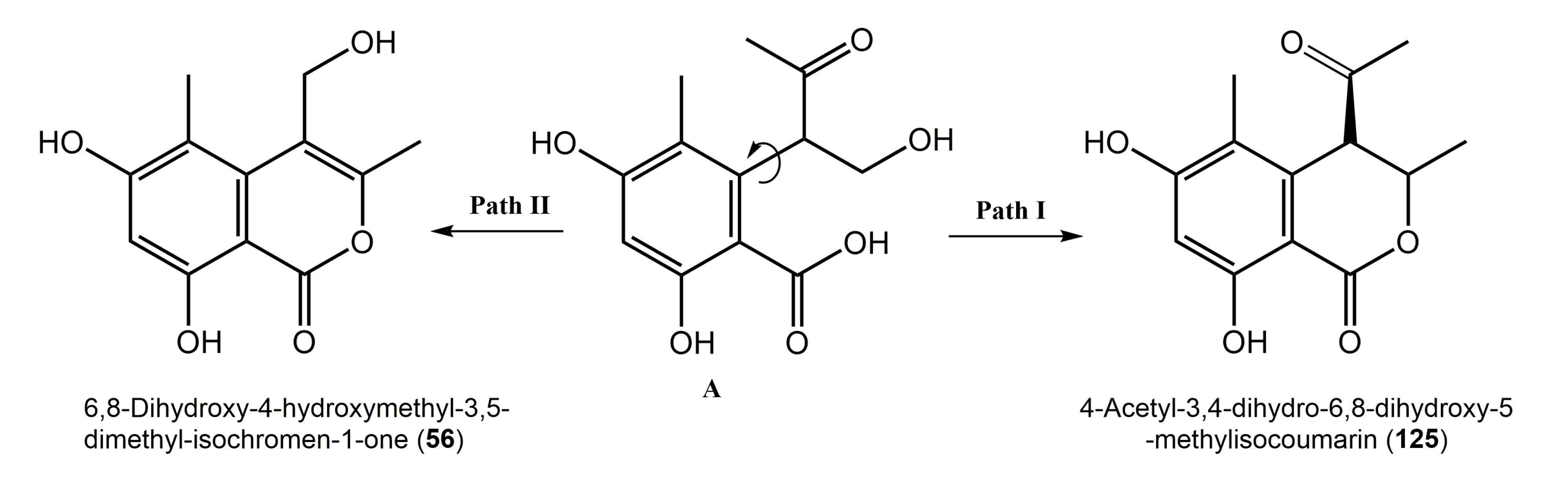
| 6,8-Dihydroxy-4-hydroxymethyl-3,5-dimethyl-isochromen-1-one (56) | Scytalidium sp. 5681 | Salix sp. (Leaves, Salicaceae) | Harz Mountains, Lower Saxony, Germany | [27] |
| Decarboxycitrinone (57) | Scytalidium sp. 5681 | Salix sp. (Leaves, Salicaceae) | Harz Mountains, Lower Saxony, Germany | [27] |
| 4-Acetyl-6,8-dihydroxy-5-methyl-2- benzopyran-1-one (58) | Scytalidium sp. 5681 | Salix sp. (Leaves, Salicaceae) | Harz Mountains, Lower Saxony, Germany | [27] |
| 6,8-Diacetoxy-3,5-dimethylisocoumarin (59) | Mycelia sterile 4567 | Canadian thistle Cirsium arvense (Asteraceae) | Lower Saxony, Germany | [20] |
| Penicilisorin (60) | Penicillium sclerotiorum PSUA13 | Garcinia atroviridis (Leaves, Clusiaceae) | Yala Province, Thailand | [124] |
| Pestalotiorin (61) | Pestalotiopsis sp. PSU-ES194 | Enhalus acoroides (Leaves, Hydrocharitaceae) | Songkla Province, Thailand | [41] |
| Tabaisocoumarin A (62) | Aspergillus versicolor 0456 | Nicotiana sanderae (Leaves, Solanaceae) | Shilin, Yunnan Province, China | [122] |
| Aspergillus oryzae | Paris polyphylla var. yunnanensis (Franch.) Hand.-Mazz. (Rhizomes, Liliaceae) | Dali, Yunnan, China | [94] | |
| 3-Acetoxyl-8-hydroxyl-isocoumarin (63) | Sarcosomataceae sp. NO.49-14-2-1 | Everniastrum nepalense (Taylor) Hale ex Sipman (Lichen, Parmeliaceae) | Panzhihua, Sichuan province, China | [121] |
| 6-Hydroxy-4-(1-hydroxyethyl)-8-methoxy-1H-isochromen-1-one (64) | Talaromyces amestolkiae | Kandelia obovata (Leaves, Rhizophoraceae) | Zhanjiang, Guangdong Province, China | [62] |
| S-(−)-5,6,8-Trihydroxy-4-(1′-hydroxyethyl)isocoumarin (65) | Penicillium sp. MWZ14-4 | Unidentified sponge GX-WZ-2008001 (Inner fresh tissues) | Weizhou, South China Sea, China | [52] |
| Talaromyces amestolkiae | Kandelia obovata (Leave, Rhizophoraceae | Zhanjiang, Guangdong Province, China | [62] | |
| Sescandelin (66) | Penicillium sp. MWZ14-4 | Unidentified sponge GX-WZ-2008001 (Inner fresh tissue) | Weizhou, South China Sea, China | [52] |
| Talaromyces amestolkiae | Kandelia obovata (Leaves, Rhizophoraceae) | Zhanjiang, Guangdong Province, China | [62] | |
| Terrecoumarin A (67) | Penicillium oxalicum 0403 | Nicotiana sanderae (Leaves, Solanaceae) | Shilin, Yunnan Province, China | [44] |
| Terrecoumarin B (68) | Penicillium oxalicum 0403 | Nicotiana sanderae (Leaves, Solanaceae) | Shilin, Yunnan Province, China | [44] |
| Terrecoumarin C (69) | Penicillium oxalicum 0403 | Nicotiana sanderae (Leaves, Solanaceae) | Shilin, Yunnan Province, China | [44] |
| Pestapyrone D (70) | Pestalotiopsis sp. | Photinia frasery (Leaves, Amygdaloideae) | Nanjing, Jiangsu, China | [22] |
| Pestapyrone E (71) | Pestalotiopsis sp. | Photinia frasery (Leaves, Amygdaloideae) | Nanjing, Jiangsu, China | [22] |
| LL-Z 1640-7 (72) | Peyronellaea glomerata XSB-01-15 | Amphimedon sp. (Sponge, Niphatidae) | Yongxin Island, Hainan Province, China | [65] |
| Aspergillspin G (73) | Aspergillus sp. SCSIO 41501 | Melitodes squamata (Gorgonian, Plexauridae) | South China Sea, Sanya Hainan Province, China | [70] |
| Acremonone E (74) | Acremonium sp. PSU-MA70 | Rhizophora apiculata (Branches, Rhizophoraceae) | Satun Province, Thailand | [125] |
| Acremonone F (75) | Acremonium sp. PSU-MA70 | Rhizophora apiculata (Branches, Rhizophoraceae) | Satun Province, Thailand | [125] |
| Acremonone G (76) | Acremonium sp. PSU-MA70 | Rhizophora apiculata (Branches, Rhizophoraceae) | Satun Province, Thailand | [125] |
| Myrothecium sp. OUCMDZ-2784 | Apocynum venetum (Leaves, Apocynaceae) | Dongying, China | [103] | |
| Acremonone H (77) | Acremonium sp. PSU-MA70 | Rhizophora apiculata (Branches, Rhizophoraceae) | Satun Province, Thailand | [125] |
| Daldiniside B (78) | Daldinia eschscholzii | Scaevola sericea Vahl (Branches, Goodeniaceae) | Hainan province, China | [54] |
| Daldiniside C (79) | Daldinia eschscholzii | Scaevola sericea Vahl (Branches, Goodeniaceae) | Hainan province, China | [54] |
| de-O-Methyldiaporthin (80) | Daldinia eschscholzii | Scaevola sericea Vahl (Branches, Goodeniaceae) | Hainan province, China | [54] |
| Penicillium coffeae MA-314 | Laguncularia racemose (Leaves, Combretaceae) | Hainan island, China | [47] | |
| Myrothelactone A (81) | Myrothecium sp. OUCMDZ-2784 | Apocynum venetum (Leaves, Apocynaceae) | Dongying, China | [103] |
| Myrothelactone B (82) | Myrothecium sp. OUCMDZ-2784 | Apocynum venetum (Leaves, Apocynaceae) | Dongying, China | [103] |
| 3-Methyl-8-hydroxyisocoumarin (83) | Sarcosomataceae sp. NO.49-14-2-1 | Everniastrum nepalense (Taylor) Hale ex Sipman (Lichen, Parmeliaceae) | Panzhihua, Sichuan province, China | [121] |
| 6,8-Dihydroxy-5-methoxy-3-methyl-1H-isochromen-1-one (84) | Talaromyces amestolkiae | Kandelia obovata (Leave, Rhizophoraceae) | Zhanjiang, Guangdong Province, China | [62] |
| Myrothelactone C (85) | Myrothecium sp. OUCMDZ-2784 | Apocynum venetum (Leaves, Apocynaceae) | Dongying, China | [103] |
| Myrothelactone D (86) | Myrothecium sp. OUCMDZ-2784 | Apocynum venetum (Leaves, Apocynaceae) | Dongying, China | [103] |
| Tubakialactone B (87) 8-Hydroxyl-3,4-bis(hydroxymethyl)-6-methoxy-4-methyl-1H-2-benzopyran-1-one | Tubakia sp. ECN-111 | Houttuynia cordata Thunb (Leaves, Saururaceae) | Chikusa-ku Nagoya city, Japan | [115] |
| Myrothecium sp. OUCMDZ-2784 | Apocynum venetum (Leaves, Apocynaceae) | Dongying, China | [103] | |
| Saccharonol A (88) 6,8-Dihydroxy-3-methylisocoumarin | Aspergillus similanensis sp. nov. KUFA 0013 | Rhabdermia sp. (Sponge, Rhabderemiidae) | Phang Nga Province, Thailand | [48] |
| Botryosphaeria sp. KcF6 | Kandelia candel (Fruits, Rhizophoraceae) | Daya Bay, Shenzhen, China | [36] | |
| Aspergillus versicolor KJ801852 | Paris polyphylla var. yunnanensis (Rhizomes, Melanthiaceae) | Dali, Yunnan, China | [126] | |
| Myrothecium sp. OUCMDZ-2784 | Apocynum venetum (Leaves, Apocynaceae) | Dongying, China | [103] | |
| Penicillium coffeae MA-314 | Laguncularia racemose (Leaves, Combretaceae) | Hainan island, China | [47] | |
| Similanpyrone B (89) 6,8-Dihydroxy-3,7-dimethylisocoumarin | Aspergillus similanensis sp. nov. KUFA 0013 | Rhabdermia sp. (Sponge, Rhabderemiidae) | Phang Nga Province, Thailand | [48] |
| Pestalotiopsis sp. HQD-6 | Rhizophora mucronata (Leaves, Rhizophoraceae) | Hainan Island, China | [126] | |
| Reticulol (90) | Aspergillus similanensis sp. nov. KUFA 0013 | Rhabdermia sp. (Sponge, Rhabderemiidae) | Phang Nga Province, Thailand | [48] |
| Biscogniauxia capnodes | Averrhoa carambola L. (Fruits, Oxalidaceae) | Kandy, Central Province, Sri Lanka | [96] | |
| 6-Hydroxy-4-hydroxymethyl-8-methoxy-3-methylisocoumarin (91) | Endophytic fungus (No. GX4-1B) | Bruguiera gymnoihiza (L.) Savigny (Branch, Rhizophoraceae) | South China Sea in Guangxi province, China | [127] |
| 6-Hydroxy-8-methoxy-3,4-dimethylisocoumarin (92) | Talaromyces amestolkiae | Kandelia obovata (Leave, Rhizophoraceae) | Zhanjiang, Guangdong Province, China | [62] |
| 3,4-Dimethyl-6,8-dihydroxyisocoumarin (93) | Talaromyces amestolkiae | Kandelia obovata (Leaves, Rhizophoraceae) | Zhanjiang, Guangdong Province, China | [62] |
| Nectria pseudotrichia 120-1NP | Gliricidia sepium (Stems, Fabaceae) | Wanagama forest of Universitas, Yogyakarta, Indonesia | [128] | |
| 6-Hydroxy-4-hydroxymethyl-8-methoxy-3-methyl-isocoumarin (94) | Talaromyces amestolkiae | Kandelia obovata (Leaves, Rhizophoraceae) | Zhanjiang, Guangdong Province, China | [62] |
| Sescandelin B (95) | Talaromyces amestolkiae | Kandelia obovata (Leaves, Rhizophoraceae) | Zhanjiang, Guangdong Province, China | [62] |
| Myrothecium sp. OUCMDZ-2784 | Apocynum venetum (Leaves, Apocynaceae) | Dongying, China | [103] | |
| 6-Hydroxy-3-hydroxymethyl-8-methoxyisocoumarin (96) | Penicillium oxalicum 0403 | Nicotiana sanderae (Leaves, Solanaceae) | Shilin, Yunnan Province, China | [44] |
| 4,6-Dihydroxy-3,9-dehydromellein (97) | Penicillium oxalicum 0403 | Nicotiana sanderae (Leaves, Solanaceae) | Shilin, Yunnan Province, China | [44] |
| Aspergillus versicolor KJ801852 | Paris polyphylla var. yunnanensis (Rhizomes, Melanthiaceae) | Dali, Yunnan, China | [126] | |
| Banksiamarin A (98) | Aspergillus banksianus sp. nov | Banksia integrifolia (Leaves, Proteaceae) | Collaroy, New South Wales, Australia | [30] |
| Banksiamarin B (99) | Aspergillus banksianus sp. nov | Banksia integrifolia (Leaves, Proteaceae) | Collaroy, New South Wales, Australia | [30] |
| 6,8-Dihydroxyisocoumarin-3-carboxylic acid (100) | Bionectria sp. | Raphia taedigera (Seeds, Arecaceae) | Haut Plateaux region, Cameroon | [82] |
| Nectria pseudotrichia 120-1NP | Gliricidia sepium (Stem, Fabaceae) | Wanagama forest of Universitas, Yogyakarta, Indonesia | [128] | |
| Aspergillus sp. HN15-5D | Acanthus ilicifolius (Leaves, Acanthaceae) | Dongzhaigang Mangrove National Nature Reserve, Hainan Island, China. | [73] | |
| Nectriapyrone A (101) | Nectria pseudotrichia 120-1NP | Gliricidia sepium (Stems, Fabaceae) | Wanagama forest of Universitas, Yogyakarta, Indonesia | [128] |
| Nectriapyrone B (102) | Nectria pseudotrichia 120-1NP | Gliricidia sepium (Stems, Fabaceae) | Wanagama forest of Universitas, Yogyakarta, Indonesia | [128] |
| 6-O-Methylreticulol (103) 8-Hydroxy-6,7-dimethoxy-3-methylisocoumarin | Xylariaceae sp. QGS 01 | Quercus gilva Blume (Stems, Fagaceae) | EhimeUniversity Garden, Ehime Prefecture, Japan | [102] |
| Biscogniauxia capnodes | Averrhoa carambola L. (Fruits, Oxalidaceae) | Home garden in Kandy, Central Province, Sri Lanka | [96] | |
| 7-Hydroxy-3,5-dimethylisochromen-1-one (104) | Phoma sp. YE3135 | Aconitum vilmorinianum (Roots, Ranunculaceae) | Yunnan University, China | [26] |
| 6,8-Dihydroxy-3-hydroxymethylisocoumarin (105) | Aspergillus versicolor KJ801852 | Paris polyphylla var. yunnanensis (Rhizomes, Melanthiaceae) | Dali, Yunnan, China | [126] |
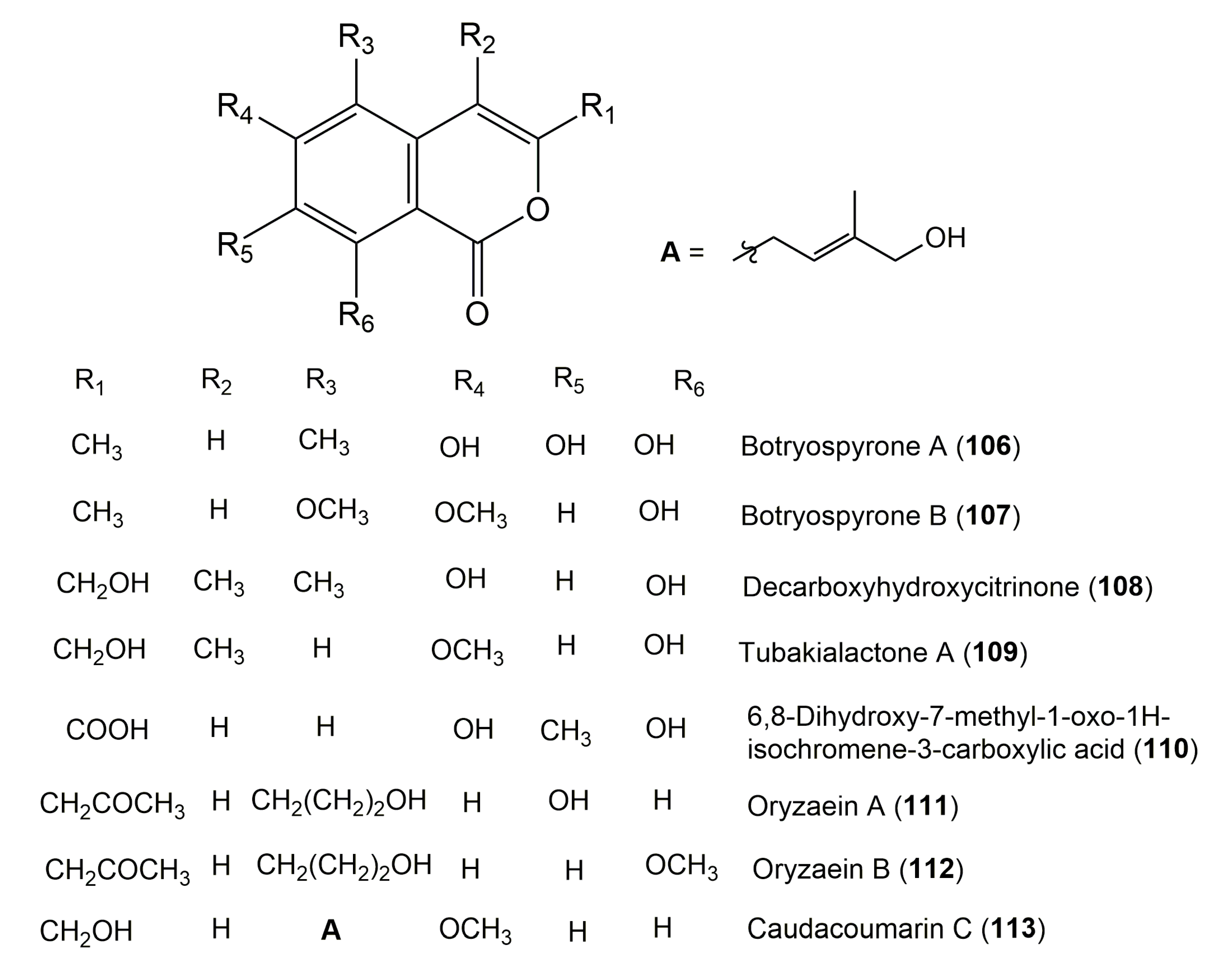
| Botryospyrone A (106) | Botryosphaeria ramosa L29 | Myoporum bontioides (Leaves, Scrophulariaceae) | Leizhou Peninsula, China | [71] |
| Botryospyrone B (107) | Botryosphaeria ramosa L29 | Myoporum bontioides (Leaves, Scrophulariaceae) | Leizhou Peninsula, China | [71] |
| Decarboxyhydroxycitrinone (108) | Arthrinium sacchari | Unidentified sponge | The coast of Atami-shi, ShizuokaPrefecture, Japan | [90] |
| Tubakialactone A (109) 8-Hydroxyl-3- hydroxymethyl-6-methoxy-4-methyl-1H-2-benzopyran-1-one | Tubakia sp. ECN-111 (Melanconidaceae) | Houttuynia cordata Thunb (Leaves, Saururaceae) | Chikusa-ku Nagoya city, Japan | [115] |
| 6,8-Dihydroxy-7-methyl-1-oxo-1H-isochromene-3-carboxylic acid (110) | Pestalotiopsis coffeae | Caryota mitis (Palm, Arecaceae) | Hainan Province, China | [129] |
| Oryzaein A (111) | Aspergillus oryzae | Paris polyphylla var. yunnanensis (Franch.) Hand-Mazz. (Rhizomes, Liliaceae) | Dali, Yunnan, China | [94] |
| Oryzaein B (112) | Aspergillus oryzae | Paris polyphylla var. yunnanensis (Franch.) Hand.-Mazz. (Rhizomes, Liliaceae) | Dali, Yunnan, China | [94] |
| Caudacoumarin C (113) | Aspergillus oryzae | Paris polyphylla var. yunnanensis (Franch.) Hand.-Mazz. (Rhizomes, Liliaceae) | Dali, Yunnan, China | [94] |
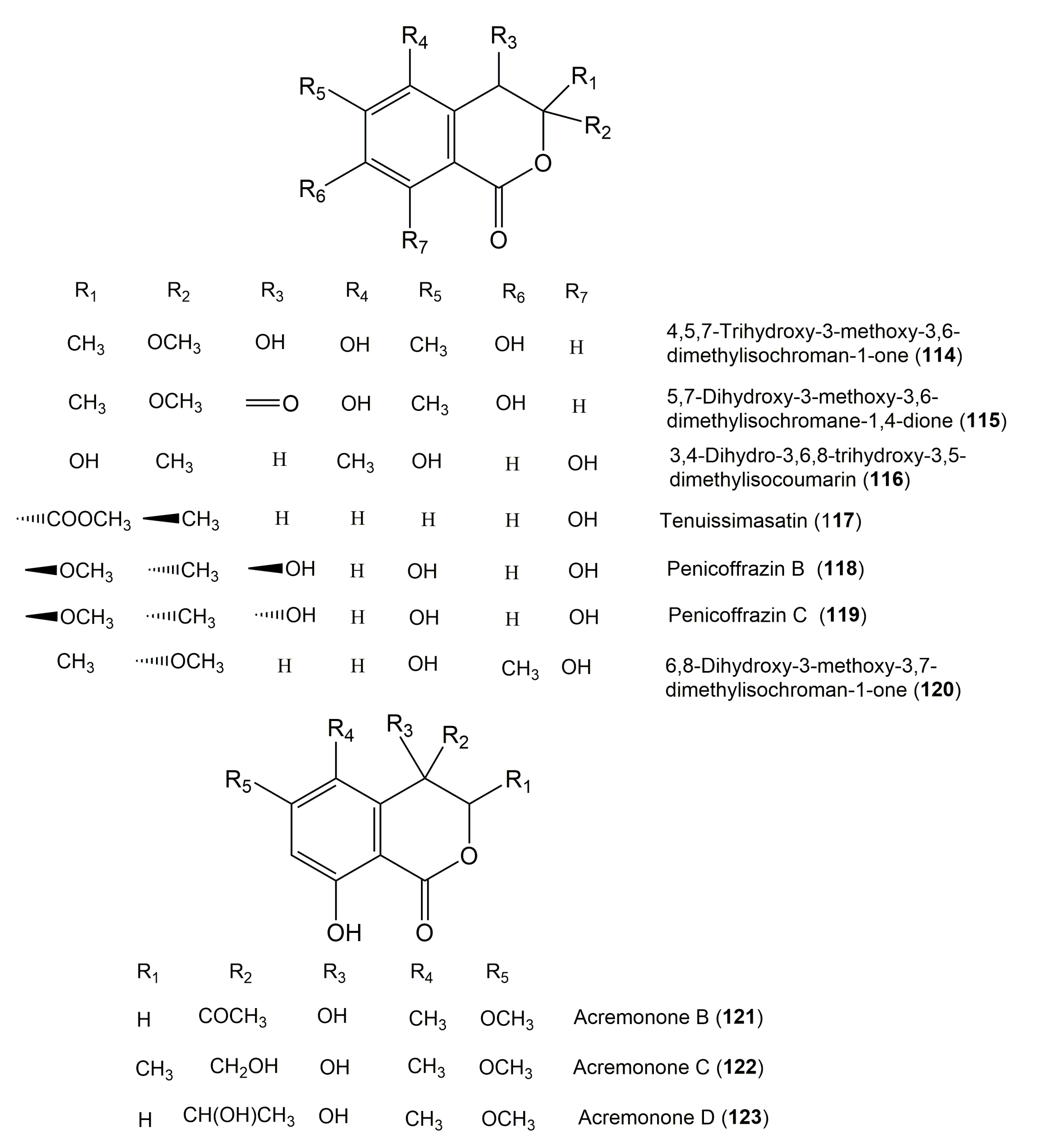
| 4,5,7-Trihydroxy-3-methoxy-3,6-dimethylisochroman-1-one (114) | Aspergillus sp. 16-5B | Sonneratia apetala (Leaves, Lythraceae) | Dongzhaigang Mangrove National Nature Reserve in Hainan Island, China | [38] |
| 5,7-Dihydroxy-3-methoxy-3,6-dimethylisochromane-1,4-dione (115) | Aspergillus sp. 16-5B | Sonneratia apetala (Leaves, Lythraceae) | Dongzhaigang Mangrove National Nature Reserve in Hainan Island, China | [38] |
| 3,4-Dihydro-3,6,8-trihydroxy-3,5-dimethylisocoumarin (116) | Mycelia sterile 4567 | Canadian thistle Cirsium arvense (Asteraceae) | Lower Saxony, Germany | [20] |
| Tenuissimasatin (117) | Alternaria tenuissima | Erythrophleum fordii (Barks, Fabaceae) | Nanning, Guangxi Province, China | [130] |
| Penicoffrazin B (118) | Penicillium coffeae MA-314 | Laguncularia racemose (Leaves, Combretaceae) | Hainan island, China | [47] |
| Penicoffrazin C (119) | Penicillium coffeae MA-314 | Laguncularia racemose (Leaves, Combretaceae) | Hainan island, China | [47] |
| 6,8-Dihydroxy-3-methoxy-3,7-dimethylisochroman-1-one (120) | Pestalotiopsis coffeae | Caryota mitis (Palm, Arecaceae) | Hainan Province, China | [129] |
| Acremonone B (121) | Acremonium sp. PSU-MA70 | Rhizophora apiculata (Branches, Rhizophoraceae) | Satun Province, Thailand | [125] |
| Acremonone C (122) | Acremonium sp. PSU-MA70 | Rhizophora apiculata (Branches, Rhizophoraceae) | Satun Province, Thailand | [125] |
| Acremonone D (123) | Acremonium sp. PSU-MA70 | Rhizophora apiculata (Branches, Rhizophoraceae) | Satun Province, Thailand | [125] |
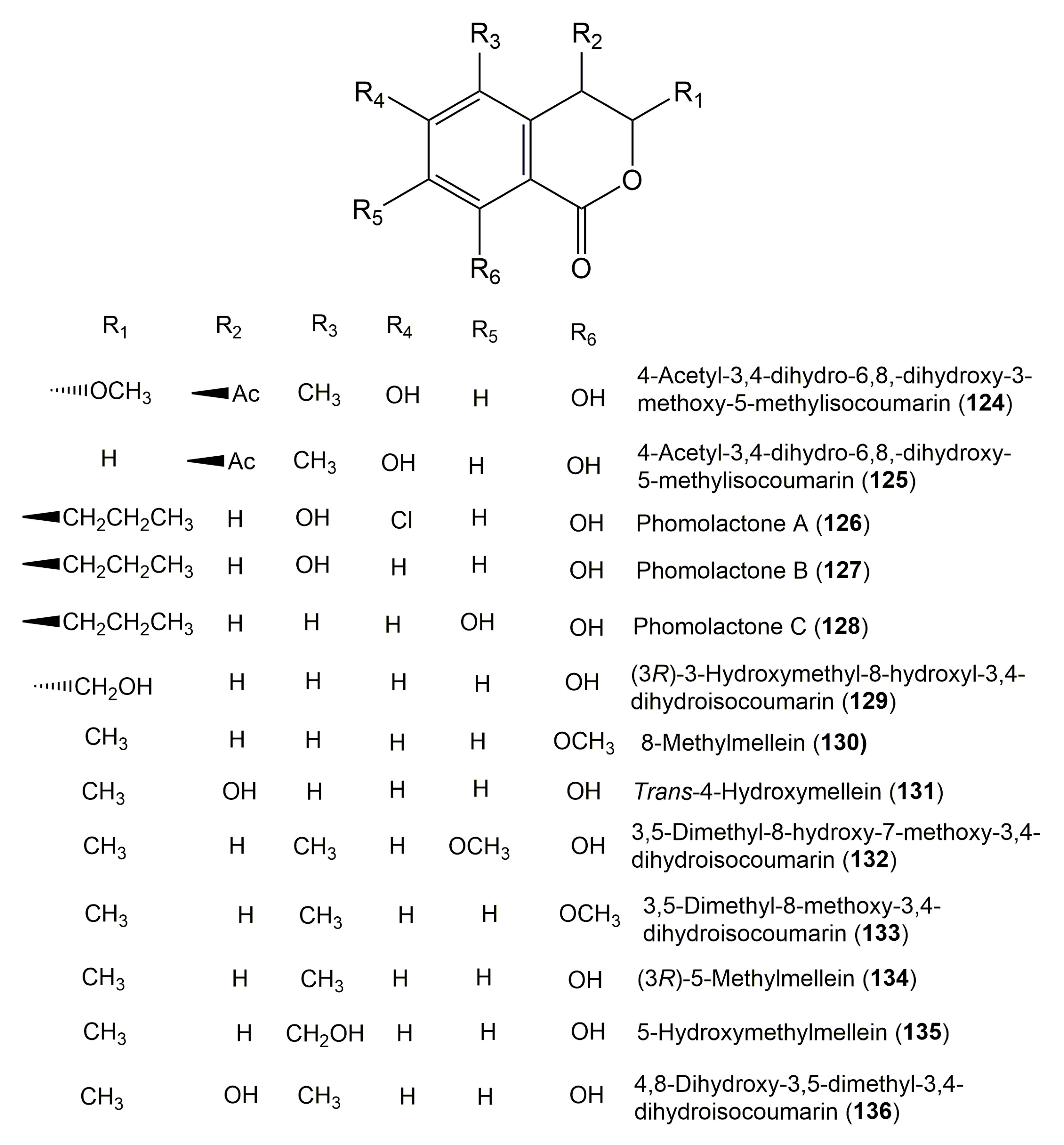
| 4-Acetyl-3,4-dihydro-6,8,-dihydroxy-3-methoxy-5-methylisocoumarin (124) | Mycelia sterile 4567 | Canadian thistle Cirsium arvense (Asteraceae) | Lower Saxony, Germany | [20] |
| 4-Acetyl-3,4-dihydro-6,8-dihydroxy-5-methylisocoumarin (125) | Mycelia sterile 4567 | Canadian thistle Cirsium arvense (Asteraceae) | Lower Saxony, Germany | [20] |
| Phomolactone A (126) | Phomopsis sp. 7233 | Laurus azorica (Leaves, Lauraceae) | Gomera, Spain | [42] |
| Aspergillus versicolor | Paris marmorata Stearn (Rhizomes, Melanthiaceae) | Dali, Yunnan, China | [93] | |
| Phomolactone B (127) | Phomopsis sp. 7233 | Laurus azorica (Leaves, Lauraceae) | Gomera, Spain | [42] |
| Aspergillus versicolor | Paris marmorata Stearn (Rhizomes, Melanthiaceae) | Dali, Yunnan, China | [93] | |
| Phomolactone C (128) | Phomopsis sp. 7233 | Laurus azorica (Leaves, Lauraceae) | Gomera, Spain | [42] |
| (3R)-3-hydroxymethyl-8-hydroxyl-3,4-dihydroisocoumarin (129) | Sarcosomataceae sp. NO.49-14-2-1 | Everniastrum nepalense (Taylor) Hale ex Sipman (Lichen, Parmeliaceae) | Panzhihua, Sichuan province, China | [121] |
| 8-Methylmellein (130) | Sarcosomataceae sp. NO.49-14-2-1 | Everniastrum nepalense (Taylor) Hale ex Sipman (Lichen, Parmeliaceae) | Panzhihua, Sichuan province, China | [121] |
| Pestalotiopsis sp. HHL101 | Rhizophora stylosa (Branches, Rhizophoraceae) | Dong Zhai Gang-Mangrove Garden, Hainan Island, China | [72] | |
| Trans-4-hydroxymellein (131) | Penicillium sp.1 and sp.2 | Alibertia macrophylla (Leaves, Rubiaceae) | Mogi-Guaçu, São Paulo, Brazil | [18] |
| Botryosphaeria sp. KcF6 | Kandelia candel (Fruits, Rhizophoraceae), | Daya Bay, Shenzhen, China | [36] | |
| Sarcosomataceae sp. NO.49-14-2-1 | Everniastrum nepalense (Taylor) Hale ex Sipman (Lichen, Parmeliaceae) | Panzhihua, Sichuan province, China | [121] | |
| Lachnum palmae | Przewalskia tangutica Maxim. (Leaves, Solanaceae) | Linzhou Country of the Tibet Autonomous Region, China | [51] | |
| 3,5-Dimethyl-8-hydroxy-7-methoxy-3,4-dihydroisocoumarin (132) | Cytospora eucalypticola SS8 | Eucalyptus perriniana (Bark, Myrtaceae) | Royal Botanic Gardens, Kew, United Kingdom | [55] |
| 3,5-dimethyl-8-methoxy-3,4-dihydroisocoumarin (133) | Cytospora eucalypticola SS8 | Eucalyptus perriniana (Barks, Myrtaceae) | Royal Botanic Gardens, Kew, United Kingdom | [55] |
| (3R)-5-Methylmellein (134) 3,4-Dihydro-(3R),5-dimethyl-8-hydroxyisocoumarin | Cytospora eucalypticola SS8 | Eucalyptus perriniana (Barks, Myrtaceae) | Royal Botanic Gardens, Kew, United Kingdom | [55] |
| Centraalbureau voor Schimmel cultures (120379) | Picea glauca (Leaves, Pinaceae) | Sussex, New Brunswick, Canada | [119] | |
| Xylaria sp. PSU-G12 | Garcinia hombroniana (Branchs, Clusiaceae) | Songkhla province, Thailand | [95] | |
| Xylaria cubensis (Xylariaceae) BCRC 09F 0035 | Litsea akoensis Hayata (Leaves, Lauraceae) | Kaohsiung, Taiwan | [109] | |
| Biscogniauxia capnodes | Averrhoa carambola L. (Fruits, Oxalidaceae) | Home garden in Kandy, Central Province, Sri Lanka | [96] | |
| 5-Hydroxymethylmellein (135) 8-Hydroxy-5-hydroxymethyl-3-methyl-3,4-dihydroisocoumarin | Cytospora eucalypticola SS8 | Eucalyptus perriniana (Barks, Myrtaceae) | Royal Botanic Gardens, Kew, United Kingdom | [55] |
| 4,8-Dihydroxy-3,5-dimethyl-3,4-dihydroisocoumarin (136) | Cytospora eucalypticola SS8 | Eucalyptus perriniana (Barks, Myrtaceae) | Royal Botanic Gardens, Kew, United Kingdom | [55] |
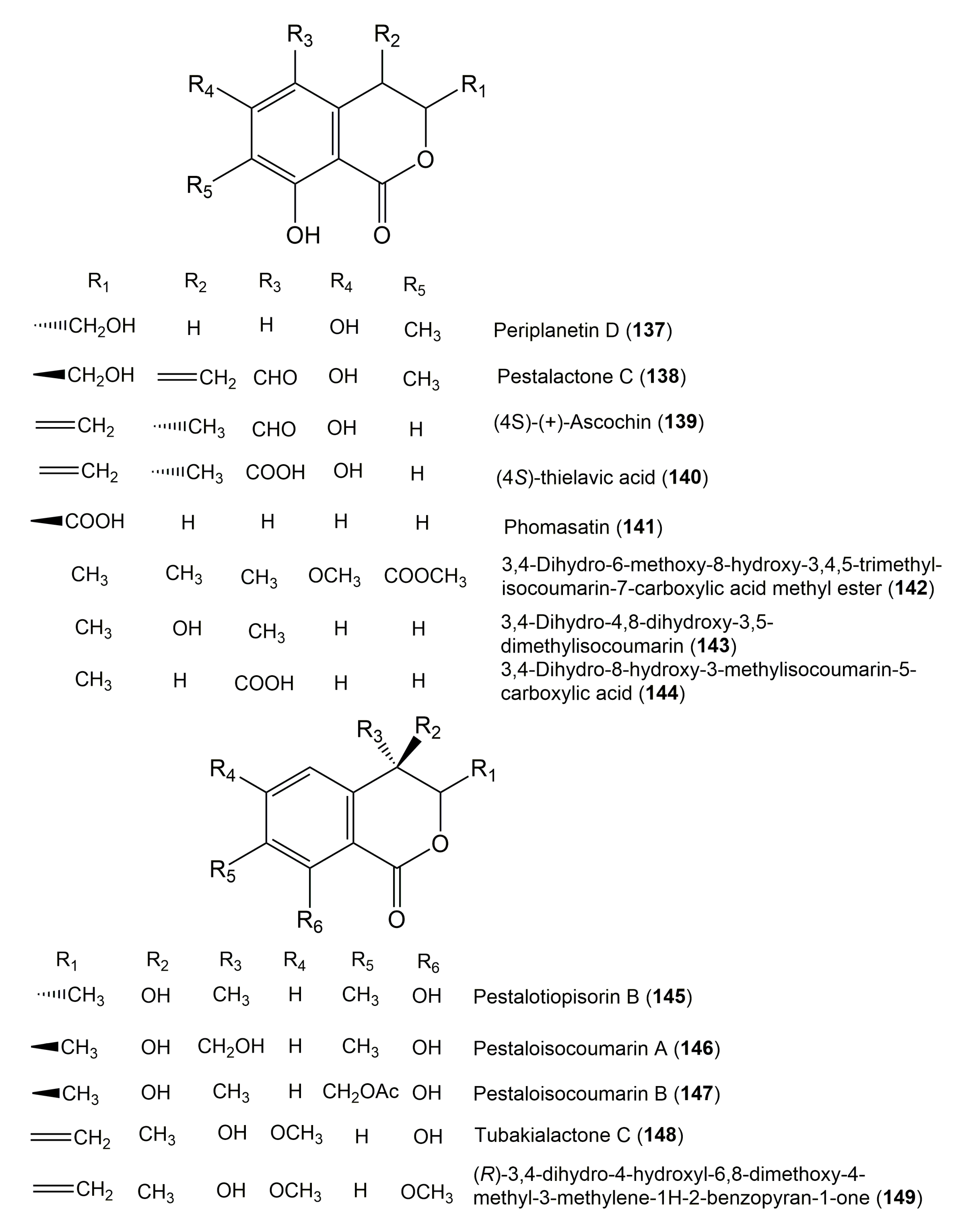
| Periplanetin D (137) | Aspergillus versicolor | Paris marmorata Stearn (Rhizomes, Melanthiaceae) | Dali, Yunnan, People’sRepublic of China, | [93] |
| Penicillium oxalicum 0403 | Nicotiana sanderae (Leaves, Solanaceae) | Shilin, Yunnan Province, China | [44] | |
| Pestalotiopsis coffeae | Caryota mitis (Palm, Arecaceae) | Hainan Province, China | [129] | |
| Pestalactone C (138) | Pestalotiopsis sp. | Photinia frasery (Leaves, Amygdaloideae) | Nanjing, Jiangsu, China | [22] |
| (4S) (+)-Ascochin (139) | Ascochyta sp. | Meliotus dentatus (Whole plant, Fabaceae) | Shores of the Baltic Sea, near Ahrenshoop, Germany | [117] |
| (4S)-Thielavic acid (140) | Thielavia sp. ECN-115 | Crassula ovata (Stems, Crassulaceae) | Chikusa-ku Nagoya city, Japan | [115] |
| Phomasatin (141) | Phoma sp. YN02-P-3 | Sumbaviopsis albicans J. J. Smith (Leaves, Euphorbiaceae) | Yunnan, China | [84] |
| 3,4-Dihydro-6-methoxy-8-hydroxy-3,4,5-trimethyl-isocoumarin-7-carboxylic acid methyl ester (142) | Fungus dz17 | Mangrove plant | South China Sea coast, China | [88] |
| 3,4-Dihydro-4,8-dihydroxy-3,5-dimethylisocoumarin (143) | Fungus dz17 | Mangrove plant | South China Sea coast, China | [88] |
| 3,4-Dihydro-8-hydroxy-3-methylisocoumarin-5-carboxylic acid (144) | Fungus dz17 | Mangrove plant | South China Sea coast, China | [88] |
| Pestalotiopisorin B (145) | Pestalotiopsis sp. HHL101 | Rhizophora stylosa (Branches, Rhizophoraceae) | Dong Zhai Gang-Mangrove Garden, Hainan Island, China | [72] |
| Pestaloisocoumarin A (146) | Pestalotiopsis heterocornis | Phakellia fusca (Sponge, Axinellidae | Xisha Islands, China | [75] |
| Pestaloisocoumarin B (147) | Pestalotiopsis heterocornis | Phakellia fusca (Sponge, Axinellidae | Xisha Islands, China | [75] |
| Tubakialactone C (148) (R)-3,4-Dihydro-4,8-dihydroxy-6-methoxy-4-methyl-3-methylene-1H-2-benzopyran-1-one | Tubakia sp. ECN-111 (Melanconidaceae) | Houttuynia cordata Thunb (Leaves, Saururaceae) | Chikusa-ku Nagoya city, Japan | [115] |
| (R)-3,4-dihydro-4-hydroxyl-6,8-dimethoxy-4-methyl-3-methylene-1H-2-benzopyran-1-one (149) | Tubakia sp. ECN-111 | Houttuynia cordata Thunb (Leaves, Saururaceae) | Chikusa-ku Nagoya city, Japan | [115] |
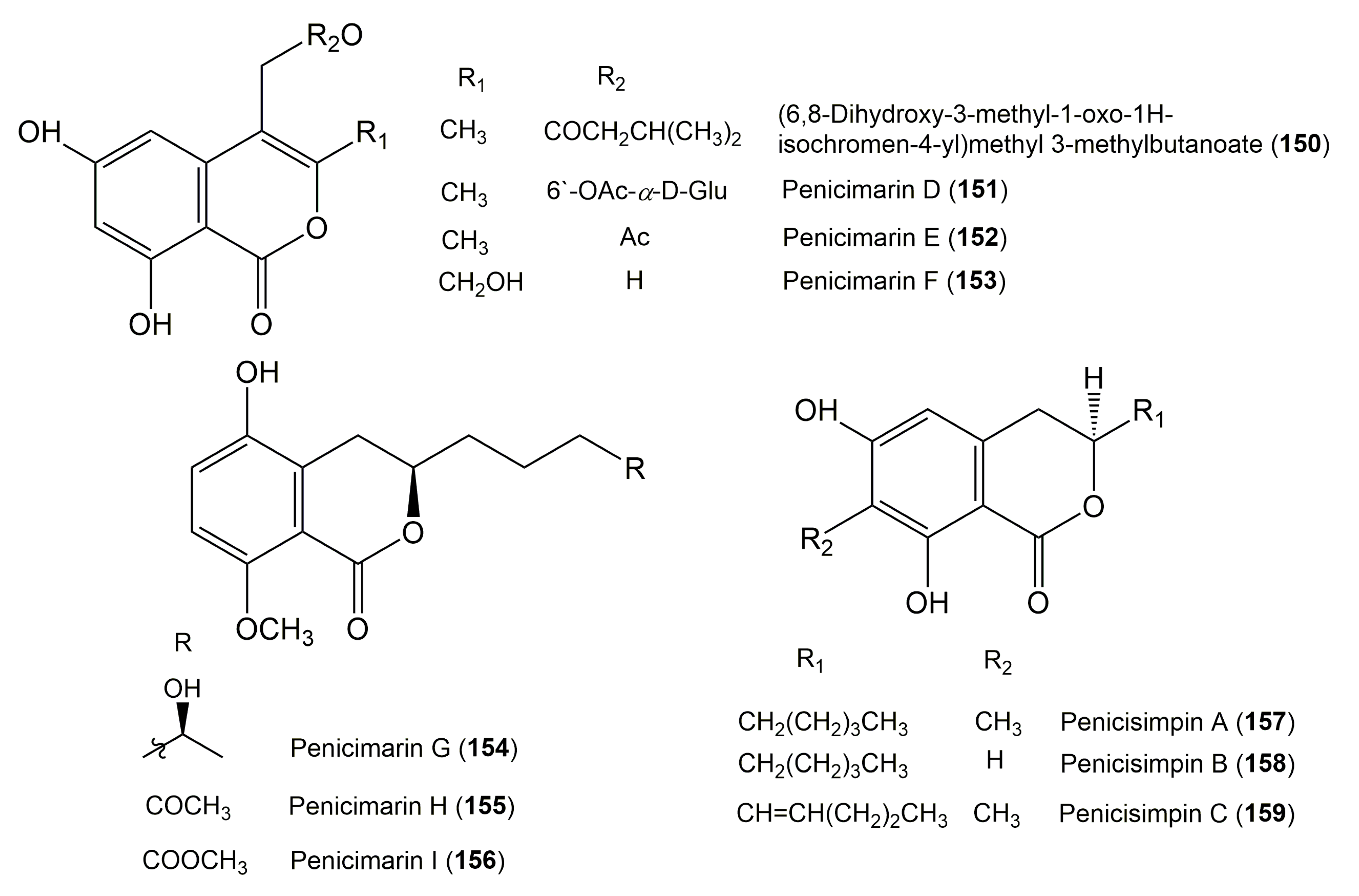
| (6,8-dihydroxy-3-methyl-1-oxo-1H-isochromen-4-yl)methyl-3-methylbutanoate (150) | Talaromyces amestolkiae | Kandelia obovata (Leaves, Rhizophoraceae) | Zhanjiang, Guangdong Province, China | [62] |
| Penicimarin D (151) | Penicillium sp. MWZ14-4 | Unidentified sponge GX-WZ-2008001 (Inner fresh tissues) | Weizhou, South China Sea, China | [52] |
| Penicimarin E (152) | Penicillium sp. MWZ14-4 | Unidentified sponge GX-WZ-2008001 (Inner fresh tissues) | Weizhou, South China Sea, China | [52] |
| Penicimarin F (153) | Penicillium sp. MWZ14-4 | Unidentified sponge GX-WZ-2008001 (Inner fresh tissues) | Weizhou, South China Sea, China | [52] |
| Aspergillus versicolor KJ801852 | Paris polyphylla var. yunnanensis (Rhizomes, Melanthiaceae) | Dali, Yunnan, China | [126] | |
| Penicimarin G (154) | Penicillium citrinum HL-5126 | Bruguiera sexangula var. rhynchopetala (Roots, Rhizophoraceae) | Hainan Island, P.R. China | [56] |
| Penicimarin H (155) | Penicillium citrinum HL-5126 | Bruguiera sexangula var. rhynchopetala (Roots, Rhizophoraceae) | Hainan Island, China | [56] |
| Penicimarin I (156) | Penicillium citrinum HL-5126 | Bruguiera sexangula var. rhynchopetala (Roots, Rhizophoraceae) | Hainan Island, China | [56] |
| Penicisimpin A (157) 3-(R)-6,8-Dihydroxy-7-methyl-3-pentylisochroman-1-one | Penicillium simplicissimum MA-332 | Bruguiera sexangula var. rhynchopetala (Roots, Rhizophoraceae) | Hainan Island, China | [58] |
| Penicisimpin B (158) 3-(R)-6,8-Dihydroxy-3-pentylisochroman-1-one | Penicillium simplicissimum MA-332 | Bruguiera sexangula var. rhynchopetala (Roots, Rhizophoraceae) | Hainan Island, China | [58] |
| Penicisimpin C (159) 3-(S)-6,8-Dihydroxy-7-methyl-3-(pent-1-enyl)isochroman-1-one | Penicillium simplicissimum MA-332 | Bruguiera sexangula var. rhynchopetala (Roots, Rhizophoraceae) | Hainan Island, China | [58] |
| Fusarentin 6-methyl ether (160) | Colletotrichum sp. CRI535-02 | Piper ornatum (Leaves, Piperaceae) | Tai Rom Yen National Park, Surat Thani Province, Thailand | [80] |
| Fusarentin 6,7-dimethyl ether (161) | Colletotrichum sp. CRI535-02 | Piper ornatum (Leaves, Piperaceae) | Tai Rom Yen National Park, Surat Thani Province, Thailand | [80] |
| 7-Butyl-6,8-dihydroxy-3(R)-pent-11-enylisochroman-1-one (162) | Geotrichum sp. | Crassocephalum crepidioides S. Moore (Stems, Asteraceae) | Songkhla Province, Southern Thailand | [74] |
| 7-But-15-enyl-6,8-dihydroxy-3(R)-pent-11-enylisochroman-1-one (163) | Geotrichum sp. | Crassocephalum crepidioides S. Moore (Stems, Asteraceae) | Songkhla Province, Southern Thailand | [74] |
| 7-Butyl-6,8-dihydroxy-3(R)-pentylisochroman-1-one (164) | Geotrichum sp. | Crassocephalum crepidioides S. Moore (Stems, Asteraceae) | Songkhla Province, Southern Thailand | [74] |
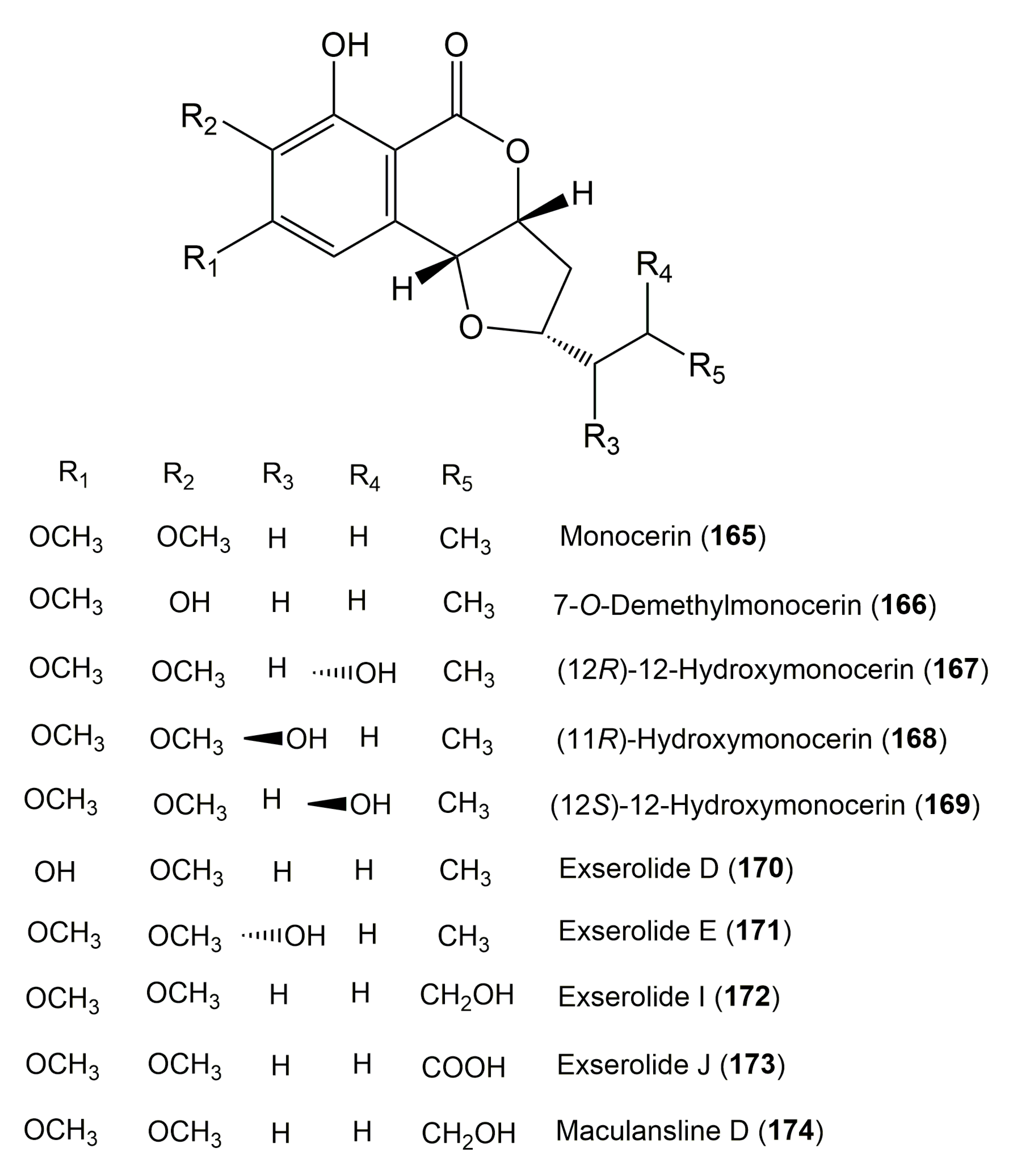
| Monocerin (165) | Microdochium bolleyi 8880 | Fagonia cretica (Leaves, Zygophyllaceae) | Gomera, Spain. | [49] |
| Exserohilum rostratum EU571210 | Stemona sp. (Leaves and roots, Stemonaceae) | Amphur Bangban, Ayutthaya Province, Thailand | [46] | |
| Colletotrichum sp. CRI535-02 | Piper ornatum (Leaves, Piperaceae) | Tai Rom Yen National Park, Surat Thani Province, Thailand | [80] | |
| Botryosphaeria sp. KcF6 | Kandelia candel (Fruits, Rhizophoraceae) | Daya Bay, Shenzhen, China | [36] | |
| Exserohilum rostratum ER1.1 | Bauhinia guianensis (Fabaceae) | Embrapa Amazônia Oriental Belém, Brazil | [59] | |
| Leptosphaena maculans | Osmanthus fragrans (Leaves, Oleaceae) | China | [104] | |
| 7-O-Demethylmonocerin (166) | Colletotrichum sp. CRI535-02 | Piper ornatum (Leaves, Piperaceae) | Tai Rom Yen National Park, Surat Thani Province, Thailand | [80] |
| Setosphaeria sp. SCSIO41009 | Callyspongia sp. (Sponge, Callyspongiidae) | Xuwen, Guangdong Province, China | [64] | |
| (12R)-Hydroxymonocerin (167) | Microdochium bolleyi 8880 | Fagonia cretica (Leaves, Zygophyllaceae) | Gomera, Spain | [49] |
| Exserohilum sp. KJ156361 | Acer truncatum (Leaves, Sapindaceae) | Dongling Mountain, Beijing, China. | [60] | |
| Setosphaeria sp. SCSIO41009 | Callyspongia sp. (Sponge, Callyspongiidae) | Guangdong Province, China | [64] | |
| Leptosphaena maculans | Osmanthus fragrans (Leaves, Oleaceae) | China | [104] | |
| (11R)-Hydroxymonocerin (168) | Exserohilum rostratum EU571210 | Stemona sp. (Leaves and roots, Stemonaceae) | Amphur Bangban, Ayutthaya Province, Thailand | [46] |
| Setosphaeria sp. SCSIO41009 | Callyspongia sp. (Sponge, Callyspongiidae) | Guangdong Province, China | [64] | |
| (12S)-Hydroxymonocerin (169) | Microdochium bolleyi 8880 | Fagonia cretica (Leaves, Zygophyllaceae) | Gomera, Spain. | [49] |
| Exserolide D (170) | Exserohilum sp. KJ156361 | Acer truncatum (Leaves, Sapindaceae) | Dongling Mountain, Beijing, China. | [60] |
| Aspergillus oryzae | Paris polyphylla var. yunnanensis (Franch.) Hand.-Mazz. (Rhizomes, Liliaceae) | Dali, Yunnan, China | [94] | |
| Exserolide E (171) | Exserohilum sp. KJ156361 | Acer truncatum (Leaves, Sapindaceae) | Dongling Mountain, Beijing, China. | [60] |
| Setosphaeria sp. SCSIO41009 | Callyspongia sp. (Sponge, Callyspongiidae) | Guangdong Province, China | [64] | |
| Exserolide I (172) | Setosphaeria sp. SCSIO41009 | Callyspongia sp. (Sponge, Callyspongiidae) | Guangdong Province, China | [64] |
| Exserolide J (173) | Setosphaeria sp. SCSIO41009 | Callyspongia sp. (Sponge, Callyspongiidae) | Guangdong Province, China | [64] |
| Maculansline D (174) Isomer of (12R)-12-hydroxymonocerin | Leptosphaena maculans | Osmanthus fragrans (Leaves, Oleaceae) | China | [104] |
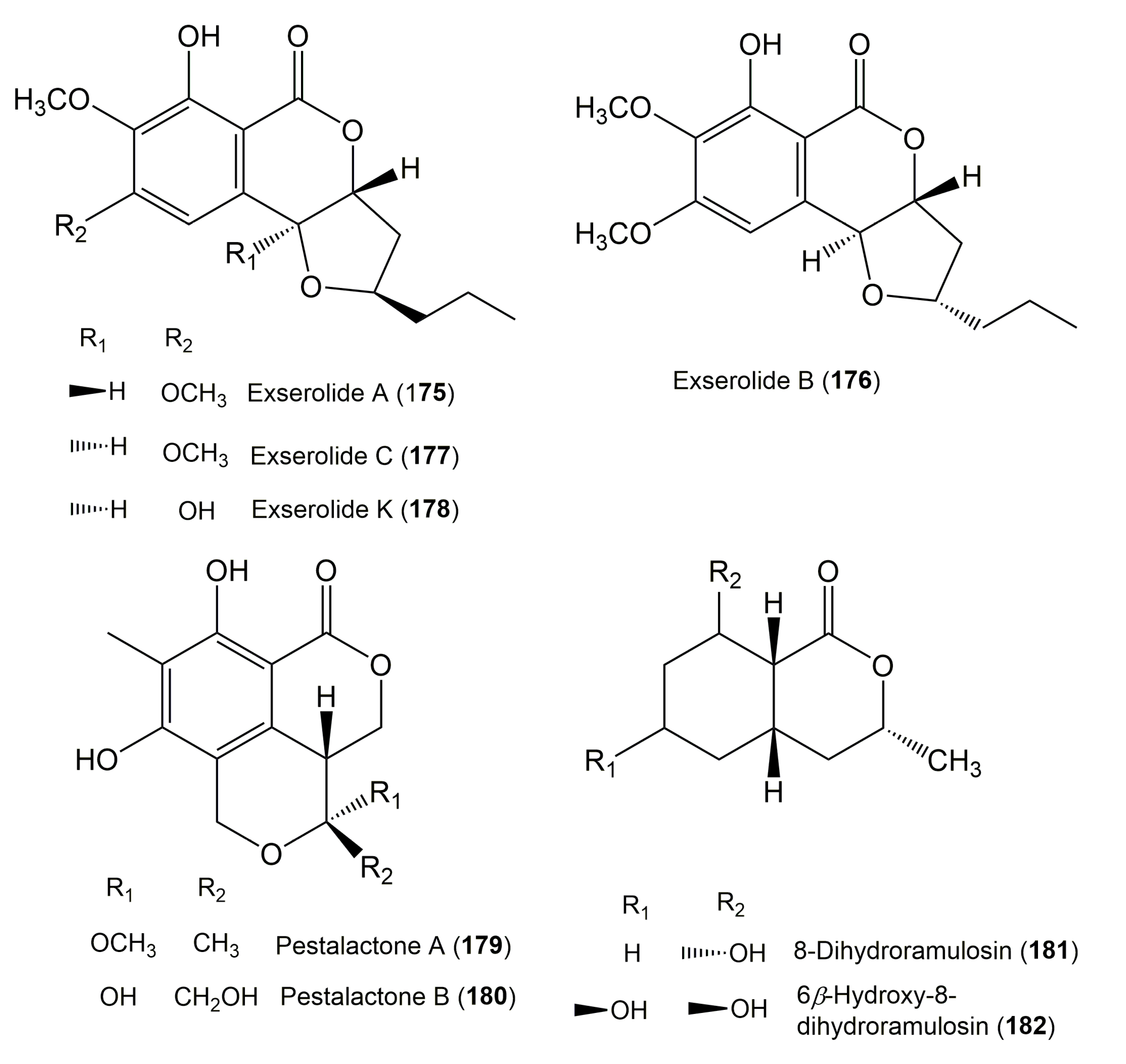
| Exserolide A (175) | Exserohilum sp. KJ156361 | Acer truncatum (Leaves, Sapindaceae) | Dongling Mountain, Beijing, China. | [60] |
| Exserolide B (176) | Exserohilum sp. KJ156361 | Acer truncatum (Leaves, Sapindaceae) | Dongling Mountain, Beijing, China. | [60] |
| Setosphaeria sp. SCSIO41009 | Callyspongia sp. (Sponge, Callyspongiidae) | Guangdong Province, China | [64] | |
| Exserolide C (177) | Exserohilum sp. KJ156361 | Acer truncatum (Leaves, Sapindaceae) | Dongling Mountain, Beijing, China | [60] |
| Setosphaeria sp. SCSIO41009 | Callyspongia sp. (Sponge, Callyspongiidae) | Guangdong Province, China | [64] | |
| Exserolide K (178) | Setosphaeria sp. SCSIO41009 | Callyspongia sp. (Sponge, Callyspongiidae) | Guangdong Province, China | [64] |
| Pestalactone A (179) | Pestalotiopsis sp. | Photinia frasery (Leaves, Amygdaloideae) | Nanjing, Jiangsu, China | [22] |
| Pestalactone B (180) | Pestalotiopsis sp. | Photinia frasery (Leaves, Amygdaloideae) | Nanjing, Jiangsu, China | [22] |
| 8-Dihydroramulosin (181) | Nigrospora sp. PSU-N24 | Garcinia nigrolineata (Branches, Clusiaceae) | Ton Nga Chang wildlife sanctuary, Songkhla province, Southern Thailand | [110] |
| Nigrospora sp. LLGLM003 | Moringa oleifera (Roots, Moringaceae) | Xiamen municipality, Fujian Province, China | [53] | |
| 6β-Hydroxy-8-dihydroramulosin (182) | Nigrospora sp. PSU-N24 | Garcinia nigrolineata (Branches, Clusiaceae) | Ton Nga Chang wildlife sanctuary, Songkhla province, Southern Thailand | [110] |
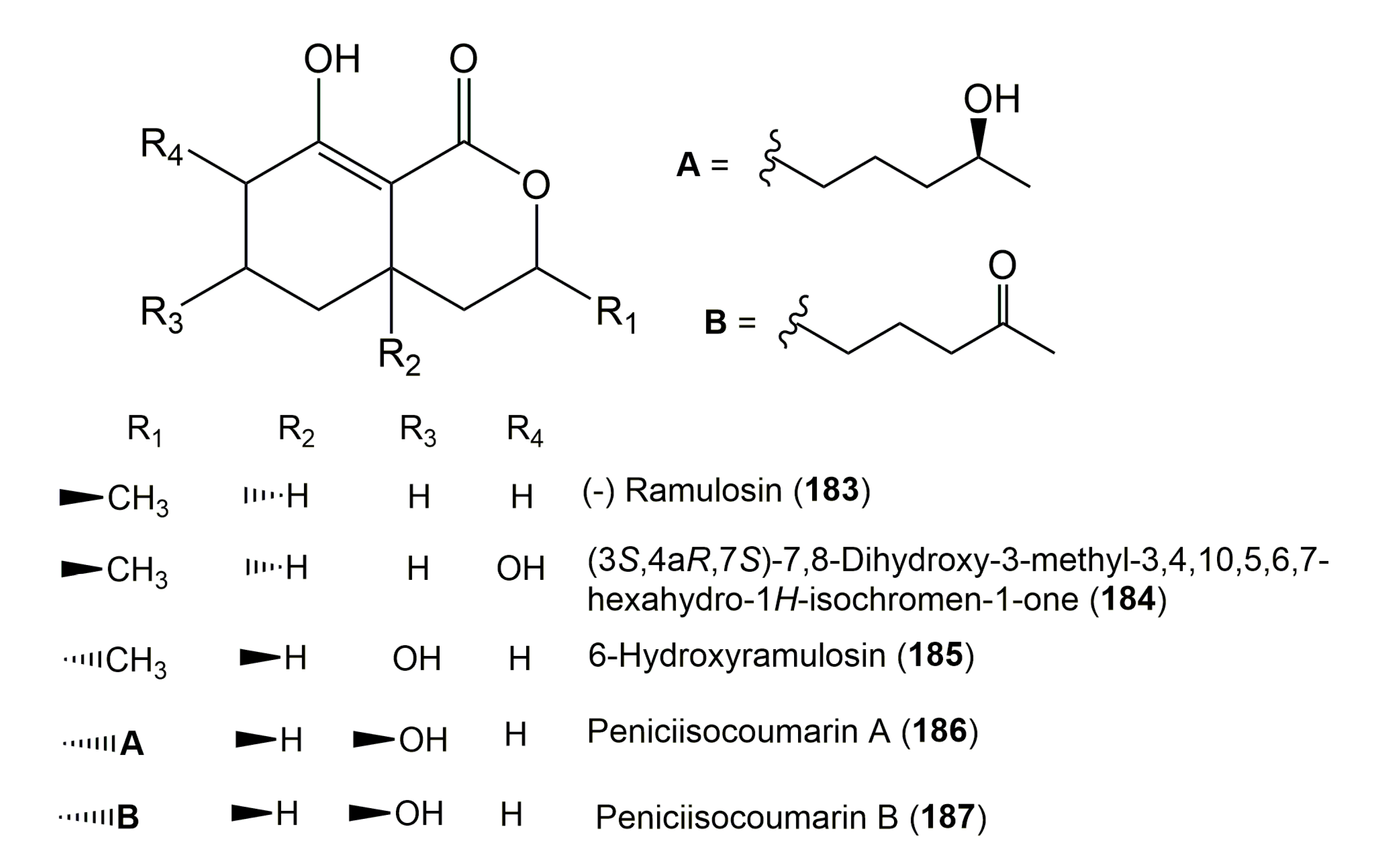
| (−) Ramulosin (183) | Talaromyces sp. JQ769262 | Cedrus deodara (Twigs, Pinaceae) | Lolab Valley in the Western Himalayas, Kashmir, India | [83] |
| (3S,4aR,7S)-7,8-Dihydroxy-3-methyl-3,4,10,5,6,7-hexahydro-1H-isochromen-1-one (184) | Talaromyces sp. JQ769262 | Cedrus deodara (Twigs, Pinaceae) | Lolab Valley in the Western Himalayas, Kashmir, India | [83] |
| 6-Hydroxyramulosin (185) | Nigrospora sp. PSU-N24 | Garcinia nigrolineata (Branches, Clusiaceae) | Ton Nga Chang wildlife sanctuary, Songkhla province, Southern Thailand | [110] |
| Peniciisocoumarin A (186) | Penicillium sp. TGM112 | Bruguiera sexangula var. rhynchopetala (Leaves, Rhizophoraceae) | South China Sea, China | [67] |
| Peniciisocoumarin B (187) | Penicillium sp. TGM112 | Bruguiera sexangula var. rhynchopetala (Leaves, Rhizophoraceae) | South China Sea, China | [67] |
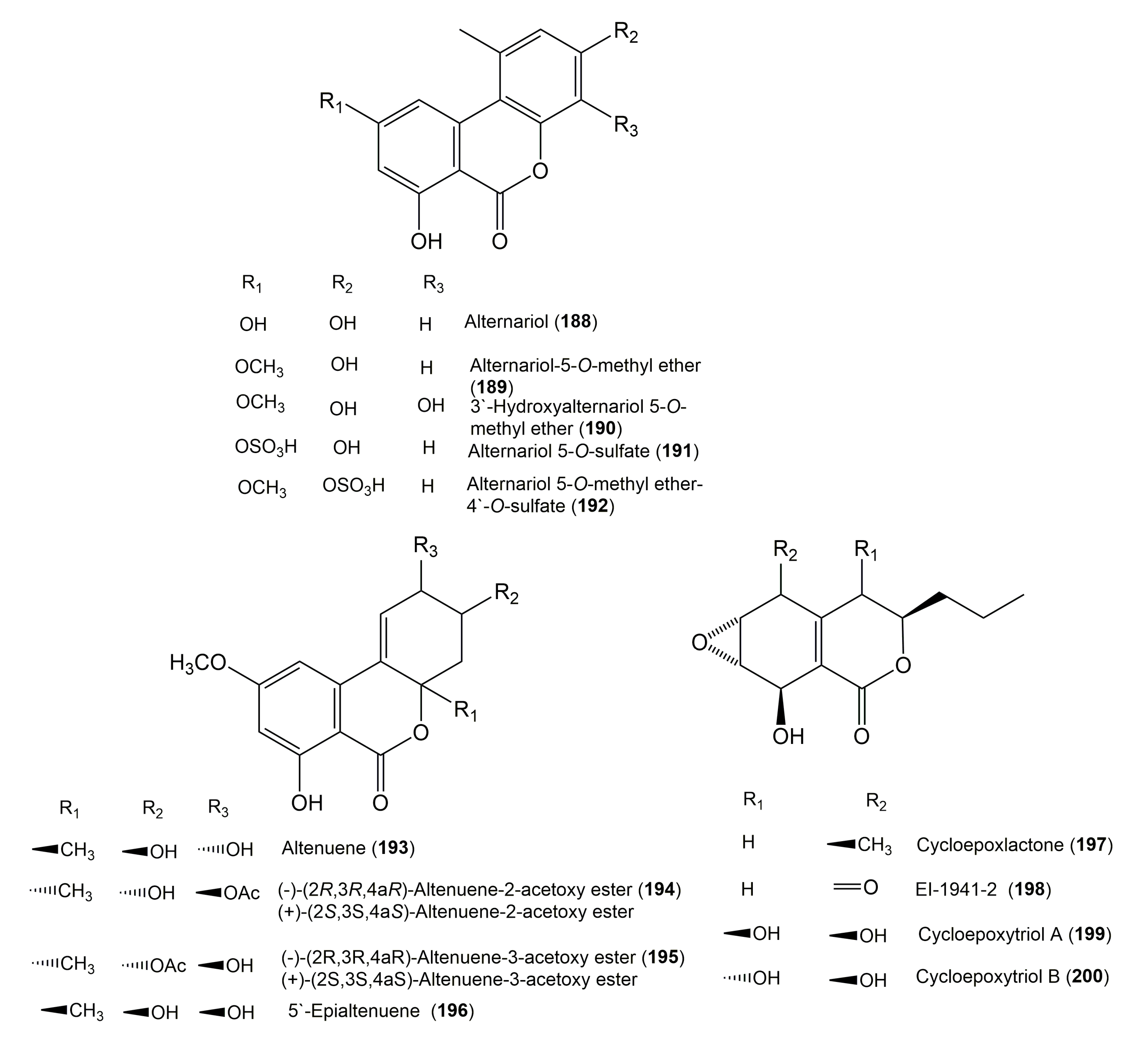
| Alternariol (188) | Alternaria sp. II2L4 | Polygonum senegalense Meisn. (Leaves, Polygonaceae) | Alexandria, Egypt | [40] |
| Alternaria tenuissima SP-07 | Salvia przewalskii (Roots, Lamiaceae) | Longxi County, Gansu Province, China | [57] | |
| Alternaria alternata | Camellia sinensis (Branches, Theaceae) | Nanjing, Jiangsu Province, China | [63] | |
| Peyronellaea glomerata XSB-01-15 | Amphimedon sp. (Sponge, Niphatidae) | Yongxin Island, Hainan Province, China | [65] | |
| Alternariol-5-O-methyl ether (189) | Alternaria sp. II2L4 | Polygonum senegalense Meisn. (Leaves, Polygonaceae) | Alexandria, Egypt | [40] |
| Alternaria tenuissima SP-07 | Salvia przewalskii (Roots, Lamiaceae) | Longxi County, Gansu Province, China | [57] | |
| Alternaria alternata | Camellia sinensis (Branches, Theaceae) | Nanjing, Jiangsu Province, China | [63] | |
| Peyronellaea glomerata XSB-01-15 | Amphimedon sp. (Sponge, Niphatidae) | Yongxin Island, Hainan Province, China | [65] | |
| 3′-Hydroxyalternariol 5-O-methyl ether (190) | Alternaria sp. II2L4 | Polygonum senegalense Meisn. (Leaves, Polygonaceae) | Alexandria, Egypt | [40] |
| Alternariol 5-O-sulfate (191) | Alternaria sp. II2L4 | Polygonum senegalense Meisn. (Leaves, Polygonaceae) | Alexandria, Egypt | [40] |
| Alternariol 5-O-methyl ether-4′-O-sulfate (192) | Alternaria sp. II2L4 | Polygonum senegalense Meisn. (Leaves, Polygonaceae) | Alexandria, Egypt | [40] |
| Altenuene (193) | Alternaria sp. II2L4 | Polygonum senegalense Meisn. (Leaves, Polygonaceae) | Alexandria, Egypt | [40] |
| Alternaria tenuissima SP-07 | Salvia przewalskii (Roots, Lamiaceae) | Longxi County, Gansu Province, China | [57] | |
| Alternaria alternata | Camellia sinensis (Branches, Theaceae) | Nanjing, Jiangsu Province, China | [63] | |
| (−)-(2R,3R,4aR)-Altenuene-2-acetoxy ester (+)-(2S,3S,4aS)-Altenuene-2-acetoxy ester (194) | Alternaria alternata | Camellia sinensis (Branches, Theaceae) | Nanjing, Jiangsu Province, China | [63] |
| (−)-(2R,3R,4aR)-Altenuene-3-acetoxy ester (+)-(2S,3S,4aS)-Altenuene-3-acetoxy ester (195) | Alternaria alternata | Camellia sinensis (Branches, Theaceae) | Nanjing, Jiangsu Province, China | [63] |
| 5′-Epialtenuene (196) | Alternaria alternata | Camellia sinensis (Branches, Theaceae) | Nanjing, Jiangsu Province, China | [63] |
| Alternaria sp. II2L4 | Polygonum senegalense Meisn. (Leaves, Polygonaceae) | Alexandria, Egypt | [40] | |
| Cycloepoxylactone (197) | Phomopsis sp. 7233 | Laurus azorica (Leaves, Lauraceae) | Gomera, Spain | [42] |
| EI-1941-2 (198) | Phomopsis sp. 7233 | Laurus azorica (Leaves, Lauraceae) | Gomera, Spain | [42] |
| Cycloepoxytriol A (199) | Phomopsis sp. 7233 | Laurus azorica (Leaves, Lauraceae) | Gomera, Spain | [42] |
| Cycloepoxytriol B (200) | Phomopsis sp. 7233 | Laurus azorica (Leaves, Lauraceae) | Gomera, Spain | [42] |
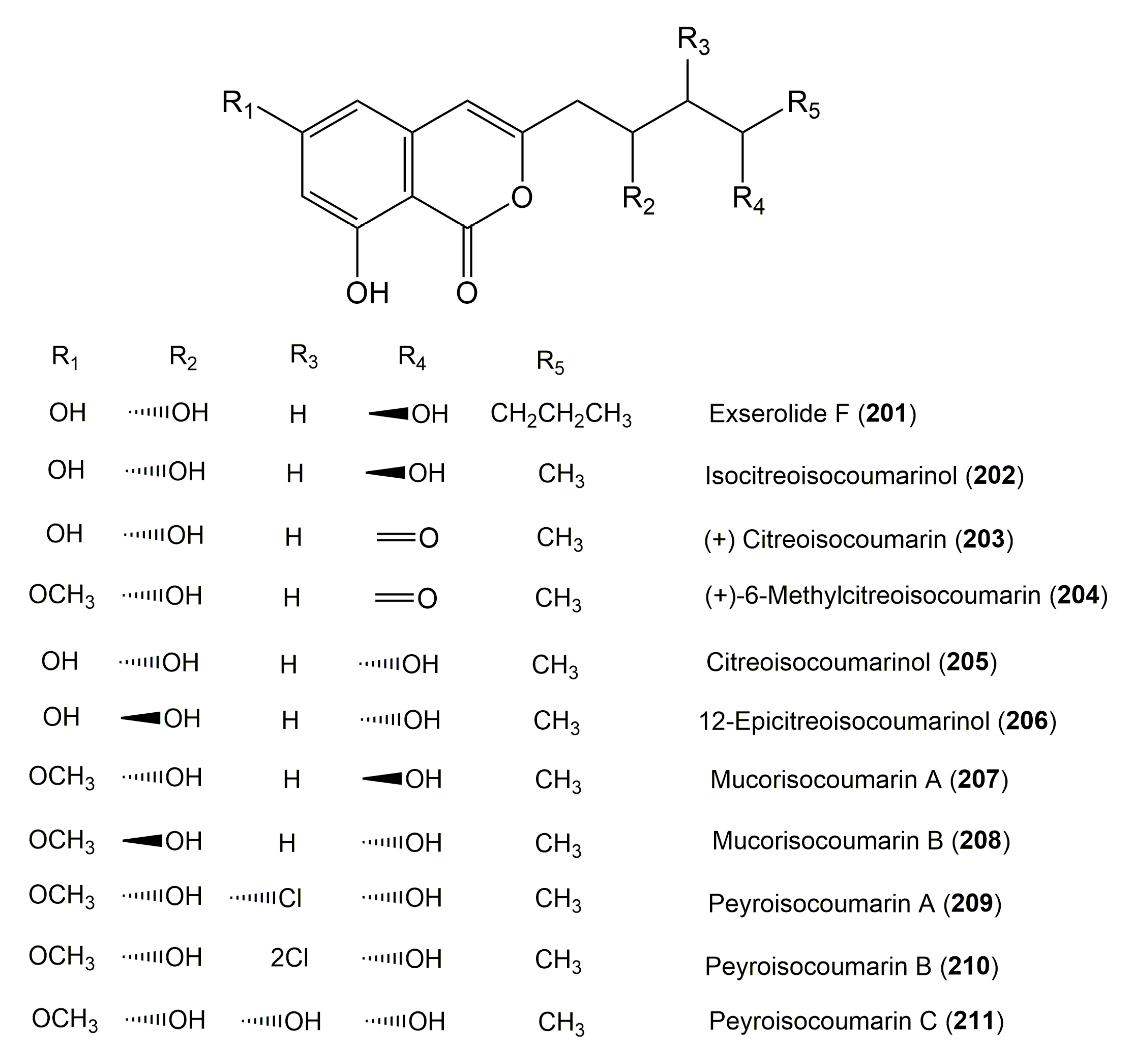
| Exserolide F (201) | Exserohilum sp. KJ156361 | Acer truncatum (Leaves, Sapindaceae) | Dongling Mountain, Beijing, China | [60] |
| Aspergillus oryzae | Paris polyphylla var. yunnanensis (Franch.) Hand.-Mazz. (Rhizomes, Liliaceae) | Dali, Yunnan, China | [94] | |
| Isocitreoisocoumarinol (202) | Peyronellaea glomerata XSB-01-15 | Amphimedon sp. (Sponge, Niphatidae) | Yongxin Island, Hainan Province, China | [65] |
| (+)-Citreoisocoumarin (203) | Ampelomyces sp. EU143251. | Urospermum picroides (Flowers, Asteraceae) | Alexandria, Egypt | [39] |
| Peyronellaea glomerata XSB-01-15 | Amphimedon sp. (Sponge, Niphatidae) | Yongxin Island, Hainan Province, China | [65] | |
| Nectria sp. HN001 | Sonneratia ovata (Branches, Lythraceae) | South China Sea in Hainan province, China | [33] | |
| Phoma sp. TA07-1 | Dichotella gemmacea GX-WZ-2008003-4 (Gorgonian, Plexauridae) | Weizhou coral reef, South China Sea, China | [131] | |
| Ascomycota sp. CYSK-4 | Pluchea indica (Branches, Asteraceae) | Shankou Mangrove Nature Reserve, Guangxi Province, China | [37] | |
| (+)-6-Methylcitreoisocoumarin (204) | Peyronellaea glomerata XSB-01-15 | Amphimedon sp. sponge (Niphatidae) | Yongxin Island, Hainan Province, China | [65] |
| Penicillium commune QQF-3 | Kandelia candel (Fruits, Rhizophoraceae) | Guangdong Province, China | [31] | |
| Citreoisocoumarinol (205) | Peyronellaea glomerata XSB-01-15 | Amphimedon sp. (Sponge, Niphatidae) | Yongxin Island, Hainan Province, China | [65] |
| Nectria sp. HN001 | Sonneratia ovata (Branches, Lythraceae) | South China Sea, Hainan province, China | [33] | |
| Phoma sp. (TA07-1) | Dichotella gemmacea GX-WZ-2008003-4 (Gorgonian, Plexauridae) | Weizhou coral reef, South China Sea, China | [131] | |
| 12-epicitreoisocoumarinol (206) | Nectria sp. HN001 | Sonneratia ovata (Branches, Lythraceae) | South China Sea, Hainan province, China | [33] |
| Mucorisocoumarin A (207) | Peyronellaea glomerata XSB-01-15 | Amphimedon sp. (Sponge, Niphatidae) | Yongxin Island, Hainan Province, China | [65] |
| Mucorisocoumarin B (208) | Peyronellaea glomerata XSB-01-15 | Amphimedon sp. (Sponge, Niphatidae) | Yongxin Island, Hainan Province, China | [65] |
| Ascomycota sp. CYSK-4 | Pluchea indica (Branches, Asteraceae) | Shankou Mangrove Nature Reserve, Guangxi Province, China | [37] | |
| Peyroisocoumarin A (209) | Peyronellaea glomerata XSB-01-15 | Amphimedon sp. (Sponge, Niphatidae) | Yongxin Island, Hainan Province, China | [65] |
| Peyroisocoumarin B (210) | Peyronellaea glomerata XSB-01-15 | Amphimedon sp. (Sponge, Niphatidae) | Yongxin Island, Hainan Province, China | [65] |
| Peyroisocoumarin C (211) | Peyronellaea glomerata XSB-01-15 | Amphimedon sp. (Sponge, Niphatidae) | Yongxin Island, Hainan Province, China | [65] |
| Aspergillumarin A (212) | Aspergillus sp. | Bruguiera gymnorrhiza (Leaves, Rhizophoraceae) | South China Sea coast, China | [61] |
| Penicillium sp. (MWZ14-4) | Unidentified sponge GX-WZ-2008001 (Inner fresh tissues) | Weizhou, South China Sea, China | [52] | |
| Talaromyces amestolkiae | Kandelia obovata (Leaves, Rhizophoraceae) | Zhanjiang, Guangdong Province, China | [62] | |
| Penicillium citrinum HL-5126 | Bruguiera sexangula var. rhynchopetala (Roots, Rhizophoraceae) | Hainan Island, China | [56] | |
| Penicillium sp. TGM112 | Bruguiera sexangula var. rhynchopetala (Leaves, Rhizophoraceae) | South China Sea, China | [67] | |
| Aspergillumarin B (213) | Aspergillus sp. | Bruguiera gymnorrhiza (Leaves, Rhizophoraceae) | South China Sea coast, China | [61] |
| Penicillium sp. (MWZ14-4) | Unidentified sponge GX-WZ-2008001 (Inner fresh tissues) | Weizhou, South China Sea, China | [52] | |
| Talaromyces amestolkiae | Kandelia obovata (Leaves, Rhizophoraceae) | Zhanjiang, Guangdong Province, China | [62] | |
| Penicimarin B (214) | Penicillium sp. (MWZ14-4) | Unidentified sponge GX-WZ-2008001 (Inner fresh tissues) | Weizhou, South China Sea, China | [52] |
| Talaromyces amestolkiae | Kandelia obovata (Leaves, Rhizophoraceae) | Zhanjiang, Guangdong Province, China | [62] | |
| Penicimarin C (215) | Penicillium sp. (MWZ14-4) | Unidentified sponge GX-WZ-2008001 (Inner fresh tissues) | Weizhou, South China Sea, China | [52] |
| Talaromyces amestolkiae | Kandelia obovata (Leaves, Rhizophoraceae) | Zhanjiang, Guangdong Province, China | [62] | |
| Penicillium sp. TGM112 | Bruguiera sexangula var. rhynchopetala (Leaves, Rhizophoraceae) | South China Sea, China | [67] | |
| (R)-3-((R)-4,5-Dihydroxypentyl)-8-hydroxyisochroman-1-one (216) | Talaromyces amestolkiae | Kandelia obovata (Leaves, Rhizophoraceae) | Zhanjiang, Guangdong Province, China | [62] |
| 5,6-Dihydroxy-3-(4-hydroxypentyl)isochroman-1-one (217) | Talaromyces amestolkiae | Kandelia obovata (Leaves, Rhizophoraceae) | Zhanjiang, Guangdong Province, China | [62] |
| Maculansline C (218) 3S, 10S-Dihydroisocoumarin, (Epimer) | Leptosphaena maculans | Osmanthus fragrans (Leaves, Oleaceae) | China | [104] |
| Desmethyldiaportinol (219) | Ampelomyces sp. EU143251. | Urospermum picroides (Flowers, Asteraceae) | Alexandria, Egypt | [39] |
| Phoma sp. (TA07-1) | Dichotella gemmacea GX-WZ-2008003-4 (Gorgonian, Plexauridae) | Weizhou coral reef, South China Sea, China | [131] | |
| Dichlorodiaportin (220) | Trichoderma sp. 09 | Myoporum bontioides (Roots, Scrophulariaceae) | Leizhou Peninsula, Guangdong Province, China | [66] |
| Trichoderma sp. 09 | Myoporum bontioides (Roots, Scrophulariaceae) | Leizhou Peninsula, Guangdong Province, China | [66] | |
| Ascomycota sp. CYSK-4 | Pluchea indica (Branches, Asteraceae) | Shankou Mangrove Nature Reserve, Guangxi Province, China | [37] | |
| Aspergillus sp. HN15-5D | Acanthus ilicifolius (Leaves, Acanthaceae) | Dongzhaigang Mangrove National Nature Reserve, Hainan Island, China | [73] | |
| Desmethyldichlorodiaportin (221) | Ampelomyces sp. EU143251. | Urospermum picroides (Flowers, Asteraceae) | Alexandria, Egypt | [39] |
| Peniisocoumarin D (222) | Penicillium commune QQF-3 | Kandelia candel (Fruit, Rhizophoraceae) | Guangdong Province, China | [31] |
| Peniisocoumarin E (223) | Penicillium commune QQF-3 | Kandelia candel (Fruits, Rhizophoraceae) | Guangdong Province, China | [31] |
| Peniisocoumarin F (224) | Penicillium commune QQF-3 | Kandelia candel (Fruits, Rhizophoraceae) | Guangdong Province, China | [31] |
| Peniisocoumarin G (225) | Penicillium commune QQF-3 | Kandelia candel (Fruits, Rhizophoraceae) | Guangdong Province, China | [31] |
| Penicillium commune QQF-3 | Kandelia candel (Fruits, Rhizophoraceae) | Guangdong Province, China | [31] | |
| Peniisocoumarin H (226) | Penicillium commune QQF-3 | Kandelia candel (Fruits, Rhizophoraceae) | Guangdong Province, China | [31] |
| Peniisocoumarin I (227) | Penicillium commune QQF-3 | Kandelia candel (Fruits, Rhizophoraceae) | Guangdong Province, China | [31] |
| Peniisocoumarin J (228) | Penicillium commune QQF-3 | Kandelia candel (Fruits, Rhizophoraceae) | Guangdong Province, China | [31] |
| 3-[(R)-3,3-Dichloro-2-hydroxypropyl]-8-hydroxy-6-methoxy-1H-isochromen-1-one (229) | Penicillium commune QQF-3 | Kandelia candel (Fruits, Rhizophoraceae) | Guangdong Province, China | [31] |
| (+)-Diaporthin (230) | Penicillium commune QQF-3 | Kandelia candel (Fruits, Rhizophoraceae) | Guangdong Province, China | [31] |
| Diaportinol (231) | Peyronellaea glomerata XSB-01-15 | Amphimedon sp. (Sponge, Niphatidae) | Yongxin Island, Hainan Province, China | [65] |
| Trichoderma sp. 09 | Myoporum bontioides (Roots, Scrophulariaceae) | Leizhou Peninsula, Guangdong Province, China | [66] | |
| Phoma sp. (TA07-1) | Dichotella gemmacea GX-WZ-2008003-4 (Gorgonian, Plexauridae) | Weizhou coral reef, South China Sea, China | [131] | |
| Ascomycota sp. CYSK-4 | Pluchea indica (Branches, Asteraceae) | Shankou Mangrove Nature Reserve, Guangxi Province, China | [37] | |
| (+)-(10R)-7-Hydroxy-3-(2-hydroxy-propyl)-5,6-dimethylisochromen-1-one (232) | Alternaria alternata | Camellia sinensis (Branches, Theaceae) | Nanjing, Jiangsu Province, China | [63] |
| Peyroisocoumarin D (233) | Peyronellaea glomerata XSB-01-15 | Amphimedon sp. (Sponge, Niphatidae) | Yongxin Island, Hainan Province, China | [65] |
| Orthosporin (234) | Peyronellaea glomerata XSB-01-15 | Amphimedon sp. (Sponge, Niphatidae) | Yongxin Island, Hainan Province, China | [65] |
| 8-Methyl-11-chlorodiaporthin (235) | Aspergillus sp. CPCC 400810 | Cetrelia sp. (Lichen, Parmeliaceae) | Laojun Mount in Yunnan Province, China | [29] |
| 8-Methyl-11,11-dichlorodiaporthin (236) | Aspergillus sp. CPCC 400810 | Cetrelia sp. (Lichen, Parmeliaceae) | Laojun Mount in Yunnan Province, China | [29] |
| 8-Hydroxy-6-methoxy-3-(2,3,3-trihydroxypropyl)-1H-isochromen-1-one (237) | Penicillium funiculosum Fes1711 | Ficus elastica (Leaves, Moraceae) | Liaocheng University Arboretum, Liaocheng, Shandong, China | [69] |
| 8-Hydroxy-6-methoxy-3-(1,2,3-trihydroxypropyl)-1H-isochromen-1-one (238) | Penicillium funiculosum Fes1711 | Ficus elastica (Leaves, Moraceae) | Liaocheng University Arboretum, Liaocheng, Shandong, China | [69] |
| Aspergisocoumrin C (239) | Aspergillus sp. HN15-5D | Acanthus ilicifolius (Leaves, Acanthaceae) | Dongzhaigang Mangrove National Nature Reserve, Hainan Island, China | [73] |
| (3R,4R,10R)-Fusarentin 4-hydroxy-6,7-dimethyl ether (240) | Microdochium bolleyi 8880 | Fagonia cretica (Leaves, Zygophyllaceae) | Gomera, Spain | [49] |
| Colletotrichum sp. CRI535-02 | Piper ornatum (Leaves, Piperaceae) | Tai Rom Yen National Park, Surat Thani Province, Thailand | [80] | |
| Colletomellein A (241) | Colletotrichum aotearoa BCRC 09F0161 | Bredia oldhamii Hook. f. (Leaves, Melastomataceae) | Mutan, Pingtung County, Taiwan | [132] |
| Colletomellein B (242) | Colletotrichum aotearoa BCRC 09F0161 | Bredia oldhamii Hook. f. (Leaves, Melastomataceae) | Mutan, Pingtung County, Taiwan | [132] |
| Peniciisocoumarin D (243) | Penicillium sp. TGM112 | Bruguiera sexangula var. rhynchopetala (Leaves, Rhizophoraceae) | South China Sea, China | [67] |
| Peniciisocoumarin F (244) | Penicillium sp. TGM112 | Bruguiera sexangula var. rhynchopetala (Leaves, Rhizophoraceae) | South China Sea, China | [67] |
| Peniciisocoumarin H (245) | Penicillium sp. TGM112 | Bruguiera sexangula var. rhynchopetala (Leaves, Rhizophoraceae) | South China Sea, China | [67] |
| 3,4-Dihydro-8-hydroxy-6-methoxy-(3R)-propylisocoumarin (246) | Centraalbureau voor Schimmel cultures (120379) | Picea glauca (Leaves, Pinaceae) | Sussex, New Brunswick, Canada | [119] |
| Peniciisocoumarin C (247) | Penicillium sp. TGM112 | Bruguiera sexangula var. rhynchopetala (Leaves, Rhizophoraceae) | South China Sea, China | [67] |
| Peniciisocoumarin E (248) | Penicillium sp. TGM112 | Bruguiera sexangula var. rhynchopetala (Leaves, Rhizophoraceae) | South China Sea, China | [67] |
| Peniciisocoumarin G (249) | Penicillium sp. TGM112 | Bruguiera sexangula var. rhynchopetala (Leaves, Rhizophoraceae) | South China Sea, China | [67] |
| (R)-3-(3-Hydroxypropyl)-8-hydroxy-3,4-dihydroisocoumarin (250) | Penicillium sp. TGM112 | Bruguiera sexangula var. rhynchopetala (Leaves, Rhizophoraceae) | South China Sea, China | [67] |
| Versicoumarin A (251) | Aspergillus versicolor | Paris marmorata Stearn (Rhizomes, Melanthiaceae) | Dali, Yunnan, China | [93] |
| Versicoumarin D (252) | Aspergillus versicolor | Paris marmorata Steam (Rhizomes, Melanthiaceae) | Dali, Yunnan, China | [89] |
| Paraphaeosphaerin A (253) | Paraphaeosphaeria quadriseptata | Opuntia leptocaulis (Rhizosphere, Cactaceae) | Tucson, Arizon | [133] |
| Paraphaeosphaerin B (254) | Paraphaeosphaeria quadriseptata | Opuntia leptocaulis (Rhizosphere, Cactaceae) | Tucson, Arizon, America | [133] |
| Chaetochiversin A (255) | Chaetomium chiversii | Ephedra fasciculata (Stems, Ephedraceae) | South mountain park, Phoenix, Arizona, America | [133] |
| Chaetochiversin B (256) | Chaetomium chiversii | Ephedra fasciculata (Stems, Ephedraceae) | South mountain park, Phoenix, Arizona, America | [133] |
| Paraphaeosphaerin C (257) | Paraphaeosphaeria quadriseptata | Opuntia leptocaulis (Rhizosphere, Cactaceae) | Tucson, Arizon, America | [133] |
| Peniisocoumarin C (258) | Penicillium commune QQF-3 | Kandelia candel (Fruits, Rhizophoraceae) | Guangdong Province, China. | [31] |
| 6,6′-Dinor-bipenicilisorin (259) | Aspergillus versicolor KU258497 | Eichhornia crassipes (Leaves, Pontederiaceae) | Mansoura, Egypt | [81] |
| 6,6′,9′-Trinor-bipenicilisorin (260) | Aspergillus versicolor KU258497 | Eichhornia crassipes (Leaves, Pontederiaceae) | Mansoura, Egypt | [81] |
| Asperisocoumarin A (261) | Aspergillus sp. 085242 | Acanthus ilicifolius (Roots, Acanthaceae) | Shankou Mangrove National Nature Reserve, Guangxi Province, China | [76] |
| Asperisocoumarin B (262) | Aspergillus sp. 085242 | Acanthus ilicifolius (Roots, Acanthaceae) | Shankou Mangrove National Nature Reserve, Guangxi Province, China | [76] |
| Asperisocoumarin C (263) | Aspergillus sp. 085242 | Acanthus ilicifolius (Roots, Acanthaceae) | Shankou Mangrove National Nature Reserve, Guangxi Province, China | [76] |
| Asperisocoumarin D (264) | Aspergillus sp. 085242 | Acanthus ilicifolius (Roots, Acanthaceae) | Shankou Mangrove National Nature Reserve, Guangxi Province, China | [76] |
| Asperisocoumarin E (265) | Aspergillus sp. 085242 | Acanthus ilicifolius (Roots, Acanthaceae) | Shankou Mangrove National Nature Reserve, Guangxi Province, China | [76] |
| Asperisocoumarin F (266) | Aspergillus sp. 085242 | Acanthus ilicifolius (Roots, Acanthaceae) | Shankou Mangrove National Nature Reserve, Guangxi Province, China | [76] |
| Peniisocoumarin A (267) | Penicillium commune QQF-3 | Kandelia candel (Fruit, Rhizophoraceae) | Guangdong Province, China | [31] |
| Peniisocoumarin B (268) | Penicillium commune QQF-3 | Kandelia candel (Fruits, Rhizophoraceae) | Guangdong Province, China | [31] |
| Sg17-1-4 (269) | Alternaria tenuis Sg17-1 | Marine alga | Zhoushan Island, Zhejiang Province, China | [85] |
| AI-77-B (270) | Alternaria tenuis Sg17-1 | Marine alga | Zhoushan Island, Zhejiang Province, China | [85] |
| AI-77-F (271) | Alternaria tenuis Sg17-1 | Marine alga | Zhoushan Island, Zhejiang Province, China | [85] |
| Similanpyrone A (272) 5-Hydroxy-8-methyl-2H,6H-pyrano [3,4-g]chromen-2,6-dione | Aspergillus similanensis sp. nov. KUFA 0013 | Rhabdermia sp. (Sponge, Rhabderemiidae) | Phang Nga Province, Thailand | [48] |
| Similanpyrone C (273) | Aspergillus similanensis KUFA 0013 | Rhabdermia sp. (Sponge, Rhabderemiidae) | Phang Nga Province, Thailand | [28] |
| Aspergisocoumrin A (274) | Aspergillus sp. HN15-5D | Acanthus ilicifolius (Leaves, Acanthaceae) | Dongzhaigang Mangrove National Nature Reserve, Hainan Island, China | [73] |
| Aspergisocoumrin B (275) | Aspergillus sp. HN15-5D | Acanthus ilicifolius (Leaves, Acanthaceae) | Dongzhaigang Mangrove National Nature Reserve, Hainan Island, China. | [73] |
| Dothideomynone A (276) | Aspergillus banksianus sp. nov | Banksia integrifolia (Leaves, Proteaceae) | Collaroy, New South Wales, Australia | [30] |
| Banksialactone A (277) | Aspergillus banksianus sp. nov | Banksia integrifolia (Leaves, Proteaceae) | Collaroy, New South Wales, Australia | [30] |
| Banksialactone F (278) | Aspergillus banksianus sp. nov | Banksia integrifolia (Leaves, Proteaceae) | Collaroy, New South Wales, Australia | [30] |
| Banksialactone E (279) | Aspergillus banksianus sp. nov | Banksia integrifolia (Leaves, Proteaceae) | Collaroy, New South Wales, Australia | [30] |
| Banksialactone B (280) | Aspergillus banksianus sp. nov | Banksia integrifolia (Leaves, Proteaceae) | Collaroy, New South Wales, Australia | [30] |
| Banksialactone C (281) | Aspergillus banksianus sp. nov | Banksia integrifolia (Leaves, Proteaceae) | Collaroy, New South Wales, Australia | [30] |
| Banksialactone D (282) | Aspergillus banksianus sp. nov | Banksia integrifolia (Leaves, Proteaceae) | Collaroy, New South Wales, Australia | [30] |
| Banksialactone G (283) | Aspergillus banksianus sp. nov | Banksia integrifolia (Leaves, Proteaceae) | Collaroy, New South Wales, Australia | [30] |
| Banksialactone H (284) | Aspergillus banksianus sp. nov | Banksia integrifolia (Leaves, Proteaceae) | Collaroy, New South Wales, Australia | [30] |
| Banksialactone I (285) | Aspergillus banksianus sp. nov | Banksia integrifolia (Leaves, Proteaceae) | Collaroy, New South Wales, Australia | [30] |
| Demethylcitreoviranol (286) | Peyronellaea glomerata XSB-01-15 | Amphimedon sp. (Sponge, Niphatidae) | Yongxin Island, Hainan Province, China | [65] |
| Citreoviranol (287) | Peyronellaea glomerata XSB-01-15 | Amphimedon sp. (Sponge, Niphatidae) | Yongxin Island, Hainan Province, China | [65] |
| Desmethyldichlorodiaportinol (288) | Ascomycota sp. CYSK-4 | Pluchea indica (Branches, Asteraceae) | Shankou Mangrove Nature Reserve, Guangxi Province, China | [37] |
| Dichlorodiaportinol A (289) | Trichoderma sp., 09 | Myoporum bontioides (Roots, Scrophulariaceae) | Leizhou Peninsula, Guangdong Province, China | [79] |
| Dichlorodiaportinolide (290) | Trichoderma sp. 09 | Myoporum bontioides (Roots, Scrophulariaceae) | Leizhou Peninsula, Guangdong Province, China | [66] |
| Dichlorodiaportintone (291) | Ascomycota sp. CYSK-4 | Pluchea indica (Branches, Asteraceae) | Shankou Mangrove Nature Reserve, Guangxi Province, China | [37] |
| Desmethyldichlorodiaportintone (292) | Ascomycota sp. CYSK-4 | Pluchea indica (Branches, Asteraceae) | Shankou Mangrove Nature Reserve, Guangxi Province, China | [37] |
| Botryoisocoumarin A (293) 3S-5,8-dihydroxy-3-hydroxymethyldihydroisocoumarin | Botryosphaeria sp. KcF6 | Kandelia candel (Fruits, Rhizophoraceae) | Daya Bay, Shenzhen, China | [36] |
| Clearanol I (294) | Aspergillus banksianus sp. nov | Banksia integrifolia (Leaves, Proteaceae) | Collaroy, New South Wales, Australia | [30] |
| Penicimarin A (295) | Penicillium sp. MWZ14-4 | Unidentified sponge GX-WZ-2008001 (Inner fresh tissues) | Weizhou, South China Sea, China | [52] |
| Isocoumarindole A (296) | Aspergillus sp. CPCC400810 | Cetrelia sp. (Lichen, Parmeliaceae) | Laojun Mount in Yunnan Province, China | [29] |
| Prochaetoviridin A (297) | Chaetomium globosum CDW7 (Chaetomiaceae) | Ginkgo biloba (Ginkgoaceae) | Jiangsu province, China | [77] |
| Fusariumin (298) | Fusarium sp. LN-10 | Melia azedarach (Leaves, Meliaceae) | Campus of Northwest A&F University, Yangling, Shaanxi province, China, | [86] |
| Aspergillus versicolor KJ801852 | Paris polyphylla var. yunnanensis (Rhizomes, Melanthiaceae) | Dali, Yunnan, China | [126] | |
| Phialophoriol (299) | Alternaria alternata | Camellia sinensis (Branches, Theaceae) | Nanjing, Jiangsu Province, China | [63] |
| (3aR,9bR)-6,9b-Dihydroxy-8-methoxy-1-methylcyclopentene[c]isochromen-3,5-dione (300) | Penicillium sp. | Riccardia multifida (L.) S. Gray (Liverwort, Aneuraceae) | Maoer Mountain, Guangxi Province, China | [116] |
| (3S,4S)-Dihydroascochin (301) | Phomopsis sp. 7233 | Laurus azorica (Leaves, Lauraceae) | Gomera, Spain | [42] |
| 3-Methoxy-6,8-dihydroxy-3-methyl-3,4-dihydroisocoumarin (302) | Penicillium coffeae MA-314 | Laguncularia racemose (Leaves, Combretaceae) | Hainan island, China | [47] |
| Cis-4,6-Dihydroxymellein (303) | Penicillium coffeae MA-314 | Laguncularia racemose (Leaves, Combretaceae) | Hainan island, China | [47] |
| 3,4-Dihydro-8-hydroxyisocoumarin-3-carboxylic methyl ether (304) | Seltsamia galinsogisoli sp. nov. SYPF 7336 | Galinsoga parviflora (Whole plant, Asteraceae) | Huludao, China | [78] |
| 3-Hydroxymethyl-6,8-dimethoxycoumarin (305) | Endophytic fungus No. GX4-1B | Bruguiera gymnoihiza (L.) Savigny (Branches, Rhizophoraceae) | South China Sea, Guangxi province, China | [127] |
| 1H-2-Benzopyran-1-one,6,8-dihydroxy-3-(2-hydroxypropyl) (306) | Seltsamia galinsogisoli sp. nov. SYPF 7336 | Galinsoga parviflora (Whole plant, Asteraceae) | Huludao, China | [78] |
| 6,8-Dihydroxy-3-hydroxymethyl-1H-2-benzopyran-1-one (307) | Penicillium coffeae MA-314 | Laguncularia racemose (Leaves, Combretaceae) | Hainan island, China | [47] |
| Compound Name | Biological Activity | Assay, Organism, or Cell Line | Biological Results | Positive Control | References | |
|---|---|---|---|---|---|---|
| Kigelin (1) (−)-(3R)-6,7-Dimethoxymellein | Antifungal | Agar tube dilution/Trichophyton longifusus | 45 (% Inhibition) | Miconazole 70 (% Inhibition) | [68] | |
| Antifungal | Agar tube dilution/A. flavus | 20 (% Inhibition) | Ampicillin 20 (% Inhibition) | [68] | ||
| Antifungal | Agar tube dilution/Microsporum canis | 50 (% Inhibition) | Miconazole 98.4 (% Inhibition) | [68] | ||
| (3R,4R)-6,7-Dimethoxy-4-hydroxymellein (2) | Antifungal | Agar tube dilution/Trichophyton longifusus | 70 (% Inhibition) | Miconazole 70 (% Inhibition) | [68] | |
| Antifungal | Agar tube dilution/Microsporum canis | 50 (% Inhibition) | Miconazole 98.4 (% Inhibition) | [68] | ||
| Antifungal | Agar tube dilution/Fusarium solani | 20 (% Inhibition) | Miconazole 73.2 (% Inhibition) | [68] | ||
| Antioxidant | XO Inhibition | 707 μM (IC50) | PG 628 μM (IC50) BHA 591 μM (IC50) | [68] | ||
| Cis-4-Acetoxyoxymellein (4) | Antibacterial | Agar diffusion/E. coli | 10 mm (GI) | Penicillin 14 mm (GI) Tetracycline 18 mm (GI) | [50] | |
| Antibacterial | Agar diffusion/Bacillus megaterium | 10 mm (GI) | Penicillin 18 mm (GI) Tetracycline 18 mm (GI) | [50] | ||
| Antifungal | Agar diffusion/Microbotryum violaceum | 8 mm (GI) | Nystatin 20 mm (IZD) Actidione 50 mm (IZD) | [50] | ||
| Antifungal | Agar diffusion/Septoria tritici | 8 mm (IZD) | [50] | |||
| Algicidal | Agar diffusion/Chlorella fusca | 7 mm (IZD) | Actidione 35 mm (IZD) | [50] | ||
| 8-Deoxy-6-hydroxy-cis-4-acetoxyoxymellein (5) | Antibacterial | Agar diffusion/E. coli | 9 mm (GI) | Penicillin 14 mm (GI) Tetracycline 18 mm (GI) | [50] | |
| Antibacterial | Agar diffusion/Bacillus megaterium | 9 mm (GI) | Penicillin 18 mm (GI) Tetracycline 18 mm (GI) | [50] | ||
| Antifungal | Agar diffusion/Microbotryum violaceum | 8 mm (GI) | Nystatin 20 mm (IZD) Actidione 50 mm (IZD) | [50] | ||
| Antifungal | Agar diffusion/Botrytis cinerea | 10 mm (IZD) | Nystatin 0 mm (IZD) Actidione 0 mm (IZD) | [50] | ||
| Antifungal | Agar diffusion/Septoria tritici | 9 mm (IZD) | [50] | |||
| Algicidal | Agar diffusion/Chlorella fusca | 8 mm (IZD) | Actidione 35 mm (IZD) | [50] | ||
| (3R,4R)-(−)-4-Hydroxymellein (3R,4R)-Cis-4-Hydroxymellein (6) | Antibacterial | Agar diffusion/Bacillus megaterium | 6 mm (GI) | Penicillin 14 mm (GI) Tetracycline 18 mm (GI) | [43] | |
| Antifungal | Agar diffusion/Microbotryum violaceum | 8 mm (IZD) | Nystatin 20 mm (IZD) Actidione 50 mm (IZD) | [43] | ||
| Algicidal | Agar diffusion/Chlorella fusca | 9 mm (IZD) | Actidione 35 mm (IZD) | [43] | ||
| (−)-6-Methoxymellein (11) | Antiviral | CPE inhibition/H1N1 virus | 20.98 µg/mL (IC50) | Arbidol 0.15 µg/mL (IC50) | [26] | |
| Botryospyrone C (13) | Antifungal | 2-Fold broth dilution method/F. oxysporum | 223 μM (MIC) | Triadimefon 340 μM (MIC) | [71] | |
| Antifungal | 2-Fold broth dilution method/F. graminearum | 223 μM (MIC) | Triadimefon 510.7 μM (MIC) | [71] | ||
| 6-(4′-Hydroxy-2′-methyl phenoxy)-(−)-(3R)-mellein (16) | Antifungal | Agar tube dilution/Trichophyton longifusus | 55 (% Inhibition) | Miconazole 70 (% Inhibition) | [68] | |
| Antifungal | Agar tube dilution/Microsporum canis | 70 (% Inhibition) | Miconazole 98.4 (% Inhibition) | [68] | ||
| Antifungal | Agar tube dilution/Fusarium solani | 30 (% Inhibition) | Miconazole 73.2 (% Inhibition) | [68] | ||
| Antioxidant | DPPH | 159 μM (IC50) | PG 30 159 μM (IC50) BHA 44 μM (IC50) | [68] | ||
| Antioxidant | XO Inhibition | 243 μM (IC50) | PG 628 μM (IC50) BHA 591 μM (IC50) | [68] | ||
| (3R,4R)-3,4-Dihydro-4,6-dihydroxy-3-methyl-1-oxo-1H-isochromene-5-carboxylic acid (34) | Antifungal | Direct Bioautography Overaly/Cladosporium cladosporioides | 10 µg (Minimum amount required for inhibition of fungi growth on TLC plates) | Nystatin 1µg (Minimum amount required for inhibition of fungi growth on TLC plates) | [17] | |
| Antifungal | Direct Bioautography Overlay/Cladosporium sphaerospermum | 25 µg (Minimum amount required for inhibition of fungi growth on TLC plates) | Nystatin 1 µg (Minimum amount required for inhibition of fungi growth on TLC plates) | [17] | ||
| Acetylcholinesterase inhibitory | TLC-based AChE inhibition | 3 µg (IC) | Galantamine 1µg (IC) | [17] | ||
| (3R)-Mellein (35) 3,4-Dihydro-(3R)-methyl-8-hydroxyisocoumarin | Antifungal | Agar diffusion/Botrytis cinerea | 49.2 µg/mL (EC50) | - | [53] | |
| (R)-7-Hydroxymellein (36) | Antifungal | Direct Bioautography Overlay/Cladosporium cladosporioides | 5 µg (Minimum amount required for inhibition of fungi growth on TLC plates) | Nystatin 1µg (Minimum amount required for inhibition of fungi growth on TLC plates) | [17] | |
| Antifungal | Direct Bioautography Overlay/Cladosporium sphaerospermum | 10 µg (Minimum amount required for inhibition of fungi growth on TLC plates) | Nystatin 1µg (Minimum amount required for inhibition of fungi growth on TLC plates) | [17] | ||
| Acetylcholinesterase inhibitory | TLC-based AChE inhibition | 10 µg (IC) | Galantamine 1µg (IC) | [17] | ||
| (3R,4R)-4,7-Dihydroxymellein (37) | Antifungal | Direct Bioautography Overlay/Cladosporium cladosporioides | 5 µg (Minimum amount required for inhibition of fungi growth on TLC plates) | Nystatin 1 µg (Minimum amount required for inhibition of fungi growth on TLC plates) | [17] | |
| Antifungal | Direct Bioautography Overlay/Cladosporium sphaerospermum | 10 µg (Minimum amount required for inhibition of fungi growth on TLC plates) | Nystatin 1 µg (Minimum amount required for inhibition of fungi growth on TLC plates) | [17] | ||
| Acetylcholinesterase inhibitory | TLC-based AChE inhibition | 10 µg (IC) | Galantamine 1µg (IC) | [17] | ||
| Periplanetin A (39) | Antivirus | Spectrophotometer/Anti-TMV | 14.6% GI (20 μM) | Ningnanmycin 28.6% GI (20 μM) | [44] | |
| (3R)-Methyl-8-hydroxy-6-(hydroxymethyl)-7-methoxydihydroisocoumarin (40) | Antiviral | Spectrophotometer/Anti-TMV | 21.8% GI (20 μM) | Ningnanmycin 32.8% GI (20 μM) | [112] | |
| (3R)-Methyl-7,8-dimethoxy-6-(hydroxymethyl) dihydroisocoumarin (41) | Antiviral | Spectrophotometer/Anti-TMV | 18.6% GI (20 μM) | Ningnanmycin 32.8% GI (20 μM) | [112] | |
| S-(-)-5-Hydroxy-8-methoxy-4-(1′-hydroxyethyl)-isocoumarin (54) | α-Glucosidase inhibitory | Chromogenic | 537.3 μM (IC50) | Acarbose 958.3 μM (IC50) | [62] | |
| S-(-)-5,6,8-Trihydroxy-4-(1′-hydroxyethyl)isocoumarin (65) | α-Glucosidase inhibitory | Chromogenic | 315.3 μM (IC50) | Acarbose 958.3 μM (IC50) | [62] | |
| Antibacterial | Colorimetric broth microdilution/S. aureus (ATCC 27154) | 12.5 μM (MIC50) | Ciprofloxacin 0.160 μM (MIC50) | [52] | ||
| Antibacterial | Colorimetric broth microdilution/B. cereus (ACCC 11077) | 6.25 μM (MIC50) | Ciprofloxacin 0.625 μM (MIC50) | [52] | ||
| Antibacterial | Colorimetric broth microdilution/Vibrio parahemolyticus (ATCC17802) | 6.25 μM (MIC50) | Ciprofloxacin 0.160 μM (MIC50) | [52] | ||
| Sescandelin (66) | α-Glucosidase inhibitory | Chromogenic | 417.8 μM (IC50) | Acarbose 958.3 μM (IC50) | [62] | |
| Terrecoumarin A (67) | Antivirus | Spectrophotometer/Anti-TMV | 25.4% GI (20 μM) | Ningnanmycin 28.6% GI (20 μM) | [44] | |
| Terrecoumarin B (68) | Antivirus | Spectrophotometer/Anti-TMV | 14.5% GI (20 μM) | Ningnanmycin 28.6% GI (20 μM) | [44] | |
| Terrecoumarin C (69) | Antivirus | Spectrophotometer/Anti-TMV | 16.3% GI (20 μM) | Ningnanmycin 28.6% GI (20 μM) | [44] | |
| LL-Z 1640-7 (72) | Antioxidant | Luciferase | 0.87 mM (IC50) | tBHQ 4.29 mM (IC50) | [65] | |
| Acremonone G (76) | α-Glucosidase inhibitory | Chromogenic | 0.37 mM (IC50) | Acarbose 0.47 mM (IC50) | [103] | |
| Myrothelactone A (81) | α-Glucosidase inhibitory | Chromogenic | 0.32 mM (IC50) | Acarbose 0.47 mM (IC50) | [103] | |
| 6,8-Dihydroxy-5-methoxy-3-methyl-1H-isochromen-1-one (84) | α-Glucosidase inhibitory | Chromogenic | 89.4 μM (IC50) | Acarbose 958.3 μM (IC50) | [62] | |
| Myrothelactone C (85) | α-Glucosidase inhibitory | Chromogenic | 0.036 mM (IC50) | Acarbose 0.47 mM (IC50) | [103] | |
| Tubakialactone B (87) 8-Hydroxyl-3,4-bis(hydroxymethyl)-6-methoxy-4-methyl-1H-2-benzopyran-1-one | α-Glucosidase inhibitory | Chromogenic | 0.026 mM (IC50) | Acarbose 0.47 mM (IC50) | [103] | |
| 6-Hydroxy-8-methoxy-3,4-dimethylisocoumarin (92) | α-Glucosidase inhibitory | Chromogenic | 585.7 μM (IC50) | Acarbose 958.3 μM (IC50) | [62] | |
| 3,4-Dimethyl-6,8-dihydroxyisocoumarin (93) | α-Glucosidase inhibitory | Chromogenic | 36.4 μM (IC50) | Acarbose 958.3 μM (IC50) | [62] | |
| 6-Hydroxy-4-hydroxymethyl-8-methoxy-3-methyl-isocoumarin (94) | α-Glucosidase inhibitory | Chromogenic | 302.6 μM (IC50) | Acarbose 958.3 μM (IC50) | [62] | |
| Sescandelin B (95) | α-Glucosidase inhibitory | Chromogenic | 17.2 μM (IC50) | Acarbose 958.3 μM (IC50) | [62] | |
| 6-Hydroxy-3-hydroxymethyl-8-methoxyisocoumarin (96) | Antivirus | Spectrophotometer/Anti-TMV | 18.7% GI (20 μM) | Ningnanmycin 28.6% GI (20 μM) | [44] | |
| 4,6-Dihydroxy-3,9-dehydromellein (97) | Antivirus | Spectrophotometer/Anti-TMV | 13.8% GI (20 μM) | Ningnanmycin 28.6% GI (20 μM) | [44] | |
| Botryospyrone A (106) | Antifungal | 2-Fold broth dilution method/F. oxysporum | 112.6 μM (MIC) | Triadimefon 340 μM (MIC) | [71] | |
| Botryospyrone B (107) | Antifungal | 2-Fold broth dilution method/F. oxysporum | 105.8 μM (MIC) | Triadimefon 340 μM (MIC) | [71] | |
| Antifungal | 2-Fold broth dilution method/F. graminearum | 211.7 μM (MIC) | Triadimefon 510.7 μM (MIC) | [71] | ||
| 4,5,7-Trihydroxy-3-methoxy-3,6-dimethylisochroman-1-one (114) | α-Glucosidase inhibitory | - | IC50 90.4 μM | Acarbose (IC50 553.7 μM) | [38] | |
| 3,5-Dimethyl-8-hydroxy-7-methoxy-3,4-dihydroisocoumarin (132) | Antifungal | TLC-autobiography/Aspergillus niger | 50 µg/mL (MIC) | Nystatin 12.5 µg/mL (MIC) | [55] | |
| TLC-autobiography/Cladosporium herbarum | 50 µg/mL (MIC) | Nystatin 12.5 µg/mL (MIC) | [55] | |||
| Antibacterial | Colorimetric broth microdilution/Bacillus subtilis | 25 µg/mL (MIC) | Chloramphenicol 3.13 µg/mL (MIC) | [55] | ||
| Colorimetric broth microdilution/Pseudomonas syringae | 100 µg/mL (MIC) | Chloramphenicol 3.13 µg/mL (MIC) | [55] | |||
| Periplanetin D (137) | Antivirus | Spectrophotometer/Anti-TMV | 15.5% GI (20 μM) | Ningnanmycin 28.6% GI (20 μM) | [44] | |
| Pestalactone C (138) | Antifungal | Colorimetric broth microdilution/Candida glabrata (ATCC 90030) | 3.49 μg/mL (MIC50) | Amphotericin B 0.25 μg/mL (MIC50) | [22] | |
| (6,8-Dihydroxy-3-methyl-1-oxo-1H-isochromen-4-yl)methyl 3-methylbutanoate (150) | α-Glucosidase inhibitory | Chromogenic | 140.8 μM (IC50) | Acarbose 958.3 μM (IC50) | [62] | |
| Penicimarin F (153) | Antibacterial | Colorimetric broth microdilution// S. aureus (ATCC 27154) | 12.5 μM (MIC) | Ciprofloxacin 0.160 μM (MIC) | [52] | |
| Penicisimpin A (157) 3-(R)-6,8-Dihydroxy-7-methyl-3-pentylisochroman-1-one | Antibacterial | Well diffusion method/E. coli | 4 µg/mL (MIC) | Chloramphenicol 2 µg/mL (MIC) | [58] | |
| Antibacterial | Well diffusion method/Micrococcus luteus | 8 µg/mL (MIC) | Chloramphenicol 1 µg/mL (MIC) | [58] | ||
| Antibacterial | Well diffusion method/Pseudomonas aeruginosa | 4 µg/mL (MIC) | Chloramphenicol 4 µg/mL (MIC) | [58] | ||
| Antibacterial | Well diffusion method/Vibrio alginolyticus | 8 µg/mL (MIC) | Chloramphenicol 0.5 µg/mL (MIC) | [58] | ||
| Antibacterial | Well diffusion method/Vibrio harveyi | 4 µg/mL (MIC) | Chloramphenicol 2 µg/mL (MIC) | [58] | ||
| Antibacterial | Well diffusion method/Vibrio parahaemolyticus | 4 µg/mL (MIC) | Chloramphenicol 2 µg/mL (MIC) | [58] | ||
| Antifungal | Well diffusion method/Colletotrichum gloeosprioides | 4 µg/mL (MIC) | Amphotericin B 8 µg/mL (MIC) | [58] | ||
| Antifungal | Well diffusion method/Phytophthora parasitica var. nicotianae | 6 µg/mL (MIC) | Amphotericin B 16 µg/mL (MIC) | [58] | ||
| Penicisimpin B (158) 3-(R)-6,8-Dihydroxy-3-pentylisochroman-1-one | Antibacterial | Well diffusion method/Aeromonas hydrophilia | 32 µg/mL (MIC) | Chloramphenicol 32 µg/mL (MIC) | [58] | |
| Antibacterial | Well diffusion method/E. coli | 32 µg/mL (MIC) | Chloramphenicol 2 µg/mL (MIC) | [58] | ||
| Antibacterial | Well diffusion method/Micrococcus luteus | 64 µg/mL (MIC) | Chloramphenicol 1 µg/mL (MIC) | [58] | ||
| Antibacterial | Well diffusion method/Pseudomonas aeruginosa | 32 µg/mL (MIC) | Chloramphenicol 4 µg/mL (MIC) | [58] | ||
| Antibacterial | Well diffusion method/Vibrio alginolyticus | 32 µg/mL (MIC) | Chloramphenicol 0.5 µg/mL (MIC) | [58] | ||
| Antibacterial | Well diffusion method/Vibrio harveyi | 16 µg/mL (MIC) | Chloramphenicol 2 µg/mL (MIC) | [58] | ||
| Antibacterial | Well diffusion method/Vibrio parahaemolyticus | 32 µg/mL (MIC) | Chloramphenicol 2 µg/mL (MIC) | [58] | ||
| Antifungal | Well diffusion method/Colletotrichum gloeosprioides | 16 µg/mL (MIC) | Amphotericin B 8 µg/mL (MIC) | [58] | ||
| Antifungal | Well diffusion method/Phytophthora parasitica var. nicotianae | 32 µg/mL (MIC) | Amphotericin B 16 µg/mL (MIC) | [58] | ||
| Penicisimpin C (159) 3-(S)-6,8-Dihydroxy-7-methyl-3-(pent-1-enyl)isochroman-1-one | Antibacterial | Well diffusion method/Aeromonas hydrophilia | 16 µg/mL (MIC) | Chloramphenicol 32 µg/mL (MIC) | [58] | |
| Antibacterial | Well diffusion method/E. coli | 8 µg/mL (MIC) | Chloramphenicol 2 µg/mL (MIC) | [58] | ||
| Antibacterial | Well diffusion method/Micrococcus luteus | 16 µg/mL (MIC) | Chloramphenicol 1 µg/mL (MIC) | [58] | ||
| Antibacterial | Well diffusion method/Pseudomonas aeruginosa | 8 µg/mL (MIC) | Chloramphenicol 4 µg/mL (MIC) | [58] | ||
| Antibacterial | Well diffusion method/Vibrio alginolyticus | 16 µg/mL (MIC) | Chloramphenicol 0.5 µg/mL (MIC) | [58] | ||
| Antibacterial | Well diffusion method/Vibrio harveyi | 8 µg/mL (MIC) | Chloramphenicol 2 µg/mL (MIC) | [58] | ||
| Antibacterial | Well diffusion method/Vibrio parahaemolyticus | 8 µg/mL (MIC) | Chloramphenicol 2 µg/mL (MIC) | [58] | ||
| Antifungal | Well diffusion method/Colletotrichum gloeosprioides | 8 µg/mL (MIC) | Amphotericin B 8 µg/mL (MIC) | [58] | ||
| Fusarentin 6,7-dimethyl ether (160) | Antioxidant | ORAC | 14.4 µM (IC50) | Trolox 1 µM (IC50) | [80] | |
| Fusarentin 6-methyl ether (161) | Antioxidant | DPPH | 16.4 μM (IC50) | Ascorbic acid 21.2 μM (IC50) | [80] | |
| Antioxidant | ORAC | 1.4 µM (IC50) | Trolox 1 µM (IC50) | [80] | ||
| Monocerin (165) | Antibacterial | Agar diffusion/E. coli | 10 mm (IZD) | Penicillin 18 mm (IZD) Tetracycline 18 mm (GI) | [49] | |
| Antibacterial | Agar diffusion/Bacillus megaterium | 6 mm (GI) | Penicillin 14 mm (IZD) Tetracycline 18 mm (GI) | [49] | ||
| Antifungal | Agar diffusion/Microbotryum violaceum | 23 mm (IZD) | Actidione 35 mm (IZD) | [49] | ||
| Antialgal | Agar diffusion/Chlorella fusca | 8 mm (IZD) | Nystatin 20 mm (IZD) Actidione 50 mm (IZD) | [49] | ||
| Antibacterial | Microbroth dilution/E. coli (ATCC 25922) | 15.62 μg/mL (MIC) | - | [59] | ||
| Antibacterial | Pseudomonas aeruginosa (ATCC 27853) | 15.62 μg/mL (MIC) | - | [59] | ||
| Antibacterial | S. aureus (ATCC 25923) | 15.62 μg/mL (MIC) | - | [59] | ||
| Antibacterial | B. subtilis (ATCC 6633) | 15.62 μg/mL (MIC) | - | [59] | ||
| Antibacterial | Salmonella Typhimurium (ATCC14028) | 31.25 μg/mL (MIC) | - | [59] | ||
| Antioxidant | ORAC | 10.8 µM (IC50) | Trolox 1 µM (IC50) | [80] | ||
| Antimalarial | Microculture radioisotope/P. falciparum (K1) | 0.68 μM (IC50) | Dihydroartemisinin 0.0004 µM (IC50) | [46] | ||
| 7-O-Demethylmonocerin (166) | Antioxidant | XXO | 52.6 μM (IC50) | - | [80] | |
| Antioxidant | DPPH | 23.4 μM (IC50) | Ascorbic acid 21.2 μM (IC50) | [80] | ||
| Antioxidant | ORAC | 11.5 µM (IC50) | Trolox 1 µM (IC50) | [80] | ||
| Antioxidant | DPPH | 38 μM (EC50) | Ascorbic acid 39 μM (EC50) | [64] | ||
| (12R)-12-Hydroxymonocerin (167) | Antifungal | Agar diffusion/Microbotryum violaceum | 7 mm (IZD) | Actidione 35 mm (IZD) | [49] | |
| Antialgal | Agar diffusion/Chlorella fusca | 6 mm (IZD) | Nystatin 20 mm (IZD) Actidione 50 mm (IZD) | [49] | ||
| Antifungal | Colorimetric broth microdilution/F. oxysporum | 20 µg/mL (MIC) | Amphotericin B 0.63 µg/mL (MIC) | [60] | ||
| (11R)-Hydroxymonocerin (168) | Antimalarial | Microculture radioisotope/P. falciparum (K1) | 7.7 μM (IC50) | Dihydroartemisinin 0.004 µM (IC50) | [46] | |
| (12S)-Hydroxymonocerin (169) | Antibacterial | Agar diffusion/E. coli | 8 mm (IZD) | Penicillin 18 mm (IZD) Tetracycline 18 mm (GI) | [49] | |
| Antibacterial | Agar diffusion/B. megaterium | 6 mm (GI) | Penicillin 14 mm (IZD) Tetracycline 18 mm (GI) | [49] | ||
| Antifungal | Agar diffusion/Microbotryum violaceum | 9 mm (IZD) | Actidione 35 mm (IZD) | [49] | ||
| Antialgal | Agar diffusion/Chlorellafusca | 10 mm (IZD) | Nystatin 20 mm (IZD) Actidione 50 mm (IZD) | [49] | ||
| Exserolide C (177) | Antifungal | Colorimetric broth microdilution/F. oxysporum | 20 µg/mL (MIC) | Amphotericin B 0.63 µg/mL (MIC) | [60] | |
| Alternariol (188) | Antimicrobial | Colorimetric broth microdilution/M. tetragenus | 50 µg/mL (MIC) | Streptomycin 3.125 µg/mL (MIC) Acheomycin 3.125 µg/mL (MIC) Ampicillin 3.125 µg/mL (MIC) | [57] | |
| Alternariol 5-O-methyl ether (189) | Antimicrobial | Colorimetric broth microdilution/B. megaterium | 12.5 µg/mL (MIC) | Streptomycin 3.125 µg/mL (MIC) Acheomycin 3.125 µg/mL (MIC) Ampicillin 3.125 µg/mL (MIC) | [57] | |
| Cycloepoxylactone (197) | Antibacterial | Agar diffusion/Bacillus megaterium | 5 mm (GI) | Penicillin 14 mm (GI) Tetracycline 18 mm (GI) | [42] | |
| Antifungal | Agar diffusion/Microbotryum violaceum | 10 mm (IZD) | Nystatin 20 mm (IZD) Actidione 50 mm (IZD) | [42] | ||
| Exserolide F (201) | Antibacterial | Colorimetric broth microdilution/B. subtilis (ATCC 6633 | 20 µg/mL (MIC) | Ampicillin 1.25 µg/mL (MIC) | [60] | |
| Antibacterial | S. aureus (CGMCC 1.2465) | 5 µg/mL (MIC) | Ampicillin 0.16 µg/mL (MIC) | [60] | ||
| Antibacterial | S. pneumoniae (CGMCC 1.1692) | 10 µg/mL (MIC) | Ampicillin 10 µg/mL (MIC) | [60] | ||
| Antibacterial | E. coli (CGMCC 1.2340) | 20 µg/mL (MIC) | Gentamicin 2.5 µg/mL (MIC) | [60] | ||
| Isocitreoisocoumarinol (202) | Antioxidant | Luciferase | 0.98 mM (IC50) | tBHQ 4.29 mM (IC50) | [65] | |
| (+) Citreoisocoumarin (203) | Antioxidant | Luciferase | 1.03 mM (IC50) | tBHQ 4.29 mM (IC50) | [65] | |
| (+)-6-Methylcitreoisocoumarin (204) | α-Glucosidase inhibitory | Chromogenic | 38% Inhibition (200 μM) | Acarbose 19% Inhibition (200 μM) | [31] | |
| Antioxidant | Luciferase | 0.98 mM (IC50) | tBHQ 4.29 mM (IC50) | [65] | ||
| Citreoisocoumarinol (205) | Antioxidant | Luciferase | 0.91 mM (IC50) | tBHQ 4.29 mM (IC50) | [65] | |
| Mucorisocoumarin A (207) | Antioxidant | Luciferase | 0.88 mM (IC50) | tBHQ 4.29 mM (IC50) | [65] | |
| Mucorisocoumarin B (208) | Antioxidant | Luciferase | 1.03 mM (IC50) | tBHQ 4.29 mM (IC50) | [65] | |
| Peyroisocoumarin A (209) | Antioxidant | Luciferase | 1.93 mM (IC50) | tBHQ 4.29 mM (IC50) | [65] | |
| Peyroisocoumarin B (210) | Antioxidant | Luciferase | 2.95 mM (IC50) | tBHQ 4.29 mM (IC50) | [65] | |
| Peyroisocoumarin C (211) | Antioxidant | Luciferase | 1.46 mM (IC50) | tBHQ 4.29 mM (IC50) | [65] | |
| Aspergillumarin A (212) | α-Glucosidase inhibitory | Chromogenic | 38.1 μM (IC50) | Acarbose 958.3 μM (IC50) | [62] | |
| Antibacterial | Colorimetric broth microdilution/S. albus (ATCC 8799) | 12.5 μM (MIC50) | Ciprofloxacin 0.312 μM (MIC50) | [52] | ||
| Aspergillumarin B (213) | α-Glucosidase inhibitory | Chromogenic | 193.1 μM (IC50) | Acarbose 958.3 μM (IC50) | [62] | |
| Antibacterial | Colorimetric broth microdilution/S. albus (ATCC 8799) | 12.5 μM (MIC50) | Ciprofloxacin 0.312 μM (MIC50) | [52] | ||
| Penicimarin B (214) | α-Glucosidase inhibitory | Chromogenic | 431.4 μM (IC50) | Acarbose 958.3 μM (IC50) | [62] | |
| Penicimarin C (215) | α-Glucosidase inhibitory | Chromogenic | 266.3 μM (IC50) | Acarbose 958.3 μM (IC50) | [62] | |
| (R)-3-(R)-4,5-Dihydroxypentyl)-8-hydroxyisochroman-1-one (216) | α-Glucosidase inhibitory | Chromogenic | 162.5 μM (IC50) | Acarbose 958.3 μM (IC50) | [62] | |
| 5,6-Dihydroxy-3-(4-ydroxypentyl)isochroman-1-one (217) | α-Glucosidase inhibitory | Chromogenic | 142.1 μM (IC50) | Acarbose 958.3 μM (IC50) | [62] | |
| Dichlorodiaportin (220) | Anti-inflammatory | Colourmetric/NO | 67.2 μM (MIC50) | Indomethacin 37.5 μM (MIC50) | [37] | |
| Antibacterial | Colorimetric broth microdilution/Staphylococcus aureus | 25 μg/mL (MIC50) | Ciprofloxacin 0.25 μg/mL (MIC50) Gentamicin 0.1 μg/mL (MIC50) | [37] | ||
| Antibacterial | Colorimetric broth microdilution/B. subtilis | 25 μg/mL (MIC50) | Ciprofloxacin 0.25 μg/mL (MIC50) Gentamicin 0.1 μg/mL (MIC50) | [37] | ||
| Antibacterial | Colorimetric broth microdilution/E. coli | 50 μg/mL (MIC50) | Ciprofloxacin 0.25 μg/mL (MIC50) Gentamicin 0.1 μg/mL (MIC50) | [37] | ||
| Antibacterial | Colorimetric broth microdilution/Klebsiella pneumoniae | 50 μg/mL (MIC50) | Ciprofloxacin 0.25 μg/mL (MIC50) Gentamicin 0.1 μg/mL (MIC50) | [37] | ||
| Antibacterial | Colorimetric broth microdilution/Acinetobacter calcoaceticus | 50 μg/mL (MIC50) | Ciprofloxacin 0.25 μg/mL (MIC50) Gentamicin 0.1 μg/mL (MIC50) | [37] | ||
| Antifungal | Broth dilution/Colletotrichum musae | 150 μg/mL (IC50) | Carbendazim 6.25 μg/mL (IC50) | [66] | ||
| Antifungal | Broth dilution/Rhizoctonia solani | 150 μg/mL (IC50) | Carbendazim 6.25 μg/mL (IC50) | [66] | ||
| Desmethyldichlorodiaportin (221) | Anti-inflammatory | Colourmetric/NO | 33.6 μM (MIC50) | Indomethacin 37.5 μM (MIC50) | [37] | |
| Antibacterial | Colorimetric broth microdilution/S. aureus | 25 μg/mL (MIC50) | Ciprofloxacin 0.25 μg/mL (MIC50) Gentamicin 0.1 μg/mL (MIC50) | [37] | ||
| Antibacterial | Colorimetric broth microdilution/B. subtilis | 25 μg/mL (MIC50) | Ciprofloxacin 0.25 μg/mL (MIC50) Gentamicin 0.1 μg/mL (MIC50) | [37] | ||
| Antibacterial | Colorimetric broth microdilution/E. coli | 25 μg/mL (MIC50) | Ciprofloxacin 0.25 μg/mL (MIC50) Gentamicin 0.1 μg/mL (MIC50) | [37] | ||
| Antibacterial | Colorimetric broth microdilution/Klebsiella pneumoniae | 25 μg/mL (MIC50) | Ciprofloxacin 0.25 μg/mL (MIC50) Gentamicin 0.1 μg/mL (MIC50) | [37] | ||
| Antibacterial | Colorimetric broth microdilution/Acinetobacter calcoaceticus | 50 μg/mL (MIC50) | Ciprofloxacin 0.25 μg/mL (MIC50) Gentamicin 0.1 μg/mL (MIC50) | [37] | ||
| Peniisocoumarin D (222) | α-Glucosidase inhibitory | Chromogenic | 41% Inhibition (200 μM) | Acarbose 19% Inhibition (200 μM) | [31] | |
| Peniisocoumarin E (223) | α-Glucosidase inhibitory | Chromogenic | 158.4 μM (IC50) | Acarbose 958.3 μM (IC50) | [31] | |
| Peniisocoumarin F (224) | α-Glucosidase inhibitory | Chromogenic | 110.3 μM (IC50) | Acarbose 958.3 μM (IC50) | [31] | |
| Peniisocoumarin G (225) | α-Glucosidase inhibitory | Chromogenic | 40.5 μM (IC50) | Acarbose 958.3 μM (IC50) | [31] | |
| Peniisocoumarin H (226) | α-Glucosidase inhibitory | Chromogenic | 43% Inhibition (200 μM) | Acarbose 19% Inhibition (200 μM) | [31] | |
| Peniisocoumarin I (227) | α-Glucosidase inhibitory | Chromogenic | 78.1 μM (IC50) | Acarbose 958.3 μM (IC50) | [31] | |
| Peniisocoumarin J (228) | α-Glucosidase inhibitory | Chromogenic | 45.1 μM (IC50) | Acarbose 958.3 μM (IC50) | [31] | |
| 3-[(R)-3,3-Dichloro-2-hydroxypropyl]-8-hydroxy- 6-methoxy-1H-isochromen-1-one (229) | α-Glucosidase inhibitory | Chromogenic | 102.4 μM (IC50) | Acarbose 958.3 μM (IC50) | [31] | |
| (+)-Diaporthin (230) | α-Glucosidase inhibitory | Chromogenic | 33% Inhibition (200 μM) | Acarbose 19% Inhibition (200 μM) | [31] | |
| Diaportinol (231) | Antioxidant | Luciferase | 0.85 mM (IC50) | tBHQ 4.29 mM (IC50) | [65] | |
| (+)-(10R)-7-Hydroxy-3-(2-hydroxy-propyl)-5,6-dimethylisochromen-1-one (232) | Antibacterial | Colorimetric broth microdilution/B. subtilis (ATCC 6633) | 19.2 μg/mL (MIC80) | Penicillin 0.9 μg/mL (MIC80) | [63] | |
| Antifungal | Colorimetric broth microdilution/Trichophyton rubrum (ATCC 28189) | 32 μg/mL (MIC80) | Fluconazole 1 μg/mL (MIC80) | [63] | ||
| Peyroisocoumarin D (233) | Antioxidant | Luciferase | 2.28 mM (IC50) | tBHQ 4.29 mM (IC50) | [65] | |
| Orthosporin (234) | Antioxidant | Luciferase | 1.58 mM (IC50) | tBHQ 4.29 mM (IC50) | [65] | |
| Versicoumarin A (251) | Anti-TMV | Half-leaf method | 28.6% (Inhibition rate) | Ningnanmycin 31.5% (Inhibition rate) | [93] | |
| Peniisocoumarin C (258) | α-Glucosidase inhibitory | Chromogenic | 95% Inhibition (200 μM) | Acarbose 19% Inhibition (200 μM) | [31] | |
| Asperisocoumarin A (261) | Antioxidant | DPPH | 125 μM (EC50) | Ascorbic acid 35 μM (EC50) | [76] | |
| Asperisocoumarin B (262) | α-Glucosidase inhibitory | Chromogenic | 87.8 μM (IC50) | Acarbose628.3 μM (IC50) | [76] | |
| Asperisocoumarin C (263) | Antioxidant | DPPH | 138 μM (EC50) | Ascorbic acid 35 μM (EC50) | [76] | |
| Asperisocoumarin E (265) | α-Glucosidase inhibitory | Chromogenic | 52.3 μM (IC50) | Acarbose 628.3 μM (IC50) | [76] | |
| Asperisocoumarin F (266) | α-Glucosidase inhibitory | Chromogenic | 95.6 μM (IC50) | Acarbose 628.3 μM (IC50) | [76] | |
| Peniisocoumarin A (267) | α-Glucosidase inhibitory | Chromogenic | 18% Inhibition (200 μM) | Acarbose 19% Inhibition (200 μM) | [31] | |
| Peniisocoumarin B (268) | α-Glucosidase inhibitory | Chromogenic | 23% Inhibition (200 μM) | Acarbose 19% Inhibition (200 μM) | [31] | |
| Demethylcitreoviranol (286) | Antioxidant | Luciferase | 1.06 mM (IC50) | tBHQ 4.29 mM (IC50) | [65] | |
| Citreoviranol (287) | Antioxidant | Luciferase | 1.44 mM (IC50) | tBHQ 4.29 mM (IC50) | [65] | |
| Dichlorodiaportinolide (290) | Antifungal | Broth dilution/Colletotrichum musae | 25 μg/mL (IC50) | Carbendazim 6.25 μg/mL (IC50) | [66] | |
| Antifungal | Broth dilution/Rhizoctonia solani | 6.25 μg/mL (IC50) | Carbendazim 6.25 μg/mL (IC50) | [66] | ||
| Dichlorodiaportintone (291) | Anti-inflammatory | Colourmetric/NO | 41.5 μM (MIC50) | Indomethacin 37.5 μM (MIC50) | [37] | |
| Antibacterial | Colorimetric broth microdilution/S. aureus | 50 μg/mL (MIC50) | Ciprofloxacin 0.25 μg/mL (MIC50) Gentamicin 0.1 μg/mL (MIC50) | [37] | ||
| Antibacterial | Colorimetric broth microdilution/E. coli | 50 μg/mL (MIC50) | Ciprofloxacin 0.25 μg/mL (MIC50) Gentamicin 0.1 μg/mL (MIC50) | [37] | ||
| Antibacterial | Colorimetric broth microdilution/Klebsiella pneumoniae | 50 μg/mL (MIC50) | Ciprofloxacin 0.25 μg/mL (MIC50) Gentamicin 0.1 μg/mL (MIC50) | [37] | ||
| Desmethyldichlorodiaportintone (292) | Anti-inflammatory | Colourmetric/NO | 15.8 μM (MIC50) | Indomethacin 37.5 μM (MIC50) | [37] | |
| Botryoisocoumarin A (293) | Anti-inflammatory | Colourmetric/COX-2 | 6.51 μM (IC50) | - | [36] | |
| Compound Name | Assay, Cell Line | Cytotoxicity Results | Positive Control | Referencs |
|---|---|---|---|---|
| Penicisimpin A (157) 3-(R)-6,8-Dihydroxy-7-methyl-3-pentylisochroman-1-one | Brine shrimp (Artemia salina) lethality | 7.7 µg/mL (LD50) | Colchicine 16.5 µg/mL (LD50) | [58] |
| Penicisimpin B (158) 3-(R)-6,8-Dihydroxy-3-pentylisochroman-1-one | 36.4 µg/mL (LD50) | Colchicine 16.5 µg/mL (LD50) | [58] | |
| Penicisimpin C (159) 3-(S)-6,8-Dihydroxy-7-methyl-3-(pent-1-enyl)isochroman-1-one | 18.6 µg/mL (LD50) | Colchicine 16.5 µg/mL (LD50) | [58] | |
| 7-O-Demethylmonocerin (166) | MTT/HepG2 | 23.7 μM (IC50) | Etoposide 15.8 μM (IC50) | [80] |
| Aspergisocoumrin A (274) | 43.70 μM (IC50) | Epirubicin 0.32 μM (IC50) | [73] | |
| Dichlorodiaportinol A (289) | 39.6 μg/mL (EC50) | Epirubicin 5.2 μg/mL(IC50) | [79] | |
| (+) Citreoisocoumarin (203) | MTT/L5178Y | 99.5% GI (10 μg/mL) | 0.1% EGMME/DMSO | [40] |
| Desmethyldiaportinol (219) | 7.3 μg/mL (EC50) | Kahalalide F 6.4 μg/mL (EC50) | [40] | |
| Desmethyldichlorodiaportin (221) | 41.4% GI (10 μg/mL) | 0.1% EGMME/DMSO | [40] | |
| 6,6′-Dinor-bipenicilisorin (259) | 13% GI (10 μg/mL) | Gerfelin 85% GI (10 μg/mL) | [81] | |
| 6,6′,9′-Trinor-bipenicilisorin (260) | 33% GI (10 μg/mL) | Gerfelin 85% GI (10 μg/mL) | [81] | |
| Versicoumarin A (251) | MTT/MCF7 | 3.8 μM (IC50) | Taxol 0.1 μM (IC50) | [93] |
| Versicoumarin D (252) | 8.0 μM (IC50) | Taxol | [89] | |
| Dichlorodiaportinol A (289) | 17.8 μg/mL (EC50) | Epirubicin 5.3 μg/mL (IC50) | [79] | |
| Peniisocoumarin G (225) | MTT/MptpB | 20.1 μM (IC50) | Oleanolic acid 22.1 µM (IC50) | [31] |
| Versicoumarin A (251) | MTT/A549 | 4.0 μM (IC50) | Taxol 0.02 μM (IC50) | [93] |
| Versicoumarin D (252) | MTT/A549 | 5.8 μM (IC50) | Taxol | [89] |
| Aspergisocoumrin A (274) | MTS/MDA-MB-435 | 5.08 μM (IC50) | Epirubicin 0.26 μM (IC50) | [73] |
| Aspergisocoumrin B (275) | 4.98 μM (IC50) | Epirubicin 0.26 μM (IC50) | [73] | |
| Aspergisocoumrin A (274) | MTS/MCF10A | 11.34 μM (IC50) | Epirubicin 0.13 μM (IC50) | [73] |
| Aspergisocoumrin B (275) | 21.40 μM (IC50) | Epirubicin 0.13 μM (IC50) | [73] | |
| Aspergisocoumrin A (274) | MTS/H460 | 21.53 μM (IC50) | Epirubicin 0.12 μM (IC50) | [73] |
| Isocoumarindole A (296) | CCK colorimetric/MIA-PACA-2 | 1.63 μM (IC50) | Gemcitabine 1.02 μM (IC50) | [29] |
| CCK colorimetric/ASPC-1 | 5.53 μM (IC50) | Gemcitabine 20.10 μM (IC50) | [29] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noor, A.O.; Almasri, D.M.; Bagalagel, A.A.; Abdallah, H.M.; Mohamed, S.G.A.; Mohamed, G.A.; Ibrahim, S.R.M. Naturally Occurring Isocoumarins Derivatives from Endophytic Fungi: Sources, Isolation, Structural Characterization, Biosynthesis, and Biological Activities. Molecules 2020, 25, 395. https://doi.org/10.3390/molecules25020395
Noor AO, Almasri DM, Bagalagel AA, Abdallah HM, Mohamed SGA, Mohamed GA, Ibrahim SRM. Naturally Occurring Isocoumarins Derivatives from Endophytic Fungi: Sources, Isolation, Structural Characterization, Biosynthesis, and Biological Activities. Molecules. 2020; 25(2):395. https://doi.org/10.3390/molecules25020395
Chicago/Turabian StyleNoor, Ahmad Omar, Diena Mohammedallam Almasri, Alaa Abdullah Bagalagel, Hossam Mohamed Abdallah, Shaimaa Gamal Abdallah Mohamed, Gamal Abdallah Mohamed, and Sabrin Ragab Mohamed Ibrahim. 2020. "Naturally Occurring Isocoumarins Derivatives from Endophytic Fungi: Sources, Isolation, Structural Characterization, Biosynthesis, and Biological Activities" Molecules 25, no. 2: 395. https://doi.org/10.3390/molecules25020395
APA StyleNoor, A. O., Almasri, D. M., Bagalagel, A. A., Abdallah, H. M., Mohamed, S. G. A., Mohamed, G. A., & Ibrahim, S. R. M. (2020). Naturally Occurring Isocoumarins Derivatives from Endophytic Fungi: Sources, Isolation, Structural Characterization, Biosynthesis, and Biological Activities. Molecules, 25(2), 395. https://doi.org/10.3390/molecules25020395