Optimization of Light Intensity, Temperature, and Nutrients to Enhance the Bioactive Content of Hyperforin and Rutin in St. John’s Wort
Abstract
:1. Introduction
2. Results and Discussion
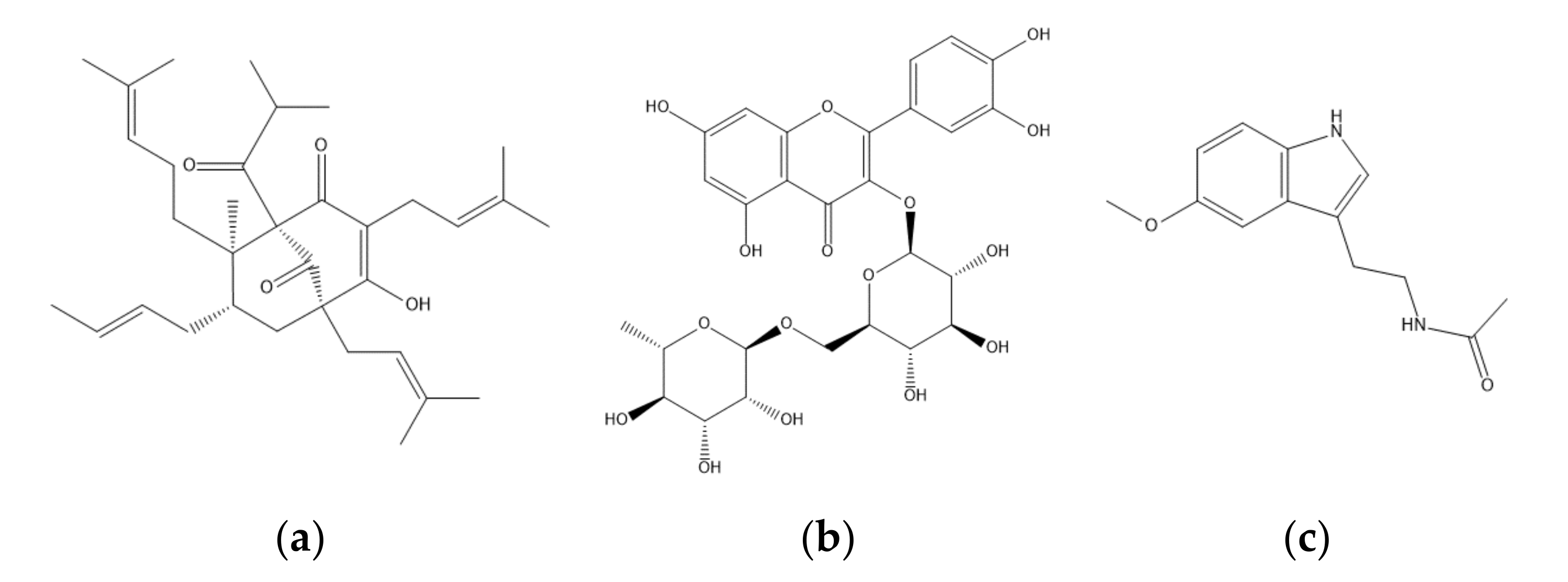
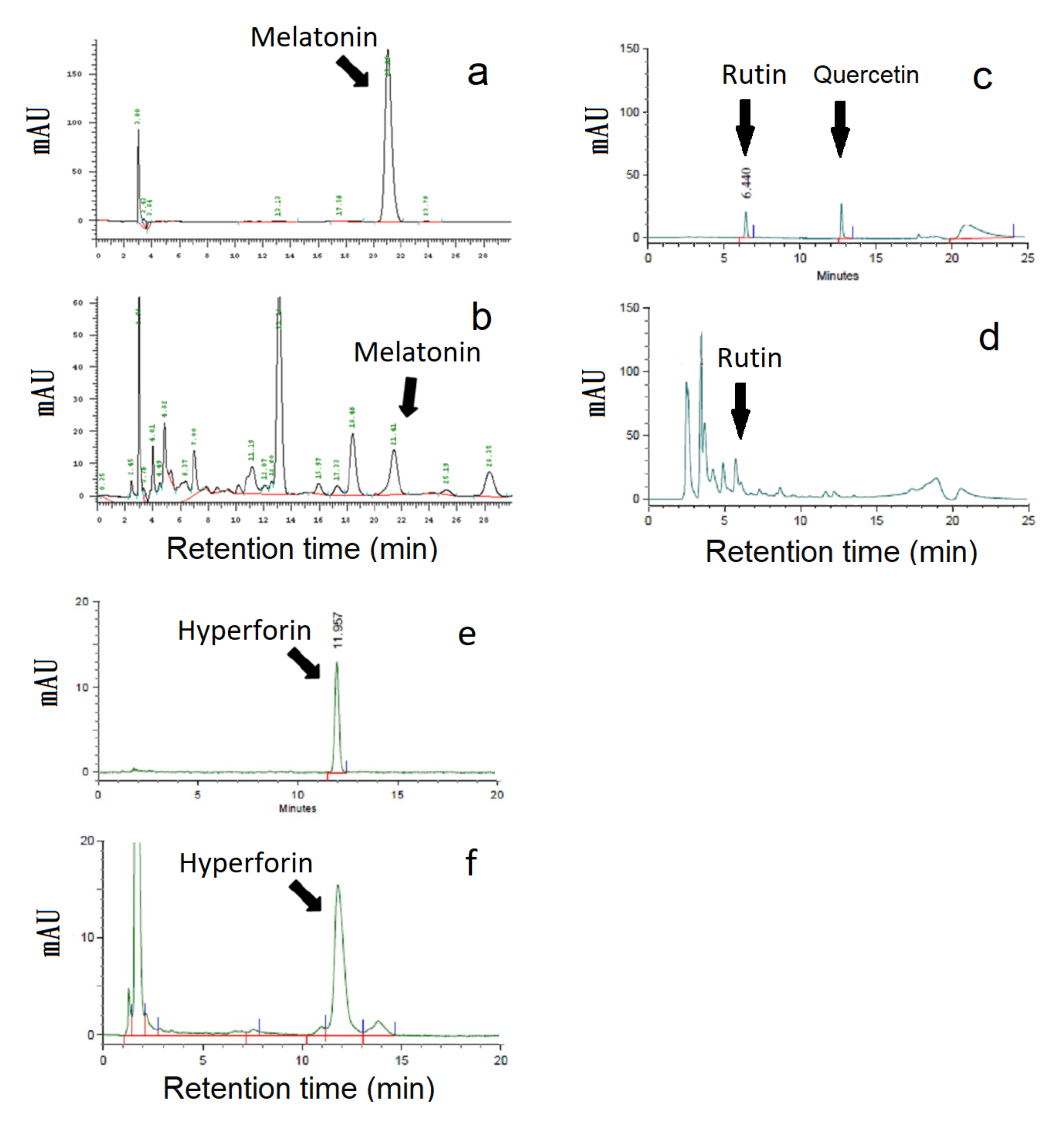
2.1. High-Performance Liquid Chromatography Traces of St. John’s Wort Leaf Extracts
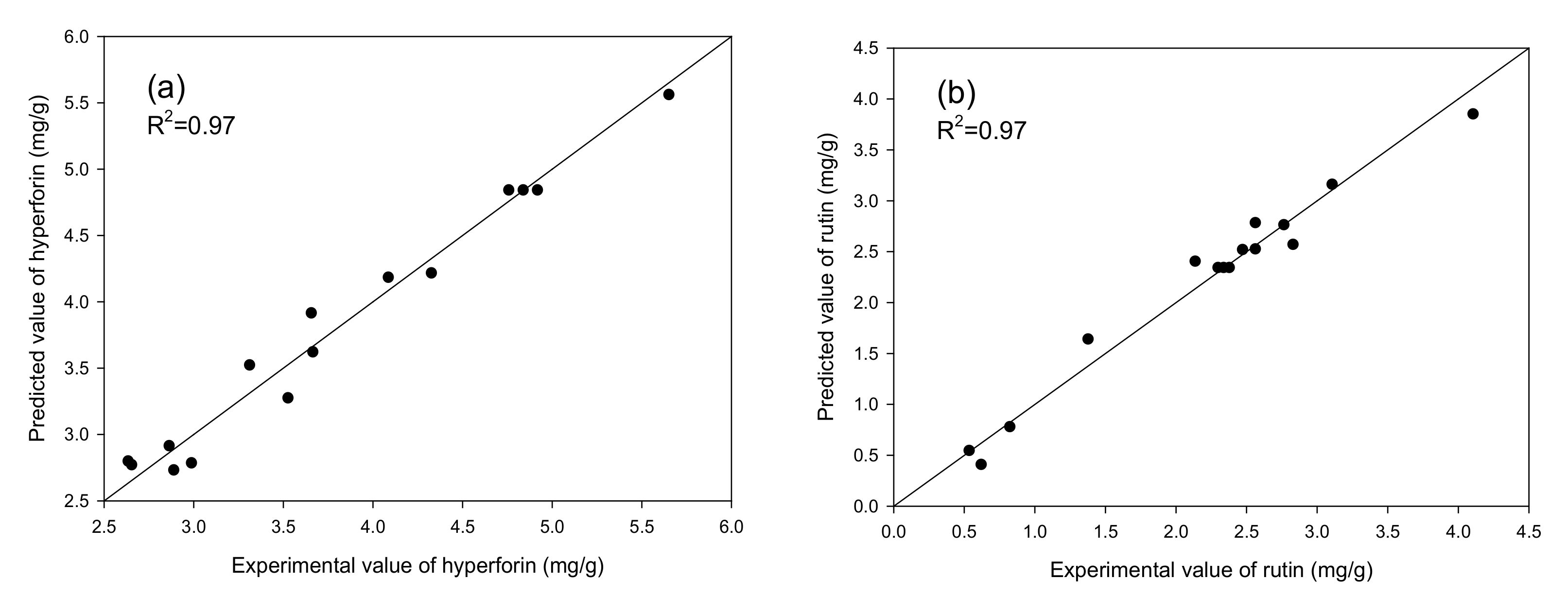
2.2. Statistical Model Fitting
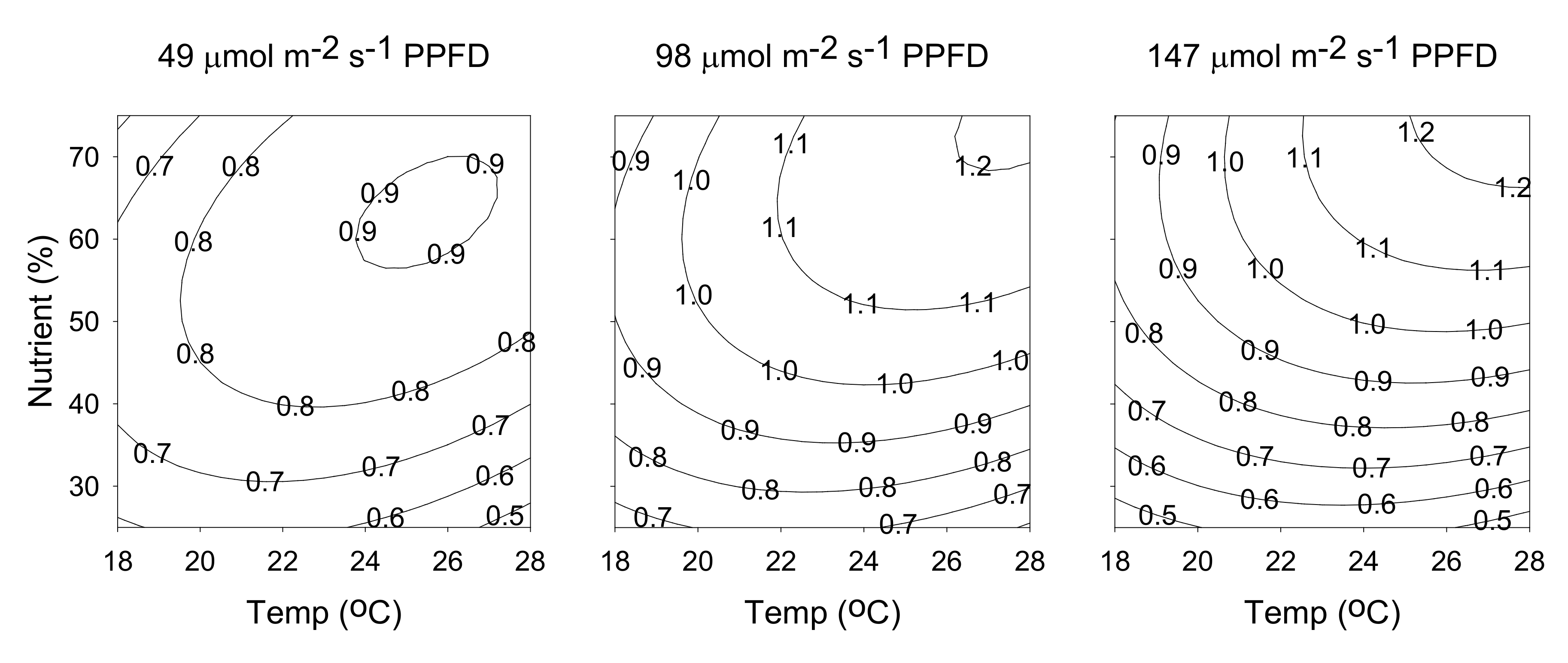
2.3. Hyperforin Content
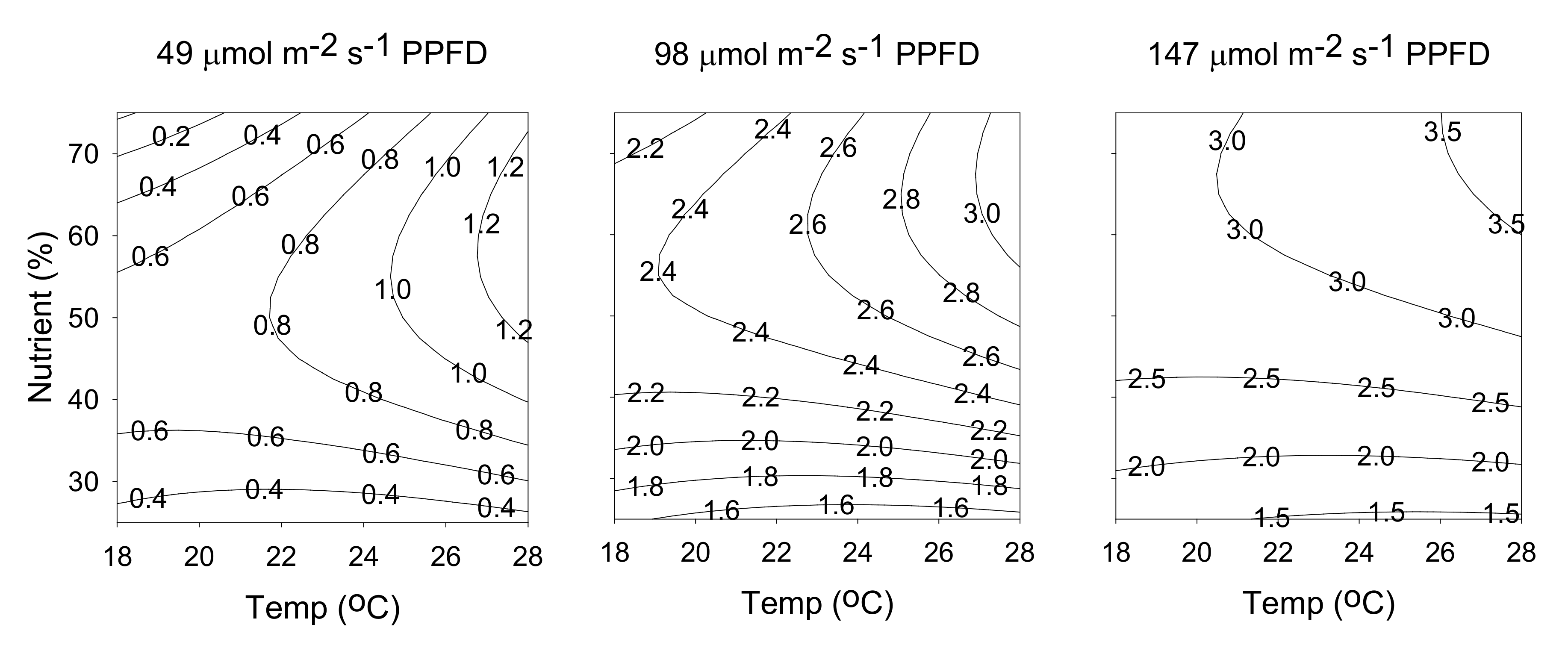
2.4. Rutin Content
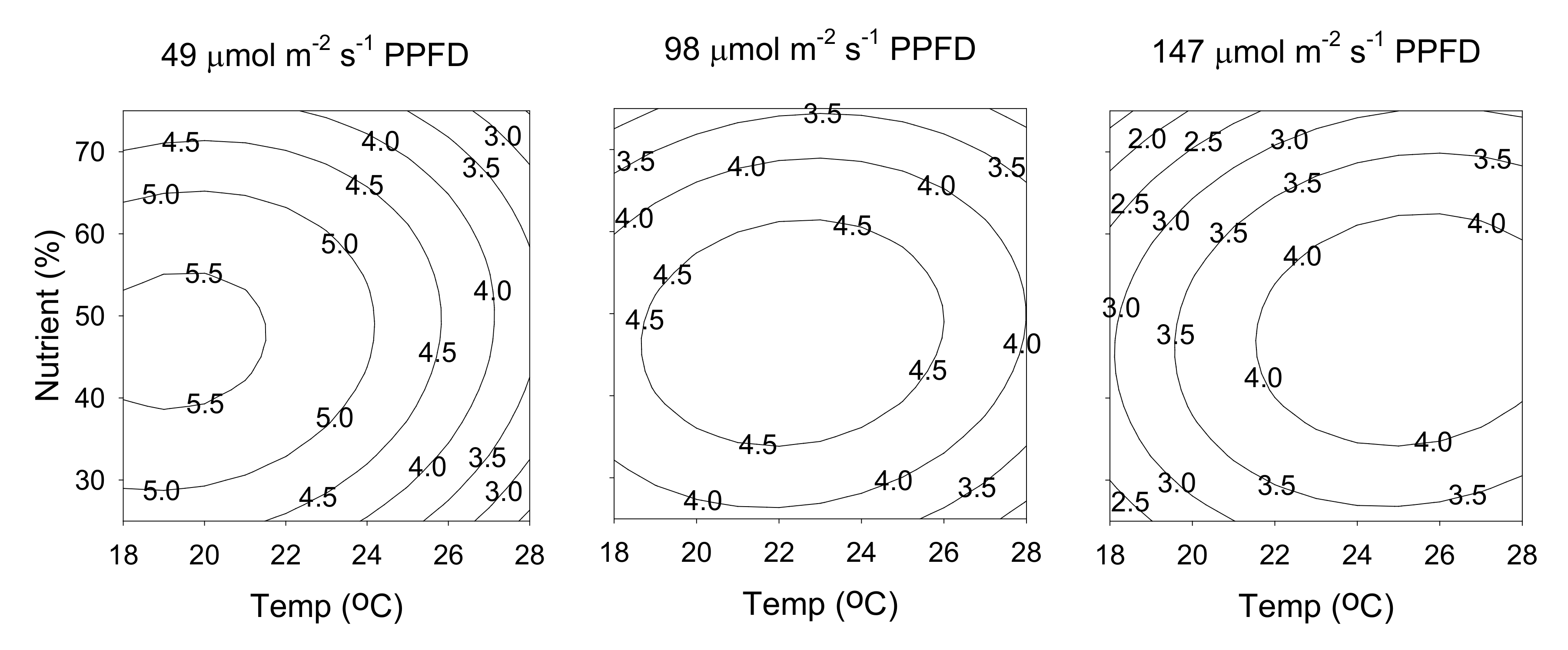
2.5. Attaining Optimal Cultivation Conditions for the Contents of Hyperforin and Rutin
2.6. Attaining Optimal Production Yields of Hyperforin and Rutin
3. Materials and Methods
3.1. Materials
3.2. Growing Conditions
3.3. Extraction of Hyperforin, Rutin and Melatonin
3.4. Experimental Design and Statistical Analysis
3.5. HPLC Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lawvere, S.; Mahoney, M.C. St. John’s wort. Am. Fam. Physician 2005, 72, 2249–2254. [Google Scholar] [PubMed]
- Bruni, R.; Sacchetti, G. Factors affecting polyphenol biosynthesis in wild and field grown St. John’s Wort (Hypericum perforatum L. Hypericaceae/Guttiferae). Molecules 2009, 14, 682–725. [Google Scholar] [PubMed] [Green Version]
- Zobayed, S.; Afreen, F.; Kozai, T. Temperature stress can alter the photosynthetic efficiency and secondary metabolite concentrations in St. John’s wort. Plant Physiol. Biochem. 2005, 43, 977–984. [Google Scholar]
- Arsić, I.; Žugić, A.; Tadić, V.; Tasić-Kostov, M.; Mišić, D.; Primorac, M.; Runjaić-Antić, D. Estimation of dermatological application of creams with St. John’s Wort oil extracts. Molecules 2012, 17, 275–294. [Google Scholar] [CrossRef] [Green Version]
- Linde, K.; Ramirez, G.; Mulrow, C.D.; Pauls, A.; Weidenhammer, W.; Melchart, D. St John’s wort for depression—An overview and meta-analysis of randomised clinical trials. BMJ 1996, 313, 253–258. [Google Scholar] [PubMed] [Green Version]
- Volz, H.-P. Controlled clinical trials of Hypericum extracts in depressed patients-An overview. Pharmacopsychiatry 2007, 30, 72–76. [Google Scholar] [CrossRef]
- Laila, U.; Akram, M.; Shariati, M.A.; Hashmi, A.M.; Akhtar, N.; Tahir, I.M.; Ghauri, A.O.; Munir, N.; Riaz, M.; Akhter, N.; et al. Role of medicinal plants in HIV/AIDS therapy. Clin. Exp. Pharmacol. Physiol. 2019, 46, 1063–1073. [Google Scholar] [CrossRef] [Green Version]
- Murch, S.; KrishnaRaj, S.; Saxena, P. Tryptophan is a precursor for melatonin and serotonin biosynthesis in in vitro regenerated St. John’s wort (Hypericum perforatum L. cv. Anthos) plants. Plant Cell Rep. 2000, 19, 698–704. [Google Scholar]
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. St John’s wort (Hypericum perforatum L.): A review of its chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 2001, 53, 583–600. [Google Scholar]
- Odabas, M.S.; Radusiene, J.; Camas, N.; Janulis, V.; Ivanauskas, L.; Cirak, C. The quantitative effects of temperature and light intensity on hyperforin and hypericins accumulation in Hypericum perforatum L. J. Med. Plants Res. 2009, 3, 519–525. [Google Scholar]
- Sosa, S.; Pace, R.; Bornanciny, A.; Morazzoni, P.; Riva, A.; Tubaro, A.; Loggia, R.D. Topical anti-inflammatory activity of extracts and compounds from Hypericum perforatum L. J. Pharm. Pharmacol. 2007, 59, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Beerhues, L. Hyperforin. Phytochemistry 2006, 67, 2201–2207. [Google Scholar] [CrossRef] [PubMed]
- García-Lafuente, A.; Guillamón, E.; Villares, A.; Rostagno, M.A.; Martínez, J.A. Flavonoids as anti-inflammatory agents: Implications in cancer and cardiovascular disease. Inflamm. Res. 2009, 58, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Ngamsuk, S.; Huang, T.-C.; Hsu, J.-L. Determination of phenolic compounds, procyanidins, and antioxidant activity in processed Coffea arabica L. Leaves. Foods 2019, 8, 389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murch, S.J.; Saxena, P.K. A melatonin-rich germplasm line of St John’s wort (Hypericum perforatum L.). J. Pineal Res. 2006, 41, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Herrera, T.; Aguilera, Y.; Rebollo-Hernanz, M.; Bravo, E.; Benítez, V.; Martínez-Sáez, N.; Arribas, S.M.; del Castillo, M.D.; Martín-Cabrejas, M.A. Teas and herbal infusions as sources of melatonin and other bioactive non-nutrient components. LWT Food Sci. Technol. 2018, 89, 65–73. [Google Scholar] [CrossRef]
- Salehi, B.; Sharopov, F.; Fokou, P.V.T.; Kobylinska, A.; Jonge, L.D.; Tadio, K.; Sharifi-Rad, J.; Posmyk, M.M.; Martorell, M.; Martins, N.; et al. Melatonin in medicinal and food plants: Occurrence, bioavailability, and health potential for humans. Cells 2019, 8, 681. [Google Scholar] [CrossRef] [Green Version]
- Bagdonaitė, E.; Mártonfi, P.; Repčák, M.; Labokas, J. Variation in concentrations of major bioactive compounds in Hypericum perforatum L. from Lithuania. Ind. Crops. Prod. 2012, 35, 302–308. [Google Scholar] [CrossRef]
- Becker, H. Boosting the quality and potency of St. John’s-wort. Agric. Res. 2000, 48, 12. [Google Scholar]
- Couceiro, M.; Afreen, F.; Zobayed, S.; Kozai, T. Variation in concentrations of major bioactive compounds of St. John’s wort: Effects of harvesting time, temperature and germplasm. Plant Sci. 2006, 170, 128–134. [Google Scholar] [CrossRef]
- Kang, J.H.; KrishnaKumar, S.; Atulba, S.L.S.; Jeong, B.R.; Hwang, S.J. Light intensity and photoperiod influence the growth and development of hydroponically grown leaf lettuce in a closed-type plant factory system. Hortic. Environ. Biotechnol. 2013, 54, 501–509. [Google Scholar] [CrossRef]
- Muneer, S.; Ko, C.H.; Wei, H.; Chen, Y.; Jeong, B.R. Physiological and proteomic investigations to study the response of tomato graft unions under temperature stress. PLoS ONE 2016, 11, e0157439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-García, F.; Huertas, M.; Mora, E.; Peña, B.; Varela, F.; González-Benito, M. Hypericum perforatum L. seed germination: Interpopulation variationand effect of light, temperature, presowing treatments and seed desiccation. Genet. Resour. Crop Evol. 2006, 53, 1187–1198. [Google Scholar] [CrossRef]
- Briskin, D.P.; Leroy, A.; Gawienowski, M. Influence of nitrogen on the production of hypericins by St. John’s wort. Plant Physiol. Biochem. 2000, 38, 413–420. [Google Scholar] [CrossRef]
- Lam, V.P.; Kim, S.J.; Park, J.S. Optimizing the electrical conductivity of a nutrient solution for plant growth and bioactive compounds of Agastache rugosa in a plant factory. Agronomy 2020, 10, 76. [Google Scholar] [CrossRef] [Green Version]
- Yoon, H.I.; Zhang, W.; Son, J.E. Optimal duration of drought stress near harvest for promoting bioactive compounds and antioxidant capacity in kale with or without UV-B radiation in plant factories. Plants 2020, 9, 295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, C.-H.; Chen, B.-Y.; Liu, Y.-C.; Chen, J.-H.; Shieh, C.-J. Production of resveratrol by piceid deglycosylation using cellulase. Catalysts 2016, 6, 32. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Lin, J.; Huang, C.-Y.; Hsieh, S.-L.; Li, S.; Kuo, J.-M.; Shieh, C.-J. Predicting sugar content of candied watermelon rind during osmotic dehydration. Food Sci. Technol. 2018. [Google Scholar] [CrossRef] [Green Version]
- Lo, C.-F.; Yu, C.-Y.; Kuan, I.-C.; Lee, S.-L. Optimization of lipase production by Burkholderia sp. using response surface methodology. Int. J. Mol. Sci. 2012, 13, 14889–14897. [Google Scholar] [CrossRef] [Green Version]
- Auwal, S.M.; Zarei, M.; Abdul-Hamid, A.; Saari, N. Optimization of bromelain-aided production of angiotensin I-converting enzyme inhibitory hydrolysates from stone fish using response surface methodology. Mar. Drugs 2017, 15, 104. [Google Scholar] [CrossRef]
- Sekoai, P.T. Modelling and optimization of operational setpoint parameters for maximum fermentative biohydrogen production using Box-Behnken design. Fermentation 2016, 2, 15. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Huang, C.-Y.; Shieh, C.-J.; Wang, H.-M.D.; Tseng, C.-Y. Hydrolysis of orange peel with cellulase and pectinase to produce bacterial cellulose using Gluconacetobacter xylinus. Waste Biomass Valorization 2019, 10, 85–93. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Abu-Ghannam, N. Fermentation-assisted extraction of isothiocyanates from brassica vegetable using Box-Behnken experimental design. Foods 2016, 5, 75. [Google Scholar] [CrossRef]
- Saha, B.; Longo, R.; Torley, P.; Saliba, A.; Schmidtke, L. SPME method optimized by Box-Behnken design for impact odorants in reduced alcohol wines. Foods 2018, 7, 127. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, M.A.; Huang, Y.; Bie, Z.; Ahmed, W.; Reiter, R.J.; Niu, M.; Hameed, S. Melatonin: Current status and future perspectives in plant science. Front. Plant Sci. 2016, 6, 1230. [Google Scholar]
- Aguilera, Y.; Herrera, T.; Benítez, V.; Arribas, S.M.; de Pablo, A.L.L.; Esteban, R.M.; Martín-Cabrejas, M.A. Estimation of scavenging capacity of melatonin and other antioxidants: Contribution and evaluation in germinated seeds. Food Chem. 2015, 170, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A new plant hormone and/or a plant master regulator? Trends Plant Sci. 2018, 24, 38–48. [Google Scholar] [CrossRef]
- Zhang, J.; Zeng, B.; Mao, Y.; Kong, X.; Wang, X.; Yang, Y.; Zhang, J.; Xu, J.; Rengel, Z.; Chen, Q. Melatonin alleviates aluminium toxicity through modulating antioxidative enzymes and enhancing organic acid anion exudation in soybean. Funct. Plant Biol. 2017, 44, 961–968. [Google Scholar] [CrossRef]
- Sarafi, E.; Tsouvaltzis, P.; Chatzissavvidis, C.; Siomos, A.; Therios, I. Melatonin and resveratrol reverse the toxic effect of high boron (B) and modulate biochemical parameters in pepper plants (Capsicum annuum L.). Plant Physiol. Biochem. 2017, 112, 173–182. [Google Scholar] [CrossRef]
- Brechner, M.; Albright, L.; Weston, L. Impact of a variable light intensity at a constant light integral: Effects on biomass and production of secondary metabolites by Hypericum perforatum. In Proceedings of the International Symposium on Medicinal and Nutraceutical Plants 756, Macon, GA, USA, 8 November 2007; pp. 221–228. [Google Scholar]
- Liu, Y.; Ren, X.; Jeong, B.R. Night temperature affects the growth, metabolism, and photosynthetic gene expression in Astragalus membranaceus and Codonopsis lanceolata plug seedlings. Plants 2019, 8, 407. [Google Scholar] [CrossRef] [Green Version]
- Soelberg, J.; Jørgensen, L.B.; Jäger, A.K. Hyperforin accumulates in the translucent glands of Hypericum perforatum. Ann. Bot. 2007, 99, 1097–1100. [Google Scholar] [PubMed] [Green Version]
- Ganzera, M.; Zhao, J.; Khan, I. Hypericum perforatum—Chemical profiling and quantitative results of St. John’s Wort products by an improved high-performance liquid chromatography method. J. Pharm. Sci. 2002, 91, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, J.R.; García-Ruíz, P.; Sánchez-Bravo, J.; Acosta, M.; Arnao, M.B. Quantitation of indole-3-acetic acid by LC with electrochemical detection in etiolated hypocotyls of Lupinus albus. J. Liq. Chromatogr. Relat. Technol. 2001, 24, 3095–3104. [Google Scholar] [CrossRef]
Sample Availability: Samples of the St. John’s wort are available from the authors. |




| Run | Independent Variable | Responses | ||||
|---|---|---|---|---|---|---|
| Temp X1 (°C) | Light Intensity X2 (μmol m−2 s−1 PPFD) | Nutrient X3 (%) | Hyperforin Content b (Y1) (mg/g) | Rutin Content (Y2) (mg/g) | Melatonin Content (Y3) (μg/g) | |
| 1 | −1 a (18) | −1 (49) | 0 (50) | 5.653 ± 0.106 | 0.825 ± 0.043 | 0.046 ± 0.040 |
| 2 | −1 (18) | 0 (98) | −1 (25) | 3.314 ± 0.062 | 2.138 ± 0.038 | 0.048 ± 0.038 |
| 3 | −1 (18) | 0 (98) | 1 (75) | 2.891 ± 0.072 | 2.474 ± 0.005 | 0.060 ± 0.048 |
| 4 | −1 (18) | 1 (147) | 0 (50) | 2.865 ± 0.059 | 4.107 ± 0.020 | 0.048 ± 0.039 |
| 5 | 0 (23) | −1 (49) | −1 (25) | 4.328 ± 0.051 | 0.622 ± 0.024 | 0.059 ± 0.042 |
| 6 | 0 (23) | −1 (49) | 1 (75) | 3.658 ± 0.074 | 0.537 ± 0.036 | 0.049 ± 0.039 |
| 7 | 0 (23) | 0 (98) | 0 (50) | 4.840 ± 0.080 | 2.340 ± 0.039 | 0.048 ± 0.039 |
| 8 | 0 (23) | 0 (98) | 0 (50) | 4.920 ± 0.102 | 2.380 ± 0.050 | 0.052 ± 0.050 |
| 9 | 0 (23) | 0 (98) | 0 (50) | 4.760 ± 0.055 | 2.300 ± 0.042 | 0.044 ± 0.043 |
| 10 | 0 (23) | 1 (147) | −1 (25) | 3.528 ± 0.086 | 2.767 ± 0.041 | 0.063 ± 0.039 |
| 11 | 0 (23) | 1 (147) | 1 (75) | 2.656 ± 0.066 | 2.565 ± 0.031 | 0.073 ± 0.039 |
| 12 | 1 (28) | −1 (49) | 0 (50) | 3.667 ± 0.072 | 1.379 ± 0.030 | 0.057 ± 0.040 |
| 13 | 1 (28) | 0 (98) | −1 (25) | 2.636 ± 0.057 | 2.565 ± 0.032 | 0.054 ± 0.039 |
| 14 | 1 (28) | 0(98) | 1 (75) | 2.990 ± 0.081 | 2.832 ± 0.021 | 0.058 ± 0.039 |
| 15 | 1 (28) | 1 (147) | 0 (50) | 4.088 ± 0.061 | 3.109 ± 0.023 | 0.052 ± 0.040 |
| Source | Hyperforin | Rutin | Melatonin | |||
|---|---|---|---|---|---|---|
| Sum of Squares | p-Value | Sum of Squares | p-Value | Sum of Squares | p-Value | |
| Linear | 2.722 | 0.0074 a | 10.572 | 0.0004 a | 0.0001 | 0.3715 b |
| Quadratic | 6.63 | 0.0010 a | 1.852 | 0.0227 a | 0.0003 | 0.1387 b |
| Crossproduct | 2.735 | 0.0073 a | 0.606 | 0.1588 b | 0.0001 | 0.4395 b |
| Total Model | 12.090 | 0.0020 a | 13.032 | 0.0023 a | 0.0006 | 0.2751 b |
| R2 = 0.9736 | R2 = 0.9717 | R2 = 0.7608 | ||||
| Factor | Hyperforin | Rutin | ||
|---|---|---|---|---|
| Sum of Squares | p-Value | Sum of Squares | p-Value | |
| Temperature (x1) | 4.6490 | 0.0037 a | 1.3564 | 0.0658 |
| Light intensity (x2) | 4.7894 | 0.0035 a | 11.8409 | 0.0006 a |
| Nutrient concentration (x3) | 5.8379 | 0.0022 a | 0.3170 | 0.4680 |
| Run | Independent Variable | Responses (Production Yield b) | ||||
|---|---|---|---|---|---|---|
| Temp X1 (°C) | Light Intensity X2 (μmol m−2 s−1 PPFD) | Nutrient X3 (%) | Total Weight of Leaves (Y4) (g/plant) | Hyperforin (Y5) (mg/plant) | Rutin (Y6) (mg/plant) | |
| 1 | −1a (18) | −1 (49) | 0 (50) | 0.709 ± 0.130 | 4.007 ± 0.734 | 0.584 ± 0.107 |
| 2 | −1 (18) | 0 (98) | −1 (25) | 0.762 ± 0.098 | 2.525 ± 0.324 | 1.629 ± 0.209 |
| 3 | −1 (18) | 0 (98) | 1 (75) | 0.860 ± 0.095 | 2.486 ± 0.274 | 2.127 ± 0.235 |
| 4 | −1 (18) | 1 (147) | 0 (50) | 0.665 ± 0.055 | 1.905 ± 0.157 | 2.731 ± 0.225 |
| 5 | 0 (23) | −1 (49) | −1 (25) | 0.531 ± 0.176 | 2.298 ± 0.762 | 0.330 ± 0.109 |
| 6 | 0 (23) | −1 (49) | 1 (75) | 0.827 ± 0.228 | 3.025 ± 0.834 | 0.444 ± 0.122 |
| 7 | 0 (23) | 0 (98) | 0 (50) | 1.160 ± 0.167 | 5.614 ± 0.808 | 2.714 ± 0.390 |
| 8 | 0 (23) | 0 (98) | 0 (50) | 1.046 ± 0.196 | 5.146 ± 0.964 | 2.489 ± 0.466 |
| 9 | 0 (23) | 0 (98) | 0 (50) | 1.010 ± 0.061 | 4.807 ± 0.290 | 2.323 ± 0.140 |
| 10 | 0 (23) | 1 (147) | −1 (25) | 0.535 ± 0.059 | 1.887 ± 0.208 | 1.480 ± 0.163 |
| 11 | 0 (23) | 1 (147) | 1 (75) | 1.201 ± 0.122 | 3.189 ± 0.324 | 3.080 ± 0.312 |
| 12 | 1 (28) | −1 (49) | 0 (50) | 0.919 ± 0.121 | 3.369 ± 0.443 | 1.267 ± 0.166 |
| 13 | 1 (28) | 0 (98) | −1 (25) | 0.561 ± 0.121 | 1.478 ± 0.318 | 1.438 ± 0.310 |
| 14 | 1 (28) | 0(98) | 1 (75) | 1.102 ± 0.135 | 3.294 ± 0.403 | 3.120 ± 0.382 |
| 15 | 1 (28) | 1 (147) | 0 (50) | 1.033 ± 0.099 | 4.222 ± 0.404 | 3.211 ± 0.307 |
| Independent Variable | Symbol | Coded Variable Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Temperature (°C) | X1 | 18 | 23 | 28 |
| Light intensity (μmol m−2 s−1 PPFD) | X2 | 49 | 98 | 147 |
| Nutrient (%) | X3 | 25 | 50 | 75 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, C.-H.; Chou, Y.-C.; Liao, K.-C.; Shieh, C.-J.; Deng, T.-S. Optimization of Light Intensity, Temperature, and Nutrients to Enhance the Bioactive Content of Hyperforin and Rutin in St. John’s Wort. Molecules 2020, 25, 4256. https://doi.org/10.3390/molecules25184256
Kuo C-H, Chou Y-C, Liao K-C, Shieh C-J, Deng T-S. Optimization of Light Intensity, Temperature, and Nutrients to Enhance the Bioactive Content of Hyperforin and Rutin in St. John’s Wort. Molecules. 2020; 25(18):4256. https://doi.org/10.3390/molecules25184256
Chicago/Turabian StyleKuo, Chia-Hung, Yi-Chin Chou, Kuo-Chun Liao, Chwen-Jen Shieh, and Tzu-Shing Deng. 2020. "Optimization of Light Intensity, Temperature, and Nutrients to Enhance the Bioactive Content of Hyperforin and Rutin in St. John’s Wort" Molecules 25, no. 18: 4256. https://doi.org/10.3390/molecules25184256
APA StyleKuo, C.-H., Chou, Y.-C., Liao, K.-C., Shieh, C.-J., & Deng, T.-S. (2020). Optimization of Light Intensity, Temperature, and Nutrients to Enhance the Bioactive Content of Hyperforin and Rutin in St. John’s Wort. Molecules, 25(18), 4256. https://doi.org/10.3390/molecules25184256








