3. Materials and Methods
3.1. General Procedures
Optical rotations were measured on a RUDOLPH Research Analytical Autopol III Automatic Polarimeter (RUDOLPH Research Analytical, Hackettstown, NJ, USA). IR spectra were recorded on a Nicolet Nexus 470 FTIR spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). High-Resolution MS were obtained on an Orbitrap mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) with electrospray ionization (ESI). NMR spectra (see
Supplementary Materials) were obtained on a Bruker Fourier 300 spectrometer (Billerica, MA, USA) in CDCl
3. The chemical shifts are given in ppm referenced to the respective solvent peak, and coupling constants are reported in Hz. Anhydrous THF and dichloromethane were purified by PureSolv MD 7 Solvent Purification System from Innovative Technologies (MB-SPS-800) (Herndon, VA, USA). All other reagents and solvents were purchased from commercial sources (Fisher Scientific, Portland, OR, USA) and were used without further purification. Silica gel column chromatography was performed using silica gel (32–63 µM). Preparative thin-layer chromatography (PTLC) separations were carried out on thin-layer chromatography plates loaded with silica gel 60 GF254 (EMD Millipore Corporation, Berlington, MA, USA). Starting materials
22 and
9 were synthesized using the procedure previously described by us [
23]. (
E)-ethyl 3-(2-fluorophenyl)acrylate (
12) was prepared from commercially available 2-fluorobenzaldehyde (
10, CAS 446-52-6) according to the reported procedure [
26]. (
E)-3-(2-Fluorophenyl)prop-2-en-1-ol (
13) was synthesized by reducing (
E)-ethyl 3-(2-fluorophenyl)acrylate (
12) with DIBAL based on the procedure reported in the literature [
27]. Its structure was confirmed by
1H NMR and high resolution mass spectrometry (HRMS) data (calculated for C
9H
10FO (M + H): 153.0716; Found: 153.0710).
3.2. Synthesis of (1R,2R)-1-(2-Fluorophenyl)propane-1,2,3-triol [(17R,18R) triol 14]
AD-mix-α (5.9 g) was dissolved in a mixture of tert-butyl alcohol (21 mL) and water (21 mL). The solution was stirred vigorously at room temperature until two clear phases were observed. At this point, the lower aqueous phase emerged as bright yellow. Methanesulfonamide (399 mg, 4.2 mmol, 1 equiv) was added and the reaction mixture was cooled down to 0 °C. A solution of 13 (640 mg, 4.2 mmol, 1 equiv) in 1 mL of tert-butyl alcohol was then added. The resulting mixture was stirred vigorously at 0 °C overnight and the reaction progress was monitored with TLC. The reaction was quenched by adding solid sodium sulfate (6.3 g) at 0 °C. The mixture was warmed to room temperature and stirred for 30–60 min. The tert-butyl alcohol was removed under vacuum, and saturated sodium bicarbonate (30 mL) and water (300 mL) were added to the residue. The mixture was stirred for additional 30 min and then extracted with ethyl acetate (150 mL × 3). The combined extracts were rinsed with brine, dried over anhydrous sodium sulfate, and concentrated. The crude product was purified through column chromatography eluting with ethyl acetate. The product after purification, with quantitative yield, still contained a trace amount of methanesulfonamide, which is good enough to be used for the next-step reaction. 1H NMR (300 MHz, CDCl3) δ. 7.38 (t, J = 7.2 Hz, 1H, aromatic H), 7.21 (dd, J = 13.5, 5.7 Hz, 1H, aromatic H), 7.06 (t, J = 7.5 Hz, 1H, aromatic H), 6.95 (t, J = 8.4 Hz, 1H, aromatic H), 4.88 (d, J = 6.3 Hz, 1H, H-18), 4.23 (br.s, 3H, 3 × OH), 3.77 (q, J = 5.1 Hz, 1H, H-17), 3.45 (d, J = 4.8 Hz, 2H, H2-16). 13C NMR (75 MHz, CDCl3) δ 159.9 (d, JCF = 243.8 Hz), 129.6, 128.5, 127.9 (d, JCF = 12.8 Hz), 124.6, 115.4 (d, JCF = 21.8 Hz), 75.3, 68.8, 63.5. IR (film) νmax: 3384, 2988, 1617, 1490, 1455, 1320, 1222, 1054 cm−1. HRMS (ESI): m/z calculated for C9H12FO3 [M + H]+: 187.0770. Found: 187.0766.
3.3. Synthesis of (2R,3R)-3-(2-Fluorophenyl)-2,3-dihydroxypropyl methanesulfonate [(17R,18R) mesylate 16]
To a solution of triol 14 (1.37 g, 7.39 mmol) in pyridine (15 mL, 0.5 M), methane sulfonyl chloride (0.57 mL, 7.39 mmol) was added dropwise at 0 °C under argon. The reaction mixture was stirred at room temperature for 20 h and the reaction progress was monitored by TLC (Hexane/EtOAc, 1:1, v/v). The reaction was quenched by diluting with ethyl acetate (800 mL), and the resulting solution was rinsed with brine (50 mL × 3) and dried over anhydrous sodium sulfate. After removing the organic solvent, the residue was subjected to column chromatography, using 40% ethyl acetate in hexane as eluent, to give the desired product. Colorless oil; 81% yield. 1H NMR (300 MHz, CDCl3) δ 7.47 (dt, J = 7.5, 1.8 Hz, 1H, aromatic H), 7.34–7.27 (m, 1H, aromatic H), 7.17 (dt, J = 7.5, 0.9 Hz, 1H, aromatic H), 7.04 (ddd, J = 10.5, 8.1, 0.9 Hz, 1H, aromatic H), 5.00 (d, J = 5.7 Hz, 1H, H-18), 4.27–4.16 (m, 2H, H2-16), 4.06–4.01 (m, 1H, H-17), 3.18 (br.s, 2H, 2 × OH), 3.03 (s, 3H, SO2CH3). 13C NMR (75 MHz, CDCl3) δ 159.9 (d, JCF = 244.3 Hz), 130.1 (d, JCF = 8.3 Hz), 128.4 (d, JCF = 3.8 Hz), 127.0 (d, JCF = 12.8 Hz), 124.8 (d, JCF = 3.8 Hz), 115.7 (d, JCF = 21.8 Hz), 72.9, 70.4, 67.9 (d, JCF = 2.3 Hz), 37.6. IR (film) νmax: 3502, 3029, 2940, 1617, 1587, 1490, 1456, 1346, 1171 cm−1. HRMS (ESI): m/z calculated for C10H14FO5S [M + H]+: 265.0546. Found: 265.0547.
3.4. Synthesis of (R)-(2-Fluorophenyl)((R)-oxiran-2-yl)methanol [(17R,18R) epoxide 18]
To a suspension of sodium hydride (275 mg, 60%, 6.87 mmol, 1.5 equiv) in anhydrous THF (36 mL), a solution of 16 (1.21 g, 4.58 mmol, 1 equiv) in anhydrous THF was added at about −30 °C under argon. The reaction mixture was stirred at 0 °C overnight and the reaction progress was monitored with TLC (hexane:EtOAc, 3:1). The mixture was filtered through a silica gel pad eluting with ethyl acetate. After concentration to remove the solvent, the residue was subjected to PTLC purification eluting with toluene:EtOAc (3:1, v/v) to furnish the desired epoxide. Colorless oil; 57% yield. 1H NMR (300 MHz, CDCl3) δ 7.57 (dd, J = 7.8, 1.8 Hz, 1H, aromatic H), 7.35–7.28 (m, 1H, aromatic H), 7.20 (dt, J = 7.5, 1.2 Hz, 1H, aromatic H), 7.06 (ddd, J = 10.5, 8.1, 1.2 Hz, 1H, aromatic H), 4.84 (t, J = 6.6 Hz, 1H, H-18), 3.26–3.22 (m, 1H, H-17), 2.92–2.85 (overlapped, 2H, H2-16), 2.47 (d, J = 5.7 Hz, 1H, OH). 13C NMR (75 MHz, CDCl3) δ 159.9 (d, JCF = 244.5 Hz), 129.6 (d, JCF = 8.3 Hz), 127.9 (d, JCF = 4.5 Hz), 127.3 (d, JCF = 13.5 Hz), 124.5 (d, JCF = 3.8 Hz), 115.4 (d, JCF = 21.8 Hz), 68.7 (d, JCF = 2.3 Hz), 55.6, 45.5 (d, J = 2.3 Hz). IR (film) νmax: 3420, 3067, 3002, 2928, 1617, 1586 1488, 1456, 1224 cm−1. HRMS (ESI): m/z calculated for C9H10FO2 [M + H]+: 169.0665. Found: 169.0657.
3.5. Synthesis of (R)-2-((R)-(2-Fluorophenyl)(methoxymethoxy)methyl)oxirane [(17R,18R)MOM ether 20]
To a solution of alcohol 18 (716 mg, 4.3 mmol) in DMF (4.2 mL), sodium hydride (204 mg, 60%, 5.1 mmol) was added at 4 °C, and the mixture was stirred at 4 °C for 30 min. Methoxymethyl chloride (0.39 mL, 5.1 mmol) followed by tetrabutylammonium iodide (157 mg, 0.43 mmol) was added to the reaction mixture. The reaction was proceeded with stirring at room temperature for 20 h. The reaction mixture was diluted with ethyl acetate (300 mL) and diethyl ether (300 mL) and then rinsed with brine (50 mL × 3). The organic layer was dried over anhydrous sodium sulfate and concentrated to give a crude mass, which was purified by column chromatography eluting with hexane/ethyl acetate (3:1, v/v) to furnish the MOM ether 20 as a pale-yellow oil in 67% yield. 1H NMR (300 MHz, CDCl3) δ 7.51 (dt, J = 7.2, 1.8 Hz, 1H, aromatic H), 7.33–7.25 (m, 1H, aromatic H), 7.17 (dt, J = 7.5, 1.2 Hz, 1H, aromatic H), 7.04 (ddd, J = 9.9, 8.1, 1.2 Hz, 1H, aromatic H), 4.75 (d, J = 6.9 Hz, 1H, OCH2OCH3), 4.67 (d, J = 6.9 Hz, 1H, OCH2OCH3), 4.62 (d, J = 6.6 Hz, 1H, H-18), 3.36 (s, 3H, OCH3), 3.26–3.22 (m, 1H, H-17), 2.73–2.72 (overlapped, 2H, H2-16). 13C NMR (75 MHz, CDCl3) δ 160.3 (d, J = 244.5 Hz), 129.8 (d, J = 8.3 Hz), 128.6 (d, J = 4.5 Hz), 125.2 (d, J = 14.3 Hz), 124.4 (d, J = 3.0 Hz), 115.5 (d, J = 21.8 Hz), 94.6, 73.0, 55.6, 54.4, 44.2 (d, J = 2.3 Hz). IR (film) νmax: 2891, 1615, 1587, 1488, 1455, 1022 cm−1. HRMS (ESI): m/z calculated for C11H14FO3 [M + H]+: 213.0927. Found: 213.0921.
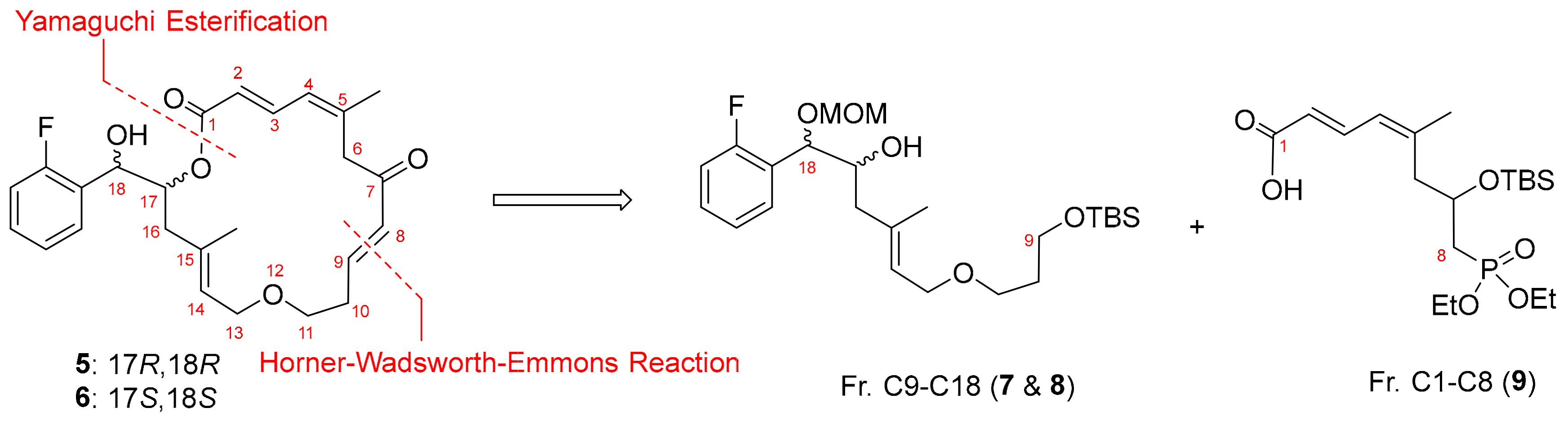
3.6. Synthesis of (5R,6R,E)-5-(2-Fluorophenyl)-8,16,16,17,17-pentamethyl-2,4,11,15-tetraoxa-16- silaoctadec-8-en-6-ol [(17R,18R) Fragment C9–C18 (7)]
To a solution of vinyliodide 22 (164 mg, 0.44 mmol; co-evaporated twice with pentane) in toluene (3 mL) at −78 °C, tert-butyllithium (0.46 mL, 1.9 M, 0.89 mmol) was added, and the mixture was stirred at −78 °C for 45 min prior to being cooled down to −90 °C. Epoxide 20 (188 mg, 0.89 mmol; co-evaporated twice with pentane) in toluene (1.5 mL) was added dropwise to make sure that interior temperature lower than −78 °C. The reaction solution was re-cooled to −90 °C before BF3•OEt2 (0.11 mL, 0.89 mmoL) was added dropwise. The resulting solution was then stirred at −78 °C overnight prior to being quenched with ethyl acetate (15 mL) and saturated aqueous sodium bicarbonate (35 mL) was added. The organic layer was separated and the aqueous layer was extracted with ethyl acetate (60 mL × 3). The combined organic layers were dried over anhydrous sodium sulfate and concentrated in Vacuum to remove ethyl acetate. The crude mass was subjected to PTLC purification over silica gel using hexane/ethyl acetate (4:1, v/v) as eluent to yield secondary alcohol 7 as a pale-yellow oil in 23% yield. 1H NMR (300 MHz, CDCl3) δ. 7.41 (dt, J = 7.2, 1.5 Hz, 1H, aromatic H), 7.32–7.24 (m, 1H, aromatic H), 7.15 (dt, J = 7.5, 0.9 Hz, 1H, aromatic H), 7.53 (ddd, J = 9.9, 8.1, 0.9 Hz, 1H, aromatic H), 5.41 (t, J = 6.6 Hz, 1H, H-14), 4.81 (d, J = 6.3 Hz, 1H, H-17), 4.62 (d, J = 6.9 Hz, 1H, OCH2OCH3), 4.58 (d, J = 6.6 Hz, 1H, OCH2OCH3), 3.94 (d, J = 6.6 Hz, 2H, H2-13), 3.67 (t, J = 6.3 Hz, 2H, H2-11), 3.46 (t, J = 6.3 Hz, 2H, H2-9), 3.36 (s, 3H), OCH3), 1.75 (quin, J = 6.3 Hz, 2H, H2-10), 1.64 (s, 3H, 15-CH3), 0.87 (s, 9H, TBS), 0.03 (s, 6H, TBS). 13C NMR (75 MHz, CDCl3) δ 160.9 (d, JCF = 244.8 Hz), 136.4, 129.8, 129.1, 128.7, 126.1 (d, JCF = 13.5 Hz), 124.5 (d, JCF = 3.8 Hz), 115.6 (d, JCF = 21.8 Hz), 95.3, 75.6, 72.5 (d, JCF = 12.1 Hz), 67.3, 67.0, 60.2, 56.2, 43.0, 33.2, 26.1, 26.07, 18.5, 16.6, 16.5. IR (film) νmax: 3420, 2929, 1715, 1647, 1615, 1488, 1029 cm−1. HRMS (ESI): m/z calculated for C24H42FO5Si [M + H]+: 457.2786. Found: 457.2780.
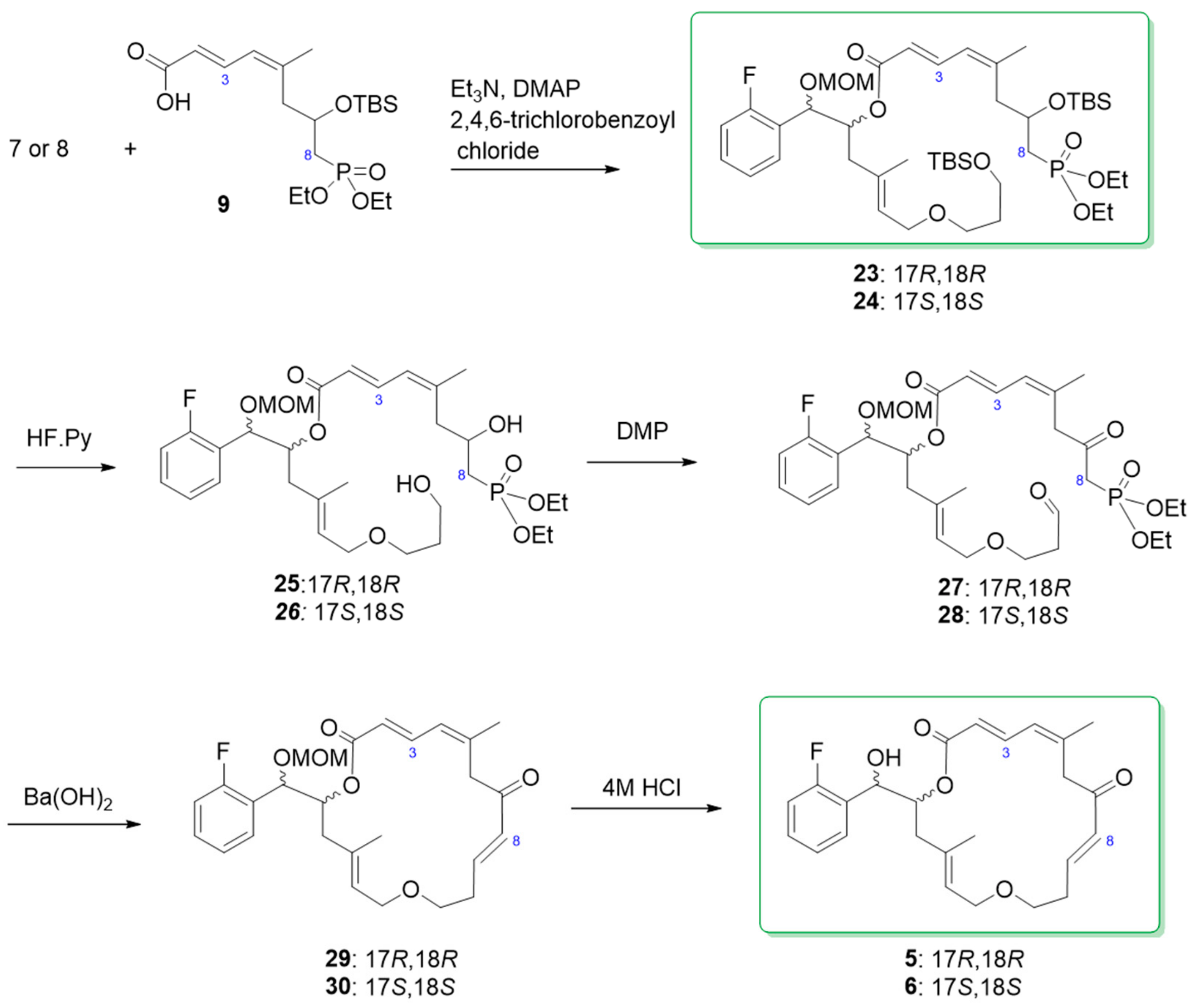
3.7. Synthesis of (2E,4Z)-(5R,6R,E)-5-(2-Fluorophenyl)-8,16,16,17,17-pentamethyl-2,4,11,15-tetraoxa-16-silaoctadec-8-en-6-yl 7-((tert-butyldimethylsilyl)oxy)-8-(diethoxyphosphoryl)-5- methylocta-2,4-dienoate [(17R,18R) ester 23]
To a solution of Fragment C1–C8 (9, 37 mg, 0.09 mmol; co-evaporated with pentane twice) in toluene (0.5 mL) at room temperature, trimethylamine (28 µL, 0.20 mmol) and 2,4,6-trichlorobenzoyl chloride (18 µL, 0.12 mmol) was sequentially added, and the reaction mixture was stirred at room temperature for 1.5 h. A solution of alcohol 7 (35 mg, 0.08 mmol) and DMAP (9.3 mg, 0.08 mmol) in toluene (0.5 mL) was then added to the reaction mixture. The reaction was allowed to proceed with stirring at room temperature for 19 h prior to being quenched with water (5 mL) and saturated aqueous sodium bicarbonate (5 mL). The mixture was extracted with ethyl acetate (5 mL × 3), the combined organic extracts were dried over anhydrous sodium sulfate and concentrated in vacuum. PTLC purification of the crude product over silica gel eluting with hexane/ethyl acetate (60:40, v/v) yielded the ester 23 as a mixture of diastereoisomers in a 1:1 ratio as a colorless oil in 77% yield. 1H NMR (300 MHz, CDCl3) δ 7.50 (dd, J = 15.0, 11.7 Hz, H-3), 7.42 (t, J = 7.5 Hz, 1H, aromatic H), 7.25–7.20 (m, 1H, aromatic H), 7.10 (t, J = 7.5 Hz, 1H, aromatic H), 7.00 (t, J = 8.4 Hz, 1H, aromatic H), 6.03 (d, J = 11.4 Hz, H-4), 5.75 (d, J = 15.3 Hz, 1H, H-2), 5.43–5.37 (m, 1H, H-17), 5.33 (t, J = 6.6 Hz, 1H, H-14), 5.08 (d, J = 5.4 Hz, 1H, H-18), 4.58 (d, J = 6.6 Hz, 1H, -OCH2OCH3), 4.51 (d, J = 6.6 Hz, 1H, OCH2OCH3), 4.18–4.04 (overlapped, 5H, 2 x OCH2CH3, H-7), 3.93–3.79 (m, 2H, H2-13), 3.64 (t, J = 6.3 Hz, 2H, H2-11), 3.40 (t, J = 6.3 Hz, 2H, H2-9), 3.32 (s, 3H, -OCH2OCH3), 2.58–2.48 (m, 2H, H2-16), 2.33–2.24 (m, 2H, H2-8), 2.00–1.92 (m, 2H, H2-6), 1.87 (s, 3H, 5-CH3), 1.72 (quin, J = 6.3 Hz, 2H, H2-10), 1.64 (1.63) (s, 3H, 15-CH3), 1.31 (t, J = 7.2 Hz, 6H, 2 × OCH2CH3), 0.86 (s, 9H, TBS), 0.81 (0.80) (s, 9H, TBS), 0.04 (0.03) (s, 3H, TBS), 0.01 (s, 6H, TBS), −0.04(−0.05) (s, 3H, TBS). 13C NMR (75 MHz, CDCl3) δ 166.8 (166.7), 160.8 (d, J = 244.5 Hz), 146.4 (d, J = 9.8 Hz), 141.6, 135.2 (d, J = 3.8 Hz), 129.7, 129.0, 126.8, 125.7 (d, J = 13.5 Hz), 125.1, 124.3, 119.3, 115.6 (115.3), 95.0, 72.9, 72.3, 67.3, 66.8, 61.7(61.6), 60.5, 60.1, 56.1, 41.8 (41.2), 36.1, 34.3, 33.1, 27.0, 26.11, 26.05, 25.90, 25.87, 25.2, 25.0, 18.5, 18.0, 16.6 (16.5), −4.6, −5.2. IR (film) νmax: 2929, 2856, 1716, 1489, 1251, 1020 cm−1. HRMS (ESI): m/z calculated for C43H77FO10PSi2 [M + H]+: 859.4777. Found: 859.4756.
3.8. Synthesis of (2E,4Z)-(1R,2R,E)-1-(2-Fluorophenyl)-6-(3-hydroxypropoxy)-1-(methoxymethoxy) -4-methylhex-4-en-2-yl 8-(diethoxyphosphoryl)-7-hydroxy-5-methylocta-2,4-dienoate [(17R,18R) diol 25]
To a solution of di-TBS ether 23 (90 mg, 0.105 mmol) in THF (4.2 mL) at 0 °C in a plastic bottle, hydrogen fluoride-pyridine complex (70%, 1.0 mL) was added dropwise. The solution was stirred at 0 °C for 5 min and then at room temperature for 16 h. Saturated sodium bicarbonate (30 mL) was added to quench the reaction and the suspension was extracted with ethyl acetate (30 mL × 3). The combined organic extracts were dried over anhydrous sodium sulfate and the dried extract was concentrated under vacuum. The crude product was purified by PTLC, eluting with dichloromethane/methanol (93:7. v/v), and the pure diol was washed out from the PTLC silica gel with acetone as a pale-yellow oil in 92% yield. 1H NMR (300 MHz, CDCl3) δ 7.50 (7.49) (dd, J = 15.3, 11.7 Hz, 1H, H-3), 7.43 (t, J = 9.0 Hz, 1H, aromatic H), 7.30–7.23 (m, 1H, aromatic H), 7.13 (7.12) (t, J = 7.5 Hz, 1H, aromatic H), 7.031 (7.028) (dd, J = 10.2, 9.0 Hz, 1H, aromatic H), 6.11 (d, J = 11.4 Hz, 1H, H-4), 5.80 (d, J = 15.3 Hz, 1H, H-2), 5.47–5.40 (m, 1H, H-17), 5.32 (t, J = 5.7 Hz, 1H, H-14), 5.09 (d, J = 5.7 Hz, 1H, H-18), 4.58 (d, J = 6.9 Hz, 1H, -OCH2OCH3), 4.51 (d, J = 6.9 Hz, 1H, OCH2OCH3), 4.18–4.06 (overlapped, 5H, 2 × OCH2CH3, H-7), 3.90 (t, J = 5.7 Hz, 2H, H2-13), 3.69 (3.68) (t, J = 5.7 Hz, 2H, H2-11), 3.48 (3.47) (t, J = 5.7 Hz, 2H, H2-9), 3.33 (3.32) (s, 3H, -OCH2OCH3), 3.03 (s, 2H, 2 × OH), 2.66–2.53 (m, 1H, H-16), 2.46–2.19 (m, 3H, H2-8 & H-16), 1.92 (S, 3H, 5-CH3), 1.96–1.88 (m, 2H, H2-6), 1.75 (quin, J = 5.7 Hz, 2H, H2-10), 1.65 (s, 3H, 15-CH3), 1.33 (t, J = 7.2 Hz, 6H, 2 × OCH2CH3). 13C NMR (75 MHz, CDCl3) δ 167.0 (166.8), 160.9 (d, J = 247.5 Hz), 146.0, 140.8 (d, J = 17.3 Hz), 135.7 (d, J = 9.0 Hz), 129.8, 129.0, 126.8, 125.6 (d, J = 12.8 Hz), 124.8 (d, J = 11.3 Hz), 124.4, 119.7 (d, J = 11.3 Hz), 115.7 (115.4), 94.9, 73.0, 72.4, 68.8, 67.4, 65.3, 62.2, 61.8, 56.1, 41.1, 34.6 (34.5), 32.7, 32.3, 25.3, 25.0, 16.6 (16.5). IR (film) νmax: 3366, 2929, 1711, 1633, 1488, 1222, 1019 cm−1. HRMS (ESI): m/z calculated for C31H49FO10P [M + H]+: 631.3048. Found: 631.3041.
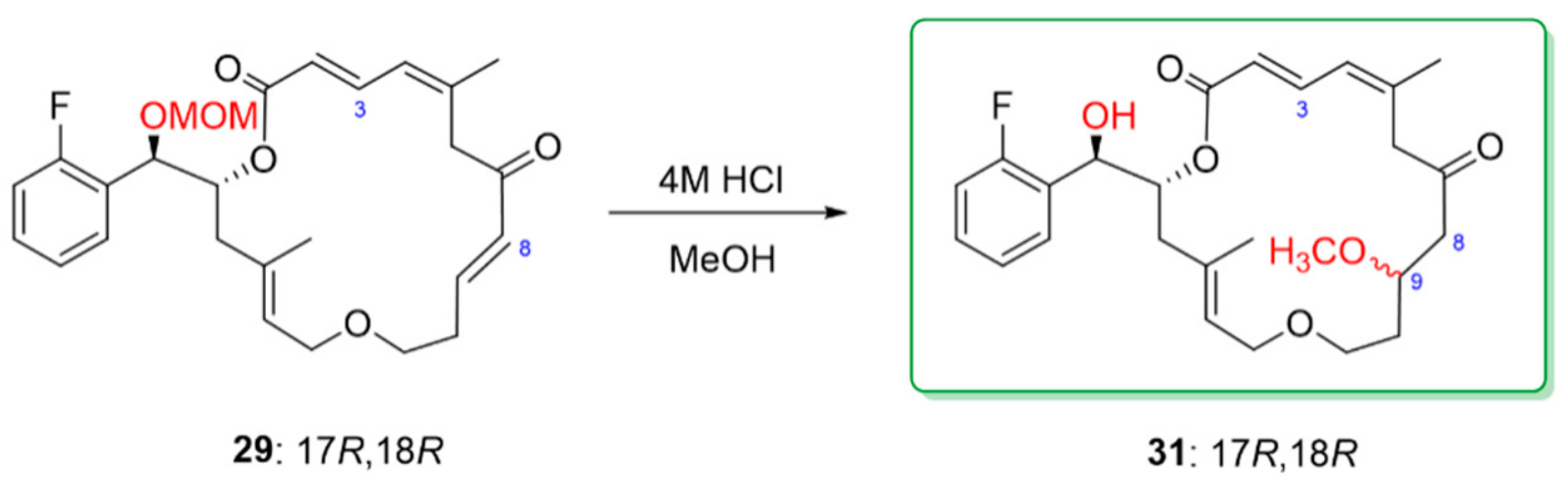
3.9. Synthesis of (R,3E,9E,11Z,15E)-6-((R)-(2-Fluorophenyl)(methoxymethoxy)methyl)-4,12- dimethyl-1,7-dioxacyclooctadeca-3,9,11,15-tetraene-8,14-dione [(17R,18R) macrolactone 29]
To a solution of diol 25 (65 mg, 0.10 mmol) in dichloromethane (6.2 mL) at room temperature, Dess–Martin periodinane (DMP, 131 mg, 0.30 mmol) was added. The reaction mixture was stirred for 30 min before a second portion of DMP (131 mg, 0.30 mmol) was added. The reaction was allowed to proceed with stirring at room temperature for an additional 1 h. The reaction mixture was poured into a stirred mixture of saturated sodium bicarbonate (30 mL) and saturated sodium thiosulfate (30 mL), and the resulting suspension was stirred for 30 min before being extracted with dichloromethane (30 mL × 3). The combined organic extracts were dried over anhydrous sodium sulfate and the dried extract was concentrated under vacuum. The crude product was used for the next reaction without further purification. To a solution of the crude ketoaldehyde (0.10 mmol) obtained above in THF (103 mL) at 0 °C was added water (2.2 mL) followed by activated barium hydroxide (15 mg, 0.08 mmol). The reaction mixture was stirred at 0 °C for 30 min and at room temperature for 2.5 h, then was filtered through a pad of sodium sulfate and a pad of silica gel that was rinsed with ethyl acetate. After evaporation of the solvent, the crude product was subjected to PTLC purification, using hexane/ethyl acetate (73:37) as eluent, to yield the pure macrolactone 29. Colorless oil, 46% yield for two steps. [α]: −15.6 (c = 0.23, MeOH). 1H NMR (300 MHz, CDCl3) δ 7.60 (dd, J = 15.0, 11.4 Hz, 1H, H-3), 7.46 (dt, J = 7.5, 1.8 Hz, 1H, aromatic H), 7.33–7.25 (m, 1H, aromatic H), 7.16 (dt, J = 7.5, 1.2 Hz, 1H, aromatic H), 7.05 (ddd, J = 9.9, 8.4, 1.2 Hz, 1H, aromatic H), 6.82 (dt, J = 16.2, 6.6 Hz, 1H, H-9), 6.12 (d, J = 11.4 Hz, 1H, H-4), 6.01 (d, J = 16.2 Hz, 1H, H-8), 5.91 (d, J = 15.0 Hz, 1H, H-2), 5.51 (ddd, J = 11.4, 6.3, 1.2 Hz, 1H, H-17), 5.27 (dd, J = 7.8, 4.8 Hz, 1H, H-14), 5.12 (d, J = 6.3 Hz, 1H, H-18), 4.60 (d, J = 6.9 Hz, 1H, O-CH2OCH3), 4.53 (d, J = 6.9 Hz, 1H, OCH2OCH3), 3.99 (dd, J = 12.0, 8.1 Hz, 1H, H-16), 3.88–3.81 (m, 2H, H2-13), 3.47–3.28 (m, 2H, H2-11), 3.34 (s, 3H, OCH2OCH3), 3.14 (d, J = 12.6 Hz, 1H, H-16), 2.43–2.36 (m, 2H, H2-10), 2.33 (d, J = 12.0 Hz, 1H, H-6), 2.05 (d, J = 12.1 Hz, 1H, H-6), 1.83 (s, 3H, 5-CH3), 1.63 (S, 3H, 15-CH3). 13C NMR (75 MHz, CDCl3) δ 197.2, 166.6, 160.9 (d, J = 244.5 Hz), 146.8, 142.5, 139.7, 138.9, 134.3, 130.3, 128.9 125.9 (d, J = 7.5 Hz), 125.6 (d, J = 13.5 Hz), 125.2 (d, J = 5.6 Hz), 124.5, 120.9 (d, J = 5.3 Hz), 115.6 (d, J = 21.8 Hz), 94.9, 72.9, 72.6, 67.9, 67.8, 56.0, 46.0, 41.4, 33.1, 24.09, 16.7. IR (film) νmax: 2931, 1713, 1633, 1489, 1358, 1226, 1030 cm−1. HRMS (ESI): m/z calculated for C27H34FO6 [M + H]+: 473.2339. Found: 473.2333.
3.10. Synthesis of (R,3E,9E,11Z,15E)-6-((R)-(2-Fluorophenyl)(hydroxy)methyl)-4,12-dimethyl- 1,7-dioxacyclooctadeca-3,9,11,15-tetraene-8,14-dione [(17R,18R) macrolactone 5]
To a solution of 29 (30 mg, 0.064 mmol) in a mixture of tetrahydrofuran/water (0.92 mL, 1:1, v/v), concentrated hydrochloric acid (130 µL) was added. The two more portions of concentrated hydrochloric acid (130 µL × 2) were added sequentially after each one-hour stirring at room temperature. The reaction mixture was stirred at room temperature overnight before the reaction was quenched by adding saturated ammonium chloride (60 mL). The subsequent mixture was extracted with ethyl acetate (40 mL × 3). The combined extracts were dried over anhydrous sodium sulfate and the solvents were evaporated in vacuum. The crude product was purified over preparative thin layer chromatography eluting with toluene/ethyl acetate (3:1, v/v) to furnish the desired product (11 mg). Colorless syrup, 41% yield. 1H NMR (300 MHz, CDCl3) δ 7.62 (dd, J = 15.0, 11.7 Hz, 1H, H-3), 7.49 (dt, J = 7.5, 1.8 Hz, 1H, aromatic H), 7.31–7.27 (m, 1H, aromatic H), 7.17 (dt, J = 7.5, 1.2 Hz, 1H), 7.05 (ddd, J = 10.2, 8.1, 0.9 Hz, 1H), 6.83 (dt, J = 15.9, 6.9 Hz, 1H, H-9), 6.12 (d, J = 11.4 Hz, 1H, H-4), 6.02 (d, J = 16.2 Hz, 1H, H-8), 5.91 (d, J = 15.0 Hz, 1H, H-2), 5.48–5.38 (m, 1H, H-17), 5.27 (t, J = 6.0 Hz, 1H, H-14), 5.10 (d, J = 6.3 Hz, 1H, H-18), 3.99 (dd, J = 12.0, 8.1 Hz, 1H, H-16), 3.86 (br.s, 1H, H-13), 3.82 (br.s, 1H, H-13), 3.51–3.36 (m, 2H, H2-11), 3.18 (d, J = 12.9 Hz, 1H, H-16), 2.44–2.34 (m, 3H, H2-10, H-6), 2.06 (d, J = 14.4 Hz, 1H, H-6), 1.83 (s, 3H, 5-CH3), 1.62 (s, 3H, 15-CH3). 13C NMR (75 MHz, CDCl3) δ 197.2, 167.0, 160.1 (d, JCF = 244.5 Hz), 146.9, 143.0, 140.1 (d, JCF = 12.8 Hz), 134.2, 130.2, 129.9, 128.8, 127.6 (d, JCF = 12.8 Hz), 126.0, 125.2, 124.6, 120.7, 115.6 (d, JCF = 21.8 Hz), 79.4, 74.4, 70.3, 68.0, 45.9, 41.4, 33.0, 24.1, 16.7. [α]: −15.2 (c = 0.16, MeOH). IR (film) νmax: 3428, 2924, 2854, 1706, 1633, 1558, 1488, 1360, 1221, 1036 cm−1. HRMS (ESI): m/z calculated for C25H30FO5 [M + H]+: 429.2077. Found: 429.2074.
3.11. Synthesis of (1S,2S)-1-(2-Fluorophenyl)propane-1,2,3-triol [(17S,18S) triol 15]
Triol 15 was prepared in 68% yield from 13 catalyzed by AD-mix-β using a similar procedure used to synthesize its antipode 14. 1H NMR (300 MHz, CDCl3) δ 7.39 (t, J = 7.5 Hz, 1H, aromatic H), 7.22 (dd, J = 13.8, 7.2 Hz, 1H, aromatic H), 7.07 (t, J = 7.5 Hz, 1H, aromatic H), 6.96 (t, J = 9.3 Hz, 1H, aromatic H), 4.90 (d, J = 6.3 Hz, 1H, H-18), 4.21 (br.s, 3H, 3 × OH), 3.78 (dd, J = 10.2, 4.8 Hz, 1H, H-17), 3.48 (d, J = 4.8 Hz, 2H, H2-16). 13C NMR (75 MHz, CDCl3) δ 160.0 (d, JCF = 244.5 Hz), 129.6, 128.5 (d, JCF = 16.5 Hz), 127.8 (d, JCF = 12.8 Hz), 124.6, 115.5 (d, JCF = 21.8 Hz), 75.4, 68.8, 63.4. IR (film) νmax: 3375, 2930, 1781, 1489, 1456, 1319 cm−1. HRMS (ESI): m/z calculated for C9H12FO3 [M + H]+: 187.0770. Found: 187.0765.
3.12. Synthesis of (2S,3S)-3-(2-Fluorophenyl)-2,3-dihydroxypropyl methanesulfonate [(17S,18S) mesylate 17]
Mesylate 17 was prepared from triol 15 as a colorless oil in 72% yield, employing a procedure similar to that used for the mesylation of triol 14. 1H NMR (300 MHz, CDCl3) δ 7.48 (dt, J = 7.5, 1.8 Hz, 1H, Aromatic H), 7.32–7.29 (m, 1H, aromatic H), 7.18 (dt, J = 8.4, 0.9 Hz, 1H, aromatic H), 7.05 (ddd, J = 9.3, 8.1, 0.9 Hz, 1H, aromatic H), 5.01 (d, J = 5.7 Hz, 1H, H-18), 4.28–4.15 (m, 2H, H2-16), 4.08–4.03 (m, 1H, H-17), 3.04 (s, 3H, SO2CH3). 13C NMR (75 MHz, CDCl3) δ 159.9 (d, JCF = 244.5 Hz, 1H), 130.1, 128.4, 127.0 (d, JCF = 12.8 Hz, 1H), 124.8, 115.7 (d, JCF = 21.8 Hz, 1H), 72.9, 70.3, 67.9, 37.6. IR (film) νmax: 3482, 3029, 2939, 1616, 1587, 1489, 1456, 1332, 1168 cm−1. HRMS (ESI): m/z calculated for C10H14FO5S [M + H]+: 265.0546. Found: 265.0542.
3.13. Synthesis of (S)-(2-Fluorophenyl)((S)-oxiran-2-yl)methanol [(17S,18S) epoxide 19]
Epoxide 19 (52%, pale yellow oil) was obtained from mesylate 17 according to the internal Williamson ether synthesis procedure employed for the conversion of mesylate 16 to epoxide 18. 1H NMR (300 MHz, CDCl3) δ 7.57 (dt, J = 7.5, 1.8 Hz, 1H, aromatic H), 7.35–7.25 (m, 1H, aromatic H), 7.20 (dt, J = 7.5, 1.2 Hz, 1H, aromatic H), 7.06 (ddd, J = 10.5, 8.1, 1.2 Hz, 1H, aromatic H), 4.83 (d, J = 5.1 Hz, 1H, H-18), 3.26–3.21 (m, 1H, H-17), 2.92–2.84 (m, 2H, H2-16). 13C NMR (75 MHz, CDCl3) δ 160.0 (d, JCF = 244.5 Hz), 129.8, 127.6 (d, JCF = 9.0 Hz), 127.4 (d, JCF = 13.5 Hz), 124.7, 115.6 (d, JCF = 21.0 Hz), 68.6, 55.4, 45.6. IR (film) νmax: 3482, 3029, 2939, 1617, 1587, 1489, 1456, 1331, 1168 cm−1. HRMS (ESI): m/z calculated for C9H10FO2 [M + H]+: 169.0665. Found: 169.0659.
3.14. Synthesis of (S)-2-((S)-(2-Fluorophenyl)(methoxymethoxy)methyl)oxirane [(17S,18S) MOM ether 21]
MOM ether 21 was prepared from epoxide 19 as a pale-yellow oil in 73% yield employing a procedure similar to that used for conversion of 18. 1H NMR (300 MHz, CDCl3) δ 7.44 (dt, J = 7.5, 1.8 Hz, 1H, aromatic H), 7.32–7.27 (m, 1H, aromatic H), 7.17 (dt, J = 7.5, 0.9 Hz, 1H, aromatic H), 7.05 (ddd, J = 10.2, 8.4, 1.2 Hz, 1H, aromatic H), 4.76 (d, J = 6.6 Hz, 1H, -OCH2OCH3), 4.68 (d, J = 6.9 Hz, 1H, H-18), 4.63 (d, J = 6.6 Hz, 1H, -OCH2OCH3), 3.37 (s, 3H, -OCH2OCH3), 3.27 – 3.23 (m, 1H, H-17), 2.75–2.73 (m, 2H, H2-16). 13C NMR (75 MHz, CDCl3) δ 160 (d, JCF = 245.3 Hz), 129.9 (d, JCF = 8.3 Hz), 128.7 (d, JCF = 3.8 Hz), 125.3 (d, JCF = 14.3 Hz), 124.5 (d, JCF = 3.8 Hz), 115.7 (d, JCF = 21.8 Hz), 94.8, 73.1, 55.8, 54.6, 44.4. IR (film) νmax: 2891, 2825, 1616, 1587, 1488, 1455, 1149, 1022 cm−1. HRMS (ESI): m/z calculated for C11H14FO3 [M + H]+: 213.0927. Found: 213.0920.
3.15. Synthesis of (5S,6S,E)-5-(2-Fluorophenyl)-8,16,16,17,17-pentamethyl-2,4,11,15-tetraoxa-16- silaoctadec-8-en-6-ol [(17S, 18S) Fragment C9–C18 (8)]
(17S,18S) Fragment C9–C18 (8) was synthesized as a colorless oil in 22% yield from MOM ether 21 in a similar way to the synthesis of its antipode (17R,18R) Fragment C9–C18 (7). 1H NMR (300 MHz, CDCl3) δ 7.41 (dt, J = 7.5, 1.8 Hz, 1H, aromatic H), 7.32–7.24 (m, 1H, aromatic H), 7.15 (dt, J = 7.5, 1.2 Hz, 1H, aromatic H), 7.03 (ddd, J = 10.2, 8.4, 1.2 Hz, 1H, aromatic H), 5.41 (t, J = 6.6 Hz, 1H, H-14), 4.81 (d, J = 6.6 Hz, 1H, H-18), 4.63 (d, J = 6.6 Hz, 1H, OCH2OCH3), 4.58 (d, J = 6.6 Hz, 1H, OCH2OCH3), 3.95 (d, J = 6.6 Hz, 2H, H2-13), 3.82 (dt, J = 10.2, 5.7 Hz, 1H, H-17), 3.67 (t, J = 6.0 Hz, 2H, H2-11), 3.46 (t, J = 6.6 Hz, 2H, H2-9), 3.36 (s, 3H, OCH3), 2.23 (dd, J = 12.6, 9.6 Hz, 1H, H-16), 2.05 (dd, J = 12.6, 2.7 Hz, 1H, H-16), 1.76 (quin, J = 6.3 Hz, 2H, H2-10), 1.64 (s, 3H, 15-CH3), 0.87 (s, 9H, TBS), 0.03 (s, 6H, TBS). 13C NMR (75 MHz, CDCl3) δ 160.9 (d, JCF = 244.7 Hz), 136.4, 129.2, 128.9, 128.6, 126.1 (d, JCF = 12.8 Hz), 124.5, 115.5, 95.3, 75.6, 72.7, 67.3, 67.0, 60.2, 56.2, 43.0, 33.2, 26.0, 18.5, 16.6, 16.5. IR (film) νmax: 3461, 2928, 2855, 1616, 1586, 1252, 1090, 1031 cm−1. HRMS (ESI): m/z calculated for C24H42FO5Si [M + H]+: 457.2786. Found: 457.2782.
3.16. Synthesis of (2E,4Z)-(5S,6S,E)-5-(2-Fluorophenyl)-8,16,16,17,17-pentamethyl-2,4,11,15- tetraoxa-16-silaoctadec-8-en-6-yl 7-((tert-butyldimethylsilyl)oxy)-8-(diethoxyphosphoryl)- 5-methylocta-2,4-dienoate [(17S,18S) ester 24]
(17S,18S) Ester 24 was obtained as a pale-yellow oil in 91% yield from (17S,18S) Fragment C9–C18 (8) and Fragment C1–C8 (9), according to the esterification procedure employed for the preparation of ester 23. 1H NMR (300 MHz, CDCl3) δ 7.50 (dd, J = 14.4, 12.0 Hz, 1H, H-3), 7.42 (t, J = 7.2 Hz, 1H, aromatic H), 7.34–7.21 (m, 1H, aromatic H), 7.10 (t, J = 7.5 Hz, 1H, aromatic H), 7.01 (t, J = 9.3 Hz, 1H, aromatic H), 6.03 (d, J = 11.7 Hz, 1H, H-4), 5.76 (d, J = 15.0 Hz, 1H, H-2), 5.45–5.37 (m, 1H, H-17), 5.33 (t, J = 6.0 Hz, 1H, H-14), 5.08 (d, J = 5.1 Hz, 1H, H-18), 4.58 (d, J = 6.9 Hz, 1H, OCH2OCH3), 4.51 (d, J = 6.6 Hz, 1H, OCH2OCH3), 4.23–4.04 (m, 5H, 2 × OCH2CH3, H-7), 3.93–3.80 (m, 2H, H2-13), 3.65 (t, J = 6.6 Hz, 2H, H2-11), 3.41 (t, J = 6.3 Hz, H2-9), 3.32 (s, 3H, OCH2OCH3), 2.58–2.54 (m, 2H, H2-16), 2.33–2.28 (m, 2H, H2-8), 2.03–1.93 (m, 2H, H2-6), 1.87 (s, 3H, 5-CH3), 1.77–1.70 (m, 2H. H2-10), 1.64 (s, 3H, 15-CH3), 1.31 (t, J = 6.9 Hz, 6H, 2 × OCH2CH3), 0.90 (0.88) (s, 3H, TBS), 0.86 (s, 6H, TBS), 0.81 (0.80) (s, 9H, TBS), 0.08 (0.04) (s, 6H, TBS), 0.02 (-0.04) (s, 6H, TBS). 13C NMR (75 MHz, CDCl3) δ 166.7, 160.8 (d, JCF = 245.3 Hz), 146.5 (146.4), 146.3 (146.2), 135.7 (135.2), 129.8, 129.0, 126.8, 125.8 (125.6),125.1 (124.7), 124.3 119.2, 115.5 (d, JCF = 27.5 Hz), 95.0, 72.9, 72.3, 68.9, 66.8, 61.8, 61.7, 60.1, 56.1, 41.7 (41.2), 36.0 (34.2), 33.1, 32.3, 29.8, 27.0, 26.0, 25.9, 25.8, 24.9, 18.5, 18.1, 18.0, 16.6, 16.5. IR (film) νmax: 2928, 2856, 1714, 1636, 1250, 1020 cm−1. HRMS (ESI): m/z calculated for C43H77FO10PSi2 [M + H]+: 859.4777. Found: 859.4774.
3.17. Synthesis of (2E,4Z)-(1S,2S,E)-1-(2-Fluorophenyl)-6-(3-hydroxypropoxy)-1-(methoxymethoxy)- 4-methylhex-4-en-2-yl 8-(diethoxyphosphoryl)-7-hydroxy-5-methylocta-2,4-dienoate [(17S,18S) diol 26]
(17S,18S) Diol 26 was obtained as a colorless syrup in 65% yield according to the TBS deprotection procedure used for the conversion of (17R,18R) ester 23 to (17R,18R) diol 25. 1H NMR (300 MHz, CD3COCD3) δ 7.63–7.55 (m, 1H, H-3), 7.52 (t, J = 7.8 Hz, 1H, aromatic H), 7.41–7.33 (m, 1H, aromatic H), 7.23 (t, J = 7.2 Hz, 1H, aromatic H), 7.14 (t, J = 9.0 Hz, 1H, aromatic H), 6.16 (d, J = 11.4 Hz, 1H, H-4), 5.80 (d, J = 15.0 Hz, 1H, H-2), 5.46 (quin, J = 5.1 Hz, 1H, H-17), 5.31 (t, J = 6.6 Hz, 1H, H-14), 5.10 (d, J = 5.7 Hz, 1H, H-18), 4.61 (d, J = 6.6 Hz, 1H, -OCH2OCH3), 4.51 (d, J = 6.6 Hz, 1H, -OCH2OCH3), 4.22–4.04 (overlapped, 5H, 2 × –OCH2CH3, H-7), 3.88 (d, J = 6.0 Hz, 2H, H2-13), 3.57 (t, J = 4.8 Hz, 2H, H2-11), 3.40 (t, J = 6.3 Hz, 2H, H2-9), 3.30 (s, 3H, -OCH2OCH3), 2.92 (br.s, 2H, 2 × OH), 2.60–2.57(overlapped, 2H, H2-16), 2.29 (d, J = 9.3 Hz, 2H, H2-8), 2.07–2.04 (m, 2H, H2-6), 1.95 (s, 3H, 5-CH3), 1.68 (quin, J = 6.0 Hz, H2-10), 1.65 (s, 3H, 15-CH3), 1.29 (t, J = 7.2 Hz, 6H, 2 × –OCH2CH3). 13C NMR (75 MHz, CDCl3) δ 166.0, 160.7 (d, JCF = 243.8 Hz), 147.0 (d, JCF = 9.8 Hz), 140.8, 134.2, 129.8, 129.3, 125.8 (d, JCF = 9.8 Hz), 125.6, 125.4 (d, JCF = 4.5 Hz), 124.3, 119.1, 115.3 (115.0), 94.7, 72.3, 66.8, 66.7, 65.5, 61.4(61.3), 61.2 (61.1), 59.1, 55.1, 41.2, 40.7, 34.6, 33.02(32.98), 32.0, 24.4, 15.91(15.86), 15.7(15.6). IR (film) νmax: 3381, 2927, 1709, 1633, 1488, 1019 cm−1. HRMS (ESI): m/z calculated for C31H49FO10P [M + H]+: 631.3048. Found: 631.3045.
3.18. Synthesis of (2E,4Z)-(1S,2S,E)-1-(2-Fluorophenyl)-1-(methoxymethoxy)-4-methyl-6- (3-oxopropoxy)hex-4-en-2-yl 8-(diethoxyphosphoryl)-5-methyl-7-oxoocta-2,4-dienoate [ketoaldehyde (17S,18S) 28]
The crude product was obtained from (17S,18S) diol 26 employing the oxidation procedure used for the synthesis of its antipode 27. After being confirmed by its 1H NMR data, the crude product was directly used for the next step reaction without further purification. 1H NMR (300 MHz, CDCl3) δ 9.72 (t, J = 1.8 Hz, 1H, CHO), 7.41 (dt, J = 7.5, 1.8 Hz, 1H, aromatic H), 7.31 (dd, J = 15.0, 11.7 Hz, 1H, H-3), 7.29–7.22 (m, 1H), 7.11 (dt, J = 7.5, 0.9 Hz, 1H), 7.51 (ddd, J = 10.2, 8.1, 0.9 Hz, 1H), 6.17 (d, J = 11.1 Hz, 1H, H-4), 5.83 (d, J = 15.0 Hz, 1H, H-2), 5.45–5.38 (m, 1H), 5.31 (t, J = 5.7 Hz, 1H, H-14), 5.07 (d, J = 5.4 Hz, 1H), 4.57 (d, J = 6.9 Hz, 1H, OCH2OCH3), 4.50 (d, J = 6.9 Hz, 1 H, OCH2OCH3), 4.20–4.11 (m, 5H, 2 × OCH2CH3, H-7), 3.90 (t, J = 7.2 Hz, 2H, H2-13), 3.65 (t, J = 6.0 Hz, 2H, H2-11), 3.31 (s, 3H, OCH3), 3.10 (d, J = 22.8 Hz, 2H, H2-8), 2.39–2.19 (m, 2H, H2-10), 1.87 (s, 3H, 5-CH3), 1.63 (s, 3H, 15-CH3).
3.19. Synthesis of (S,3E,9E,11Z,15E)-6-((S)-(2-Fluorophenyl)(methoxymethoxy)methyl)-4,12- dimethyl-1,7-dioxacyclooctadeca-3,9,11,15-tetraene-8,14-dione [(17S,18S) macrolide 30]
(17S,18S) Macrolide 30 was synthesized according to the HWE ring closing procedure employed for the conversion of 27 to 29. Colorless syrup, 68% yield for two steps. [α]: +8.1 (c = 0.22, MeOH). 1H NMR (300 MHz, CDCl3) δ 7.59 (dd, J = 15.0, 11.4 Hz, 1H, H-3), 7.46 (dt, J = 7.5, 1.8 Hz, 1H, aromatic H), 7.36–7.25 (m, 1H, aromatic H), 7.15 (dt, J = 7.5, 1.2 Hz, 1H, aromatic H), 7.04 (ddd, J = 9.9, 8.1, 1.2 Hz, 1H, aromatic H), 6.81 (dt, J = 15.9, 6.9 Hz, 1H, H-9), 6.11 (d, J = 11.4 Hz, 1H, H-4), 6.00 (d, J = 16.2 Hz, 1H, H-8), 5.90 (d, J = 15.0 Hz, 1H, H-2), 5.50 (dd, J = 11.4, 6.3 Hz, 1H, H-17), 5.26 (dd, J = 7.5, 4.5 Hz, 1H, H-14), 5.11 (d, J = 6.3 Hz, 1H, H-18), 4.59 (d, J = 6.9 Hz, 1H,–OCH2OCH3), 4.52 (d, J = 6.6 Hz, 1H, –OCH2OCH3), 3.98 (dd, J = 12.0, 8.1 Hz, 1H, H-16), 3.88 – 3.81 (overlapped, 2H, H2-13), 3.46 – 3.31 (overlapped, 2H, H2-11), 3.33 (s, 3H, OCH3), 3.14 (d, J = 12.6 Hz, 1H, H-16), 2.42–2.35 (m, 2H, H2-10), 2.33 (d, J = 11.7 Hz, 1H, H-6), 2.04 (d, J = 11.7 Hz, 1H, H-6), 1.82 (s, 3H, 5-CH3), 1.63 (s, 3H, 15-CH3). 13C NMR (75 MHz, CDCl3) δ 197.1, 166.6, 160.9 (d, JCF = 244.5 Hz), 146.8, 142.5, 139.8, 134.3, 130.2, 129.9, 129.0, 125.9 (d, JCF = 7.5 Hz), 125.5 (d, J = 13.5 Hz), 125.2 (d, JCF = 4.5 Hz), 124.5, 120.9 (d, JCF = 3.8 Hz), 115.6 (d, JCF = 21.0 Hz), 94.9, 72.9, 72.6, 67.9, 67.8, 56.0, 45.9, 41.4, 33.1, 24.1, 16.7. IR (film) νmax: 2925, 2854, 1714, 1669, 1634, 1489, 1359, 1280 cm−1. HRMS (ESI): m/z calculated for C27H34FO6 [M + H]+: 473.2339. Found: 473.2332.
3.20. Synthesis of (S,3E,9E,11Z,15E)-6-((S)-(2-Fluorophenyl)(hydroxy)methyl)-4,12-dimethyl- 1,7-dioxacyclooctadeca-3,9,11,15-tetraene-8,14-dione [(17S,18S) zampanolide mimic 6]
(17S,18S) Zampanolide mimic 6 was obtained according to the MOM deprotection procedure used for the conversion of (17R,18R) macrolide 29 to (17S,18S) zampanolide mimic 5. Colorless syrup, 45% yield. [α]: +15.6 (c = 0.15, MeOH). 1H NMR (300 MHz, CD3COCD3) δ 7.61 (dd, J = 15.0, 11.1 Hz, 1H, H-3), 7.34 (dd, J = 14.4, 7.2 Hz, 1H, aromatic H), 7.21 (t, J = 7.5 Hz, 1H, aromatic H), 7.10 (dd, J = 10.5, 8.1 Hz, 1H, aromatic H), 6.83 (dt, J = 16.2, 6.3 Hz, 1H, H-9), 6.20 (d, J = 11.1 Hz, 1H, H-4), 6.06 (d, J = 15.9 Hz, H-8), 5.88 (d, J = 12.6 Hz, 1H, H-2), 5.44 (dd, J = 11.1, 5.4 Hz, 1H, H-17), 5.27 (t, J = 5.4 Hz, 1H, H-14), 5.17 (t, J = 5.4 Hz, 1H, H-18), 4.81 (d, J = 5.4 Hz, 1H, OH), 4.07–3.97 (m, 1H, H-16), 3.90 (d, J = 12.9 Hz, 1H, H-13), 3.83 (dd, J = 12.3, 4.5 Hz, 1H, H-13), 3.48– 3.32 (m, 2H, H2-11), 3.17 (d, J = 12.6 Hz, 1H, H-16), 2.42–2.33 (overlapped, 3H, H2-10, H-6), 2.19 (d, J = 13.8 Hz, 1H, H-6), 1.81 (s, 3H, 5-CH3), 1.64 (s, 3H, 15-CH3). 13C NMR (75 MHz, CD3COCD3) δ 196.5, 166.5, 160.5 (d, JCF = 242.3 Hz), 146.6, 142.7, 139.8, 134.7, 130.6, 129.7, 129.5, 126.3, 126.2, 125.7, 124.8, 121.7, 115.6, 73.8, 69.0, 68.9, 68.2, 45.8, 41.6, 33.2, 23.8, 16.3. IR (film) νmax: 3447, 2916, 2855, 1710, 1669, 1633, 1489, 1456, 1359, 1281, 1148 cm−1. HRMS (ESI): m/z calculated for C25H30FO5 [M + H]+: 429.2077. Found: 429.2073.
3.21. Cell Culture
All cell lines were initially purchased from American Type Culture Collection (ATCC). The PC-3, PC-3/DTX, and LNCaP prostate cancer cell lines were routinely cultured in RPMI-1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin. Cultures were maintained in a high humidity environment supplemented with 5% carbon dioxide at a temperature of 37 °C. The DU145 and DU145/DTX prostate cancer cells were routinely cultured in Eagle’s Minimum Essential Medium (EMEM) supplemented with 10% FBS and 1% penicillin/streptomycin.
The procedure illustrated in the literature [
29,
30] was adapted to establish docetaxel-resistant prostate cancer cell lines. Specifically, docetaxel-resistant DU145 and PC-3 cell lines (DU145/DTX and PC-3/DTX) were developed over a period of one year by stepwise increased concentrations of docetaxel. Cells were repeatedly conserved in an appropriate concentration of docetaxel, starting with IC
50 value of the respective parent cell lines. Docetaxel-containing media will be replaced every 2–3 days. The concentration of docetaxel was increased when cells exhibited resistance to treatments.
3.22. WST-1 Cell Proliferation Assay
PC-3, PC-3/DTX, DU145, DU145/DTX, or LNCaP cells were placed in 96-well plates at a density of 3200 cells each well in 200 µL of culture medium. The cells were then treated with synthesized mimics, or positive reference separately at different doses for 3 days, while equal treatment volumes of DMSO were used as vehicle control. After the cells were cultured in a CO2 incubator at 37 °C for three days, the premixed WST-1 cell proliferation reagent (10 µL, Clontech) was added to each well. The cells were incubated for additional 3 h at 37 °C before mixing gently for one minute on an orbital shaker to ensure homogeneous distribution of color. A microplate-reader (Synergy HT, BioTek) was used to measure the absorbance of each well at a wavelength of 430 nm. The half-maximal inhibitory concentration (IC50 value) is the concentration of each compound that inhibits cell proliferation by 50% under the experimental conditions. Each of IC50 value is represented as the average from triplicate determinations that were reproducible and statistically significant. The IC50 values were calculated based on dose–response curves from at least five dosages for each compound.
3.23. Statistical Analysis
All data are represented as the mean ± standard deviation (S.D.) for the number of experiments indicated. Other differences between treated and control groups were analyzed using the Student’s t-test. A p-value < 0.05 was considered statistically significant.