Physicochemical and Antioxidative Characteristics of Potato Protein Isolate Hydrolysate
Abstract
1. Introduction
2. Results and Discussion
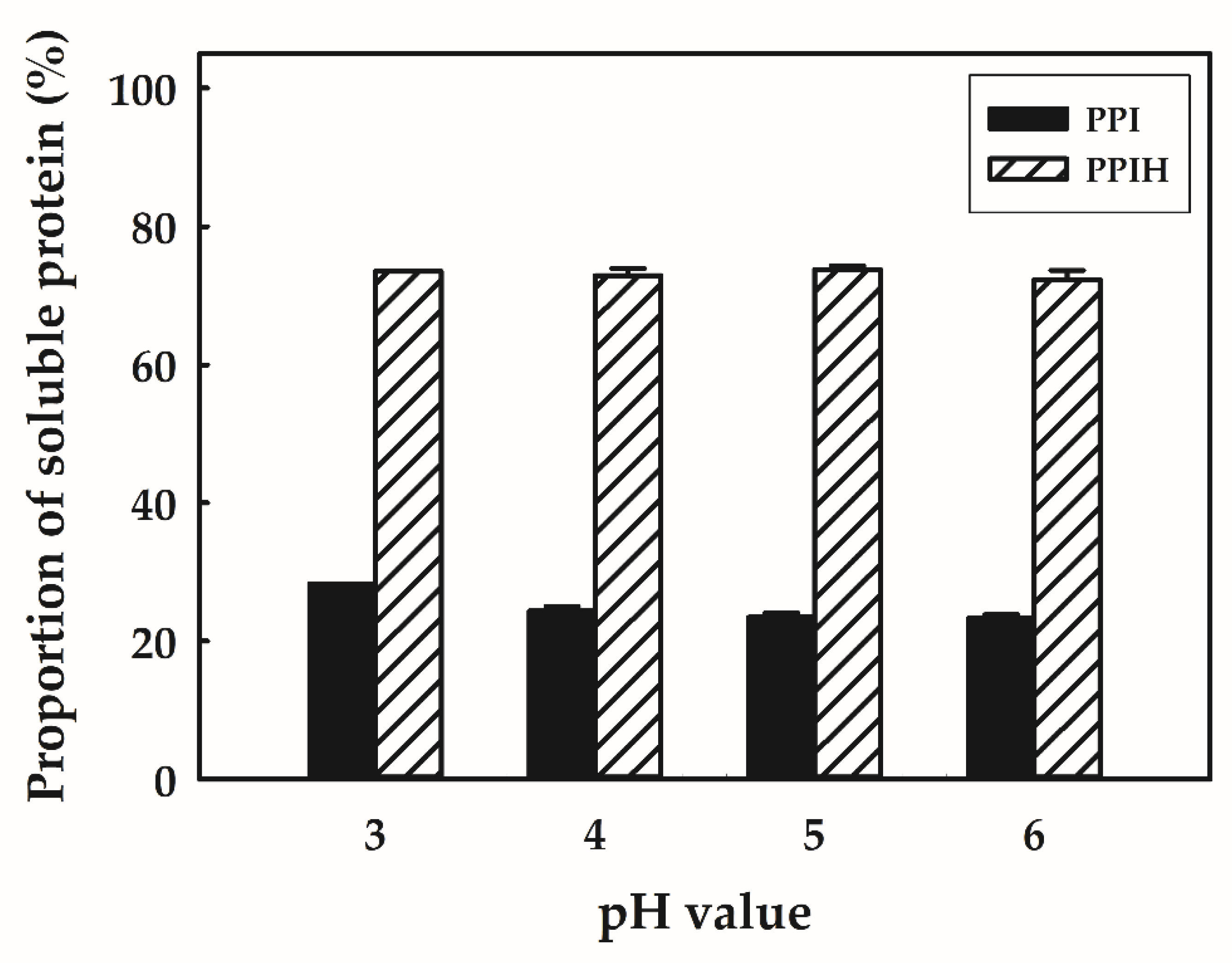
2.1. Solubility of PPI and PPIH Samples under Acidic Conditions
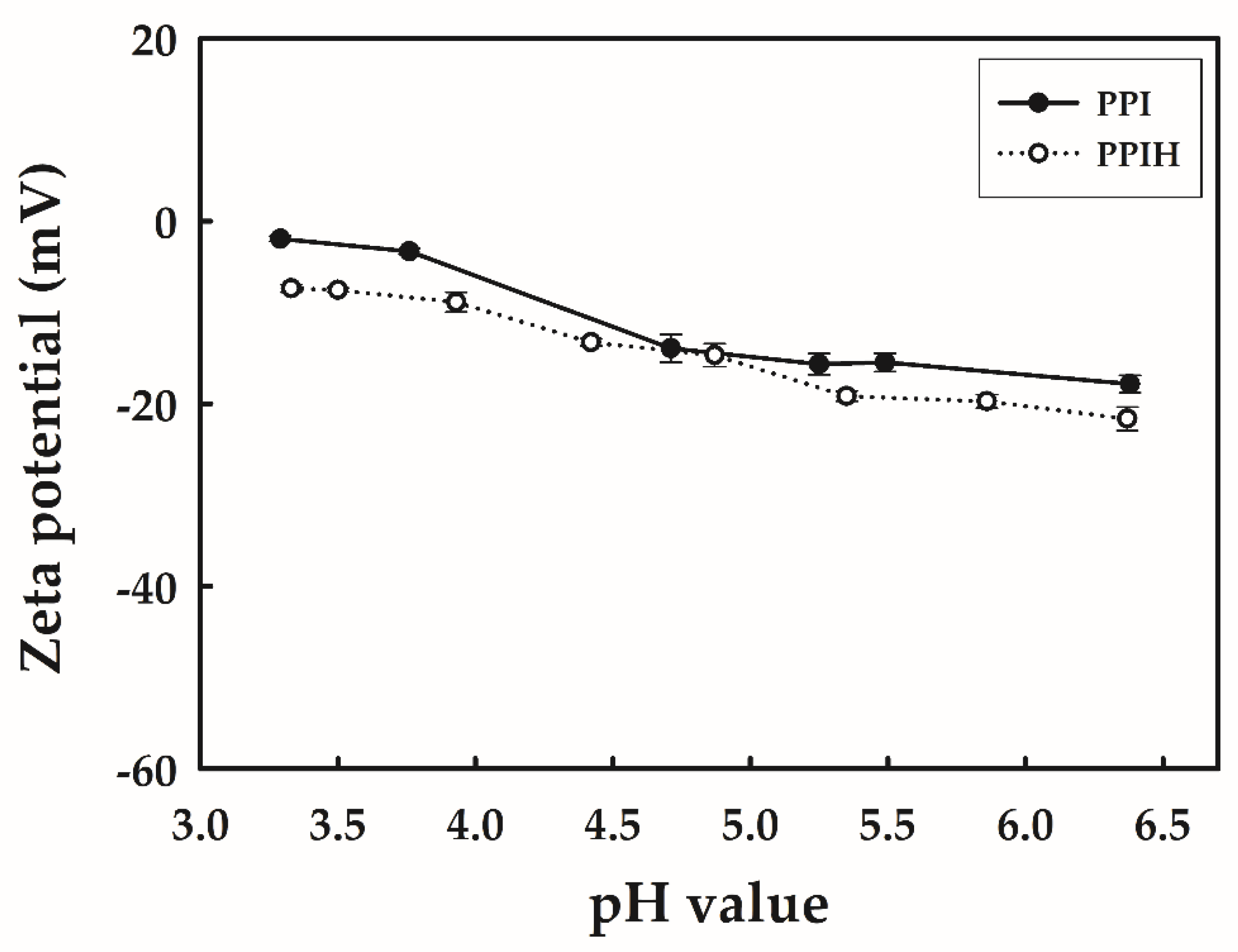
2.2. Zeta Potential of PPI and PPIH Samples under Acidic Conditions
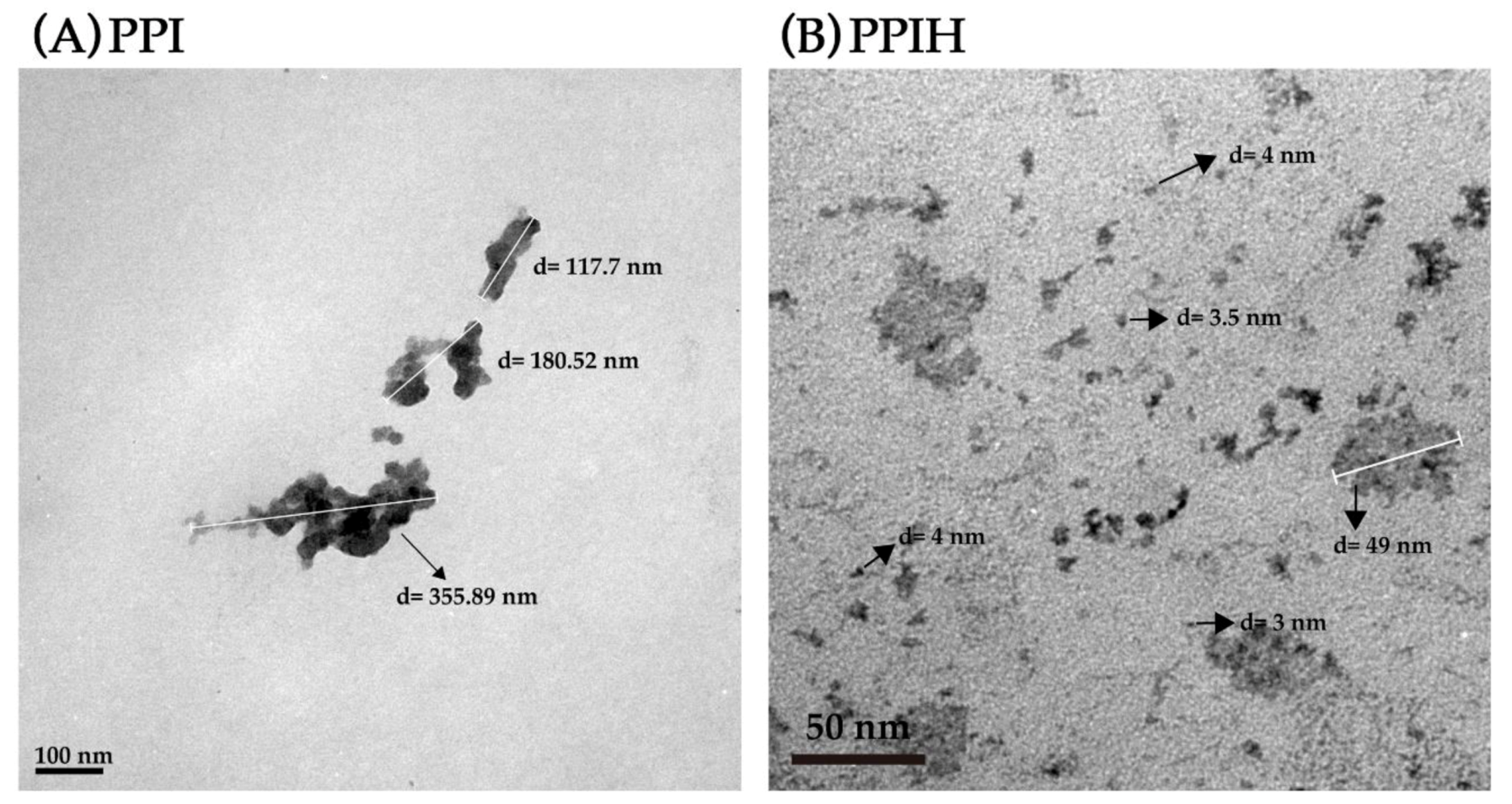
2.3. Particle Size and TEM Analysis of PPI and PPIH Samples
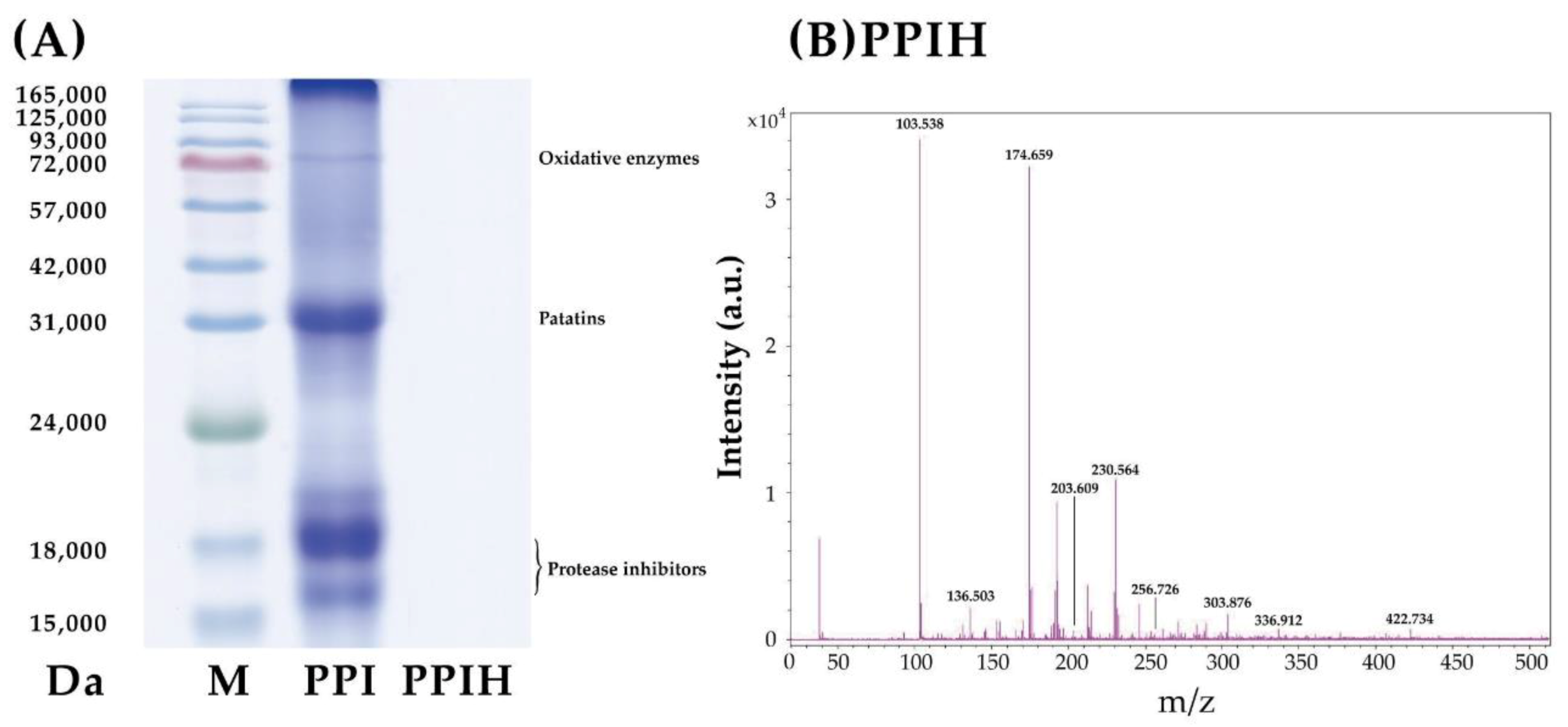
2.4. SDS-PAGE and Mass Spectrometry Analysis of PPI and PPIH
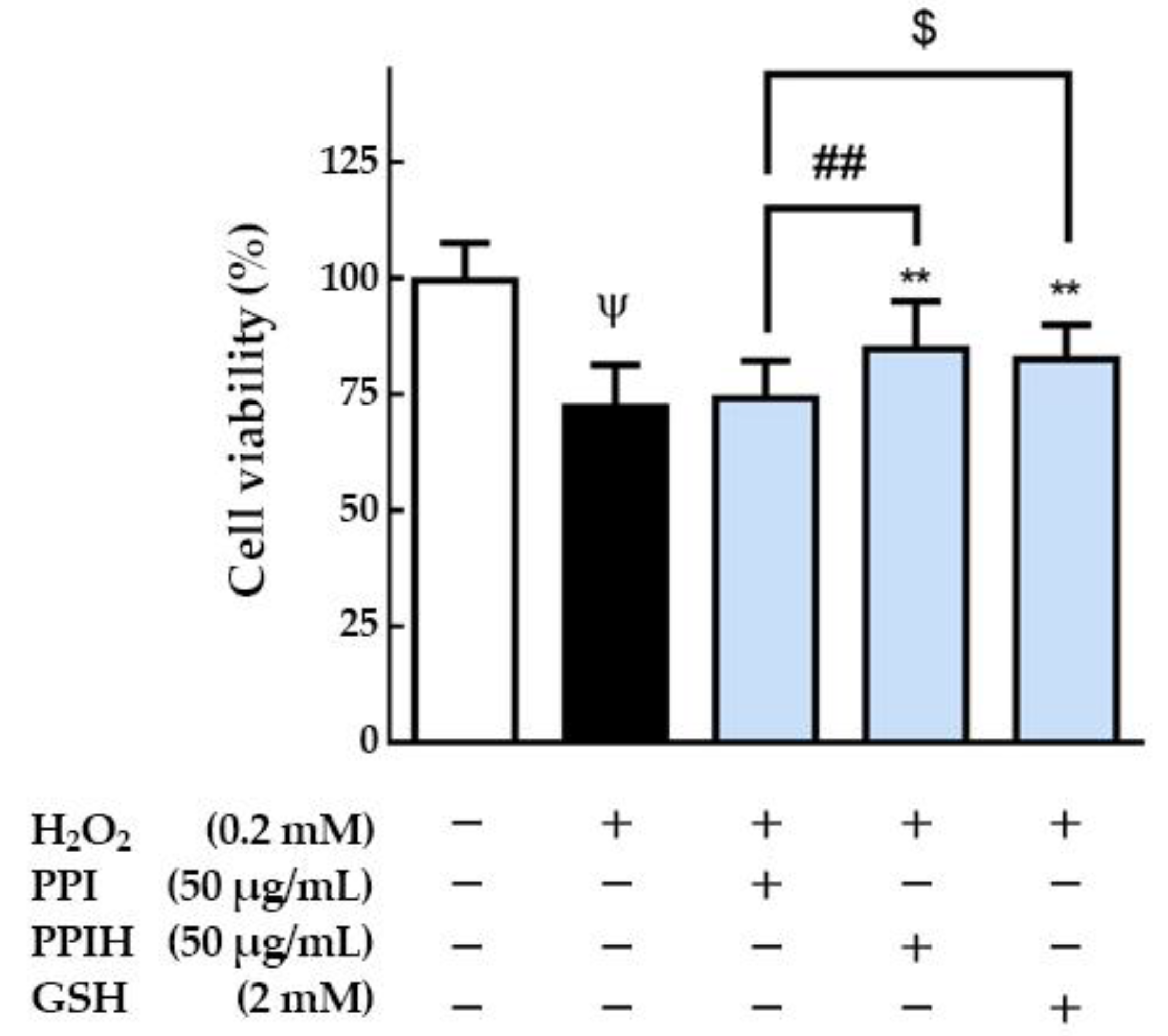
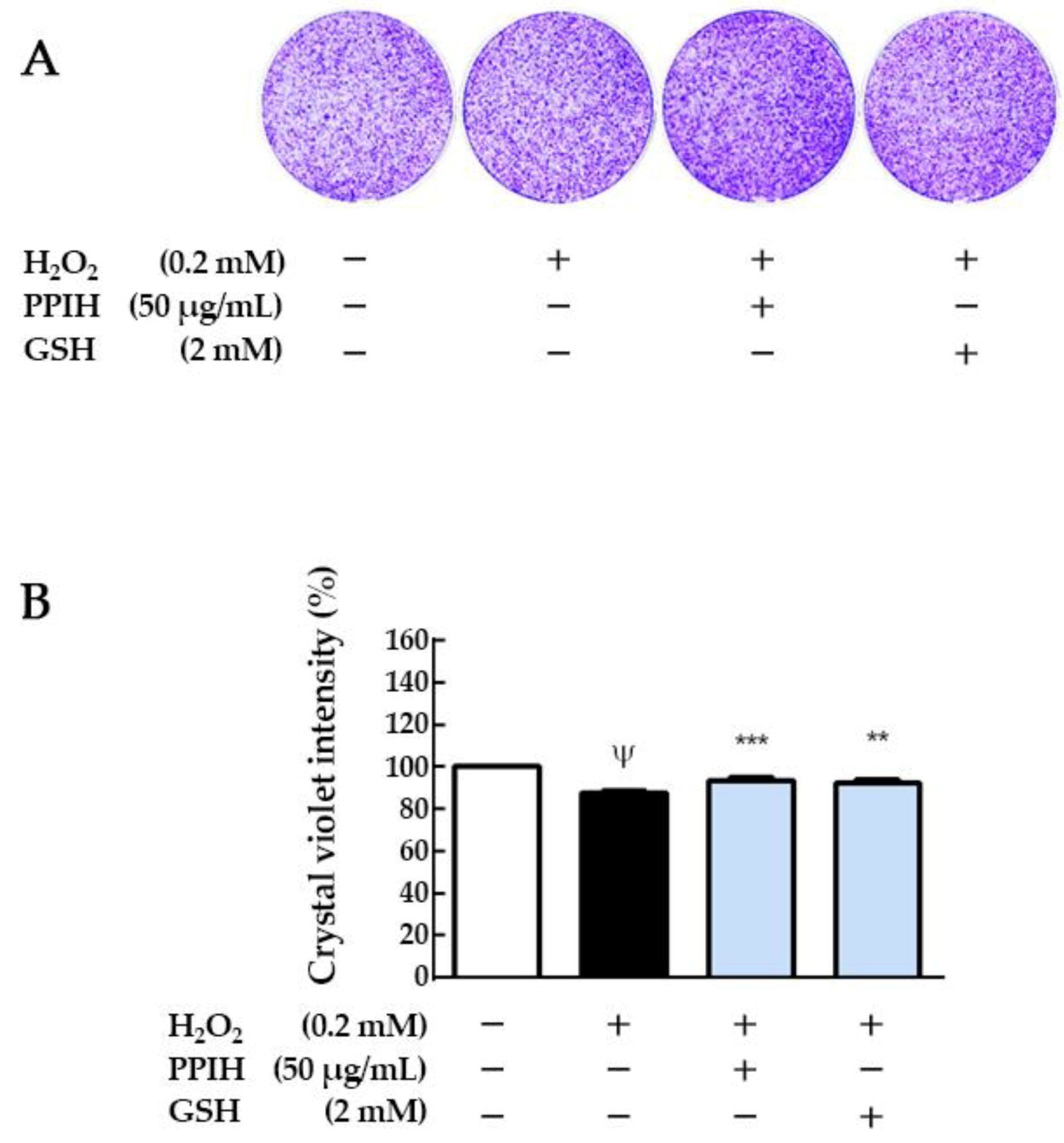
2.5. Antioxidant Effects of PPIH on Muscle C2C12 Cells
3. Materials and Methods
3.1. Preparation of PPI and PPIH
3.2. Solubility of PPI and PPIH under Acidic Conditions
3.3. Determination of the MW Distributions of PPI and PPIH
3.4. The Particle Size of PPI and PPIH
3.5. Microstructure of PPI and PPIH
3.6. Zeta Potential Measurements
3.7. ABTS Radical-Scavenging Assay
3.8. Cell Culture
3.9. Cell Viability Assay
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chung, I.M.; Kim, J.K.; Jin, Y.I.; Oh, Y.T.; Prabakaran, M.; Youn, K.J.; Kim, S.H. Discriminative study of a potato (Solanum tuberosum L.) cultivation region by measuring the stable isotope ratios of bio-elements. Food Chem. 2016, 212, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Waglay, A.; Karboune, S.; Alli, I. Potato protein isolates: Recovery and characterization of their properties. Food Chem. 2014, 142, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Camire, M.E.; Kubow, S.; Donnelly, D.J. Potatoes and human health. Crit. Rev. Food Sci. Nutr. 2009, 49, 823–840. [Google Scholar] [CrossRef] [PubMed]
- Miedzianka, J.; Pęksa, A.; Aniołowska, M. Properties of acetylated potato protein preparations. Food Chem. 2012, 133, 1283–1291. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, W.N.; Soladoye, O.P. Towards potato protein utilisation: Insights into separation, functionality and bioactivity of patatin. Int. J. Food Sci. Technol. 2020, 55, 2314–2322. [Google Scholar] [CrossRef]
- Elias, R.J.; Kellerby, S.S.; Decker, E.A. Antioxidant activity of proteins and peptides. Crit. Rev. Food Sci. Nutr. 2008, 48, 430–441. [Google Scholar] [CrossRef]
- Pihlanto, A.; Akkanen, S.; Korhonen, H.J. ACE-inhibitory and antioxidant properties of potato (Solanum tuberosum). Food Chem. 2008, 109, 104–112. [Google Scholar] [CrossRef]
- Gambuti, A.; Rinaldi, A.; Moio, L. Use of patatin, a protein extracted from potato, as alternative to animal proteins in fining of red wine. Eur. Food Res. Technol. 2012, 235, 753–765. [Google Scholar] [CrossRef]
- Spelbrink, R.E.; Lensing, H.; Egmond, M.R.; Giuseppin, M.L. Potato patatin generates short-chain fatty acids from milk fat that contribute to flavour development in cheese ripening. Appl. Biochem. Biotechnol. 2015, 176, 231–243. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Udechukwu, M.C.; Yiridoe, C.; Gibson, A.; Gong, M. Antioxidant mechanism of potato protein hydrolysates against in vitro oxidation of reduced glutathione. J. Funct. Foods 2016, 20, 195–203. [Google Scholar] [CrossRef]
- Akbari, N.; Mohammadzadeh Milani, J.; Biparva, P. Functional and conformational properties of proteolytic enzyme-modified potato protein isolate. J. Sci. Food Agric. 2020, 100, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- Asokan, S.M.; Wang, T.; Wang, M.F.; Lin, W.T. A novel dipeptide from potato protein hydrolysate augments the effects of exercise training against high-fat diet-induced damages in senescence-accelerated mouse-prone 8 by boosting pAMPK/SIRT1/PGC-1α/pFOXO3 pathway. Aging (Albany NY) 2020, 12, 7334–7349. [Google Scholar] [CrossRef] [PubMed]
- Evangelho, J.A.D.; Vanier, N.L.; Pinto, V.Z.; Berrios, J.J.; Dias, A.R.G.; Zavareze, E.D.R. Black bean (Phaseolus vulgaris L.) protein hydrolysates: Physicochemical and functional properties. Food Chem. 2017, 214, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Kudo, K.; Onodera, S.; Takeda, Y.; Benkeblia, N.; Shiomi, N. Antioxidative activities of some peptides isolated from hydrolyzed potato protein extract. J. Funct. Foods 2009, 1, 170–176. [Google Scholar] [CrossRef]
- Moure, A.; Sineiro, J.; Domínguez, H.; Parajó, J.C. Functionality of oilseed protein products: A review. Food Res. Int. 2006, 39, 945–963. [Google Scholar] [CrossRef]
- Corrochano, A.R.; Sariçay, Y.; Arranz, E.; Kelly, P.M.; Buckin, V.; Giblin, L. Comparison of antioxidant activities of bovine whey proteins before and after simulated gastrointestinal digestion. J. Dairy Sci. 2019, 102, 54–67. [Google Scholar] [CrossRef] [PubMed]
- López-García, B.; Hernández, M.; Segundo, B.S. Bromelain, a cysteine protease from pineapple (Ananas comosus) stem, is an inhibitor of fungal plant pathogens. Lett. Appl. Microbiol. 2012, 55, 62–67. [Google Scholar] [CrossRef]
- Merz, M.; Eisele, T.; Berends, P.; Appel, D.; Rabe, S.; Blank, I.; Stressler, T.; Fischer, L. Flavourzyme, an enzyme preparation with industrial relevance: Automated nine-step purification and partial characterization of eight enzymes. J. Agric. Food Chem. 2015, 63, 5682–5693. [Google Scholar] [CrossRef]
- Hou, Y.; Wu, Z.; Dai, Z.; Wang, G.; Wu, G. Protein hydrolysates in animal nutrition: Industrial production, bioactive peptides, and functional significance. J. Anim. Sci. Biotechnol. 2017, 8, 24. [Google Scholar] [CrossRef]
- Pęksa, A.; Miedzianka, J. Amino acid composition of enzymatically hydrolysed potato protein preparations. Czech J. Food Sci. 2014, 32, 265–272. [Google Scholar] [CrossRef]
- Cheng, Y.; Xiong, Y.L.; Chen, J. Antioxidant and emulsifying properties of potato protein hydrolysate in soybean oil-in-water emulsions. Food Chem. 2010, 120, 101–108. [Google Scholar] [CrossRef]
- Schmidt, J.M.; Damgaard, H.; Greve-Poulsen, M.; Larsen, L.B.; Hammershøj, M. Foam and emulsion properties of potato protein isolate and purified fractions. Food Hydrocoll. 2018, 74, 367–378. [Google Scholar] [CrossRef]
- Chuacharoen, T.; Sabliov, C.M. Stability and controlled release of lutein loaded in zein nanoparticles with and without lecithin and pluronic F127 surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2016, 503, 11–18. [Google Scholar] [CrossRef]
- Mahobia, S.; Bajpai, J.; Bajpai, A.K. An in-vitro investigation of swelling controlled delivery of insulin from egg albumin nanocarriers. Iran. J. Pharm. Res. 2016, 15, 695–711. [Google Scholar] [PubMed]
- Klompong, V.; Benjakul, S.; Kantachote, D.; Shahidi, F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2007, 102, 1317–1327. [Google Scholar] [CrossRef]
- Midelfort, K.S.; Wittrup, K.D. Context-dependent mutations predominate in an engineered high-affinity single chain antibody fragment. Protein Sci. 2006, 15, 324–334. [Google Scholar] [CrossRef]
- Ripple, D.C.; Dimitrova, M.N. Protein particles: What we know and what we do not know. J. Pharm. Sci. 2012, 101, 3568–3579. [Google Scholar] [CrossRef]
- Ryan, K.N.; Vardhanabhti, B.; Jaramillo, D.P.; van Zanten, J.H.; Coupland, J.N.; Foegeding, E.A. Stability and mechanism of whey protein soluble aggregates thermally treated with salts. Food Hydrocoll. 2012, 27, 411–420. [Google Scholar] [CrossRef]
- Pouvreau, L.; Gruppen, H.; Piersma, S.R.; van den Broek, L.A.; van Koningsveld, G.A.; Voragen, A.G.J. Relative abundance and inhibitory distribution of protease inhibitors in potato juice from cv. Elkana. J. Agric. Food Chem. 2001, 49, 2864–2874. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.R.; Wang, B.; Chi, C.F.; Gong, Y.; Luo, H.Y.; Ding, G.F. Influence of average molecular weight on antioxidant and functional properties of cartilage collagen hydrolysates from Sphyrna lewini, Dasyatis akjei and Raja porosa. Food Res. Int. 2013, 51, 283–293. [Google Scholar] [CrossRef]
- Sánchez, A.; Vázquez, A. Bioactive peptides: A review. Food Qual. Saf. 2017, 1, 29–46. [Google Scholar] [CrossRef]
- Cheng, Y.; Xiong, Y.L.; Chen, J. Fractionation, separation, and identification of antioxidative peptides in potato protein hydrolysate that enhance oxidative stability of soybean oil emulsions. J. Food Sci. 2010, 75, 760–765. [Google Scholar] [CrossRef] [PubMed]
- De Castro, R.J.S.; Sato, H.H. Comparison and synergistic effects of intact proteins and their hydrolysates on the functional properties and antioxidant activities in a simultaneous process of enzymatic hydrolysis. Food Bioprod. Process. 2014, 92, 80–88. [Google Scholar] [CrossRef]
- Hood, D.A.; Uguccioni, G.; Vainshtein, A.; D’souza, D. Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle: Implications for health and disease. Compr. Physiol. 2011, 1, 1119–1134. [Google Scholar]
- Nishida, H.; Ichikawa, H.; Konishi, T. Shengmai-san enhances antioxidant potential in C2C12 myoblasts through the induction of intracellular glutathione peroxidase. J. Pharmacol. Sci. 2007, 105, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Kerasioti, E.; Stagos, D.; Priftis, A.; Aivazidis, S.; Tsatsakis, A.M.; Hayes, A.W.; Kouretas, D. Antioxidant effects of whey protein on muscle C2C12 cells. Food Chem. 2014, 155, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.X.; Ling, Y.F.; Sun, Z.; Zhang, L.; Yu, H.X.; Kamau, S.M.; Lu, R.R. Protective effect of whey protein hydrolysates against hydrogen peroxide-induced oxidative stress on PC12 cells. Biotechnol. Lett. 2012, 34, 2001–2006. [Google Scholar] [CrossRef] [PubMed]
- Intiquilla, A.; Jiménez-Aliaga, K.; Zavaleta, A.I.; Arnao, I.; Peña, C.; Chávez-Hidalgo, E.L.; Hernández-Ledesma, B. Erythrina edulis (Pajuro) seed protein: A new source of antioxidant peptides. Nat. Prod. Commun. 2016, 11, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids. 2018, 50, 1685–1695. [Google Scholar] [CrossRef]
- Shen, Y.R.; Kuo, M.I. Effects of different carrageenan types on the rheological and water holding properties of tofu. LWT-Food Sci. Technol. 2017, 78, 122–128. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Win, K.Y.; Feng, S.S. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials 2005, 26, 2713–2722. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Wu, L.C.; Lu, S.Y.; Chang, L.L.; Teng, I.T.; Yang, C.M.; Ho, J.A. Biofunctionalized phospholipid-capped mesoporous silica nanoshuttles for targeted drug delivery: Improved water suspensibility and decreased nonspecific protein binding. ACS Nano 2010, 4, 4371–4379. [Google Scholar] [CrossRef]
- Tang, C.H.; Sun, X. Physicochemical and structural properties of 8S and/or 11S globulins from mungbean [Vigna radiata (L.) Wilczek] with various polypeptide constituents. J. Agric. Food Chem. 2010, 58, 6395–6402. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Bahuguna, A.; Khan, I.; Bajpai, V.K.; Kang, S.C. MTT assay to evaluate the cytotoxic potential of a drug. Bangladesh J. Pharmacol. 2017, 12, 115–118. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-Y.; Jin, J.-D.; Chang, H.-L.; Huang, K.-C.; Chiang, Y.-F.; Hsia, S.-M. Physicochemical and Antioxidative Characteristics of Potato Protein Isolate Hydrolysate. Molecules 2020, 25, 4450. https://doi.org/10.3390/molecules25194450
Chang C-Y, Jin J-D, Chang H-L, Huang K-C, Chiang Y-F, Hsia S-M. Physicochemical and Antioxidative Characteristics of Potato Protein Isolate Hydrolysate. Molecules. 2020; 25(19):4450. https://doi.org/10.3390/molecules25194450
Chicago/Turabian StyleChang, Chiung-Yueh, Jinn-Der Jin, Hsiao-Li Chang, Ko-Chieh Huang, Yi-Fen Chiang, and Shih-Min Hsia. 2020. "Physicochemical and Antioxidative Characteristics of Potato Protein Isolate Hydrolysate" Molecules 25, no. 19: 4450. https://doi.org/10.3390/molecules25194450
APA StyleChang, C.-Y., Jin, J.-D., Chang, H.-L., Huang, K.-C., Chiang, Y.-F., & Hsia, S.-M. (2020). Physicochemical and Antioxidative Characteristics of Potato Protein Isolate Hydrolysate. Molecules, 25(19), 4450. https://doi.org/10.3390/molecules25194450






