Anthocyanin Intake and Physical Activity: Associations with the Lipid Profile of a US Working Population
Abstract
1. Introduction
2. Results
2.1. Study Participants
2.2. Characteristics of Anthocyanin Intake
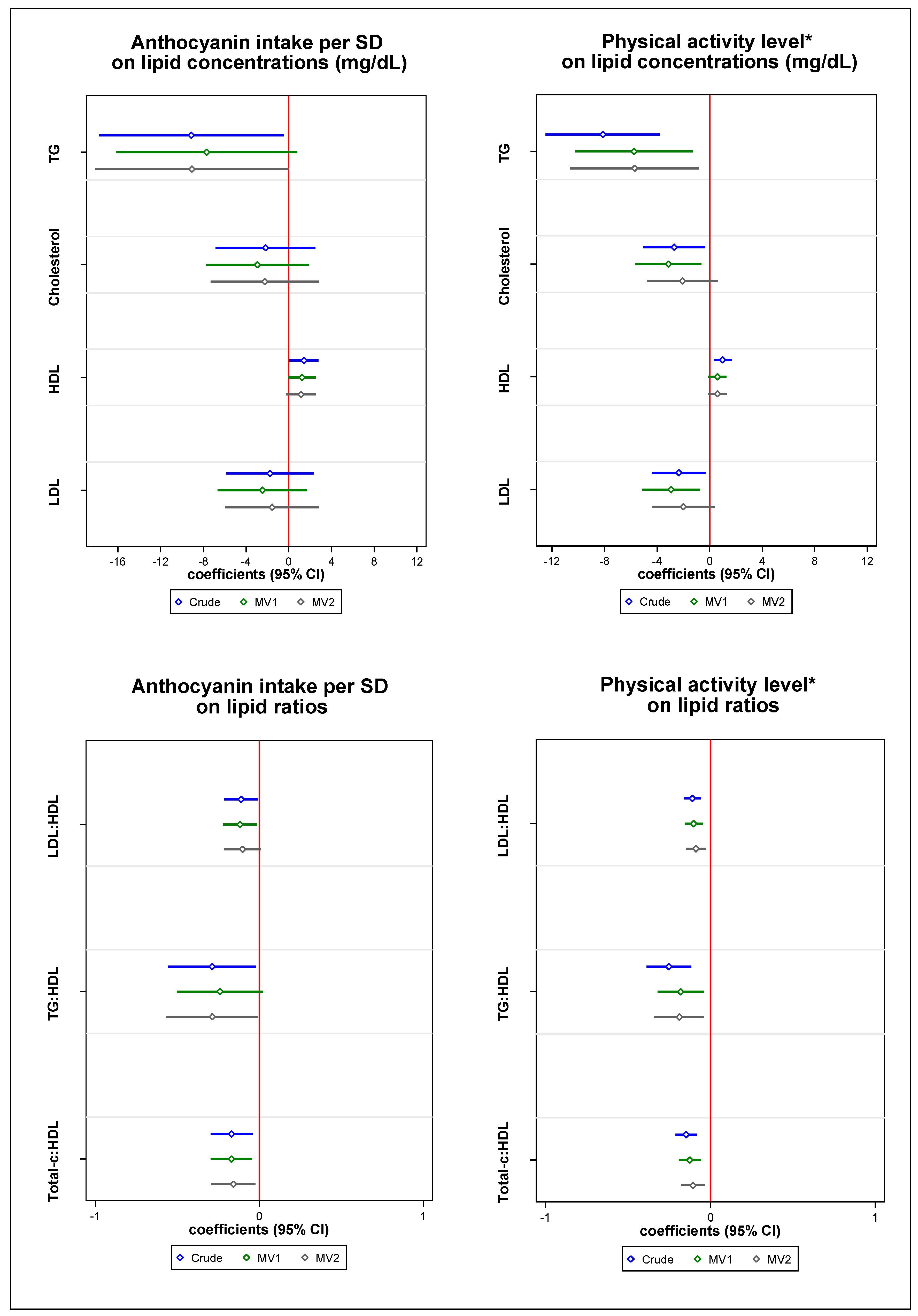
2.3. Individual Associations between Anthocyanin Intake and Physical Activity on Lipid Profile
2.4. The Combined Effect, Stratification, and Interaction Analyses between Anthocyanin Intake and Physical Activity on HDL Cholesterol <40 mg/dL
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Dietary Assessment
4.3. Physical Activity
4.4. Outcome Assessment
4.5. Covariate Assessment
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, D.L.; Graham, E.; Stewart, D.; Mathias, K.C. Cardiovascular Disease Risk Factor Changes over 5 Years Among Male and Female US Firefighters. J. Occup. Env. Med. 2020, 62, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Kales, S.N.; Smith, D.L. Firefighting and the Heart. Circulation 2017, 135, 1296–1299. [Google Scholar] [CrossRef] [PubMed]
- Soteriades, E.S.; Smith, D.L.; Tsismenakis, A.J.; Baur, D.M.; Kales, S.N. Cardiovascular Disease in US Firefighters: A Systematic Review. Cardiol. Rev. 2011, 19, 202–215. [Google Scholar] [CrossRef]
- Yang, J.; Teehan, D.; Farioli, A.; Baur, D.M.; Smith, D.; Kales, S.N. Sudden Cardiac Death among Firefighters ≤45 Years of Age in the United States. Am. J. Cardiol. 2013, 112, 1962–1967. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.L.; Haller, J.M.; Korre, M.; Fehling, P.C.; Sampani, K.; Grossi Porto, L.G.; Christophi, C.A.; Kales, S.N. Pathoanatomic Findings Associated With Duty-Related Cardiac Death in US Firefighters: A Case–Control Study. J. Am. Heart Assoc. 2018, 7, e009446. [Google Scholar] [CrossRef]
- Tena, N.; Martín, J.; Asuero, A.G. State of the Art of Anthocyanins: Antioxidant Activity, Sources, Bioavailability, and Therapeutic Effect in Human Health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef]
- Wallace, T.; Slavin, M.; Frankenfeld, C. Systematic Review of Anthocyanins and Markers of Cardiovascular Disease. Nutrients 2016, 8, 32. [Google Scholar] [CrossRef]
- Cassidy, A.; Bertoia, M.; Chiuve, S.; Flint, A.; Forman, J.; Rimm, E.B. Habitual Intake of Anthocyanins and Flavanones and Risk of Cardiovascular Disease in Men. Am. J. Clin. Nutr. 2016, 104, 587–594. [Google Scholar] [CrossRef]
- Cook, M.D.; Willems, M.E.T. Dietary Anthocyanins: A Review of the Exercise Performance Effects and Related Physiological Responses. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 322–330. [Google Scholar] [CrossRef]
- Kashi, D.S.; Shabir, A.; Da Boit, M.; Bailey, S.J.; Higgins, M.F. The Efficacy of Administering Fruit-Derived Polyphenols to Improve Health Biomarkers, Exercise Performance and Related Physiological Responses. Nutrients 2019, 11, 2389. [Google Scholar] [CrossRef]
- García-Conesa, M.-T.; Chambers, K.; Combet, E.; Pinto, P.; Garcia-Aloy, M.; Andrés-Lacueva, C.; de Pascual-Teresa, S.; Mena, P.; Konic Ristic, A.; Hollands, W.; et al. Meta-Analysis of the Effects of Foods and Derived Products Containing Ellagitannins and Anthocyanins on Cardiometabolic Biomarkers: Analysis of Factors Influencing Variability of the Individual Responses. Int. J. Mol. Sci. 2018, 19, 694. [Google Scholar] [CrossRef] [PubMed]
- Kimble, R.; Keane, K.M.; Lodge, J.K.; Howatson, G. Dietary Intake of Anthocyanins and Risk of Cardiovascular Disease: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Crit. Rev. Food Sci. Nutr. 2019, 59, 3032–3043. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, Z.; Zhao, H.; Wang, X.; Pang, J.; Li, Q.; Yang, Y.; Ling, W. Anthocyanin Supplementation Improves Anti-Oxidative and Anti-Inflammatory Capacity in a Dose–Response Manner in Subjects with Dyslipidemia. Redox Biol. 2020, 32, 101474. [Google Scholar] [CrossRef] [PubMed]
- Luís, Â.; Domingues, F.; Pereira, L. Association between Berries Intake and Cardiovascular Diseases Risk Factors: A Systematic Review with Meta-Analysis and Trial Sequential Analysis of Randomized Controlled Trials. Food Funct. 2018, 9, 740–757. [Google Scholar] [CrossRef]
- Zhu, Y.; Ling, W.; Guo, H.; Song, F.; Ye, Q.; Zou, T.; Li, D.; Zhang, Y.; Li, G.; Xiao, Y.; et al. Anti-Inflammatory Effect of Purified Dietary Anthocyanin in Adults with Hypercholesterolemia: A Randomized Controlled Trial. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 843–849. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Li, D.; Wang, P.; Luo, Y.; Zhao, M.; Chen, F. Health Benefits of Anthocyanins and Molecular Mechanisms: Update from Recent Decade. Crit. Rev. Food Sci. Nutr. 2017, 57, 1729–1741. [Google Scholar] [CrossRef]
- Yang, L.; Ling, W.; Du, Z.; Chen, Y.; Li, D.; Deng, S.; Liu, Z.; Yang, L. Effects of Anthocyanins on Cardiometabolic Health: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2017, 8, 684–693. [Google Scholar] [CrossRef]
- Kokkinos, P.; Myers, J. Exercise and Physical Activity: Clinical Outcomes and Applications. Circulation 2010, 122, 1637–1648. [Google Scholar] [CrossRef]
- Zwald, M.L.; Akinbami, L.J.; Fakhouri, T.H.I.; Fryar, C.D. Prevalence of Low High-Density Lipoprotein Cholesterol Among Adults, by Physical Activity: United States, 2011–2014. Age 2017, 20, 39. [Google Scholar]
- Wang, Y.; Xu, D. Effects of Aerobic Exercise on Lipids and Lipoproteins. Lipids Health Dis. 2017, 16, 132. [Google Scholar] [CrossRef] [PubMed]
- Kraus, W.E.; Houmard, J.A.; Duscha, B.D.; Knetzger, K.J.; Wharton, M.B.; McCartney, J.S.; Bales, C.W.; Henes, S.; Samsa, G.P.; Otvos, J.D.; et al. Effects of the Amount and Intensity of Exercise on Plasma Lipoproteins. N. Engl. J. Med. 2002, 347, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Van Der Weele, T.J.; Knol, M.J. A Tutorial on Interaction. Epidemiol. Methods 2014, 3, 33–72. [Google Scholar] [CrossRef]
- Knol, M.J.; VanderWeele, T.J.; Groenwold, R.H.H.H.; Klungel, O.H.; Rovers, M.M.; Grobbee, D.E. Estimating Measures of Interaction on an Additive Scale for Preventive Exposures. Eur. J. Epidemiol. 2011, 26, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Şahin, M.A.; Bilgiç, P.; Montanari, S.; Willems, M.E.T. Intake Duration of Anthocyanin-Rich New Zealand Blackcurrant Extract Affects Metabolic Responses during Moderate Intensity Walking Exercise in Adult Males. J. Diet. Suppl. 2020, 24, 1–12. [Google Scholar] [CrossRef]
- Krga, I.; Milenkovic, D. Anthocyanins: From Sources and Bioavailability to Cardiovascular-Health Benefits and Molecular Mechanisms of Action. J. Agric. Food Chem. 2019, 67, 1771–1783. [Google Scholar] [CrossRef]
- Willett, W.C.; Sampson, L.; Stampfer, M.J.; Rosner, B.; Bain, C.; Witschi, J.; Hennekens, C.H.; Speizer, F.E. Reproducibility and Validity of a Semiquantitative Food Frequency Questionnaire. Am. J. Epidemiol. 1985, 122, 51–65. [Google Scholar] [CrossRef]
- Rothman, K.; Greenland, S.; Lash, T. Modern Epidemiology, 3rd ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Rothman, K.J.; Gallacher, J.E.J.; Hatch, E.E. Why Representativeness Should Be Avoided. Int. J. Epidemiol. 2013, 42, 1012–1014. [Google Scholar] [CrossRef]
- Estruch, R.; Camafort, M. The Mediterranean Diet and Plasma Lipid Profile | Dieta Mediterránea y Perfil Lipídico Plasmático. Rev. Esp. Cardiol. 2015, 68, 279–281. [Google Scholar] [CrossRef]
- Sotos-Prieto, M.; Christophi, C.; Black, A.; Furtado, J.D.; Song, Y.; Magiatis, P.; Papakonstantinou, A.; Melliou, E.; Moffatt, S.; Kales, S.N. Assessing Validity of Self-Reported Dietary Intake within a Mediterranean Diet Cluster Randomized Controlled Trial among US Firefighters. Nutrients 2019, 11, 2250. [Google Scholar] [CrossRef] [PubMed]
- Vanderweele, T.J. Sample Size and Power Calculations for Additive Interactions. Epidemiol. Methods 2012, 1, 159–188. [Google Scholar] [CrossRef] [PubMed]
- Vanderweele, T. Power and Sample-Size Claculations for Interaction Analysis. In Explanation in Causal Inference: Methods for Mediation and Interaction; Oxford University Press: New York, NY, USA, 2015; pp. 346–368. [Google Scholar]
- Welsh, C.; Celis-Morales, C.A.; Brown, R.; MacKay, D.F.; Lewsey, J.; Mark, P.B.; Gray, S.R.; Ferguson, L.D.; Anderson, J.J.; Lyall, D.M.; et al. Comparison of Conventional Lipoprotein Tests and Apolipoproteins in the Prediction of Cardiovascular Disease Data from UK Biobank. Circulation 2019, 140, 542–552. [Google Scholar] [CrossRef] [PubMed]
- LeFevre, M.L. Behavioral Counseling to Promote a Healthful Diet and Physical Activity for Cardiovascular Disease Prevention in Adults With Cardiovascular Risk Factors: U.S. Preventive Services Task Force Recommendation Statement. Ann. Intern. Med. 2014, 161, 587. [Google Scholar] [CrossRef]
- Grossman, D.C.; Bibbins-Domingo, K.; Curry, S.J.; Barry, M.J.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W.; Kemper, A.R.; Krist, A.H.; Kurth, A.E.; et al. Behavioral Counseling to Promote a Healthful Diet and Physical Activity for Cardiovascular Disease Prevention in Adults Without Cardiovascular Risk Factors. JAMA 2017, 318, 167. [Google Scholar] [CrossRef]
- Huang, H.; Chen, G.; Liao, D.; Zhu, Y.; Xue, X. Effects of Berries Consumption on Cardiovascular Risk Factors: A Meta-Analysis with Trial Sequential Analysis of Randomized Controlled Trials. Sci. Rep. 2016, 6, 23625. [Google Scholar] [CrossRef]
- Feeding America’s Bravest: Mediterranean Diet-Based Interventions to Change Firefighters’ Eating Habits. NCT02941757. Available online: https://clinicaltrials.gov/show/NCT02941757 (accessed on 22 September 2020).
- Sotos-Prieto, M.; Cash, S.B.; Christophi, C.; Folta, S.; Moffatt, S.; Muegge, C.; Korre, M.; Mozaffarian, D.; Kales, S.N. Rationale and Design of Feeding America’s Bravest: Mediterranean Diet-Based Intervention to Change Firefighters’ Eating Habits and Improve Cardiovascular Risk Profiles. Contemp. Clin. Trials 2017, 61, 101–107. [Google Scholar] [CrossRef]
- Yang, J.; Farioli, A.; Korre, M.; Kales, S.N.S.S.N.; Kales, S.N.S.S.N. Modified Mediterranean Diet Score and Cardiovascular Risk in a North American Working Population. PLoS ONE 2014, 9, e87539. [Google Scholar] [CrossRef]
- Cassidy, A.; O’Reilly, É.J.; Kay, C.; Sampson, L.; Franz, M.; Forman, J.P.; Curhan, G.; Rimm, E.B. Habitual Intake of Flavonoid Subclasses and Incident Hypertension in Adults. Am. J. Clin. Nutr. 2011, 93, 338–347. [Google Scholar] [CrossRef]
- Bhagwat, S.; Haytowitz, D.; Holden, J. USDA Database for the Flavonoid Content of Selected Foods, Release 3.1. Available online: https://data.nal.usda.gov/dataset/usda-database-flavonoid-content-selected-foods-release-31-may-2014/resource/b8de90f1-d035 (accessed on 22 September 2020).
- Cassidy, A. Berry Anthocyanin Intake and Cardiovascular Health. Mol. Aspects Med. 2018, 61, 76–82. [Google Scholar] [CrossRef]
- Speer, H.; D’Cunha, N.M.; Alexopoulos, N.I.; McKune, A.J.; Naumovski, N. Anthocyanins and Human Health—A Focus on Oxidative Stress, Inflammation and Disease. Antioxidants 2020, 9, 366. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.S.; Blair, S.N.; Mahar, M.T.; Wier, L.T.; Ross, R.M.; Stuteville, J.E. Prediction of Functional Aerobic Capacity without Exercise Testing. Med. Sci. Sports Exerc. 1990, 22, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Knol, M.J.; VanderWeele, T.J. Recommendations for Presenting Analyses of Effect Modification and Interaction. Int. J. Epidemiol. 2012, 41, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Zou, G. A Modified Poisson Regression Approach to Prospective Studies with Binary Data. Am. J. Epidemiol. 2004, 159, 702–706. [Google Scholar] [CrossRef]
- VanderWeele, T.J. An Introduction to Interaction Analysis. In Explanation in Causal Inference: Methods for Mediation and Interaction; Oxford University Press: New York, NY, USA, 2015; pp. 255–257. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
| Anthocyanin Intake (SD) a | Physical Activity Level b | |||||
|---|---|---|---|---|---|---|
| Low | High | p-Value | Low | High | p-Value | |
| N | 124 | 125 | 95 | 154 | ||
| Women (%) | 4.8 | 5.6 | 0.79 | 2.11 | 7.14 | 0.08 |
| Age (yrs) | 47.2 (7.4) | 46.3 (7.5) | 0.30 | 48.7 (6.9) | 45.5 (7.6) | 0.002 |
| BMI (kg/m2) | 30 (4.5) | 29.7 (4.2) | 0.59 | 31.7 (4.4) | 28.7 (3.9) | <0.001 |
| Total energy intake (kcal/d) | 2244 (941) | 2491 (852) | 0.03 | 2423 (993) | 2334 (846) | 0.45 |
| mMDS † (pts) | 22.3 (6.6) | 25.3 (6.9) | <0.001 | 21 (7.6) | 25.6 (5.8) | <0.001 |
| Flavonoids (mg/d) | 286 (212.0) | 460 (282.0) | <0.001 | 383 (306.0) | 367 (238.0) | 0.65 |
| Anthocyanins (mg/d) | 10.9 (6.0) | 53 (40.2) | <0.001 | 29.7 (35.5) | 33.4 (35.7) | 0.43 |
| Protein intake (g/d) | 97 (43.0) | 110 (38.0) | 0.01 | 104 (46.0) | 103 (37.0) | 0.95 |
| Carbohydrate intake (g/d) | 243 (106.0) | 267 (99.0) | 0.07 | 262 (106.0) | 251 (101.0) | 0.40 |
| Whole grains (g/d) | 33.2 (18.7) | 39.9 (23.2) | 0.01 | 35.7 (17.9) | 37 (23.2) | 0.64 |
| Total fiber intake (g/d) | 21 (8.7) | 27.7 (9.2) | <0.001 | 23.7 (8.8) | 24.8 (10.0) | 0.39 |
| Added sugar (g/d) | 65.2 (48.7) | 54.2 (35.2) | 0.04 | 66.1 (47.7) | 55.7 (39.0) | 0.06 |
| Fat intake (g/d) | 93.5 (45.8) | 101.4 (39.3) | 0.09 | 101.2 (47.8) | 95.1 (39.3) | 0.27 |
| Saturated fat (g/d) | 31.4 (17.3) | 31.8 (12.9) | 0.85 | 33.3 (17.1) | 30.6 (13.9) | 0.18 |
| Polyunsaturated fat (g/d) | 19.4 (9.3) | 22 (9.1) | 0.02 | 21.6 (9.9) | 20.2 (8.9) | 0.27 |
| Monounsaturated fat (g/d) | 35.2 (17.6) | 39.4 (16.8) | 0.05 | 38.5 (18.8) | 36.6 (16.3) | 0.39 |
| Alcohol (g/d) | 9.1 (12.4) | 15.6 (25.5) | 0.01 | 11.2 (18.8) | 13.1 (21.2) | 0.48 |
| Nondrinkers (%) | 18.5 | 16.8 | 0.72 | 17.9 | 17.5 | 0.94 |
| Smoking status (%) | 0.29 | 0.54 | ||||
| never | 57.3 | 52.8 | 54.7 | 55.2 | ||
| current | 18.5 | 14.4 | 13.7 | 18.2 | ||
| former | 24.2 | 32.8 | 31.6 | 26.6 | ||
| Education (%) | 0.38 | 0.28 | ||||
| Technical school/some college/associates degree Bachelor’s degree or higher | ||||||
| 66.9 | 61.6 | 68.4 | 61.7 | |||
| 33.1 | 38.4 | 31.6 | 38.3 | |||
| Marital status (%) | 0.41 | 0.92 | ||||
| married | 79.2 | 83.4 | 80 | 80.5 | ||
| single | 21.8 | 17.6 | 20 | 19.5 | ||
| Multivitamin use (%) | 38.7 | 38.4 | 0.96 | 31.6 | 42.9 | 0.08 |
| Supplement use (proteins, glutamine, amino acids, etc.) (%) | 24.2 | 39.2 | 0.01 | 20.0 | 39.0 | 0.002 |
| Sitting (hrs/wk) | 19.5 (13.0) | 18.7 (16.8) | 0.64 | 22.4 (19.4) | 17.1 (11.1) | 0.01 |
| TV, computer, and driving (hrs/wk) | 8.02 (3.8) | 7.7 (4.0) | 0.52 | 8.33 (4.4) | 7.56 (3.5) | 0.14 |
| Sleep (hrs/d) | 6.57 (1.1) | 6.38 (0.9) | 0.13 | 6.41 (1.1) | 6.51 (1.0) | 0.42 |
| Prevalent hypertension (%) | 5.65 | 6.4 | 0.80 | 7.37 | 5.19 | 0.48 |
| Prevalent dyslipidemia (%) | 20.2 | 7.2 | 0.003 | 10.5 | 15.6 | 0.26 |
| Prevalent type 2 diabetes (%) | 1.61 | 1.6 | 0.99 | 1.05 | 1.95 | 0.51 |
| Sources (Serving Size) | mg/Serving | Cumulative R2 | Change in R² | Contribution (%) |
|---|---|---|---|---|
| Blueberries (1/2 cup) | 120.8 | 0.961 | --- | 43 |
| Strawberries (1/2 cup) | 20.5 | 0.981 | 0.020 | 11 |
| Red wine (5 oz. glass) | 28.3 | 0.989 | 0.008 | 11 |
| Apple or pears (1 fresh) | 6.05 * | 0.994 | 0.005 | 8 |
| Raisins or grapes (1 oz or small pack) or (1/2 cup) | 36.5 | 0.997 | 0.003 | 15 |
| Lipid Profile | Anthocyanin Intake (Per SD) | |
|---|---|---|
| Low PA * (n = 95) | High PA * (n = 154) | |
| Triglycerides | ||
| Age, sex, and energy adjusted model (95% CI) | −8.47 (−22.38 to 5.45) | −8.95 (−19.64 to 1.74) |
| Multivariable adjusted model 1 (95% CI) a | −7.47 (−23.34 to 8.40) | −7.83 (−18.21 to 2.56) |
| Multivariable adjusted model 2 (95% CI) b | −11.29 (−27.19 to 4.61) | −6.57 (−18.32 to 5.19) |
| Total cholesterol | ||
| Age, sex, and energy adjusted model (95% CI) | −3.75 (−11.07 to 3.56) | −0.97 (−6.94 to 5.00) |
| Multivariable adjusted model 1 (95% CI) a | −2.34 (−10.62 to 5.95) | −1.94 (−7.97 to 4.09) |
| Multivariable adjusted model 2 (95% CI) b | −4.38 (−12.74 to 3.97) | 2.80 (−3.79 to 9.39) |
| HDL cholesterol | ||
| Age, sex, and energy adjusted model (95% CI) | 0.52 (−1.51 to 2.55) | 1.97 (0.25 to 3.69) |
| Multivariable adjusted model 1 (95% CI) a | 1.09 (−1.08 to 3.25) | 1.55 (−0.07 to 3.17) |
| Multivariable adjusted model 2 (95% CI) b | 0.83 (−1.39 to 3.05) | 1.73 (−0.10 to 3.57) |
| LDL cholesterol | ||
| Age, sex, and energy adjusted model (95% CI) | −2.16 (−8.61 to 4.30) | −1.31 (−6.59 to 3.97) |
| Multivariable adjusted model 1 (95% CI) a | −1.39 (−8.66 to 5.87) | −2.09 (−7.47 to 3.29) |
| Multivariable adjusted model 2 (95% CI) b | −2.66 (−10.17 to 4.86) | 2.16 (−3.72 to 8.04) |
| LDL:HDL | ||
| Age, sex, and energy adjusted model (95% CI) | −0.10 (−0.28 to 0.08) | −0.11 (−0.23 to 0.01 |
| Multivariable adjusted model 1 (95% CI) a | −0.12 (−0.32 to 0.08) | −0.11 (−0.23 to 0.02) |
| Multivariable adjusted model 2 (95% CI) b | −0.13 (−0.33 to 0.07) | −0.04 (−0.18 to 0.09) |
| TG:HDL | ||
| Age, sex, and energy adjusted model (95% CI) | −0.22 (−0.69 to 0.25) | −0.31 (−0.62 to 0.00) |
| Multivariable adjusted model 1 (95% CI) a | −0.23 (−0.75 to 0.30) | −0.27 (−0.57 to 0.03) |
| Multivariable adjusted model 2 (95% CI) b | −0.31 (−0.84 to 0.22) | −0.28 (−0.62 to 0.07) |
| Total cholesterol:HDL | ||
| Age, sex, and energy adjusted model (95% CI) | −0.16 (−0.37 to 0.06) | −0.17 (−0.32 to −0.01) |
| Multivariable adjusted model 1 (95% CI) a | −019 (−0.42 to 0.05) | −0.15 (−0.30 to 0.00) |
| Multivariable adjusted model 2 (95% CI) b | −0.21 (−0.44 to 0.03) | −0.09 (−0.26 to 0.08) |
| Lipid Profile | Physical Activity Level (Per Unit) | |
|---|---|---|
| Low Anthocyanin Intake * (n = 124) | High Anthocyanin Intake * (n = 125) | |
| Triglycerides | ||
| Age, sex, and energy adjusted model (95% CI) | −6.19 (−13.03 to 0.65) | −8.88 (−14.53 to −3.23) |
| Multivariable adjusted model 1 (95% CI) a | −4.33 (−11.34 to 2.68) | −5.34 (−11.50 to 0.81) |
| Multivariable adjusted model 2 (95% CI) b | −4.96 (−12.45 to 2.53) | −4.79 (−11.62 to 2.04) |
| Total Cholesterol | ||
| Age, sex, and energy adjusted model (95% CI) | −2.69 (−6.01 to 0.64) | 2.70 (−6.21 to 0.82) |
| Multivariable adjusted model 1 (95% CI) a | −3.39 (−6.93 to 0.15) | −2.79 (−6.75 to 1.16) |
| Multivariable adjusted model 2 (95% CI) b | −2.86 (−6.65 to 0.93) | −0.65 (−4.99 to 3.69) |
| HDL Cholesterol | ||
| Age, sex, and energy adjusted model (95% CI) | 0.82 (−0.15 to 1.80) | 0.86 (−0.11 to 1.83) |
| Multivariable adjusted model 1 (95% CI) a | 0.73 (−0.29 to 1.75) | 0.11 (−0.89 to 1.11) |
| Multivariable adjusted model 2 (95% CI) b | 0.65 (−0.44 to 1.74) | 0.35 (−0.77 to 1.46) |
| LDL Cholesterol | ||
| Age, sex, and energy adjusted model (95% CI) | −2.24 (−5.08 to 0.60) | −2.47 (−5.68 to 0.74) |
| Multivariable adjusted model 1 (95% CI) a | −3.34 (−6.28 to −0.20) | −2.63 (−6.25 to 0.98) |
| Multivariable adjusted model 2 (95% CI) b | −2.56 (−5.81 to 0.68) | −0.99 (−4.98 to 3.01) |
| LDL:HDL | ||
| Age, sex, and energy adjusted model (95% CI) | −0.10 (−0.17 to −0.03) | −0.11 (−0.19 to −0.03) |
| Multivariable adjusted model 1 (95% CI) a | −0.12 (−0.19 to −0.04) | −0.08 (−0.17 to 0.01) |
| Multivariable adjusted model 2 (95% CI) b | −0.10 (−0.18 to −0.02) | −0.06 (−0.16 to 0.04) |
| TG:HDL | ||
| Age, sex, and energy adjusted model (95% CI) | −0.18 (−0.38 to 0.02) | −0.29 (−0.48 to −0.09) |
| Multivariable adjusted model 1 (95% CI) a | −0.14 (−3.35 to 0.07) | −0.17 (−0.38 to 0.035) |
| Multivariable adjusted model 2 (95% CI) b | −0.15 (−0.37 to 0.06) | −0.18 (−0.41 to 0.05) |
| Total cholesterol:HDL | ||
| Age, sex, and energy adjusted model (95% CI) | −0.14 (−0.23 to −0.05) | −0.14 (−0.23 to −0.04) |
| Multivariable adjusted model 1 (95% CI) a | −0.15 (−0.24 to −0.05) | −0.08 (−0.18 to 0.02) |
| Multivariable adjusted model 2 (95% CI) b | −0.13 (−0.23 to −0.03) | −0.06 (−0.18 to 0.05) |
| HDL Cholesterol < 40 mg/dL | Anthocyanin Intake (SD) | RR (95% CI) c for Anthocyanin Intake Stratified by Physical Activity | ||||
|---|---|---|---|---|---|---|
| High a | Low b | |||||
| Prevalence (%) | RR (95% CI)c | Prevalence (%) | RR (95% CI) c | |||
| Physical Activity | High a | 12.66 | 1 Ref. | 18.67 | 1.46 (0.68 to 3.11); p = 0.33 | 1.46 (0.67 to 3.18); p = 0.34 |
| Low b | 32.61 | 2.36 (1.15 to 4.83); p = 0.02 | 38.78 | 2.83 (1.42 to 5.67); p = 0.003 | 1.21 (0.70 to 2.08); p = 0.50 | |
| RR (95% CI) d for physical activity stratified by anthocyanin intake | 2.19 (1.07 to 4.49); p = 0.03 | 1.99 (1.10 to 3.60); p = 0.02 | ||||
| Measure of interaction on multiplicative scale: Ratio of RR (95% CI) | p = 0.72 | |||||
| Measure of interaction on additive scale: RERI (95% CI) | 0.02 (−1.63 to 1.66); p = 0.98 | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hershey, M.S.; Sotos-Prieto, M.; Ruiz-Canela, M.; Martinez-Gonzalez, M.A.; Cassidy, A.; Moffatt, S.; Kales, S.N. Anthocyanin Intake and Physical Activity: Associations with the Lipid Profile of a US Working Population. Molecules 2020, 25, 4398. https://doi.org/10.3390/molecules25194398
Hershey MS, Sotos-Prieto M, Ruiz-Canela M, Martinez-Gonzalez MA, Cassidy A, Moffatt S, Kales SN. Anthocyanin Intake and Physical Activity: Associations with the Lipid Profile of a US Working Population. Molecules. 2020; 25(19):4398. https://doi.org/10.3390/molecules25194398
Chicago/Turabian StyleHershey, Maria S., Mercedes Sotos-Prieto, Miguel Ruiz-Canela, Miguel Angel Martinez-Gonzalez, Aedin Cassidy, Steven Moffatt, and Stefanos N. Kales. 2020. "Anthocyanin Intake and Physical Activity: Associations with the Lipid Profile of a US Working Population" Molecules 25, no. 19: 4398. https://doi.org/10.3390/molecules25194398
APA StyleHershey, M. S., Sotos-Prieto, M., Ruiz-Canela, M., Martinez-Gonzalez, M. A., Cassidy, A., Moffatt, S., & Kales, S. N. (2020). Anthocyanin Intake and Physical Activity: Associations with the Lipid Profile of a US Working Population. Molecules, 25(19), 4398. https://doi.org/10.3390/molecules25194398






