Synthesis, Crystal Structure, and Biological Activity of a Multidentate Calix[4]arene Ligand Doubly Functionalized by 2-Hydroxybenzeledene-Thiosemicarbazone
Abstract
1. Introduction
2. Results and Discussion
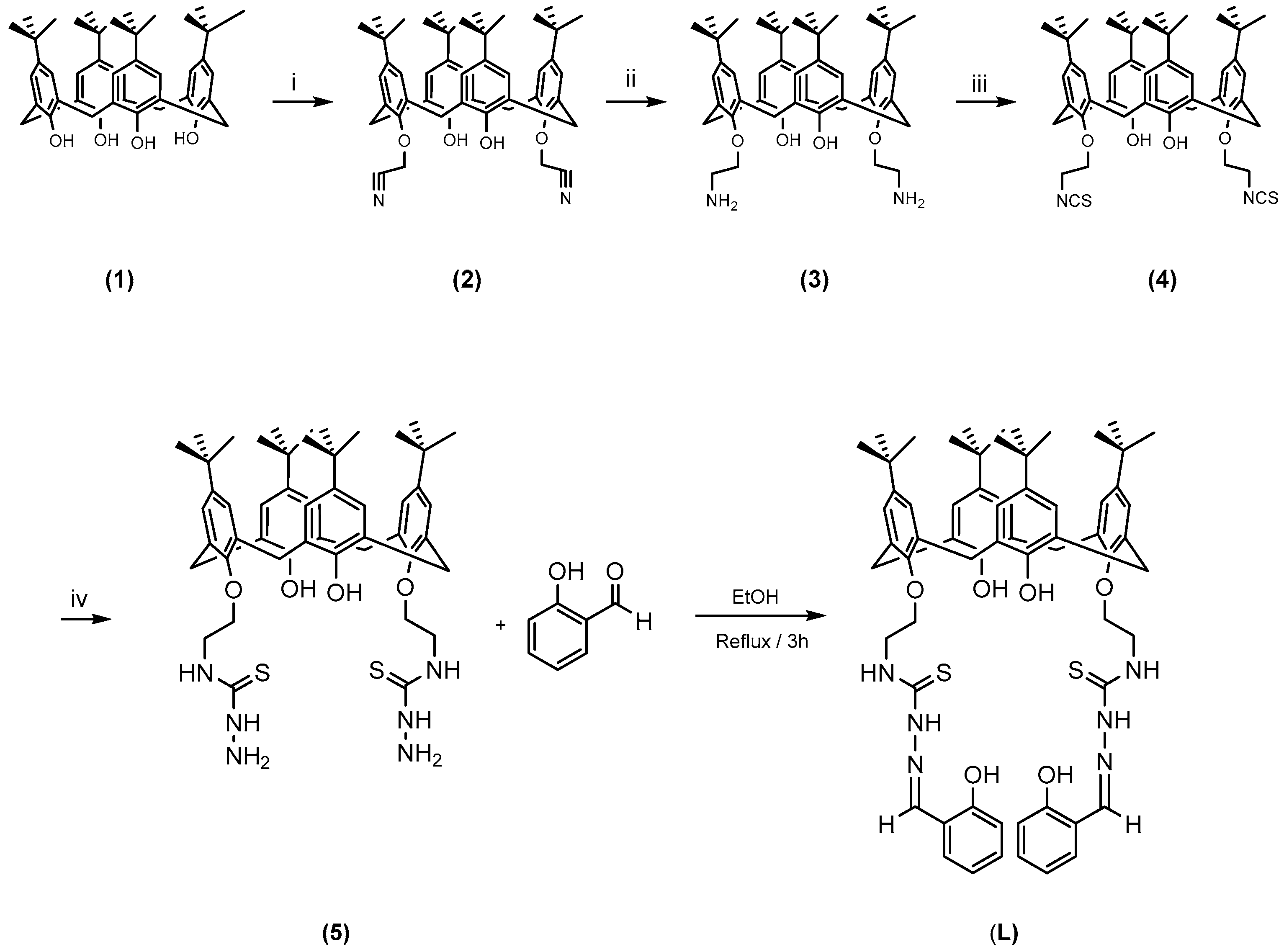
2.1. Synthesis of the Ligand and Metal Derivatives
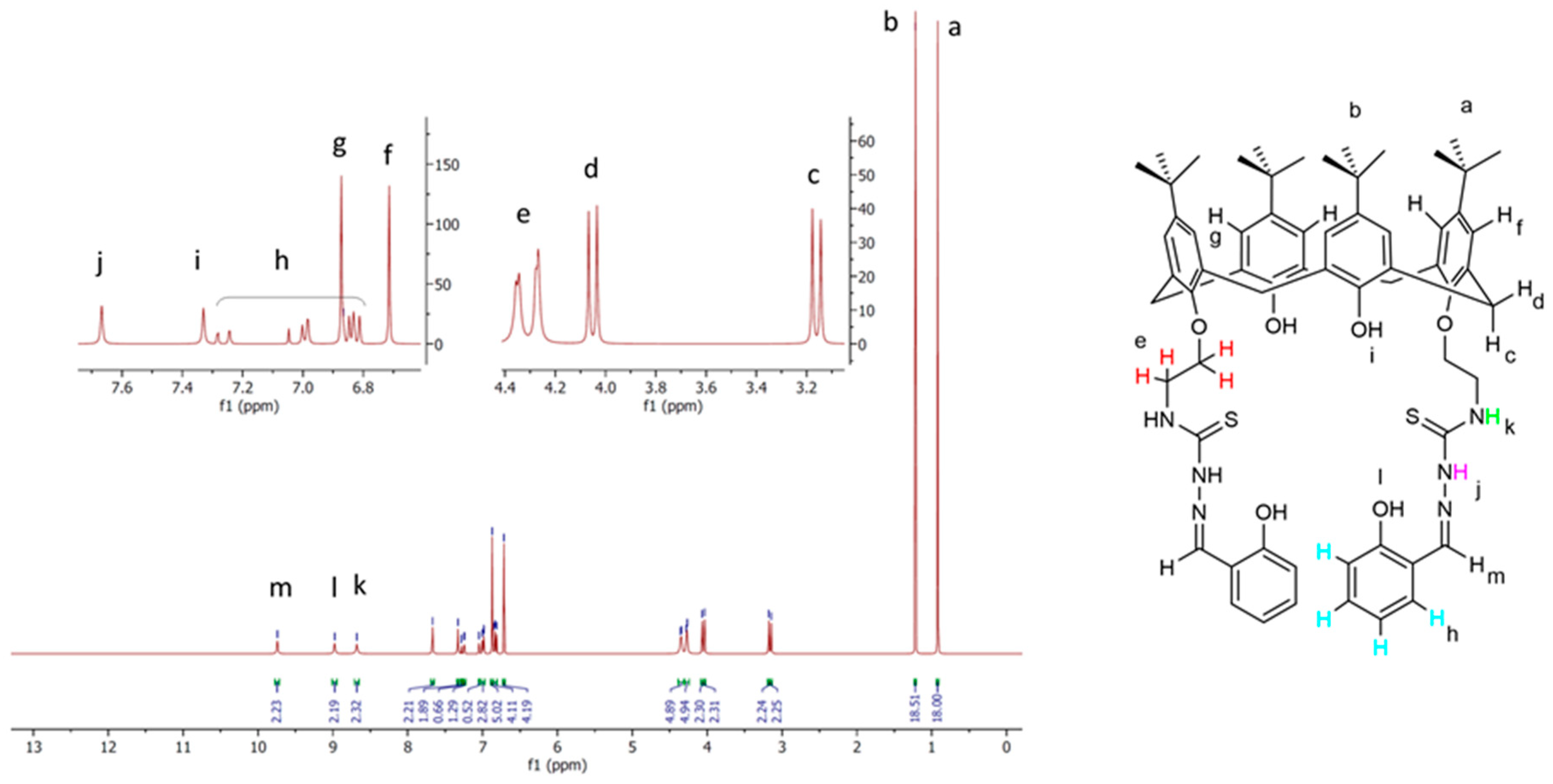
2.2. Characterization
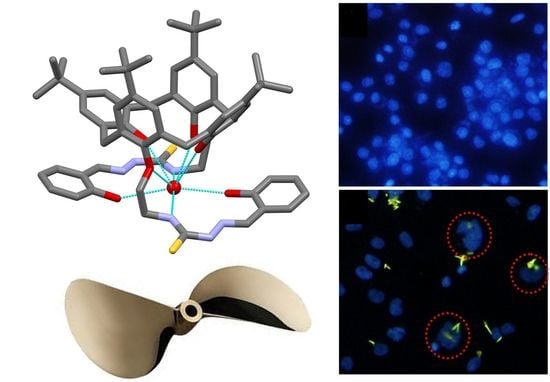
2.3. Solid-State Structure of L
2.4. Determination of Antimicrobial and Antifungal Activities
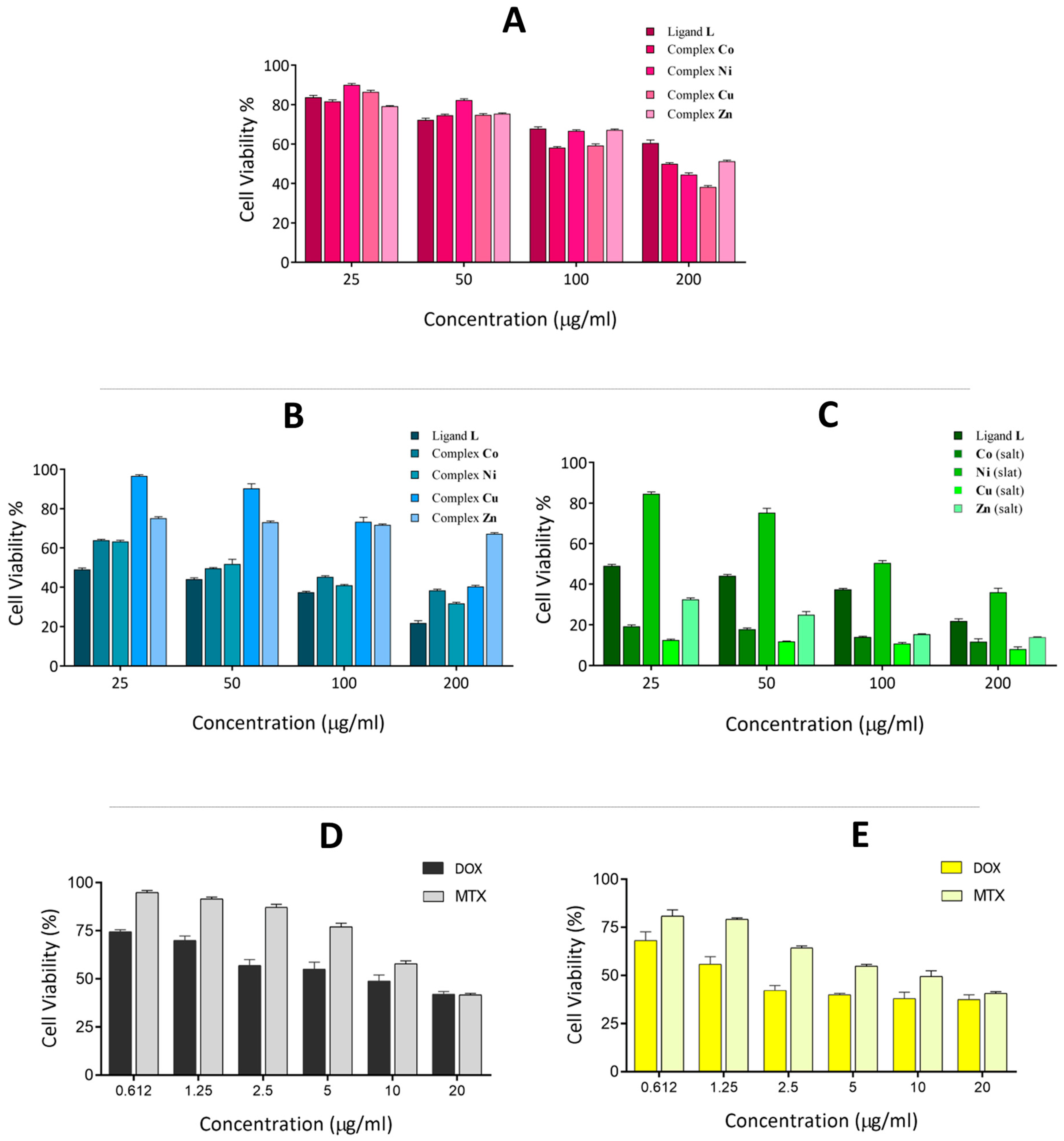
2.5. Cytotoxicity Assay
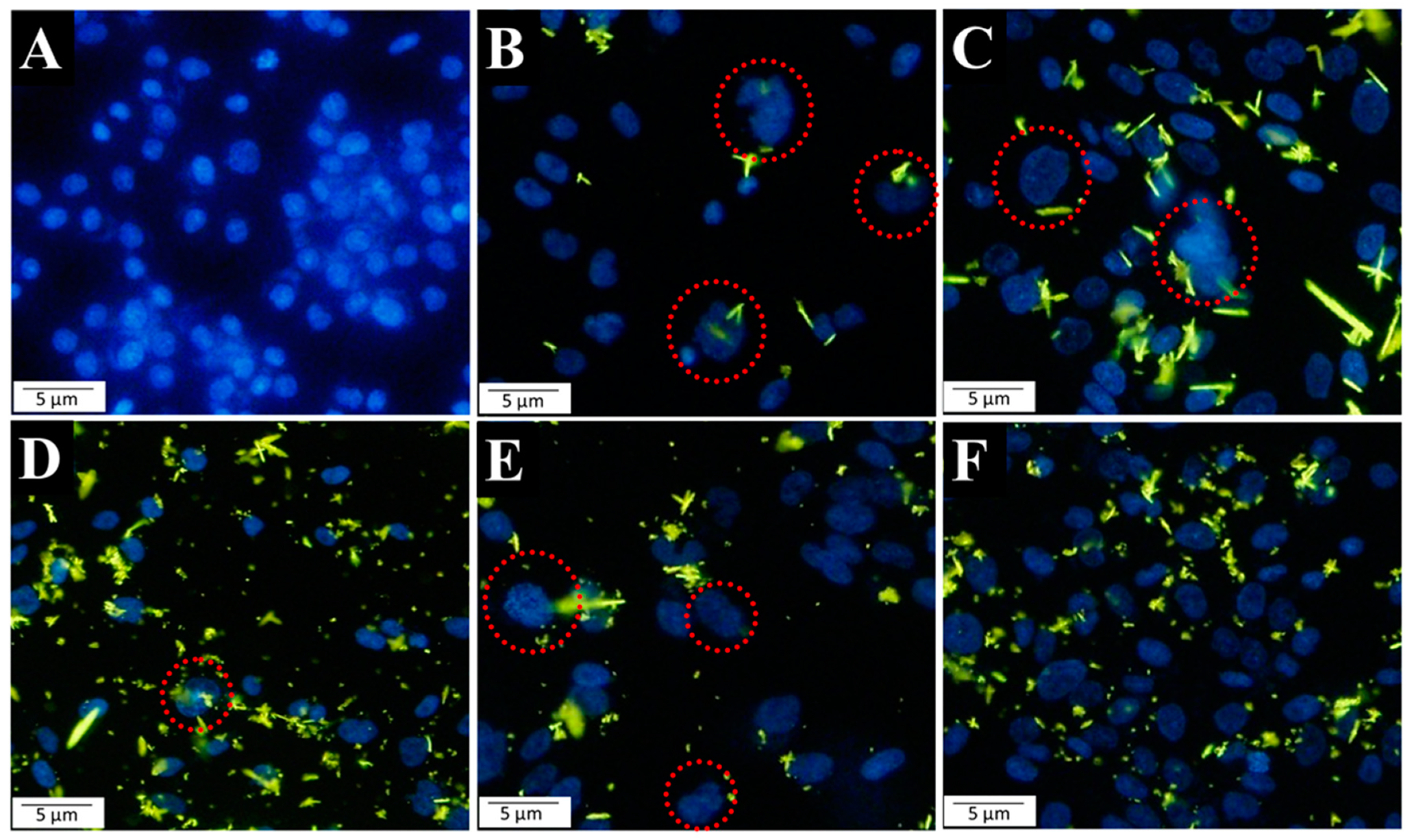
2.6. Morphology of Cell Nuclei
2.7. Biocompatibility Evaluation
3. Materials and Methods
3.1. Reagents
3.2. Instrumentation
3.3. Synthesis
3.3.1. Preparation of Ligand
3.3.2. Preparation of Metal Derivatives
Cobalt Derivative:
Nickel Derivative:
Copper Derivative:
Zinc Derivative:
3.4. Crystal Structure Determination and Refinement of L
3.5. Antimicrobial Studies
3.5.1. Preparation of Microorganism’s Suspensions
3.5.2. Broth Microdilution Method
3.6. Cell Culture and MTT Assay
3.7. DAPI Staining
3.8. Erythrocytes Hemolysis Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gutsche, C.D.; Levine, J.A. Calixarenes 6 Synthesis of a Functionalizable Calix[4]arene in a Conformationally Rigid Cone Conformation. J. Am. Chem. Soc. 1982, 104, 2652–2653. [Google Scholar] [CrossRef]
- Erdemir, S.; Tabakci, B.; Tabakci, M. A highly selective fluorescent sensor based on calix [4] arene appended benzothiazole units for Cu2+, S2− and HSO4− ions in aqueous solution. Sens. Actuators B Chem. 2016, 228, 109–116. [Google Scholar] [CrossRef]
- Bauer, A.; Jäschke, A.; Azzam, S.S.A.; Glasneck, F.; Ullmann, S.; Kersting, B.; Brendler, V.; Schmeide, K.; Stumpf, T. Multidentate extracting agents based on calix [4] arene scaffold–UVI/EuIII separation studies. Sep. Purif. Technol. 2019, 213, 246–254. [Google Scholar] [CrossRef]
- Shirakawa, S.; Shimizu, S. Inherently Chiral Calix [4] arenes as Supramolecular Catalysts. In Designed Molecular Space in Material Science and Catalysis; Springer: Berlin, Germany, 2018; pp. 51–68. [Google Scholar]
- De Rosa, M.; La Manna, P.; Soriente, A.; Gaeta, C.; Talotta, C.; Hickey, N.; Geremia, S.; Neri, P. A Simple Tetraminocalix[4]arene as a Highly Efficient Catalyst under “On-Water” Conditions through Hydrophobic Amplification of Weak Hydrogen Bonds. Chem. Eur. J. 2017, 23, 7142–7151. [Google Scholar]
- Rahimi, M.; Karimian, R.; Mostafidi, E.; Noruzi, E.B.; Taghizadeh, S.; Shokouhi, B.; Kafil, H.S. Highly branched amine-functionalized p-sulfonatocalix [4] arene decorated with human plasma proteins as a smart, targeted, and stealthy nano-vehicle for the combination chemotherapy of MCF7 cells. New J. Chem. 2018, 42, 13010–13024. [Google Scholar]
- Rahimi, M.; Karimian, R.; Noruzi, E.B.; Ganbarov, K.; Zarei, M.; Kamounah, F.S.; Yousefi, B.; Bastami, M.; Yousefi, M.; Kafil, H.S. Needle-shaped amphoteric calix [4] arene as a magnetic nanocarrier for simultaneous delivery of anticancer drugs to the breast cancer cells. Int. J. Nanomed. 2019, 14, 2619–2636. [Google Scholar] [CrossRef]
- Böhmer, V.; Rathay, D.; Kämmerer, H. The t-butyl group as a possible protective group in the synthesis of oligo [hydroxy-1, 3-phenylene] methylenes. Org. Prep. Proc. Int. 1978, 10, 113–121. [Google Scholar] [CrossRef]
- Sengupta, A.; Godbole, S.V.; Mohapatra, P.K.; Iqbal, M.; Huskens, J.; Verboom, W. Judd–Ofelt parameters of diglycolamide-functionalized calix [4] arene Eu3+ complexes in room temperature ionic liquid for structural analysis: Effects of solvents and ligand stereochemistry. J. Lumin. 2014, 148, 174–180. [Google Scholar] [CrossRef]
- Dessingou, J.; Tabbasum, K.; Mitra, A.; Hinge, V.K.; Rao, C.P. Lower rim 1, 3-di {4-antipyrine} amide conjugate of calix [4] arene: Synthesis, characterization, and selective recognition of Hg2+ and its sensitivity toward pyrimidine bases. J. Org. Chem. 2012, 77, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Gubbuk, I.H.; Gungor, O.; Alpoguz, H.K.; Ersoz, M.; Yılmaz, M. Kinetic study of mercury (II) transport through a liquid membrane containing calix [4] arene nitrile derivatives as a carrier in chloroform. Desalination 2010, 261, 157–161. [Google Scholar] [CrossRef]
- Bozkurt, S.; Turkmen, M.B.; Soykan, C. Synthesis of new chiral calix [4] arene thiourea derivatives for enantiomeric recognition of carboxylate anions. J. Incl. Phenom. Macrocyclic Chem. 2016, 84, 35–41. [Google Scholar] [CrossRef]
- Sap, A.; Tabakci, B.; Yilmaz, A. Calix [4] arene-based Mannich and Schiff bases as versatile receptors for dichromate anion extraction: Synthesis and comparative studies. Tetrahedron 2012, 68, 8739–8745. [Google Scholar] [CrossRef]
- Iuliano, V.; Talotta, C.; Gaeta, C.; Soriente, A.; De Rosa, M.; Geremia, S.; Hickey, N.; Mennucci, B.; Neri, P. Negative Solvatochromism in a N-Linked p-Pyridiniumcalix[4]arene Derivative. Org. Lett. 2019, 21, 2704–2707. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, M.; Picron, J.-F.; Mattiuzzi, A.; Lascaux, A.; De Cesco, S.; Brugnara, A.; Thiabaud, G.; Darbost, U.; Coquiere, D.; Colasson, B. Ipso-nitration of calix [6] azacryptands: Intriguing effect of the small rim capping pattern on the large rim substitution selectivity. J. Org. Chem. 2012, 77, 3838–3845. [Google Scholar] [CrossRef]
- Kim, S.K.; Sessler, J.L.; Gross, D.E.; Lee, C.-H.; Kim, J.S.; Lynch, V.M.; Delmau, L.H.; Hay, B.P. A calix [4] arene strapped calix [4] pyrrole: An ion-pair receptor displaying three different cesium cation recognition modes. J. Am. Chem. Soc. 2010, 132, 5827–5836. [Google Scholar] [CrossRef]
- Durso, A.; Brancatelli, G.; Hickey, N.; Farnetti, E.; De Zorzi, R.; Bonaccorso, C.; Purrello, R.; Geremia, S. Interactions of a water-soluble calix[4]arene with spermine: Solution and solid-state characterisation. Supramol. Chem. 2016, 28, 499–505. [Google Scholar] [CrossRef]
- Kim, S.K.; Lee, J.; Williams, N.J.; Lynch, V.M.; Hay, B.P.; Moyer, B.A.; Sessler, J.L. Bipyrrole-Strapped Calix [4] pyrroles: Strong Anion Receptors That Extract the Sulfate Anion. J. Am. Chem. Soc. 2014, 136, 15079–15085. [Google Scholar] [CrossRef]
- Teixeira, F.A.; Marcos, P.M.; Ascenso, J.R.; Brancatelli, G.; Hickey, N.; Geremia, S. Selective Binding of Spherical and Linear Anions by Tetraphenyl(thio)urea-Based Dihomooxacalix[4]arene Receptors. J. Org. Chem. 2017, 82, 11383–11390. [Google Scholar] [CrossRef]
- Miranda, A.S.; Serbetci, D.; Marcos, P.M.; Ascenso, J.R.; Berberan-Santos, M.N.; Hickey, N.; Geremia, S. Ditopic Receptors Based on Dihomooxacalix[4]arenes Bearing Phenylurea Moieties with Electron-Withdrawing Groups for Anions and Organic Ion Pairs. Front. Chem. 2019, 7, 758. [Google Scholar] [CrossRef]
- Gattuso, G.; Notti, A.; Parisi, M.F.; Pisagatti, I.; Marcos, P.M.; Ascenso, J.R.; Brancatelli, G.; Geremia, S. Selective recognition of biogenic amine hydrochlorides by heteroditopic dihomooxacalix[4]arenes. New, J. Chem. 2015, 39, 817–821. [Google Scholar] [CrossRef]
- Gao, F.; Cui, L.; Song, Y.; Li, Y.-Z.; Zuo, J.-L. Calix [4] arene-supported mononuclear lanthanide single-molecule magnet. Inorg. Chem. 2013, 53, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Cante-Mota, I.; Moreno-Alcántar, G.; Flores-Alamo, M.; Castillo, I. Benzimidazole-derived calix [4] arenes with polymerizable styrene groups and their Cu (II) complexes. Inorg. Chim. Acta 2013, 407, 11–18. [Google Scholar] [CrossRef]
- Li, L.; Gu, W.-W.; Yan, C.-G. Syntheses, Crystal Structures and Complexing Properties of 1, 3-Distal Calix [4] arene Schiff Bases. Chem. Res. Chin. Univ. 2010, 26, 38–45. [Google Scholar]
- Sgarlata, C.; Brancatelli, G.; Fortuna, C.G.; Sciotto, D.; Geremia, S.; Bonaccorso, C. Three-dimensional network structures based on pyridylcalix[4]arene metal complexes. Chem. Plus Chem. 2017, 82, 1341–1350. [Google Scholar]
- Meninno, S.; Parrella, A.; Brancatelli, G.; Geremia, S.; Gaeta, C.; Talotta, C.; Neri, P.; Lattanzi, A. Polyoxomolybdate-Calix[4]arene Hybrid: A Catalyst for Sulfoxidation Reactions with Hydrogen Peroxide. Org. Lett. 2015, 17, 5100–5103. [Google Scholar] [CrossRef]
- Brancatelli, G.; De Zorzi, R.; Hickey, N.; Siega, P.; Zingone, G.; Geremia, S. New Multicomponent Porous Architecture of Self-Assembled Porphyrins/Calixarenes Driven by Nickel Ions. Cryst. Growth Des. 2012, 12, 5111–5117. [Google Scholar] [CrossRef]
- Casas, J.S.; García-Tasende, M.S.; Sordo, J. Main group metal complexes of semicarbazones and thiosemicarbazones. A structural review. Coord. Chem. Rev. 2000, 209, 197–261. [Google Scholar] [CrossRef]
- De Oliveira, R.B.; de Souza-Fagundes, E.M.; Soares, R.P.P.; Andrade, A.A.; Krettli, A.U.; Zani, C.L. Synthesis and antimalarial activity of semicarbazone and thiosemicarbazone derivatives. Eur. J. Med. Chem. 2008, 43, 1983–1988. [Google Scholar]
- Shn Moorthy, N.; Mfsa Cerqueira, N.; Ramos, M.J.; Fernandes, P.A. Aryl-and heteroaryl-thiosemicarbazone derivatives and their metal complexes: A pharmacological template. Recent Pat. Anti-Cancer Drug Discov. 2013, 8, 168–182. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Al-Majedy, Y.K.; Abdulreazak, H.; Abood, H. Synthesis, characterization, theoretical crystal structure, and antibacterial activities of some transition metal complexes of the thiosemicarbazone (Z)-2-(pyrrolidin-2-ylidene) hydrazinecarbothioamide. Bioinorg. Chem. Appl. 2011, 2011, 483101. [Google Scholar] [CrossRef]
- Pathan, A.H.; Bakale, R.P.; Naik, G.N.; Frampton, C.S.; Gudasi, K.B. Synthesis, crystal structure, redox behavior and comprehensive studies on DNA binding and cleavage properties of transition metal complexes of a fluoro substituted thiosemicarbazone derived from ethyl p yruvate. Polyhedron 2012, 34, 149–156. [Google Scholar] [CrossRef]
- Netalkar, P.P.; Netalkar, S.P.; Revankar, V.K. Transition metal complexes of thiosemicarbazone: Synthesis, structures and invitro antimicrobial studies. Polyhedron 2015, 100, 215–222. [Google Scholar] [CrossRef]
- Quiroga, A.G.; Ranninger, C.N. Contribution to the SAR field of metallated and coordination complexes: Studies of the palladium and platinum derivatives with selected thiosemicarbazones as antitumoral drugs. Coord. Chem. Rev. 2004, 248, 119–133. [Google Scholar] [CrossRef]
- Melha, K.S.A. In-vitro antibacterial, antifungal activity of some transition metal complexes of thiosemicarbazone Schiff base (HL) derived from N4-(7′-chloroquinolin-4′-ylamino) thiosemicarbazide. J. Enzyme Inhib. Med. Chem. 2008, 23, 493–503. [Google Scholar] [CrossRef]
- Elsayed, S.A.; El-Hendawy, A.M.; Mostafa, S.I.; Jean-Claude, B.J.; Todorova, M.; Butler, I.S. Antineoplastic activity of new transition metal complexes of 6-methylpyridine-2-carbaldehyde-n (4)-ethylthiosemicarbazone: X-Ray crystal structures of [VO2 (mpETSC)] and [Pt (mpETSC) Cl]. Bioinorg. Chem. Appl. 2010, 2010, 149149. [Google Scholar] [CrossRef]
- Santini, C.; Pellei, M.; Gandin, V.; Porchia, M.; Tisato, F.; Marzano, C. Advances in copper complexes as anticancer agents. Chem. Rev. 2013, 114, 815–862. [Google Scholar] [CrossRef]
- Glisoni, R.J.; Cuestas, M.L.; Mathet, V.L.; Oubiña, J.R.; Moglioni, A.G.; Sosnik, A. Antiviral activity against the hepatitis C virus (HCV) of 1-indanone thiosemicarbazones and their inclusion complexes with hydroxypropyl-β-cyclodextrin. Eur. J. Pharm. Sci. 2012, 47, 596–603. [Google Scholar] [CrossRef]
- Lessa, J.A.; Soares, M.A.; Dos Santos, R.G.; Mendes, I.C.; Salum, L.B.; Daghestani, H.N.; Andricopulo, A.D.; Day, B.W.; Vogt, A.; Beraldo, H. Gallium (III) complexes with 2-acetylpyridine-derived thiosemicarbazones: Antimicrobial and cytotoxic effects and investigation on the interactions with tubulin. Biometals 2013, 26, 151–165. [Google Scholar] [CrossRef]
- Kalaivani, P.; Prabhakaran, R.; Ramachandran, E.; Dallemer, F.; Paramaguru, G.; Renganathan, R.; Poornima, P.; Padma, V.V.; Natarajan, K. Influence of terminal substitution on structural, DNA, protein binding, anticancer and antibacterial activities of palladium (II) complexes containing 3-methoxy salicylaldehyde-4 (N) substituted thiosemicarbazones. Dalton Trans. 2012, 41, 2486–2499. [Google Scholar] [CrossRef]
- Li, M.X.; Chen, C.L.; Zhang, D.; Niu, J.Y.; Ji, B.S. Mn (II), Co (II) and Zn (II) complexes with heterocyclic substituted thiosemicarbazones: Synthesis, characterization, X-ray crystal structures and antitumor comparison. Eur. J. Med. Chem. 2010, 45, 3169–3177. [Google Scholar] [CrossRef]
- Chawla, H.M.; Sahu, S.N.; Shrivastava, R.; Kumar, S. Calix [4] arene-based ditopic receptors for simultaneous recognition of fluoride and cobalt (II) ions. Tetrahedron Lett. 2012, 53, 2244–2247. [Google Scholar] [CrossRef]
- Chawla, H.M.; Singh, S.P. Calix [4] arene based neutral receptor for dihydrogen phosphate anion. Tetrahedron 2008, 64, 741–748. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, D.; Wang, J.; Sun, J.; Yan, C. Synthesis and crystal structure of p-tert-butylcalix [4] arene 1, 3-distal and monosubstituted semicarbazones and thiosemicarbazones. Chem. Res. Chin. Univ. 2014, 30, 415–419. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Venkataraghavan, R. The C= S stretching frequency and the “-NC= S bands” in the infrared. Spectrochim. Acta 1962, 18, 541–547. [Google Scholar] [CrossRef]
- Mostafa, M.M. Spectroscopic studies of some thiosemicarbazide compounds derived from Girard’s T and P. Spectrochim. Acta A 2007, 66, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Viñuelas-Zahínos, E.; Luna-Giles, F.; Torres-García, P.; Fernández-Calderón, M.C. Co (III), Ni (II), Zn (II) and Cd (II) complexes with 2-acetyl-2-thiazoline thiosemicarbazone: Synthesis, characterization, X-ray structures and antibacterial activity. Eur. J. Med. Chem. 2011, 46, 150–159. [Google Scholar] [CrossRef]
- Quiroga-Campano, C.; Gomez-Machuca, H.; Moris, S.; Jara, P.; De la Fuente, J.; Pessoa-Mahana, H.; Jullian, C.; Saitz, C. Synthesis of bifunctional receptor for fluoride and cadmium based on calix [4] arene with thiourea moieties. J. Mol. Struct. 2017, 1141, 133–141. [Google Scholar] [CrossRef]
- Bahojb Noruzi, E.; Kheirkhahi, M.; Shaabani, B.; Geremia, S.; Hickey, N.; Asaro, F.; Nitti, P.; Kafil, H.S. Design of a thiosemicarbazide functionalized calix [4] arene ligand and related transition metal complexes: Synthesis, characterization and biological studies. Front. Chem. 2019, 7, 663. [Google Scholar] [CrossRef]
- Zhong, Z.; Aotegen, B.; Xu, H.; Zhao, S. Structure and antimicrobial activities of benzoyl phenyl-thiosemicarbazone-chitosans. Int. J. Biol. Macromol. 2012, 50, 1169–1174. [Google Scholar] [CrossRef]
- Kovala-Demertzi, D.; Demertzis, M.A.; Miller, J.R.; Papadopoulou, C.; Dodorou, C.; Filousis, G. Platinum(II) complexes with 2-acetyl pyridine thiosemicarbazone: Synthesis, crystal structure, spectral properties, antimicrobial and antitumour activity. J. Inorg. Biochem. 2001, 86, 555–563. [Google Scholar] [CrossRef]
- Hall, I.H.; Lackey, C.B.; Kistler, T.D.; Durham, R.W.; Jouad, E.M.; Khan, M.; Thanh, X.D.; Djebbar-Sid, S.; Benali-Baitich, O.; Bouet, G.M. Cytotoxicity of copper and cobalt complexes of furfural semicarbazone and thiosemicarbazone derivatives in murine and human tumor cell lines. Pharmazie 2000, 55, 937–941. [Google Scholar] [PubMed]
- Baldini, M.; Belicchi-Ferrari, M.; Bisceglie, F.; Pelosi, G.; Pinelli, S.; Tarasconi, P. Cu(II) Complexes with Heterocyclic Substituted Thiosemicarbazones: The Case of 5-Formyluracil. Synthesis, Characterization, X-ray Structures, DNA Interaction Studies, and Biological Activity. Inorg. Chem. 2003, 42, 2049–2055. [Google Scholar] [CrossRef]
- Das, D.; Rameshbabu, A.P.; Ghosh, P.; Patra, P.; Dhara, S.; Pal, S. Biocompatible nanogel derived from functionalized dextrin for targeted delivery of doxorubicin hydrochloride to MG 63 cancer cells. Carbohydr. Polym. 2017, 171, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Chai, D.; Hao, B.; Hu, R.; Zhang, F.; Yan, J.; Sun, Y.; Huang, X.; Zhang, Q.; Jiang, H. Delivery of Oridonin and Methotrexate via PEGylated Graphene Oxide. ACS Appl. Mater. Interfaces 2019, 11, 22915–22924. [Google Scholar] [CrossRef] [PubMed]
- Meshkini, A.; Oveisi, H. Methotrexate-F127 conjugated mesoporous zinc hydroxyapatite as an efficient drug delivery system for overcoming chemotherapy resistance in osteosarcoma cells. Colloids Surf. B 2017, 158, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.I.P.; Sousa, A.I.; Silva, J.C.; Ferreira, I.M.M.; Novo, C.M.M.; Borges, J.P. Chitosan-based nanoparticles as drug delivery systems for doxorubicin: Optimization and modelling. Carbohydr. Polym. 2016, 147, 304–312. [Google Scholar] [CrossRef]
- Rahimi, M.; Shojaei, S.; Safa, K.D.; Ghasemi, Z.; Salehi, R.; Yousefi, B.; Shafiei-Irannejad, V. Biocompatible magnetic tris (2-aminoethyl) amine functionalized nanocrystalline cellulose as a novel nanocarrier for anticancer drug delivery of methotrexate. New J. Chem. 2017, 41, 2160–2168. [Google Scholar] [CrossRef]
- Gutsche, C.D.; Iqbal, M.; Stewart, D. Calixarenes 18. Syntheses procedures for p-tert-butylcalix [4] arene. J. Org. Chem. 1986, 51, 742–745. [Google Scholar] [CrossRef]
- Zhang, W.-C.; Huang, Z.-T. Synthesis of 4-tert-butylcalix [4] arenes bearing two schiff-base units at the lower rim. Synthesis 1997, 1997, 1073–1076. [Google Scholar] [CrossRef]
- Kabsch, W. XDS. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 125–132. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallog. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallog. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallog. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Skovgaard, S.; Larsen, M.H.; Nielsen, L.N.; Skov, R.L.; Wong, C.; Westh, H.; Ingmer, H. Recently introduced qacA/B genes in Staphylococcus epidermidis do not increase chlorhexidine MIC/MBC. J. Antimicrob. Chemother. 2013, 68, 2226–2233. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Rahimi, M.; Shafiei-Irannejad, V.; Safa, K.D.; Salehi, R. Multi-branched ionic liquid-chitosan as a smart and biocompatible nano-vehicle for combination chemotherapy with stealth and targeted properties. Carbohydr. Polym. 2018, 196, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Shafiei-Irannejad, V.; Rahimi, M.; Zarei, M.; Dinparast-isaleh, R.; Bahrambeigi, S.; Alihemmati, A.; Shojaei, S.; Ghasemi, Z.; Yousefi, B. Polyelectrolyte Carboxymethyl Cellulose for Enhanced Delivery of Doxorubicin in MCF7 Breast Cancer Cells: Toxicological Evaluations in Mice Model. Pharm. Res. 2019, 36, 68. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compound L are available from the authors. |
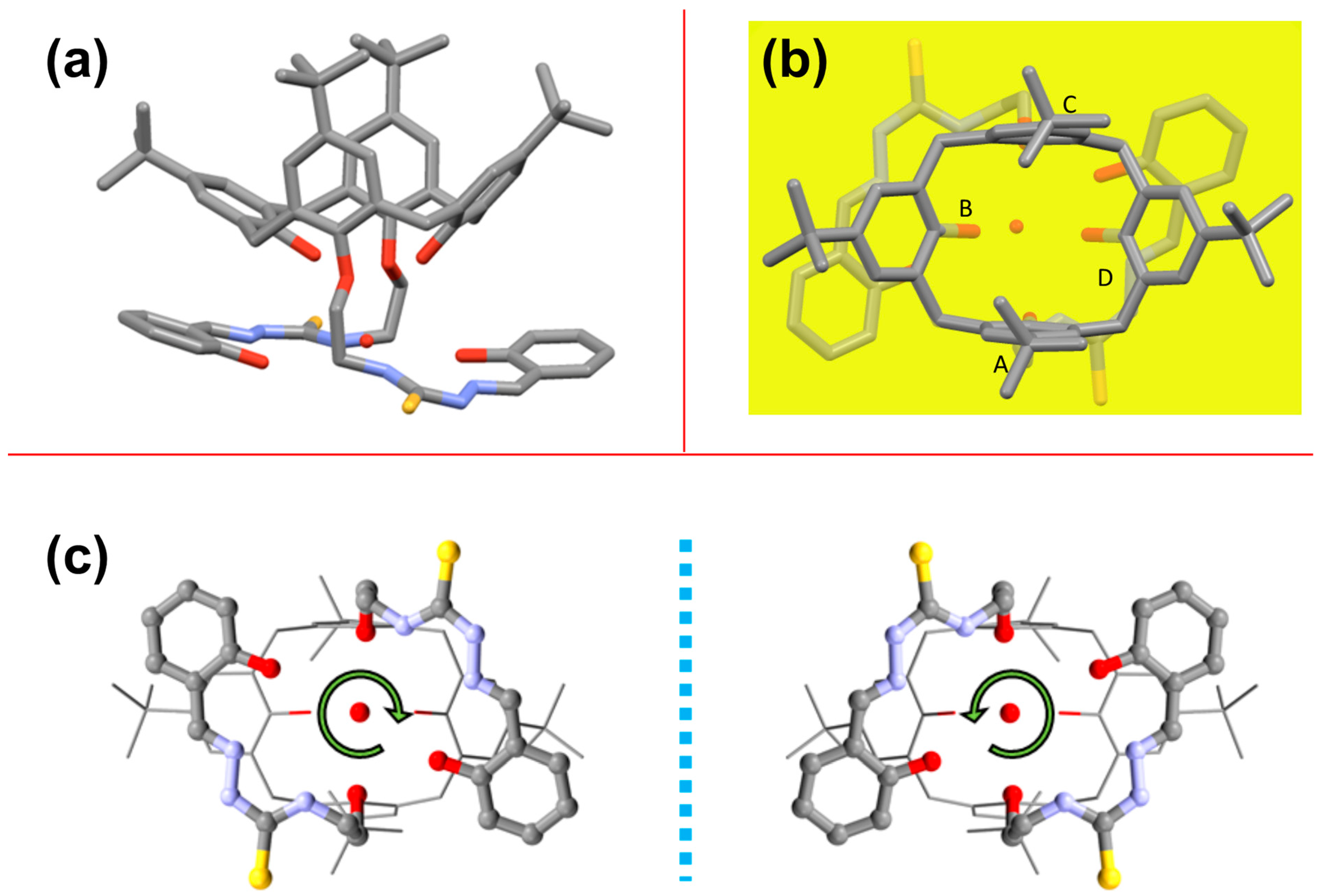

| D •••A a | d (D•••A) (Å) |
|---|---|
| Intramolecular H-Bonds on Calix[4]arene Lower Rim | |
| O(1B)•••O(1A) | 2.871 |
| O(1D)•••O(1C) | 2.922 |
| Intramolecular H-Bonds on Thiosemicarbazone Substituents | |
| O(2A)•••N(3A) | 2.710 |
| O(2C)•••N(3C) | 2.742 |
| Interactions between Water Molecule and Thiosemicarbazone Substituents b | |
| N(1A)•••O(1W) | 2.978 |
| N(1C)•••O(1W) | 3.185 |
| O(2A)•••O(1W) | 2.850 (phenol hydroxy) |
| O(2C)•••O(1W) | 3.051 (phenol hydroxy) |
| Interactions between Water Molecule and Calix[4]arene Lower Rim Oxygen Atoms b | |
| O(1D)•••O(1W) | 2.872 (hydroxy) |
| O(1B)•••O(1W) | 2.986 (hydroxy) |
| O(1C)•••O(1W) | 3.094 (alkoxy) |
| O(1A)•••O(1W) | 3.258 (alkoxy) |
| Microorganism | S. aureus | B. subtilis | E. coli | P. aeruginosa | C. albicans | C. glabrata |
|---|---|---|---|---|---|---|
| Ligand L | - | 31.25 | 31.25/31.25 | 31.25/- | 31.25/125 | - |
| Complex Co | - | 31.25/1000 | 31.25/31.25 | 31.25/250 | 62.5/- | - |
| Complex Ni | - | 31.25 | 31.25/31.25 | 31.25/2000 | - | - |
| Complex Cu | 31.25 | 31.25 | 31.25/31.25 | 31.25/500 | - | - |
| Complex Zn | - | 31.25 | 31.25/31.25 | 31.25/31.25 | - | - |
| Gentamicin * | 0.12/0.25 | 2/2 | 0.5/0.5 | 2/2 | - | - |
| Nystatin * | - | - | - | - | 1.25 | 0.625 |
| Compound | Saos-2 | MG-63 |
|---|---|---|
| Ligand L | <25 | >200 |
| Complex Co | 80 | 195 |
| Complex Ni | 62 | 173 |
| Complex Cu | 43 | 140 |
| Complex Zn | >200 | >200 |
| MTX | 7.9 | 14.5 |
| DOX | 2.1 | 10.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahojb Noruzi, E.; Shaabani, B.; Geremia, S.; Hickey, N.; Nitti, P.; Kafil, H.S. Synthesis, Crystal Structure, and Biological Activity of a Multidentate Calix[4]arene Ligand Doubly Functionalized by 2-Hydroxybenzeledene-Thiosemicarbazone. Molecules 2020, 25, 370. https://doi.org/10.3390/molecules25020370
Bahojb Noruzi E, Shaabani B, Geremia S, Hickey N, Nitti P, Kafil HS. Synthesis, Crystal Structure, and Biological Activity of a Multidentate Calix[4]arene Ligand Doubly Functionalized by 2-Hydroxybenzeledene-Thiosemicarbazone. Molecules. 2020; 25(2):370. https://doi.org/10.3390/molecules25020370
Chicago/Turabian StyleBahojb Noruzi, Ehsan, Behrouz Shaabani, Silvano Geremia, Neal Hickey, Patrizia Nitti, and Hossein Samadi Kafil. 2020. "Synthesis, Crystal Structure, and Biological Activity of a Multidentate Calix[4]arene Ligand Doubly Functionalized by 2-Hydroxybenzeledene-Thiosemicarbazone" Molecules 25, no. 2: 370. https://doi.org/10.3390/molecules25020370
APA StyleBahojb Noruzi, E., Shaabani, B., Geremia, S., Hickey, N., Nitti, P., & Kafil, H. S. (2020). Synthesis, Crystal Structure, and Biological Activity of a Multidentate Calix[4]arene Ligand Doubly Functionalized by 2-Hydroxybenzeledene-Thiosemicarbazone. Molecules, 25(2), 370. https://doi.org/10.3390/molecules25020370