Application of Conducting Polymer Nanostructures to Electrochemical Biosensors
Abstract
1. Introduction
2. NSCP-Integrated Electrochemical Biosensors
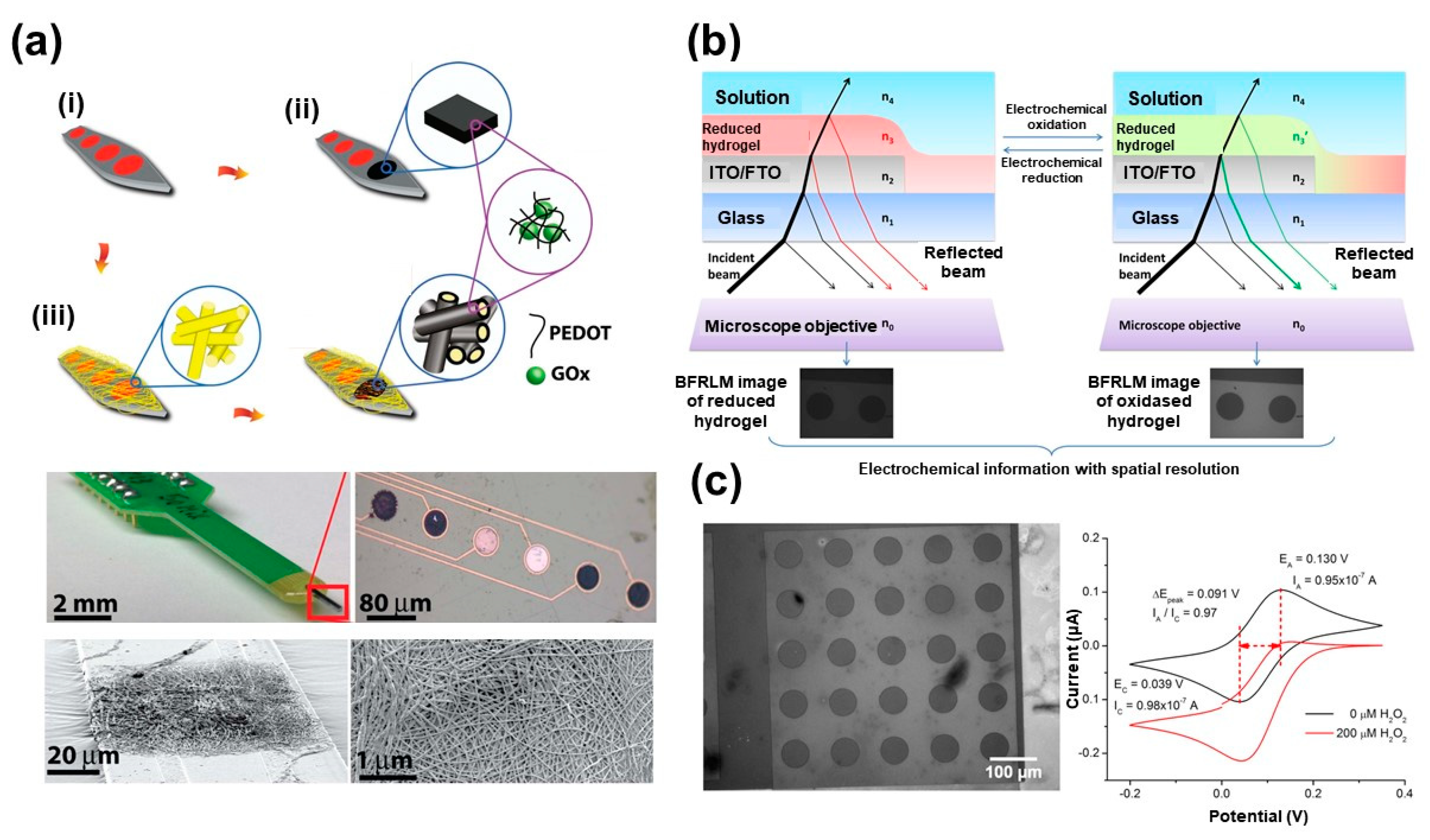
2.1. Nscps for Electrochemical Detection of Glucose and H2O2
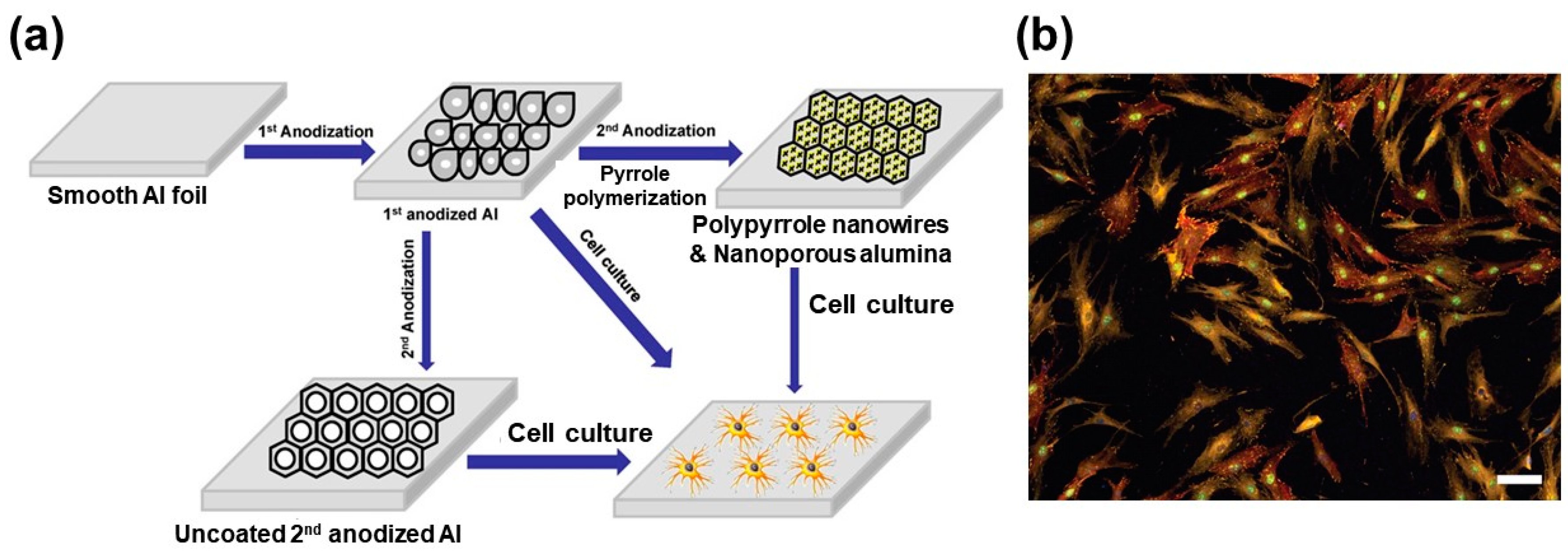
2.2. Nscps for Cell-Based Chip Applications
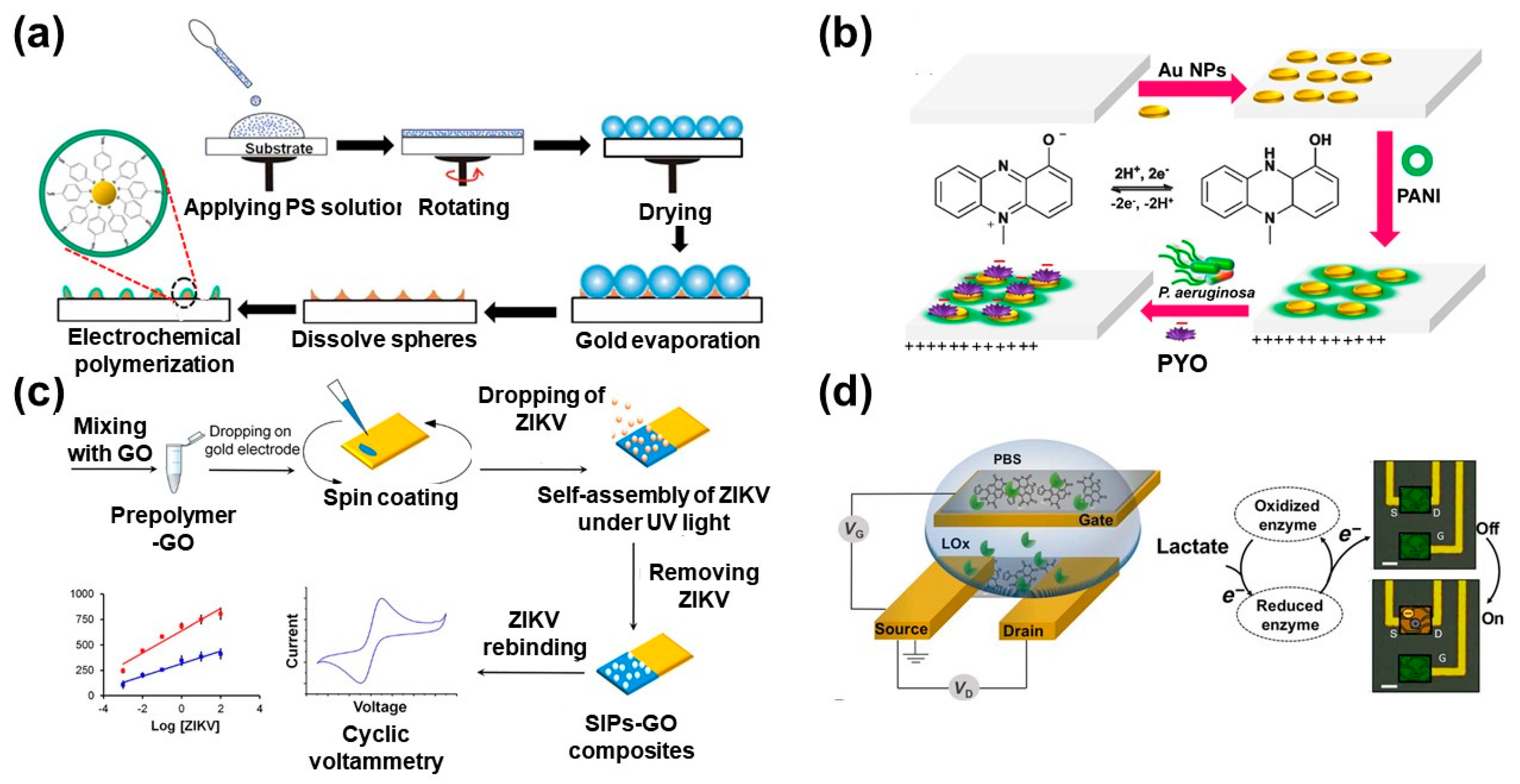
2.3. Nscps for Different Biosensor Applications
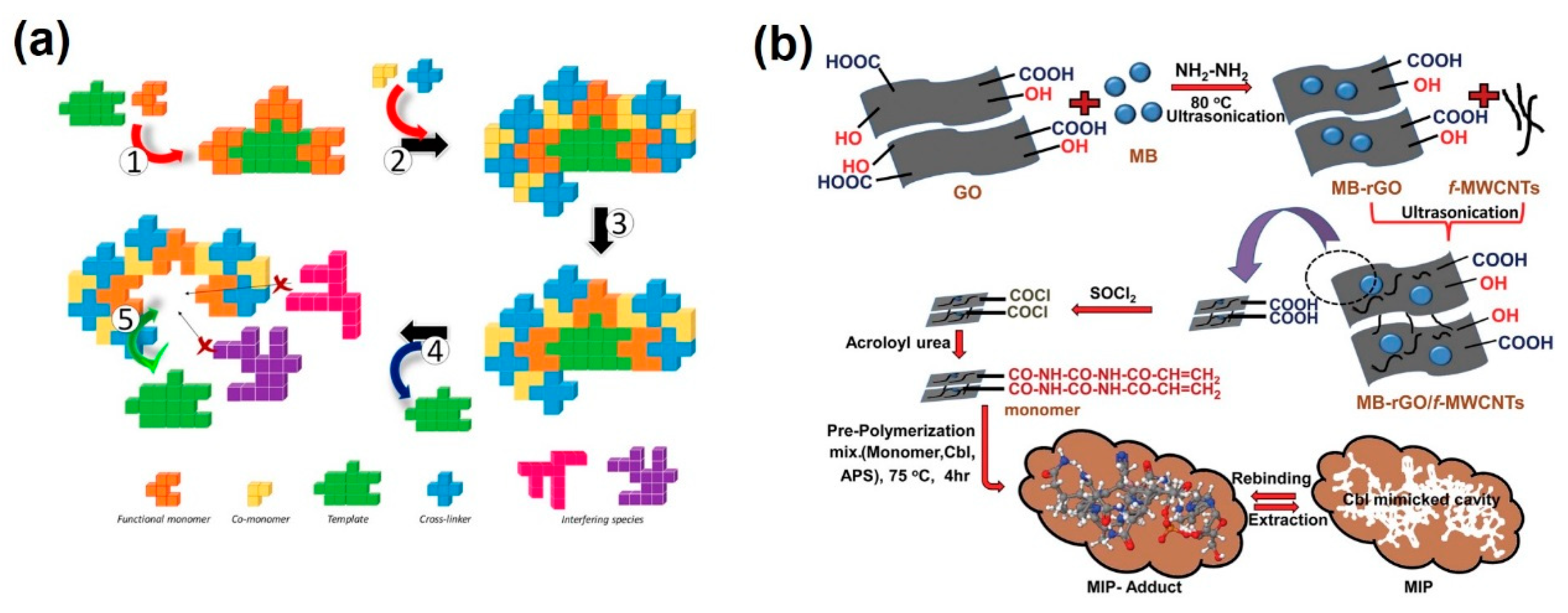
3. Molecularly Imprinted Polymers as Selective Biosensors
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, Y.; Kim, A.; Wan, G.; Tee, B.C. Design and applications of stretchable and self-healable conductors for soft electronics. Nano Converg. 2019, 6, 25. [Google Scholar] [CrossRef]
- Navya, P.N.; Kaphle, A.; Srinivas, S.P.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019, 6, 23. [Google Scholar] [CrossRef]
- Kim, J.Y.; O’Hare, D. Monolithic nano-porous polymer in microfluidic channels for lab-chip liquid chromatography. Nano Converg. 2018, 5, 19. [Google Scholar] [CrossRef]
- Soylemez, S.; Kanik, F.E.; Uzun, S.D.; Hacioglu, S.O.; Toppare, L. Development of an efficient immobilization matrix based on a conducting polymer and functionalized multiwall carbon nanotubes: Synthesis and its application to ethanol biosensors. J. Mater. Chem. B 2014, 2, 511–521. [Google Scholar] [CrossRef]
- Dhand, C.; Singh, S.P.; Arya, S.K.; Datta, M.; Malhotra, B.D. Cholesterol biosensor based on electrophoretically deposited conducting polymer film derived from nano-structured polyaniline colloidal suspension. Anal. Chim. Acta 2007, 602, 244–251. [Google Scholar] [CrossRef]
- Aydemir, N.; Malmström, J.; Travas-Sejdic, J. Conducting Polymer Based Electrochemical Biosensors. Phys. Chem. Chem. Phys. 2016, 18, 8264–8277. [Google Scholar] [CrossRef] [PubMed]
- Solanki, P.R.; Kaushik, A.; Ansari, A.A.; Tiwari, A.; Malhotra, B.D. Multi-walled carbon nanotubes/sol–gel-derived silica/chitosan nanobiocomposite for total cholesterol sensor. Sens. Actuators B 2009, 137, 727–735. [Google Scholar] [CrossRef]
- Park, Y.M.; Lim, S.Y.; Jeong, S.W.; Song, Y.; Bae, N.H.; Hong, S.B.; Choi, B.G.; Lee, S.J.; Lee, K.G. Flexible nanopillar-based electrochemical sensors for genetic detection of foodborne pathogens. Nano Converg. 2018, 5, 15. [Google Scholar] [CrossRef]
- Nandi, M.A.; Gangopadhyay, R.B.; Bhaumik, A. Mesoporous polyaniline having high conductivity at room temperature. Microporous Mesoporous Mater. 2007, 109, 239. [Google Scholar] [CrossRef]
- Moulton, S.E.; Innis, P.C.; Kane-Maguire, L.A.P.; Ngamna, O.; Wallace, G.G. Polymerisation and characterisation of conducting polyaniline nanoparticle dispersions. Curr. Appl. Phys. 2004, 4, 402–406. [Google Scholar] [CrossRef]
- Mazur, M.; Tagowska, M.; Pałys, B.; Jackowska, K. Template synthesis of polyaniline and poly (2-methoxyaniline) nanotubes: Comparison of the formation mechanisms. Electrochem. Commun. 2003, 5, 403. [Google Scholar] [CrossRef]
- Huang, J.; Kaner, R.B. A General Chemical Route to Polyaniline Nanofibers. J. Am. Chem. Soc. 2004, 126, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.P.; Gopalan, A.; Komathi, S.; Raghupathy, D. Polyaniline-based nanocomposites: Preparation, properties and applications. In Physical Properties and Applications of Polymer Nanocomposites, 1st ed.; Tjong, S.C., Mai, Y.-W., Eds.; Woodhead Publishing: Cambridge, UK, 2010. [Google Scholar]
- Sawall, D.D.; Villahermosa, R.M.; Lipeles, R.A.; Hopkins, A.R. Interfacial Polymerization of Polyaniline Nanofibers Grafted to Au Surfaces. Chem. Mater. 2004, 16, 1606. [Google Scholar] [CrossRef]
- Saini, D.; Chauhan, R.; Basu, T. Fabrication of biosensors based on nanostructured conducting polymer (NSCP). Int. Res. J. Biotechnol. 2011, 2, 145–156. [Google Scholar]
- Che, S.; Bennett, A.E.G.; Yokoi, T.; Sakamoto, K.; Kunieda, H.; Terasaki, O.; Tatsumi, T. A novel anionic surfactant templating route for synthesizing mesoporous silica with unique structure. Nat. Mater. 2003, 2, 801–805. [Google Scholar] [CrossRef]
- Moreno, K.J.; Moggio, I.; Arias, E.; Llarena, I.; Moya, S.E.; Ziolo, R.F.; Barrientos, H. Silver nanoparticles functionalized in situ with the conjugated polymer (PEDOT: PSS). J. Nanosci. Nanotechnol 2009, 9, 3987–3992. [Google Scholar] [CrossRef]
- Melendez, R.G.; Moreno, K.J.; Moggio, I.; Arias, E.; Ponce, A.; Llanera, I.; Moya, S.E. On the influence of silver nanoparticles size in the electrical conductivity of PEDOT:PSS. Mater. Sci. Forum 2010, 644, 85–90. [Google Scholar] [CrossRef]
- Hussain, I.; Brust, M.; Papworth, A.J.; Cooper, A.I. Preparation of acrylate-stabilized gold and silver hydrosols and gold-polmer composite films. Langmuir 2003, 19, 4831–4835. [Google Scholar] [CrossRef]
- Cong, H.; Radosz, M.; Towler, B.F.; Shen, Y. Polymer-inorganic nanocomposite membranes for gas separation. Sep. Purif. Technol. 2007, 55, 281–291. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, T.; Zhang, W.; Jiang, C.; Jiao, K. Enhanced sensitivity for deoxyribonucleic acid electrochemical impedance sensor: Gold nanoparticle/polyaniline nanotube membranes. Anal. Chim. Acta. 2008, 616, 144–151. [Google Scholar] [CrossRef]
- Balamurugan, A.; Chen, S.M. Silver nanograins incorporated PEDOT modified electrode for electrocatalytic sensing of hydrogen peroxide. Electroanalysis 2009, 21, 1419–1423. [Google Scholar] [CrossRef]
- Paulraj, P.; Janaki, N.; Sandhya, S.; Pandian, K. Single pot synthesis of polyaniline protected silver nanoparticles by interfacial polymerization and study its application on electrochemical oxidation of hydrazine. Colloids Surf. A. 2011, 377, 28–34. [Google Scholar] [CrossRef]
- Dutse, S.W.; Yusof, N.A.; Ahmad, H.; Hussein, M.Z.; Zainal, Z. An electrochemical DNA biosensor for Ganodermaboninense pathogen of oil palm utilizing a new ruthenium complex, [Ru(dppz)2(qtpy)]Cl2. Int. J. Electrochem. Sci. 2012, 7, 8105–8115. [Google Scholar]
- Yang, S.; Li, G.; Wang, D.; Qiao, Z.; Qu, L. Synthesis of nanoneedle-like copper oxide on N-doped reduced graphene oxide: A three-dimensional hybrid for nonenzymatic glucose sensor. Sens. Actuator B 2017, 238, 588–595. [Google Scholar] [CrossRef]
- Zhu, H.; Li, L.; Zhou, W.; Shao, Z.; Chen, X. Advances in non-enzymatic glucose sensors based on metal oxides. J. Mater. Chem. B 2016, 4, 7333–7349. [Google Scholar] [CrossRef]
- Elkholy, A.E.; Heakal, F.E.-T.; El-Said, W.A. Improving the electrocatalytic performance of Pd nanoparticles supported on indium/tin oxide substrates towards glucose oxidation. Appl. Catal. A: Gen. 2019, 580, 28–33. [Google Scholar] [CrossRef]
- Lee, J.-H.; El-Said, W.A.; Oh, B.-K.; Choi, J.-W. Enzyme-free glucose sensor based on Au nanobouquet fabricated indium tin oxide electrode. J. Nanosci. Nanotechnol. 2014, 14, 8432–8438. [Google Scholar] [CrossRef]
- Salazar, P.; Rico, V.; González-Elipe, A.R. Nickel-copper bilayer nanoporous electrode prepared by physical vapor deposition at oblique angles for the non-enzymatic determination of glucose. Sens. Actuator B 2016, 226, 436–443. [Google Scholar] [CrossRef]
- Yang, G.; Kampstra, K.L.; Abidian, M.R. High-Performance Conducting Polymer Nanofiber Biosensors for Detection of Biomolecules. Adv. Mater. 2014. [Google Scholar] [CrossRef]
- Soganci, T.; Soyleyici, H.C.; Demirko, D.O.; Ak, M.; Timur, S. Use of Super-Structural Conducting Polymer as Functional Immobilization Matrix in Biosensor Design. J. Electrochem. Soc. 2018, 165, B22–B26. [Google Scholar] [CrossRef]
- Munteanu, R.-E.; Ye, R.; Polonschii, C.; Ruff, A.; Gheorghiu, M.; Gheorghiu, E.; Boukherroub, R.; Schuhmann, W.; Melinte, S.; Gáspár, S. High spatial resolution electrochemical biosensing using reflected light microscopy. Sci. Rep. 2019, 9, 15196. [Google Scholar] [CrossRef] [PubMed]
- Yea, C.-H.; Lee, B.; Kim, H.-H.; Kim, S.-U.; El-Said, W.A.; Minc, J.-H.; Oh, B.-K.; Choi, J.-W. The immobilization of animal cells using the cysteine-modified RGD oligopeptide. Ultramicroscopy 2008, 108, 1144–1147. [Google Scholar] [CrossRef] [PubMed]
- El-Said, W.A.; Yea, C.-H.; Kwon, Il-K.; Choi, J.-W. Fabrication of Electrical Cell Chip for the Detection of Anticancer Drugs and Environmental Toxicants Effect. Biochip. J. 2009, 3, 105–112. [Google Scholar]
- El-Said, W.A.; Yea, C.-H.; Choi, J.-W.; Kwon, Il-K. Ultrathin polyaniline film coated on an indium–tin oxide cell-based chip for study of anticancer effect. Thin Solid Film. 2009, 518, 661–667. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, J.-W.; Schmidt, C.E. Neuroactive conducting scaffolds: Nerve growth factor conjugation on active ester-functionalized polypyrrole. J. R. Soc. Interface 2009, 6, 801–810. [Google Scholar] [CrossRef]
- El-Said, W.A.; Yea, C.-H.; Jung, M.; Kim, H.-C.; Choi, J.-W. Analysis of effect of nanoporous alumina substrate coated with polypyrrole nanowire on cell morphology based on AFM topography. Ultramicroscopy 2010, 110, 676–681. [Google Scholar] [CrossRef]
- Strover, L.T.; Malmström, J.; Laita, O.; Reynisson, J.; Aydemir, N.; Nieuwoudt, M.K.; Williams, D.E.; Dunbar, P.R.; Brimble, M.A.; Travas-Sejdic, J. A new precursor for conducting polymer-based brush interfaces with electroactivity in aqueous solution. Polymer 2013, 54, 1305–1317. [Google Scholar] [CrossRef]
- El-Said, W.A.; Choi, J.-W. Electrochemical Biosensor consisted of conducting polymer layer on gold nanodots patterned Indium Tin Oxide electrode for rapid and simultaneous determination of purine bases. Electrochim. Acta 2014, 123, 51–57. [Google Scholar] [CrossRef]
- Aksoy, B.; Paşahan, A.; Güngör, Ö.; Köytepe, S.; Seçkin, T. A novel electrochemical biosensor based on polyimide-boron nitride composite membranes. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 203–212. [Google Scholar] [CrossRef]
- El-Said, W.A.; Yea, C.-H.; Kim, H.; Choi, J.-W. Fabrication of self-assembled RGD layer for cell chip to detect anticancer drug effect on HepG2 cells. Curr. Appl. Phys. 2009, 9, e76–e80. [Google Scholar] [CrossRef]
- Elkhawaga, A.A.; Khalifa, M.M.; El-badawy, O.; Hassan, M.A.; El-Said, W.A. Rapid and highly sensitive detection of pyocyanin biomarker in different Pseudomonas aeruginosa infections using gold nanoparticles modified sensor. PLoS ONE 2019. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, M.M.; Elkhawaga, A.A.; Hassan, M.A.; Zahran, A.M.; Fathalla, A.M.; El-Said, W.A.; El-Badawy, O. Highly specific Electrochemical Sensing of Pseudomonas aeruginosa in patients suffering from corneal ulcers: A comparative study. Sci. Rep. 2019, 9, 18320. [Google Scholar] [CrossRef] [PubMed]
- Tancharoen, C.; Sukjee, W.; Thepparit, C.; Jaimipuk, T.; Auewarakul, P.; Thitithanyanont, A.; Sangma, C. Electrochemical Biosensor Based on Surface Imprinting for Zika Virus Detection in Serum. Acs Sens. 2019, 4, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Pappa, A.M.; Ohayon, D.; Giovannitti, A.; Maria, I.P.; Savva, A.; Uguz, I.; Rivnay, J.; McCulloch, I.; Owens, R.M.; Inal, S. Direct metabolite detection with an n-type accumulation mode organic electrochemical transistor. Sci Adv. 2018, 4, eaat0911. [Google Scholar] [CrossRef] [PubMed]
- Rico-Yuste, A.; Carrasco, S. Molecularly Imprinted Polymer-Based Hybrid Materials for the Development of Optical Sensors. Polymers 2019, 11, 1173. [Google Scholar] [CrossRef] [PubMed]
- Öndeş¸, B.; Soysal, M. Determination of Diuron by Using Electrochemical Sensor Based on Molecularly Imprinted Polymer Film. J. Electrochem. Soc. 2019, 166, B395–B401. [Google Scholar] [CrossRef]
- Essousi, H.; Barhoumi, H.; Bibani, M.; Ktari, N.; Wendler, F.; Al-Hamry, A.; Kanoun, O. Ion-Imprinted Electrochemical Sensor Based on Copper Nanoparticles-Polyaniline Matrix for Nitrate Detection. J. Sens. 2019, 2019. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, X.; Zeng, Y.; Wang, H.; Lu, Y.; Zhang, J.; Yin, Z.; Chen, Z.; Yang, Y.; Li, L. An Electrochemical Sensor for Diphenylamine Detection Based on Reduced Graphene Oxide/Fe3O4-Molecularly Imprinted Polymer with 1,4-Butanediyl-3,30-bis-l-vinylimidazolium Dihexafluorophosphate Ionic Liquid as Cross-Linker. Polymers 2018, 10, 1329. [Google Scholar] [CrossRef]
- Samari, F.; Hemmateenejad, B.; Rezaei, Z.; Shamsipur, M. A Novel Approach for Rapid Determination of Vitamin B12 in Pharmaceutical preparations using BSA-modified Gold. Anal. Methods 2012, 4, 4155–4160. [Google Scholar] [CrossRef]
- Nakos, M.; Pepelanova, I.; Beutel, S.; Krings, U.; Berger, R.G.; Scheper, T. Isolation and Analysis of Vitamin B12 from Plant Samples. Food Chem. 2017, 216, 301–308. [Google Scholar] [CrossRef]
- Singh, R.; Jaiswal, S.; Singh, K.; Fatma, S.; Prasad, B.B. Biomimetic Polymer-Based Electrochemical Sensor Using Methyl Blue-Adsorbed Reduced Graphene Oxide and Functionalized Multiwalled Carbon Nanotubes for Trace Sensing of Cyanocobalamin. ACS Appl. Nano Mater. 2018, 1, 4652–4660. [Google Scholar] [CrossRef]
- Langford, K.H.; Scrimshaw, M.D.; Birkett, J.W.; Lester, J.N. Degradation of nonylphenolic surfactants in activated sludge batch tests. Water Res. 2005, 39, 870–876. [Google Scholar] [CrossRef] [PubMed]
- El-Shafei, G.M.S.; Yehia, F.Z.; Eshaq, G.; Elmetwally, A.E. Enhanced degradation of nonylphenol at neutral pH by ultrasonic assisted- heterogeneous Fenton using nano zero valent metals. Sep. Purif. Technol. 2017, 178, 122–129. [Google Scholar] [CrossRef]
- Shan, J.; Jiang, B.Q.; Yu, B.; Li, C.L.; Sun, Y.Y.; Guo, H.Y.; Wu, J.C.; Klumpp, E.; Schäffer, A.; Ji, R. Isomer-Specific Degradation of Branched and Linear 4-Nonylphenol Isomers in an Oxic Soil. Env.. Sci. Technol. 2011, 45, 8283–8289. [Google Scholar] [CrossRef]
- Liu, B.; Yan, J.; Wang, M.; Wu, X. Electrochemical Sensor Based on Molecularly Imprinted Polymer for Determination of Nonylphenol. Int. J. Electrochem. Sci. 2018, 13, 11953–11960. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Said, W.A.; Abdelshakour, M.; Choi, J.-H.; Choi, J.-W. Application of Conducting Polymer Nanostructures to Electrochemical Biosensors. Molecules 2020, 25, 307. https://doi.org/10.3390/molecules25020307
El-Said WA, Abdelshakour M, Choi J-H, Choi J-W. Application of Conducting Polymer Nanostructures to Electrochemical Biosensors. Molecules. 2020; 25(2):307. https://doi.org/10.3390/molecules25020307
Chicago/Turabian StyleEl-Said, Waleed A., Muhammad Abdelshakour, Jin-Ha Choi, and Jeong-Woo Choi. 2020. "Application of Conducting Polymer Nanostructures to Electrochemical Biosensors" Molecules 25, no. 2: 307. https://doi.org/10.3390/molecules25020307
APA StyleEl-Said, W. A., Abdelshakour, M., Choi, J.-H., & Choi, J.-W. (2020). Application of Conducting Polymer Nanostructures to Electrochemical Biosensors. Molecules, 25(2), 307. https://doi.org/10.3390/molecules25020307






