Biocatalytic Potential of Native Basidiomycetes from Colombia for Flavour/Aroma Production
Abstract
1. Introduction
2. Results and Discussion
2.1. Fungal Strains
2.2. Screening Basidiomycetes via the Oxidation of β,β-Carotene
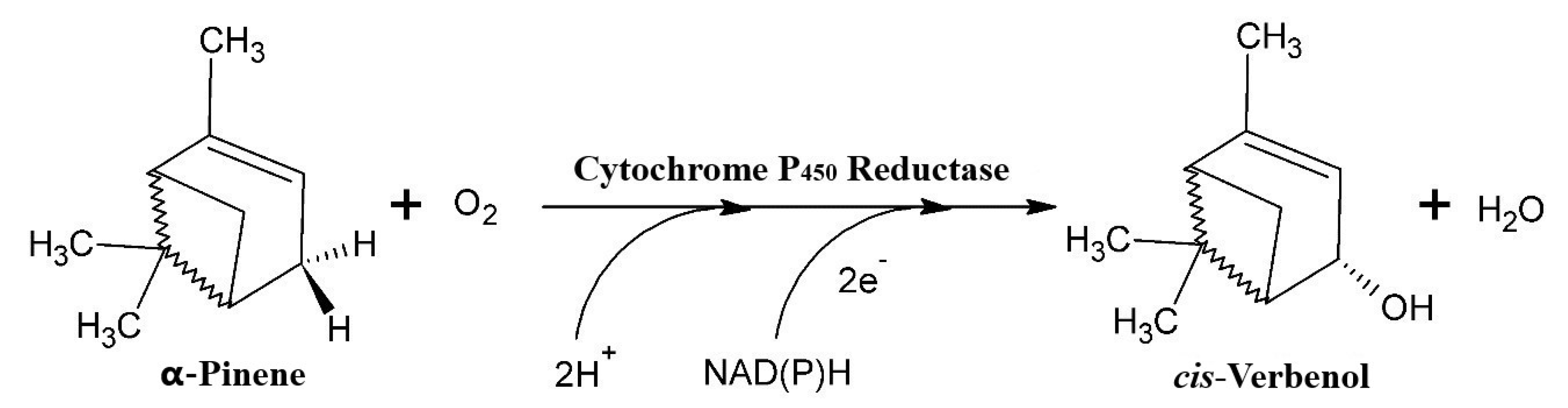
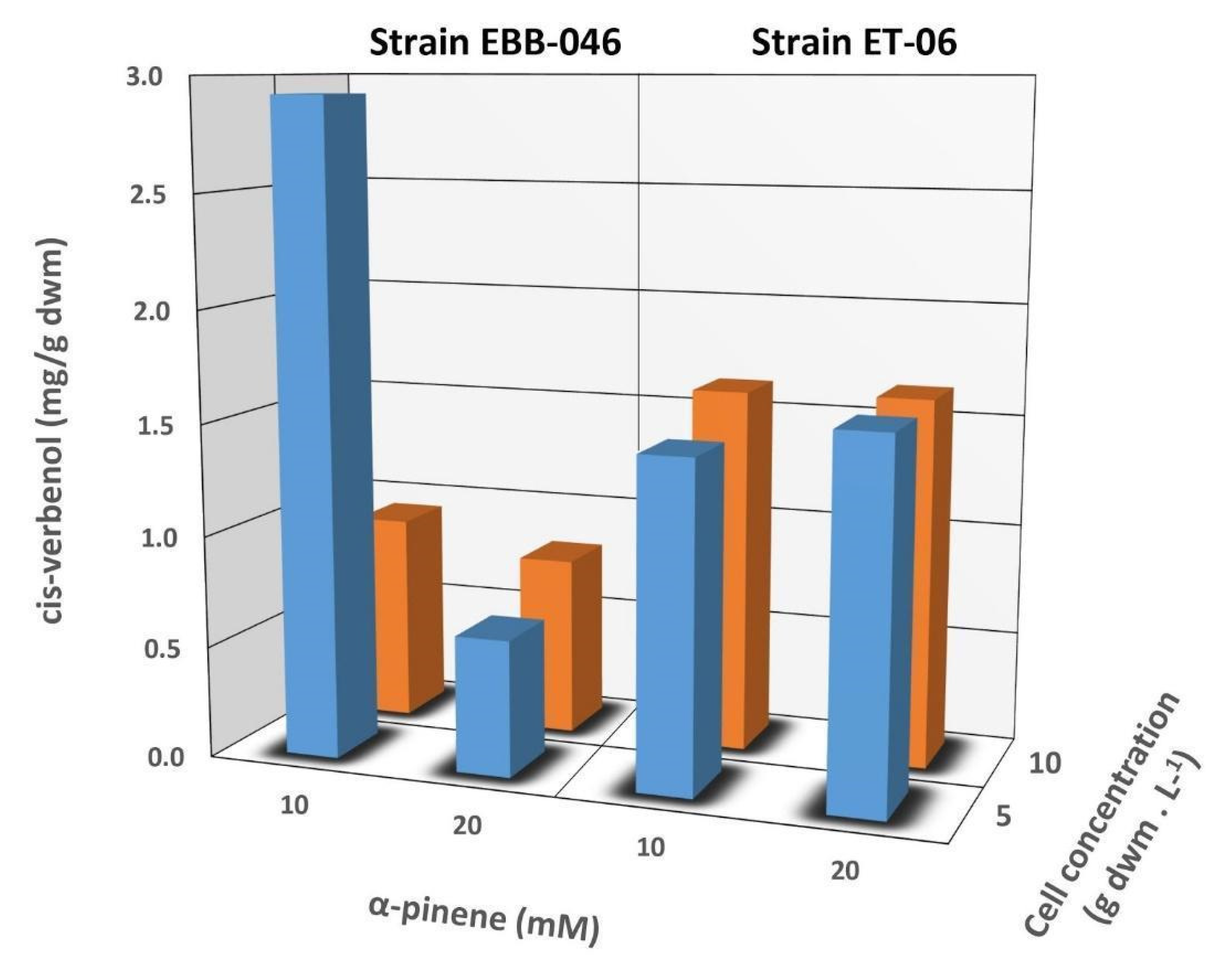
2.3. Biotransformation of α-Pinene
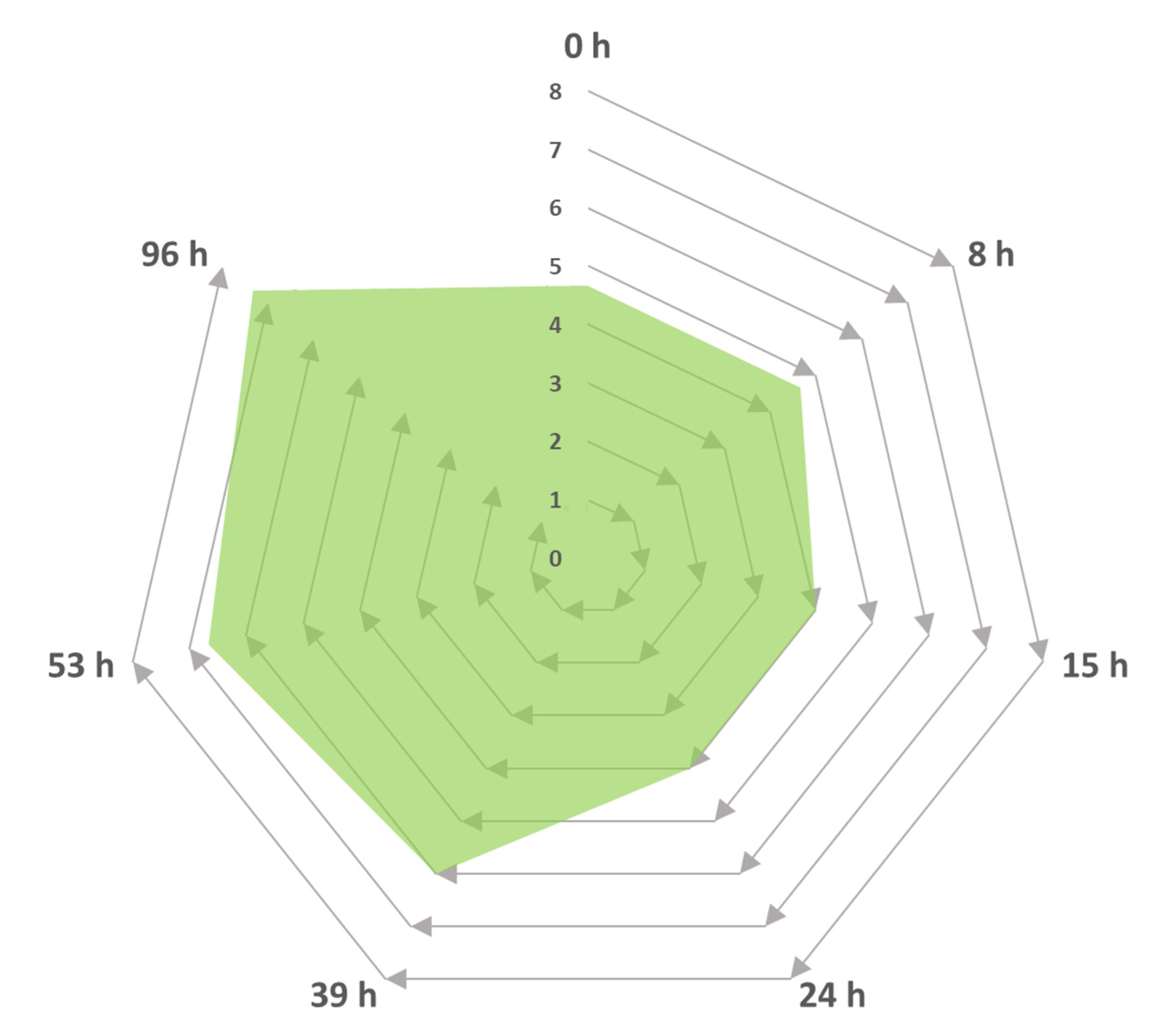
2.4. Identifying the Volatile Products of the Trametes Elegans Strains in Submerged Cultures
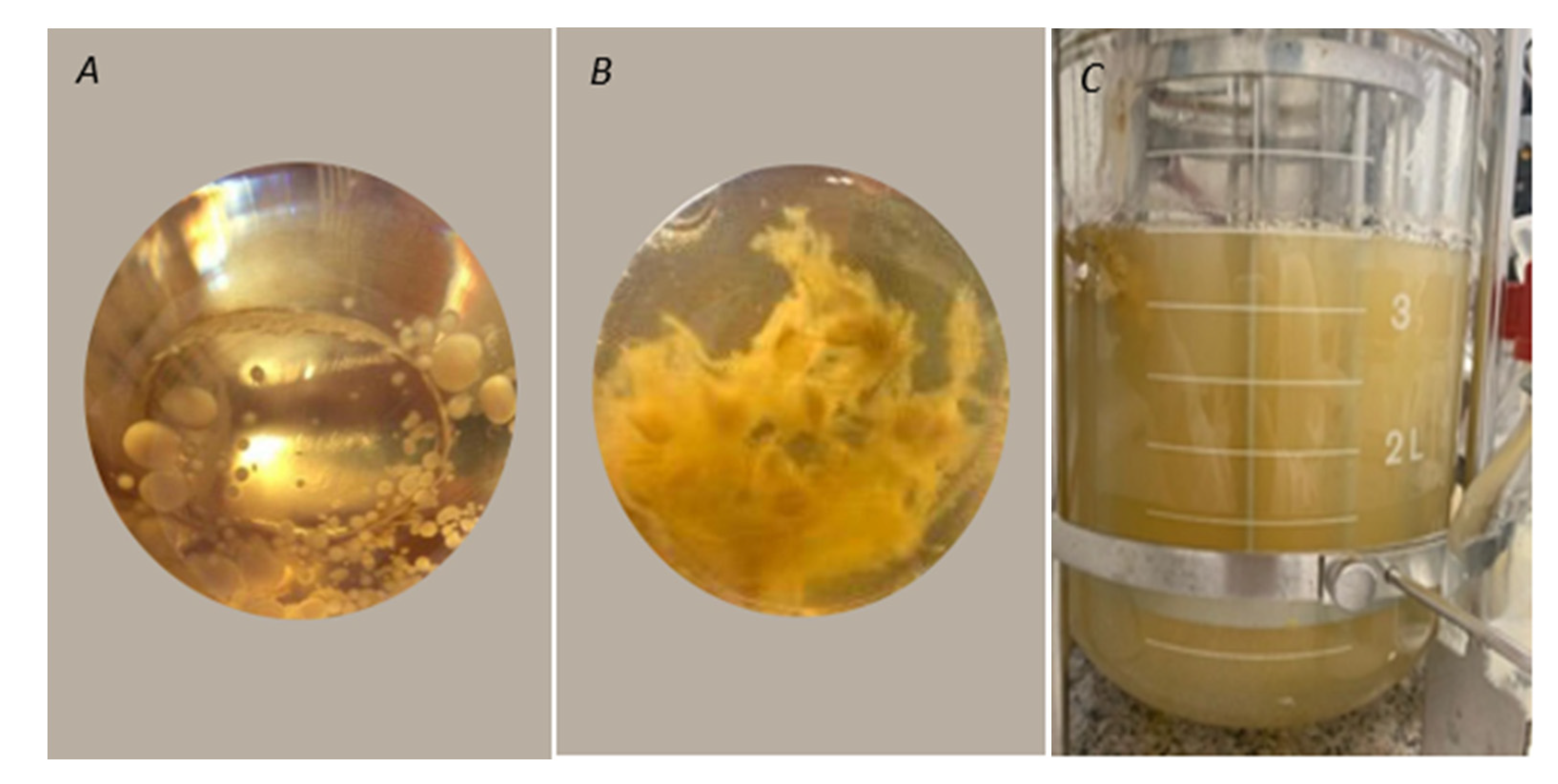
2.4.1. Submerged Culture
2.4.2. Sensory Evaluation
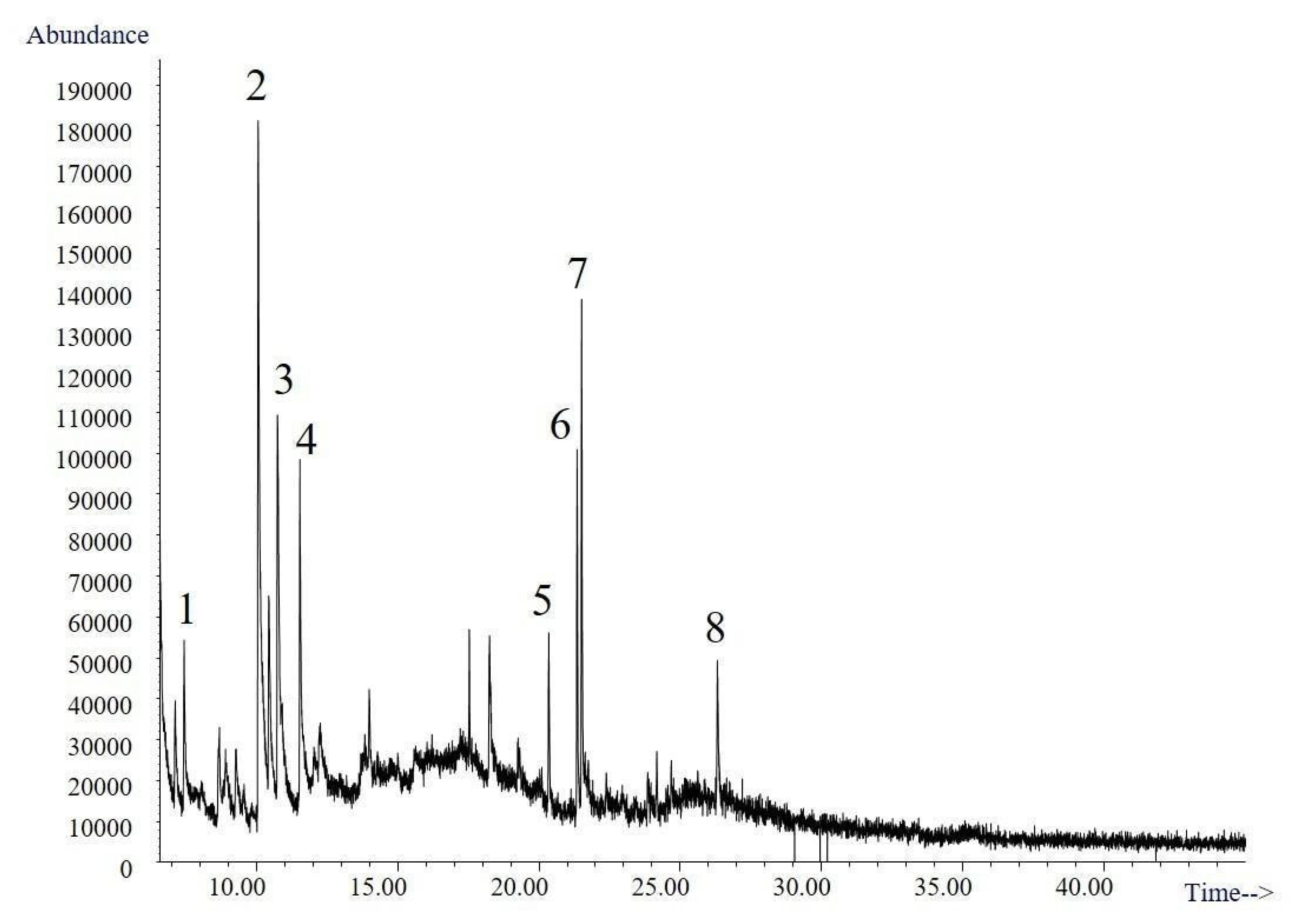
2.4.3. Gas Chromatography
3. Materials and Methods
3.1. Isolating the Microorganisms and Conducting Molecular Identification
3.2. Screening the Catalytic Action of Basidiomycetes Using β,β-Carotene Oxidation
3.3. Biocatalytic Transformation of α-Pinene
3.3.1. Inoculum Development
3.3.2. Producing the Mycelial Biomass for Biotransformation Reactions
3.3.3. Biocatalytic Transformation Reactions of α-Pinene using the Trametes Elegans Mycelia
3.3.4. Quantitative Analysis of the cis-Verbenol Content via GC
3.4. Identifying Volatile Compounds Derived from Trametes Elegans (EBB-046) in Submerged Cultures
3.4.1. Submerged Culture
3.4.2. Sensory Evaluation of the Culture
3.4.3. Gas Chromatography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dubal, S.A.; Tilkari, Y.; Momin, S.A.; Borkar, I.V. Biotechnological routes in flavor industries. Adv. Biotech. 2008, 8, 20–31. [Google Scholar]
- Ibrahimi, H.; Gadzovska-Simic, S.; Tusevski, O.; Haziri, A. Generation of flavor compounds by biotransformation of genetically modified hairy roots of Hypericum perforatum (L.) with basidiomycetes. Food. Sci. Nutr. 2020, 8, 2809–2816. [Google Scholar] [CrossRef] [PubMed]
- Akacha, N.; Gargouri, N. Microbial and enzymatic technologies used for the production of natural aroma compounds: Synthesis, recovery modeling, and bioprocesses. Food Bioprod. Process. 2015, 94, 675–706. [Google Scholar] [CrossRef]
- Borges, K.B.; de Souza Borges, W.; Durán-Patrón, R.; Pupo, M.T.; Bonato, P.S.; Collado, I.G. Stereoselective biotransformations using fungi as biocatalysts. Tetrahedron. Asymmetry 2009, 20, 385–397. [Google Scholar] [CrossRef]
- European Parliament and Council. Regulation (EC) No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EC) No 1601/91. Regulations (EC) No 2232 and (EC) No 110/2008 and Directive 2000/13/EC (text with EEA relevance). Off. J. Eur. Union 2008, 354, 34–50. [Google Scholar]
- Berger, R.G. Biotechnology as a source of natural volatile flavours. Curr. Opin. Food. Sci. 2015, 1, 38–43. [Google Scholar] [CrossRef]
- Longo, M.A.; Sanromán, M.A. Production of food aroma compounds: Microbial and enzymatic methodologies. Food Technol. Biotechnol. 2006, 44, 335–353. [Google Scholar]
- Bicas, J.L.; Dionísio, A.P.; Pastore, G.M. Bio-oxidation of Terpenes: An Approach for the Flavor Industry. Chem. Rev. 2009, 109, 4518–4531. [Google Scholar] [CrossRef]
- Abraham, B.G.; Berger, R.G. Higher fungi for generating aroma components through novel biotechnologies. J. Agric. Food Chem. 1994, 42, 2344–2348. [Google Scholar] [CrossRef]
- Wang, X.L.; Han, W.J.; Zhou, G.Y. Analysis of Volatile Flavor Compounds in Submerged Cultured Ganoderma Sinense Mycelium by Headspace Gas Chromatography. In Proceedings of the 2nd International Conference on Biomedical Engineering and Informatics, Tianjin, China, 17–19 October 2009; pp. 1–4. [Google Scholar] [CrossRef]
- Lu, Z.M.; Tao, W.Y.; Xu, H.Y.; Lim, J.; Zhang, X.M.; Wang, L.P.; Chen, J.H.; Xu, Z.H. Analysis of volatile compounds of Antrodia camphorata in submerged culture using headspace solid-phase microextraction. Food. Chem. 2011, 127, 662–668. [Google Scholar] [CrossRef]
- Goretti, M.; Ponzoni, C.; Caselli, E.; Marchegiani, E.; Cramarossa, M.R.; Turchetti, B.; Forti, L.; Buzzini, P. Bioreduction of α, β-unsaturated ketones and aldehydes by non-conventional yeast (N.C.Y.) whole-cells. Bioresour. Technol. 2011, 102, 3993–3998. [Google Scholar] [CrossRef] [PubMed]
- Negoi, A.; Parvulescu, V.; Tudorache, M. Peroxidase-based biocatalysis in a two-phase system for allylic oxidation of α-pinene. Catal. Today 2018, 306, 199–206. [Google Scholar] [CrossRef]
- Rozenbaum, H.F.; Patitucci, M.L.; Antunes, O.A.C.; Pereira, N., Jr. Production of aromas and fragrances through microbial oxidation of monoterpenes. Braz. J. Chem. Eng. 2006, 23, 273–279. [Google Scholar] [CrossRef]
- Serra, S. Recent advances in the synthesis of carotenoid-derived flavours and fragrances. Molecules 2015, 20, 12817–12840. [Google Scholar] [CrossRef]
- Serra, S.; De Simeis, D. Fungi-mediated biotransformation of the isomeric forms of the apocarotenoids ionone, damascone and theaspirane. Molecules 2019, 24, 19. [Google Scholar] [CrossRef]
- Vespermann, K.A.; Paulino, B.N.; Barcelos, M.C.; Pessôa, M.G.; Pastore, G.M.; Molina, G. Biotransformation of alpha and beta-pinene into flavor compounds. App. Microbiol. Biotechnol. 2017, 101, 1805–1817. [Google Scholar] [CrossRef]
- Zorn, H.; Langhoff, S.; Scheibner, M.; Nimtz, M.; Berger, R.G. A peroxidase from Lepista irina cleaves β, β-carotene to flavor compounds. Biol. Chem. 2003, 384, 1049–1056. [Google Scholar] [CrossRef]
- Busmann, D.; Günter, R. Oxyfunctionalization of a- and ß-Pinene by Selected Basidiomycetes. Z. Naturforsch. 1994, 49, 545–552. [Google Scholar] [CrossRef]
- Zelena, K.; Hardebusch, B.; Huülsdau, B.; Berger, R.G.; Zorn, H. Generation of norisoprenoid flavors from carotenoids by fungal peroxidases. J. Agric. Food. Chem. 2009, 57, 9951–9955. [Google Scholar] [CrossRef]
- Wu, S. Volatile Compounds Generated by Basidiomycetes. Ph.D. Thesis, Universität Hannover, Hannover, Germany, 2005. [Google Scholar]
- Carrau, F.; Boido, E.; Dellacassa, E. Yeast Diversity and Flavor Compounds. In Fungal Metabolites; Mérillon, J.M., Ramawat, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 569–597. ISBN 978-3-319-25001-4. [Google Scholar]
- Cánepa, A.L.; Herrero, E.R.; Crivello, M.E.; Eimer, G.A.; Casuscelli, S.G. H2O2 based α-pinene oxidation over Ti-MCM-41. A kinetic study. J. Mol. Catal. A Chem. 2011, 347, 1–7. [Google Scholar] [CrossRef]
- Choi, I.Y.; Lim, J.H.; Hwang, S.; Lee, J.C.; Cho, G.S.; Kim, W.K. Anti-ischemic and anti-inflammatory activity of (S)-cis-verbenol. Free Radic. Res. 2010, 44, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Trytek, M.; Jędrzejewski, K.; Fiedurek, J. Bioconversion of α-pinene by a novel cold-adapted fungus Chrysosporium pannorum. J. Ind. Microbiol. Biotechnol. 2015, 42, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients; C.R.C. Press: Boka Raton, FL, USA, 2016. [Google Scholar]
- Biotechnology for flavors, Fragances and Functional Ingredients; Symposium Introduction. In Proceedings of the Bioflavour Conference, Frankfurt, Germany, 18–21 September 2018.
- Rodríguez-Bustamante, E.; Sánchez, S. Microbial production of C13-norisoprenoids and other aroma compounds via carotenoid cleavage. Crit. Rev. Microbiol. 2007, 33, 211–230. [Google Scholar] [CrossRef] [PubMed]
- Duetz, W.A.; Bouwmeester, H.; Van Beilen, J.B.; Witholt, B. Biotransformation of limonene by bacteria, fungi, yeasts, and plants. Appl. Microbiol. Biotechnol. 2003, 61, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Bier, M.C.J.; Medeiros, A.B.P.; Soccol, C.R. Biotransformation of limonene by an endophytic fungus using synthetic and orange residue-based media. Fungal. Biol. 2017, 121, 137–144. [Google Scholar] [CrossRef]
- Zorn, H.; Langhoff, S.; Scheibner, M.; Berger, R.G. Cleavage of β, β-carotene to flavor compounds by fungi. Appl. Microbiol. Biotechnol. 2003, 62, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Martinez, H.; Jaime, A.; Camacho, J. Biotechnology profile analysis in Colombia. Scientometrics 2014, 101, 1789–1804. [Google Scholar] [CrossRef]
- Gamboa-Gaitán, M.Á. Presence of Aspergillus and other fungal symbionts in coffee beans from Colombia. Acta. Biol. Colomb. 2012, 17, 39–50. [Google Scholar]
- Chanagá Vera, X.; Escobar, J.P.; Marín Montoya, M.; Yepes Pérez, M.D.S. Hongos nativos con potencial degradador de tintes industriales en el valle de aburrá, Colombia. Rev. Fac. Nal. Agric. Medellín. 2012, 65, 6817–6827. [Google Scholar]
- Miles, L.A.; Lopera, C.A.; González, S.; de García, M.C.; Franco, A.E.; Restrepo, S. Exploring the biocontrol potential of fungal endophytes from an Andean Colombian Paramo ecosystem. BioControl 2012, 57, 697–710. [Google Scholar] [CrossRef]
- Mosquera, W.G.; Criado, L.Y.; Sierra, B.E.G. Actividad antimicrobiana de hongos endófitos de plantas medicinales Mammea americana (Calophyllaceae) y Moringa oleífera (Moringaceae). Biomédica 2020, 40, 55–71. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arboleda, C.; Mejia, A.I.; Franco-Molano, A.E.; Jimenez, G.A.; Penninckx, M.J. Autochthonous white rot fungi from the tropical forest of Colombia for dye decolourisation and ligninolytic enzymes production. Sydowia 2008, 60, 165–180. [Google Scholar]
- Montoya, S.; Sánchez, O.J.; Levin, L. Evaluation of endoglucanase, exoglucanase, laccase, and lignin peroxidase activities on ten white-rot fungi. Biotecnol. Sect. Agropecu. Agroind. 2014, 12, 115–124. [Google Scholar]
- Bravo, K.; Pereañez, J.A. Colombian biodiversity, an opportunity for the strengthening of the pharmaceutical and cosmetic industries. Vitae 2016, 23, 163–165. [Google Scholar] [CrossRef]
- Vasco-Palacios, A.M.; Franco-Molano, A.E. Diversity of Colombian macrofungi (Ascomycota-Basidiomycota). Mycotaxon 2013, 21, 100–158. [Google Scholar]
- Taskin, H.; Kafkas, E.; Çakiroglu, Ö.; Büyükalaca, S. Determination of volatile aroma compounds of Ganoderma lucidum by gas chromatography mass spectrometry (HS-GC/MS). Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Sandargo, B.; Chepkirui, C.; Cheng, T.; Chaverra-Muñoz, L.; Thongbai, B.; Stadler, M.; Hüttel, S. Biological and chemical diversity go hand in hand: Basidiomycota as source of new pharmaceuticals and agrochemicals. Biotechnol. Adv. 2019, 37, 107344. [Google Scholar] [CrossRef]
- Maciel, M.J.M.; Ribeiro, H.C.T. Industrial and biotechnological applications of ligninolytic enzymes of the basidiomycota: A review. Electron. J. Biotechnol. 2010, 13, 14–15. [Google Scholar]
- Yao, L.; Zhu, L.P.; Xu, X.Y.; Tan, L.L.; Sadilek, M.; Fan, H.; Hu, F.; Shen, X.T.; Yang, J.; Qiao, B.; et al. Discovery of novel xylosides in co-culture of basidiomycetes Trametes versicolor and Ganoderma applanatum by integrated metabolomics and bioinformatics. Sci. Rep. 2016, 6, 33237. [Google Scholar] [CrossRef]
- Mendez, M.J.; Caicedo, N.H.; Salamanca, C. Trametes elegans: A fungal endophytic isolate from Otoba gracilipes as biocatalyst for natural flavors production. New Biotech. 2018, 44. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, K.; Ke, Z.; Wu, Y.; Li, X. Optimisation of medium and culture conditions for the production of antifungal substances to Colletotrichum musae by Trametes elegans SR06. Biocontrol. Sci. Technol. 2016, 26, 1538–1551. [Google Scholar] [CrossRef]
- Thiers, B.M. The World’s Herbaria 2017: A Summary Report Based on Data from Index Herbariorum: William and Lynda Steere Herbarium; The New York Botanical Garden: Bronx, NY, USA, 2016. [Google Scholar]
- Scheibner, M.; Hülsdau, B.; Zelena, K.; Nimtz, M.; de Boer, L.; Berger, R.G.; Zorn, H. Novel peroxidases of Marasmius scorodonius degrade betacarotene. Appl. Microbiol. Biotechnol. 2008, 77, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Van der Werf, M.J.; de Bont, J.A.; Leak, D.J. Opportunities in Microbial Biotransformation of Monoterpenes. In Biotechnology of Aroma Compounds; Springer: Berlin/Heidelberg, 1997; Volume 55, pp. 147–177. [Google Scholar]
- Rogawansamy, S.; Gaskin, S.; Taylor, M.; Pisaniello, D. An evaluation of antifungal agents for the treatment of fungal contamination in indoor air environments. Int. J. Environ. Res. Public Health 2015, 12, 6319–6332. [Google Scholar] [CrossRef]
- Rojas, J.P.; Perea, J.A.; Ortiz, C.C. Evaluation of the Biotransformation of Geraniol and (R)-(+)-α-Pinene Using Cell of Rhodococcus Opacus Dsm 44313. Biotecnol. Sect. Agropecu. Agroind. 2009, 7, 104–112. [Google Scholar]
- Renninger, N.S. Production of Isoprenoids. U.S. Patent 7.659.097, 25 May 2007. [Google Scholar]
- Lin, G.; Bai, X.; Duan, W.; Cen, B.; Huang, M.; Lu, S. High Value-Added Application of Sustainable Natural Forest Product α-Pinene: Synthesis of Myrtenal Oxime Esters as Potential KARI Inhibitors. ACS. Sustain. Chem. Eng. 2019, 7, 7862–7868. [Google Scholar] [CrossRef]
- Hunt, D.W.A.; Borden, J.H.; Lindgren, B.S.; Gries, G. The role of autoxidation of α-pinene in the production of pheromones of Dendroctonus ponderosae (Coleoptera: Scolytidae). Can. J. Res. 1989, 19, 1275–1282. [Google Scholar] [CrossRef]
- Jakoby, W.; Ziegle, D.M. The enzyme of detoxification. J. Biol. Chem. 1990, 265, 20715–20719. [Google Scholar]
- Ancel, J.E.; Maksimchuk, N.V.; Simakova, I.L.; Semikolenov, V.A. Kinetic peculiarities of α-pinene oxidation by molecular oxygen. Appl. Catal. A Gen. 2004, 272, 109–114. [Google Scholar] [CrossRef]
- Krings, U.; Lehnert, N.; Fraatz, M.A.; Hardebusch, B.; Zorn, H.; Berger, R.G. Autoxidation versus biotransformation of α-pinene to flavors with Pleurotus sapidus: Regioselective hydroperoxidation of α-pinene and stereoselective dehydrogenation of verbenol. J. Agric. Food Chem. 2009, 57, 9944–9950. [Google Scholar] [CrossRef]
- Williams, P.A.; Cosme, J.; Sridhar, V.; Johnson, E.F.; McRee, D.E. Mammalian microsomal cytochrome P450 monooxygenase: Structural adaptations for membrane binding and functional diversity. Mol. Cell. 2000, 5, 121–131. [Google Scholar] [CrossRef]
- Schulz, B.; Boyle, C.; Draeger, S.; Römmert, A.K.; Krohn, K. Endophytic fungi: A source of novel biologically active secondary metabolites. Mycol. Res. 2002, 106, 996–1004. [Google Scholar] [CrossRef]
- Joint Genome Institute. Same Fungus, Different Strains. Available online: https://phys.org/news/2011-05-fungus-strains.html (accessed on 10 June 2020).
- Molina, G.; Pimentel, M.R.; Bertucci, T.C.; Pastore, G.M. Application of fungal endophytes in biotechnological processes. Chem. Eng. Trans. 2012, 27, 289–294. [Google Scholar]
- Trytek, M.; Fiedurek, J.; Gromada, A. Effect of some abiotic stresses on the biotransformation of α-pinene by a psychrotrophic Chrysosporium pannorum. Biochem. Eng. J. 2016, 112, 86–93. [Google Scholar] [CrossRef]
- Bisswanger, H. Enzyme assays. Perspect. Sci. 2014, 1, 41–55. [Google Scholar] [CrossRef]
- Moore, R.N.; Golumbic, C.; Fisher, G.S. Autoxidation of R-pinene. J. Am. Chem. Soc. 1956, 78, 1173–1176. [Google Scholar] [CrossRef]
- Lueangjaroenkit, P.; Teerapatsakul, C.; Chitradon, L. Morphological characteristic regulation of ligninolytic enzyme produced by Trametes polyzona. Mycobiology 2018, 46, 396–406. [Google Scholar] [CrossRef]
- Bosse, A.K.; Fraatz, M.A.; Zorn, H. Formation of complex natural flavours by biotransformation of apple pomace with basidiomycetes. Food Chem. 2013, 141, 2952–2959. [Google Scholar] [CrossRef]
- Böker, A.; Fischer, M.; Berger, R.G. Raspberry ketone from submerged cultured cells of the basidiomycete Nidula niveo-tomentosa. Biotechnol. Prog. 2001, 17, 568–572. [Google Scholar] [CrossRef]
- Gallois, A.; Gross, B.; Langlois, D.; Spinnler, H.-E.; Brunerie, P. Influence of culture conditions on production of flavour compounds by 29 lignolytic Basidomycetes. Mycol. Res. 1990, 94, 494–504. [Google Scholar] [CrossRef]
- Altunay, N.; Gürkan, R.; Orhan, U. Indirect determination of the flavor enhancer maltol in foods and beverages through flame atomic absorption spectrometry after ultrasound assisted-cloud point extraction. Food Chem. 2017, 235, 308–317. [Google Scholar] [CrossRef]
- Evans, J.A.; Eyre, C.A.; Rogers, H.J.; Boddy, L.; Müller, C.T. Changes in volatile production during interspecific interactions between four wood rotting fungi growing in artificial media. Fungal Ecol. 2008, 1, 57–68. [Google Scholar] [CrossRef]
- Mohamed, E.M.; Farghaly, F. Bioactive Compounds of Fresh and Dried Pleurotus ostreatus Mushroom. Int. J. Biotechnol. Wellness Ind. 2014, 3, 4–14. [Google Scholar] [CrossRef]
- Grab, W. Blended Flavourings. In Flavourings: Production, Composition, Applications, Regulations, 2nd ed.; Ziegler, H., Ed.; Wiley VCH: Weinheim, Germany, 2007. [Google Scholar]
- Caicedo, N.H.; Davalos, A.F.; Puente, P.A.; Rodríguez, A.Y.; Caicedo, P.A. Antioxidant activity of exo-metabolites produced by Fusarium oxysporum: An endophytic fungus isolated from leaves of Otoba gracilipes. Microbiol. Open 2019, 8, e903. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W. Taxonomy and Phylogeny of the Genus Mycosphaerella and Its Anamorphs. Ph. D. Thesis, University of Pretoria, Pretoria, Gauteng, South Africa, 2010. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.J.W.T.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic press: Cambridge, MA, USA, 1990; Volume 18, pp. 315–322. [Google Scholar]
- Blast. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 25 May 2020).
- Cape Gooseberries. FatSecret South Africa. Available online: https://www.fatsecret.co.za/calories-nutrition/woolworths/cape-gooseberries/100g (accessed on 15 May 2019).
- Stashenko, E.E.; Jaramillo, B.E.; Martínez, J.R. Comparison of different extraction methods for the analysis of volatile secondary metabolites of Lippia alba (Mill.) NE Brown, grown in Colombia, and evaluation of its in vitro antioxidant activity. J. Chromatogr. A 2004, 1025, 93–103. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Taxa | Strain | Voucher Information * | GenBank Accession Numbers | Degradation Ability * |
|---|---|---|---|---|
| Ganoderma chocoense J.A. Flores, C.W. Barnes & Ordoñez | EBB-083 | EBB-083 (Icesi); Colombia, Valle del Cauca, Reserva La Carolina | MT945608 | ++ |
| Ganoderma gibbosum (Cooke) Pat. | CM-UDEA 10 | 2543 AMV (HUA); Colombia, Antioquia, Medellin, on trunk | MT945606 | + |
| Ganoderma stipitatum (Murrill) Murril | CM-UDEA 110 | 2526 AMV (HUA); Colombia, Antioquia, San Luis, Reserva Río Claro | MT945605 | ++ |
| Ganoderma weberianum (Bres. & Henn. ex Sacc.) Steyaert | CM-UDEA 111 | 2531 AMV (HUA); Colombia, Antioquia, San Luis, Reserva Río Claro | MT953467 | ++ |
| Laetiporus gilbertsonii Burds. | CM-UDEA 1 | 2483 AMV (HUA); Colombia, Antioquia, Medellin, on trunk | MT945604 | - |
| Trametes elegans (Spreng.) Fr. | ET-06 | Endophytic isolated from Otoba gracilipes (Myristicaceae) | MT941002 | - |
| Trametes elegans (Spreng.) Fr. | EBB-046 | 2472 AMV (HUA); Colombia, Antioquia, Santafé de Antioquia on trunk | MT945607 | + |
| Cell Concentration (g dwm L−1) | Concentration of cis-Verbenol (mM) | |||
|---|---|---|---|---|
| T. elegans ET-06 | T. elegans EBB-046 | |||
| α-pinene 10 mM | α-pinene 20 mM | α-pinene 10 mM | α-pinene 20 mM | |
| 5 | 0.048 ± 0.011 | 0.086 ± 0.001 | 0.107 ± 0.002 | 0.103 ± 0.038 |
| 10 | 0.052 ± 0.001 | 0.095 ± 0.004 | 0.107 ± 0.011 | 0.078 ± 0.019 |
| 0 (control) | 0.030 ± 0.005 | 0.037 ± 0.004 | 0.030 ± 0.005 | 0.037 ± 0.004 |
| No | tR (min) | Presumptive Identification | MW |
|---|---|---|---|
| 1 | 7.44 | 2,3-Dihydro-3,5-dihydroxy-methyl-4H-pyran-4-one | 144 |
| 2 | 10.13 | Maltol | 126 |
| 3 | 10.78 | 2,3-Dihydro-3,5-dihydroxy-methyl- 4H-pyran-4-one (isomer) | 144 |
| 4 | 11.54 | Methyl-3-methoxy-4H-pyran-4-one | 140 |
| 5 | 20.32–20.36 | Hexahydro-3-(methylpropyl)-pyrrolo[1,2-C-a]pyrazine-1,4-dione | 210 |
| 6 | 21.34 | Hexahydro-3-(methylpropyl)-pyrrolo[1,2-C-a]pyrazine-1,4-dione (isomer) | 210 |
| 7 | 21.35 | Hexahydro-3-(methylpropyl)-pyrrolo[1,2-a]pyrazine-1,4-dione (isomer) | 210 |
| 8 | 26.30 | Hexahydro-3-(methylphenyl)-pyrrolo[1,2-a]pyrazine-1,4-dione | 244 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaramillo, D.A.; Méndez, M.J.; Vargas, G.; Stashenko, E.E.; Vasco-Palacios, A.-M.; Ceballos, A.; Caicedo, N.H. Biocatalytic Potential of Native Basidiomycetes from Colombia for Flavour/Aroma Production. Molecules 2020, 25, 4344. https://doi.org/10.3390/molecules25184344
Jaramillo DA, Méndez MJ, Vargas G, Stashenko EE, Vasco-Palacios A-M, Ceballos A, Caicedo NH. Biocatalytic Potential of Native Basidiomycetes from Colombia for Flavour/Aroma Production. Molecules. 2020; 25(18):4344. https://doi.org/10.3390/molecules25184344
Chicago/Turabian StyleJaramillo, David A., María J. Méndez, Gabriela Vargas, Elena E. Stashenko, Aída-M. Vasco-Palacios, Andrés Ceballos, and Nelson H. Caicedo. 2020. "Biocatalytic Potential of Native Basidiomycetes from Colombia for Flavour/Aroma Production" Molecules 25, no. 18: 4344. https://doi.org/10.3390/molecules25184344
APA StyleJaramillo, D. A., Méndez, M. J., Vargas, G., Stashenko, E. E., Vasco-Palacios, A.-M., Ceballos, A., & Caicedo, N. H. (2020). Biocatalytic Potential of Native Basidiomycetes from Colombia for Flavour/Aroma Production. Molecules, 25(18), 4344. https://doi.org/10.3390/molecules25184344