The Emulsifying Properties of Hydrogenated Rosin Xylitol Ester as a Biomass Surfactant for Food: Effect of pH and Salts
Abstract
1. Introduction
2. Results and Discussion
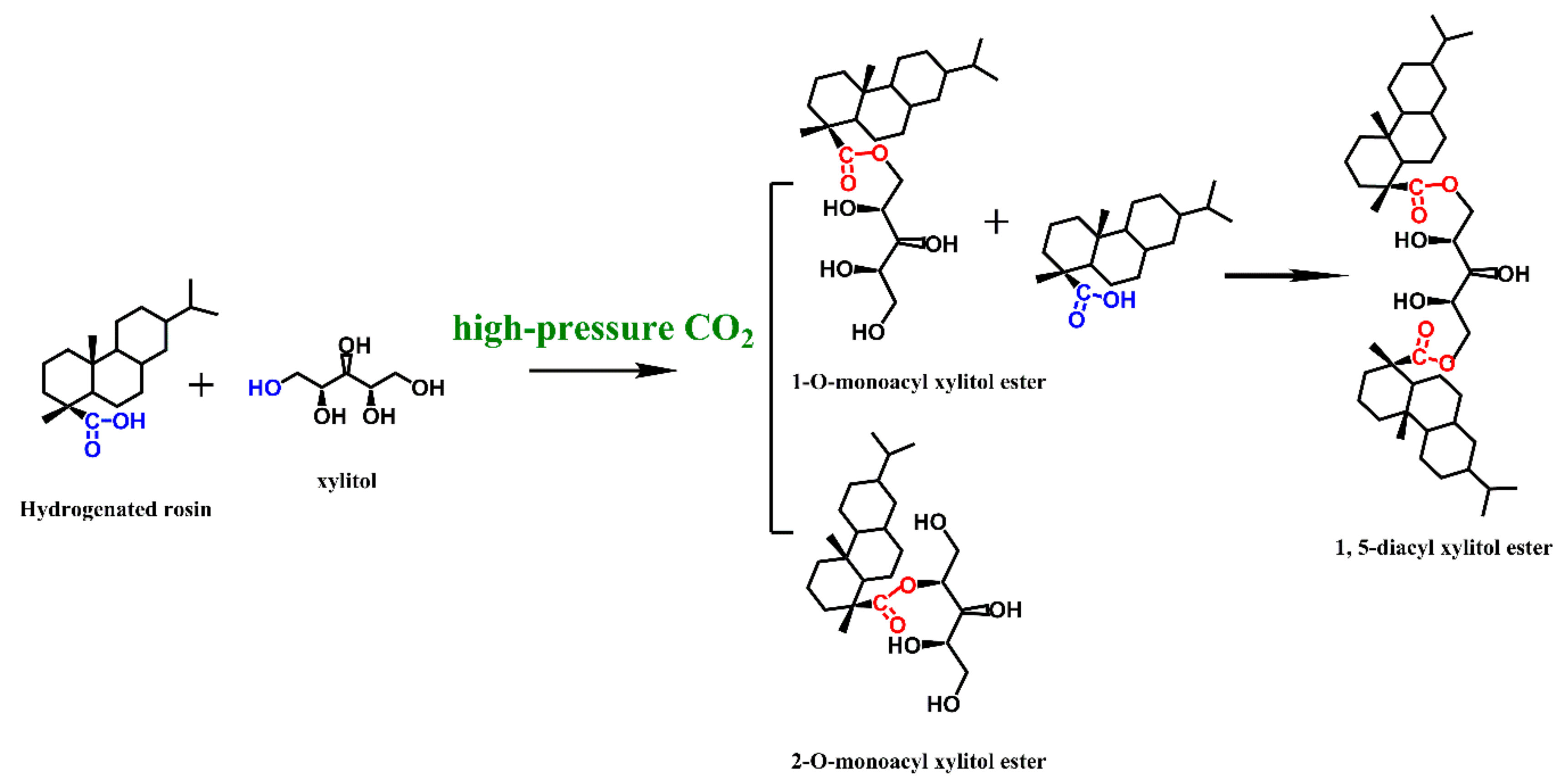
2.1. Preparation
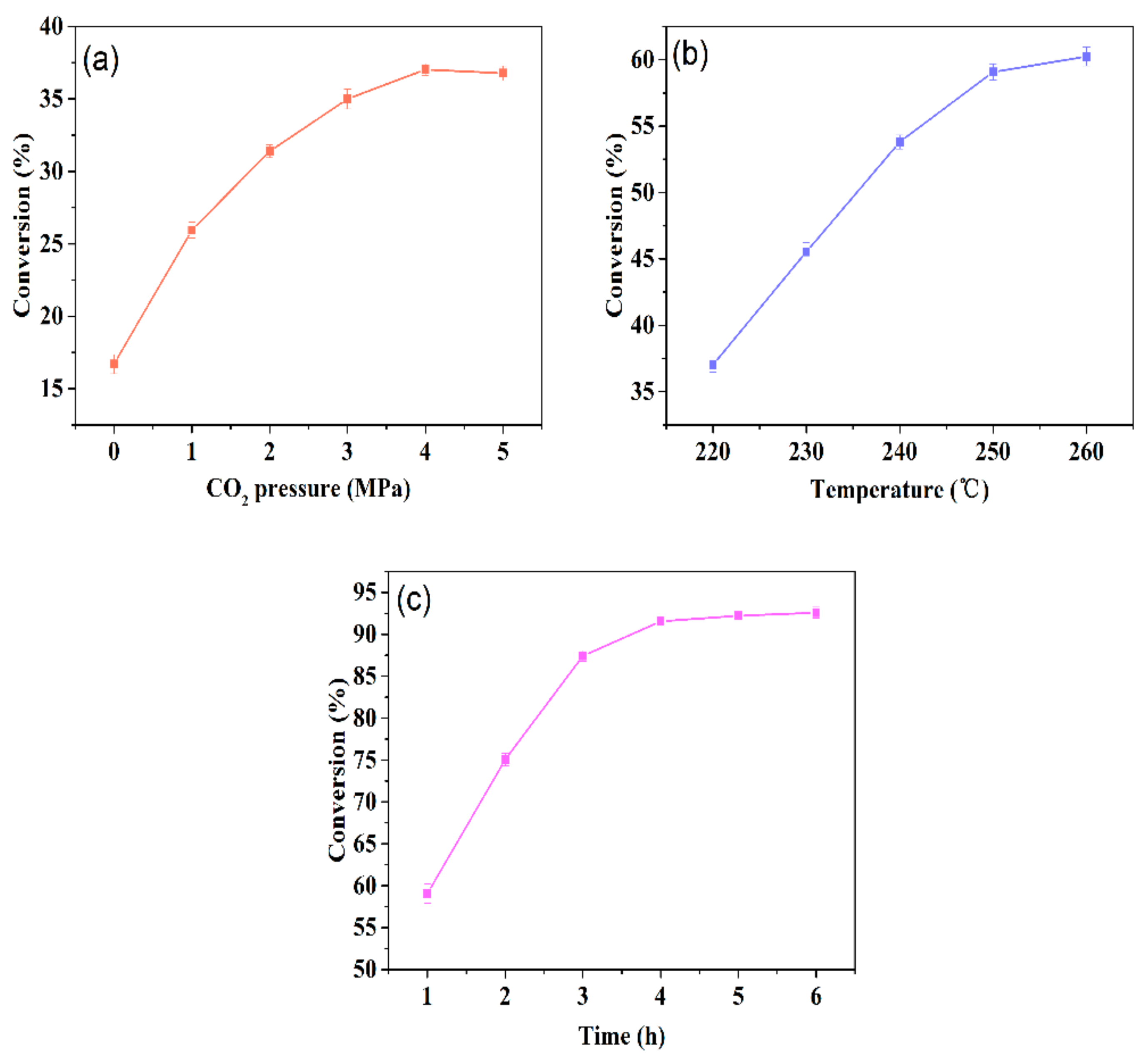
2.1.1. Conversion Values
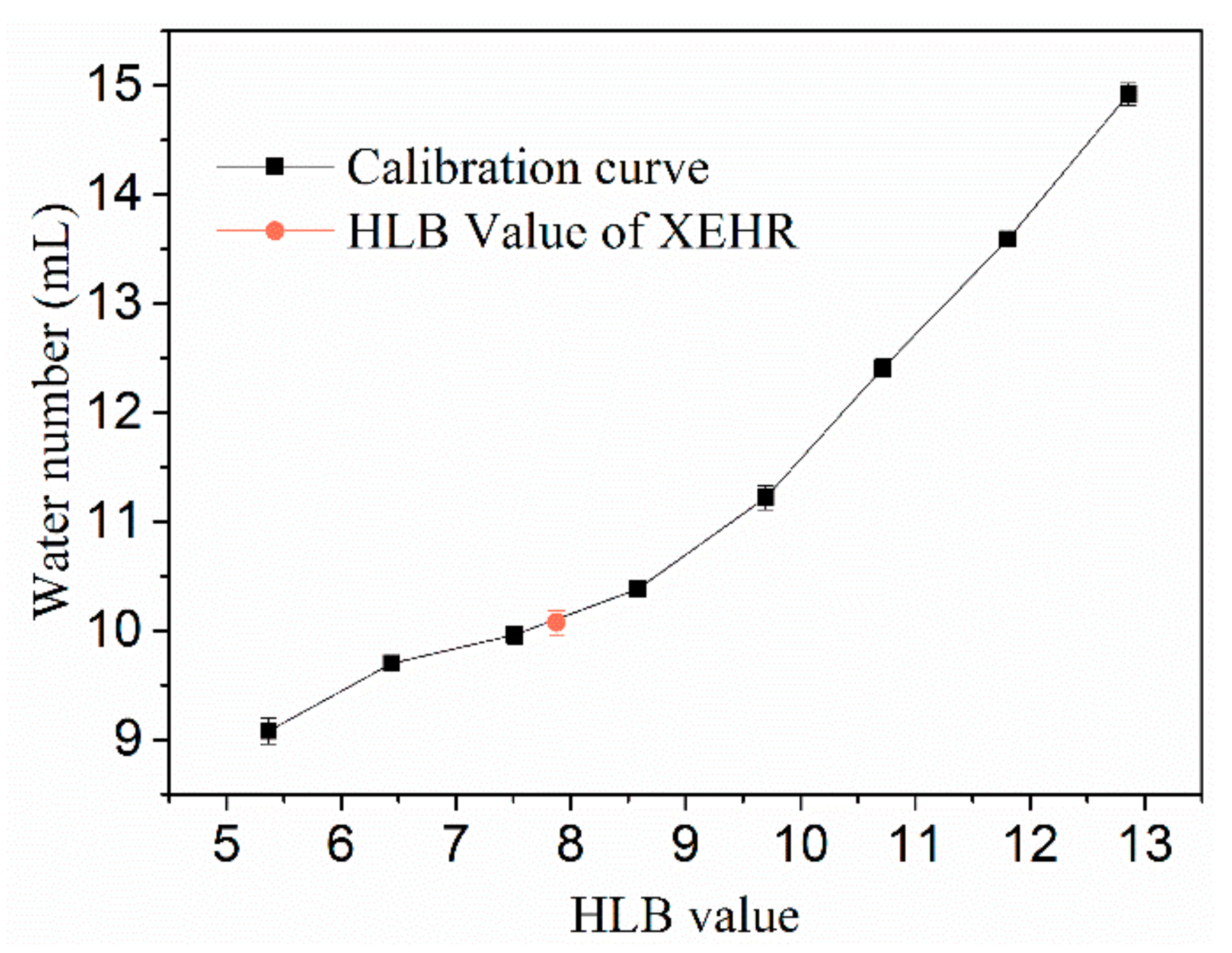
2.1.2. Hydrophilic–Lipophilic Balance (HLB) Value
2.1.3. ICP-AES
2.2. Characterization
2.2.1. FTIR
2.2.2. NMR
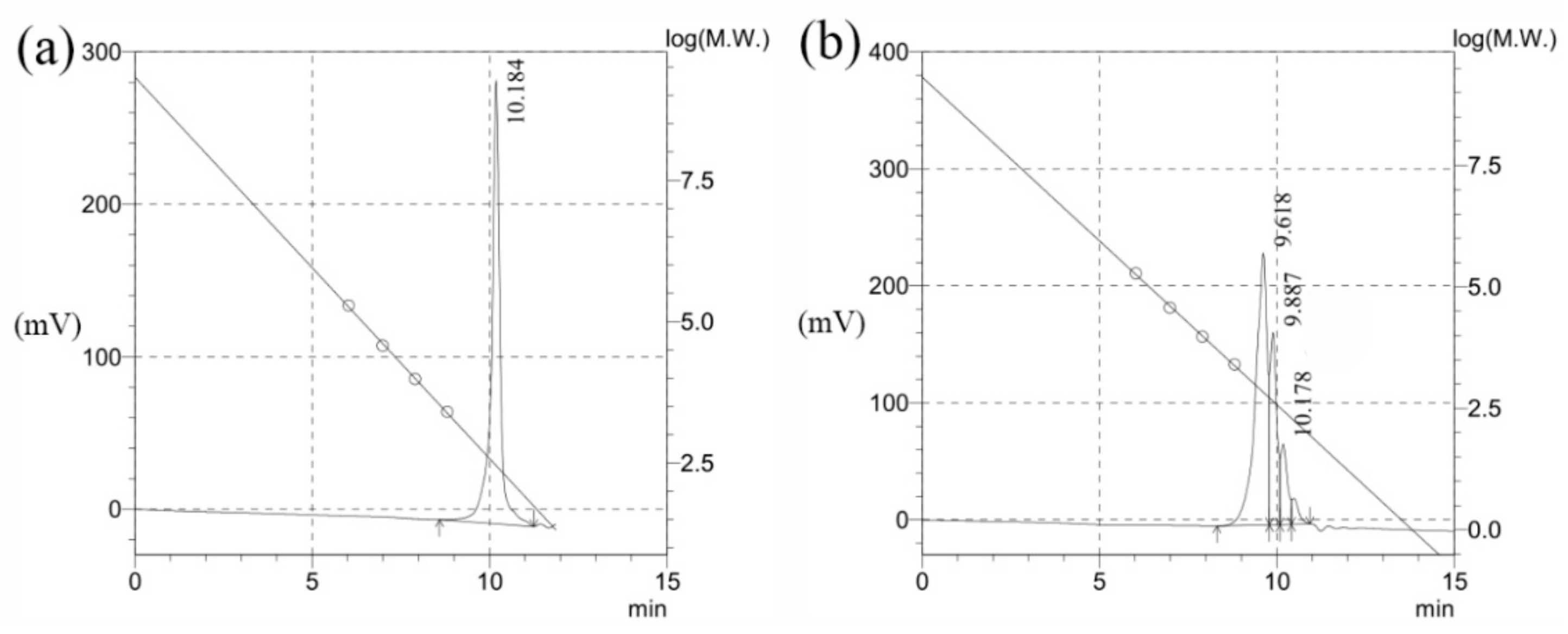
2.2.3. GPC
2.3. Emulsification Properties
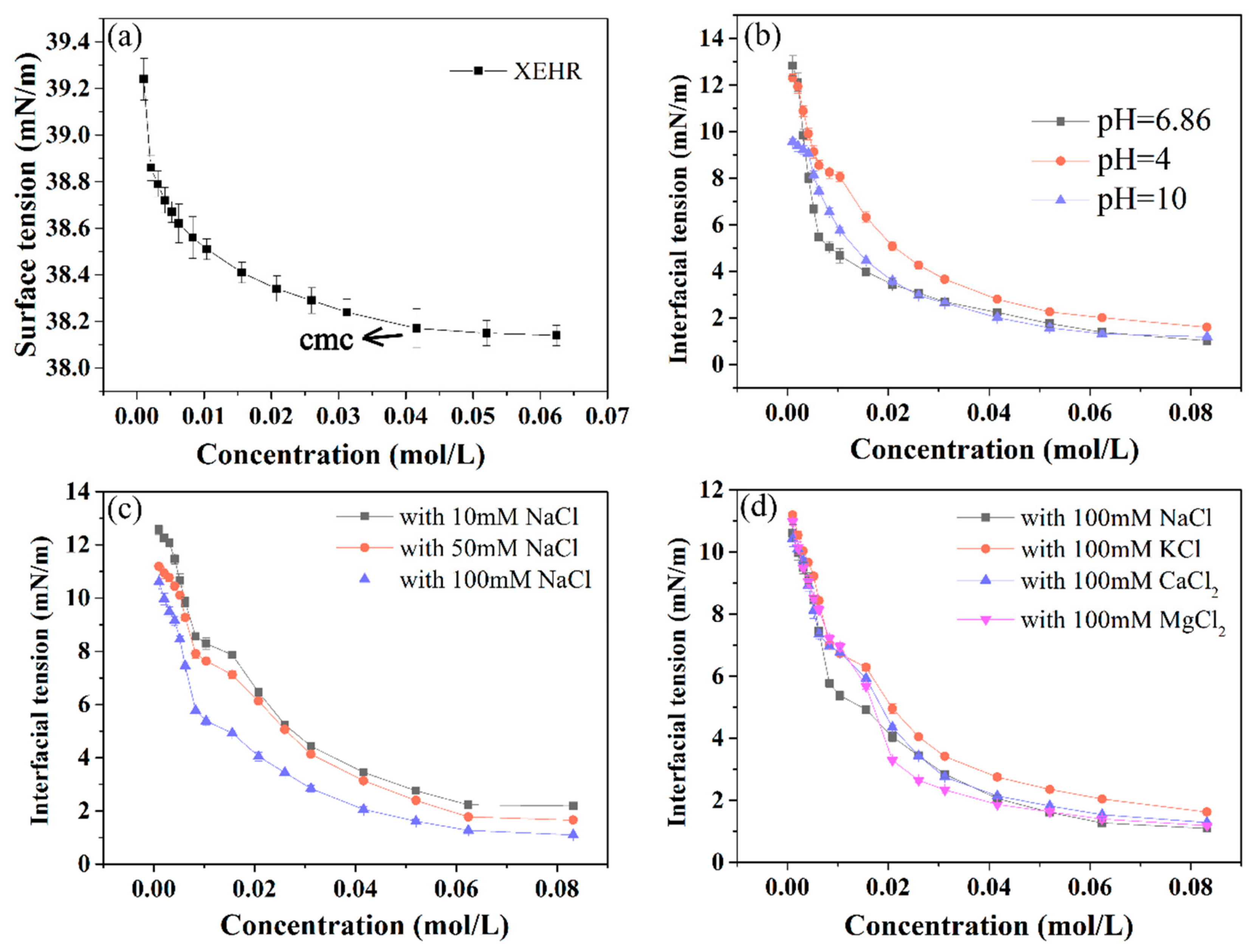
2.3.1. Surface and Interfacial Tensions
2.3.2. Emulsifying Activity
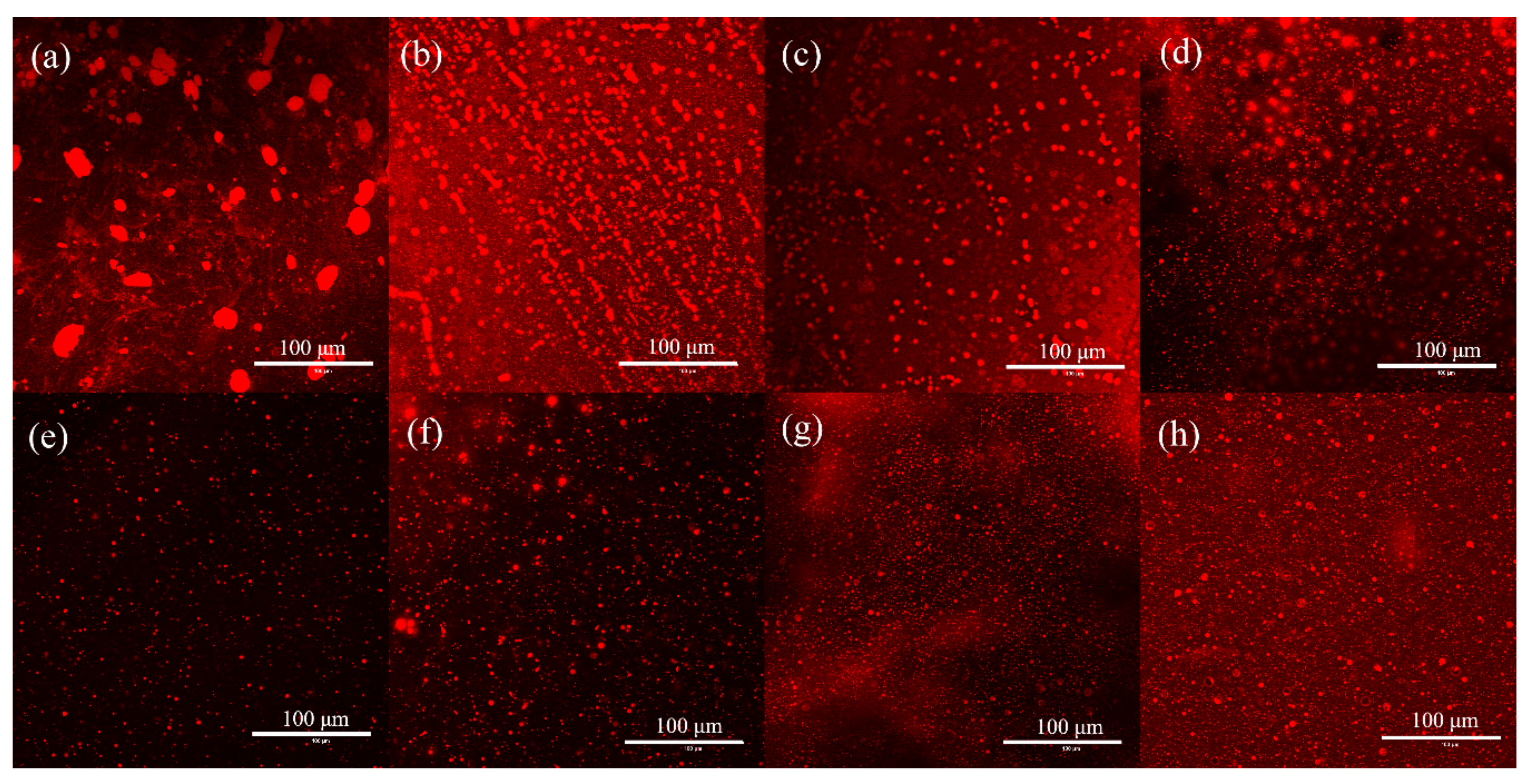
2.3.3. Microstructures of Emulsions
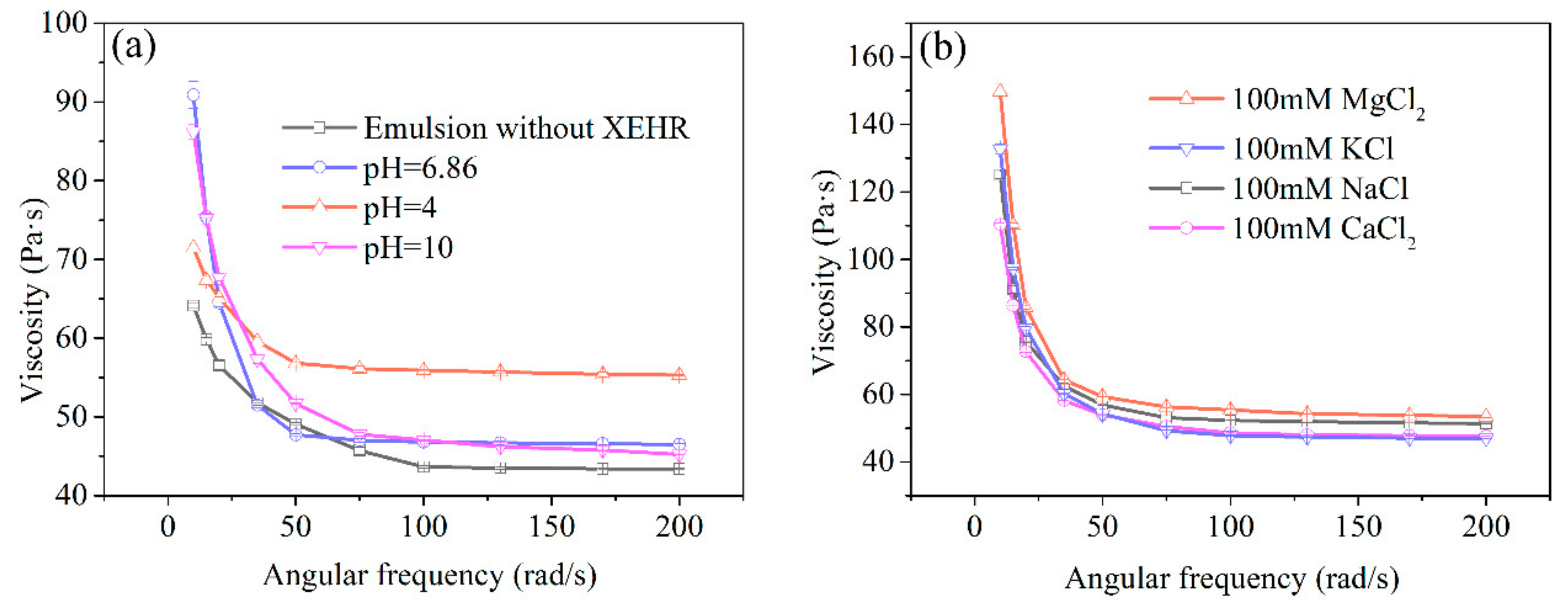
2.3.4. Emulsion Rheology
3. Materials and Methods
3.1. Materials
3.2. Preparation and Purification of XEHR
3.2.1. Use High-Pressure CO2 as Catalyst
3.2.2. Use ZnO as Catalyst
3.2.3. Without Using Any Catalysts
3.3. Fundamental Properties of XEHR
3.3.1. Determination of Conversion Values
3.3.2. HLB Values
3.3.3. ICP-AES
3.4. Characterization of XEHR
3.4.1. Fourier Transform Infrared Spectroscopy
3.4.2. NMR
3.4.3. GPC
3.5. Emulsion Preparation
3.5.1. Surface and Interfacial Tensions
3.5.2. Emulsification Properties
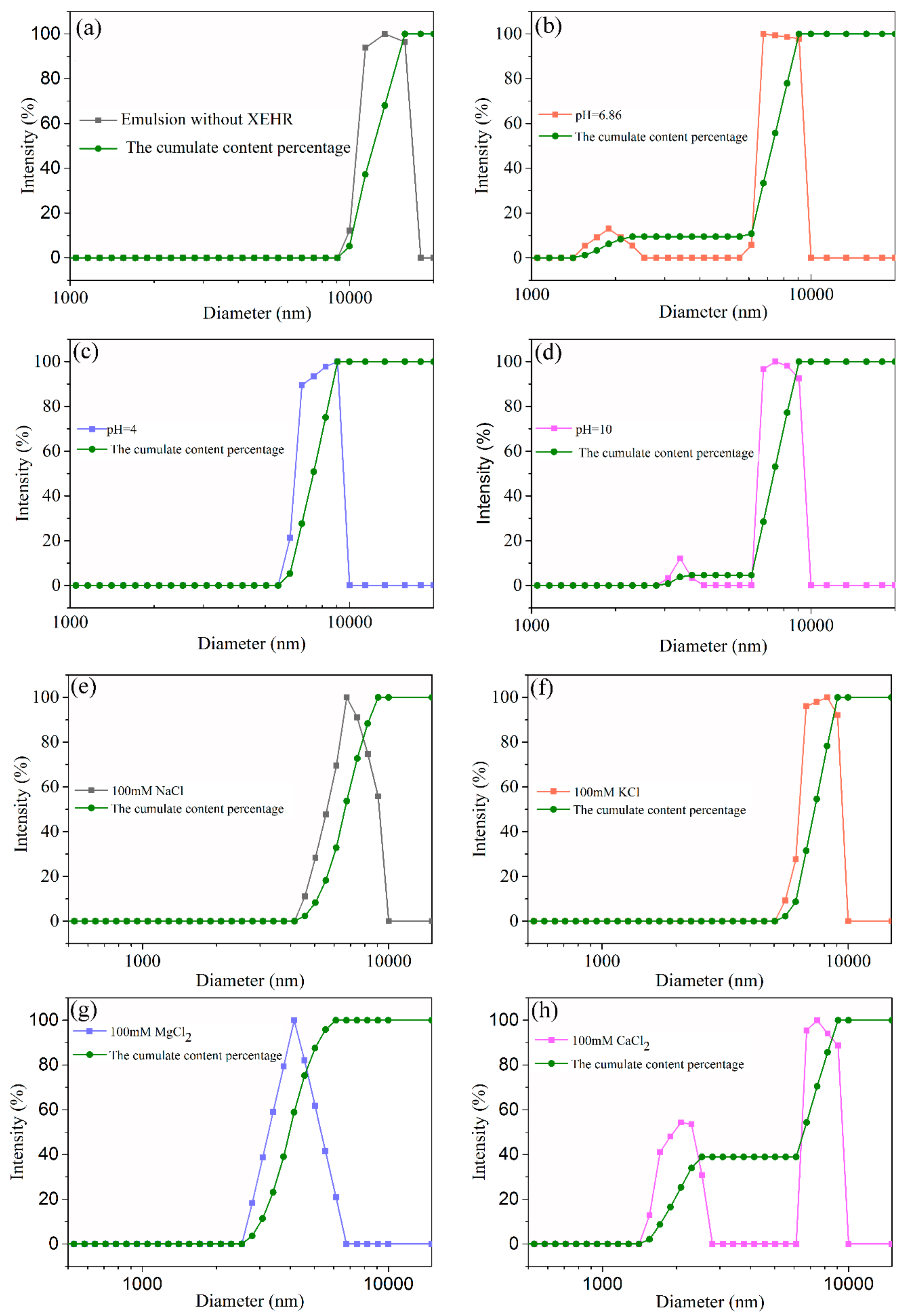
3.5.3. Droplet Size Distribution and Zeta Potentials
3.5.4. Confocal Laser Scanning Microscopy
3.5.5. Rheological Behaviors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hay, W.T.; Fanta, G.F.; Felker, F.C.; Peterson, S.C.; Skory, C.D.; Hojilla-Evangelista, M.P.; Biresaw, G.; Selling, G.W. Emulsification properties of amylose-fatty sodium salt inclusion complexes. Food Hydrocoll. 2019, 90, 490–499. [Google Scholar] [CrossRef]
- Koliastasi, A.; Kompothekra, V.; Giotis, C.; Moustakas, A.K.; Skotti, E.P.; Gerakis, A.; Kalogianni, E.P.; Georgiou, D.; Ritzoulis, C. Novel emulsifiers from olive mill compost. Food Hydrocoll. 2020, 99, 105373. [Google Scholar] [CrossRef]
- Gaur, V.K.; Regar, R.K.; Dhiman, N.; Gautam, K.; Srivastava, J.K.; Patnaik, S.; Kamthan, M.; Manickam, N. Biosynthesis and characterization of sophorolipid biosurfactant by Candida spp.: Application as food emulsifier and antibacterial agent. Bioresour. Technol. 2019, 285, 121314. [Google Scholar] [CrossRef] [PubMed]
- Duprat-de-Paule, S.; Guilbot, J.; Roso, A.; Cambos, S.; Pierre, A. Augmented bio-based lipids for cosmetics. OCL 2018, 25, D503. [Google Scholar] [CrossRef]
- Zhang, M.; Wei, L.; Chen, H.; Du, Z.; Binks, B.P.; Yang, H. Compartmentalized droplets for continuous flow Liquid-Liquid interface catalysis. J. Am. Chem. Soc. 2016, 138, 10173–10183. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.; Zhang, P.; Zhang, Z.; Liu, S.; Han, B. Efficient emulsifying properties of glycerol-based surfactant. Colloids Surf. A: Physicochem. Eng. Asp. 2018, 553, 225–229. [Google Scholar] [CrossRef]
- Nakauma, M.; Funami, T.; Noda, S.; Ishihara, S.; Al-Assaf, S.; Nishinari, K.; Phillips, G.O. Comparison of sugar beet pectin, soybean soluble polysaccharide, and gum arabic as food emulsifiers. 1. Effect of concentration, pH, and salts on the emulsifying properties. Food Hydrocoll. 2008, 22, 1254–1267. [Google Scholar]
- Hosseini-Parvar, S.H.; Osano, J.P.; Matia-Merino, L. Emulsifying properties of basil seed gum: Effect of pH and ionic strength. Food Hydrocoll. 2016, 52, 838–847. [Google Scholar] [CrossRef]
- Habulin, M.; Šabeder, S.; Knez, Ž. Enzymatic synthesis of sugar fatty acid esters in organic solvent and in supercritical carbon dioxide and their antimicrobial activity. J. Supercrit. Fluids 2008, 45, 338–345. [Google Scholar] [CrossRef]
- Acevedo-Fani, A.; Soliva-Fortuny, R.; Martín-Belloso, O. Nanostructured emulsions and nanolaminates for delivery of active ingredients: Improving food safety and functionality. Trends Food Sci. Tech. 2017, 60, 12–22. [Google Scholar] [CrossRef]
- Tang, S.; Yu, J.; Lu, L.; Fu, X.; Cai, Z. Interfacial and enhanced emulsifying behavior of phosphorylated ovalbumin. Int. J. Biol. Macromol. 2019, 131, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Burger, T.G.; Zhang, Y. Recent progress in the utilization of pea protein as an emulsifier for food applications. Trends Food Sci. Tech. 2019, 86, 25–33. [Google Scholar] [CrossRef]
- Agyare, K.K.; Addo, K.; Xiong, Y.L. Emulsifying and foaming properties of transglutaminase-treated wheat gluten hydrolysate as influenced by pH, temperature and salt. Food Hydrocoll. 2009, 23, 72–81. [Google Scholar] [CrossRef]
- Fink, J.K. Reactive Polymers: Fundamentals and Applications: A Concise Guide to Industrial Polymers; William Andrew: Norwich, NY, USA, 2017. [Google Scholar]
- Wang, J.; Lu, C.; Liu, Y.; Wang, C.; Chu, F. Preparation and characterization of natural rosin stabilized nanoparticles via miniemulsion polymerization and their pressure-sensitive adhesive applications. Ind. Crop. Prod. 2018, 124, 244–253. [Google Scholar] [CrossRef]
- Karlberg, A.T.; Boman, A.; Nilsson, J.L.G. Hydrogenation reduces the allergenicity of colophony (rosin). Contact Dermat. 1988, 19, 22–29. [Google Scholar] [CrossRef]
- Huang, Z.; Wei, X.J.; Chen, X.P.; Zheng, Q. Microwave-assisted synthesis of hydrogenated rosin-based quaternary ammonium surfactant and its properties. Chem. Ind. For. Prod. 2012, 32, 41–45. [Google Scholar]
- Jia, W.; Rao, X.; Song, Z.; Shang, S. Microwave-assisted synthesis and properties of a novel cationic gemini surfactant with the hydrophenanthrene structure. J. Surfactants Deterg. 2009, 12, 261–267. [Google Scholar] [CrossRef]
- Kugler, S.; Ossowicz, P.; Malarczyk-Matusiak, K.; Wierzbicka, E. Advances in Rosin-Based chemicals: The latest recipes, applications and future trends. Molecules 2019, 24, 1651. [Google Scholar] [CrossRef]
- Code of Federal Regulations; US Food and Drug Administration: Silver Spring, MD, USA, 2012.
- El Ministerio de Sanidad, Política Social e Igualdad Publica el Primer Documento de Análisis de Situación de las Terapias Naturales; Ministerio de Sanidad, Política Social e Igualdad: Madrid, Spain, 2011.
- Leitner, W. Supercritical carbon dioxide as a green reaction medium for catalysis. Acc. Chem. Res. 2002, 35, 746–756. [Google Scholar] [CrossRef]
- Albuquerque, T.L.D.; Da Silva, I.J.; de Macedo, G.R.; Rocha, M.V.P. Biotechnological production of xylitol from lignocellulosic wastes: A review. Process. Biochem. 2014, 49, 1779–1789. [Google Scholar] [CrossRef]
- Zhang, X.; Nie, K.; Wang, M.; Liu, L.; Li, K.; Wang, F.; Tan, T.; Deng, L. Site-specific xylitol dicaprate ester synthesized by lipase from Candida sp. 99-125 with solvent-free system. J. Mol. Catal. B: Enzym. 2013, 89, 61–66. [Google Scholar] [CrossRef]
- Brunzell, J.D. Use of fructose, xylitol, or sorbitol as a sweetener in diabetes mellitus. Diabetes Care 1978, 1, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Ly, K.A.; Milgrom, P.; Rothen, M. Xylitol, sweeteners, and dental caries. Pediatric Dent. 2006, 28, 154–163. [Google Scholar]
- Piao, J.; Kishi, S.; Adachi, S. Surface tensions of aqueous solutions of 1- O-monoacyl sugar alcohols. Colloids Surf. A: Physicochem. Eng. Asp. 2006, 277, 15–19. [Google Scholar] [CrossRef]
- Ferrer, M.; Soliveri, J.; Plou, F.J.; López-Cortés, N.; Reyes-Duarte, D.; Christensen, M.; Copa-Patiño, J.L.; Ballesteros, A. Synthesis of sugar esters in solvent mixtures by lipases from Thermomyces lanuginosus and Candida antarctica B, and their antimicrobial properties. Enzym. Microb. Tech. 2005, 36, 391–398. [Google Scholar] [CrossRef]
- Food Additives Permitted for Direct Addition to Food for Human Consumption; Code of Federal Regulations; Food and Drug Administration, US Department of Health and Human Services: Silver Spring, MD, USA, 2016.
- Gadhave, A. Determination of hydrophilic-lipophilic balance value. Int. J. Sci. Res. 2014, 3, 573–575. [Google Scholar]
- Hemmings, P.N.; Wang, L. Rosin Ester Derivative as Surfactants. U.S. Patent No. 5,552,519, 3 September 1996. [Google Scholar]
- Dhanorkar, V.T.; Gawande, R.S.; Gogte, B.B.; Dorle, A.K. Development and characterization of rosin-based polymer and its application as a cream base. J. Cosmet. Sci. 2002, 53, 199–208. [Google Scholar]
- Atta, A.M.; Ramadan, A.M.; Shaffei, K.A.; Nassar, A.M.; Ahmed, N.S.; Fekry, M. Synthesis and properties of nonionic surfactants from rosin-imides maleic anhydride adduct. J. Dispers. Sci. Technol. 2009, 30, 1100–1110. [Google Scholar] [CrossRef]
- Pa, D.; Gogte, B.B. Synthesized and chacterisation polymeric materials based on coconut oil, rosin & maleic anhydrides. Int. J. Knowl. Eng. 2012, 1, 77–79. [Google Scholar]
- Xu, Z.; Lou, W.; Zhao, G.; Zhang, M.; Hao, J.; Wang, X. Pentaerythritol rosin ester as an environmentally friendly multifunctional additive in vegetable oil-based lubricant. Tribol. Int. 2019, 135, 213–218. [Google Scholar] [CrossRef]
- Biçer, A.; Sarı, A. Synthesis and thermal energy storage properties of xylitol pentastearate and xylitol pentapalmitate as novel solid–liquid PCMs. Sol. Energ. Mat. Sol. C. 2012, 102, 125–130. [Google Scholar] [CrossRef]
- Cramer, J.F.; Dueholm, M.S.; Nielsen, S.B.; Pedersen, D.S.; Wimmer, R.; Pedersen, L.H. Controlling the degree of esterification in lipase catalysed synthesis of xylitol fatty acid esters. Enzym. Microb. Tech. 2007, 41, 346–352. [Google Scholar] [CrossRef]
- Albarrán-Preza, E.; Corona-Becerril, D.; Vigueras-Santiago, E.; Hernández-López, S. Sweet polymers: Synthesis and characterization of xylitol-based epoxidized linseed oil resins. Eur. Polym. J. 2016, 75, 539–551. [Google Scholar] [CrossRef]
- Sinzato, Y.Z.; Sousa Dias, N.J.; Cunha, F.R. An experimental investigation of the interfacial tension between liquid-liquid mixtures in the presence of surfactants. Exp. Therm. Fluid Sci. 2017, 85, 370–378. [Google Scholar] [CrossRef]
- Guo, L.; Li, Q.; Jin, X. Synthesis and surface activities of organic solvent-soluble fluorinated surfactants. J. Fluor. Chem. 2009, 130, 674–681. [Google Scholar]
- Yang, X.; Nisar, T.; Hou, Y.; Gou, X.; Sun, L.; Guo, Y. Pomegranate peel pectin can be used as an effective emulsifier. Food Hydrocoll. 2018, 85, 30–38. [Google Scholar] [CrossRef]
- Zhang, Q.; Cai, B.; Xu, W.; Gang, H.; Liu, J.; Yang, S.; Mu, B. Novel zwitterionic surfactant derived from castor oil and its performance evaluation for oil recovery. Colloids Surf. A: Physicochem. Eng. Asp. 2015, 483, 87–95. [Google Scholar] [CrossRef]
- Kakati, A.; Sangwai, J.S. Effect of monovalent and divalent salts on the interfacial tension of pure hydrocarbon-brine systems relevant for low salinity water flooding. J. Petrol. Sci. Eng. 2017, 157, 1106–1114. [Google Scholar] [CrossRef]
- Tokle, T.; McClements, D.J. Physicochemical properties of lactoferrin stabilized oil-in-water emulsions: Effects of pH, salt and heating. Food Hydrocoll. 2011, 25, 976–982. [Google Scholar] [CrossRef]
- Liu, X.; Ji, L.; Zhang, T.; Xue, Y.; Xue, C. Effects of pre-emulsification by three food-grade emulsifiers on the properties of emulsified surimi sausage. J. Food Eng. 2019, 247, 30–37. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, S.; Chen, Q.; Liu, Q.; Kong, B. Antioxidant activities and emulsifying properties of porcine plasma protein hydrolysates modified by oxidized tannic acid and oxidized chlorogenic acid. Process. Biochem. 2019, 79, 105–113. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, X.; Zhong, J.; Tan, L.; Liu, C. Effect of pH on emulsification performance of a new functional protein from jackfruit seeds. Food Hydrocoll. 2019, 93, 325–334. [Google Scholar] [CrossRef]
- Cabezas, D.M.; Ortiz, M.P.; Wagner, J.R.; Porfiri, M.C. Effect of salt content and type on emulsifying properties of hull soy soluble polysaccharides at acidic pH. Food Res. Int. 2017, 97, 62–70. [Google Scholar] [CrossRef]
- Joshi, M.; Adhikari, B.; Aldred, P.; Panozzo, J.F.; Kasapis, S.; Barrow, C.J. Interfacial and emulsifying properties of lentil protein isolate. Food Chem. 2012, 134, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hu, X.; Ye, Y.; Wang, M.; Wang, J. Emulsifying properties of wheat germ: Influence of pH and NaCl. Food Hydrocoll. 2020, 100, 105431. [Google Scholar] [CrossRef]
- Ladero, M.; de Gracia, M.; Tamayo, J.J.; Ahumada, I.L.D.; Trujillo, F.; Garcia-Ochoa, F. Kinetic modelling of the esterification of rosin and glycerol: Application to industrial operation. Chem. Eng. J. 2011, 169, 319–328. [Google Scholar] [CrossRef]
- Shi, Y.; Yan, F.; Jia, Q.; Wang, Q. Norm descriptors for predicting the hydrophile-lipophile balance (HLB) and critical micelle concentration (CMC) of anionic surfactants. Colloids Surf. A: Physicochem. Eng. Asp. 2019, 583, 123967. [Google Scholar] [CrossRef]
- Mozafarpour, R.; Koocheki, A.; Milani, E.; Varidi, M. Extruded soy protein as a novel emulsifier: Structure, interfacial activity and emulsifying property. Food Hydrocoll. 2019, 93, 361–373. [Google Scholar] [CrossRef]
- Cha, Y.; Wu, F.; Zou, H.; Shi, X.; Zhao, Y.; Bao, J.; Du, M.; Yu, C. High-Pressure homogenization Pre-Treatment improved functional properties of oyster protein isolate hydrolysates. Molecules 2018, 23, 3344. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available to qualified researchers from the authors. |
| Samples | Conversion (%) |
|---|---|
| XEHR (without catalyst) | 63.65 ± 0.05 c |
| XEHR (ZnO as catalyst) | 78.52 ± 0.06 b |
| XEHR (high-pressure CO2 as catalyst) | 91.54 ± 0.03 a |
| Samples | Cu (%) | Fe (%) | Ni (%) | Pb (%) | Zn (%) |
|---|---|---|---|---|---|
| Hydrogenated rosin | <0.0004 | <0.0004 | <0.0004 | <0.0004 | 0.0013 |
| XEHR (ZnO as catalyst) | <0.0004 | <0.0004 | <0.0004 | <0.0004 | 0.20 |
| XEHR (high-pressure CO2 as catalyst) | <0.0004 | <0.0004 | <0.0004 | <0.0004 | 0.0018 |
| Retention Time (min) | Mn | Mw | Polydispersion Index |
|---|---|---|---|
| 10.184 | 276 | 319 | 1.15367 |
| 9.618 | 772 | 844 | 1.09319 |
| 9.887 | 427 | 433 | 1.01454 |
| 10.178 | 266 | 271 | 1.01699 |
| Samples | Emulsifying Activity Index (m2/g) | Emulsifying Stability Index (%) |
|---|---|---|
| Emulsion without XEHR | 4.60 ± 0.31 g | 70.0 ± 0.64 g |
| pH = 6.86 | 7.94 ± 0.09 c | 84.69 ± 0.38 bc |
| pH = 4 | 7.11 ± 0.11 f | 75.56 ± 1.57 ef |
| pH = 10 | 7.92 ± 0.18 cd | 82.65 ± 1.28 cd |
| 10 mM NaCl | 7.33 ± 0.35 ef | 72.71 ± 0.70 f |
| 50 mM NaCl | 7.64 ± 0.39 de | 78.56 ± 1.75 de |
| 100 mM NaCl | 9.33 ± 0.20 a | 84.36 ± 0.29 bc |
| 100 mM KCl | 8.37 ± 0.33 bc | 89.26 ± 3.08 ab |
| 100 mM MgCl2 | 9.52 ± 0.13 a | 94.53 ± 1.53 a |
| 100 mM CaCl2 | 8.75 ± 0.13 b | 80.20 ± 1.12 cd |
| Samples | Zeta Potentials (mV) | Samples | Zeta Potentials (mV) |
|---|---|---|---|
| Emulsion without XEHR | −16.41 ± 3.09 c | 100 mM NaCl | −37.58 ± 1.01 a |
| pH = 6.86 | −35.72 ± 2.42 d | 100 mM KCl | −39.45 ± 5.36 d |
| pH = 4 | 27.88 ± 1.41 b | 100 mM MgCl2 | −41.75 ± 4.72 d |
| pH = 10 | −37.51 ± 5.29 d | 100 mM CaCl2 | −32.61 ± 3.26 ab |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, H.; Chen, X.; Wei, X.; Liang, J.; Zhou, D.; Wang, L. The Emulsifying Properties of Hydrogenated Rosin Xylitol Ester as a Biomass Surfactant for Food: Effect of pH and Salts. Molecules 2020, 25, 302. https://doi.org/10.3390/molecules25020302
Qiu H, Chen X, Wei X, Liang J, Zhou D, Wang L. The Emulsifying Properties of Hydrogenated Rosin Xylitol Ester as a Biomass Surfactant for Food: Effect of pH and Salts. Molecules. 2020; 25(2):302. https://doi.org/10.3390/molecules25020302
Chicago/Turabian StyleQiu, Hong, Xiaopeng Chen, Xiaojie Wei, Jiezhen Liang, Dan Zhou, and Linlin Wang. 2020. "The Emulsifying Properties of Hydrogenated Rosin Xylitol Ester as a Biomass Surfactant for Food: Effect of pH and Salts" Molecules 25, no. 2: 302. https://doi.org/10.3390/molecules25020302
APA StyleQiu, H., Chen, X., Wei, X., Liang, J., Zhou, D., & Wang, L. (2020). The Emulsifying Properties of Hydrogenated Rosin Xylitol Ester as a Biomass Surfactant for Food: Effect of pH and Salts. Molecules, 25(2), 302. https://doi.org/10.3390/molecules25020302




