Oxidations of Benzhydrazide and Phenylacetic Hydrazide by Hexachloroiridate(IV): Reaction Mechanism and Structure–Reactivity Relationship
Abstract
1. Introduction
2. Results
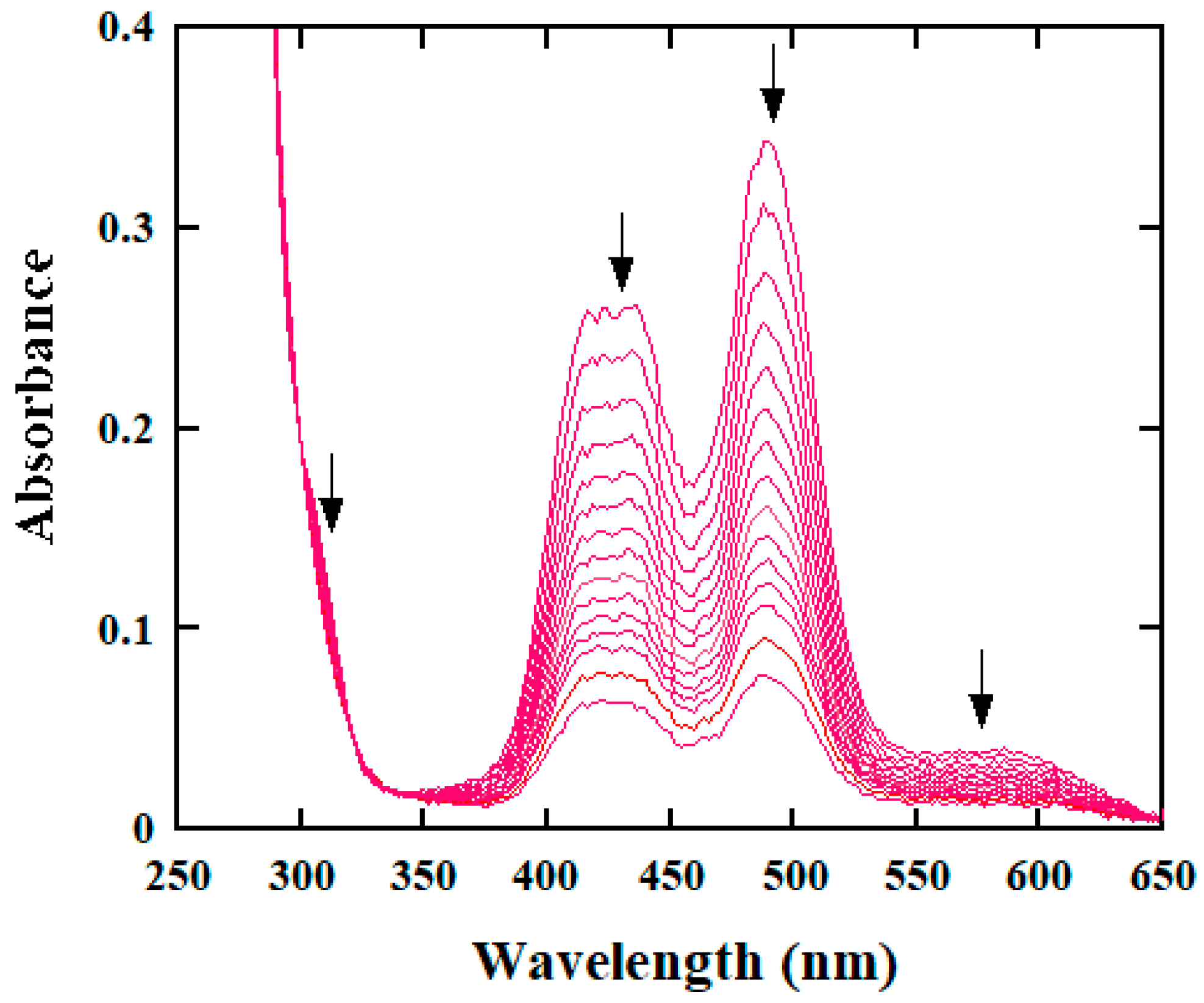
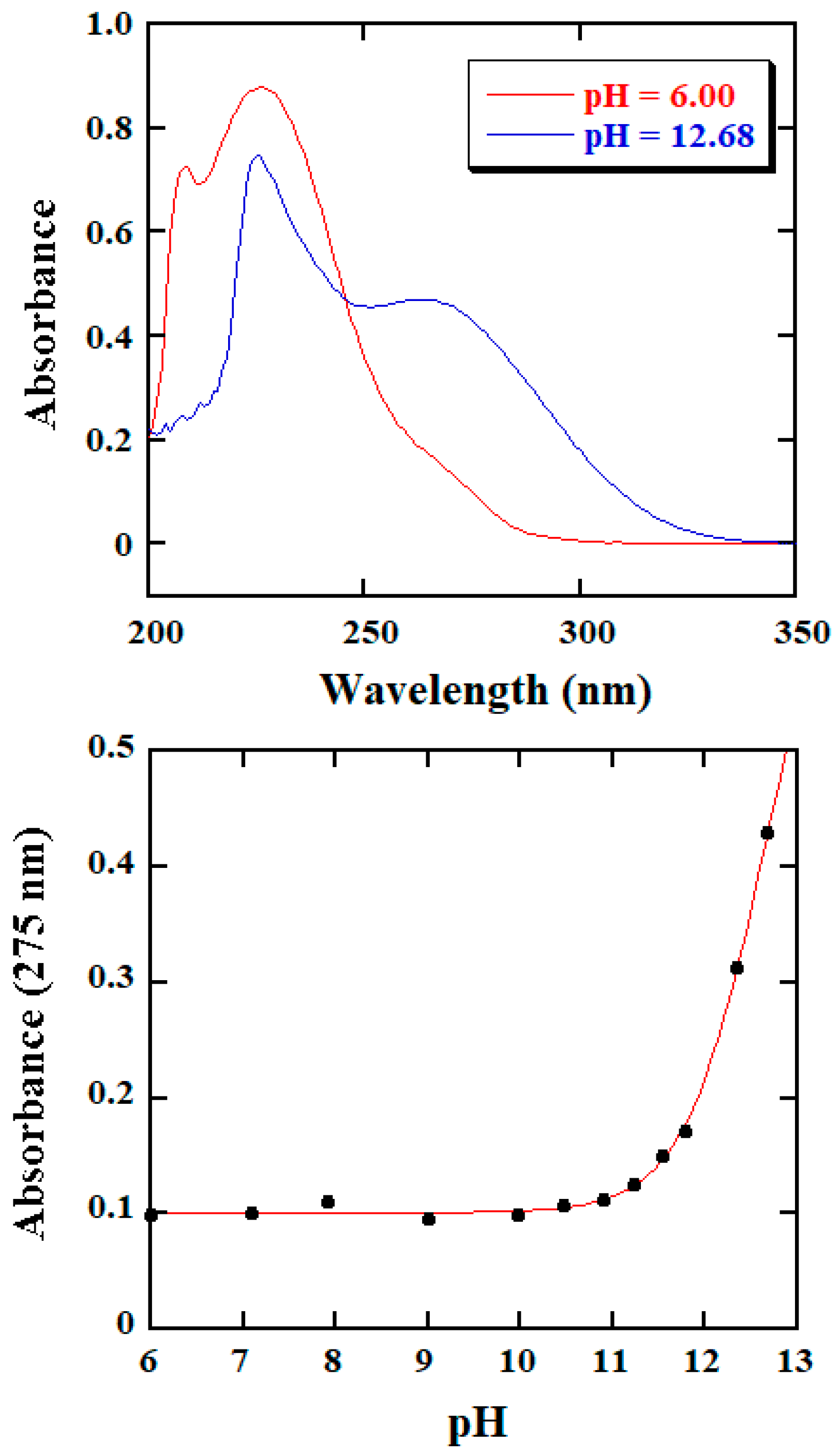
2.1. Spectral Analysis of Reaction Course
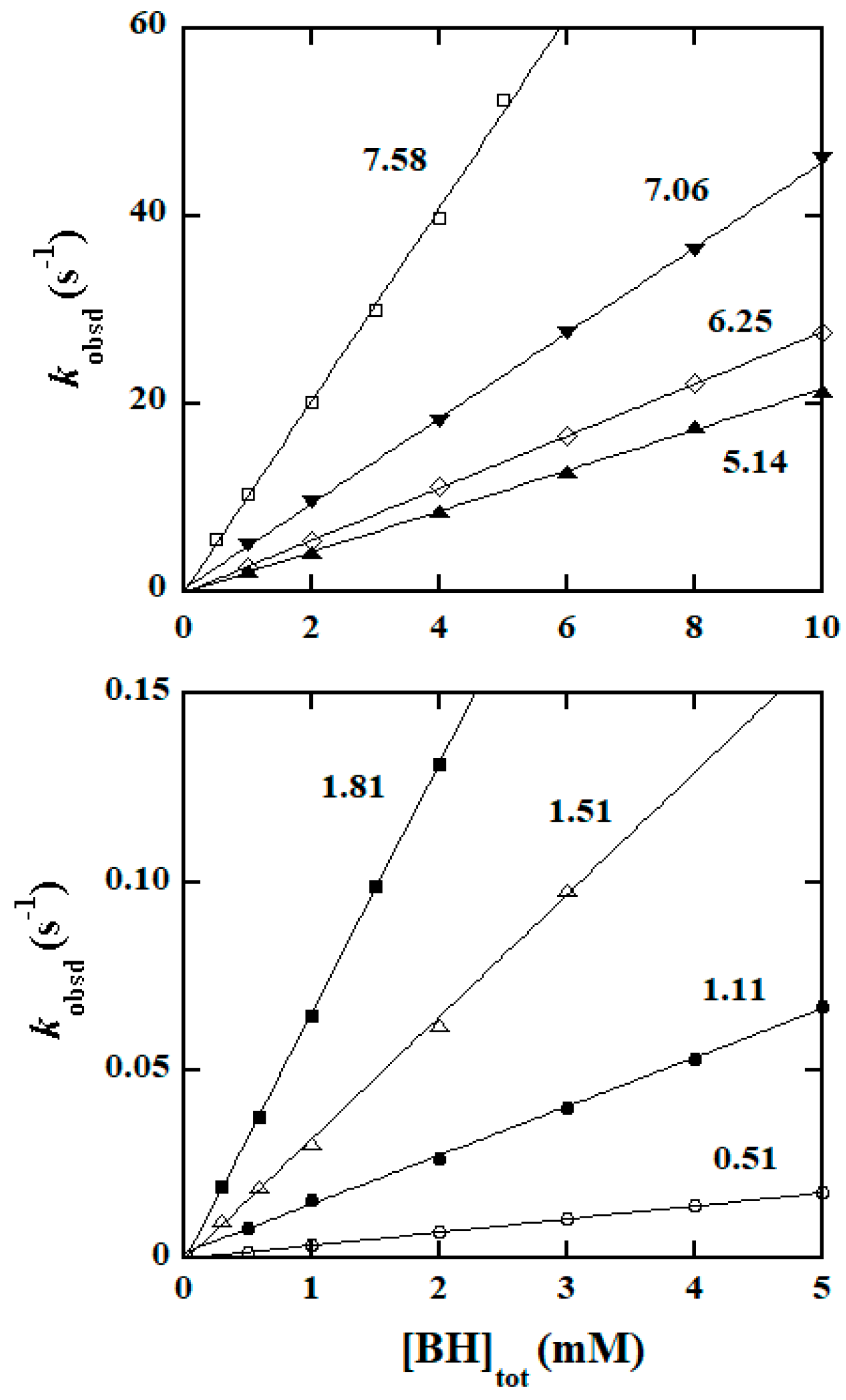
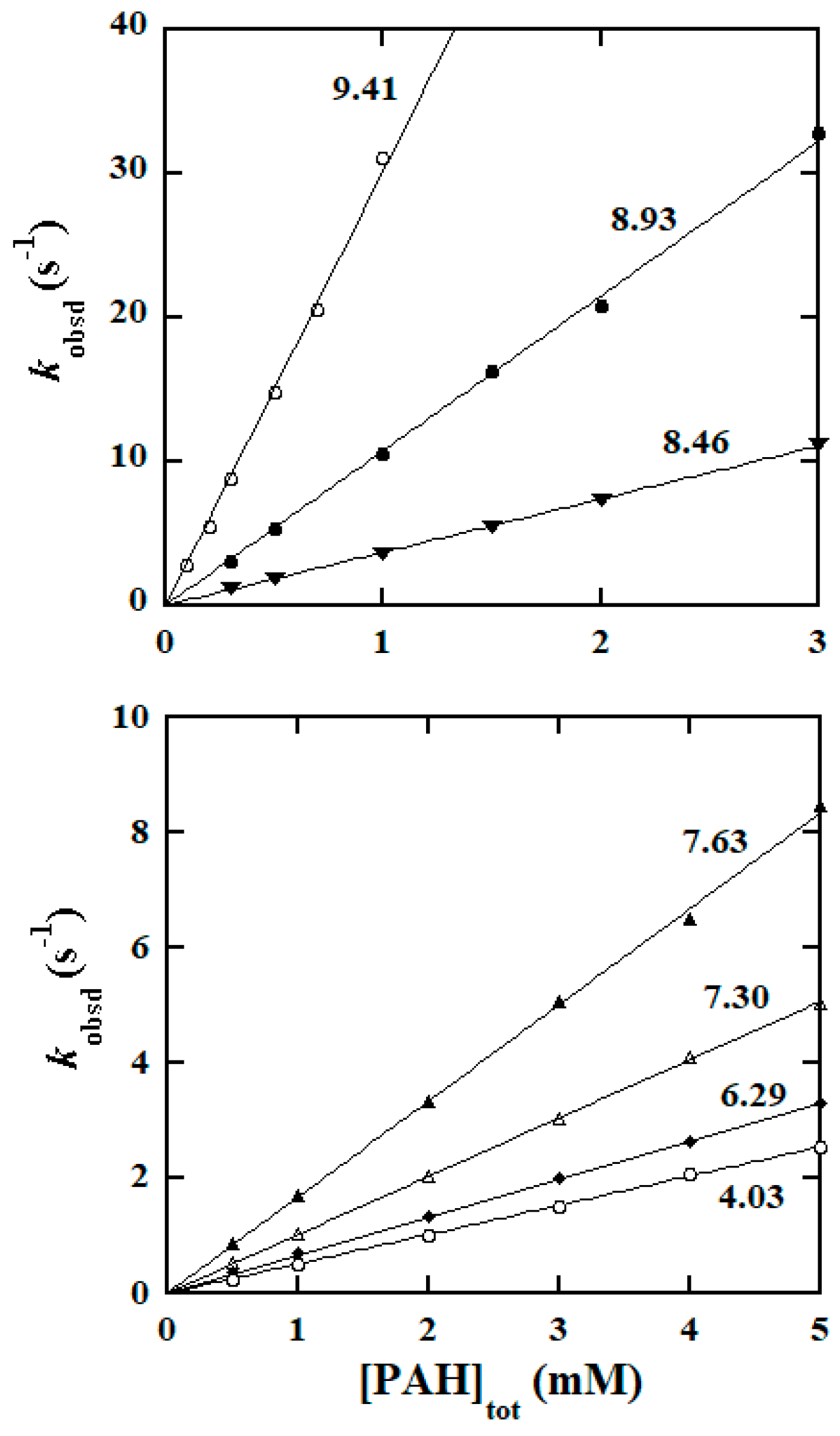
2.2. Empirical Rate Law and Kinetic Data Collection
2.3. Evaluation of the Reaction Stoichiometry
2.4. Product Analysis
3. Discussion
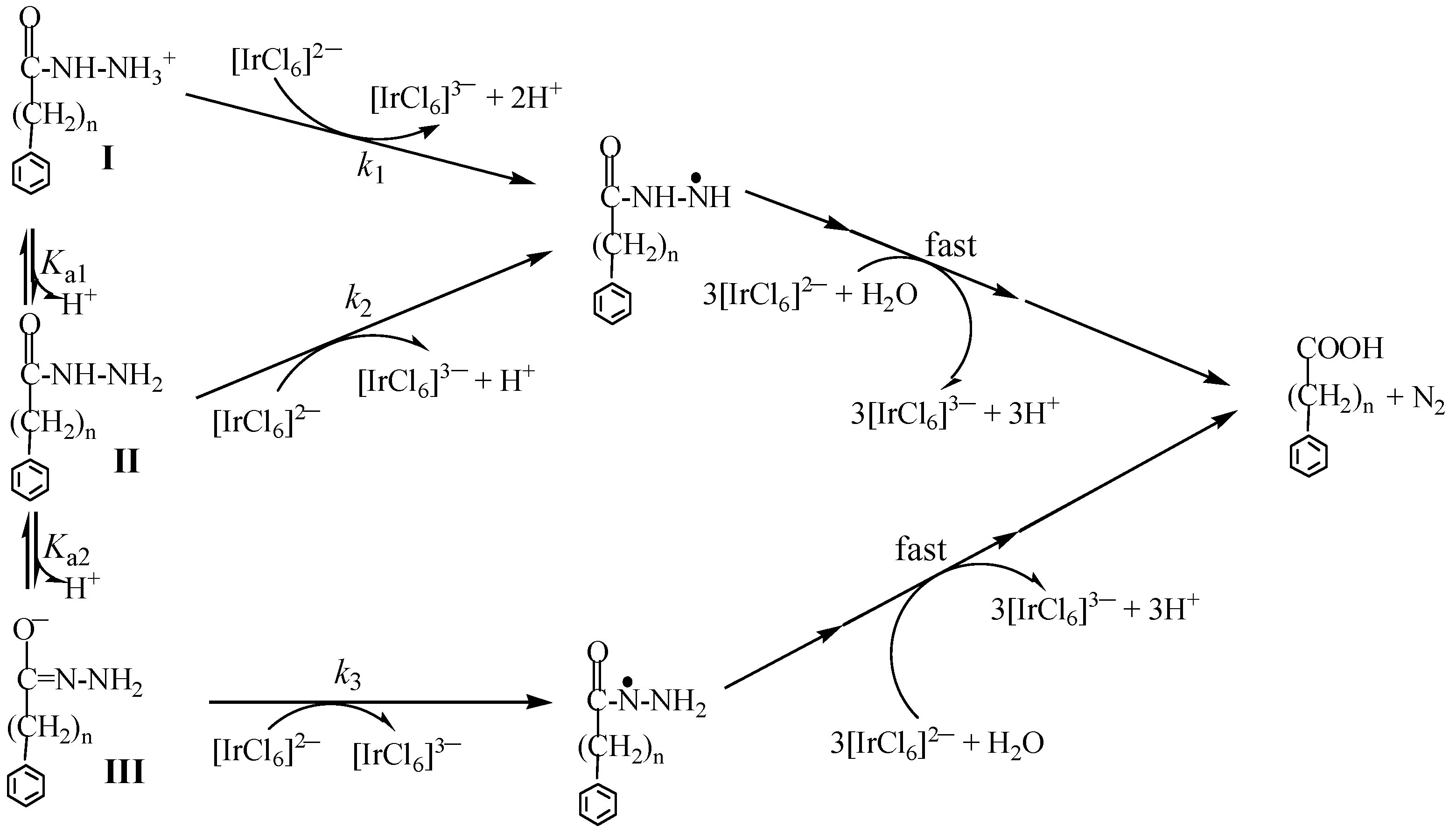
3.1. Mechanistic Analysis
−d[IrCl62−]/dt = _______________________________________[Hydrazide]tot[IrCl62−]
aH2 + Ka1aH + Ka1Ka2
k′ = _______________________________________
aH2 + Ka1aH + Ka1Ka2
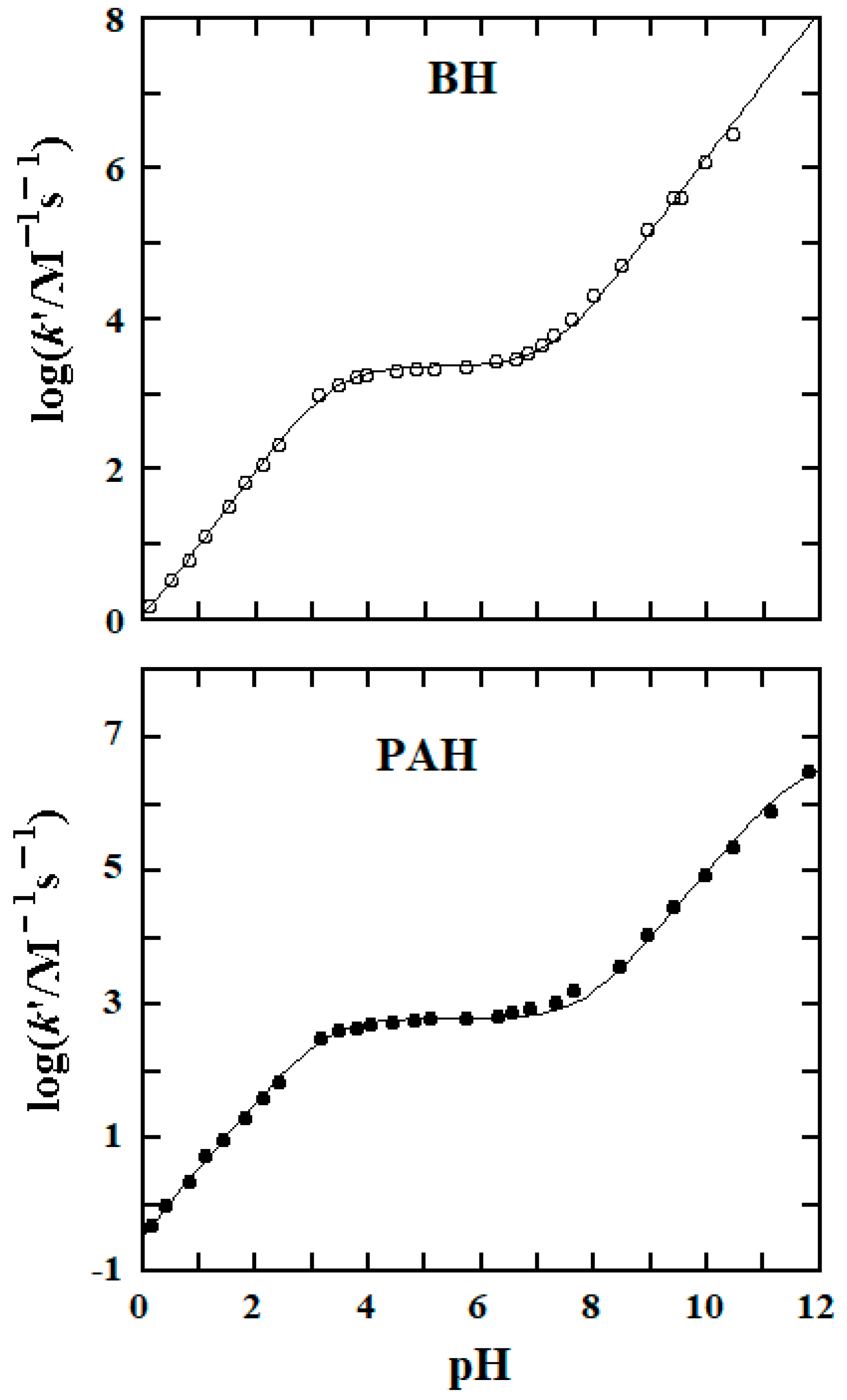
3.2. pKa Values and Rate Constants of the Rate-Determining Steps
3.3. Probing the Activation Process
3.4. Comparison of the Rate Constants
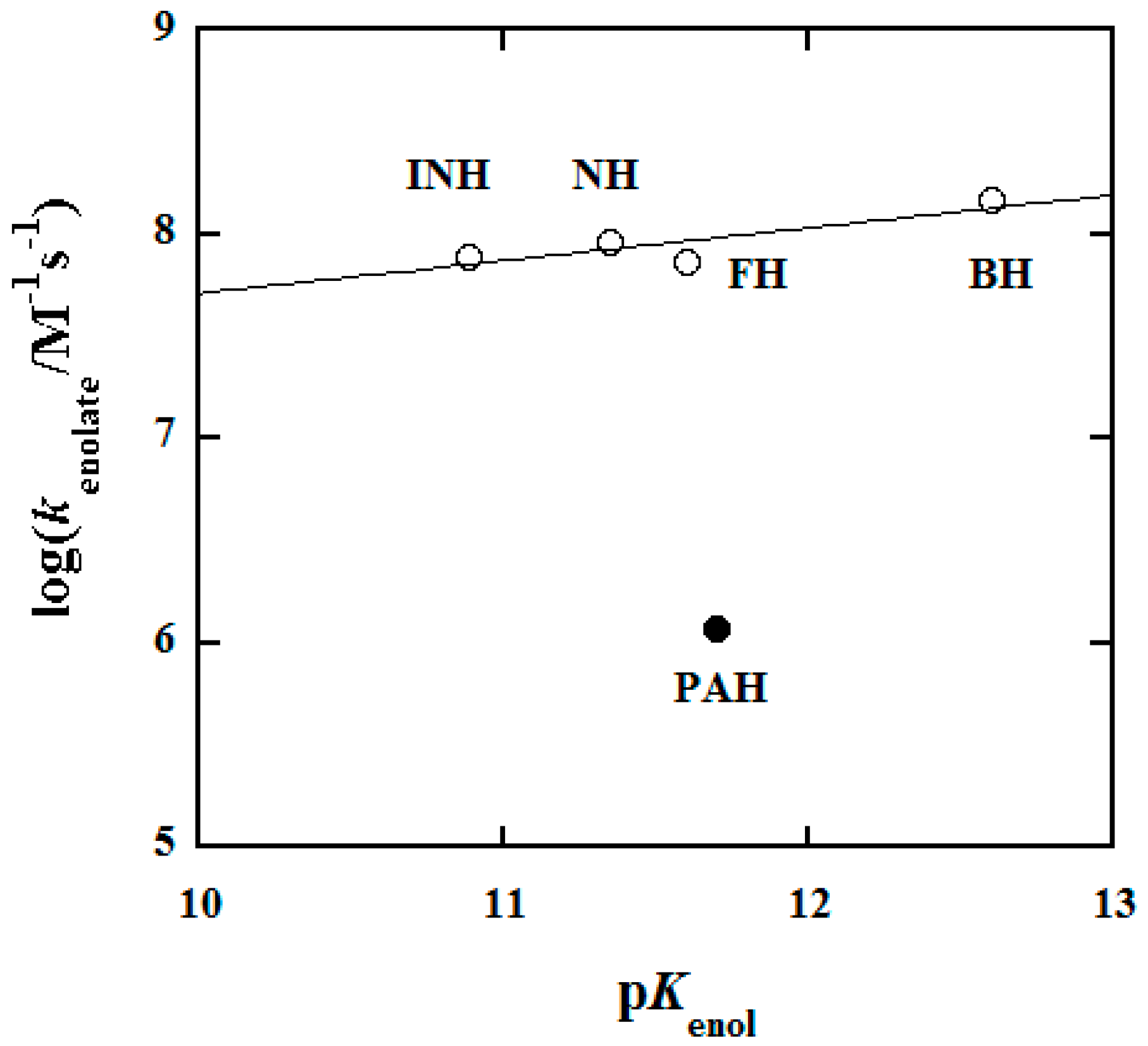
3.5. Structure–Reactivity Relationship
4. Materials and Methods
4.1. Chemicals
4.2. Buffers and Reaction Media
4.3. Stoichiometric Investigation
4.4. Product Analysis by RP-HPLC and NMR Spectra
4.5. Kinetic Measurements
5. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- Majumdar, P.; Pati, A.; Patra, M.; Behera, R.K.; Behera, A.K. Acid hydrazides, potent reagents for synthesis of oxygen-, nitrogen-, and/or sulfur-containing heterocyclic rings. Chem. Rev. 2014, 114, 2942–2977. [Google Scholar] [CrossRef]
- Narang, R.; Narasimhan, B.; Sharma, S. A review on biological activities and chemical synthesis of hydrazide derivatives. Curr. Med. Chem. 2012, 19, 569–612. [Google Scholar] [CrossRef]
- Rivers, E.C.; Mancera, R.L. New anti-tuberculosis drugs in clinical trials with novel mechanisms of action. Drug Discov. Today 2008, 13, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Metushi, I.G.; Cai, P.; Zhu, X.; Nakagawa, T.; Uetrecht, J.P. A fresh look at the mechanism of isoniazid--induced hepatotoxicity. Clin. Pharmacol. Therap. 2011, 89, 911–914. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Pradhan, K.; Zhong, X.; Mao, X. Isoniazid metabolism and hepatotoxicity. Acta Pharm. Sinica B 2016, 6, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Bedia, K.K.; Elçin, O.; Seda, U.; Fatma, K.; Nathaly, S.; Sevim, R.; Dimoglo, A. Synthesis and characterization of novel hydrazide–hydrazones and the study of their structure-antituberculosis activity. Eur. J. Med. Chem. 2006, 41, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Rollas, S.; Küçükgüzel, S.G. Biological activities of hydrazine derivatives. Molecules 2007, 12, 1910–1939. [Google Scholar] [CrossRef] [PubMed]
- Eldehna, W.M.; Fares, M.; Abdel-Aziz, M.M.; Abdel-Aziz, H.A. Design, synthesis and antitubercular activity of certain nicotinic acid hydrazides. Molecules 2015, 20, 8800–8815. [Google Scholar] [CrossRef] [PubMed]
- Kumari, D.; Bansal, H. Benzohydrazides: As potential bio-active agents. Pharma Innov. J. 2018, 7, 543–550. [Google Scholar]
- Abbas, A.; Ali, B.; Kanwal, K.M.; Khan, K.M.; Iqbal, J.; Ur Rahman, S.; Zaib, S.; Perveen, S. Synthesis and in vitro urease inhibitory activity of benzohydrazide derivatives, in silico and kinetic studies. Bioorg. Chem. 2019, 82, 163–177. [Google Scholar] [CrossRef]
- Timmins, G.S.; Deretic, V. Mechanisms of action of isoniazid. Mol. Microb. 2006, 62, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Wengenack, N.L.; Rusnak, F. Evidence for isoniazid-dependent free radical generation catalyzed by Mycobacterium tuberculosis KatG and the isoniazid-resistant mutant KatG(S315T). Biochemistry 2001, 40, 8990–8996. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R.; Morgan, A.G.M.; Michail, K.; Srivastava, N.; Whittal, R.M.; Aljuhani, N.; Siraki, A.G. Metabolism of isoniazid by neutrophil myeloperoxidase leads to isoniazid-NAD+ adduct formation: A comparison of the reactivity of isoniazid with its known human metabolites. Biochem. Pharmacol. 2016, 106, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Ren, Y.; Sun, S.; Yang, J.; Nan, C.; Shi, H.; Xu, J.; Duan, J.; Shi, T.; Elding, L.I. Kinetics and mechanism of oxidation of the anti-tubercular prodrug isoniazid and its analog by iridium (IV) as models for biological redox systems. Dalton Trans. 2017, 46, 8377–8386. [Google Scholar] [CrossRef]
- Pelizzetti, E.; Mentasti, E.; Baiocchi, C. Kinetics and mechanism of oxidation of quinols by hexachloroiridate(IV) in aqueous acidic perchlorate media. J. Phys. Chem. 1976, 80, 2979–2982. [Google Scholar] [CrossRef]
- Pelizzetti, E.; Mentasti, E.; Pramauro, E. Outer-sphere oxidation of ascorbic acid. Inorg. Chem. 1978, 17, 1181–1186. [Google Scholar] [CrossRef]
- Stanbury, D.M. Reactions involving the hydrazinium free radical; oxidation of hydrazine by hexachloroiridate. Inorg. Chem. 1984, 23, 2879–2882. [Google Scholar] [CrossRef]
- Hubbard, C.D.; Gerhard, A.; van Eldik, R. Electrostriction and counter ion effects in an outer-sphere electron transfer reaction. Kinetics of the reduction of hexachloroiridate(IV) by iodide ion. Dalton Trans. 2001, 1069–1075. [Google Scholar] [CrossRef]
- Bhattari, N.; Stanburry, D.M. Oxidation of glutathione by hexachloroiridate(IV), dicyanobis(bipyridine) iron(III), and tetracyano (bipyridine)iron(III). Inorg. Chem. 2012, 51, 13303–13311. [Google Scholar] [CrossRef]
- Kim, E.; Winkler, T.E.; Kitchen, C.; Kang, M.; Banis, G.; Bentley, W.E.; Kelly, D.L.; Ghodssi, R.; Payne, G.F. Redox probing for chemical information of oxidative stress. Anal. Chem. 2017, 89, 1583–1592. [Google Scholar] [CrossRef]
- Katouaha, H.A.; Al-Fahemia, J.H.; Elghalbana, M.G.; Saada, F.A.; Althagafia, I.A.; El-Metwaly, N.M.; Khedra, A.M. Synthesis of new Cu(II)-benzohydrazide nanometer complexes, spectral, modeling, CT-DNA binding with potential anti-inflammatory and anti-allergic theoretical feature. Mater. Sci. Eng. C: Mater. Biol. Appl. 2019, 96, 740–756. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Pulido, S.B.; Linares-Ordóñez, F.M.; Martínez-Martos, J.M.; Moreno-Carretero, M.N.; Quirós-Olozábal, M.; Ramírez-Expósito, M.J. Metal complexes with the ligand derived from 6-acetyl-1,3,7-trimethyllumazine and benzohydrazide. Molecular structures of two new Co(II) and Rh(III) complexes and analysis of in vitro antitumor activity. J. Inorg. Biochem. 2008, 102, 1677–1683. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Lim, L.H.; Chuanprasit, P.; Hirao, H.; Zhou, J. Nickel--catalyzed enantioselective reductive amination of ketones with both arylamines and benzhydrazide. Angew. Chem. Int. Ed. 2016, 55, 12083–12087. [Google Scholar] [CrossRef] [PubMed]
- Shewale, S.A.; Phadkule, A.N.; Gokavi, G.S. Kinetics and mechanism of oxidation of benzohydrazide by bromate catalyzed by vanadium (IV) in aqueous acidic medium. Int. J. Chem. Kinet. 2008, 40, 151–159. [Google Scholar] [CrossRef]
- Kadam, S.D.; Supale, A.R.; Gokavi, G.S. Kinetics and mechanism of oxidation of benzoic acid hydrazide by bromate catalyzed by octamolybdomanganate(II). Transit. Met. Chem. 2008, 33, 989–994. [Google Scholar] [CrossRef]
- Burner, U.; Obinger, C.; Paumann, M.; Furtmuller, P.G.; Kettle, A.J. Transient and steady-state kinetics of the oxidation of substituted benzoic acid hydrazides by myeloperoxidase. J. Biol. Chem. 1999, 274, 9494–9502. [Google Scholar] [CrossRef] [PubMed]
- Aitken, S.M.; Quellet, M.; Percival, M.D.; English, A.M. Mechanism of horseradish peroxidase inactivation by benzhydrazide: A critical evaluation of arylhydrazides as peroxidase inhibitors. Biochem. J. 2003, 375, 613–621. [Google Scholar] [CrossRef][Green Version]
- Nan, C.; Dong, J.; Tian, H.; Shi, H.; Shen, S.; Xu, J.; Li, X.; Shi, T. Oxidations of hydrazine and substituted hydrazines by hexachloroiridate (IV) in aqueous solution: Kinetic and mechanistic analyses. J. Mol. Liq. 2018, 256, 489–496. [Google Scholar] [CrossRef]
- Wang, J.; Yao, H.; Lu, T.; Dong, J.; Xu, B.; Liu, Y.; Zhou, L.; Liu, C.; Shi, T. Spectroscopic, kinetic, and theoretical analyses of oxidation of dl-ethionine by Pt(IV) anticancer model compounds. Spectrochim. Acta Part A 2019, 223, 117328. [Google Scholar] [CrossRef]
- Manoussakis, G.; Haristos, D.; Youri, C. Halogen ring monosubstituted benzoic acid hydrazides as ligands. II. Ultraviolet spectra and pK determination. Can. J. Chem. 1973, 51, 811–814. [Google Scholar] [CrossRef]
- Huo, S.; Shen, S.; Liu, D.; Shi, T. Oxidation of 3, 6-dioxa-1, 8-octanedithiol by platinum(IV) anticancer prodrug and model complex: Kinetic and mechanistic studies. J. Phys. Chem. B 2012, 116, 6522–6528. [Google Scholar] [CrossRef] [PubMed]
- Belkheiri, N.; Bouguerne, B.; Bedos-Belval, F.; Duran, H.; Bernis, C.; Salvayre, R.; Nègre-Salvayre, A.; Baltas, M. Synthesis and antioxidant activity evaluation of a syringic hydrazones family. Eur. J. Med. Chem. 2010, 45, 3019–3026. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Tian, H.; Xu, L.; Xia, Y.; Zhou, L.; Liu, C.; Shi, T. Kinetic and mechanistic analysis of oxidation of 2-furoic hydrazide by hexachloroirradate(IV) in a wide pH range. Transit. Met. Chem. 2019, 44, 771–777. [Google Scholar] [CrossRef]
- Kimura, M.; Yamamoto, M.; Yamabe, S. Kinetics and mechanism of the oxidation of L-ascorbic acid by tris(oxalato)cobaltate(III) and tris(1,10-phenanthroline)iron(III) complexes in aqueous solution. J. Chem. Soc., Dalton Trans. 1982, 423–427. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds BH, PAH, and Na2IrCl6·6H2O are available from the author. |
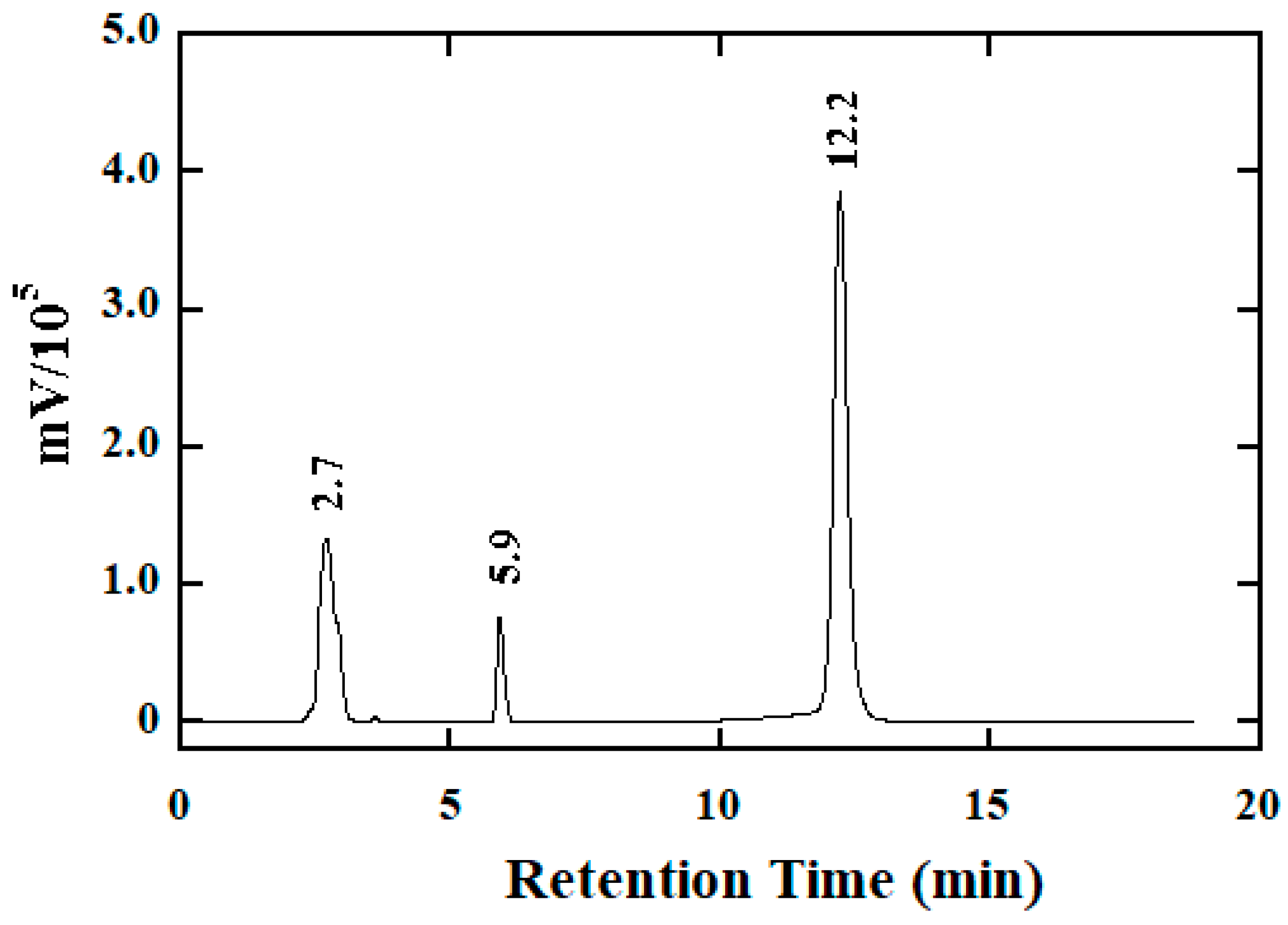
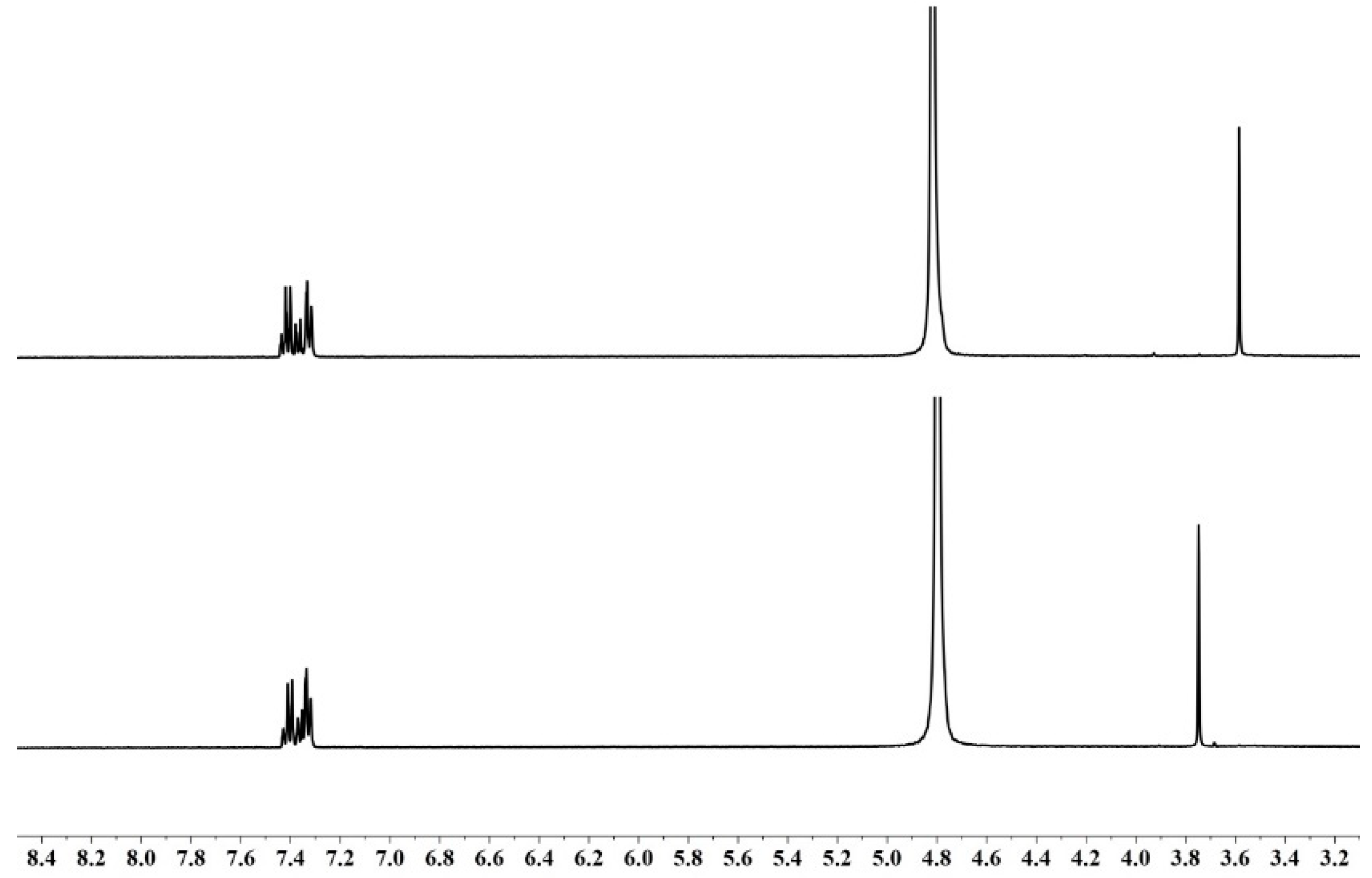
| Reductant | ∆[Ir(IV)]:∆[Hydrazide]tot | Reaction Medium (Reaction Time) |
|---|---|---|
| BH | 4.0:1.03 ± 0.03 | pH 6.31 phosphate buffer (5 min) |
| 4.0:1.05 ± 0.03 | 0.010 M HClO4 (2 h) | |
| PAH | 4.0:0.097 ± 0.03 | pH 6.31 phosphate buffer (30 min) |
| 4.0:1.00 ± 0.03 | 0.010 M HClO4 (2 h) |
| Hydrazide | km/pKam | Values |
|---|---|---|
| BH | k1 | 0.046 ± 0.013 M−1s−1 |
| k2 | 597 ± 9 M−1s−1 | |
| k3 | (1.47 ± 0.05) × 108 M−1s−1 | |
| pKa1 | 3.37 ± 0.09 | |
| pKa2 | 12.6 ± 0.1 | |
| PAH | k1 | 0 (or indeterminate) |
| k2 | 157 ± 5 M−1s−1 | |
| k3 | (1.19 ± 0.06) × 106 M−1s−1 | |
| pKa1 | 3.24 ± 0.08 | |
| pKa2 | 11.7 ± 0.2 |
| Hydrazide | t/°C | k2/M−1s−1 | ∆H2‡/kJ∙mol−1 | ∆S2‡/J∙K−1∙mol−1 |
|---|---|---|---|---|
| BH | 15.0 | 297 ± 9 | 36.2 ± 0.7 | −72 ± 5 |
| 20.0 | 397 ± 15 | |||
| 25.0 | 521 ± 20 | |||
| 30.0 | 654 ± 25 | |||
| 35.0 | 857 ± 27 | |||
| PAH | 15.0 | 86 ± 2 | 40.7 ± 0.9 | −66 ± 4 |
| 20.0 | 119 ± 3 | |||
| 25.0 | 158 ± 4 | |||
| 30.0 | 205 ± 5 | |||
| 35.0 | 283 ± 9 |
| Hydrazide | pKa a | k/M−1s−1 b | pKenol c | kenolate/M−1s−1 d | Ref. |
|---|---|---|---|---|---|
| INH | 3.67 | 1.13 × 103 | 10.89 | 7.9 × 107 | [14] |
| NH | 3.49 | 261 | 11.35 | 9.1 × 107 | [14] |
| FH | 3.04 | 620 | 11.6 | 7.3 × 107 | [34] |
| BH | 3.37 | 597 | 12.6 | 1.47 × 108 | This Work |
| PAH | 3.24 | 157 | 11.7 | 1.19 × 106 | This Work |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X. Oxidations of Benzhydrazide and Phenylacetic Hydrazide by Hexachloroiridate(IV): Reaction Mechanism and Structure–Reactivity Relationship. Molecules 2020, 25, 308. https://doi.org/10.3390/molecules25020308
Zhang X. Oxidations of Benzhydrazide and Phenylacetic Hydrazide by Hexachloroiridate(IV): Reaction Mechanism and Structure–Reactivity Relationship. Molecules. 2020; 25(2):308. https://doi.org/10.3390/molecules25020308
Chicago/Turabian StyleZhang, Xiaolai. 2020. "Oxidations of Benzhydrazide and Phenylacetic Hydrazide by Hexachloroiridate(IV): Reaction Mechanism and Structure–Reactivity Relationship" Molecules 25, no. 2: 308. https://doi.org/10.3390/molecules25020308
APA StyleZhang, X. (2020). Oxidations of Benzhydrazide and Phenylacetic Hydrazide by Hexachloroiridate(IV): Reaction Mechanism and Structure–Reactivity Relationship. Molecules, 25(2), 308. https://doi.org/10.3390/molecules25020308





