Lipid Profile Modulates Cardiometabolic Risk Biomarkers Including Hypertension in People with Type-2 Diabetes: A Focus on Unbalanced Ratio of Plasma Polyunsaturated/Saturated Fatty Acids
Abstract
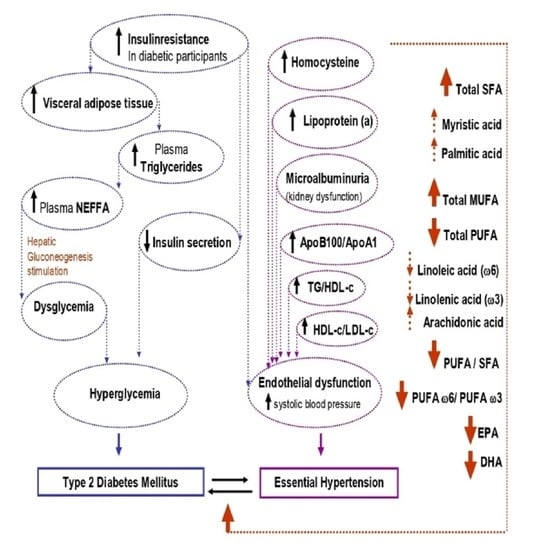
1. Introduction
2. Results
2.1. Cardiometabolic Risk Profile
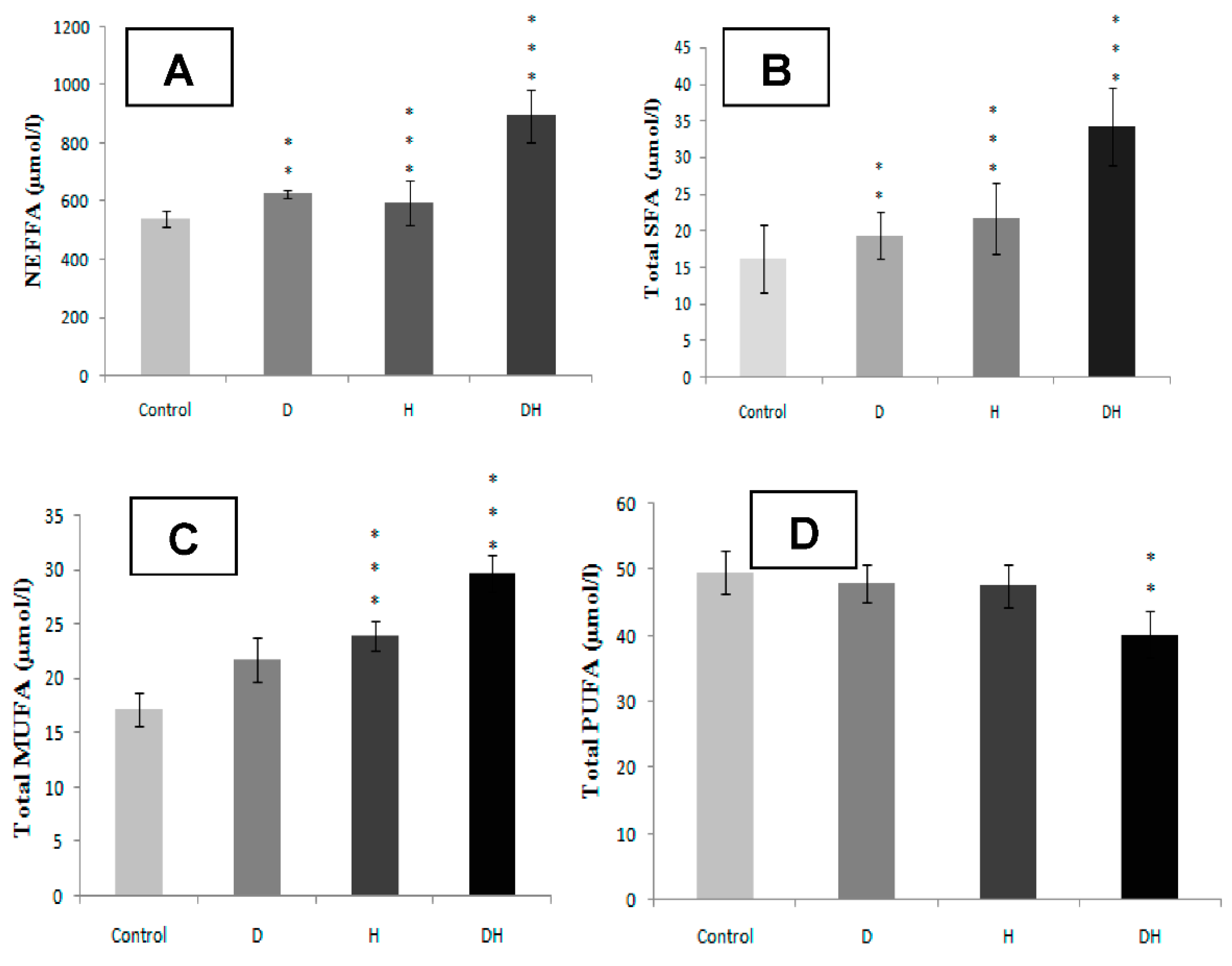
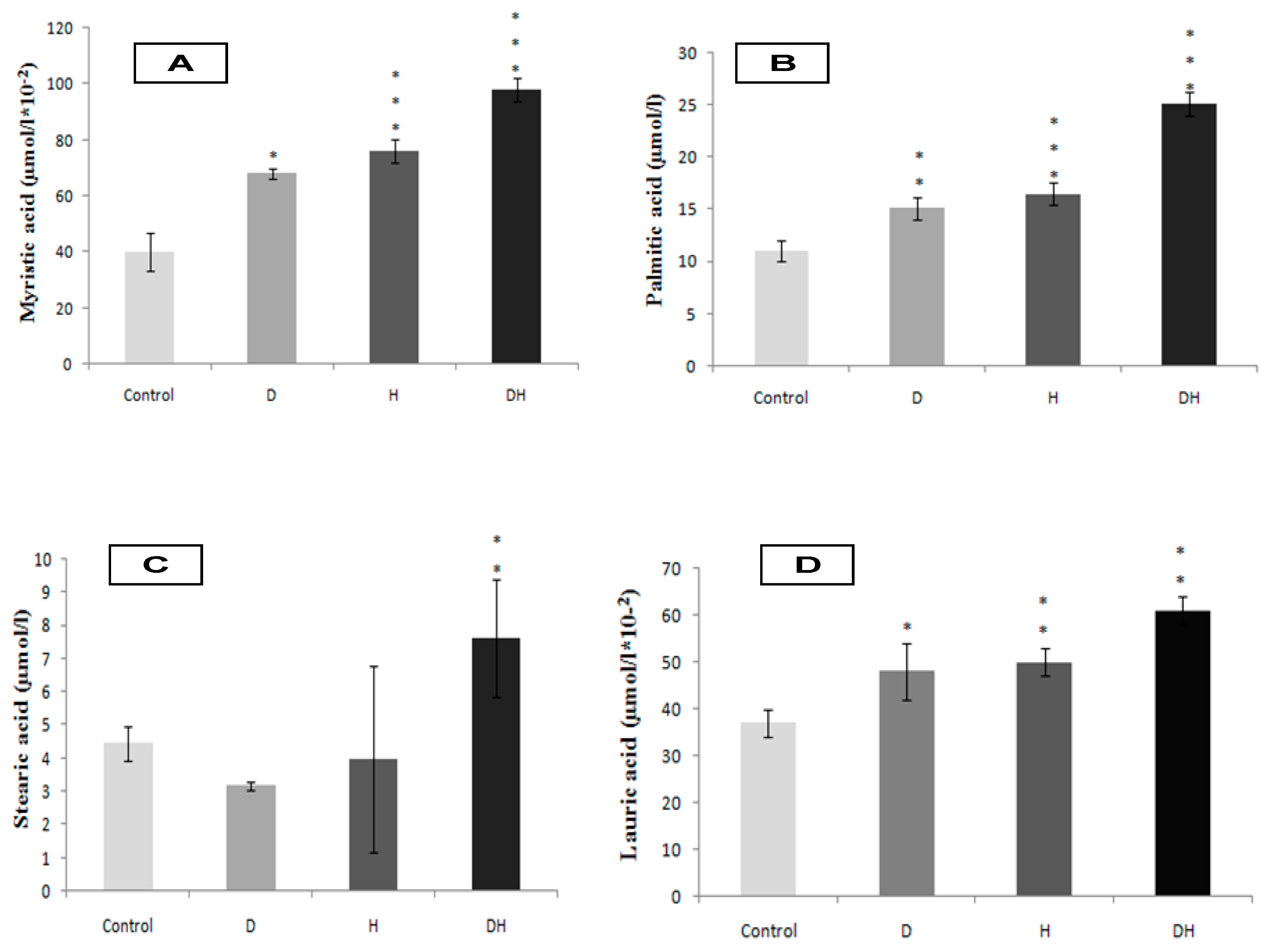
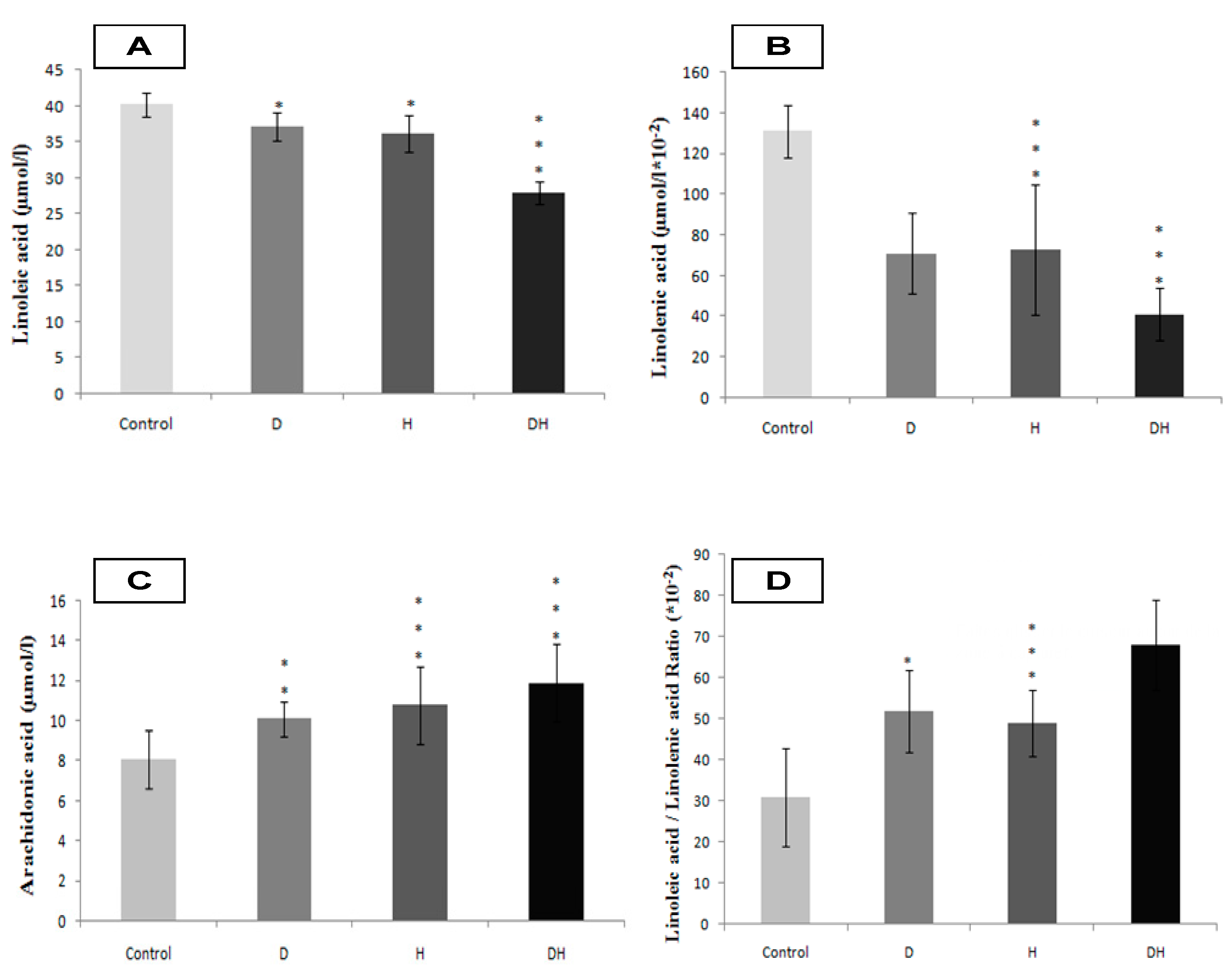
2.2. Plasma Fatty Acids Profile
2.3. Feeding Patterns of the Participants
2.3.1. Dietary Saturated Fatty Acids (SFA) Intake
2.3.2. Dietary Monounsaturated Fatty Acids (MUFA) Intake
2.3.3. Dietary Polyunsaturated Fatty Acids (PUFA) Intake
2.4. Physical Activity Levels of the Participants
3. Patients and Methods
3.1. Participant’s Inclusion and Protocol Design
3.2. Feeding Pattern Participants
3.3. Evaluation of Physical Activity Levels in Participants
3.4. Cadiometabolic Syndrome (CMS) Screening
3.5. Blood Samples and Analyses
3.6. Plasma Fatty Acids Determination
3.7. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 8th ed.; International Diabetes Federation: Brussels, Belgium, 2017. [Google Scholar]
- Siri-Tarino, P.W.; Krauss, R.M. Diet, lipids, and cardiovascular disease. Curr. Opin. Lipidol. 2016, 27, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Randle, P.J.; Garland, P.B.; Hales, C.N.; Newsholme, E.A. The glucose fatty-acid cycle: Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963, 1, 785–789. [Google Scholar] [CrossRef]
- Carmena, R. Type 2 diabetes, dyslipidemia, and vascular risk: Rationale and evidence for correcting the lipid imbalance. Am. Heart J. 2005, 150, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.; Lemaitre, R.N.; King, I.B.; Song, X.; Psaty, B.M.; Siscovick, D.S.; Mozaffarian, D. Circulating omega-6 polyunsaturated fatty acids and total and cause-specific mortality: The Cardiovascular Health Study. Circulation 2014, 130, 1245–1253. [Google Scholar] [CrossRef]
- Steffen, B.T.; Steffen, L.M.; Zhou, X.; Ouyang, P.; Weir, N.L.; Tsai, M.Y. n-3 Fatty acids attenuate the risk of diabetes associated with elevated serum non esterified fatty acids: The multi-ethnic study of atherosclerosis. Diabetes Care 2015, 38, 575–580. [Google Scholar] [PubMed]
- Grimsgaard, S.; Bonaa, K.; Hansen, J.; Nordoy, A. Highly purified eicosapentaenoicacid and docosahexaenoic acid in humans have similar triacylglycerol-lowering effects but divergent effects on serum fatty acids. Am. J. Clin. Nutr. 1997, 66, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Colussi, G.; Catena, C.; Mos, L.; Sechi, L.A. The Metabolic Syndrome and the Membrane Content of Polyunsaturated Fatty Acids in Hypertensive Patients. Metab. Syndr. Relat. Disord. 2015, 13, 343–351. [Google Scholar] [CrossRef]
- Williams, C.M.; Salter, A. Saturated fatty acids and coronary heart disease risk: The debate goes on. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 97–102. [Google Scholar] [CrossRef]
- Nordoy, A.; Goodnight, S.H. Dietary lipids and thrombosis. Relationships to atherosclerosis. Arteriosclerosis 1990, 10, 149–163. [Google Scholar] [CrossRef]
- Kassab, A.; Ajmi, T.; Issaoui, M.; Chaeib, L.; Miled, A.; Hammami, M. Homocysteine enhances LDL fatty acid peroxidation, promoting microalbuminuria in type 2 diabetes. Ann. Clin. Biochem. 2008, 45, 476–480. [Google Scholar] [CrossRef]
- Haring, B.; von Ballmoos, M.C.W.; Appel, L.J.; Sacks, F.M. Healthy Dietary Interventions and Lipoprotein (a) Plasma Levels: Results from the Omni Heart Trial. PLoS ONE 2014, 9, e114859. [Google Scholar] [CrossRef] [PubMed]
- Weisser, B.; Mengden, T.; Düsing, R.; Vetter, H.; Vetter, W. Normal values of blood pressure self-measurement in view of the 1999 World health organization-international society of hypertension guidelines. Am. J. Hypertens. 2000, 13, 940–943. [Google Scholar] [CrossRef][Green Version]
- Belfki, H.; Ben Ali, S.; Aounallah-Skhiri, H.; Traissac, P.; Bougatef, S.; Maire, B.; Delpeuch, F.; Achour, N.; Ben Romdhane, H. Prevalence and determinants of the metabolic syndrome among Tunisian adults: Results of the Transition and Health Impact in North Africa (TAHINA) project. Public Health Nutr. 2013, 16, 582–590. [Google Scholar] [CrossRef] [PubMed]
- El Kinany, K.; Garcia-Larsen, V.; Khalis, M.; Deoula, M.M.S.; Benslimane, A.; Ibrahim, A.; Benjelloun, M.C.; El Rhazi, K. Adaptation and validation of a food frequency questionnaire (FFQ) to assess dietary intake in Moroccan adults. Nutr. J. 2018, 17, 61. [Google Scholar] [CrossRef]
- Favier, J.C.; Ireland-Ripert, J.; Toque, C.; Findberg, M. Répertoire Général des Aliments, Table de Composition; Tec&Doc, INRA, CNEVA CIQUAL: Paris, France, 1995. [Google Scholar]
- Hagströmer, M.; Oja, P.; Sjöström, M. The international physical activity questionnaire (IPAQ): A study of concurrent and construct validity. Public Health Nutr. 2005, 9, 755–762. [Google Scholar] [CrossRef]
- Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Bonora, E.; Targher, G.; Alberiche, M.; Bonadonna, R.C.; Saggiani, F.; Zenere, M.B.; Monauni, T.; Muggeo, M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: Studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 2000, 23, 57–63. [Google Scholar] [CrossRef]
- Deurenberg, P.; Westrate, J.A.; Seidell, J.C. Body mass index as a measure of body fatness: Age- and sex-specific prediction formulas. Br. J. Nutr. 1991, 65, 105–111. [Google Scholar] [CrossRef]
- O’Brien, E.; Mee, F.; Atkins, N.; Thomas, M. Evaluation of three devices for self measurement of blood pressure according to the revised British Hypertension Society Protocol: The Omron HEM-705CP, Philips HP5332, and Nissei DS-175. Blood Press. Monit. 1996, 1, 55–61. [Google Scholar]
- Knopfholz, J.; Disserol, C.C.; Pierin, A.J.; Schirr, F.L.; Streisky, L.; Takito, L.L.; Massucheto Ledesma, P.; Faria-Neto, J.R.; Olandoski, M.; da Cunha, C.L.; et al. Validation of the Friedewald formula in patients with metabolic syndrome. Cholesterol 2014, 2014, 261878. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Dole, V.P.; Meinertz, H. Micro-determination of long chain fatty acids in plasma and tissues. J. Biol. Chem. 1960, 235, 2595–2599. [Google Scholar] [PubMed]
- Duncombe, W.G.; Rising, T.J. Quantitative extraction and determination of non esterified fatty acids in plasma. J. Lipid Res. 1973, 14, 258–261. [Google Scholar]
- Masson, L.F.; McNeill, G.; Tomany, J.O.; Simpson, J.A.; Peace, H.S.; Wei, L.; Grubb, D.A.; Bolton-Smith, C. Statistical approaches for assessing the relative validity of a food-frequency questionnaire: Use of correlation coefficients and the kappa statistic. Public Health Nutr. 2003, 6, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakos, D.B.; Pitsavos, C.; Stefanadis, C. Dietary patterns: A Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Griffin, B.A. How relevant is the ratio of dietary n-6 to n-3 polyunsaturated fatty acids to cardiovascular disease risk? Evidence from the OPTILIP study. Curr. Opin. Lipidol. 2008, 19, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Hayashino, Y.; Jackson, J.L.; Fukumori, N.; Nakamura, F.; Fukuhara, S. Effects of supervised exercise on lipid profiles and blood pressure control in people with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. Diabetes Res. Clin. Pract. 2012, 98, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Tchernof, A.; Després, J.P. Pathophysiology of human visceral obesity: An update. Physiol. Rev. 2013, 93, 359–404. [Google Scholar] [CrossRef]
- Samuel, V.T.; Petersen, K.F.; Shulman, G.I. Lipid-induced insulin resistance: Unravelling the mechanism. Lancet 2010, 375, 2267–2277. [Google Scholar] [CrossRef]
- Guo, S.X.; Yan, Y.Z.; Mu, L.T.; Niu, Q.; He, J.; Liu, J.M.; Rui, D.S. Association of serum free fatty acids with hypertension and insulin resistance among rural Uyghur adults in far Western China. Int. J. Environ. Res. Public Health 2015, 12, 6582–6590. [Google Scholar] [CrossRef]
- Xu, S.; Yang, G.; Yang, M.; Li, S.; Liu, H.; Li, L. Elevated adipose triglyceride lipase in newly diagnosed type 2 diabetes mellitus with hypertension. Am. J. Med. Sci. 2011, 342, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Tanamas, S.K.; Wong, E.; Backholer, K.; Abdullah, A.; Wolfe, R.; Barendregt, J.; Peeters, A. Duration of obesity and incident hypertension in adults from the Framingham Heart study. J. Hypertens. 2015, 33, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Massiera, F.; Bloch-Faure, M.; Ceiler, D.; Murakami, K.; Fukamizu, A.; Gasc, J.M.; Quignard-Boulange, A.; Negrel, R.; Ailhaud, G.; Seydoux, J.; et al. Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. FASEB J. 2001, 15, 2727–2729. [Google Scholar] [CrossRef] [PubMed]
- Kamijo, A.; Kimura, K.; Sugaya, T.; Yamanouchi, M.; Hase, H.; Kaneko, T.; Hirata, Y.; Goto, A.; Fujita, T.; Omata, M. Urinary free fatty acids bound to albumin aggravate tubulo interstitial damage. Kidney Int. 2002, 62, 1628–1637. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.A.; Kellum, J.A.; Haase, M.; Müller, C.; Damman, K.; Murray, P.T.; Cruz, D.; House, A.A.; Schmidt-Ott, K.M.; Vescovo, G.; et al. Pathophysiology of the Cardiorenal Syndromes: Executive Summary from the Eleventh Consensus Conference of the Acute Dialysis Quality Initiative (ADQI). Contrib. Nephrol. 2013, 182, 82. [Google Scholar]
- Levey, A.S.; Coresh, J.; Balk, E.; Kausz, A.T.; Levin, A.; Steffes, M.W.; Hogg, R.J.; Perrone, R.D.; Lau, J.; Eknoyan, G. National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann. Intern. Med. 2003, 139, 137–147. [Google Scholar] [CrossRef]
- Gueguen, Y.; Rouas, C.; Leblond, F.A. Kidney injury biomarkers. Nephrol. Ther. 2012, 8, 146–155. [Google Scholar] [CrossRef]
- Sowers, J.R. Insulin resistance and hypertension. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, 11597–11602. [Google Scholar] [CrossRef]
- Borai, A.; Livingstone, C.; Abdelaal, F.; Bawazeer, A.; Keti, V.; Ferns, G. The relationship between glycosylated haemoglobin (HbA1c) and measures of insulin resistance across a range of glucose tolerance. Scand. J. Clin. Lab. Investig. 2011, 71, 168–172. [Google Scholar] [CrossRef]
- Song, F.; Schmidt, A.M. Glycation and Insulin Resistance: Novel Mechanisms and Unique Targets? Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1760–1765. [Google Scholar] [CrossRef]
- Vasdev, S.; Gill, V.; Singal, P. Role of Advanced Glycation End Products in Hypertension and Atherosclerosis: Therapeutic Implications. Cell Biochem. Biophys. 2007, 49, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, F.; Steinmetz, A.; Kostner, G.M.; Dieplinger, H. Lipoprotein(a) in health and disease. Crit. Rev. Clin. Lab. Sci. 1996, 33, 495–543. [Google Scholar] [CrossRef] [PubMed]
- Zoubiri, H.; Kacimi, G.; Haffaf, E.M.; Oudjit, B.; Adjroud, N.; Koceir, E.A. Cardiometabolic and cardiorenal yndromes interactions in Algerian diabetic-hypertensive patient: Interest of predictive multi-biomarkers strategy to renal dysfunction. Ann. Biol. Clin. 2015, 73, 443–453. [Google Scholar]
- Antonicelli, R.; Testa, R.; Bonfigli, A.R.; Sirolla, C.; Pieri, C.; Marra, M.; Marcovina, S.M. Relationship between lipoprotein (a) levels, oxidative stress, and blood pressure levels in patients with essential hypertension. Clin. Exp. Med. 2001, 1, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Bjorkbacka, H. Multiple roles of toll-like receptor signaling in atherosclerosis. Curr. Opin. Lipidol. 2006, 17, 527–533. [Google Scholar] [CrossRef]
- Walton, K.W.; Hitchens, J.; Magnani, H.N.; Khan, M. A study of methods of identification and estimation of Lp (a) lipoprotein and of its significance in health, hyperlipidaemia and atherosclerosis. Atherosclerosis 1974, 20, 323–346. [Google Scholar] [CrossRef]
- Schlaich, M.P.; John, S.; Langenfeld, M.R.; Lackner, K.J.; Schmitz, G.; Schmieder, R.E. Does Lipoprotein (a) Impair Endothelial Function. J. Am. Coll. Cardiol. 1998, 31, 359–365. [Google Scholar] [CrossRef][Green Version]
- Takagi, H.; Umemoto, T. Atorvastatin decreases lipoprotein (a): A meta-analysis of randomized trials. Int. J. Cardiol. 2012, 154, 183–186. [Google Scholar] [CrossRef]
- Keys, A.; Menotti, A.; Karvonen, M.J.; Aravanis, C.; Blackburn, H.; Buzina, R.; Djordjevic, B.S.; Dontas, A.S.; Fidanza, F.; Keys, M.H.; et al. The diet and 15 year death rate in the seven countries study. Am. J. Epidemiol. 1986, 124, 903–915. [Google Scholar] [CrossRef]
- De Souza, R.J.; Mente, A.; Maroleanu, A.; Cozma, A.I.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schünemann, H.; Beyene, J.; et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ 2015, 351, h3978. [Google Scholar] [CrossRef]
- Karupaiah, T.; Tan, C.H.; Chinna, K.; Sundram, K. The chain length of dietary saturated fatty acids affects human postprandial lipemia. J. Am. Coll. Nutr. 2011, 30, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.J.; Billett, M.A.; Saler, A.M.; Mangiapane, E.H.; Bruce, J.S.; Anderton, K.L.; Marenah, C.B.; Lawson, N.; White, D.A. Modulation of hepatic apolipoprotein B, 3-hydroxy-3-methyglutaryl-CoA reductase and low-density lipoprotein receptor mRNA and plasma lipoprotein concentrations by defined dietary fats. Biochem. J. 1995, 311, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Straczkowski, M.; Kowalska, I.; Nikolajuk, A.; Dzienis-Straczkowska, S.; Kinalska, I.; Baranowski, M.; Zendzian-Piotrowska, M.; Brzezinska, Z.; Gorski, J. Relationship between insulin sensitivity and sphingomyelin signaling pathway in human skeletal muscle. Diabetes 2004, 53, 1215–1221. [Google Scholar] [CrossRef]
- Hoogeveen, R.C.; Gaubatz, J.W.; Sun, W.; Dodge, R.C.; Crosby, J.R.; Jiang, J.; Couper, D.; Virani, S.S.; Kathiresan, S.; Boerwinkle, E.; et al. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: The Atherosclerosis Risk In Communities (ARIC) study. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Mirza, S.; Hossain, M.; Mathews, C.; Martinez, P.; Pino, P.; Gay, J.L.; Rentfro, A.; McCormick, J.B.; Fisher-Hoch, S.P. Type 2-diabetes is associated with elevated levelsof TNF-alpha, IL-6 and adiponectin and low levels of leptin in a population of Mexican Americans: A cross-sectional study. Cytokine 2012, 57, 136–142. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Tangney, C.C.; Levine, D.M.; Parker, T.S.; Gordon, B.R. Elevated soluble tumor necrosis factor receptor levels in non-obese adults with the atherogenic dyslipoproteinemia. Atherosclerosis 2004, 177, 77–81. [Google Scholar] [CrossRef]
- Ciapaite, J.; van Bezu, J.; van Eikenhorst, G.; Bakker, S.J.; Teerlink, T.; Diamant, M.; Heine, R.J.; Krab, K.; Westerhoff, H.V.; Schalkwijk, C.G. Palmitate and oleate have distinct effects on the inflammatory phenotype of human endothelial cells. Biochim. Biophys. Acta 2007, 1771, 147–154. [Google Scholar] [CrossRef]
- Weigert, C.; Brodbeck, K.; Staiger, H.; Kausch, C.; Machicao, F.; Haring, H.U.; Schleicher, E.D. Palmitate, but not unsaturated fatty acids, induces the expression of interleukin-6 in human myotubes through proteasome-dependent activation of nuclear factor-kappaB. J. Biol. Chem. 2004, 279, 23942–23952. [Google Scholar] [CrossRef]
- Jove, M.; Planavila, A.; Sanchez, R.M.; Merlos, M.; Laguna, J.C.; Vazquez-Carrera, M. Palmitate induces tumor necrosis factor-alpha expresión in C2C12 skeletal muscle cells by a mechanism involving protein kinase C and nuclear factor-kappaB activation. Endocrinology 2006, 147, 552–561. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Griel, A.E.; Psota, T.L.; Gebauer, S.K.; Zhang, J.; Etherton, T.D. Dietary stearic acid and risk of cardiovascular disease: Intake, sources, digestion, and absorption. Lipids 2005, 40, 1193–1200. [Google Scholar] [CrossRef]
- Nagai, T.; Honda, Y.; Sugano, Y.; Nishimura, K.; Nakai, M.; Honda, S.; Iwakami, N.; Okada, A.; Asaumi, Y.; Aiba, T.; et al. NaDEF investigators. Circulating Omega-6, But Not Omega-3 Polyunsaturated Fatty Acids, Are Associated with Clinical Outcomes in Patients with Acute Decompensated Heart Failure. PLoS ONE 2016, 11, e0165841. [Google Scholar] [CrossRef] [PubMed]
- Crandell, J.R.; Tartaglia, C.; Tartaglia, J. Lipid effects of switching from prescription EPA+DHA (omega-3-acid ethyl esters) to prescription EPA only (icosapent ethyl) in dyslipidemic patients. Postgrad. Med. 2016, 128, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Giles, T.D.; Sander, G.E.; Nossaman, B.D.; Kadowitz, P.J. Impaired vasodilation in the pathogenesis of hypertension: Focus on nitric oxide, endothelial-derived hyperpolarizing factors, and prostaglandins. J. Clin. Hypertens. 2012, 14, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Wachira, J.K.; Larson, M.K.; Harris, W.S. n-3 Fatty acids affect haemostasis but do not increase the risk of bleeding: Clinical observations and mechanistic insights. Br. J. Nutr. 2014, 111, 1652–1662. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Khaza’ai, H.; Patimah, I.; Rahmat, A.; Abed, Y. Effect of long chain omega-3 polyunsaturated fatty acids on inflammation and metabolic markers in hypertensive and/or diabetic obese adults: A randomized controlled trial. Food. Nutr. Res. 2016, 60, 29268. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Kothapalli, K.S.; Brenna, J.T. Desaturase and elongase-limiting endogenous long-chain polyunsaturated fatty acid biosynthesis. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 103–110. [Google Scholar] [CrossRef]
- Leonarduzzi, G.; Gamba, P.; Gargiulo, S.; Biasi, F.; Poli, G. Inflammation-related gene expression by lipid oxidation-derived products in the progression of atherosclerosis. Free Radic. Biol. Med. 2012, 52, 19–34. [Google Scholar] [CrossRef]
- Erkan, L.G.; Guvenc, G.; Altinbas, B.; Niaz, N.; Yalcin, M. The effects of centrally injected arachidonic acid on respiratory system: Involvement of cyclooxygenase to thromboxane signaling pathway. Respir. Physiol. Neurobiol. 2016, 225, 1–7. [Google Scholar] [CrossRef]
- Meng, H.; McClendon, C.L.; Dai, Z.; Li, K.; Zhang, X.; He, S.; Shang, E.; Liu, Y.; Lai, L. Discovery of Novel 15-Lipoxygenase Activators To Shift the Human Arachidonic Acid Metabolic Network toward Inflammation Resolution. J. Med. Chem. 2016, 59, 4202–4209. [Google Scholar] [CrossRef]
- Tretjakovs, P.; Kalnins, U.; Dabina, I.; Erglis, A.; Dinne, I.; Jurka, A.; Latkovskis, G.; Zvaigzne, A.; Pirags, V. Nitric oxide production and arachidonic acid metabolism in platelet membranes of coronary heart disease patients with and without diabetes. Med. Princ. Pract. 2003, 12, 10–16. [Google Scholar] [CrossRef]
- Shin, M.J.; Blanche, P.J.; Rawlings, R.S.; Fernstrom, H.S.; Krauss, R.M. Increased plasma concentrations of lipoprotein (a) during a low-fat, high-carbohydrate diet are associated with increased plasma concentrations of apolipoprotein C-III bound to apolipoprotein B-containing lipoproteins. Am. J. Nutr. 2007, 85, 1527–1532. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hu, X.; Yang, X.; Li, X.; Luo, D.; Zhou, Y.; Dong, H. Lipoprotein (a) as a residual risk factor for atherosclerotic renal artery stenosis in hypertensive patients: A hospital-basedcross-sectional study. Lipids Health Dis 2020, 19, 173. [Google Scholar] [CrossRef] [PubMed]
- Maehre, H.K.; Jensen, I.J.; Elvevoll, E.O.; Eilertsen, K.E. ω-3 Fatty Acids and Cardiovascular Diseases: Effects, Mechanisms and Dietary Relevance. Int. J. Mol. Sci. 2015, 16, 22636–22661. [Google Scholar] [CrossRef] [PubMed]
- Colussi, G.; Catena, C.; Novello, M.; Bertin, N.; Sechi, L.A. Impact of omega-3 polyunsaturated fatty acids on vascular function and blood pressure: Relevance for cardiovascular outcomes. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Warnakula, S.; Kunutsor, S.; Crowe, F.; Ward, H.A.; Johnson, L.; Franco, O.H.; Butterworth, A.S.; Forouhi, N.G.; Thompson, S.G.; et al. Association of Dietary, Circulating, and Supplement Fatty Acids With Coronary Risk:A Systematic Review and Meta-analysis. Ann. Intern. Med. 2014, 160, 398–406. [Google Scholar] [CrossRef] [PubMed]
- de Assis, A.M.; Rech, A.; Longoni, A.; da Silva Morrone, M.; de Bittencourt Pasquali, M.A.; Perry, M.L.; Souza, D.O.; Moreira, J.C. Dietary n-3 polyunsaturated fatty acids revert renal responses induced by a combination of 2 protocols that increase the amounts of advanced glycation end product in rats. Nutr. Res. 2015, 35, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.; Marchioli, R.; Silletta, M.G.; Masson, S.; Sellke, F.W.; Libby, P.; Milne, G.L.; Brown, N.J.; Lombardi, F.; Damiano, R.J., Jr.; et al. Oxidative Stress Biomarkers and Incidence of Postoperative Atrial Fibrillation in the Omega-3 Fatty Acids for Prevention ofPostoperative Atrial Fibrillation (OPERA) Trial. J. Am. Heart Assoc. 2015, 4, e001886. [Google Scholar] [CrossRef]
- Clevidence, B.A.; Judd, J.T.; Schaefer, E.J.; Jenner, J.L.; Lichtenstein, A.H.; Muesing, R.A.; Wittes, J.; Sunkin, M.E. Plasma lipoprotein (a) levels in men and women consuming diets enriched in saturated, cis-, or trans-monounsaturated fatty acids. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1657–1661. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

| p/G | Control | D | H | DH |
|---|---|---|---|---|
| (N = 50) | (N = 100) | (N = 316) | (N = 368) | |
| Age (year) | 44 ± 3 | 48 ± 2 | 51 ± 2 | 45 ± 3 |
| BMI (Kg/m2) | 23.5 ± 2 | 34 ± 2 ***(a) | 22 ± 2 ***(a,b) | 34 ± 2 ***(a,b,c) |
| WC (cm) | 77 ± 2 (F) | 106 ± 6 (F)***(a) | 105 ± 5 (F)***(a,b) | 111 ± 5 (F)***(a,b,c) |
| 73 ± 1 (M) | 91 ± 3 (M) | 100 ± 7 (M)***(a,b) | 110 ± 7 (M)***(a,b,c) | |
| WC/WH ratio | 0.83 ± 0.03 (F) | 1.09 ± 0.02 (F)***(a) | 0.97 ± 0.01 (F)***(a,b) | 1.11 ± 0.01 (F)***(a,b,c) |
| 0.86 ± 0.01 (M) | 1.03 ± 0.01 (M) | 0.91 ± 0.02 (M)***(a,b) | 1.08 ± 0.02 (M)***(a,b,c) | |
| BF (%) | 12.0 ± 0.61 (F) | 46.6 ± 0.2 (F)*** | 30.51 ± 3.29 (F)***(a,b) | 52.01 ± 0.01 (F)***(a,b,c) |
| 8.22 ± 0.60 (M) | 42.1 ± 0.9 (M)***(a) | 28.80 ± 1.10 (M)***(a,b) | 48.22 ± 0.06 (M)***(a,b,c) |
| p/G | Control | D | H | DH |
|---|---|---|---|---|
| Glycemia (mmol/L) | 4.71 ± 0.12 | 7.55 ± 0.8 **(a) | 5.63 ± 0.8 ***(a,b) | 8.33 ± 0.6 ***(a,b,c) |
| Insulinemia (pmol/L) | 66 ± 1.7 | 147 ± 2.2 **(a) | 136 ± 3.1 ***(a,b) | 164 ± 8 |
| Homa Index | 1.98 ± 0.07 | 4.19 ± 0.10 ***(a) | 5.95 ± 0.64 ***(a,b) | 6.97 ± 0.87 ***(a,b,c) |
| HbA1C (%) | 5.1 ± 0.59 | 6.33 ± 0.12 **(a) | 5.94 ± 0.10 ***(a,b) | 9.21 ± 0.40 ***(a,b,c) |
| Triglycerides (mmol/L) | 1.16 ± 0.31 | 1.82 ± 0.22 ***(a) | 1.89 ± 0.45 ***(a,b) | 1.98 ± 0.26 ***(a,b,c) |
| Total Cholesterol (mmol/L) | 4.05 ± 0.1 | 5.27 ± 0.6 ***(a) | 5.11 ± 0.7 ***(a,b) | 5.61 ± 0.8 ***(a,b,c) |
| HDL-C (mmol/L) | 1.52 ± 0.2 (F) | 1.08 ± 0.3 (F) | 1.10 ± 0.4 (F)*(a,b) | 1.04 ± 0.1 (F)*(a,b,c) |
| 1.24 ± 0.1 (M) | 1.06 ± 0.2 (M) | 1.09 ± 0.1 (M)*(a,b) | 0.98 ± 0.1 (M)*(a,b,c) | |
| LDL-C (mmol/L) | 2.45 ± 0.5 | 3.48 ± 0.6 **(a) | 3.23 ± 0.5 ***(a,b) | 3.69 ± 0.3 ***(a,b,c) |
| SBP (mm Hg) | 121 ± 12 | 129 ± 6 | 147 ± 3 ***(a,b) | 159 ± 3 ***(a,b,c) |
| DBP (mm Hg) | 73 ± 5 | 80 ± 4 | 92 ± 1 ***(a,b) | 98 ± 1 ***(a,b,c) |
| Hs-CRP (mg/L) | 3.5 ± 1.2 | 5.6 ± 0.1 **(a) | 5.4 ± 0.7 ***(a,b) | 7.7 ± 0.6 ***(a,b,c) |
| Fibrinogen (mg/L) | 2.97 ± 0.41 | 3.38 ± 0.13 | 3.19 ± 0.11 | 3.42 ± 0.1 |
| Microalbuminuria (mg/24h) | 14.7 ± 5 | 29.9 ± 14 ***(a) | 34.1 ± 8.2 ***(a,b) | 44.8 ± 7 ***(a,b,c) |
| p/G | Control | D | H | DH |
|---|---|---|---|---|
| HDL-c/LDL-c | 0.56 ± 0.04 | 0.31 ± 0.01 **(a) | 0.33 ± 0.02 ***(a,b) | 0.27 ± 0.02 ***(a,b,c) |
| TG/HDL-c | 0.86 ± 0.01 | 1.69 ± 0.05 **(a) | 1.68 ± 0.22 ***(a,b) | 1.96 ± 0.06 ***(a,b,c) |
| ApoA1 (g/L) | 1.69 ± 0.03 | 1.38 ± 0.07 | 0.87 ± 0.02 ***(a,b) | 0.90 ± 0.05 ***(a,b,c) |
| ApoB100 (g/L) | 0.85 ± 0.01 | 0.92 ± 0.08 | 0.90 ± 0.05 | 0.98 ± 0.08 |
| ApoB100/ApoA1 | 0.50 ± 0.01 | 0.66 ± 0.01 | 1.03 ± 0.02 ***(a,b) | 1.08 ± 0.06 ***(a,b,c) |
| Lp (a) (g/L) | 0.20 ± 0.07 | 0.44 ± 0.06 **(a) | 0.79 ± 0.03 ***(a,b) | 0.83 ± 0.01 ***(a,b,c) |
| tHcy (µmol/L) | 10.4 ± 0.99 | 10.7 ± 0.06 *(a) | 15.2 ± 0.35 **(a,b) | 17.1 ± 0.15 **(a,b,c) |
| TNF-α (pg/mL) | 26.1 ± 2.21 | 55.8 ± 3.27 *(a) | 85.5 ± 5.13 **(a,b) | 149 ± 17.5 ***(a,b) |
| IL-6 (pg/mL) | 42.6 ± 1.34 | 107 ± 8.5 **(a) | 113 ± 7.61 **(a) | 228 ± 32 ***(a,b,c) |
| IL-1β (pg/mL) | 32.5 ± 4.5 ** | 82.1 ± 2.5 *(a) | 109 ± 21.2 **(a,b) | 135 ± 19.4 ***(a,b,c) |
| p/G | Group D | Group H | Group DH | |||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| PUFA/SFA ratio—Homa-IR | −0.74 | 0.001 | −0.85 | 0.001 | −0.55 | 0.05 |
| PUFA/SFA ratio—%BF(F) | 0.92 | 0.001 | −0.21 | 0.049 | −0.59 | 0.05 |
| PUFA/SFA ratio—%BF(M) | −0.34 | 0.055 | 0.56 | 0.05 | −0.42 | 0.024 |
| PUFA/SFA ratio—WC (F) | −0.32 | 0.058 | −0.48 | 0.032 | −0.23 | 0.05 |
| PUFA/SFA ratio—WC (M) | −0.89 | 0.031 | −0.78 | 0.024 | −0.78 | 0.001 |
| PUFA/SFA ratio—Triglycerides | −0.35 | 0.03 | −0.2 | 0.055 | −0.85 | 0.001 |
| PUFA/SFA ratio—Total Cholesterol | −0.4 | 0.05 | −0.6 | 0.01 | −0.71 | 0.001 |
| PUFA/SFA ratio—HDL-c/LDL-c | 0.45 | 0.05 | −0.24 | 0.05 | 0.66 | 0.001 |
| PUFA/SFA ratio—TG/HDL-c | 0.41 | 0.04 | −0.12 | 0.005 | −0.68 | 0.001 |
| PUFA/SFA ratio—ApoB100/ApoA1 | −0.85 | 0.001 | −0.45 | 0.05 | −0.71 | 0.001 |
| PUFA/SFA ratio—NEFFA | −0.75 | 0.001 | 0.52 | 0.03 | −0.2 | 0.02 |
| PUFA/SFA ratio—Lp (a) | −0.57 | 0.02 | −0.64 | 0.001 | −0.5 | 0.01 |
| PUFA/SFA ratio—tHcy | −0.42 | 0.05 | −0.5 | 0.02 | −0.59 | 0.01 |
| PUFA/SFA ratio—Hs-CRP | −0.75 | 0.001 | −0.23 | 0.051 | −0.43 | 0.04 |
| PUFA/SFA ratio—EPA + DHA | 0.14 | 0.006 | 0.21 | 0.075 | 0.54 | 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gouaref, I.; Bouazza, A.; Abderrhmane, S.A.; Koceir, E.-A. Lipid Profile Modulates Cardiometabolic Risk Biomarkers Including Hypertension in People with Type-2 Diabetes: A Focus on Unbalanced Ratio of Plasma Polyunsaturated/Saturated Fatty Acids. Molecules 2020, 25, 4315. https://doi.org/10.3390/molecules25184315
Gouaref I, Bouazza A, Abderrhmane SA, Koceir E-A. Lipid Profile Modulates Cardiometabolic Risk Biomarkers Including Hypertension in People with Type-2 Diabetes: A Focus on Unbalanced Ratio of Plasma Polyunsaturated/Saturated Fatty Acids. Molecules. 2020; 25(18):4315. https://doi.org/10.3390/molecules25184315
Chicago/Turabian StyleGouaref, Ines, Asma Bouazza, Samir Ait Abderrhmane, and Elhadj-Ahmed Koceir. 2020. "Lipid Profile Modulates Cardiometabolic Risk Biomarkers Including Hypertension in People with Type-2 Diabetes: A Focus on Unbalanced Ratio of Plasma Polyunsaturated/Saturated Fatty Acids" Molecules 25, no. 18: 4315. https://doi.org/10.3390/molecules25184315
APA StyleGouaref, I., Bouazza, A., Abderrhmane, S. A., & Koceir, E.-A. (2020). Lipid Profile Modulates Cardiometabolic Risk Biomarkers Including Hypertension in People with Type-2 Diabetes: A Focus on Unbalanced Ratio of Plasma Polyunsaturated/Saturated Fatty Acids. Molecules, 25(18), 4315. https://doi.org/10.3390/molecules25184315