Bioactive Compounds and Health-Promoting Properties of Pear (Pyrus communis L.) Fruits
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties of Pear Fruits
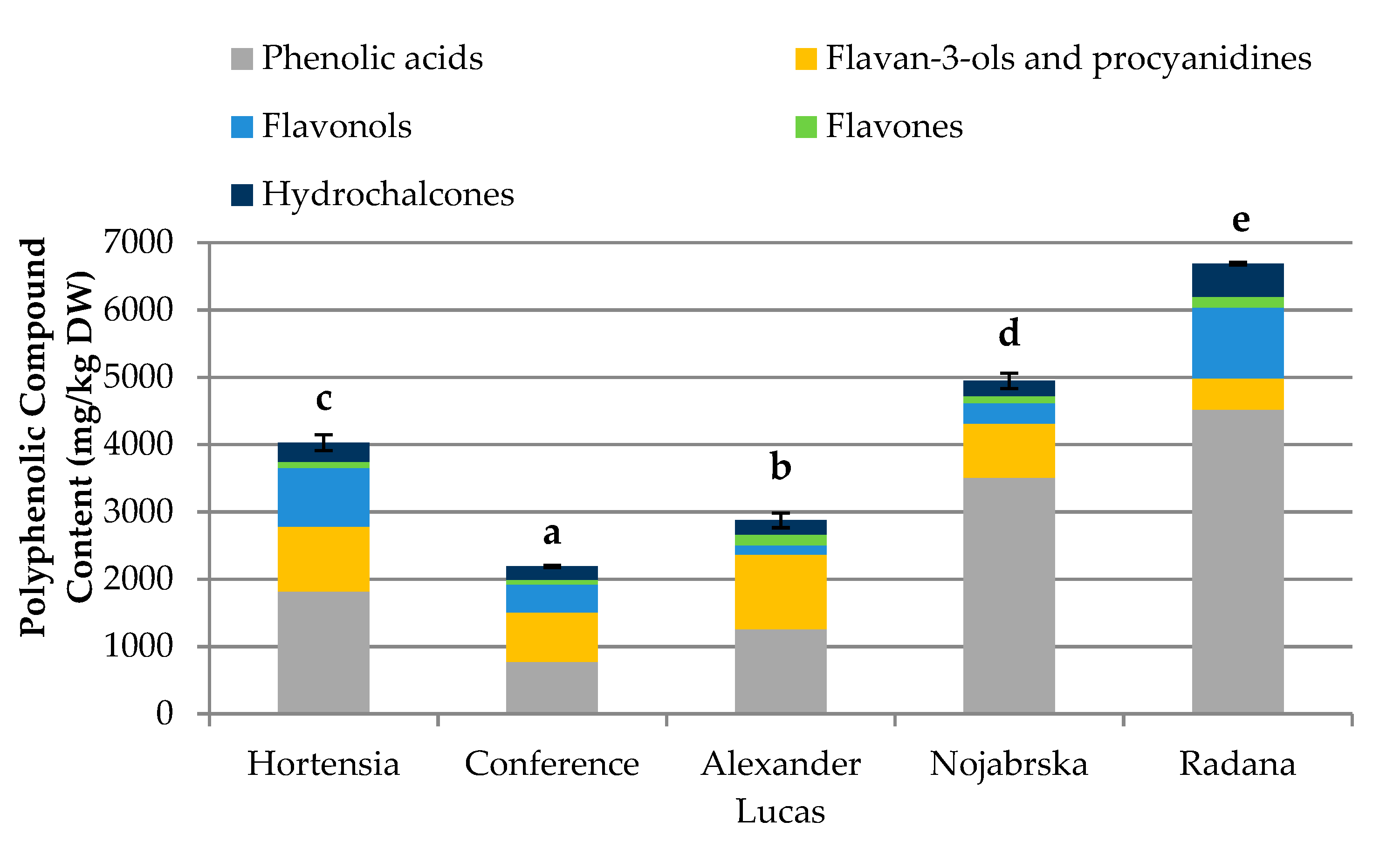
2.2. Quantification of the Phenolic Compounds in the Pear Fruits
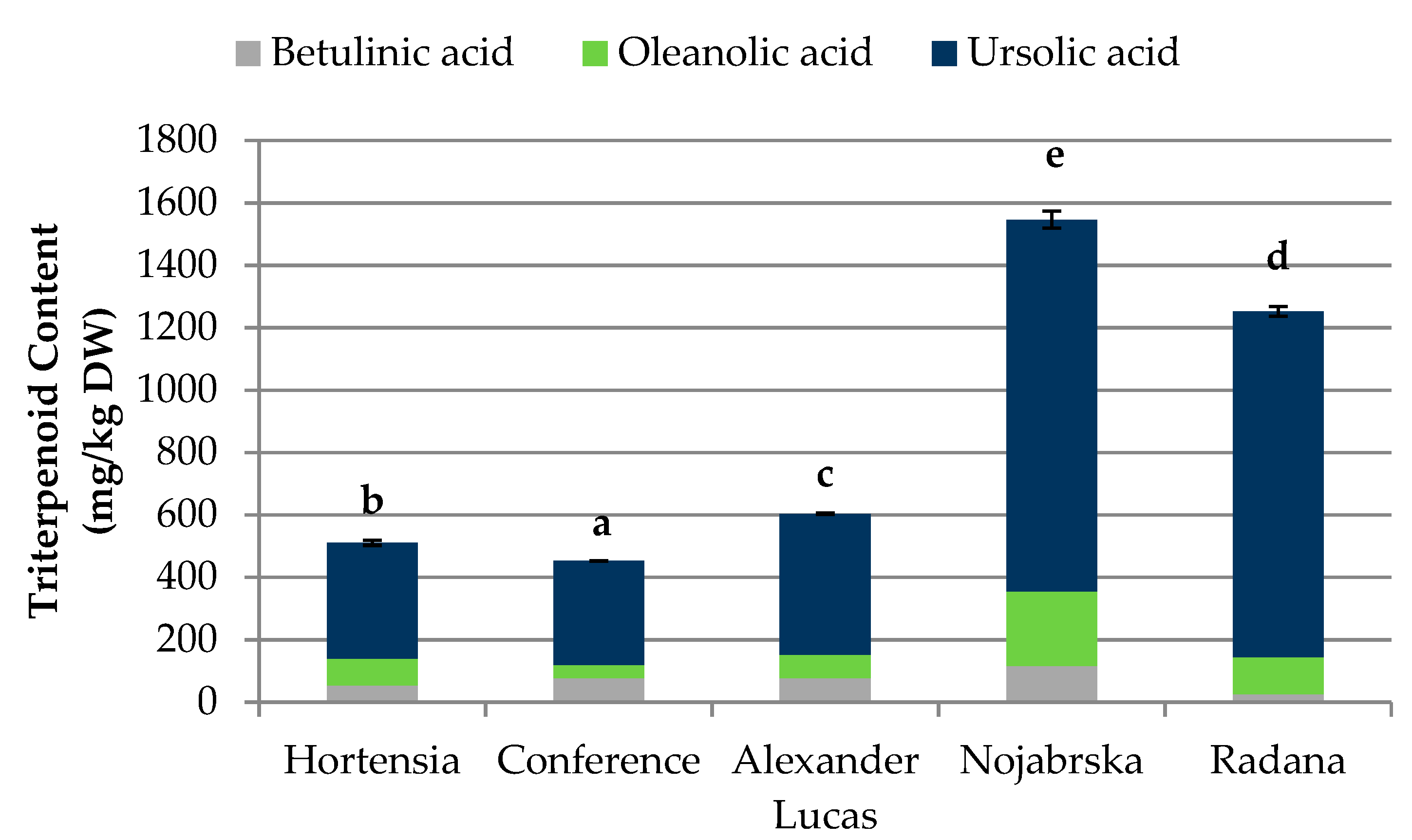
2.3. Quantification of the Triterpenoids in Pear Fruits
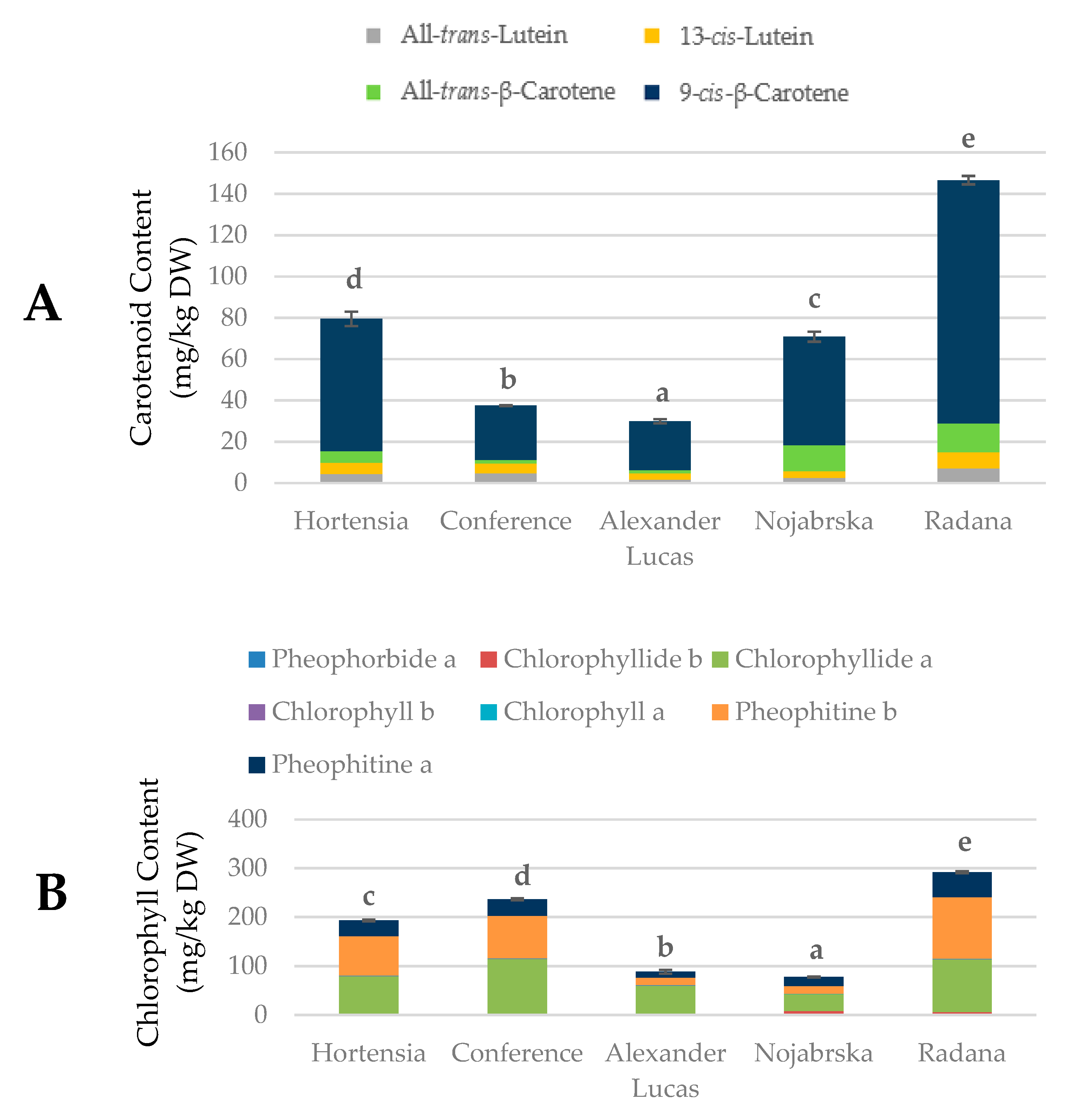
2.4. Quantification of the Carotenoids and Chlorophylls in Pear Fruits
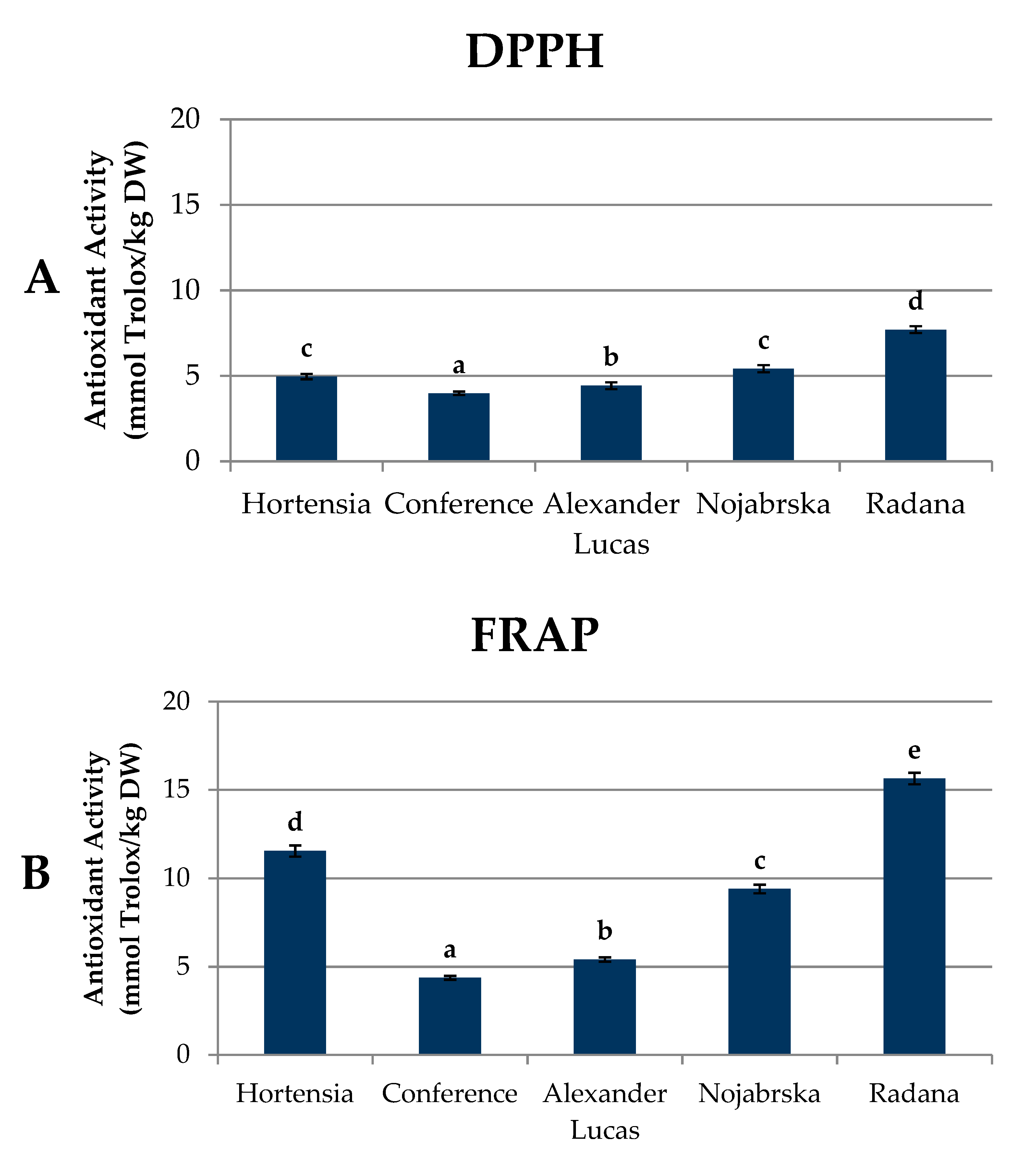
2.5. Antioxidant Capacity of Pear Fruits
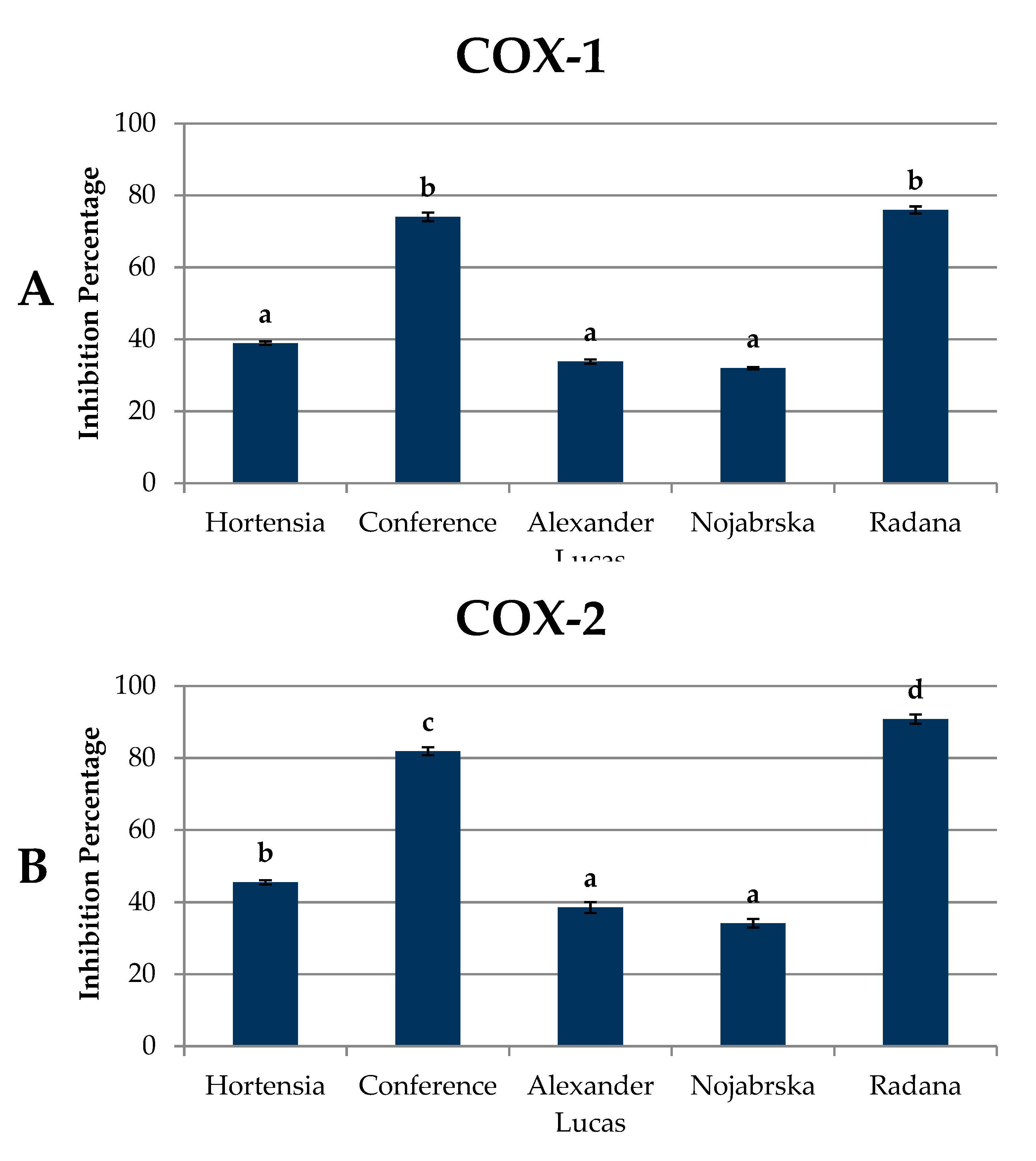
2.6. Anti-Inflammatory Activity of Pear Fruits
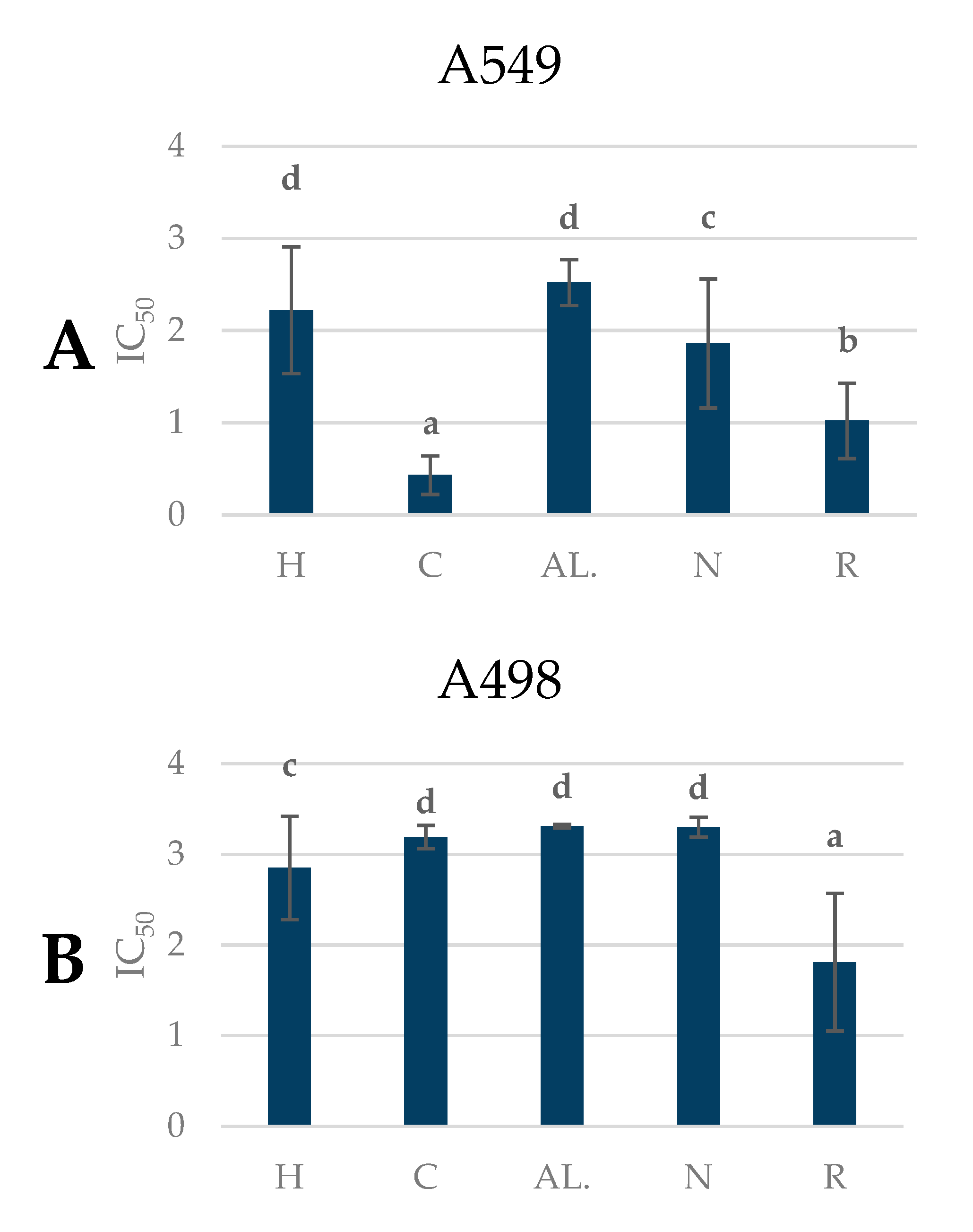
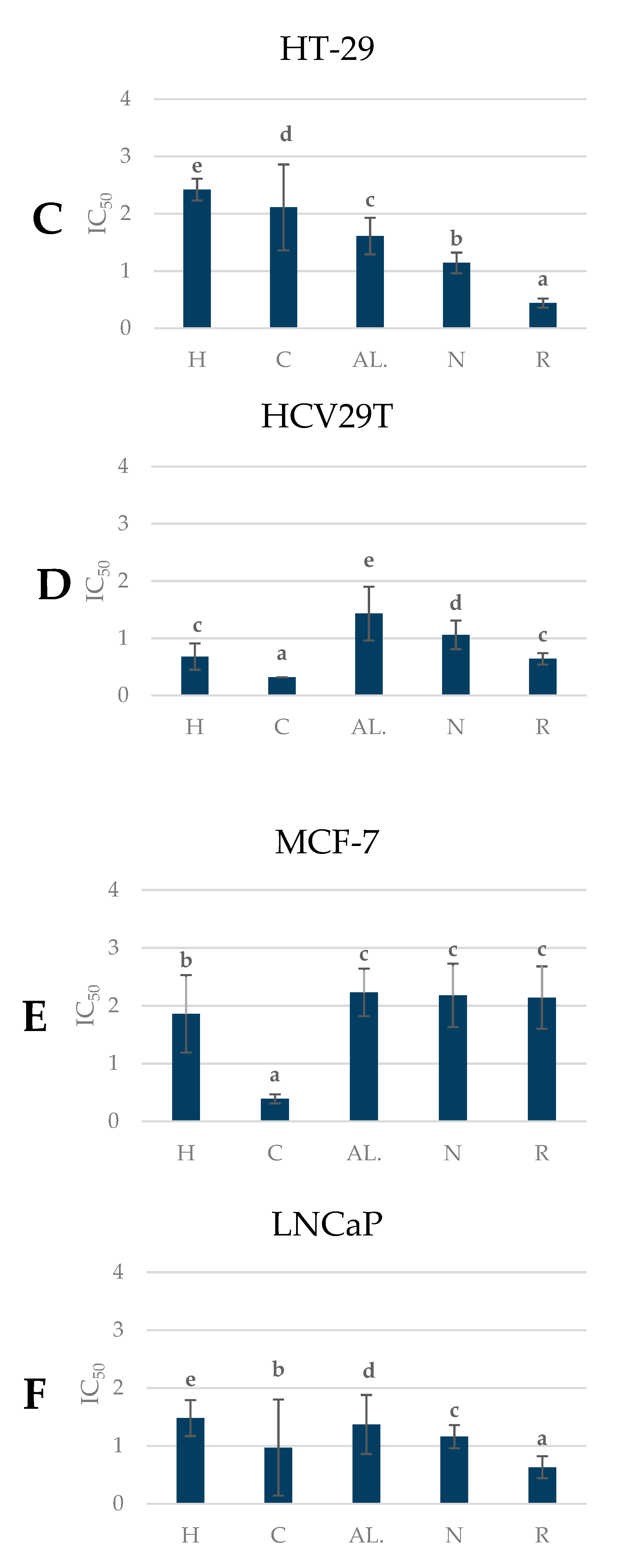
2.7. Antiproliferative Activity of Pear Fruits
3. Materials and Methods
3.1. Reagents and Standards
3.2. Plant Material
3.3. Physicochemical Analyses
3.4. Identification and Quantification of the Polyphenols Using the UPLC-PDA-MS Method
3.5. Analysis of the Proanthocyanidins Using the Phloroglucinolysis Method
3.6. Analysis of the Triterpenoids Using the UPLC-PDA-MS Method
3.7. Analysis of the Carotenoids and Chlorophylls Using the UPLC-PDA-MS Method
3.8. Analysis of the Antioxidant Activity
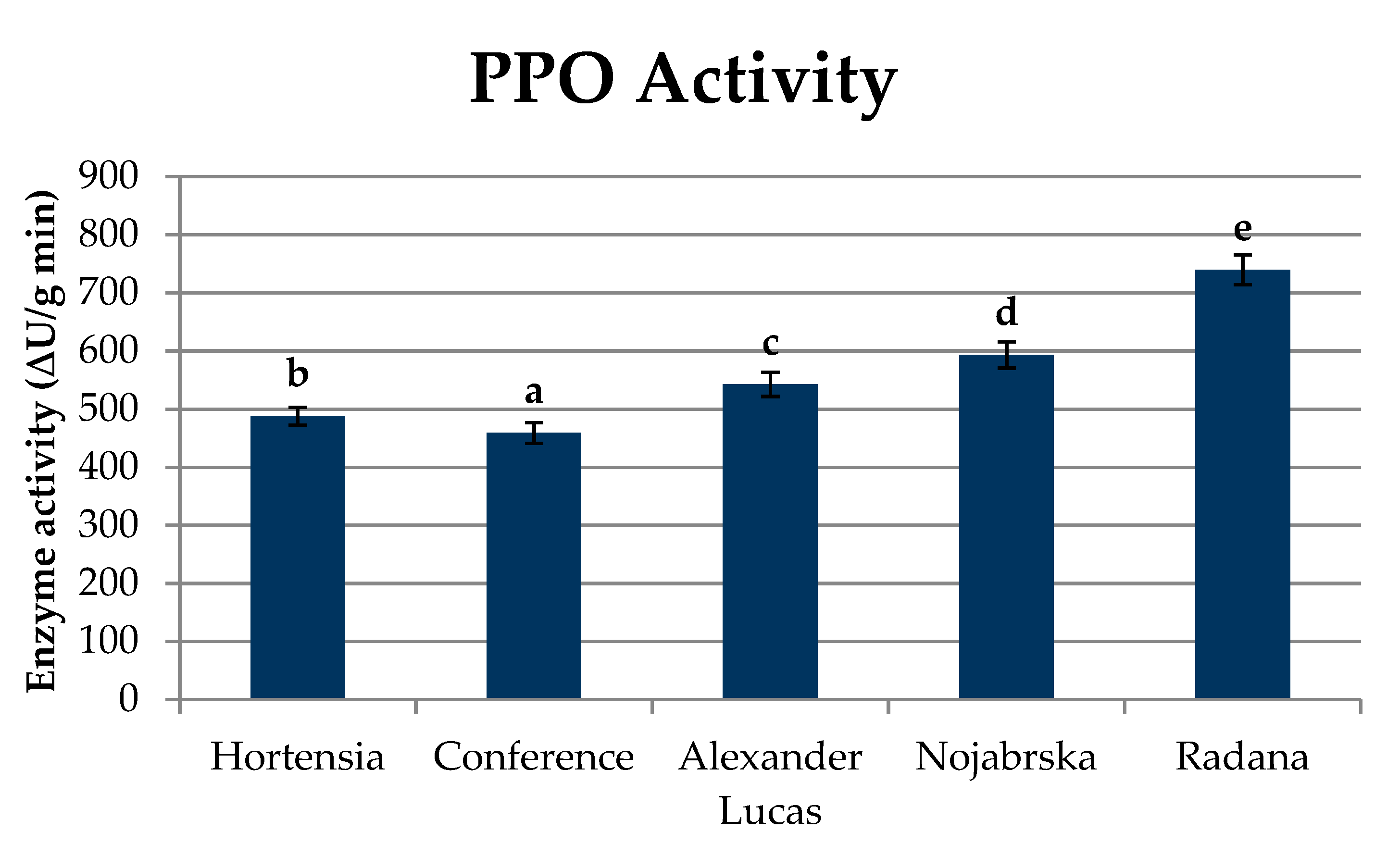
3.9. Analysis of the Polyphenol Oxidase Activity
3.10. Anti-Inflammatory Activity
3.11. Antiproliferative Assay In Vitro
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pawlowska, E.; Szczepanska, J.; Koskela, K.; Kaarniranta, K.; Blasiak, J. Dietary polyphenols in age-related macular degeneration: Protection against oxidative stress and beyond. Oxid. Med. Cell. Longev. 2019, 9682318. [Google Scholar] [CrossRef] [PubMed]
- Gayer, B.A.; Avendano, E.E.; Edelson, E.; Nirmala, A.; Johnson, E.J.; Raman, G. Effects of intake of apples, pears, or their products on cardiometabolic risk factors and clinical outcomes: A systematic review and meta-Analysis. Curr. Dev. Nutr. 2019, 3, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Borges, G.; Mullen, W.; Crozier, A. Comparison of the polyphenolic composition and antioxidant activity of European commercial fruit juices. Food Funct. 2010, 1, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Proc. 2011, 89, 217–233. [Google Scholar] [CrossRef]
- Pandareesh, M.D.; Mythri, R.B.; Srinivas Bharath, M.M. Bioavailability of dietary polyphenols: Factors contributing to their clinical application in CNS diseases. Neurochem. Int. 2015, 89, 198–208. [Google Scholar] [CrossRef]
- Ayeleso, T.B.; Matumba, M.G.; Mukwevho, E. Oleanolic acid and its derivatives: Biological activities and therapeutic potential in chronic diseases. Molecules 2017, 22, 1915. [Google Scholar] [CrossRef]
- Chakravarti, B.; Maurya, R.; Siddiqui, J.A.; Bid, H.K.; Rajendran, S.M.; Yadav, P.P.; Konwar, R. In vitro anti-breast cancer activity of ethanolic extract of Wrightia tomentosa: Role of pro-apoptotic effects of oleanolic acid and urosolic acid. J. Ethnopharmacol. 2012, 142, 72–79. [Google Scholar] [CrossRef]
- Michaud, D.S.; Feskanich, D.; Rimm, E.B.; Colditz, G.A.; Speizer, F.E.; Willett, W.C. Intake of specific carotenoids and risk of lung cancer in 2 prospective US cohorts. Am. J. Clin. Nutr. 2000, 72, 990–997. [Google Scholar] [CrossRef]
- Sathasivam, R.; Ki, J.-S. A review of the biological activities of microalgal carotenoids and their potential use in healthcare and cosmetic industries. Mar. Drugs 2018, 16, 26. [Google Scholar] [CrossRef]
- Paprstein, F. Prospective Pear Cultivars Bred in the Czech Republic. Proc. Xth IS on Pear. Eds.: A.D. Webster and C.M. Oliveira. Acta Hort. 2008, 800, 343–348. [Google Scholar] [CrossRef]
- Konopacka, D.; Rutkowski, K.P.; Kruczyńska, D.E.; Skorupińska, A.; Płocharski, W. Quality potential of some new pear cultivars – hoe to obtain fruit of the best sensory characteristics? J. Hort. Res. 2014, 22, 71–84. [Google Scholar] [CrossRef]
- Wawrzyńczak, A.; Rutkowski, K.P.; Kruczyńska, D. Changes in fruit quality in pears during CA storage. J. Fruit Ornam. Plant Res. 2006, 14, 77–84. [Google Scholar]
- Wawrzyńczak, A.; Rutkowski, K.P.; Kruczynska, D. Ripening of ‘Radana’ and ‘Conference’ pears as influenced by cold storage duration. Proc. Xth IS on Pear. Eds.: A.D. Webster and C.M. Oliveira. Acta Hort. 2008, 800, 1091–1098. [Google Scholar] [CrossRef]
- Kappel, F.; Fisher-Fleming, R.; Hogue, E.J. Ideal Pear Sensory Attributes and Fruit Characteristics. Hort. Sci. 1995, 30, 988–993. [Google Scholar] [CrossRef]
- Li, X.; Wang, T.; Zhou, B.; Gao, W.; Cao, J.; Huang, L. Chemical composition and antioxidant and anti-inflammatory potential of peels and flesh from 10 different pear varieties (Pyrus spp.). Food Chem. 2014, 152, 531–538. [Google Scholar] [CrossRef]
- Özturk, A.; Demirsoy, L.; Demirsoy, H.; Asan, A.; Gül, O. Phenolic compounds and chemical characteristics of pears (Pyrus communis L.). Int. J Food Prop. 2015, 18, 536–546. [Google Scholar] [CrossRef]
- Cebulak, T.; Oszmiański, J.; Kapusta, I.; Lachowicz, S. Efect of abiotic stress factors on polyphenolic content in the skin and fesh of pear by UPLC-PDA-Q/TOF-MS. Eur. Food Res. Int. 2019, 245, 2715–2725. [Google Scholar] [CrossRef]
- Liaudanskas, M.; Zymonè, K.; Viškelis, J.; Klevinskas, A.; Janulis, V. Determination of the phenolic composition and nntioxidant activity of Pear extracts. J. Chem. 2017, 7856521. [Google Scholar] [CrossRef]
- Górnaś, P.; Mišina, I.; Olšteine, A.; Krasnova, I.; Pugajeva, I.; Lācis, G.; Siger, A.; Michalak, M.; Soliven, A.; Seglina, D. Phenolic compounds in different fruit parts of crab apple: Dihydrochalcones as promising quality markers of industrial apple pomace by-products. Ind. Crop. Prod. 2015, 74, 607–612. [Google Scholar] [CrossRef]
- Teleszko, M.; Wojdyło, A. Comparison of phenolic compounds and antioxidant potential between selected edible fruits and their leaves. J. Funct. Foods 2015, 14, 736–746. [Google Scholar] [CrossRef]
- Fevrier, H.; Le Quere, J.-M.; Le Bail, G.; Guyot, S. Polyphenol profile, PPO activity and pH variation in relation to colour changes in a series of red-fleshed apple juices. LWT Food Sci. Technol. 2016, 85, 353–362. [Google Scholar] [CrossRef]
- Podsędek, A.; Wilska-Jeszka, J.; Anders, B.; Markowski, J. Compositional characterisation of some apple varieties. Eur. Food Res. Technol. 2000, 210, 268–272. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Bielecki, P. Polyphenolic composition, antioxidant activity, and polyphenol oxidase (PPO) activity of quince (Cydonia oblonga Miller) varieties. J. Agric. Food Chem. 2013, 61, 2762–2772. [Google Scholar] [CrossRef] [PubMed]
- Amiot, M.J.; Tacchini, M.; Aubert, S.; Nicolas, J. Phenolic composition and browning susceptibility of various apple cultivars at maturity. J. Food Sci. 1999, 57, 958–962. [Google Scholar] [CrossRef]
- Sun, L.; Tao, S.; Zhang, S. Characterization and quantification of polyphenols and triterpenoids in thinned young fruits of ten pear varieties by UPLC-Q TRAP-MS/MS. Molecules 2019, 24, 159. [Google Scholar] [CrossRef] [PubMed]
- Kolniak-Ostek, J. Chemical composition and antioxidant capacity of different anatomical parts of pear (Pyrus communis L.). Food Chem. 2016, 203, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, X.; Zhou, B.; Li, H.; Zeng, J.; Gao, W. Antidiabetic activity in type 2 diabetic mice and α-glucosidase inhibitory, antioxidant and anti-inflammatory potential of chemically profiled pear peel and pulp extracts (Pyrus spp.). J. Funct. Foods. 2015, 13, 276–288. [Google Scholar] [CrossRef]
- Chudzik, M.; Korzonek-Szlacheta, I.; Król, W. Triterpenes as potentially cytotoxic compounds. Molecules 2015, 20, 1610–1625. [Google Scholar] [CrossRef]
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A. Betulin and betulinic acid in cancer research. J. Pre-Clin. Clin. Res. 2018, 12, 72–75. [Google Scholar] [CrossRef]
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A.; Kurzepa, J. Betulin and betulinic acid: Triterpenoids derivatives with a powerful biological potential. Phytochem. Rev. 2019, 18, 929–951. [Google Scholar] [CrossRef]
- Delgado-Pelayo, R.; Gallardo-Guerrero, L.; Hornero-Méndez, D. Chlorophyll and carotenoid pigments in the peel and flesh of commercial apple fruit varieties. Food Res. Int. 2014, 65, 272–281. [Google Scholar] [CrossRef]
- Martínez-Valdivieso, D.; Font, R.; Blanco-Díaz, M.T.; Moreno-Rojas, J.M.; Gómez, P.; Alonso-Moraga, Á.; Del Rio-Celestino, M. Application of near-infrared reflectance spectroscopy for predicting carotenoid content in summer squash fruit. Comp. Electr. Agric. 2014, 108, 71–79. [Google Scholar] [CrossRef]
- Charoenchongsuk, N.; Matsumoto, D.; Itai, A.; Murayama, H. Ripening characteristics and pigment changes in Russeted pear fruit in response to ethylene and 1-MCP. Horticulturae 2018, 4, 22. [Google Scholar] [CrossRef]
- Miller, E.; Malinowska, K.; Gałęcka, E.; Mrowicka, M.; Kędziora, J. The role of flavonoids as antioxidants in the human body. Polish Med. Merc. 2008, 24, 556–560. (In Polish) [Google Scholar]
- Dudonne, S.; Vitrac, X.; Coutiere, P.; Woillez, M.; Merillon, J.-M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Eberhardt, M.V.; Lee, C.Y.; Liu, R.H. Antioxidant activity of fresh apples. Nature 2000, 405, 903–904. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J.; Oszmiański, J.; Wojdyło, A. Effect of apple leaves addition on physicochemical properties of cloudy beverages. Ind. Crop. Prod. 2013, 44, 413–420. [Google Scholar] [CrossRef]
- Tian, B.; Xu, Z.; Sun, Z.; Lin, J.; Hua, Y. Evaluation of the antioxidant effects of carotenoids from Deinococcus radiodurans through targeted mutagenesis, chemiluminescence, and DNA damage analyses. Biochim. Biophys. Acta 2007, 1770, 902–911. [Google Scholar] [CrossRef]
- Cefarelli, G.; D’Abrosca, B.; Fiorentino, A.; Izzo, A.; Mastellone, C.; Pacifico, S.; Piscopo, V. Free-radical-scavenging and antioxidant activities of secondary metabolites from reddened cv. Annurca apple fruits. J. Agric. Food Chem. 2006, 54, 803–809. [Google Scholar] [CrossRef] [PubMed]
- D’Abrosca, B.; Fiorentino, A.; Monaco, P.; Oriano, P.; Pacifico, S. Annurcoic acid: A new antioxidant ursane triterpene from fruits of cv. Annurca apple. Food Chem. 2006, 98, 285–290. [Google Scholar] [CrossRef]
- Vareed, S.K.; Reddy, M.K.; Schutzki, R.E.; Nair, M.G. Anthocyanins in Cornus alternifolia, Cornus controversa, Cornus kousa and Cornus florida fruits with health benefits. Life Sci. 2006, 78, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Momin, R.A.; Nair, M.G.; Bourquin, L.D. Cyclooxygenase inhibitory and antioxidant cyanidin glycosides in cherries and berries. Phytomedicine 2001, 8, 362–369. [Google Scholar] [CrossRef]
- Živković, J.; Šavikin, K.; Stanisavljević, N.; Zdunić, G.; Stanojković, T.; Samardžić, J. Chemical composition and antiproliferative potential of dried wild apple and pear tea before and after in vitro simulated digestion. J. Serb. Chemic. Soc. 2018, 83, 1315–1326. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; El-Tantawi, M.E.; Kirollos, F.N.; Hammam, W.E. Chemical composition, in vitro cytotoxic and antimicrobial activities of volatile constituents from Pyrus communis L. and Malus domestica Borkh. fruits cultivated in Egypt. J. Essent. Oil Bear. Plants 2018, 21, 1642–1651. [Google Scholar] [CrossRef]
- Gerhauser, C. Cancer chemopreventive potential of apples, apple juice, and apple components. Planta Med. 2008, 74, 1608–1624. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J. Content of bioactive compounds and antioxidant capacity in skin tissues of pear. J. Funct. Foods 2016, 23, 40–51. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Yen, G.C.; Chen, H.Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 2015, 43, 27–32. [Google Scholar] [CrossRef]
- Gonzales, E.M.; de Ancos, B.; Cano, A.P. Partial Characterization of Polyphenol Oxidase Activity in Raspberry Fruits. J. Agric. Food Chem. 1999, 47, 4068–4072. [Google Scholar] [CrossRef] [PubMed]
- Mizgier, P.; Kucharska, A.Z.; Sokół-Łętowska, A.; Kolniak-Ostek, J.; Kidoń, M.; Fecka, I. Characterization of phenolic compounds and antioxidant and anti-inflammatory properties of red cabbage and purple carrot extracts. J. Funct. Foods. 2016, 21, 133–146. [Google Scholar] [CrossRef]
- Wietrzyk, J.; Chodyński, M.; Fitak, H.; Wojdat, E.; Kutner, A.; Opolski, A. Antitumor properties of diastereomeric and geometric analogs of vitamin D3. Anti Cancer Drug. 2007, 18, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Klopotowska, D.; Matuszyk, J.; Wietrzyk, J. Steroid hormone calcitriol and its analog tacalcitol inhibit miR-125b expression in a human breast cancer MCF-7 cell line. Steroids 2019, 141, 70–75. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples are not available from the authors. |
| Features | Hortensia | Conference | Alexander Lucas | Nojabrska | Radana | Minimum | Maximum | Mean |
|---|---|---|---|---|---|---|---|---|
| Fresh weight (g) | 183.92 a1 | 213.59 b | 216.41 b | 324.15 c | 165.21 a | 165.21 | 324.15 | 220.75 |
| Total soluble solids (°Brix) | 9.35 a | 12.64 c | 11.10 b | 12.43 c | 10.69 b | 9.35 | 12.64 | 11.22 |
| Titratable acidity (%) | 0.39 d | 0.15 a | 0.31 c | 0.24 b | 0.47 e | 0.15 | 0.47 | 0.31 |
| Blush (%) | 30.33 b | 0.00 a | 0.00 a | 0.00 a | 46.67 c | 0.00 | 46.67 | 15.61 |
| Firmness (N) | 46.36 b | 50.42 c | 42.80 a | 59.57 d | 73.02 e | 42.80 | 73.02 | 54.49 |
| Ethylene (µL∙kg−1∙h−1) | 0.28 a | 9.98 b | 0.08 a | 0.04 a | 0.16 a | 0.04 | 9.98 | 3.50 |
| CO2 (µL∙g−1∙h−1) | 3.41 a | 10.63 d | 5.40 b | 4.84 b | 7.94 c | 3.41 | 10.63 | 7.64 |
| Starch index (1–10) | 9.80 d | 10.00 d | 8.73 c | 7.80 b | 5.53 a | 5.53 | 10.00 | 8.36 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolniak-Ostek, J.; Kłopotowska, D.; Rutkowski, K.P.; Skorupińska, A.; Kruczyńska, D.E. Bioactive Compounds and Health-Promoting Properties of Pear (Pyrus communis L.) Fruits. Molecules 2020, 25, 4444. https://doi.org/10.3390/molecules25194444
Kolniak-Ostek J, Kłopotowska D, Rutkowski KP, Skorupińska A, Kruczyńska DE. Bioactive Compounds and Health-Promoting Properties of Pear (Pyrus communis L.) Fruits. Molecules. 2020; 25(19):4444. https://doi.org/10.3390/molecules25194444
Chicago/Turabian StyleKolniak-Ostek, Joanna, Dagmara Kłopotowska, Krzysztof P. Rutkowski, Anna Skorupińska, and Dorota E. Kruczyńska. 2020. "Bioactive Compounds and Health-Promoting Properties of Pear (Pyrus communis L.) Fruits" Molecules 25, no. 19: 4444. https://doi.org/10.3390/molecules25194444
APA StyleKolniak-Ostek, J., Kłopotowska, D., Rutkowski, K. P., Skorupińska, A., & Kruczyńska, D. E. (2020). Bioactive Compounds and Health-Promoting Properties of Pear (Pyrus communis L.) Fruits. Molecules, 25(19), 4444. https://doi.org/10.3390/molecules25194444