Evaluation of the Perceptual Interactions among Aldehydes in a Cheddar Cheese Matrix According to Odor Threshold and Aroma Intensity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Thresholds of Aldehydes in the Cheese Matrix
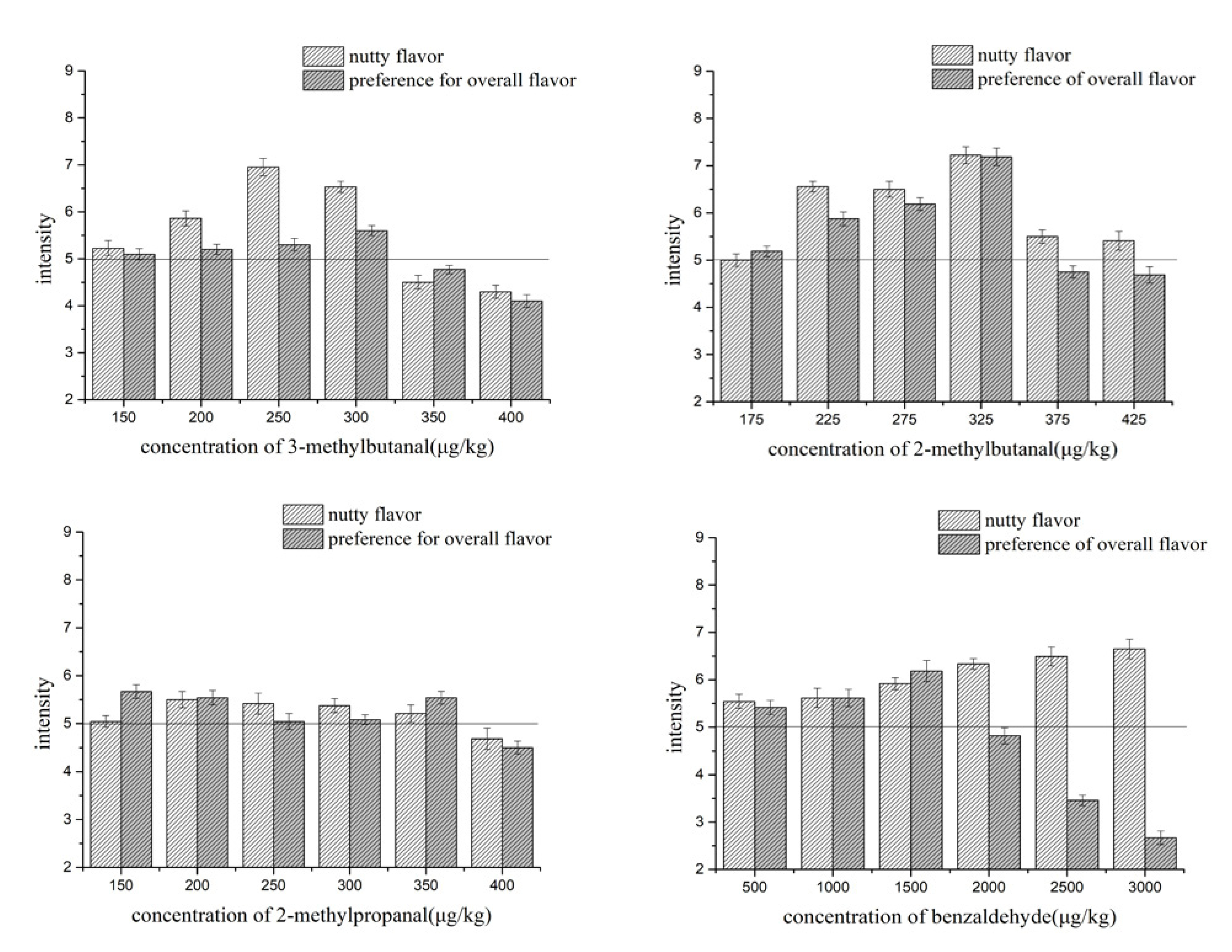
2.2. The Optimal Concentration Ranges of Aldehydes in a Cheese Matrix
2.3. Evaluation of the Perceptual Interactions among Aldehydes in the Cheese Matrix Using the Odor Threshold
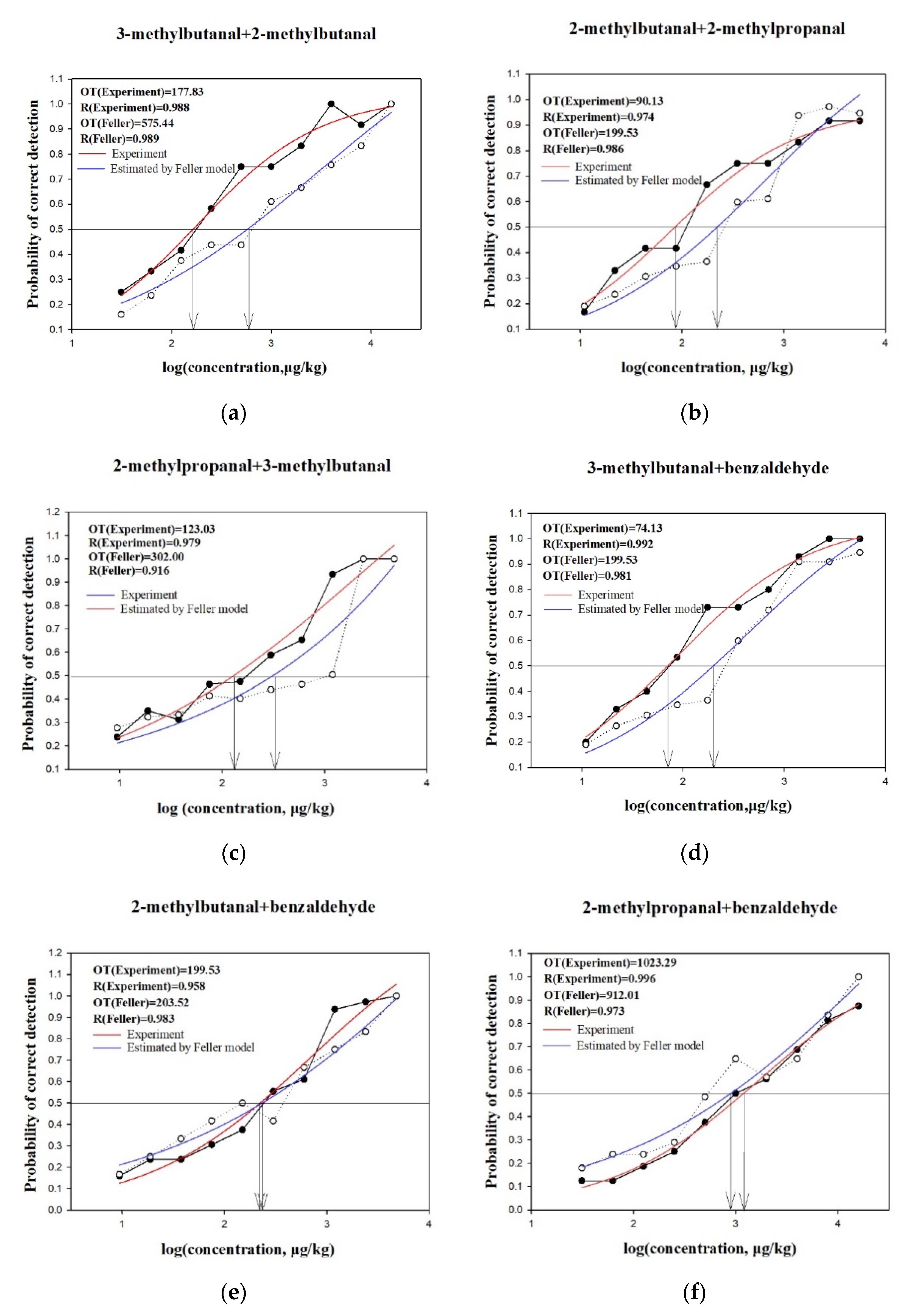
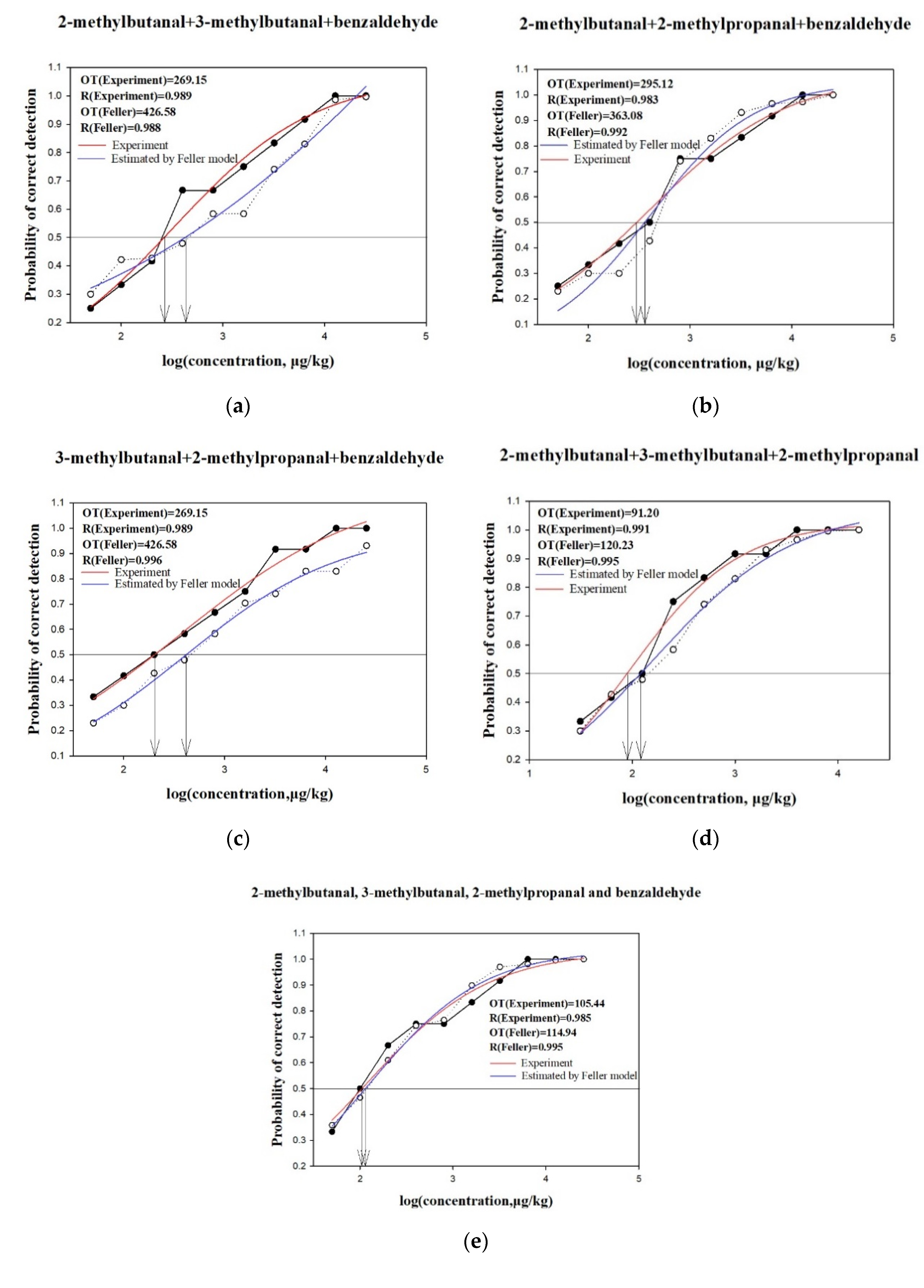
2.4. Analysis of Interactions Using Feller’s Additive Model
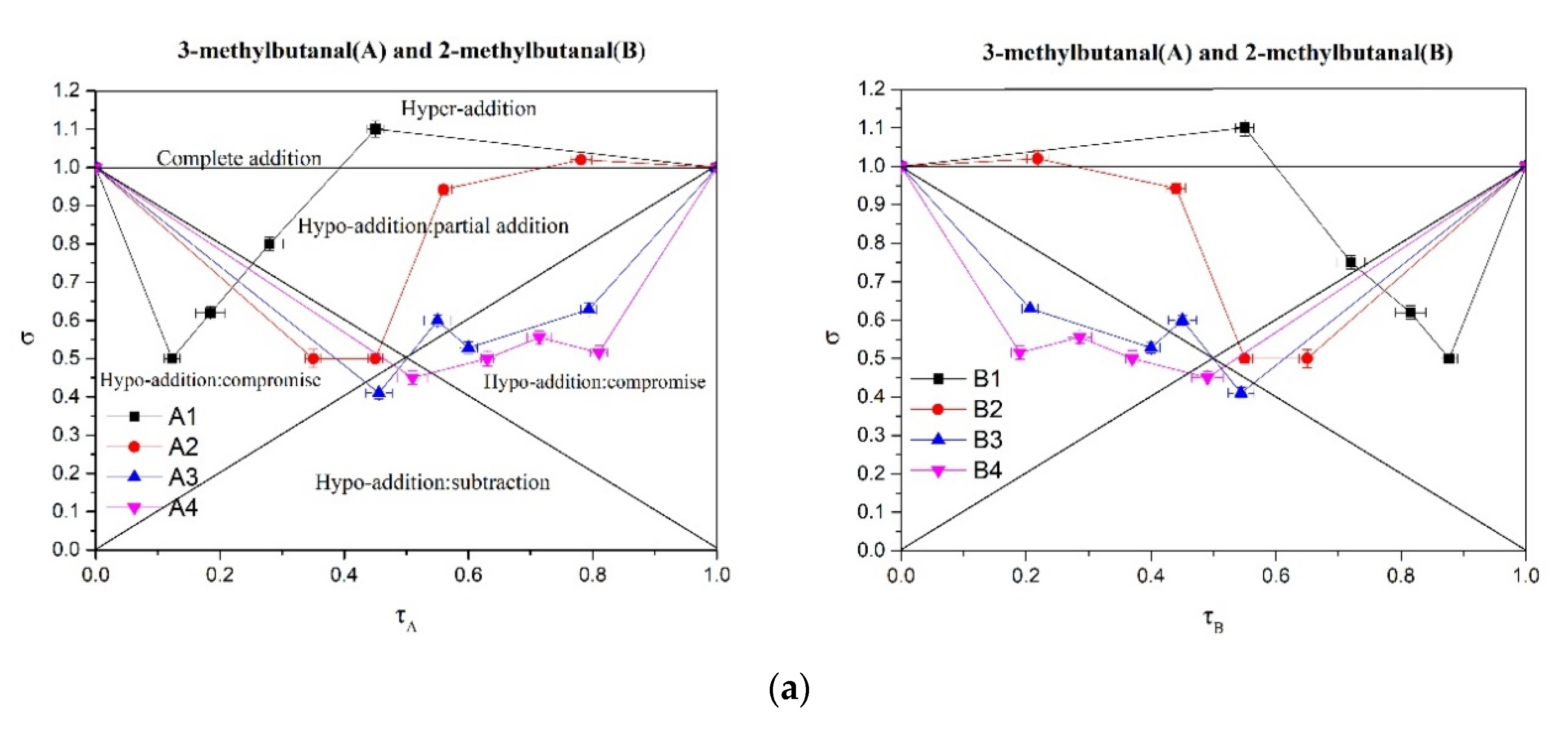
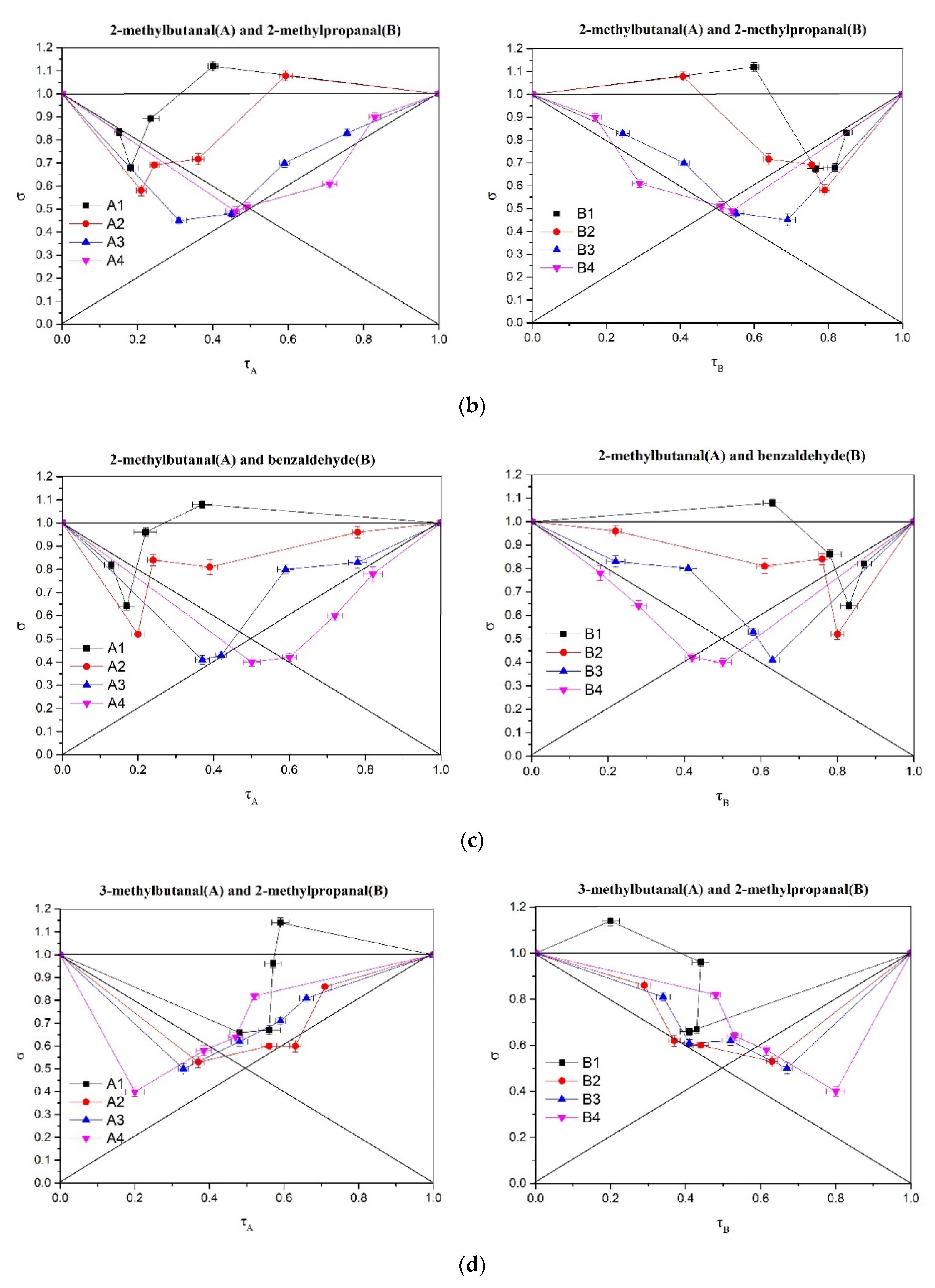
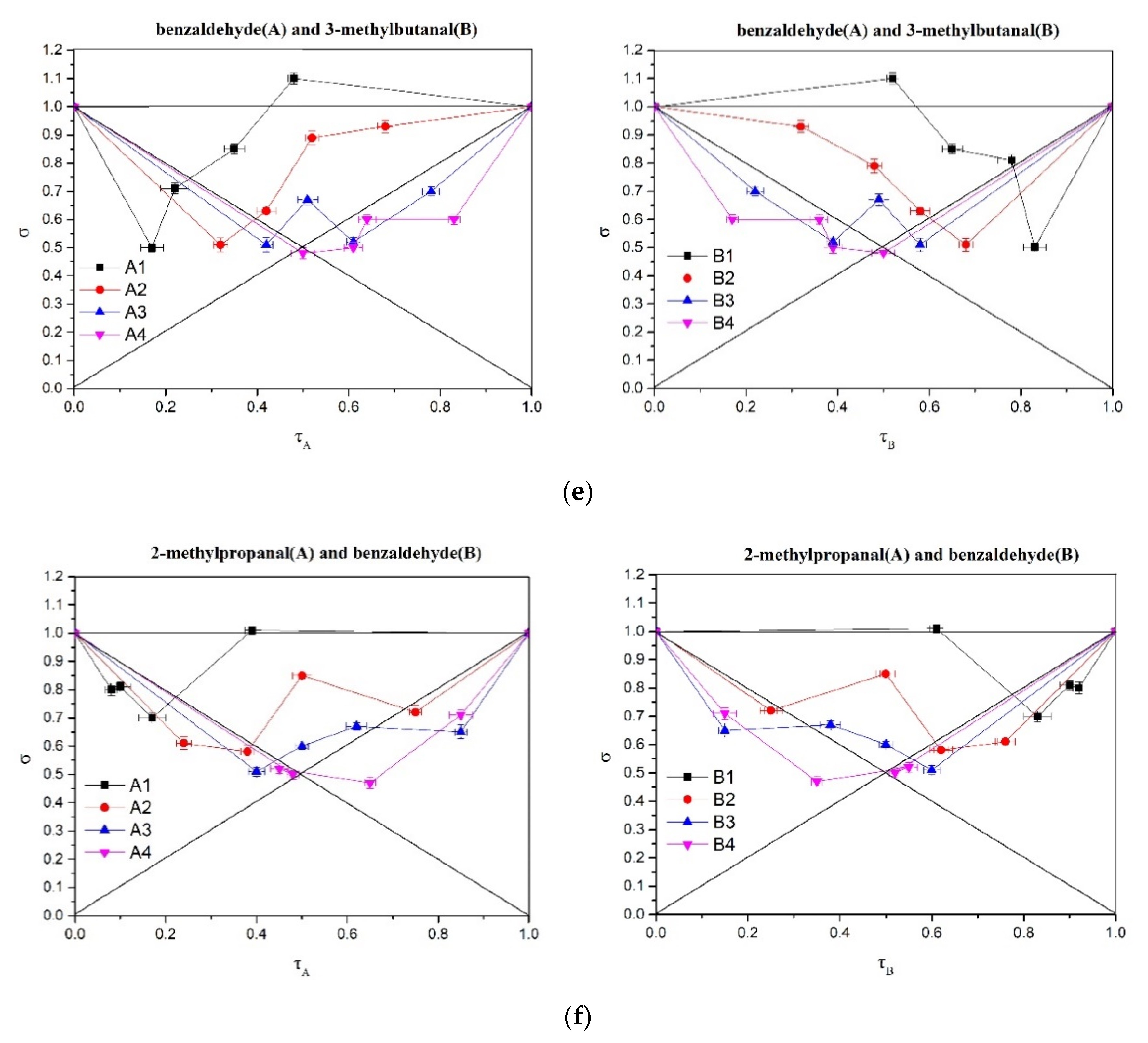
2.5. Analysis of Perceptual Interactions of Nutty Aroma Compounds Using σ-τ Diagrams
3. Material and Methods
3.1. Chemicals
3.2. Matrix Preparation
3.3. Sensory Evaluation
3.4. Measurement of Odor Thresholds in the Cheddar Cheese Matrix
3.5. Determination of the Optimal Concentration Ranges of Aldehydes in a Cheese Matrix
3.6. Perceptual Interaction Analysis
3.6.1. Interactions between Aroma Compounds Using the Threshold Approach
3.6.2. Perceptual Interactions of Binary, Ternary, and Quaternary Mixtures Determined Using Feller’s Additive Model
3.6.3. σ-τ Plot Analysis
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Murtaza, M.A.; Ur-Rehman, S.; Anjum, F.M.; Huma, N.; Hafiz, I. Cheddar cheese ripening and flavor characterization: A review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1309–1321. [Google Scholar] [CrossRef] [PubMed]
- Batool, M.; Nadeem, M.; Imran, M.; Gulzar, N.; Shahid, M.Q.; Shahbaz, M.; Ajmal, M.; Khan, I.T. Impact of vitamin E and selenium on antioxidant capacity and lipid oxidation of Cheddar cheese in accelerated ripening. Lipids Health Dis. 2018, 17, 79–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Togay, S.O.; Guneser, O.; Yuceer, Y.K. Evaluation of physicochemical, microbiological, sensory properties and aroma profiles of goat cheeses provided from Canakkale. Int. J. Dairy Technol. 2017, 70, 514–525. [Google Scholar] [CrossRef]
- Khattab, A.R.; Guirguis, H.A.; Tawfik, S.M.; Farag, M.A. Cheese ripening: A review on modern technologies towards flavor enhancement, process acceleration and improved quality assessment. Trends Food Sci. Technol. 2019, 88, 343–360. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, H.; Zhao, L.; Sun, W.; Zeng, S.; Lu, X.; Cao, X.; Ren, F. Sensory profile and Beijing youth preference of seven cheese varieties. Food Qual. Prefer. 2011, 22, 101–109. [Google Scholar] [CrossRef]
- Curioni, P.; Bosset, J. Key odorants in various cheese types as determined by gas chromatography-olfactometry. Int. Dairy J. 2002, 12, 959–984. [Google Scholar] [CrossRef]
- Frank, D.C.; Owen, C.M.; Patterson, J. Solid phase microextraction (SPME) combined with gas-chromatography and olfactometry-mass spectrometry for characterization of cheese aroma compounds. LWT-Food Sci. Technol. 2004, 37, 139–154. [Google Scholar] [CrossRef]
- Avsar, Y.K.; Karagul-Yuceer, Y.; Drake, M.A.; Singh, T.K.; Yoon, Y.; Cadwallader, K.R. Characterization of nutty flavor in Cheddar cheese. J. Dairy Sci. 2004, 87, 1999–2010. [Google Scholar] [CrossRef] [Green Version]
- Afzal, M.I.; Ariceaga, C.C.; Boulahya, K.A.; Jacquot, M.; Delaunay, S.; Cailliez-Grimal, C. Biosynthesis and role of 3-methylbutanal in cheese by lactic acid bacteria: Major metabolic pathways, enzymes involved, and strategies for control. Crit. Rev. Food Sci. Nutr. 2017, 57, 399–406. [Google Scholar] [CrossRef]
- Smit, B.A.; Engels, W.J.M.; Smit, G. Branched chain aldehydes: Production and breakdown pathways and relevance for flavour in foods. Appl. Microbiol. Biotechnol. 2008, 81, 987–999. [Google Scholar] [CrossRef] [Green Version]
- Whetstine, M.C.; Drake, M.A.; Broadbent, J.R.; Mcmahon, D. Enhanced nutty flavor formation in cheddar cheese made with a malty Lactococcus lactis adjunct culture. J. Dairy Sci. 2006, 89, 3277–3284. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Zhou, W.; Yu, H.; Yuan, J.; Tian, H. Characterization of major odor-active compounds responsible for nutty flavor in Cheddar cheese according to Chinese taste. Flavour Fragr. J. 2020, in press. [Google Scholar]
- Grosch, W. Evaluation of the key odorants of foods by dilution experiments, aroma models and omission. Chem. Senses 2001, 26, 533–545. [Google Scholar] [CrossRef]
- Van Gemert, L. Compilations of Odour Threshold Values in Air, Water and Other Media; Boelens Aroma Chemical Information Service: Huizen, The Netherlands, 2003. [Google Scholar]
- Culleré, L.; Cacho, J.; Ferreira, V. An assessment of the role played by some oxidation-related aldehydes in wine aroma. J. Agric. Food Chem. 2007, 55, 876–881. [Google Scholar] [CrossRef]
- van Aardt, M.; Duncan, S.E.; Bourne, D.; Marcy, J.E.; Long, T.E.; Hackney, C.R.; Heisey, C. Flavor threshold for acetaldehyde in milk, chocolate milk, and spring water using solid phase microextraction gas chromatography for quantification. J. Agric. Food Chem. 2001, 49, 1377–1381. [Google Scholar] [CrossRef]
- Adhikari, K.; Hein, K.; Elmore, J.; Heymann, H.; Willott, A.M.Y. Flavor threshold as affected by interaction among three dairy-related flavor compounds. J. Sens. Stud. 2006, 21, 626–643. [Google Scholar] [CrossRef]
- Afzal, M.I.; Delaunay, S.; Paris, C.; Borges, F.; Revol-Junelles, A.-M.; Cailliez-Grimal, C. Identification of metabolic pathways involved in the biosynthesis of flavor compound 3-methylbutanal from leucine catabolism by Carnobacterium maltaromaticum LMA 28. Int. J. Food Microbiol. 2012, 157, 332–339. [Google Scholar] [CrossRef]
- Molimard, P.; Spinnler, H. Compounds involved in the flavor of surface mold-ripened cheeses: Origins and properties. J. Dairy Sci. 1996, 79, 169–184. [Google Scholar] [CrossRef]
- Ferreira, V. Revisiting psychophysical work on the quantitative and qualitative odour properties of simple odour mixtures: A flavour chemistry view. Part 1: Intensity and detectability. A review. Flavour Fragr. J. 2012, 27, 124–140. [Google Scholar] [CrossRef]
- Niu, Y.; Yao, Z.; Xiao, Z.; Zhu, G.; Zhu, J.; Chen, J. Sensory evaluation of the synergism among ester odorants in light aroma-type liquor by odor threshold, aroma intensity and flash GC electronic nose. Food Res. Int. 2018, 113, 102–114. [Google Scholar] [CrossRef]
- Wu, K.N.; Tan, B.K.; Howard, J.D.; Conley, D.B.; Gottfried, J.A. Olfactory input is critical for sustaining odor quality codes in human orbitofrontal cortex. Nat. Neurosci. 2012, 15, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Gallego, R.; Hernández-Hierro, J.M.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Sensory evaluation of bitterness and astringency sub-qualities of wine phenolic compounds: Synergistic effect and modulation by aromas. Food Res. Int. 2014, 62, 1100–1107. [Google Scholar] [CrossRef] [Green Version]
- Lytra, G.; Tempere, S.; Le Floch, A.; de Revel, G.; Barbe, J.-C. Study of sensory interactions among red wine fruity esters in a model solution. J. Agric. Food Chem. 2013, 61, 8504–8513. [Google Scholar] [CrossRef]
- Cameleyre, M.; Lytra, G.; Tempere, S.; Barbe, J.-C. Olfactory impact of higher alcohols on red wine fruity ester aroma expression in model solution. J. Agric. Food Chem. 2015, 63, 9777–9788. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, F.; Wang, L.; Niu, Y.; Xiao, Z. Evaluation of the synergism among volatile compounds in Oolong tea infusion by odour threshold with sensory analysis and E-nose. Food Chem. 2017, 221, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Xu, X.; Sun, X.; Chen, C.; Yu, H. Evaluation of the perceptual interaction among key aroma compounds in milk fan by gas chromatography−olfactometry, odor threshold, and sensory analyses. J. Dairy Sci. 2020, 103, 5863–5873. [Google Scholar] [CrossRef]
- Meilgaard, M.C. Prediction of flavor differences between beers from their chemical composition. J. Agric. Food Chem. 1982, 30, 1009–1017. [Google Scholar] [CrossRef]
- Ortigosa, M.; Torre, P.; Izco, J. Effect of pasteurization of ewe’s milk and use of a native starter culture on the volatile components and sensory characteristics of Roncal cheese. J. Dairy Sci. 2001, 84, 1320–1330. [Google Scholar] [CrossRef]
- Niimi, J.; Eddy, A.; Overington, A.; Silcock, P.; Bremer, P.; Delahunty, C. Sensory interactions between cheese aroma and taste. J. Sens. Stud. 2015, 30, 247–257. [Google Scholar] [CrossRef]
- Dunn, H.C.; Lindsay, R. Evaluation of the role of microbial Strecker-derived aroma compounds in unclean-type flavors of Cheddar cheese. J. Dairy Sci. 1985, 68, 2859–2874. [Google Scholar] [CrossRef]
- Guadagni, D.G.; Buttery, R.G.; Okano, S.; Burr, H.K. Additive effect of sub-threshold concentrations of some organic compounds associated with food aromas. Nature 1963, 200, 1288–1289. [Google Scholar] [CrossRef] [PubMed]
- Saison, D.; De Schutter, D.P.; Uyttenhove, B.; Delvaux, F.; Delvaux, F.R. Contribution of staling compounds to the aged flavour of lager beer by studying their flavour thresholds. Food Chem. 2009, 114, 1206–1215. [Google Scholar] [CrossRef]
- Cometto-Muñiz, J.E.; Abraham, M.H. Dose–response functions for the olfactory, nasal trigeminal, and ocular trigeminal detectability of airborne chemicals by humans. Chem. Senses 2015, 41, 3–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Xie, T.; Xie, J.; Chen, C.; Ai, L.; Tian, H. Aroma perceptual interactions of benzaldehyde, furfural, and vanillin and their effects on the descriptor intensities of Huangjiu. Food Res. Int. 2020, 129, 108808. [Google Scholar] [CrossRef]
- Stevens, J.C. Detection of very complex taste mixtures: Generous integration across constituent compounds. Physiol. Behav. 1997, 62, 1137–1143. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, R.; Xiao, Z.; Zhu, J.; Sun, X.; Wang, P. Characterization of ester odorants of apple juice by gas chromatography-olfactometry, quantitative measurements, odour threshold, aroma intensity and electronic nose. Food Res. Int. 2019, 120, 92–101. [Google Scholar] [CrossRef]
- AOAC. Offcial Methods of Analysis. In The Association of Official Analytical Chemists, 17th ed.; AOAC International: Washington, DC, USA, 2000. [Google Scholar]
- Kim, M.; Drake, S.; Drake, M. Evaluation of key flavor compounds in reduced-and full-fat Cheddar cheeses using sensory studies on model systems. J. Sens. Stud. 2011, 26, 278–290. [Google Scholar] [CrossRef]
- Drake, M.A. Invited review: Sensory analysis of dairy foods. J. Dairy Sci. 2007, 90, 4925–4937. [Google Scholar] [CrossRef] [Green Version]
- Lytra, G.; Cameleyre, M.; Tempere, S.; Barbe, J.-C. Distribution and organoleptic impact of ethyl 3-hydroxybutanoate enantiomers in wine. J. Agric. Food Chem. 2015, 63, 10484–10491. [Google Scholar] [CrossRef]
- ATSM International. Standard Practice for Defining and Calculating Individual and Group Sensory Thresholds from Forced-Choice Data Sets of Intermediate Size. ATSM Standard E1432–04; ATSM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- ISO-13301. Sensory analysis–methodology general guidance for measuring odour, flavour and test detection thresholds by a three-alternative forced choice (3-AFC) procedure. In Analyse Sensorielle; AFNOR: Paris, France, 2002.
- Whetstine, M.E.C.; Cadwallader, K.R.; Maryanne, D. Characterization of aroma compounds responsible for the rosy/floral flavor in Cheddar cheese. J. Agric. Food Chem. 2005, 53, 3126–3132. [Google Scholar] [CrossRef]
- Borges, A.R.; Pires, A.F.; Marnotes, N.G.; Gomes, D.G.; Henriques, M.F.; Pereira, C.D. Dairy by-products concentrated by ultrafiltration used as ingredients in the production of reduced fat washed curd cheese. Foods 2020, 9, 1020. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Yu, B.; Yu, H.; Chen, C. Evaluation of the synergistic olfactory effects of diacetyl, acetaldehyde, and acetoin in a yogurt matrix using odor threshold, aroma intensity, and electronic nose analyses. J. Dairy Sci. 2020, 103, 7957–7967. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Gallagher, M.; Preti, G.; Wise, P. Synergistic mixture interactions in detection of perithreshold odors by humans. Chem. Senses 2008, 33, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Feller, W. An Introduction to Probability Theory and Its Applications. In Wiley Series in Probability and Mathematical Statistics, 3rd ed.; John Wiley and Sons: Hoboken, NJ, USA, 1968; Volume 1. [Google Scholar]
- Patte, F.; Laffort, P. An alternative model of olfactory quantitative interaction in binary mixtures. Chem. Senses 1979, 4, 267–274. [Google Scholar] [CrossRef]
- Frijters, J.E.R. Psychophysical models for mixtures of tastants and mixtures of odorants. Ann. N. Y. Acad. Sci. 2006, 510, 67–78. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
| Compound | Aroma Description | Threshold (Determined, μg/kg) | Threshold in Water (Literature, μg/kg) | Ratio (Determined/Literature) |
|---|---|---|---|---|
| 3-Methylbutanal | Malty, nutty, almond, cocoa | 150.3 | 1.1 | 136.7 |
| 2-Methylbutanal | Malty, almond, cacao, apple-like | 175.4 | 1.0 | 175.4 |
| 2-Methylpropanal | Pungent, varnish, fruity | 150.7 | 1.5 | 100.2 |
| Benzaldehyde | Almond, cherry stone | 500.2 | 350.0 | 1.4 |
| 9 | 2 Compounds | Flavor Description | TH of Mixture a (%) | SD b |
|---|---|---|---|---|
| 1 | 2-methylbutanal + 3-methylbutanal | Nutty, malty | 29.29% | 1.90 |
| 2 | 2-methylbutanal + 2-methylpropanal | Milky, malty | 49.08% | 2.10 |
| 3 | 2-methylbutanal + benzaldehyde | Milky, almond | 46.59% | 2.50 |
| 4 | 3-methylbutanal + 2-methylpropanal | Flowery, caramel, fruity, malty | 40.90% | 3.50 |
| 5 | 3-methylbutanal + benzaldehyde | Nutty, milky | 38.36% | 5.60 |
| 6 | 2-methylpropanal + benzaldehyde | Malty | 148.20% | 10.30 |
| Sample | Origin | Maturity | Moisture% | Fat% | Protein% | Salt% | Ash% | pH |
|---|---|---|---|---|---|---|---|---|
| Fresh cheese | China | 0 month | 43.21 ± 0.67 | 22.10 ± 0.91 | 28.01 ± 0.63 | 1.34 ± 0.25 | 3.38 ± 0.19 | 5.29 ± 0.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Zhou, W.; Yu, H.; Yuan, J.; Tian, H. Evaluation of the Perceptual Interactions among Aldehydes in a Cheddar Cheese Matrix According to Odor Threshold and Aroma Intensity. Molecules 2020, 25, 4308. https://doi.org/10.3390/molecules25184308
Chen C, Zhou W, Yu H, Yuan J, Tian H. Evaluation of the Perceptual Interactions among Aldehydes in a Cheddar Cheese Matrix According to Odor Threshold and Aroma Intensity. Molecules. 2020; 25(18):4308. https://doi.org/10.3390/molecules25184308
Chicago/Turabian StyleChen, Chen, Wenya Zhou, Haiyan Yu, Jiajie Yuan, and Huaixiang Tian. 2020. "Evaluation of the Perceptual Interactions among Aldehydes in a Cheddar Cheese Matrix According to Odor Threshold and Aroma Intensity" Molecules 25, no. 18: 4308. https://doi.org/10.3390/molecules25184308
APA StyleChen, C., Zhou, W., Yu, H., Yuan, J., & Tian, H. (2020). Evaluation of the Perceptual Interactions among Aldehydes in a Cheddar Cheese Matrix According to Odor Threshold and Aroma Intensity. Molecules, 25(18), 4308. https://doi.org/10.3390/molecules25184308






