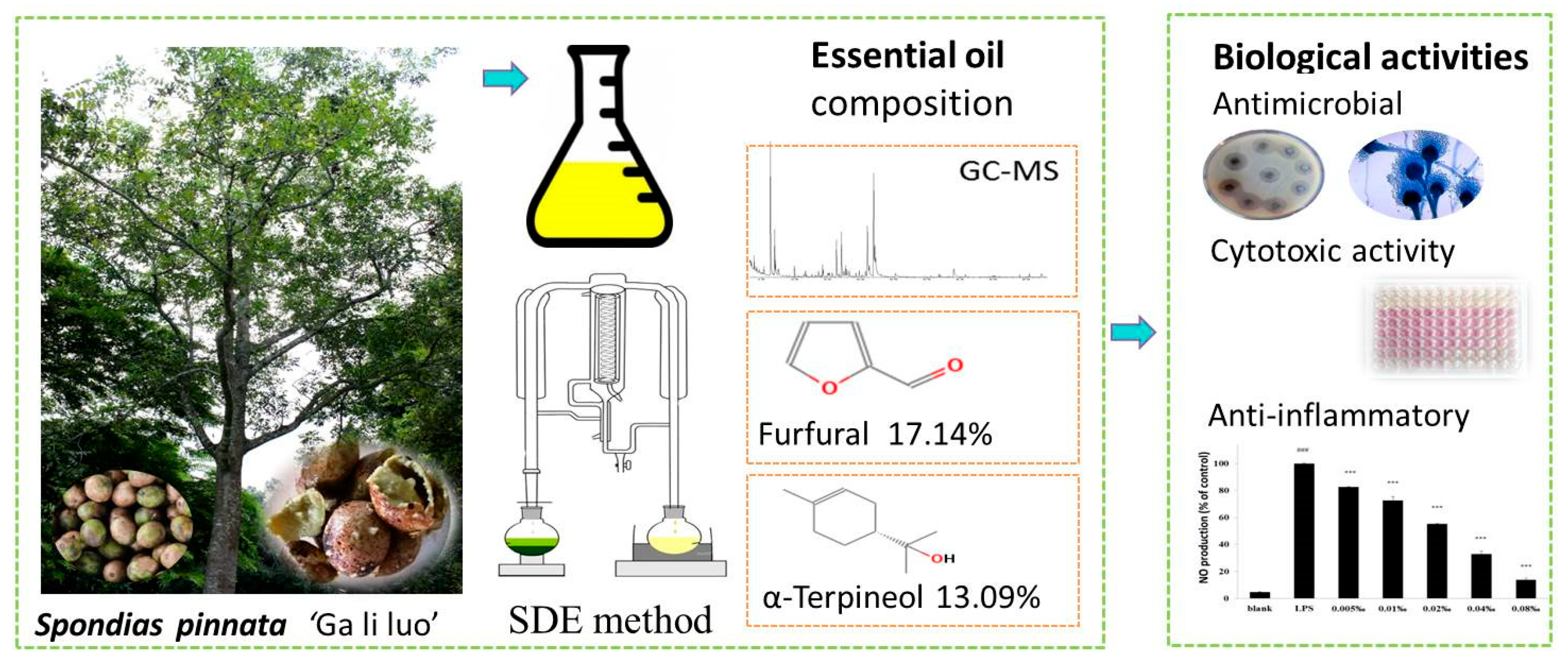
Chemical Composition and the Cytotoxic, Antimicrobial, and Anti-Inflammatory Activities of the Fruit Peel Essential Oil from Spondias pinnata (Anacardiaceae) in Xishuangbanna, Southwest China
Abstract
1. Introduction
2. Result and Discussion.
2.1. Essential Oil Composition
2.2. Cytotoxicity Activity
2.3. Antimicrobial Activity
2.4. Anti-Inflammatory Activity
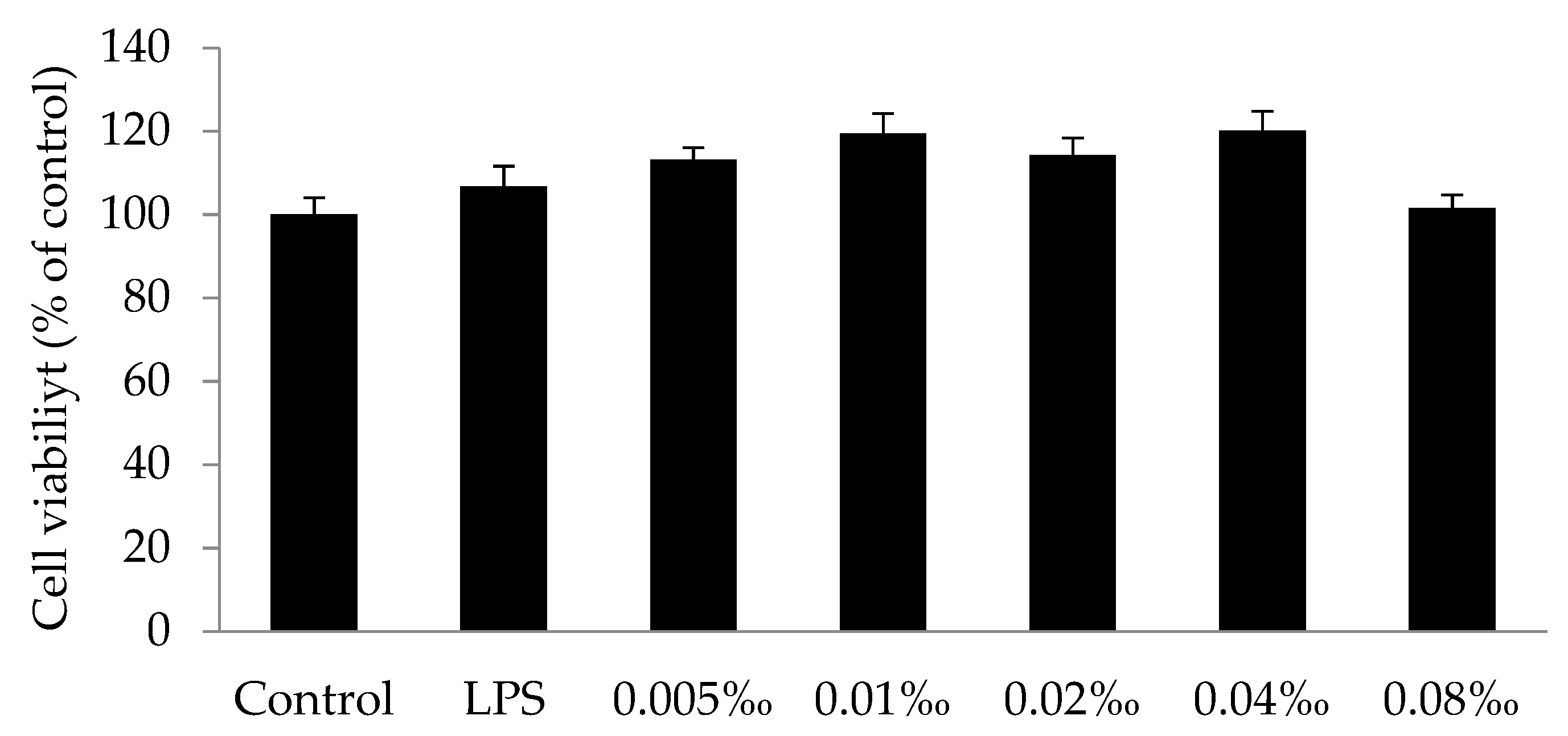
2.4.1. Effect of Essential Oil on Cell Viability
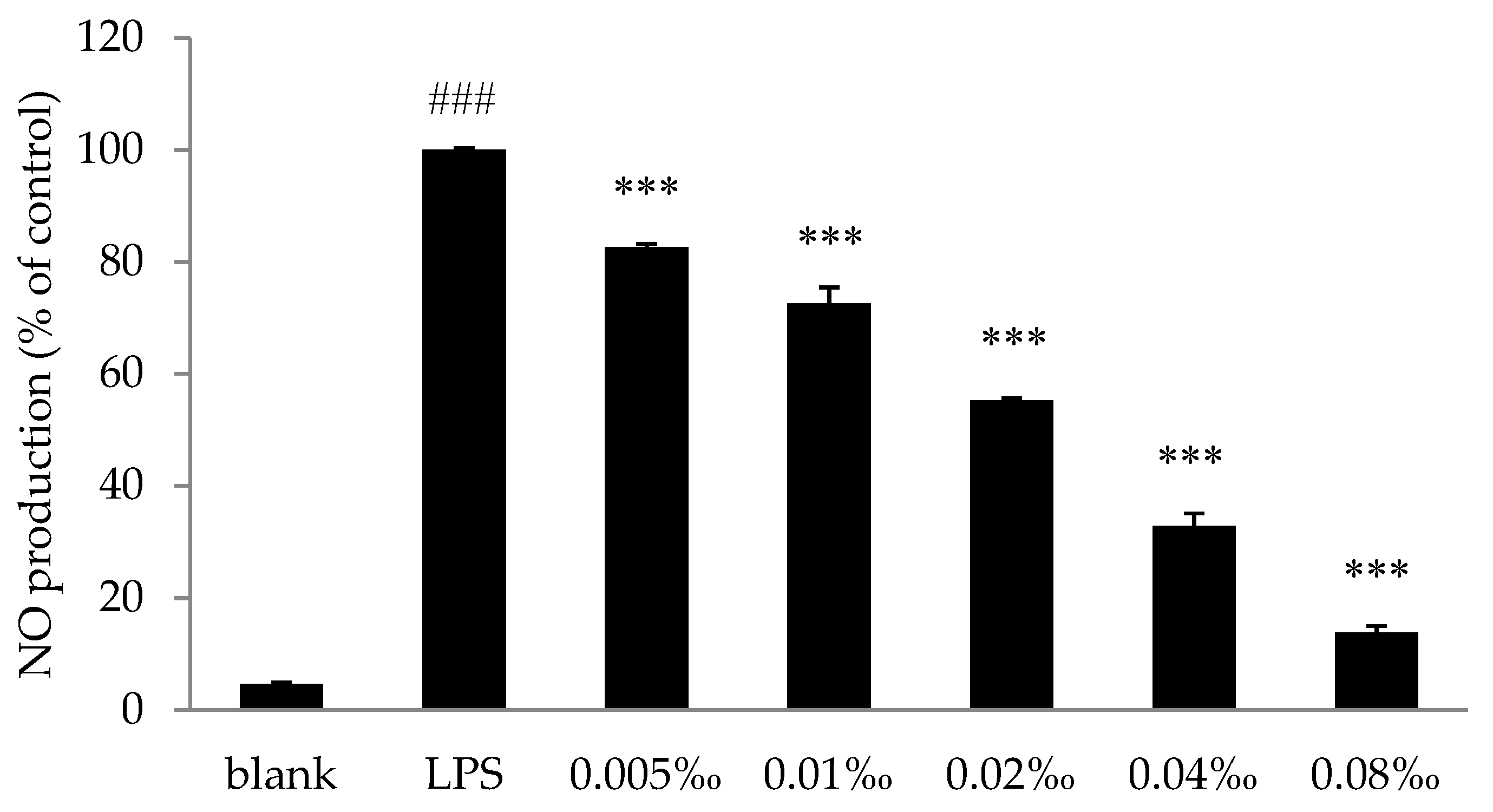
2.4.2. Effect of Essential Oil on NO Production
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Materials and Essential Oil Extraction
3.3. Gas Chromatography/Mass Spectrometry (GC-MS) Analysis
3.4. Identification of the Components
3.5. Cytotoxicity Assays
3.6. Antimicrobial Activity Assay
3.6.1. Microbial Strains and Culture Media
3.6.2. Minimal Inhibitory, Bactericidal, and Fungicidal Concentration (MIC, MBC, and MFC) Assay
3.7. Anti-Inflammatory Activity Assay
3.7.1. Cell Culture
3.7.2. Cell Viability Assay
3.7.3. Measurement of NO Production
3.8. Statistical Analysis.
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Combrinck, S.; Regnier, T.; Kamatou, G.P.P. In Vitro activity of eighteen essential oils and some major components against common postharvest fungal pathogens of fruit. Ind. Crop. Prod. 2011, 33, 344–349. [Google Scholar] [CrossRef]
- Shaaban, H.A.E.; El-Ghorab, A.H.; Shibamoto, T. Bioactivity of essential oils and their volatile aroma components: Review. J. Essent. Oil Res. 2012, 24, 203–212. [Google Scholar] [CrossRef]
- Bassole, I.H.N.; Juliani, H.R. Essential oils in combination and their antimicrobial properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.G. Antioxidant and anti-inflammatory activities of essential Oils: A short review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef] [PubMed]
- Drummond, R.A. Aspergillus fumigatus. Available online: https://www.immunology.org/public-information/bitesized-immunology/pathogens-and-disease/aspergillus-fumigatus (accessed on 25 September 2019).
- Rybak, J.M.; Fortwendel, J.R.; Rogers, P.D. Emerging threat of triazole-resistant Aspergillus fumigatus. J. Antimicrob. Chemother. 2019, 74, 835–842. [Google Scholar] [CrossRef]
- Tianlu, M.; Barfod, A. Anacardiaceae. In Flora of China; Flora of China Editorial Committee; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2008; Volume 11, pp. 335–357. [Google Scholar]
- Hosni, K.; Jemli, M.; Dziri, S.; M’Rabet, Y.; Ennigrou, A.; Sghaier, A.; Casabianca, H.; Vulliet, E.; Ben Brahim, N.; Sebei, H. Changes in phytochemical, antimicrobial and free radical scavenging activities of the Peruvian pepper tree (Schinus molle L.) as influenced by fruit maturation. Ind. Crop. Prod. 2011, 34, 1622–1628. [Google Scholar] [CrossRef]
- Marcetic, M.; Bozic, D.; Milenkovic, M.; Malesevic, N.; Radulovic, S.; Kovacevic, N. Antimicrobial, antioxidant and anti-inflammatory activity of young shoots of the smoke tree, Cotinus coggygria Scop. Phytother. Res. 2013, 27, 1658–1663. [Google Scholar] [CrossRef]
- Montanari, R.M.; Barbosa, L.C.A.; Demuner, A.J.; Silva, C.J.; Andrade, N.J.; Ismail, F.M.D.; Barbosa, M.C.A. Exposure to Anacardiaceae volatile oils and their constituents induces lipid peroxidation within food-borne bacteria cells. Molecules 2012, 17, 9728–9740. [Google Scholar] [CrossRef]
- Chen, J.; Sun, Y.C.; Chen, G.Q.; Wang, W.D. Ethnobotanical studies on wild edible fruits in Southern Yunnan: Folk names; nutritional value and uses. Econ. Bot. 1999, 53, 2–14. [Google Scholar]
- Xu, Y.K.; Tao, G.D.; Liu, H.M.; Yan, K.L.; Dao, X.S. Wild vegetable resources and market survey in Xishuangbanna, southwest China. Econ. Bot. 2004, 58, 647–667. [Google Scholar]
- Pei, S.J. Preliminary study of ethnobotany in xishuang banna, People’s Republic of China. J. Ethnopharmacol. 1985, 13, 121–137. [Google Scholar] [CrossRef]
- Satpathy, G.; Tyagi, Y.K.; Gupta, R.K. Preliminary evaluation of nutraceutical and therapeutic potential of raw Spondias pinnata K., an exotic fruit of India. Food Res. Int. 2011, 44, 2076–2087. [Google Scholar] [CrossRef]
- Maisuthisakul, P.; Suttajit, M.; Pongsawatmanit, R. Assessment of phenolic content and free radical-scavenging capacity of some Thai indigenous plants. Food Chem. 2007, 100, 1409–1418. [Google Scholar] [CrossRef]
- Hazra, B.; Biswas, S.; Mandal, N. Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complement. Altern. Med. 2008, 8, 63. [Google Scholar] [CrossRef]
- Sulaiman, S.F.; Sajak, A.A.B.; Ooi, K.L.; Seow, E.M. Effect of solvents in extracting polyphenols and antioxidants of selected raw vegetables. J. Food Compos. Anal. 2011, 24, 506–515. [Google Scholar] [CrossRef]
- State Administration of Traditional Chinese Medicine (SATSACM). Traditional Chinese Meteria Medica of Dai Nationality; Shanghai Science and Technology Press: Shanghai, China, 2005; Volume 34, pp. 264–265. [Google Scholar]
- Lin, Y.F.; Yi, Z.; Zhao, Y.H. Chinese Dai Medicine Colorful Illustrations; The Nationalities Publishing House of Yunnan: Kunming, China, 2003; p. 688. [Google Scholar]
- Kirtikar, K.R.; Basu, B.D. Indian Medicinal Plants; Bishen singh Mahendra Pal Singh: Dehra Dun, India, 1975; Volume 1, pp. 672–675. [Google Scholar]
- Acharyya, S.; Dash, G.K.; Dash, S.K. Pharmacognostic studies on the root of Spondias mangifera Willd. J. Nat. Remedies 2011, 11, 150–157. [Google Scholar]
- Judprasong, K.; Charoenkiatkul, S.; Thiyajai, P.; Sukprasansap, M. Nutrients and bioactive compounds of Thai indigenous fruits. Food Chem. 2013, 140, 507–512. [Google Scholar] [CrossRef]
- Andola, H.C.; Purohit, V.K. Evaluation of nutritive and mineral value in ripe fruits of Spondias pinnata from two location of Western Himalaya, India. Med. Plants Int. J. Phytomedicines Relat. Ind. 2010, 2, 233–236. [Google Scholar] [CrossRef]
- Debnath, P.K.; Bezbaruah, B.K.; Devi, D. To evaluate the hypoglycemic effect of the fruit pulp Extract of spondias pinnata linn. Kurz on experimental Model of diabetes mellitus. Indian J. Pharmacol. 2013, 45, S74. [Google Scholar]
- Sameh, S.; Al-Sayed, E.; Labib, R.M.; Singab, A.N.B. Comparative metabolic profiling of essential oils from Spondias pinnata (Linn. F.) Kurz and characterization of their antibacterial activities. Ind. Crop. Prod. 2019, 137, 468–474. [Google Scholar] [CrossRef]
- Panda, S.K.; Mohanta, Y.K.; Padhi, L.; Park, Y.-H.; Mohanta, T.K.; Bae, H. Large scale screening of ethnomedicinal plants for identification of potential antibacterial compounds. Molecules 2016, 21, 293. [Google Scholar] [CrossRef] [PubMed]
- Daduang, J.; Vichitphan, S.; Daduang, S.; Hongsprabhas, P.; Boonsiri, P. High phenolics and antioxidants of some tropical vegetables related to antibacterial and anticancer activities. Afr. J. Pharm. Pharmacol. 2011, 5, 608–615. [Google Scholar] [CrossRef]
- Sai, K.; Thapa, R.; Devkota, H.P.; Joshi, K.R. Phytochemical screening, free fadical scavenging and alpha-amylase inhibitory activities of selected medicinal plants from western Nepal. Medicines 2019, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, D.; Ghate, N.B.; Singh, S.S.; Mandal, N. Methyl gallate isolated from Spondias pinnata exhibits anticancer activity against human glioblastoma by induction of apoptosis and sustained extracellular signal-regulated kinase 1/2 activation. Pharmacogn. Mag. 2015, 11, 269–276. [Google Scholar] [PubMed]
- Chaudhuri, D.; Ghate, N.B.; Panja, S.; Mandal, N. Role of phenolics from Spondias pinnata bark in amelioration of iron overload induced hepatic damage in Swiss albino mice. BMC Pharmacol. Toxicol. 2016, 17, 34. [Google Scholar] [CrossRef]
- Iqbal, S.S.; Mujahid, M.; Kashif, S.M.; Khalid, M.; Badruddeen Arif, M.; Bagga, P.; Akhtar, J.; Rahman, M.A. Protection of hepatotoxicity using Spondias pinnata by prevention of ethanol-induced oxidative stress, DNA-damage and altered biochemical markers in Wistar rats. Integr. Med. Res. 2016, 5, 267–275. [Google Scholar] [CrossRef]
- Ghate, N.B.; Chaudhuri, D.; Panja, S.; Singh, S.S.; Gupta, G.; Lee, C.Y.; Mandal, N. In vitro mechanistic study of the anti-inflammatory activity of a quinoline isolated from Spondias pinnata Bark. J. Nat. Prod. 2018, 81, 1956–1961. [Google Scholar] [CrossRef]
- Lukevits, E.Y.; Erchak, N.; Demicheva, L.; Verovskii, V.N.; Augustane, I. Synthesis and cytotoxicity of furfural derivatives. Pharm. Chem. J. 1992, 26, 59–63. [Google Scholar] [CrossRef]
- Hassan, S.B.; Gali-Muhtasib, H.; Goransson, H.; Larsson, R. Alpha terpineol: A potential anticancer agent which acts through suppressing NF-kappaB signalling. Anticancer Res. 2010, 30, 1911–1919. [Google Scholar]
- Muhammad, A.; Rahman, M.S.; Kabir, A.H.; Kabir, S.; Hossain, M.K. Antibacterial and cytotoxic activities of Spondias pinnata (Linn. f.) Kurz fruit extract. Indian J. Nat. Prod. 2011, 2, 265–267. [Google Scholar]
- Manik, M.K.; Islam, S.M.A.; Wahid, M.A.; Morshed, M.M.; Kamal, S.; Islam, M.S.; Ahmed, K.T. Investigation of In vitro antioxidant, antimicrobial and thrombolytic activity of the exocarp of Spondias pinnata (Anacardiaceae). Can. Chem. Trans. 2013, 1, 191–201. [Google Scholar]
- Chai, W.M.; Liu, X.; Hu, Y.H.; Feng, H.L.; Jia, Y.L.; Guo, Y.J.; Zhou, H.T.; Chen, Q.X. Antityrosinase and antimicrobial activities of furfuryl alcohol, furfural and furoic acid. Int. J. Biol. Macromol. 2013, 57, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Krist, S.; Buchbauer, G. Antimicrobial effect of vapours of geraniol, (R)-(-)-linalool, terpineol, gamma-terpinene and 1,8-cineole on airborne microbes using an airwasher. Flavour Fragr. J. 2007, 22, 435–437. [Google Scholar] [CrossRef]
- Zhou, H.E.; Tao, N.G.; Jia, L. Antifungal activity of citral, octanal and alpha-terpineol against Geotrichum citri-aurantii. Food Control 2014, 37, 277–283. [Google Scholar] [CrossRef]
- Muroi, H.; Kubo, A.; Kubo, I. Antimicrobial activity of cashew apple flavor compounds. J. Agric. Food Chem. 1993, 41, 1106–1109. [Google Scholar] [CrossRef]
- Kadariya, J.; Smith, T.C.; Thapaliya, D. Staphylococcus aureus and staphylococcal food-borne disease: An ongoing challenge in public health. Biomed Res. Int. 2014, 2014, 827965. [Google Scholar] [CrossRef]
- Latgé, J.-P. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 1999, 12, 310–350. [Google Scholar] [CrossRef]
- De Oliveira, M.G.B.; Marques, R.B.; de Santana, M.F.; Santos, A.B.D.; Brito, F.A.; Barreto, E.O.; De Sousa, D.P.; Almeida, F.R.C.; Badaue-Passos, D.; Antoniolli, A.R.; et al. Alpha-terpineol reduces mechanical hypernociception and inflammatory response. Basic Clin. Pharmacol. Toxicol. 2012, 111, 120–125. [Google Scholar]
- Andrade, L.N.; de Sousa, D.P. A review on anti-inflammatory activity of monoterpenes. Molecules 2013, 18, 1227–1254. [Google Scholar]
- Held, S.; Schieberle, P.; Somoza, V. Characterization of alpha-terpineol as an anti-inflammatory component of orange juice by in vitro studies using oral buccal cells. J. Agric. Food Chem. 2007, 55, 8040–8046. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, R.; Sun, L.; Huang, C.; Wang, C.; Zhang, D.M.; Zhang, T.T.; Du, G.H. Anti-inflammatory activity of methyl salicylate glycosides isolated from Gaultheria yunnanensis (Franch.) Rehder. Molecules 2011, 16, 3875–3884. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Akaike, T. Nitric oxide and oxygen radicals in infection, inflammation, and cancer. Biochemistry 1998, 63, 854–865. [Google Scholar] [PubMed]
- Likens, S.T.; Nickerson, G.B. Detection of Certain Hop Oil Constituents in Brewing Products. Proc. Annu. Meet. Am. Soc. Brew. Chem. 1964, 22, 5–13. [Google Scholar] [CrossRef]
- Hochmuth, D. Retention Index Guide. Available online: https://massfinder.com/wiki/Retention_index_guide (accessed on 20 September 2019).
- NIST Chemistry Web Book, NIST Standard Reference Database Number 69. Available online: http://webbook.nist.gov/chemistry/ (accessed on 23 September 2019).
- Reed, L.J.; Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- National Committee for Clinical Laboratory Standards. Methods for Determining Bactericidal Activity of Antimicrobial Agents; Approved Guideline, Document M26-A; CLSI (formerly NCCLS): Wayne, PA, USA, 1999. [Google Scholar]
- National Committee for Clinical Laboratory Standards. Minutes US NCCLS Antifungal Susceptibility Subcommittee Meeting on Interpretive Breakpoints; CLSI (formerly NCCLS): Wayne, PA, USA, 2002. [Google Scholar]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard-Seventh Edition, Document M7-A7; CLSI: Wayne, PA, USA, 2006. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing—17th Informational Supplement; Approved Standard, Document M100-S17; CLSI: Wayne, PA, USA, 2007. [Google Scholar]
- Li, R.; Hu, H.B.; Li, X.F.; Zhang, P.; Xu, Y.K.; Yang, J.J.; Wang, Y.F. Essential oils composition and bioactivities of two species leaves used as packaging materials in Xishuangbanna, China. Food Control 2015, 51, 9–14. [Google Scholar] [CrossRef]
- Chae, S.Y.; Lee, M.; Kim, S.W.; Bae, Y.H. Protection of insulin secreting cells from nitric oxide induced cellular damage by crosslinked hemoglobin. Biomaterials 2004, 25, 843–850. [Google Scholar] [CrossRef]
Sample Availability: Samples are available from the authors. |
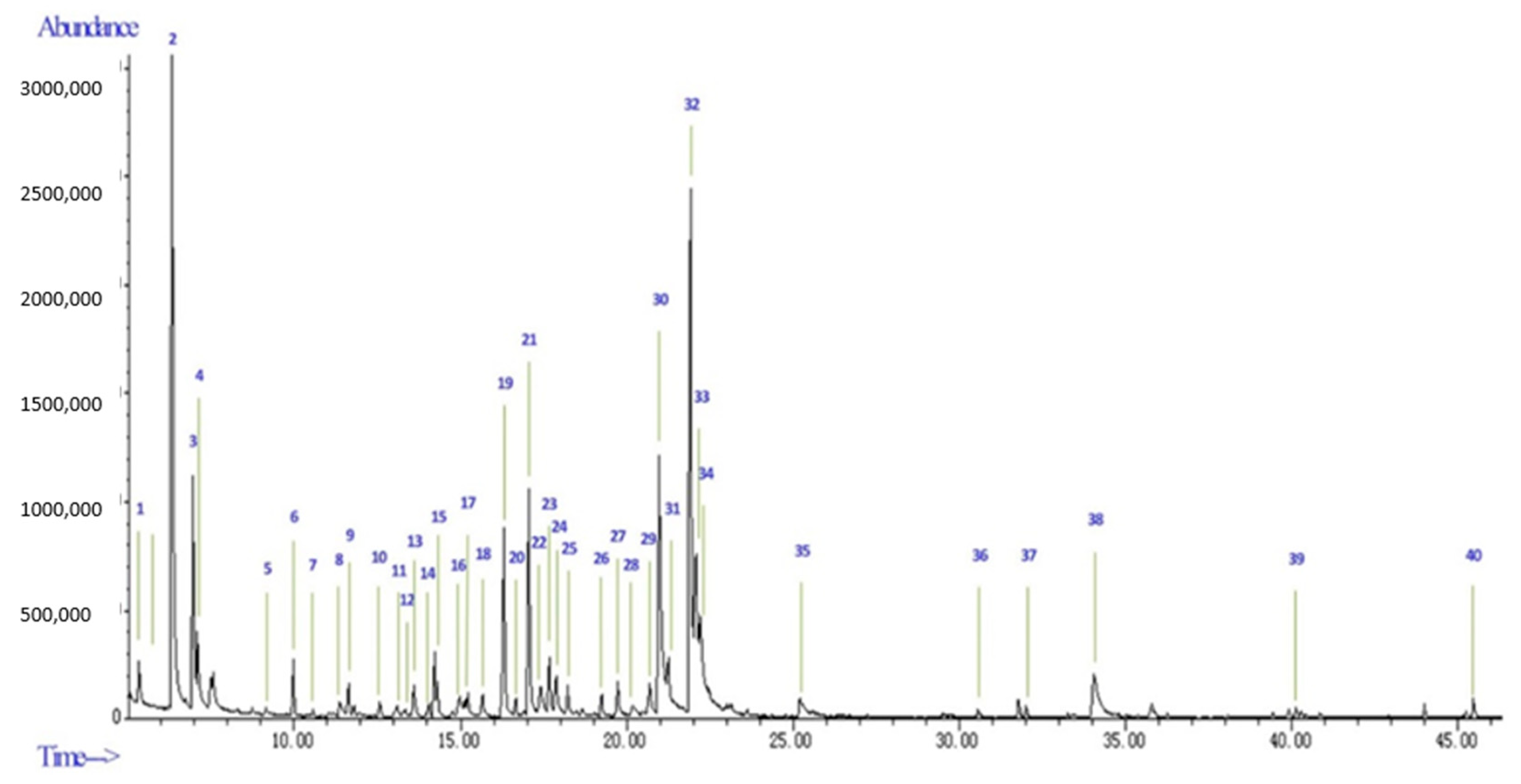
| No | RT | RI cal | RI lit | Compound | Area (%) |
|---|---|---|---|---|---|
| 1 | 5.34 | 799 | 800 | Hexanal | 0.81 |
| 2 | 6.32 | 830 | 835 | Furfural | 17.14 |
| 3 | 6.96 | 850 | 853 | 2-Hexenal | 4.17 |
| 4 | 7.09 | 854 | 855 | (Z)-3-Hexen-1-ol, | 4.88 |
| 5 | 9.16 | 914 | 918 | Ketone, 2-furyl methyl | 0.22 |
| 6 | 9.98 | 932 | 930 | α-Pinene | 0.93 |
| 7 | 10.58 | 946 | 945 | Camphene | 0.21 |
| 8 | 11.38 | 964 | 964 | 5-Methyl-2-furaldehyde | 0.57 |
| 9 | 11.81 | 974 | 978 | β-Pinene | 0.19 |
| 10 | 12.58 | 991 | 993 | β-Myrcene | 0.33 |
| 11 | 13.10 | 1003 | 1005 | 3-Carene | 0.40 |
| 12 | 13.34 | 1008 | 1008 | (3E)-Hexenyl acetate | 0.28 |
| 13 | 13.60 | 1014 | 1016 | Isocineole | 0.89 |
| 14 | 14.05 | 1023 | 1025 | p-Cymene | 0.32 |
| 15 | 14.22 | 1027 | 1028 | Limonene | 2.04 |
| 16 | 14.99 | 1043 | 1043 | Benzeneacetaldehyde | 0.76 |
| 17 | 15.23 | 1048 | 1048 | (E)-β-Ocimene | 0.99 |
| 18 | 15.67 | 1058 | 1060 | γ-Terpinene | 0.58 |
| 19 | 16.31 | 1072 | 1068 | Linalool oxide | 4.38 |
| 20 | 16.67 | 1079 | 1078 | 2-Furaldehyde diethyl acetal | 0.39 |
| 21 | 17.06 | 1087 | 1088 | (E)-Linalool oxide, furanoid | 5.33 |
| 22 | 17.41 | 1095 | 1092 | Benzoic acid, methyl ester | 1.25 |
| 23 | 17.68 | 1101 | 1103 | Linalool | 1.45 |
| 24 | 17.88 | 1105 | 1105 | Nonanal | 1.28 |
| 25 | 18.24 | 1112 | 1110 | 2-Fenchanol | 0.68 |
| 26 | 19.25 | 1134 | 1138 | 1-Terpineol | 0.52 |
| 27 | 19.74 | 1144 | 1149 | β-Terpineol | 0.94 |
| 28 | 20.19 | 1154 | 1153 | Ocimenol | 0.44 |
| 29 | 20.71 | 1164 | 1163 | Isoborneol | 1.04 |
| 30 | 20.99 | 1170 | 1072 | Ethyl benzoate | 9.05 |
| 31 | 21.25 | 1176 | 1177 | Terpinen-4-ol | 2.66 |
| 32 | 21.92 | 1190 | 1190 | α-Terpineol | 13.09 |
| 33 | 22.08 | 1193 | 1197 | Methyl salicylate | 5.88 |
| 34 | 22.22 | 1196 | 1201 | γ-Terpineol | 5.55 |
| 35 | 25.23 | 1262 | 1260 | (E)-2-Decenal | 0.78 |
| 36 | 30.57 | 1382 | 1388 | β-(E)-Damascenone | 0.26 |
| 37 | 32.00 | 1416 | 1418 | Caryophyllene | 0.33 |
| 38 | 34.04 | 1467 | 1462 | Ethyl cinnamate | 3.55 |
| 39 | 39.90 | 1637 | 1635 | γ-Eudesmole | 0.17 |
| 40 | 45.46 | 1996 | 1999 | Ethyl hexadecanoate | 0.44 |
| Total identified | 95.19 | ||||
| Aliphatic alcohols | 39.42 | ||||
| Monoterpene hydrocarbons | 29.62 | ||||
| Aromatics | 22.03 | ||||
| Oxygenated monoterpenes | 3.62 | ||||
| Sesquiterpene hydrocarbons | 0.50 | ||||
| Compound | HL-60 | SMMC-7721 | A-549 | MCF-7 | SW480 |
|---|---|---|---|---|---|
| EOSP | 13.29 | 44.67 | 34.43 | 48.60 | 50.21 |
| Cisplatin | 0.50 | 2.08 | 2.22 | 3.25 | 2.97 |
| Microbial Strain | EOSP | Positive Control b | |||
|---|---|---|---|---|---|
| Gram-positive bacteria | MIC | MBC/MFC | MIC | MBC/MFC | |
| S. aureus | 512 | 512 | 0.25 | 0.5 | |
| Gram-negative bacteria | A. baumannii | 512 | >512 | 0.5 | 1 |
| E. coli | >512 | ND | 0.05 | 0.25 | |
| K. pneumonia | >512 | ND | 256 | 512 | |
| P. aeruginosa | 128 | 128 | 0.25 | 1 | |
| Fungi | A. fumigatus | 16 | 32 | 512 | 1024 |
| C. albicans | 128 | 256 | 0.5 | 1 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Yang, J.-J.; Song, X.-Z.; Wang, Y.-F.; Corlett, R.T.; Xu, Y.-K.; Hu, H.-B. Chemical Composition and the Cytotoxic, Antimicrobial, and Anti-Inflammatory Activities of the Fruit Peel Essential Oil from Spondias pinnata (Anacardiaceae) in Xishuangbanna, Southwest China. Molecules 2020, 25, 343. https://doi.org/10.3390/molecules25020343
Li R, Yang J-J, Song X-Z, Wang Y-F, Corlett RT, Xu Y-K, Hu H-B. Chemical Composition and the Cytotoxic, Antimicrobial, and Anti-Inflammatory Activities of the Fruit Peel Essential Oil from Spondias pinnata (Anacardiaceae) in Xishuangbanna, Southwest China. Molecules. 2020; 25(2):343. https://doi.org/10.3390/molecules25020343
Chicago/Turabian StyleLi, Ren, Jing-Jing Yang, Xing-Zhen Song, Yuan-Fei Wang, Richard T. Corlett, You-Kai Xu, and Hua-Bin Hu. 2020. "Chemical Composition and the Cytotoxic, Antimicrobial, and Anti-Inflammatory Activities of the Fruit Peel Essential Oil from Spondias pinnata (Anacardiaceae) in Xishuangbanna, Southwest China" Molecules 25, no. 2: 343. https://doi.org/10.3390/molecules25020343
APA StyleLi, R., Yang, J.-J., Song, X.-Z., Wang, Y.-F., Corlett, R. T., Xu, Y.-K., & Hu, H.-B. (2020). Chemical Composition and the Cytotoxic, Antimicrobial, and Anti-Inflammatory Activities of the Fruit Peel Essential Oil from Spondias pinnata (Anacardiaceae) in Xishuangbanna, Southwest China. Molecules, 25(2), 343. https://doi.org/10.3390/molecules25020343







