1. Introduction
Energetic materials (EMs) are widely used in military and civilian applications, such as weaponry, aerospace explorations and fireworks. However, both the power and safety of energetic materials are the most concern in the field of energetic materials application, but there is an essential contradiction between them: the highly energetic materials are often not safe, and, at present, the rareness of pure low-sensitive and highly energetic explosive has been found [
1,
2,
3]. For a long time, in order to obtain EMs with lower sensitivity, the modifications of existing explosives have often focused mainly on recrystallizing with solution and coating with polymer [
4,
5,
6]. However, these traditional methods cannot markedly reduce the sensitivities of existing explosives by only modifying morphology or diluting power, due to the unchanging inherent structures of explosive molecules [
7,
8]. Due to the stringent requirements for both low-sensitivity and high-power simultaneously, the cocrystallization of explosive, a technique by which a multi-component crystal of several neutral explosive molecules forms in a defined ratio through non-covalent interactions (e.g., H-bond, electrostatic interaction, etc.) [
9,
10], has attracted great interest since it can alleviate, to a certain extent, the power-safety contradiction [
11,
12]. The new energetic cocrystals can potentially exhibit decreased sensitivity, higher performance through increased packing efficiency or oxygen balance, or improve aging due to altered bonds to specific groups on constituent molecules [
13,
14,
15]. Recently a lot of cocrystal explosives have been synthesized and characterized [
16,
17,
18,
19,
20]. An evaluation of the power and safety of energetic cocrystals has been carried out, and cocrystallization is becoming increasingly hot in the field of energetic materials [
21]. It was found that the stability (sensitivity) and detonation performance (energy, detonation velocity, and detonation pressure, etc.) of cocrystal explosive could be influenced by the molar ratio of molecular combination. Generally, when a cocrystal has too much content of high-energy explosives, the packing density and detonation performance will be increased, with possible increase in explosive sensitivity. On the contrary, the sensitivity will be decreased in a cocrystal explosive with a high content of low-energetic or non-energetic explosives. The searches for stable insensitive and highly-energetic explosive is the primary goal in the field of energetic material chemistry. Therefore, the molar ratios of two or more kinds of explosive components should be controlled in a reasonable scope, and it is very necessary to clarify the influence of the ratio of molecular combination on the stability and detonation performance of cocrystal explosives, such as packing density, oxygen balance, detonation velocity, and detonation pressure, etc. [
22].
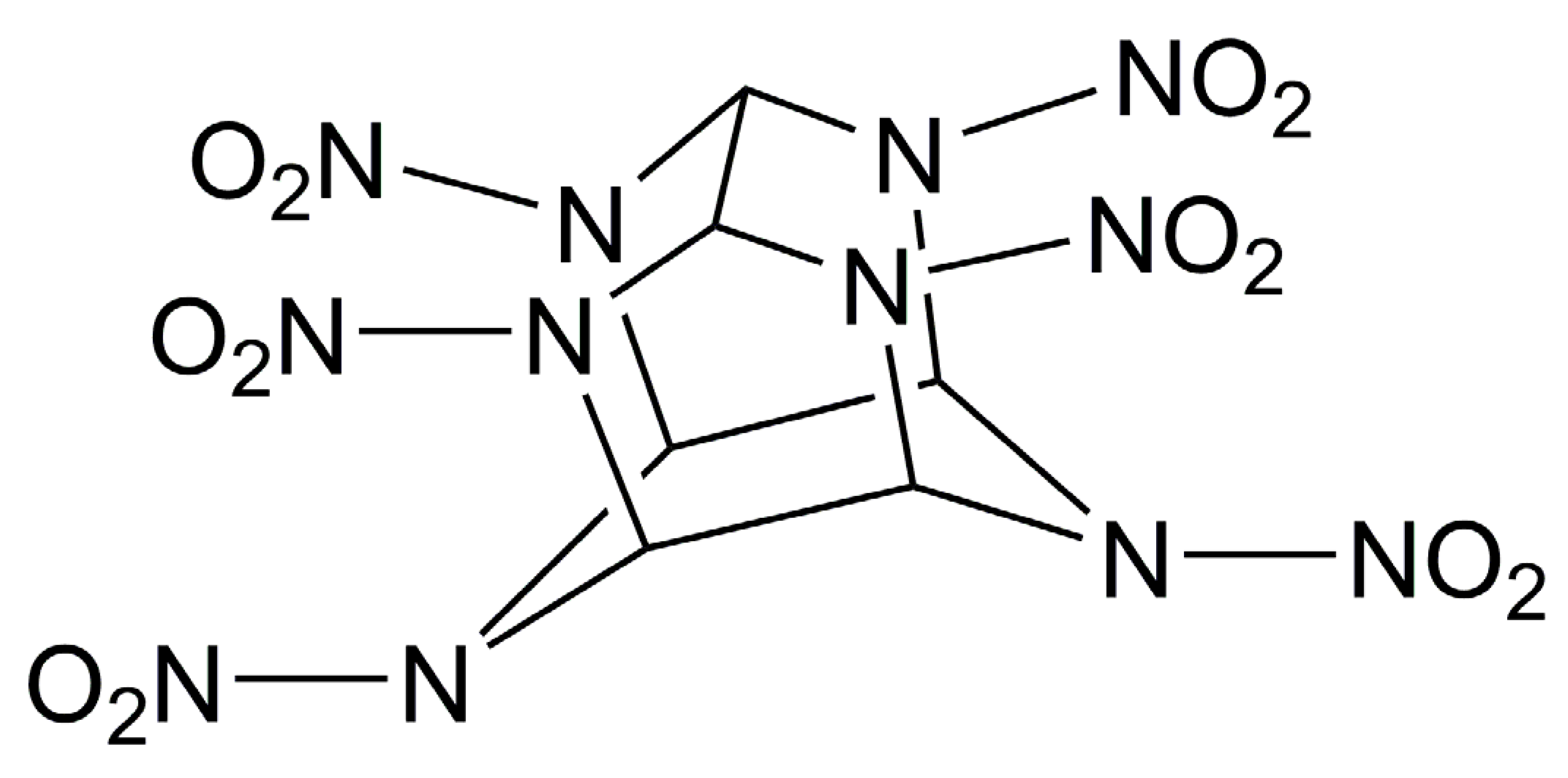
The compound 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane (CL-20) is currently known to be one of the most powerful explosives [
23], and it is also a superior alternative to HMX for applications in low signature rocket propellants [
24,
25,
26] and gun propellants [
27]. However, the disadvantage of CL-20 is its increased sensitivity to mechanical stimuli and cocrystallization of CL-20 with other compounds, which may provide a way to decrease its sensitivity [
28]. Moreover, the relationship of the reaction kinetic process with temperatures and densities for pyrolysis of CL-20/TNT cocrystal was investigated using a reactive force field (ReaxFF) molecular dynamics simulation. The evolution distribution of potential energy and total species, decay kinetics and kinetic parameters for thermal decomposition reaction of CL-20 and TNT were analyzed. It was shown that the breaking of −NO
2 bond from CL-20 molecules is the initial reaction pathway for the thermal decomposition of the cocrystal. With increasing the cocrystal density, the reaction energy barrier of CL-20 and TNT molecule decomposition increases correspondingly. The decomposition process of TNT has an inhibition action on the decomposition of CL-20 [
29]. Meanwhile, the combustion behavior, flame structure, and thermal decomposition of bi-molecular crystals of CL-20 with glycerol triacetate (GTA), tris [
1,
2,
5]oxadiazolo [3,4-b:3′,4′-d:3′’,4′’-f]azepine-7-amine (ATFAz), 4,4′’-dinitro-ter-furazan (BNTF), oxepino [2,3-c:4,5-c’:6,7-c’’] trisfurazan (OTF), oxepino [2,3-c:4,5-c’:6,7-c’’] trisfurazan-1-oxide (OTFO) were studied [
30]. It was found that the introduction of volatile and thermally stable compounds into the composition with CL-20 decreased the thermal stability of CL-20. The combustion mechanism of the CL-20 cocrystal depends both on the burning rate of the second component and its volatility. While there are few overview papers on CL-20-based cocrystal’s simulation, preparation and characterization. Thus, in this paper, the achievements in the intermolecular interaction of five types of CL-20-based cocrystals (CL-20/TNT, CL-20/HMX, CL-20/FOX-7, CL-20/TKX-50 and CL-20/DNB) by using molecular dynamic simulation are reviewed. The preparation and performance of CL-20-based cocrystals are emphatically analyzed, and the existing problems and challenges in the future work are reviewed. The aim of this work is mainly to explore the nature of the formation of the different CL-20-based cocrystal morphology and clarify the influence of the ratio of molecular combination on the stability and detonation performance of CL-20-based cocrystal explosives.
3. Preparation
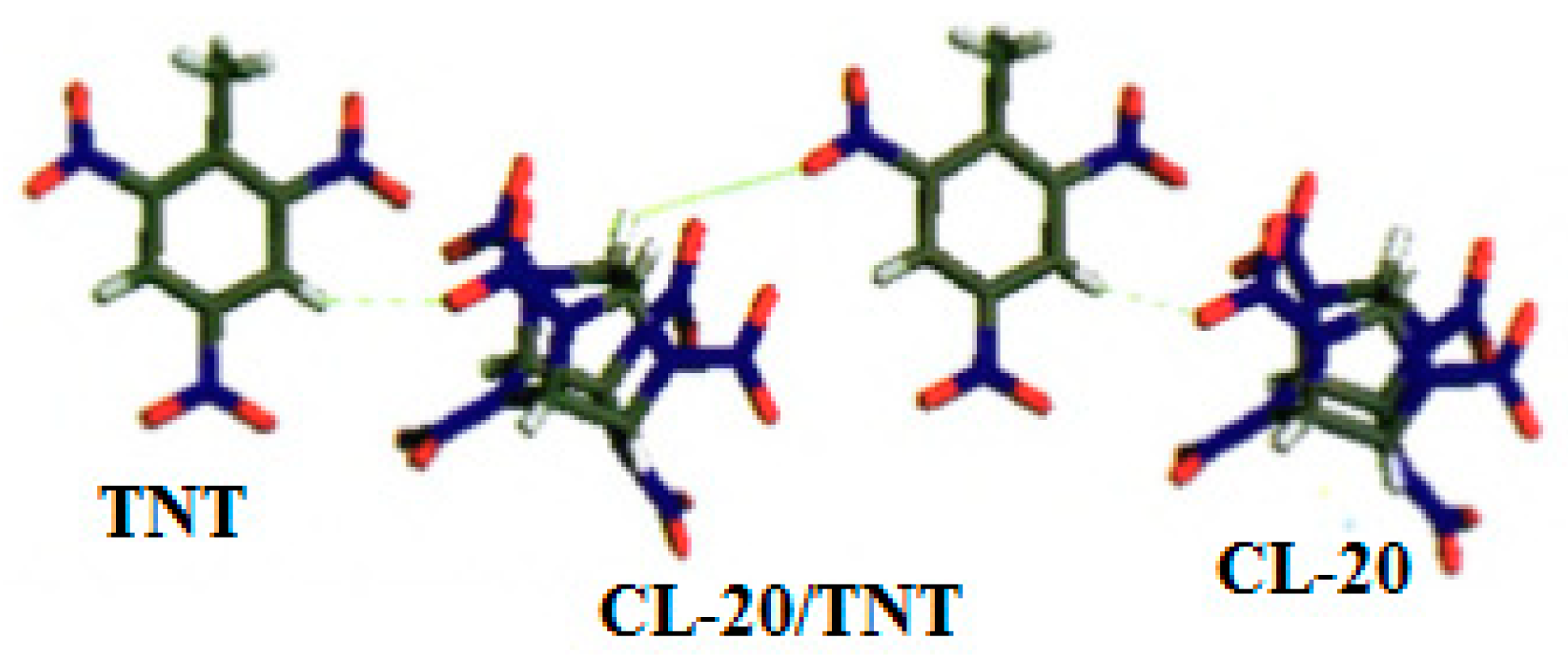
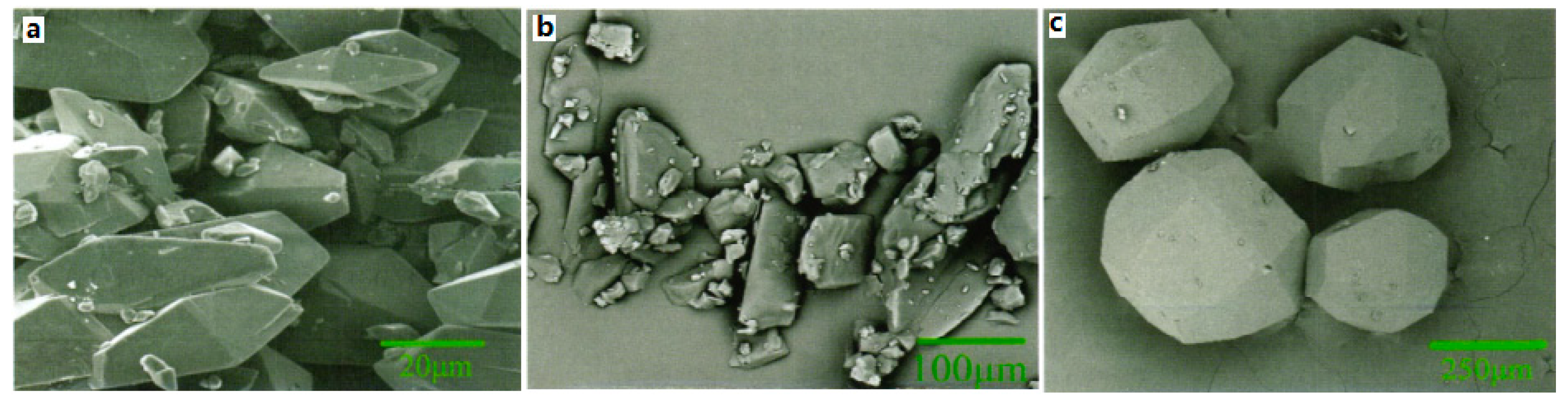
For CL-20/TNT cocrystal, the CL-20/TNT cocrystal with 1:1 mole ratio was prepared by means of the recrystallization method at room temperature, and with ethyl acetate as solvent [
8]. It was found that the morphology of CL-20/TNT cocrystal is different from that of CL-20 and TNT. The crystal of CL-20 is “spindle” shape and TNT is an irregular block crystal. However, CL-20/TNT cocrystal is a prismatic crystal with a smooth and complete surface and uniform size (
Figure 6). The average particle size is 270 μm, indicating that the designing and preparation of cocrystal can effectively change the shape and size of explosive cocrystals.
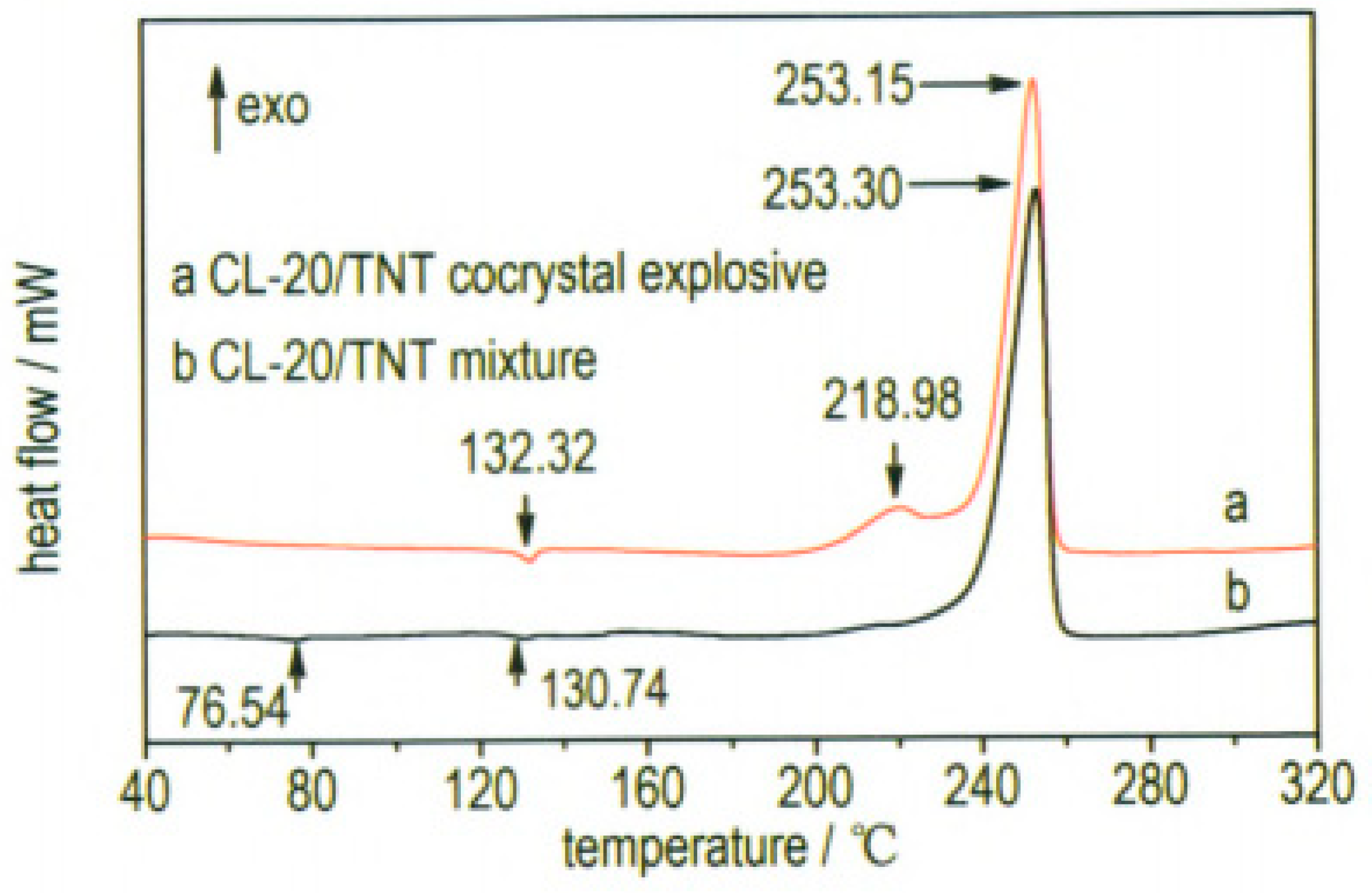
At the same time, ultrafine CL-20/TNT cocrystal explosive was prepared by a spray-drying method [
20]. Results show that the prepared samples are not the mix of CL-20 and TNT but rather ultrafine CL-20/TNT cocrystal explosives. The particle sizes of the cocrystal explosives were lower than 1 μm and they can aggregate into many microparticles, which are spherical in shape and 1–10 μm in size (
Figure 7). The thermal decomposition process can be divided into two stages. The peak temperatures of exothermic decomposition for the first and second stages are 218.98 °C and 253.15 °C, respectively (
Figure 8). The characteristic drop height of CL-20/TNT cocrystal explosives is 49.3 cm, which increases by 36.2 cm compared with that of raw CL-20 (
Table 7).
The ultrafine CL-20/HMX cocrystal explosive was prepared by an ultra-highly efficient mixing method [
6]. The prepared samples were regular block-like ultrafine CL-20/HMX cocrystal explosives with a uniform particle size of less than 1 μm (
Figure 9), which appeared new stronger diffraction peaks at 11.558°, 13.264°, 18.601°, 24.474°, 33.785°, 36.269°. The purity of the CL-20/HMX cocrystal explosive was 92.6%.
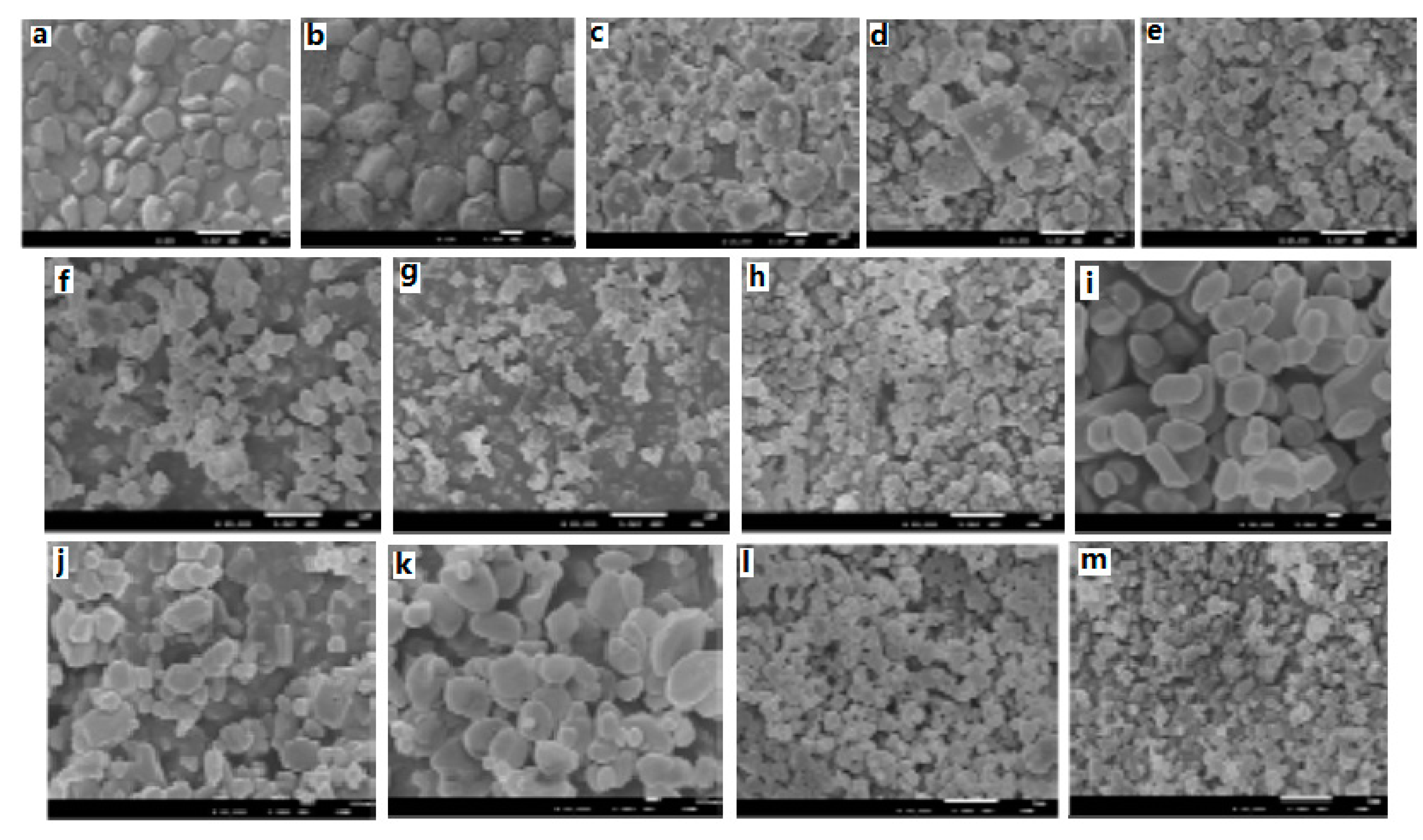
Moreover, the micro/nano CL-20/HMX energetic cocrystal materials were prepared by mechanical ball milling, and characterized using SEM [
7]. Results show that after a milling time of 120 min, CL-20/HMX cocrystals prepared under optimal conditions are spherical in shape and the particle size is 80–250 nm. Compared with respective energetic monomers, the prepared micro/nano CL-20/HMX energetic cocrystal materials exhibit unique crystal structure and thermal decomposition properties (
Figure 10).
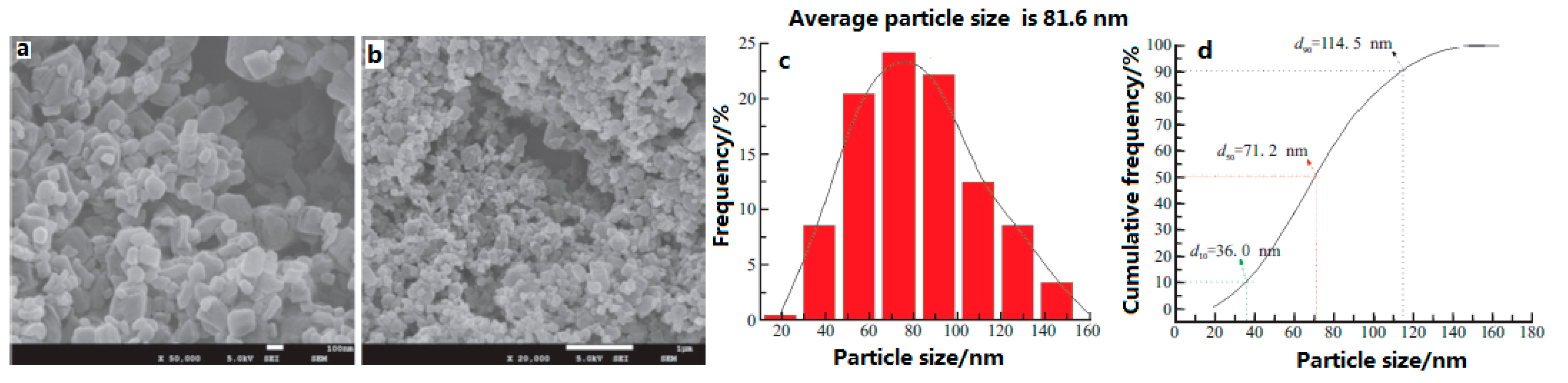
In addition, nano-sized CL-20/HMX cocrystals with a mean particle size of 81.6 nm were prepared under the conditions of the 0.3 mm diameter of milling balls [
9]. The impact sensitivity was conducted to evaluate the impact safety performance of the CL-20/HMX cocrystal (
Table 7). The micro-morphology of the explosives is near-spherical and the particle size reveals a normal distribution (
Figure 11). Before and after milling, the element composition and molecular structure of CL-20/HMX did not change in comparison to the raw CL-20 and HMX, while there are new crystal phases in the formed cocrystal. The characteristic drop height (
H50) of CL-20/HMX was 32.62 cm with 5 kg hammer, which is lower than that of raw materials and mixture, indicating that the mechanical ball milling method is not simply to mix the two explosives physically, but to form the cocrystal between the explosive molecules through the hydrogen bond. Meanwhile, the crystal technology can improve the impact safety of explosives.
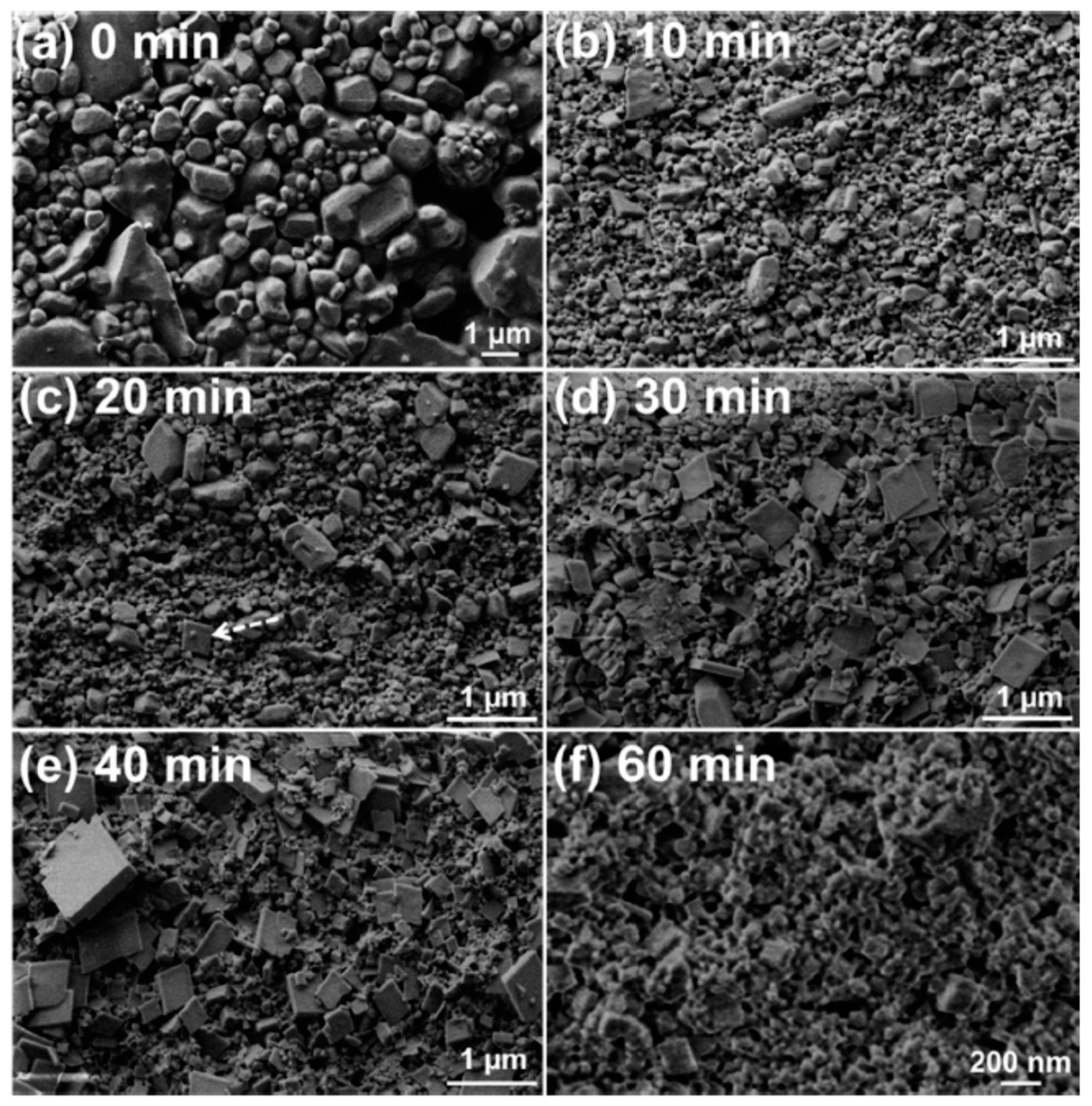
Nano-scale CL-20/HMX cocrystal of CL-20 and HMX in 2:1 molar ratio with a mean size below 200 nm was prepared by bead milling [
36].
Figure 12 shows the morphological evolution of the crystal particles. It was found that most of the coformer crystal particles are ~1 μm (
Figure 12a). The mean particle size of the discrete coformers substantially decreased after 10 min of milling (
Figure 12b), with no conversion to the cocrystalline material. Plate-like crystal particles with dimensions less than 500 nm started to appear in the specimen being milled for 20 min, as indicated by the arrow in
Figure 12c. The plate-like particles were assigned to the 2CL-20·HMX cocrystal as (1) the 2CL-20·HMX cocrystal is known to have a plate-like morphology; (2) the appearance of these particles and the diffraction peaks of the 2CL-20·HMX cocrystal in the XRD pattern occurred at the same time; and (3) more plate-like particles were observed in the specimens upon further milling (
Figure 12d and e, respectively). The observation of these relatively large cocrystal particles seems to be contradicting to the intensive collisions between the grinding media and particles and between the particles themselves occurring during the milling process. It is possible that some growth occurs during the drying of the sampled specimens.
Preparation procedure: 438.0 mg (1 mmol) CL-20 and 472.3 mg (2 mmol) TKX-50 were dried in a beaker at 40 °C for 3 h, 30 mL DMF was added to the beaker, heat to 80 °C in the water bath, and ultrasonic for 30 min until the drug is completely dissolved. Then, 100 mL chloroform was put into the flask. The completely dissolved solution was dropped into the flask at the rate of 0.8 mL/min, and the magnetic stirring speed was kept at 1000 r/min. After dropping, keep the original speed and continue stirring for 1 h. After standing for 3 h and filtering, the filtered samples were dried in a vacuum for 3 h to obtain CL-20/TKX-50 ultrafine cocrystal samples. The performance was compared with that of CL-20/TKX-50 mechanical mixture in the same mole ratio.
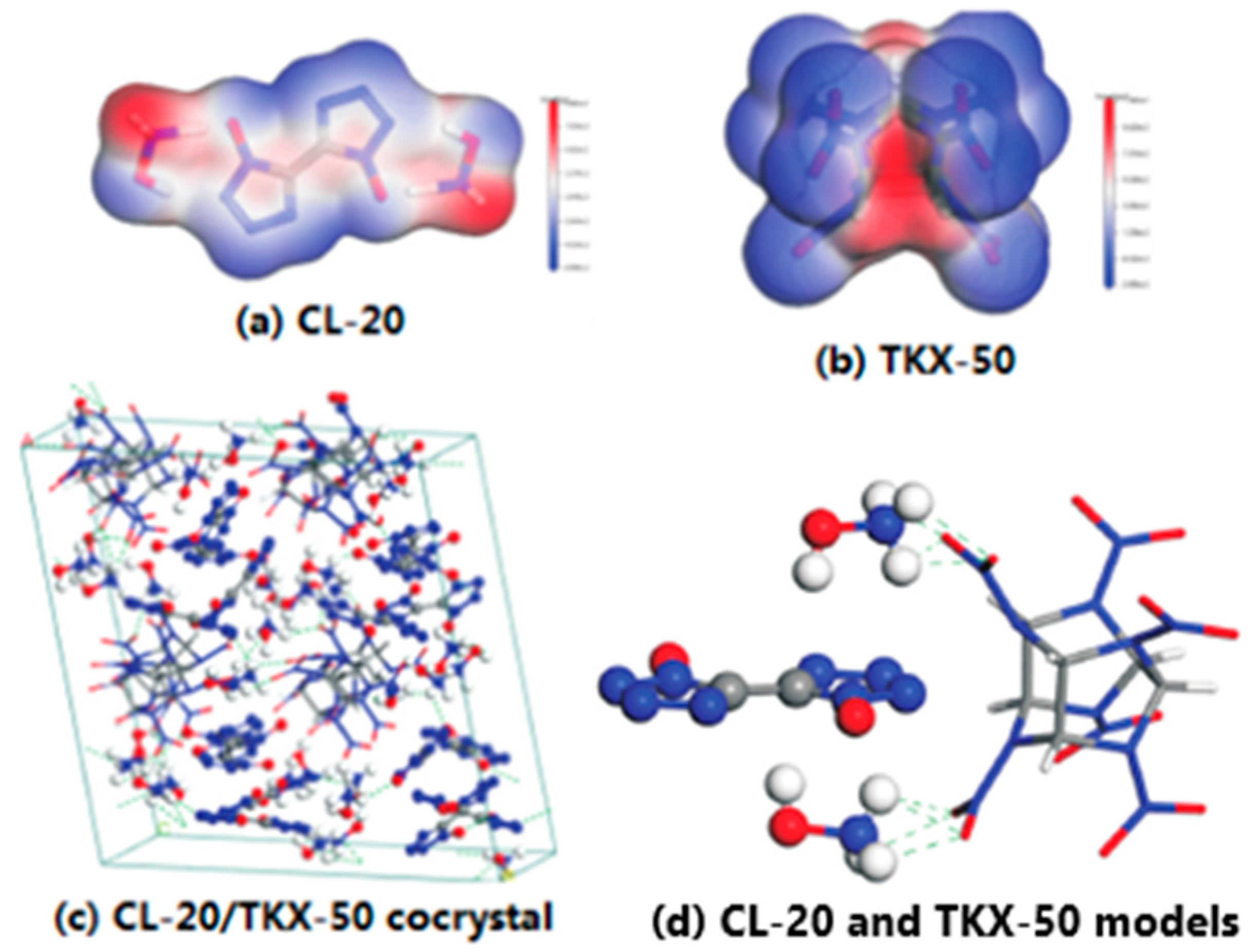
To decrease the sensitivity of CL-20, the CL-20/TKX-50 cocrystal explosive was prepared by solvent-nonsolvent method. The surface electrostatic potentials of CL-20 and TKX-50 were analyzed and the possible non-covalent bonding between cocrystal molecules was predicted [
34]. Results shown that the prepared CL-20/TKX-50 cocrystal has a flat sheet shape, the formation, disappearance, shift and change of intensity of peaks been proved the formation of a new lattice structure. The crystal morphology of CL-20 and TKX-50 is nearly spherical shape, and the particle size distribution is relatively uniform, and the particle size is 1 μm. However, the morphology of CL-20/TKX-50 cocrystal shows a long and thin lamellar structure, which is quite different from that of CL-20 and TKX-50, and the particle size of CL-20/TKX-50 cocrystal is 10 μm, indicating that the cocrystal process can not only change the morphology of the original crystal, but also prove the formation of a new crystal (
Figure 13).
4. Energetic Performance
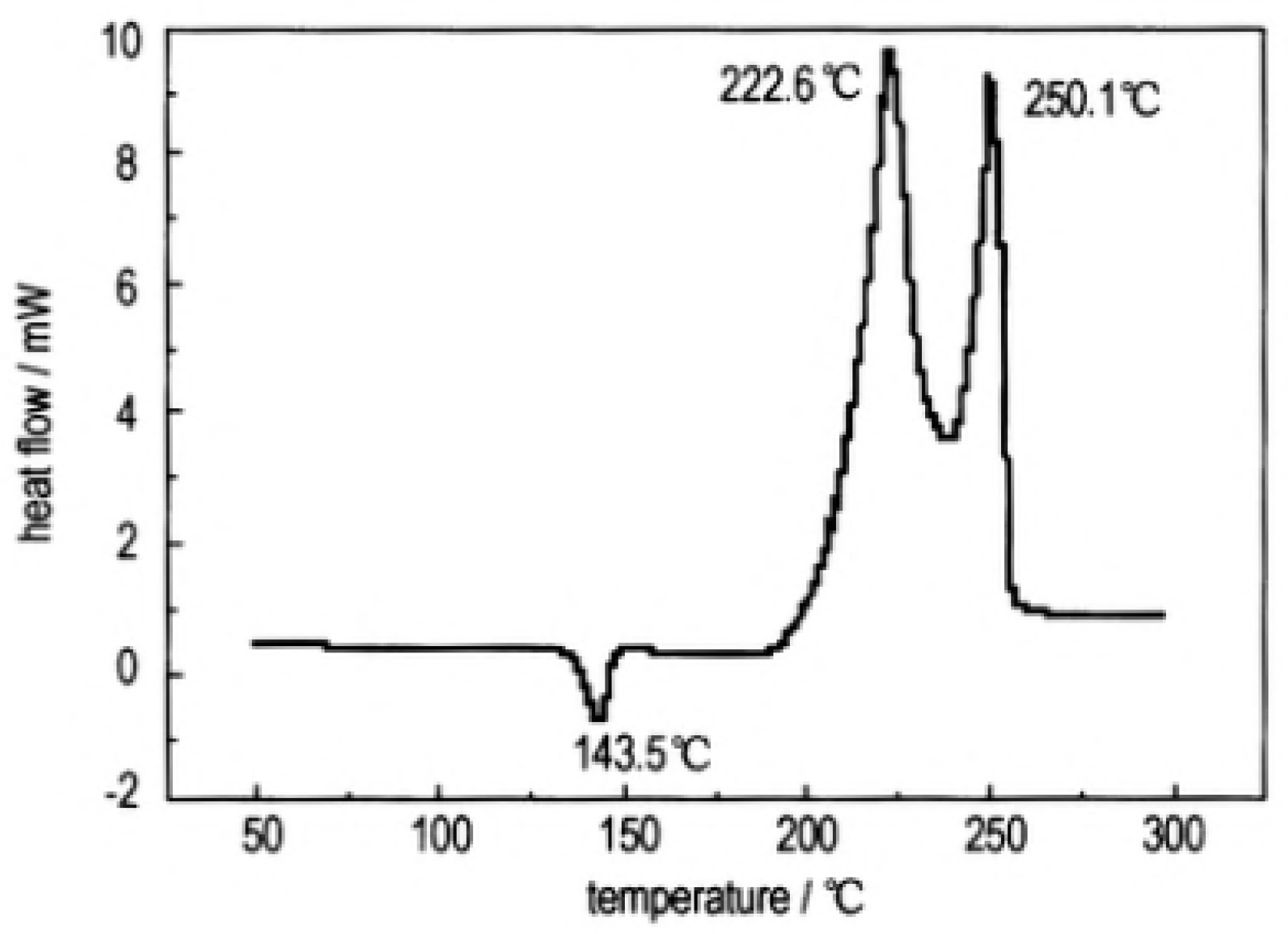
In order to study the thermal decomposition properties of CL-20/TNT cocryatal explosives, the samples of CL-20/TNT cocryatals were tested by DSC (TA Instruments, New Castle, DE, USA) at the heating rate of 10 °C·min
−1, the curve shown in
Figure 14. As is shown there are three stages of the thermal decomposition process of CL-20/TNT cocrystal, the maximum endothermic peak temperature is 143.5 °C, which is higher than that of the melting point of TNT (81 °C [
37]). With the increase of temperature, the hydrogen bond between the cocrystal molecules breaks and the molecular structure is destroyed [
38]. There are two processes of exothermic decomposition at 222.6 °C and 250.1 °C, respectively. Compared with the maximum exothermic peak value of CL-20 (321.5 °C [
33]) and TNT (245 °C [
39]), the exothermic peak of CL-20/TNT cocrystal shifts, indicating that the cocrystal changes the thermal decomposition characteristics of the raw materials and endows the cocrystal with unique thermal decomposition behavior.
The impact sensitivity value of CL-20/TNT cocrystal is H50 = 28 cm. Compared with CL-20 (H50 = 15 cm), the impact sensitivity of CL-20 is significantly reduced by 87%. Low-sensitivity TNT and high-sensitivity CL-20 combine to form a cocrystal at the molecular scale through eutectic technology, which changes the internal composition and crystal structure of explosives compared with traditional methods. In addition, due to the intermolecular hydrogen bond, on the one hand, it increases the stability of the molecular system of the cocrystal, on the other hand, it improves the anti-vibration of the cocrystal molecule to the mechanical external force, so the desensitization effect is obvious. Through the eutectic technology, it can effectively realize the desensitization of high sensitive explosive and improve its safety performance.
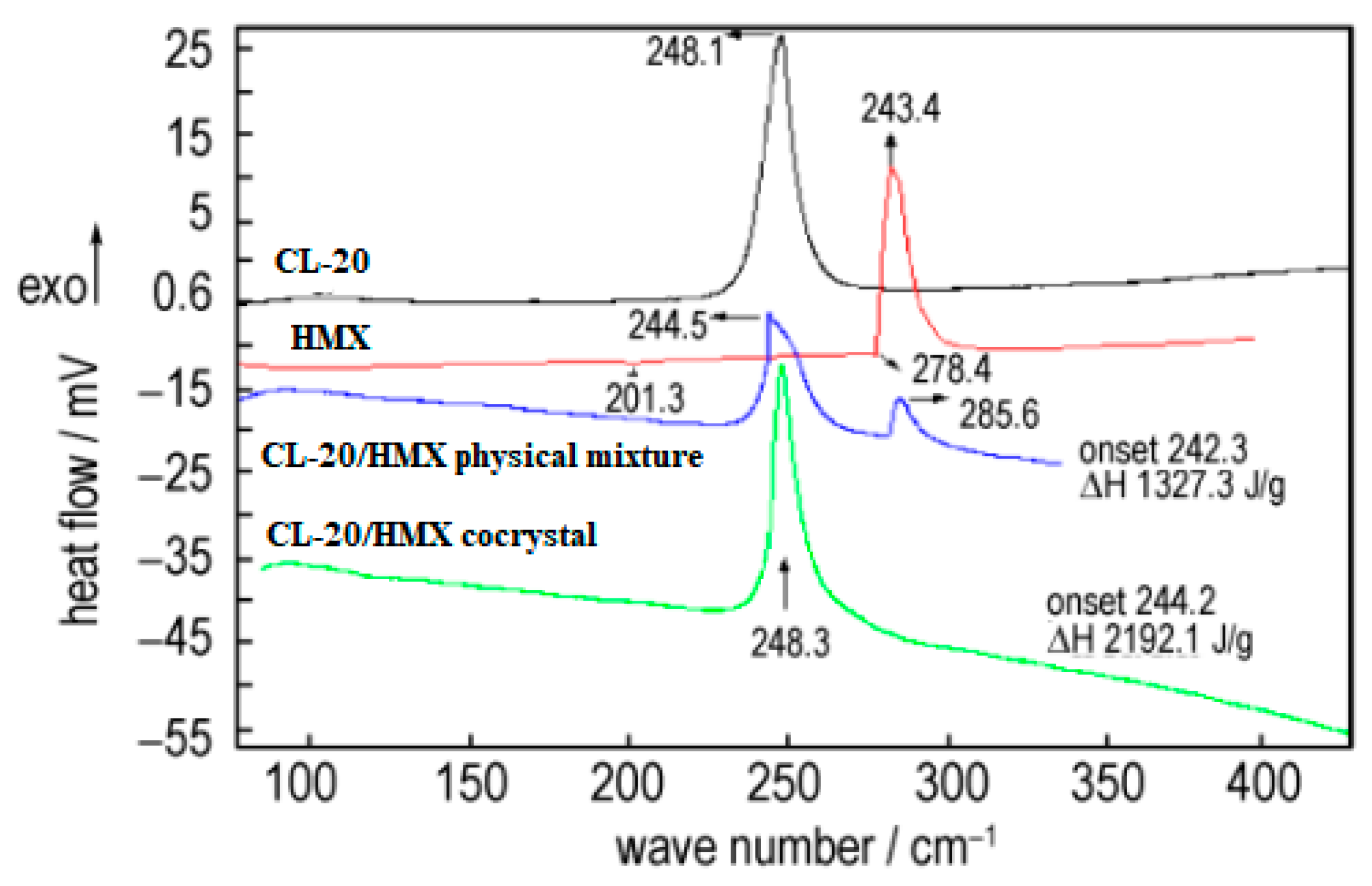
The crystal morphology, particle size and sensitivity of prepared CL-20/HMX cocrystal were characterized [
6]. The thermal decomposition process of cocrystal explosives had only one exothermic decomposition stage with peak temperatures of 248.3 °C. The enthalpy for the exothermic decomposition of the cocrystal (2912.1 J·g
−1) was remarkably higher than that of the physical mixture of CL-20 and HMX (1327.3 J·g
−1) (
Figure 15). The friction sensitivity of CL-20/HMX cocrystal explosive was 84%, which was decreased by 16% compared with original CL-20, and the characteristic height of the cocrystal was increased by 28.6 cm and 11.5 cm compared with original CL-20 and HMX, respectively (
Table 8). The compatibility of CL-20/HMX cocrystal with components of solid propellant shown that the prepared CL-20/HMX cocrystal was compatible with NG/BTTN, AP and Al powder, while incompatible with HGAP, N-100.
At the same time, the DSC curves of CL-20/HMX cocrystal were prepared by means of mechanical ball milling, and compared with the CL-20/HMX mixture [
7]. The mechanical sensitivity of the micro/nano CL-20/HMX energetic cocrystal materials was reduced obviously compared to that of raw HMX, while the energy output property was equivalent to that of raw CL-20.
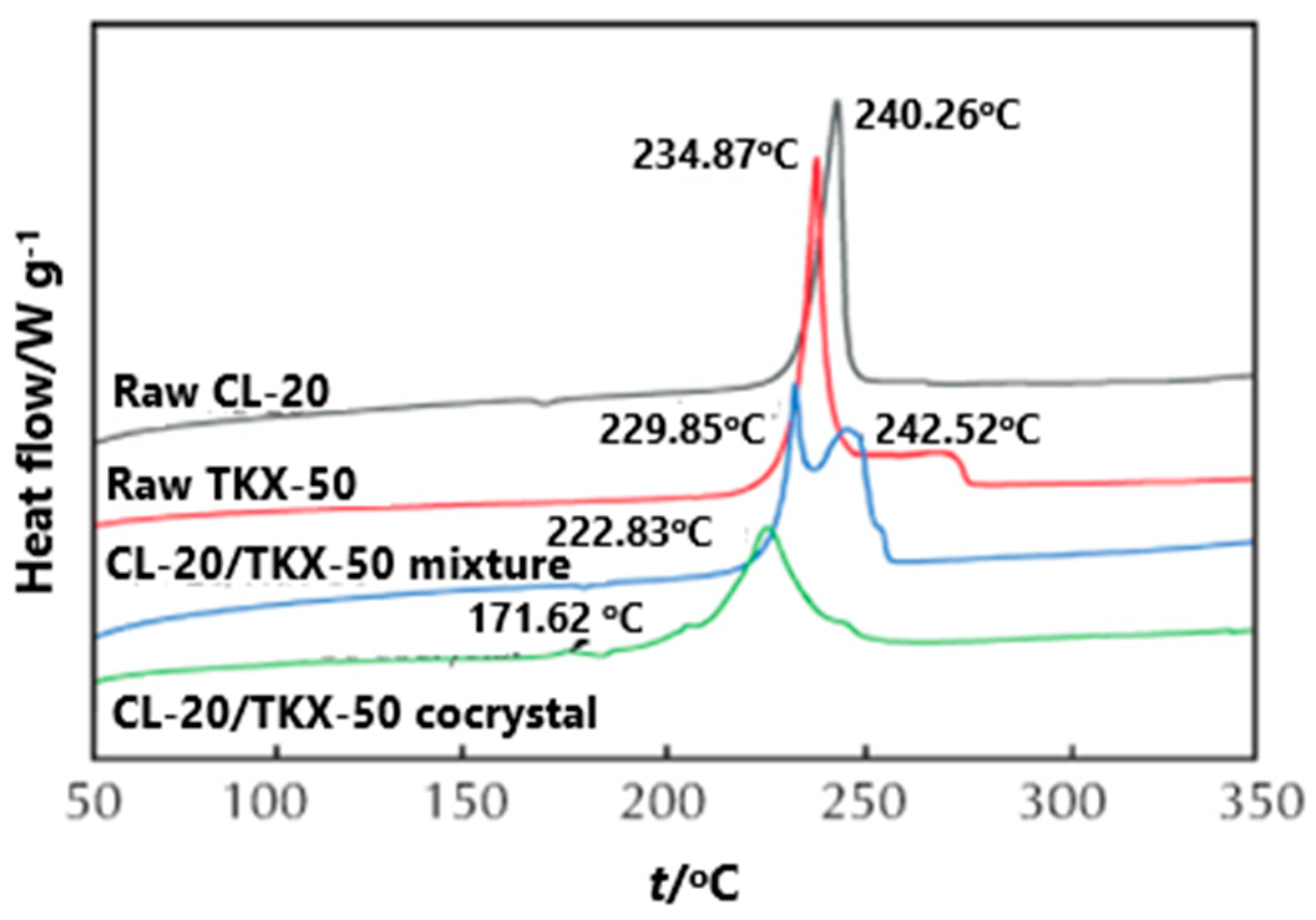
In order to compare the effect of the prepared CL-20/TKX-50 cocrystal on the thermal decomposition of each component, the DSC curves of CL-20, TKX-50, CL-20/TKX-50 mixture and CL-20/TKX-50 cocrystal were investigated [
34] (
Figure 16). The exothermic decomposition peaks of CL-20 and TKX-50 were 240.2 and 234.8 °C, respectively, and there were two exothermic decomposition peaks in the decomposition process of the CL-20/TKX-50 mixture, indicating that the decomposition process of the CL-20/TKX-50 mixture is obviously a simple superposition of CL-20 and TKX-50. The first decomposition peak appears at 229.8 °C, corresponding to the exothermic decomposition of part of TKX-50; the second peak appears at 242.5 °C, corresponding to the exothermic decomposition of CL-20. For the CL-20/TKX-50 cocrystal decomposition, with the increase of temperature, the first exothermic decomposition peak of CL-20/TKX-50 cocrystal appears at 171.6 °C, which can be attributed to the destruction of hydrogen in the cocrystal structure and the decomposition of a small amount of TKX-50. Subsequently, a large number of cocrystal products begin to decompose, and the second exothermic decomposition peak appeared at 222.8 °C, indicating the formation of hydrogen bond and the existence of a new structure between CL-20/TKX-50 cocrystal molecules.
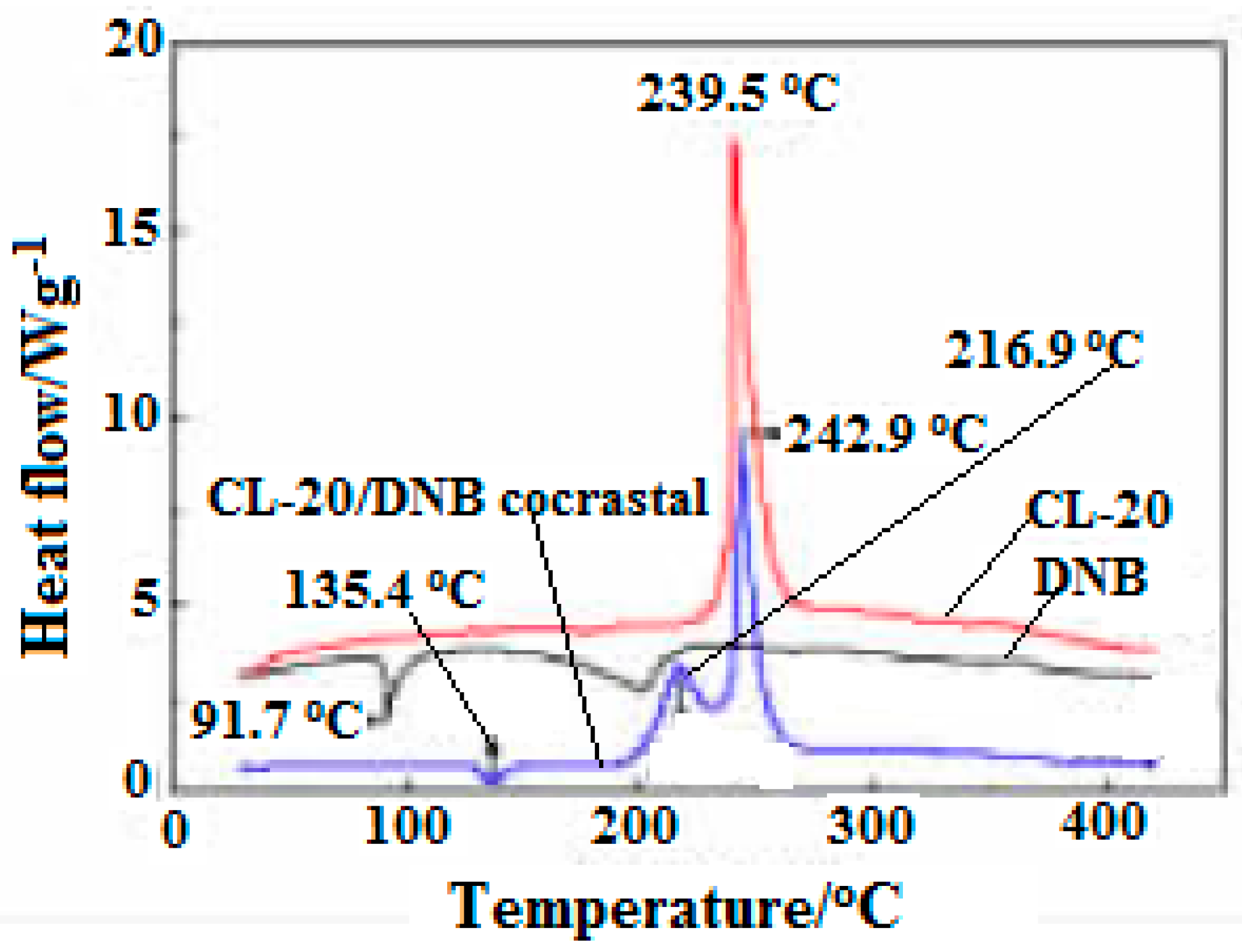
In addition, the DSC curves of CL-20, DNB, CL-20/DNB cocrystal were shown in
Figure 17. There are three (one is endothermic melting and two exothermic decompositions) thermal decomposition stages. The melting temperature of cocrystal is 136.4 °C, 44.7 °C higher than that of raw material DNB (91.7 °C) in the melting stage, indicating that the cocrystal decomposes into liquid DNB at this point. There are two exothermic peaks at 216.9 °C and 242.9 °C in the exothermic decomposition stage, which was attributed to the exothermic decomposition of two single components after cocrystal decomposition. Moreover, the crystal density of CL-20/DNB is higher than that of CL-20/TNT, and the sensitivity of DNB is lower than that of TNT. Thus, the sensitivity of CL-20/DNB is lower than that of CL-20/TNT. In addition, the cost of the DNB is significantly lower than that of TNT. Therefore, the cocrystal may become an excellent explosive with high-energy, insensitive and low-cost prospective ingredients in explosives and propellants.
The impact sensitivity, the calculated detonation of the CL-20, TKX-50, CL-20/TKX-50 mixture and CL-20/TKX-50 cocrystal are shown in
Table 8. The characteristic drop height of CL-20/TKX-50 cocrystal is lower than that of TKX-50, but significantly higher than that of CL-20, β-HMX and CL-20/TKX-50 mixture, indicating that the impact sensitivity of CL-20/TKX-50 cocrystal is much lower. The detonation velocity and detonation pressure of CL-20/TKX-50 cocrystal are slightly lower than that of CL-20, but the detonation performance is obviously improved compared with β-HMX, indicating CL-20/TKX-50 cocrystal has good detonation performance.