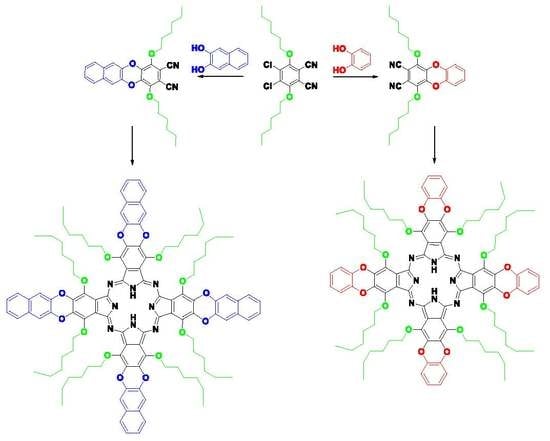
α- and β-Substituted Metal-Free Phthalocyanines: Synthesis, Photophysical and Electrochemical Properties
Abstract
1. Introduction
2. Results and Discussion
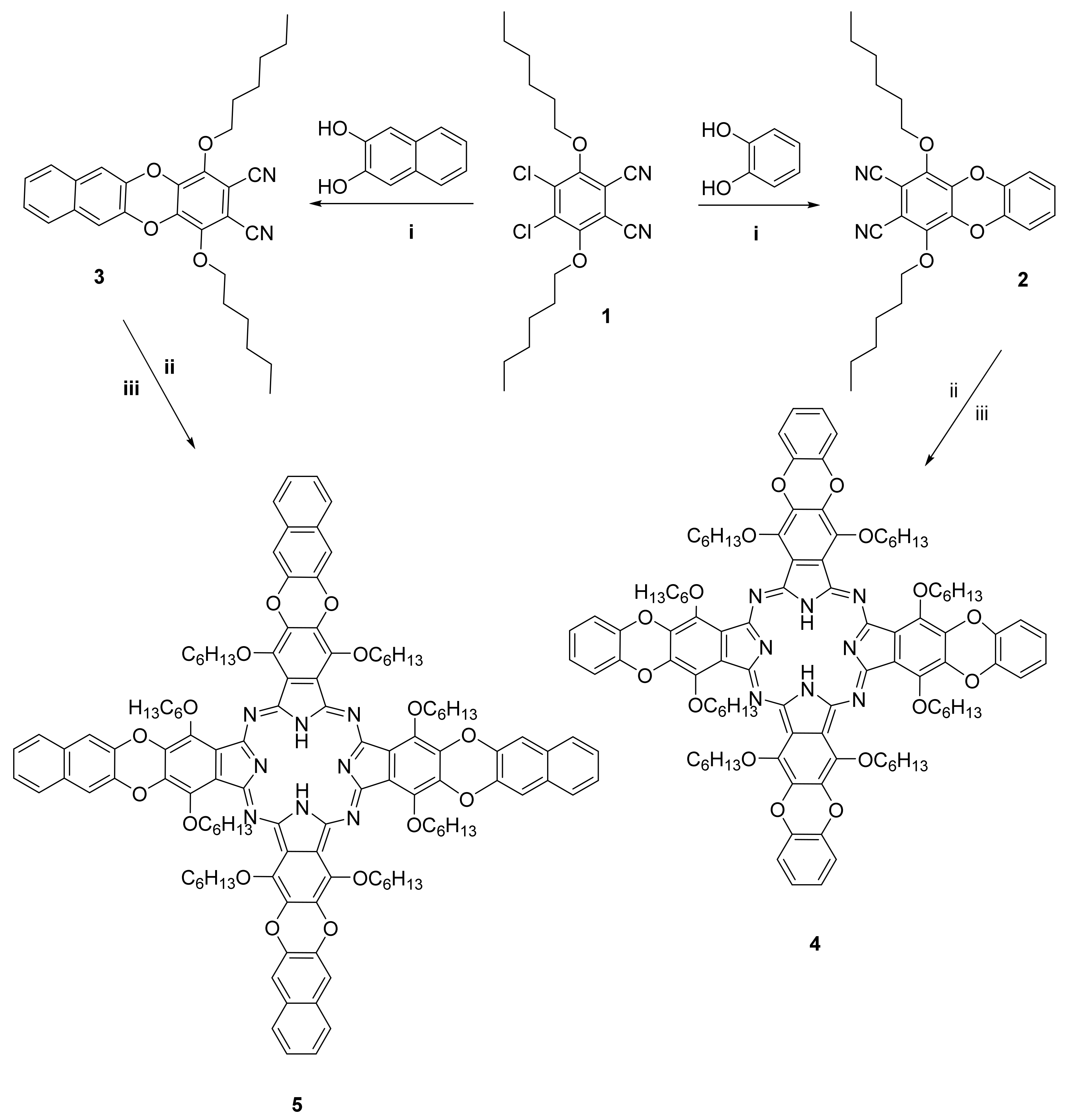
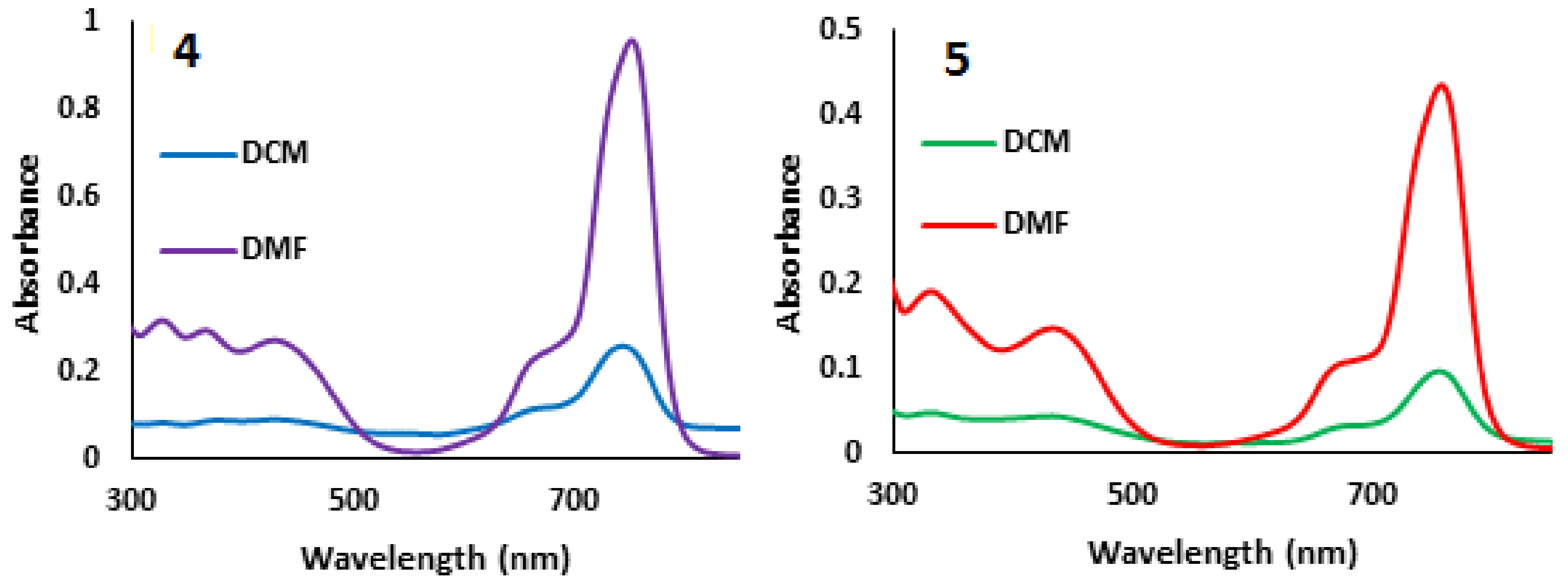
2.1. Synthesis and Characterization
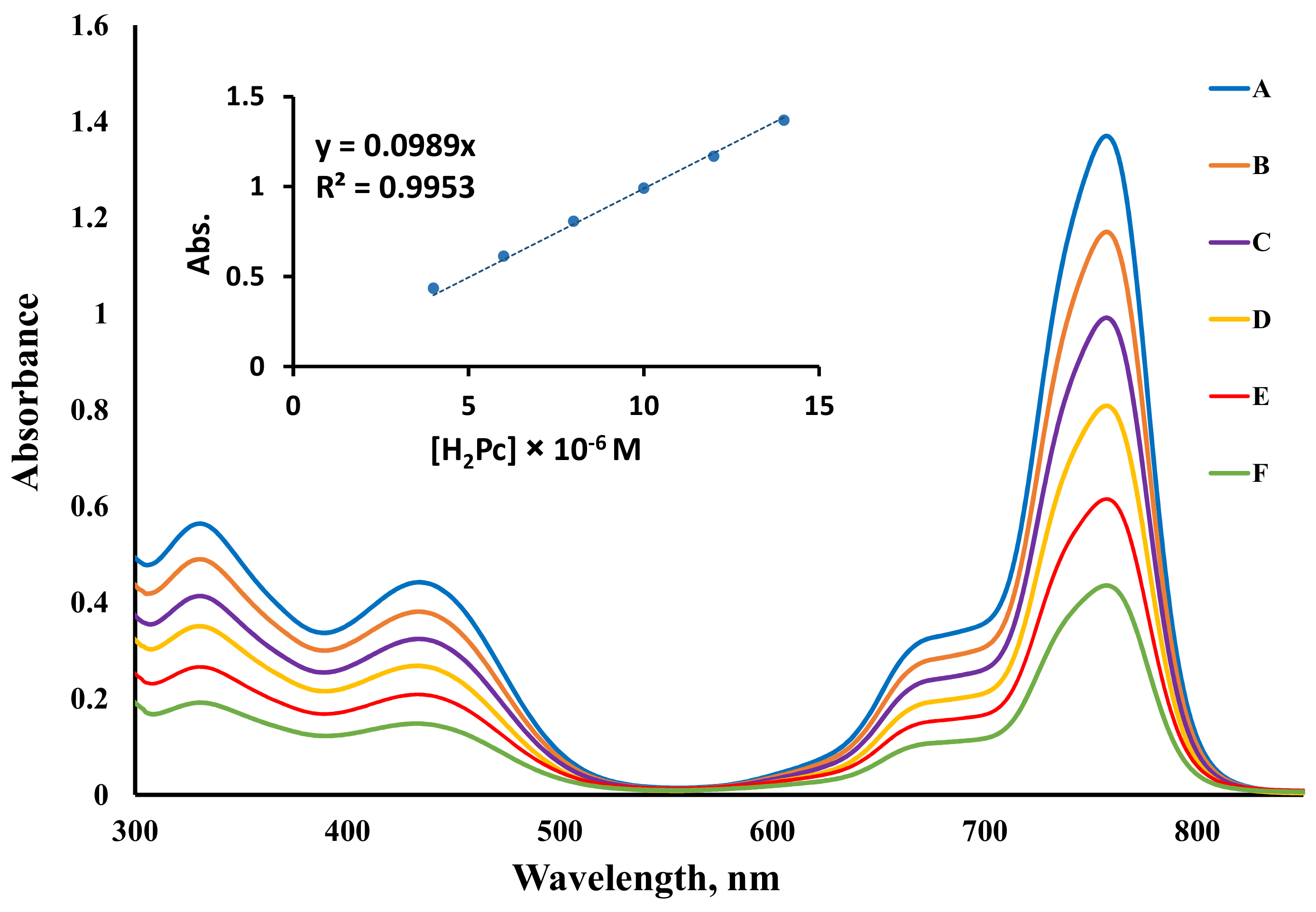
2.2. Fluorescence Studies
2.3. Benzoquinone (BQ)-Based Fluorescent Quenching
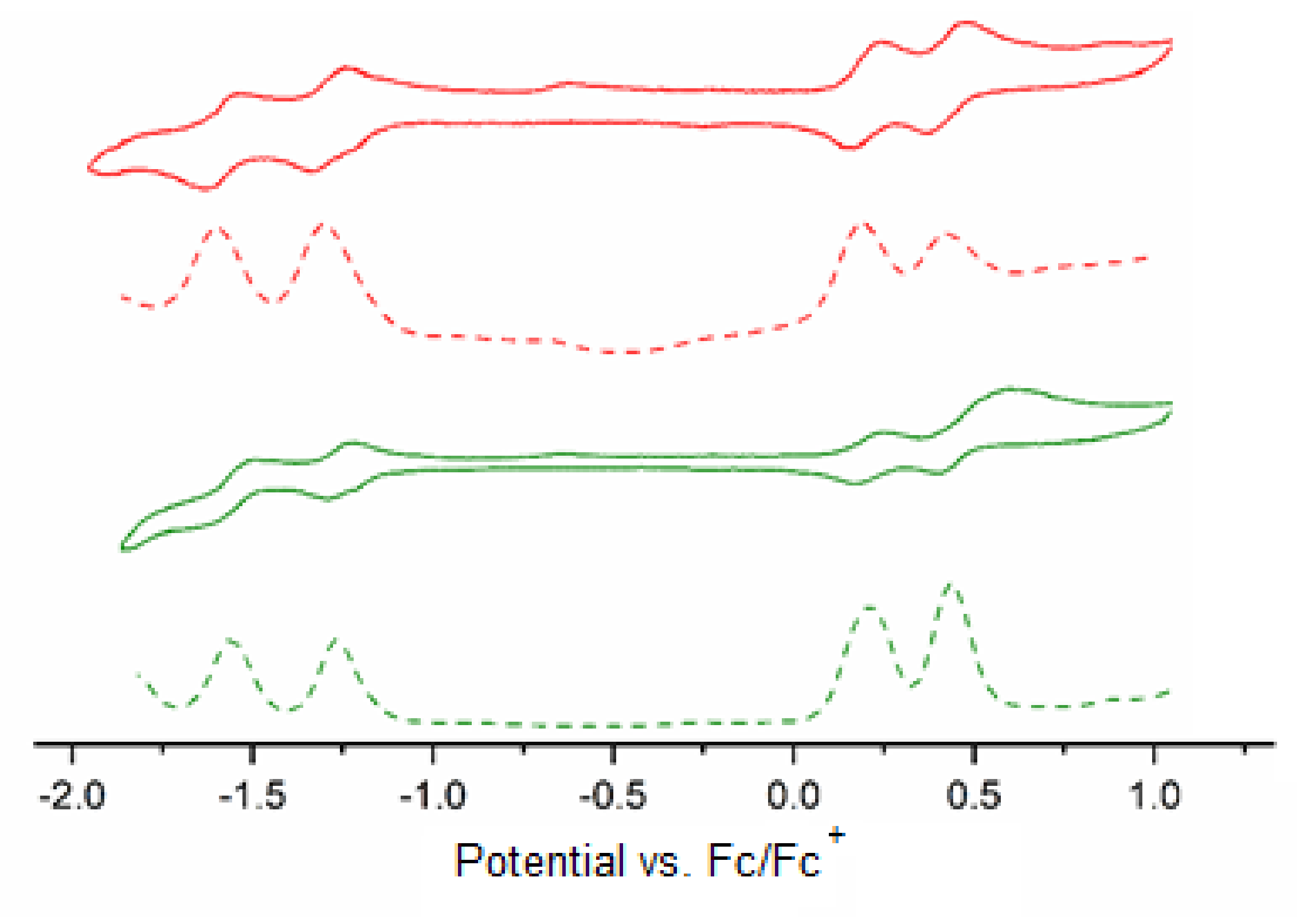
2.4. Electrochemical Measurements
3. Materials and Methods
3.1. Materials and Equipment
3.2. Chemistry
3.2.1. 1,4-Bis(hexyloxy)dibenzodioxin-2,3-dicarbonitrile (2)
3.2.2. 1,4-Bis(hexyloxy)benzo[b]naphtho[2–e]dioxin-2,3-dicarbonitrile (3)
3.2.3. 1,4,8,11,15,18,22,25-Octakis(Hexyloxy)-tetra(benzodioxin) Phthalocyanine (4)
3.2.4. 1,4,8,11,15,18,22,25-Octakis(Hexyloxy)-tetra(naphthodioxin) Phthalocyanine (5)
3.3. Photophysics
3.3.1. Fluorescent Quantum Yields and Lifetimes
3.3.2. Fluorescent Quenching Based on Benzoquinone (BQ)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Leznoff, C.C.L. Phthalocyanines, Properties and Applications; VCH Publishers Inc.: New York, NY, USA, 1989. [Google Scholar]
- Kadish, K.; Smith, K.M.; Guilard, R. The Porphyrin Handbook, Phthalocyanines: Spectroscopic and Electrochemical Characterization; Academic Press: San Diego, CA, USA, 2003. [Google Scholar]
- Bonnett, R. Chemical Aspects of Photodynamic Therapy; Gordon and Breach Science Publishers: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Klyamer, D.; Sukhikh, A.; Gromilov, S.; Krasnov, P.; Basova, T. Fluorinated Metal Phthalocyanines: Interplay between Fluorination Degree, Films Orientation, and Ammonia Sensing Properties. Sensors 2018, 18, 2141. [Google Scholar] [CrossRef]
- Boileau, N.T.; Cranston, R.; Mirka, B.; Melville, O.A.; Lessard, B.H. Metal phthalocyanine organic thin-film transistors: Changes in electrical performance and stability in response to temperature and environment. RSC Adv. 2019, 9, 21478–21485. [Google Scholar] [CrossRef]
- Dualeh, A.; Moehl, T.; Nazeeruddin, M.K.; Grätzel, M. Temperature Dependence of Transport Properties of Spiro-MeOTAD as a Hole Transport Material in Solid-State Dye-Sensitized Solar Cells. ACS Nano 2013, 7, 2292–2301. [Google Scholar] [CrossRef] [PubMed]
- Nar, I.; Atsay, A.; Pekbelgin Karaoglu, H.; Altindal, A.; Hamuryudan, E. pi-Extended hexadeca-substituted cobalt phthalocyanine as an active layer for organic field-effect transistors. Dalton Trans 2018, 47, 15017–15023. [Google Scholar] [CrossRef] [PubMed]
- Nar, I.; Atsay, A.; Altındal, A.; Hamuryudan, E.; Koçak, M.B.; Gül, A. Ferrocenyl Phthalocyanine as Donor in Non-Poly(3-hexylthiophen-2,5-diyl) Bulk Heterojunction Solar Cell. Chem. A Eur. J. 2018, 24, 6946–6949. [Google Scholar] [CrossRef]
- Li, X.; Peng, X.-H.; Zheng, B.-D.; Tang, J.; Zhao, Y.; Zheng, B.-Y.; Ke, M.-R.; Huang, J.-D. New application of phthalocyanine molecules: From photodynamic therapy to photothermal therapy by means of structural regulation rather than formation of aggregates. Chem. Sci. 2018, 9, 2098–2104. [Google Scholar] [CrossRef]
- Nar, I.; Atsay, A.; Gumrukcu, S.; Karazehir, T.; Hamuryudan, E. Low-Symmetry Phthalocyanine Cobalt Bis(dicarbollide) Conjugate for Hydrogen Reduction. Eur. J. Inorg. Chem. 2018, 34, 3878–3882. [Google Scholar] [CrossRef]
- Kabwe, K.P.; Louzada, M.; Britton, J.; Olomola, T.O.; Nyokong, T.; Khene, S. Nonlinear optical properties of metal free and nickel binuclear phthalocyanines. Dye Pigment. 2019, 168, 347–356. [Google Scholar] [CrossRef]
- Bouvet, M.; Gaudillat, P.; Suisse, J.-M. Phthalocyanine-based hybrid materials for chemosensing. J. Porphyr. Phthalocyanines 2013, 17, 913–919. [Google Scholar] [CrossRef]
- Kurt, Ö.; Koca, A.; Gül, A.; Koçak, M.B. Synthesis, electrochemistry and in situ spectroelectrochemistry of novel hexadeca-substituted phthalocyanines with three different groups. Synth. Met. 2015, 206, 72–83. [Google Scholar] [CrossRef]
- Atsay, A.; Gül, A.; Koçak, M.B. A new hexadeca substituted non-aggregating zinc phthalocyanine. Dye. Pigment. 2014, 100, 177–183. [Google Scholar] [CrossRef]
- Hassan, B.M.; Li, H.; McKeown, N.B. The control of molecular self-association in spin-coated films of substituted phthalocyanines. J. Mater. Chem. 2000, 10, 39–45. [Google Scholar] [CrossRef]
- McKeown, N.B.; Li, H.; Helliwell, M. A non-planar, hexadeca-substituted, metal-free phthalocyanine. J. Porphyr. Phthalocyanines 2005, 9, 841–845. [Google Scholar] [CrossRef]
- Machacek, M.; Cidlina, A.; Novakova, V.; Svec, J.; Rudolf, E.; Miletin, M.; Kučera, R.; Simunek, T.; Zimcik, P. Far-Red-Absorbing Cationic Phthalocyanine Photosensitizers: Synthesis and Evaluation of the Photodynamic Anticancer Activity and the Mode of Cell Death Induction. J. Med. Chem. 2015, 58, 1736–1749. [Google Scholar] [CrossRef]
- Awaji, A.I.; Köksoy, B.; Durmuş, M.; Aljuhani, A.; Alraqa, S.Y. Novel Hexadeca-Substituted Metal Free and Zinc(II) Phthalocyanines; Design, Synthesis and Photophysicochemical Properties. Molecules 2018, 24, 77. [Google Scholar] [CrossRef] [PubMed]
- Safonova, E.A.; Martynov, A.G.; Nefedov, S.E.; Kirakosyan, G.A.; Gorbunova, Y.G.; Tsivadze, A.Y. A Molecular Chameleon: Reversible pH- and Cation-Induced Control of the Optical Properties of Phthalocyanine-Based Complexes in the Visible and Near-Infrared Spectral Ranges. Inorg. Chem. 2016, 55, 2450–2459. [Google Scholar] [CrossRef] [PubMed]
- Muto, T.; Temma, T.; Kimura, M.; Hanabusa, K.; Shirai, H. Elongation of the π-System of Phthalocyanines by Introduction of Thienyl Substituents at the Peripheral β Positions. Synthesis and Characterization. J. Org. Chem. 2001, 66, 6109–6115. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Kudo, C.; Nakajo, S. Preparation and Electrochemical and Optical Properties of α -Alkoxyphthalocyanines with β -Pyridylthio Groups. Eur. J. Inorg. Chem. 2019, 2019, 4006–4013. [Google Scholar] [CrossRef]
- Lyubimtsev, A.; Iqbal, Z.; Crucius, G.; Syrbu, S.; Taraymovich, E.S.; Ziegler, T.; Hanack, M. Aggregation behavior and UV-vis spectra of tetra- and octaglycosylated zinc phthalocyanines. J. Porphyr. Phthalocyanines 2011, 15, 39–46. [Google Scholar] [CrossRef]
- Soldatova, A.V.; Kim, J.; Peng, X.; Rosa, A.; Ricciardi, G.; Kenney, M.E.; Rodgers, M.A. Effects of benzoannulation and alpha-octabutoxy substitution on the photophysical behavior of nickel phthalocyanines: A combined experimental and DFT/TDDFT study. Inorg. Chem. 2007, 46, 2080–2093. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, Y.; Li, R.; Bian, Y.; Li, X.; Jiang, J. Nonperipherally octa(butyloxy)-substituted phthalocyanine derivatives with good crystallinity: Effects of metal-ligand coordination on the molecular structure, internal structure, and dimensions of self-assembled nanostructures. Chem. Eur. J. 2009, 15, 13241–13252. [Google Scholar] [CrossRef] [PubMed]
- Honda, T.; Kojima, T.; Fukuzumi, S. Control of electron-transfer reduction by protonation of zinc octabutoxyphthalocyanine assisted by intramolecular hydrogen bonding. Chem. Commun. 2011, 47, 7986. [Google Scholar] [CrossRef] [PubMed]
- Kurt, Ö.; Özçeşmeci, I.; Koca, A.; Gül, A.; Koçak, M.B. Synthesis, photophysical and electrochemical properties of novel hexadeca-substituted phthalocyanines bearing naphthoxy groups. Dye Pigment. 2017, 137, 236–243. [Google Scholar] [CrossRef]
- Güzel, E.; Atsay, A.; Nalbantoglu, S.; Şaki, N.; Dogan, A.L.; Gul, A.; Koçak, M.B. Synthesis, characterization and photodynamic activity of a new amphiphilic zinc phthalocyanine. Dye Pigment. 2013, 97, 238–243. [Google Scholar] [CrossRef]
- Karaoğlu, H.P.; Atsay, A.; Nar, I.; McKee, V.; Koçak, M.B.; Hamuryudan, E.; Gül, A. Near-infrared absorbing π-extended hexadeca substituted phthalocyanines. J. Mol. Struct. 2019, 1197, 736–741. [Google Scholar] [CrossRef]
- Kobayashi, N.; Furuyama, T.; Satoh, K. Rationally Designed Phthalocyanines Having Their Main Absorption Band beyond 1000 nm. J. Am. Chem. Soc. 2011, 133, 19642–19645. [Google Scholar] [CrossRef]
- Furuyama, T.; Satoh, K.; Kushiya, T.; Kobayashi, N. Design, Synthesis, and Properties of Phthalocyanine Complexes with Main-Group Elements Showing Main Absorption and Fluorescence beyond 1000 nm. J. Am. Chem. Soc. 2014, 136, 765–776. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, F.; Li, Z.; Huang, L.; Tang, Y.; Zhang, F.; Tung, C.-H. A Novel Self-aggregates of Phthalocyanine Based on Zn–O Coordination. Chem. Lett. 2007, 36, 108–109. [Google Scholar] [CrossRef]
- Weitman, H.; Schatz, S.; Gottlieb, H.E.; Kobayashi, N.; Ehrenberg, B. Spectroscopic Probing of the Acid-Base Properties and Photosensitization of a Fluorinated Phthalocyanine in Organic Solutions and Liposomes. Photochem. Photobiol. 2007, 73, 473–481. [Google Scholar] [CrossRef]
- Manaa, H.; Al Mulla, A.; Makhseed, S.; Al-Sawah, M.; Samuel, J. Fluorescence and nonlinear optical properties of non-aggregating hexadeca-substituted phthalocyanine. Opt. Mater. 2009, 32, 108–114. [Google Scholar] [CrossRef]
- Cerqueira, A.F.R.; Almodôvar, V.A.S.; Neves, M.G.P.M.S.; Tomé, A.C. Coumarin–Tetrapyrrolic Macrocycle Conjugates: Synthesis and Applications. Molecules 2017, 22, 994. [Google Scholar] [CrossRef]
- Catalan, J.; Díaz, C.; López, V.; Pérez, P.; De Paz, J.-L.G.; Rodríguez, J.G. A Generalized Solvent Basicity Scale: The Solvatochromism of 5-Nitroindoline and Its Homomorph 1-Methyl-5-nitroindoline. Eur. J. Org. Chem. 1996, 1996, 1785–1794. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, C.; Zhang, Z.; Li, Z.; Niu, L.; Bin, Y.; Zhang, F. Photoresponsive J-Aggregation Behavior of a Novel Azobenzene−Phthalocyanine Dyad and Its Third-Order Optical Nonlinearity. J. Phys. Chem. B 2008, 112, 7387–7394. [Google Scholar] [CrossRef]
- Islam, M.R.; Sundararajan, P.R. Tubular or Subsurface Morphology of Octabutoxyphthalocyanine upon Self-Assembly in Polymer Matrices: Effect of the Casting Solvent. Chem. A Eur. J. 2011, 17, 6098–6108. [Google Scholar] [CrossRef] [PubMed]
- Donzello, M.P.; Ercolani, C.; Gaberkorn, A.A.; Kudrik, E.V.; Meneghetti, M.; Marcolongo, G.; Rizzoli, C.; Stuzhin, P.A. Synthesis, X-ray Crystal Structure, UV/Visible Linear and Nonlinear (Optical Limiting) Spectral Properties of Symmetrical and Unsymmetrical Porphyrazines with Annulated 1,2,5-Thiadiazole and 1,4-Diamyloxybenzene Moieties. Chem. A Eur. J. 2003, 9, 4009–4024. [Google Scholar] [CrossRef] [PubMed]
- Ogunsipe, A.; Nyokong, T. Effects of substituents and solvents on the photochemical properties of zinc phthalocyanine complexes and their protonated derivatives. J. Mol. Struct. 2004, 689, 89–97. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Wang, C.; Zhu, C.-H.; Zhang, Z.-H.; Xue, J.-P. Preparation and In Vitro Photodynamic Activity of Glucosylated Zinc(II) Phthalocyanines as Underlying Targeting Photosensitizers. Molecules 2017, 22, 845. [Google Scholar] [CrossRef]
- Karaoğlu, H.R.P.; Yenilmez, H.Y.; Koçak, M.B. Phthalocyanines formed from several precursors: Synthesis, characterization, and comparative fluorescence and quinone quenching. J. Co-ord. Chem. 2018, 71, 2340–2357. [Google Scholar] [CrossRef]
- Du, H.; Fuh, R.-C.A.; Li, J.; Corkan, L.A.; Lindsey, J.S. PhotochemCAD: A Computer-Aided Design and Research Tool in Photochemistry. Photochem. Photobiol. 1998, 68, 141–142. [Google Scholar]
- Zorlu, Y.; Dumoulin, F.; Durmuş, M.; Ahsen, V. Comparative studies of photophysical and photochemical properties of solketal substituted platinum(II) and zinc(II) phthalocyanine sets. Tetrahedron 2010, 66, 3248–3258. [Google Scholar] [CrossRef]
- Li, R.; Zhang, X.; Zhu, P.; Ng, D.K.P.; Kobayashi, N.; Jiang, J. Electron-Donating or -Withdrawing Nature of Substituents Revealed by the Electrochemistry of Metal-Free Phthalocyanines. Inorg. Chem. 2006, 45, 2327–2334. [Google Scholar] [CrossRef] [PubMed]
- L’Her, M.; Pondaven, A. 104-Electrochemistry of Phthalocyanines. In The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: Amsterdam, The Netherlands, 2003; pp. 117–169. [Google Scholar]
- Djurovich, P.I.; Mayo, E.I.; Forrest, S.R.; Thompson, M.E. Measurement of the lowest unoccupied molecular orbital energies of molecular organic semiconductors. Org. Electron. 2009, 10, 515–520. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, X.; Bian, Y.; Jiang, J. Organic Semiconductors of Phthalocyanine Compounds for Field Effect Transistors (FETs). In Functional Phthalocyanine Molecular Materials; Jiang, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 275–321. [Google Scholar]
Sample Availability: Samples of the compounds 2–5 are available from the authors. |
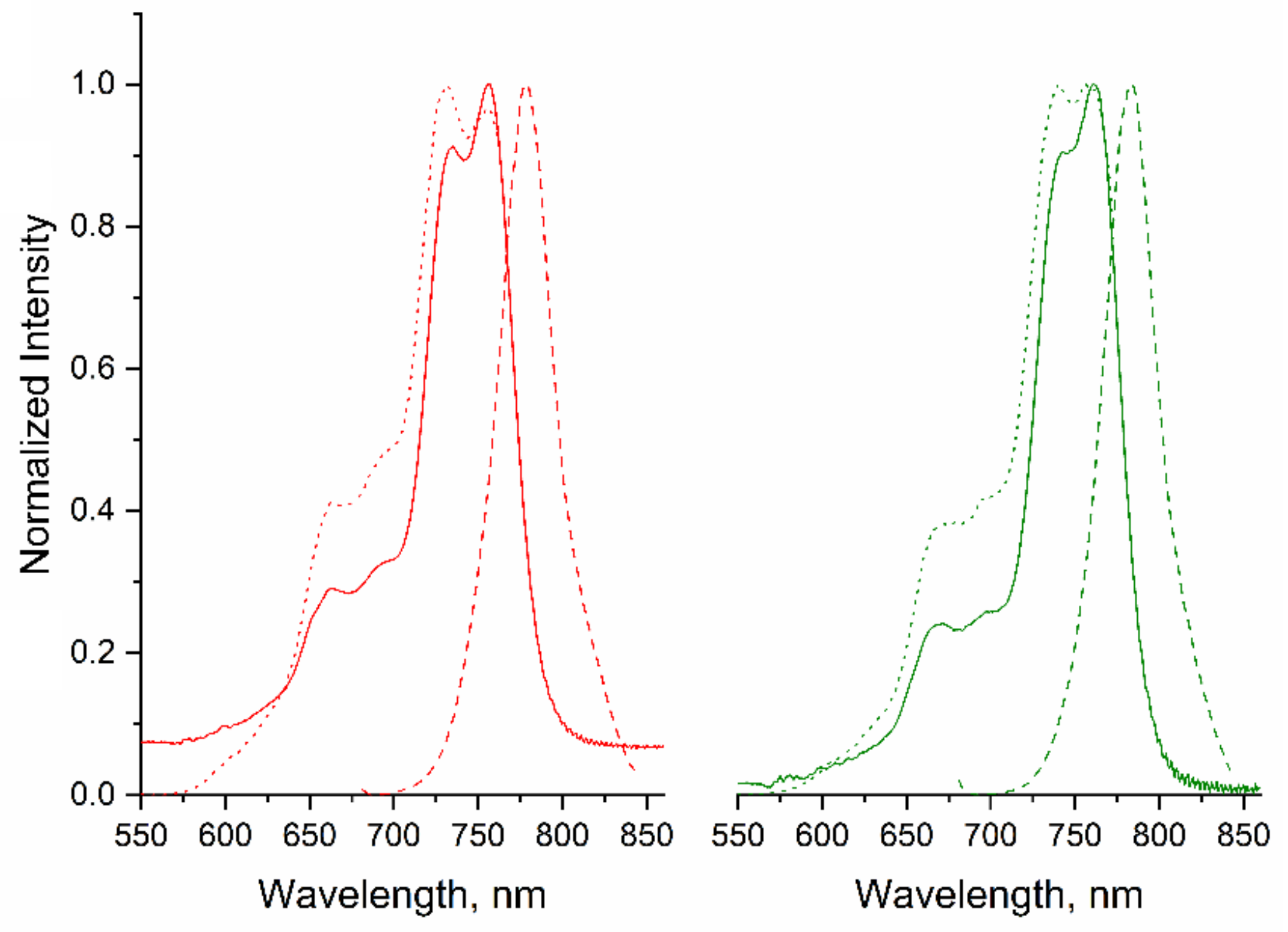
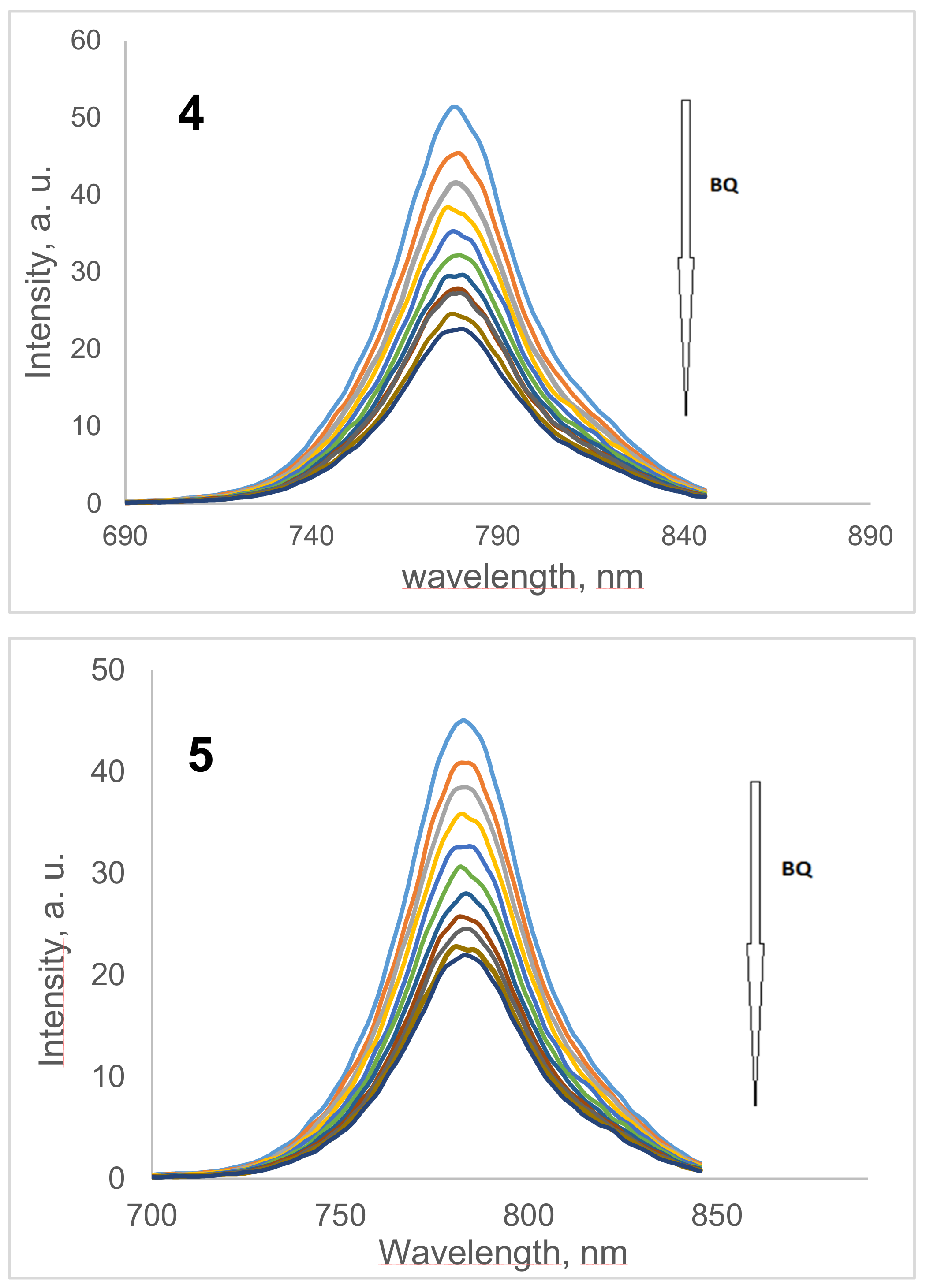
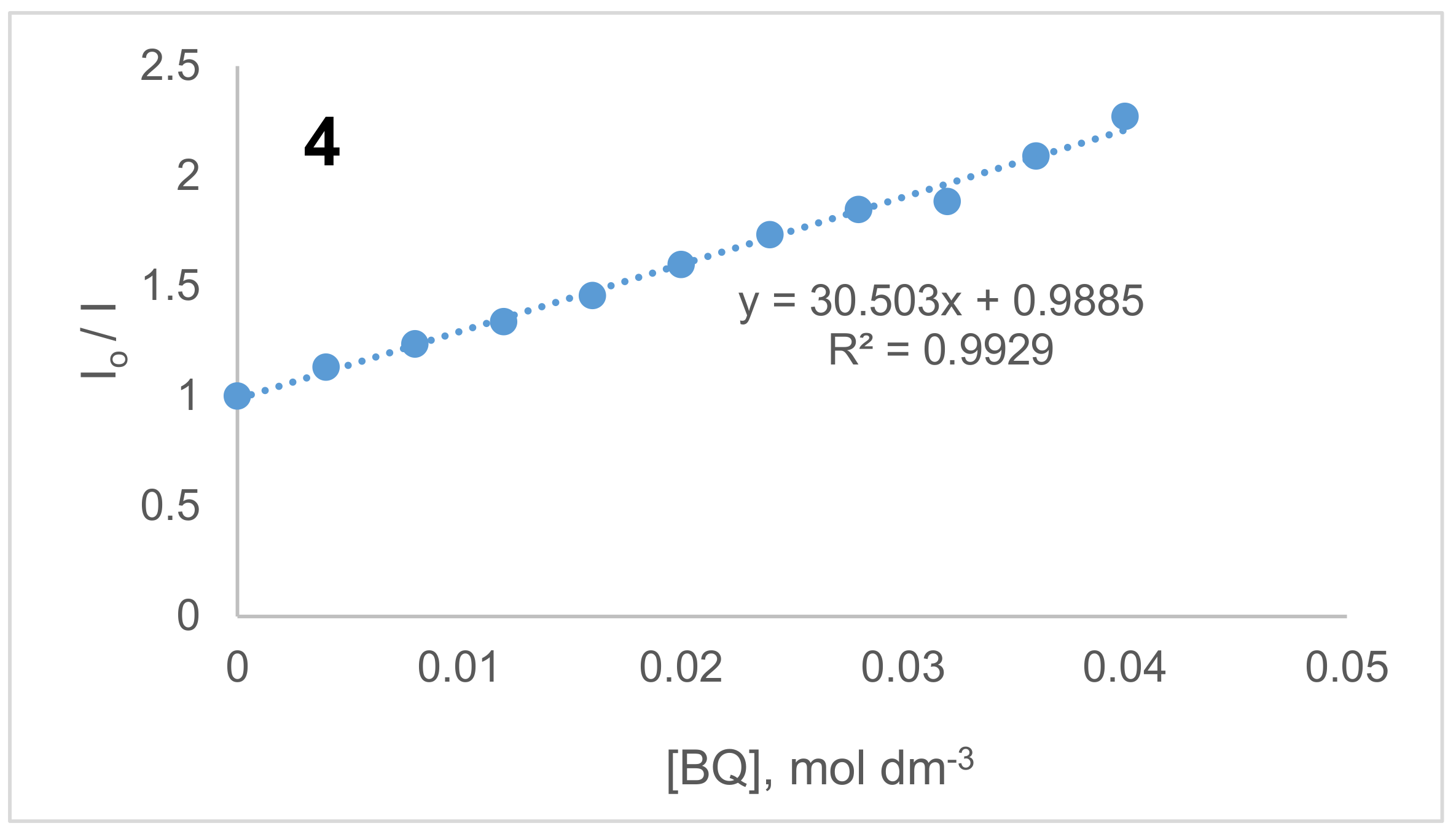
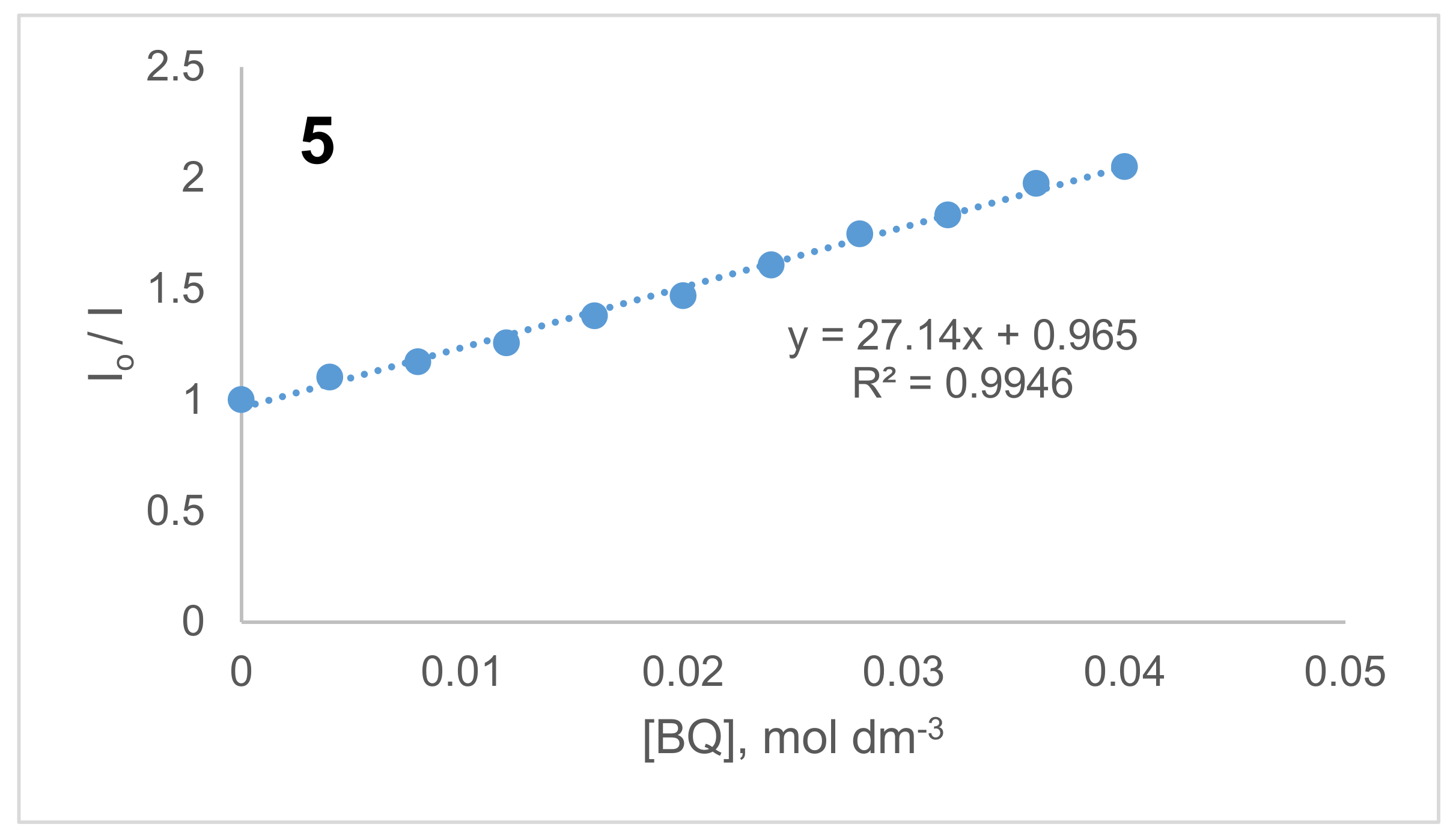
| Compound | Excitation λEx (nm) | Emission λEm (nm) | Stokes’ Shift ΔStokes (nm) | ΦF | τF (ns) | τo (ns) | kF (s1) (×108) a | Ksv c | kq (×1010 s−1) c |
|---|---|---|---|---|---|---|---|---|---|
| 4 | 731,755 | 779 | 23 | 0.29 | 2.09 | 7.12 | 1.40 | 30.50 | 1.46 |
| 5 | 740,756 | 783 | 22 | 0.44 | 4.25 | 9.59 | 1.04 | 27.14 | 0.64 |
| ZnPcb | 670 | 676 | 6 | 0.17 | 1.03 | 6.05 | 16.53 | 57.60 | 5.59 |
| Compound | E2 red (V) | E1 red (V) | E1 Ox (V) | E2 Ox (V) | HOMO a (eV) | LUMO c (eV) | Eg b (eV) | Eg d (eV) |
|---|---|---|---|---|---|---|---|---|
| 4 | −1.58 | −1.3 | 0.20 | 0.44 | −5.0 | −3.42 | 1.58 | 1.50 |
| 5 | −1.54 | −1.25 | 0.26 | 0.48 | −5.06 | −3.50 | 1.56 | 1.51 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pekbelgin Karaoğlu, H.; Kalkan Burat, A. α- and β-Substituted Metal-Free Phthalocyanines: Synthesis, Photophysical and Electrochemical Properties. Molecules 2020, 25, 363. https://doi.org/10.3390/molecules25020363
Pekbelgin Karaoğlu H, Kalkan Burat A. α- and β-Substituted Metal-Free Phthalocyanines: Synthesis, Photophysical and Electrochemical Properties. Molecules. 2020; 25(2):363. https://doi.org/10.3390/molecules25020363
Chicago/Turabian StylePekbelgin Karaoğlu, Hande, and Ayfer Kalkan Burat. 2020. "α- and β-Substituted Metal-Free Phthalocyanines: Synthesis, Photophysical and Electrochemical Properties" Molecules 25, no. 2: 363. https://doi.org/10.3390/molecules25020363
APA StylePekbelgin Karaoğlu, H., & Kalkan Burat, A. (2020). α- and β-Substituted Metal-Free Phthalocyanines: Synthesis, Photophysical and Electrochemical Properties. Molecules, 25(2), 363. https://doi.org/10.3390/molecules25020363