Fate of Antioxidative Compounds within Bark during Storage: A Case of Norway Spruce Logs
Abstract
1. Introduction
2. Results and Discussion
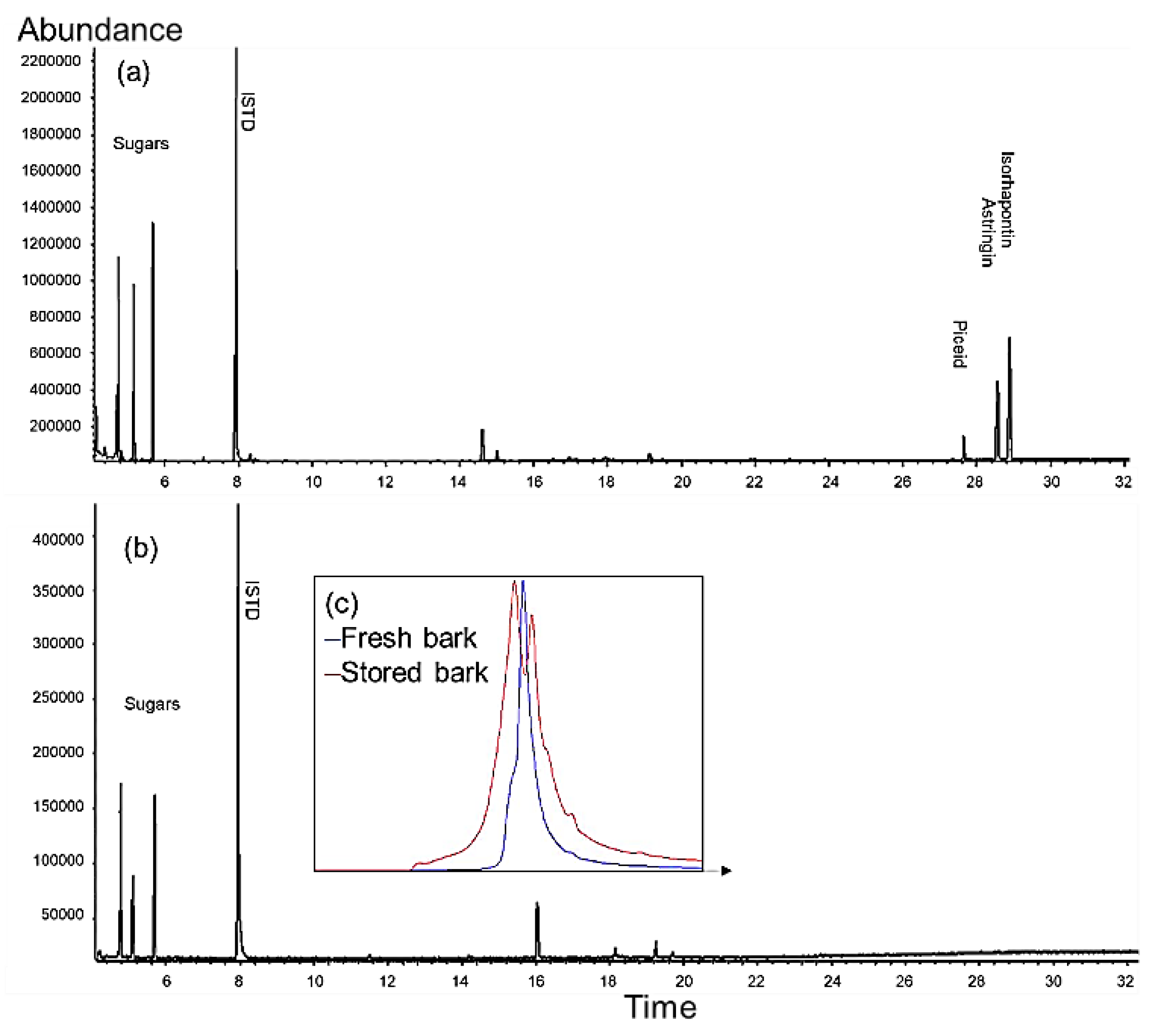
2.1. Pre-Trial: Stilbene Glycosides of Bark at Sawmill during Storage
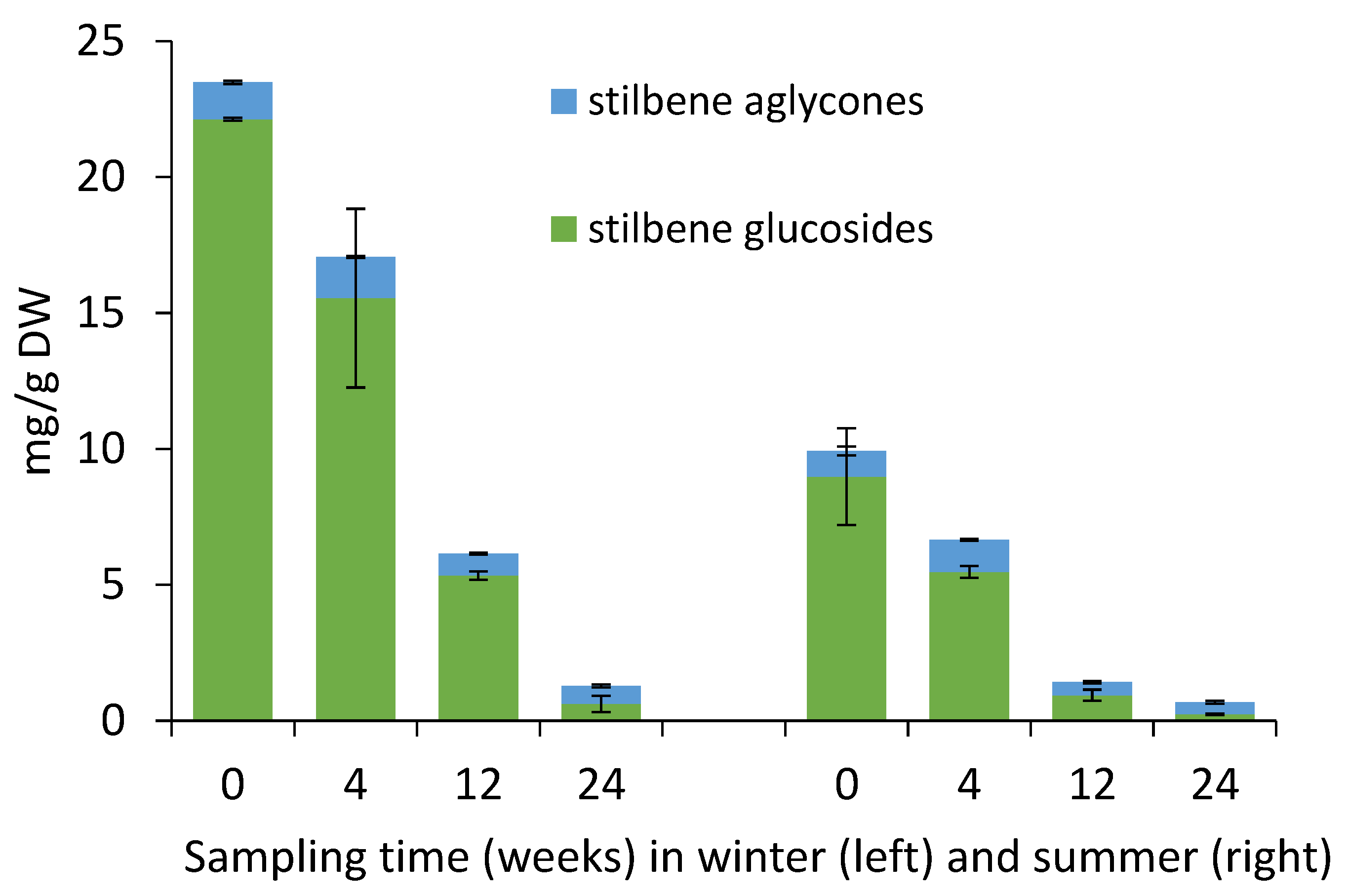
2.2. Yield of Stilbene Glycosides in Bark during Storage Experiment
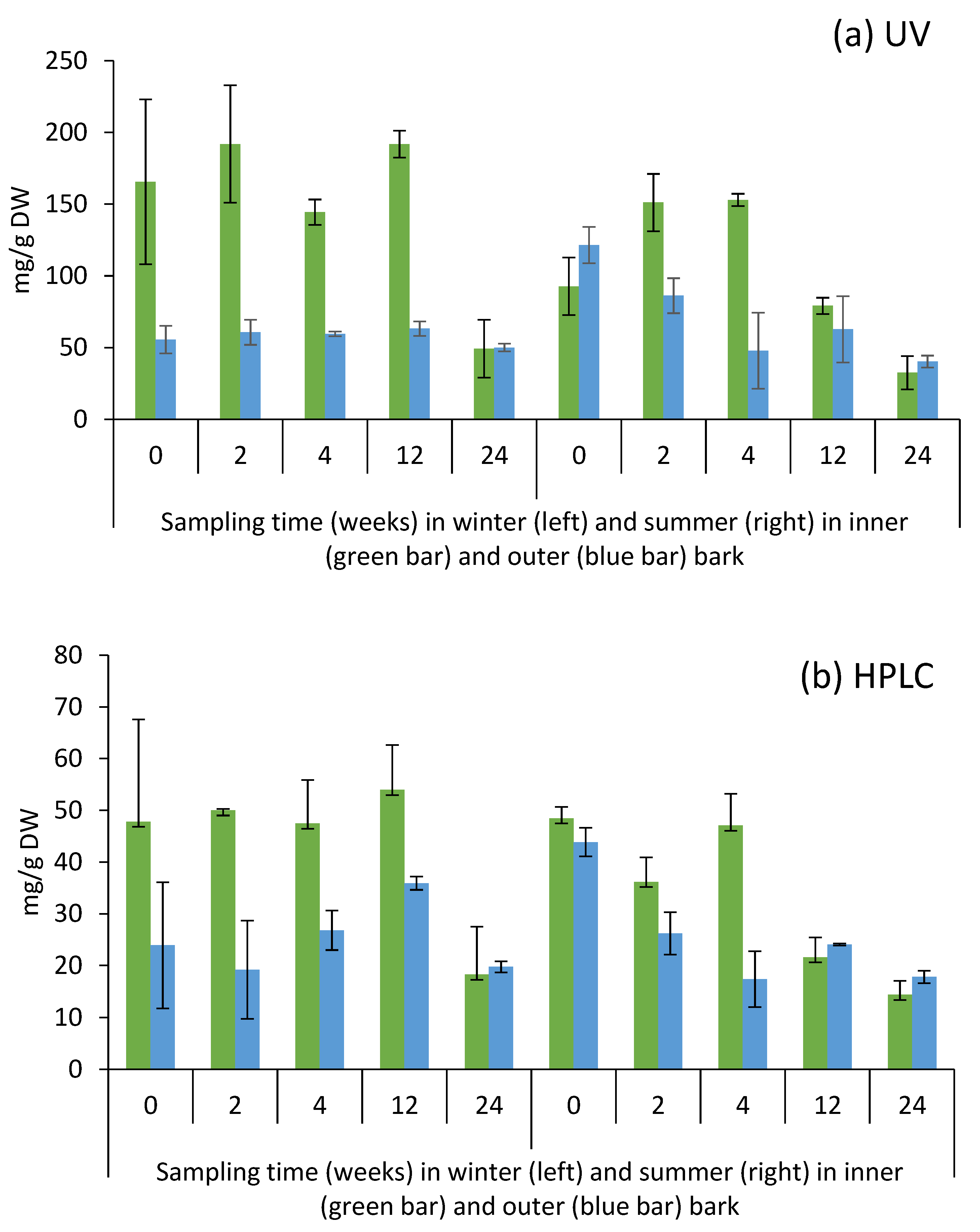
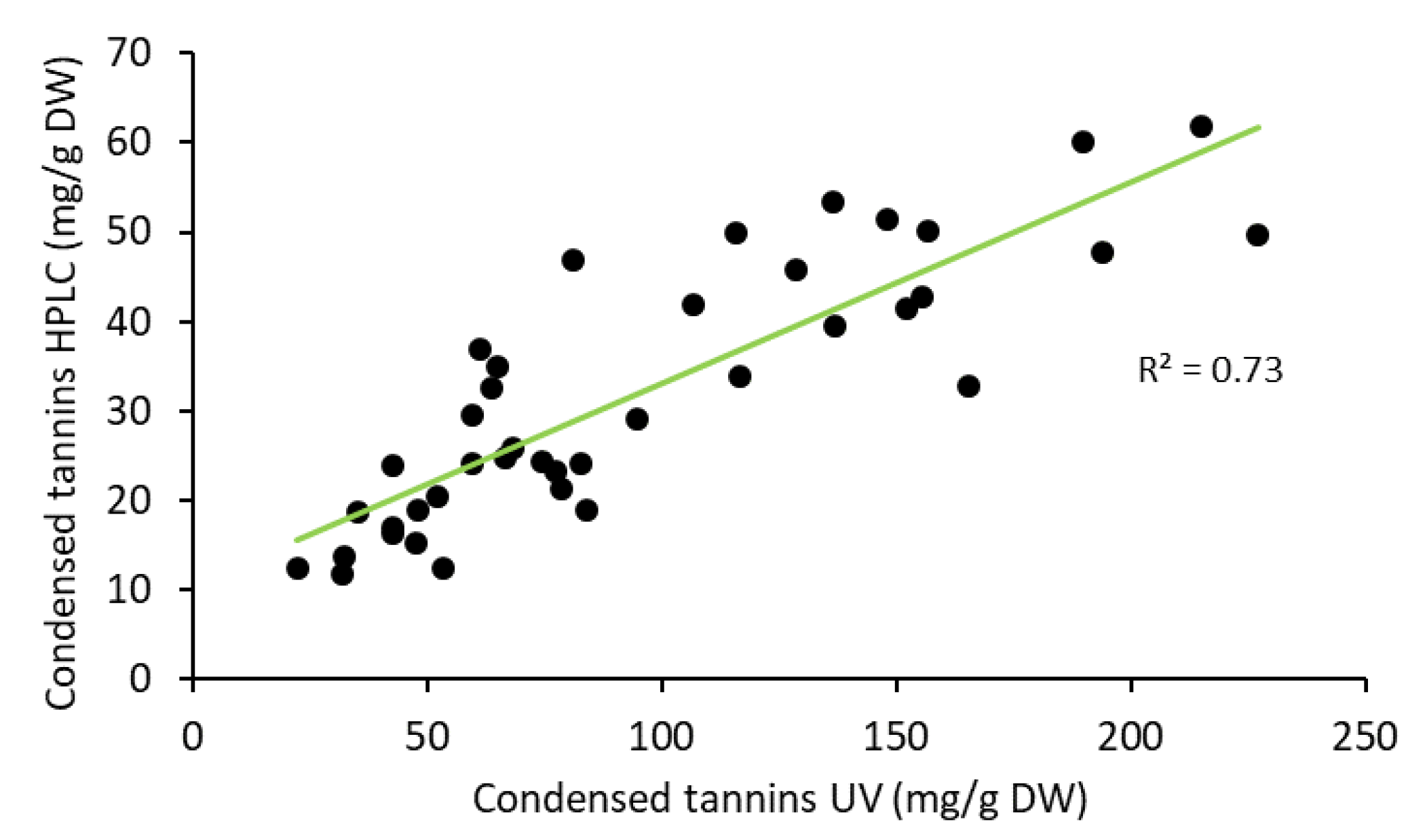
2.3. Yield of Condensed Tannins in Bark during Storage Experiment
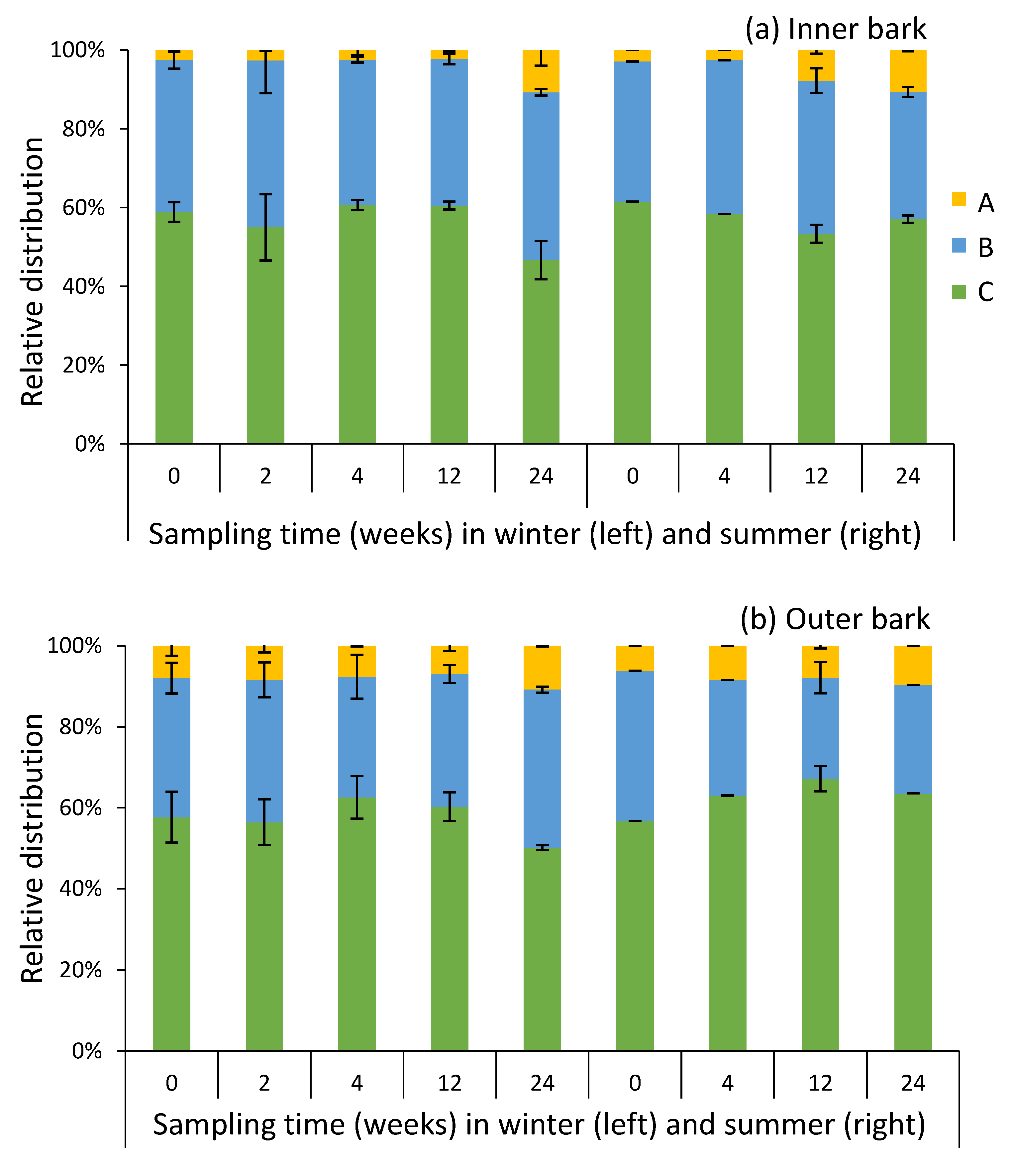
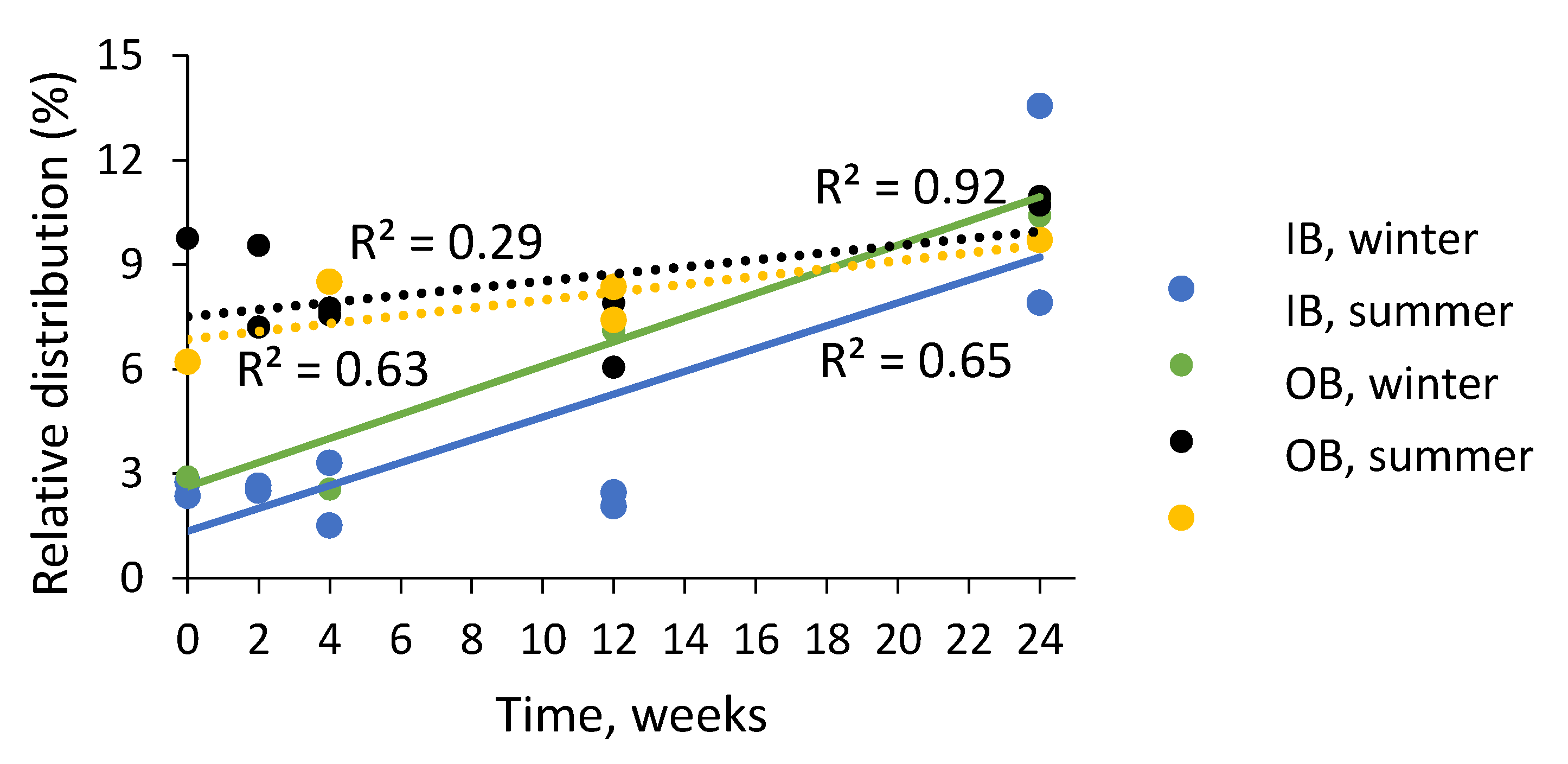
2.4. Composition of Condensed Tannins in Bark during Storage Experiment
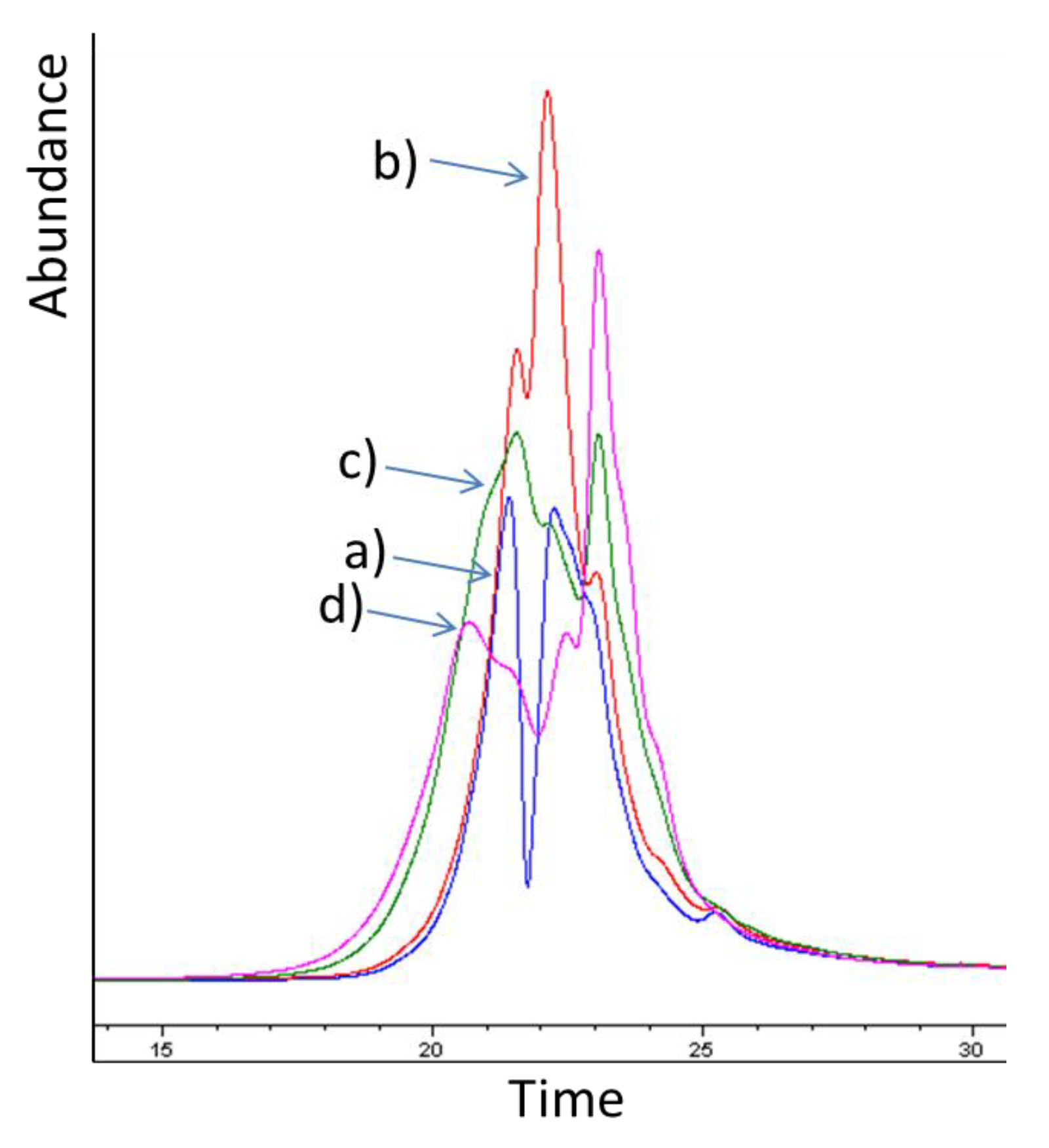
2.5. Molar Mass Distribution of Bark Extracts during Storage Experiment
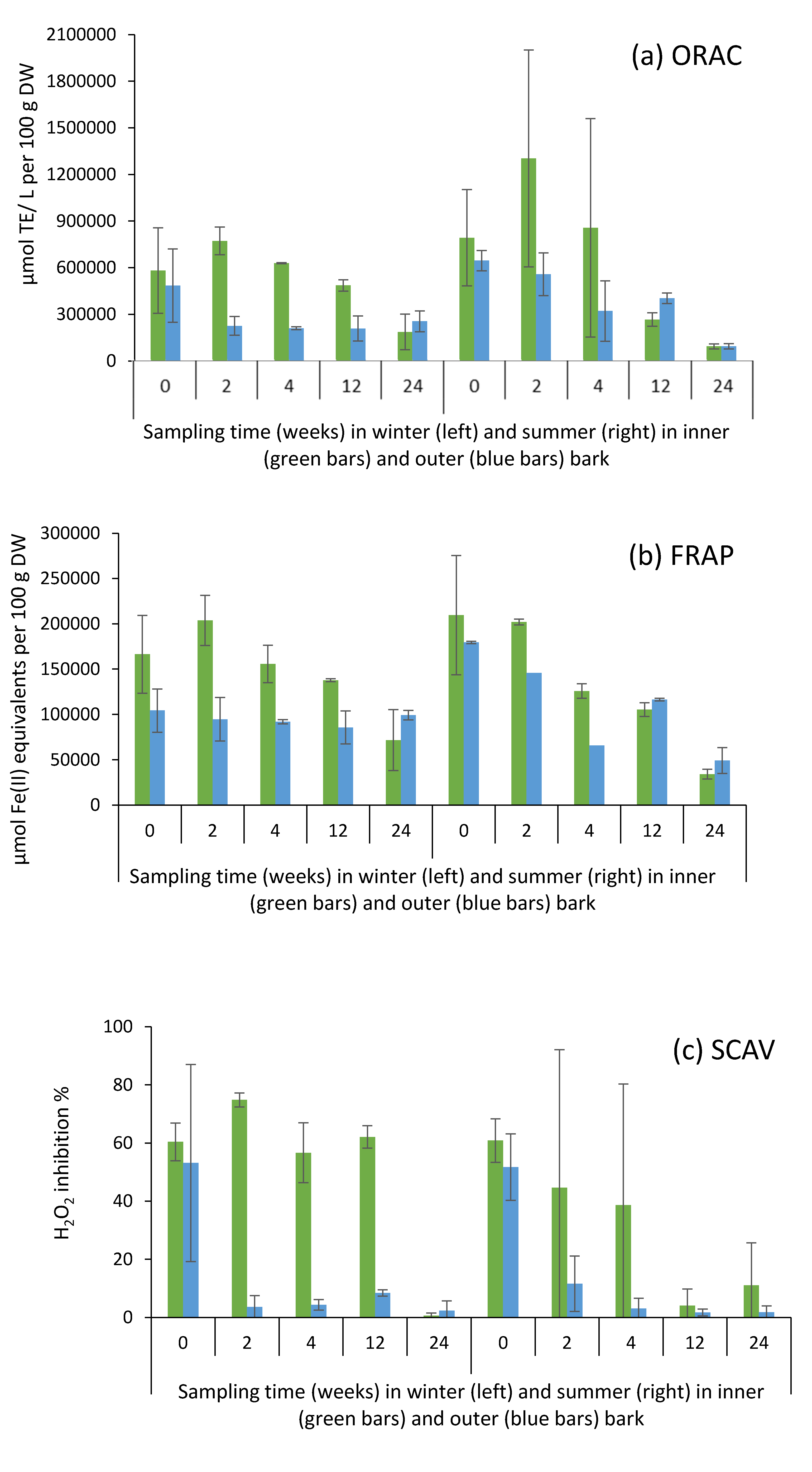
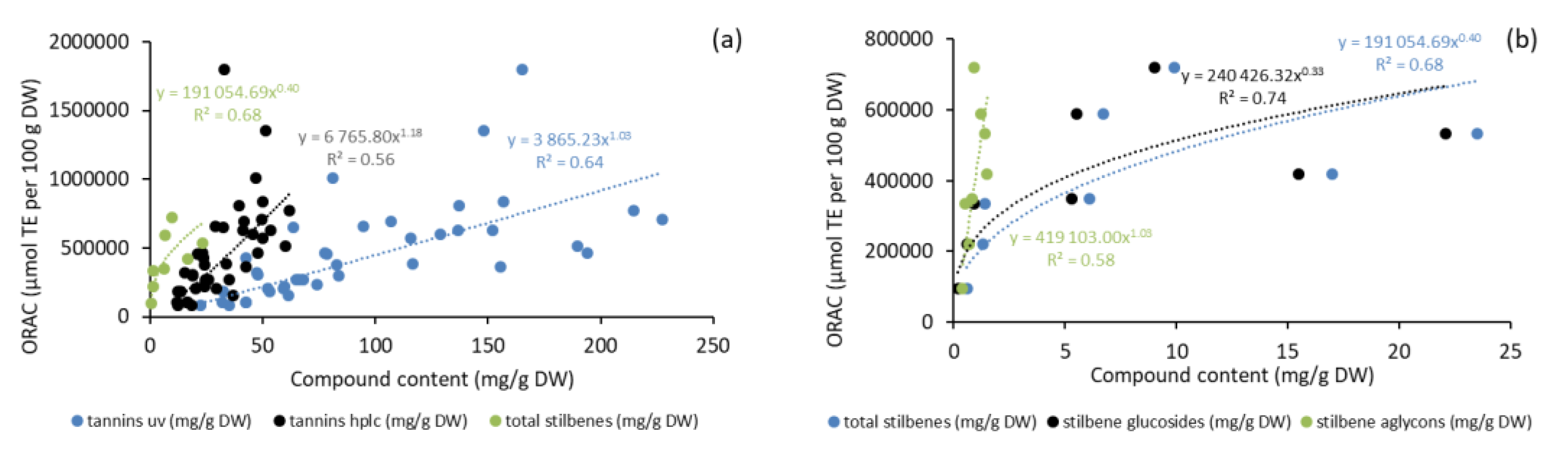
2.6. Antioxidative Activity of Bark Extracts during Storage Experiment
3. Materials and Methods
3.1. Bark Material
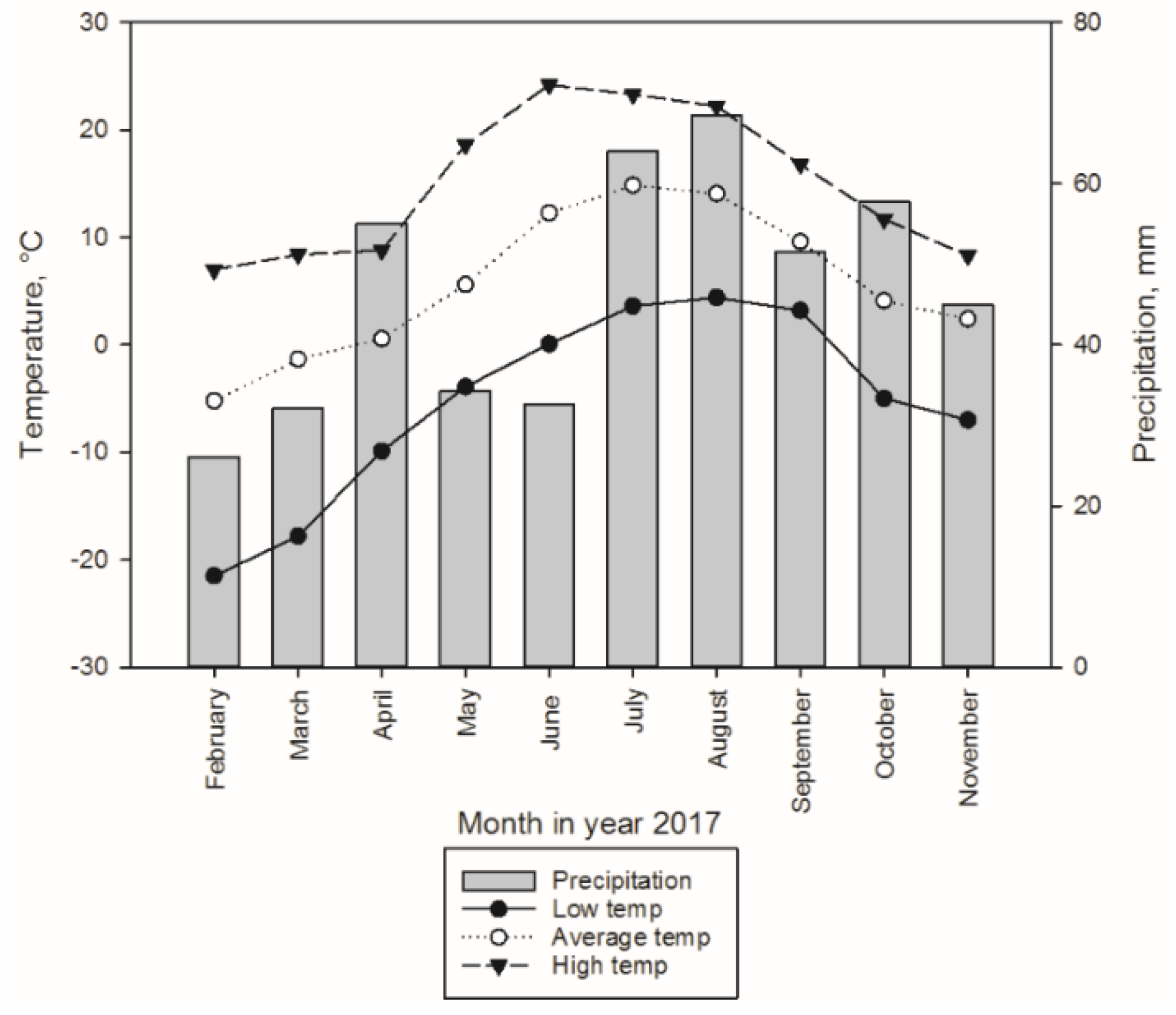
3.1.1. Sample Trees and Experimental Design for Storage Treatments
3.1.2. Industrial Bark
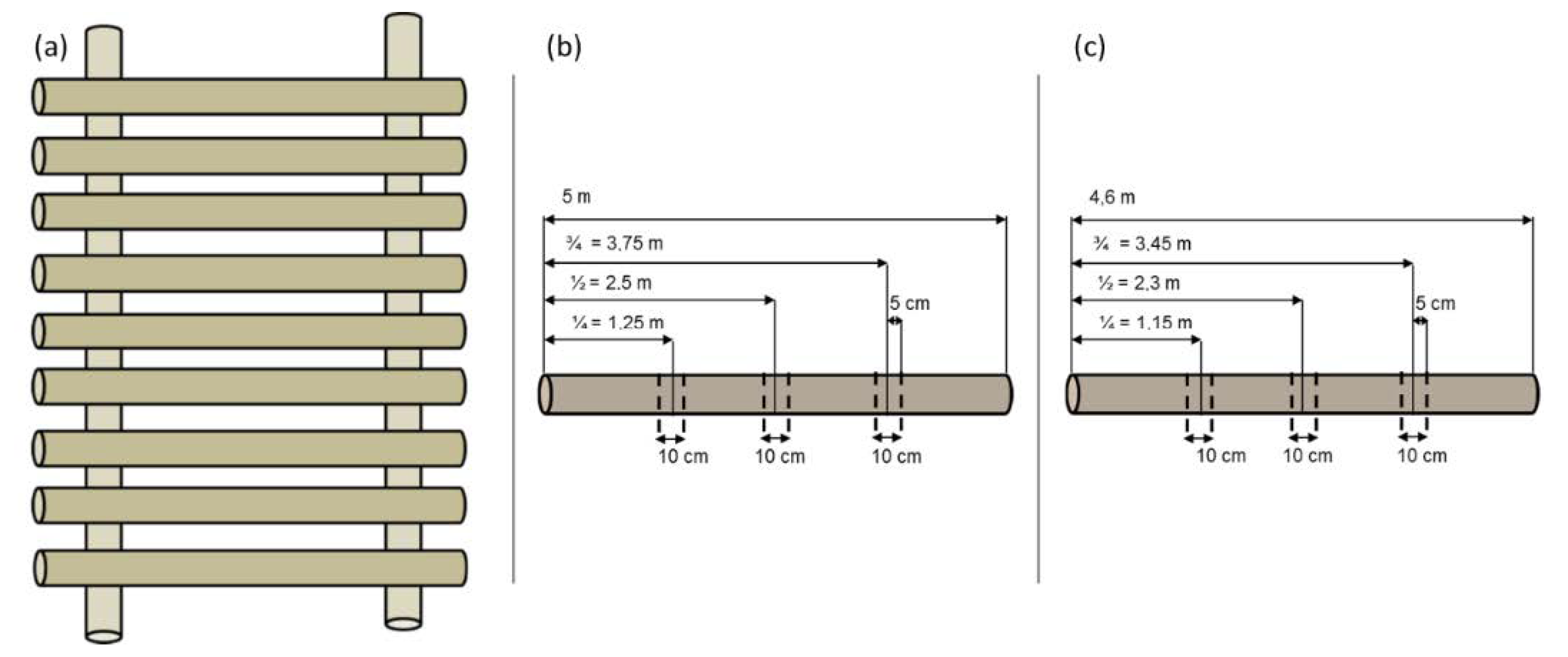
3.2. Sampling Design in Storage Treatments
3.3. Preparation of Bark Extracts
3.4. Chemical Analysis of Extracts
3.4.1. Yield of Condensed Tannins
3.4.2. Chemical Composition of Condensed Tannins
3.4.3. Yield of Stilbene Glycosides
3.4.4. Analysis of Molar Mass Distribution of Extracts
3.5. Antioxidative Analysis of Extracts
3.5.1. FRAP
3.5.2. ORAC
3.5.3. SCAV/FOX Reagent Method
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Statistics Finland (OSF); Natural Resources Institute Finland. Forest Industries’ Wood Consumption; Natural Resources Institute Finland: Helsinki, Finland, 2020; Available online: https://stat.luke.fi/en/wood-consumption (accessed on 27 July 2020).
- Björklund, L. Bark på Massaved–en Studie över Barkhalten i Travar med Massaved; The Swedish Timber Measurement Council (VMR) Virkesmätning och Redovisning, Reports; SKOGSNARINGENS IT-FÖRETAG: Sundsvall, Sweden, 2004. [Google Scholar]
- Franceschi, V.R.; Krokene, P.; Christiansen, E.; Krekling, T. Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytol. 2005, 167, 353–376. [Google Scholar] [CrossRef] [PubMed]
- Krokene, P. Conifer defense and resistance to bark beetles. In Biology and Ecology of Native and Invasive Species; Vega, F.E., Hofstetter, R.W., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2015; pp. 177–207. [Google Scholar]
- Gabaston, J.; Richard, T.; Biais, B.; Waffo-Teguo, P.; Pedrot, E.; Jourdes, M.; Corio-Costet, M.-F.; Mérillon, J.-M. Stilbenes from common spruce (Picea abies) bark as natural antifungal agent against downy mildew (Plasmopara viticola). Ind. Crops Prod. 2017, 103, 267–273. [Google Scholar] [CrossRef]
- Metsämuuronen, S.; Siren, H. Antibacterial compounds in predominant trees in Finland: Review. J. Bioprocess. Biotechnol. 2014, 4, 167. [Google Scholar] [CrossRef]
- Reinisalo, M.; Kårlund, A.; Koskela, A.; Kaarniranta, K.; Karjalainen, R.O. Polyphenol stilbenes: Molecular mechanisms of defence against oxidative stress and aging-related diseases. Oxid. Med. Cell. Longev. 2015, 340520. [Google Scholar] [CrossRef] [PubMed]
- Jansone, Z.; Muizniece, I.; Blumberga, D. Analysis of wood bark use opportunities. Energy Procedia 2017, 128, 268–274. [Google Scholar] [CrossRef]
- Latva-Mäenpää, H. Bioactive and Protective Polyphenolics from Roots and Stumps of Conifer Trees (Norway Spruce and Scots Pine). Ph.D. Thesis, University of Helsinki, Helsinki, Finland, June 2017. [Google Scholar]
- Routa, J.; Brännström, H.; Anttila, P.; Mäkinen, M.; Jänis, J.; Asikainen, A. Wood extractives of Finnish pine, spruce and birch–availability and optimal sources of compounds. Nat. Resour. Bioecon. Stud. 2017, 73, 55. [Google Scholar]
- Varila, T.; Brännström, H.; Kilpeläinen, P.; Hellström, J.; Romar, H.; Nurmi, J.; Lassi, U. From norway spruce bark to carbon foams: Characterization, and applications. BioResources 2020, 15, 3651–3666. [Google Scholar]
- Sánchez-Martín, J.; Beltrán-Heredia, J.; Pizarro-Rebollo, A. Cationic tannins: New and effective coagulant/flocculant agents. In Handbook of Wastewater Treatment: Biological Methods, Technology, and Environmental Impact; Valdez, C.J., Maradona, E.M., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2013; pp. 221–247. [Google Scholar]
- Bianchi, S. Extraction and Characterization of Bark Tannins from Domestic Softwood Species. Ph.D. Thesis, University of Hamburg, Hamburg, Germany, January 2017. [Google Scholar]
- Tanac-Water Treatment. Available online: http://www.tanac.com.br/en/products/water-treatment (accessed on 11 September 2020).
- Raitanen, J.-E.; Järvenpää, E.; Korpinen, R.; Mäkinen, S.; Hellström, J.; Kilpeläinen, P.; Liimatainen, J.; Ora, A.; Tupasela, T.; Jyske, T. Tannins of conifer bark as nordic piquancy—Sustainable preservative and aroma? Molecules 2020, 25, 567. [Google Scholar] [CrossRef]
- Singh, A.P.; Singh, R.; Verma, S.S.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health benefits of resveratrol: Evidence from clinical studies. Med. Res. Rev. 2019, 39, 1851–1891. [Google Scholar] [CrossRef]
- Välimaa, A.-L.; Raitanen, J.-E.; Tienaho, J.; Sarjala, T.; Nakayama, E.; Korpinen, R.; Mäkinen, S.; Eklund, P.; Willför, S.; Jyske, T. Enhancement of Norway spruce bark side-streams: Modification of bioactive and protective properties of stilbenoid-rich extracts by UVA-irradiation. Ind. Crops Prod. 2020, 145, 17. [Google Scholar] [CrossRef]
- Jyske, T.; Laakso, T.; Latva-Mäenpää, H.; Tapanila, T.; Saranpää, P. Yield of stilbene glucosides from the bark of young and old Norway spruce stems. Biomass Bioenergy 2014, 71, 216–227. [Google Scholar] [CrossRef]
- Jyske, T.; Kuroda, K.; Suuronen, J.P.; Pranovich, A.; Roig Juan, S.; Aoki, D.; Fukushima, K. In planta localization of stilbenes within Picea abies phloem. Plant Physiol. 2016, 172, 913–928. [Google Scholar] [CrossRef] [PubMed]
- Jyske, T.; Kuroda, K.; Keriö, S.; Pranovich, A.; Linnakoski, R.; Hayashi, N.; Aoki, D.; Fukushima, K. Localization of (+)-catechin in Picea abies phloem: Responses to wounding and fungal inoculation. Molecules 2020, 25, 2952. [Google Scholar] [CrossRef]
- Ekman, R. Resin during storage and in biological treatment. In Pitch Control, Wood Resin and Deresination; Back, E.L., Allen, L.H., Eds.; Tappi Press: Atlanta, GA, USA, 2000; pp. 185–204. [Google Scholar]
- Krogell, J.; Holmbom, B.; Pranovich, A.; Hemming, J.; Willför, S. Extraction and chemical characterization of Norway spruce inner and outer bark. Nord. Pulp Pap. Res. J. 2012, 27, 6–17. [Google Scholar] [CrossRef]
- Krigstin, S.; Wetzel, S. A review of mechanisms responsible for changes to stored woody biomass fuels. Fuel 2016, 175, 75–86. [Google Scholar] [CrossRef]
- Bhat, T.K.; Singh, B.; Sharma, O.P. Microbial degradation of tannins–a current perspective. Biodegradation 1998, 9, 343–357. [Google Scholar] [CrossRef]
- Hedmark, Å.; Scholz, M. Review of environmental effects and treatment of runoff from storage and handling of wood. Bioresour. Technol. 2008, 99, 5997–6009. [Google Scholar] [CrossRef]
- Zhang, L.; Gellerstedt, G. Reactive structures in wood and high-yield pulps. IV. Daylight-induced oxidation of stilbene structures in the solid state. Acta Chem. Scand. 1994, 48, 490–497. [Google Scholar] [CrossRef]
- Zahri, S.; Belloncle, C.; Charrier, F.; Pardon, P.; Quideau, S.; Charrier, B. UV light impact on ellagitannins and wood surface colour of European oak (Quercus petraea and Quercus robur). Appl. Surf. Sci. 2007, 253, 4985–4989. [Google Scholar] [CrossRef]
- Kovacs, A.; Vasas, A.; Hohmann, J. Natural phenanthrenes and their biological activity. Phytochemistry 2008, 69, 1084–1110. [Google Scholar] [CrossRef]
- Matthews, S.; Mila, I.; Scalbert, A.; Donnelly, D.M. Extractable and non-extractable proanthocyanidins in barks. Phytochemistry 1997, 45, 405–410. [Google Scholar] [CrossRef]
- Bianchi, S.; Gloess, A.N.; Kroslakova, I.; Mayer, I.; Pichelin, F. Analysis of the structure of condensed tannins in water extracts from bark tissues of Norway spruce (Picea abies [Karst.]) and Silver fir (Abies alba [Mill.]) using MALDI-TOF mass spectrometry. Ind. Crops Prod. 2014, 61, 430–437. [Google Scholar] [CrossRef]
- Hammerbacher, A.; Paetz, C.; Wright, L.P.; Fischer, T.C.; Bohlmann, J.; Davis, A.J.; Fenning, T.M.; Gershenzon, J.; Schmidt, A. Flavan-3-ols in Norway spruce: Biosynthesis, accumulation, and function in response to attack by the bark beetle-associated fungus Ceratocystis polonica. Plant Physiol. 2014, 164, 2107–2122. [Google Scholar] [CrossRef]
- Bianchi, S.; Koch, G.; Janzon, R.; Mayer, I.; Saake, B.; Pichelin, F. Hot water extraction of Norway spruce (Picea abies [Karst.]) bark: Analyses of the influence of bark aging and process parameters on the extract composition. Holzforschung 2016, 70, 619–631. [Google Scholar] [CrossRef]
- Buckles, R.E. Illumination of cis- and trans-stilbenes in dilute solutions. J. Am. Chem. Soc. 1955, 77, 1040–1041. [Google Scholar] [CrossRef]
- Mallory, F.B.; Wood, C.S.; Gordon, J.T. Photochemistry of stilbenes. III. Some aspects of the mechanism of photocyclization to phenanthrenes. J. Am. Chem. Soc. 1964, 86, 3094–3102. [Google Scholar] [CrossRef]
- Rodríguez-Bonilla, P.; Gandía-Herrero, F.; Matencio, A.; García-Carmona, F.; López-Nicolás, J.M. Comparative study of the antioxidant capacity of four stilbenes using ORAC, ABTS+, and FRAP techniques. Food Anal. Methods 2017, 10, 2994–3000. [Google Scholar] [CrossRef]
- Co, M.; Fagerlund, A.; Engman, L.; Sunnerheim, K.; Sjöberg, P.J.R.; Turner, C. Extraction of antioxidants from spruce (Picea abies) bark using ecofriendly solvents. Phytochem. Anal. 2012, 23, 1–11. [Google Scholar] [CrossRef]
- Hubert, J.; Angelis, A.; Aligiannis, N.; Rosalia, M.; Abedini, A.; Bakiri, A.; Reynaud, R.; Nuzillard, J.-M.; Gangloff, S.C.; Skaltsounis, A.-L.; et al. In vitro dermo-cosmetic evaluation of bark extracts from common temperate trees. Planta. Med. 2016, 82, 1351–1358. [Google Scholar] [CrossRef]
- Halmemies, E.; Brännström, H.; Nurmi, J.; Alén, R. The degradation of bark extractives-derived phenolics during storage. In NWBC 2018, Proceedings of the 8th Nordic Wood Biorefinery Conference, Helsinki, Finland, 23–25 October 2018; Hytönen, E., Vepsäläinen, J., Eds.; VTT Technical Research Centre of Finland Ltd.: Espoo, Finland, 2018. [Google Scholar]
- Tamminen, T.; Ruuskanen, M.; Grönqvist, S. The influence of softwood bark origin on tannin recovery by hot-water extraction. In Proceedings of the 19th International Symposium on Wood, Fibre and Puling Chemistry, Porto Seguro, Brazil, 28 August–1 September 2017. [Google Scholar]
- Rasi, S.; Kilpeläinen, P.; Rasa, K.; Korpinen, R.; Raitanen, J.-E.; Vainio, M.; Kitunen, V.; Pulkkinen, H.; Jyske, T. Cascade processing of softwood bark with hot water extraction, pyrolysis and anaerobic digestion. Bioresour. Technol. 2019, 292, 7. [Google Scholar] [CrossRef]
- Venäläinen, A.; Tuomenvirta, H.; Pirinen, P.; Drebs, A. A basic Finnish climate data set 1961–2000–description and illustrations. Finn. Meteorol. Inst. Rep. 2005, 5, 1–27. [Google Scholar]
- Mattila, P.H.; Pihlava, J.-M.; Hellström, J.; Nurmi, M.; Eurola, M.; Mäkinen, S.; Jalava, T.; Pihlanto, A. Contents of phytochemicals and antinutritional factors in commercial protein-rich plant products. Food Qual. Saf. 2018, 2, 213–219. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORAC(FL))) of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef]
- Hazra, B.; Biswas, S.; Mandal, N. Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complement. Altern. Med. 2008, 8, 1–10. [Google Scholar] [CrossRef]
- Jiang, Z.-Y.; Woollard, A.C.; Wolff, S.P. Hydrogen peroxide production during experimental protein glycation. FEBS Lett. 1990, 268, 69–71. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| p-Values, Saw Logs | |||||||
|---|---|---|---|---|---|---|---|
| Factor | Stilbene Glycosides | Stilbene Aglycones | CTs HPLC | CTs UV | ORAC | FRAP | SCAV |
| Storage time | 0.003 | 0.007 | 0.005 | 0.000 | 0.001 | 0.000 | 0.031 |
| Bark layer | n.d. | n.d. | 0.000 | 0.000 | 0.001 | 0.001 | 0.025 |
| Season | 0.009 | 0.010 | 0.140 | 0.052 | 0.526 | 0.386 | 0.275 |
| Storage time × Bark layer | n.d. | n.d. | 0.001 | 0.003 | 0.145 | 0.002 | 0.465 |
| Storage time × Season | n.d. | n.d. | 0.061 | 0.152 | 0.089 | 0.006 | 0.737 |
| Bark layer × Season | n.d. | n.d. | 0.003 | 0.001 | 0.177 | 0.287 | 0.543 |
| Storage time × Bark layer × Season | n.d. | n.d. | 0.021 | 0.030 | 0.730 | 0.399 | 0.563 |
| Season | Time Weeks | Sample | DP 1 | PC 2 (%) | PD 3 (%) | A-type 4 (%) |
|---|---|---|---|---|---|---|
| W | 0 | IB | 8.2 ± 0.7 | 100.0 | n.d. | n.d. |
| W | 2 | IB | 8.7 ± 0.8 | 100.0 | n.d. | n.d. |
| W | 4 | IB | 7.4 ± 1.0 | 99.9 | 0.2 | n.d. |
| W | 12 | IB | 8.0 ± 1.8 | 100.0 | n.d. | n.d. |
| W | 24 | IB | 8.3 ± 2.8 | 95.5 | 4.5 | n.d. |
| W | 0 | OB | 6.1 ± 0.4 | 83.4 | 16.6 | 1.5 |
| W | 2 | OB | 6.4 ± 0.4 | 90.5 | 9.5 | n.d. |
| W | 4 | OB | 6.2 ± 0.8 | 83.8 | 16.2 | n.d. |
| W | 12 | OB | 6.9 ± 1.8 | 84.8 | 14.2 | n.d. |
| W | 24 | OB | 6.6 ± 0.4 | 86.0 | 14.1 | 1.3 |
| S | 0 | IB | 7.1 ± 0.1 | 100.0 | n.d. | 2.8 |
| S | 2 | IB | 5.6 ± 0.6 | 99.2 | 1.6 | 5.6 |
| S | 4 | IB | 6.2 ± 0.7 | 100.0 | n.d. | 3.6 |
| S | 12 | IB | 8.0 ± 0.4 | 97.1 | 2.9 | 2.2 |
| S | 24 | IB | 6.2 ± 0.3 | 95.2 | 4.8 | n.d. |
| S | 0 | OB | 5.9 ± 0.4 | 93.6 | 6.5 | 2.6 |
| S | 2 | OB | 5.5 ± 0.6 | 91.2 | 8.8 | 2.7 |
| S | 4 | OB | 7.0 ± 0.2 | 80.4 | 19.7 | 1.6 |
| S | 12 | OB | 7.5 ± 0.5 | 84.1 | 16.0 | n.d. |
| S | 24 | OB | 6.7 ± 0.1 | 81.7 | 18.3 | n.d. |
| p-Values, Saw Logs | |||
|---|---|---|---|
| Factor | A | B | C |
| Storage time | 0.002 | 0.568 | 0.143 |
| Bark layer | 0.001 | 0.002 | 0.138 |
| Season | 0.337 | 0.141 | 0.257 |
| Storage time × Bark layer | 0.059 | 0.192 | 0.382 |
| Storage time × Season | 0.171 | 0.279 | 0.248 |
| Bark layer × Season | 0.186 | 0.540 | 0.350 |
| Storage time × Bark layer × Season | 0.452 | 0.225 | 0.257 |
| p-Values, Pulpwood | |||
|---|---|---|---|
| Factor | ORAC | FRAP | SCAV |
| Storage time | 0.000 | 0.000 | 0.018 |
| Bark layer | 0.323 | 0.225 | 0.006 |
| Season | 0.014 | 0.001 | 0.399 |
| Storage time × Bark layer | 0.644 | 0.357 | 0.412 |
| Storage time × Season | 0.001 | 0.546 | 0.052 |
| Bark layer × Season | 0.765 | 0.577 | 0.043 |
| Storage time × Bark layer × Season | 0.421 | 0.452 | 0.385 |
| Storage Sample | Sampling Date | Storage Time | Tree Age | Tree Height | D1.3 m | Log Length | Log Diameter | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Butt End | Middle | Top | ||||||||||||
| Number | Weeks | Years | dm | mm | mm | dm | mm | mm | mm | mm | mm | mm | ||
| Winter Storage | 3 | 7.2.2017 | 0 | 119 | 225 | 362 | 345 | 47 | 310 | 319 | 290 | 306 | 261 | 270 |
| 33 | 7.2.2017 | 0 | 96 | 223 | 321 | 272 | 46 | 273 | 255 | 242 | 249 | 215 | 227 | |
| 12 | 21.2.2017 | 2 | 95 | 221 | 282 | 291 | 45 | 257 | 275 | 235 | 251 | 221 | 223 | |
| 15 | 21.2.2017 | 2 | 110 | 222 | 323 | 345 | 46 | 302 | 291 | 293 | 277 | 255 | 243 | |
| 1 | 7.3.2017 | 4 | 97 | 210 | 301 | 299 | 47 | 260 | 258 | 234 | 238 | 216 | 219 | |
| 2 | 7.3.2017 | 4 | 94 | 215 | 280 | 277 | 46 | 282 | 279 | 257 | 263 | 235 | 232 | |
| 30 | 2.5.2017 | 12 | 73 | 206 | 260 | 263 | 46 | 234 | 226 | 204 | 207 | 191 | 185 | |
| 34 | 2.5.2017 | 12 | 56 | 213 | 362 | 362 | 45 | 317 | 305 | 282 | 290 | 254 | 262 | |
| 14 | 25.7.2017 | 24 | 96 | 224 | 323 | 341 | 46 | 264 | 269 | 238 | 244 | 207 | 210 | |
| 31 | 25.7.2017 | 24 | 78 | 256 | 305 | 299 | 46 | 282 | 281 | 256 | 261 | 247 | 240 | |
| Summer Storage | 41 | 30.5.2017 | 0 | 67 | 243 | 307 | 310 | 48 | 279 | 272 | 262 | 254 | 243 | 237 |
| 51 | 30.5.2017 | 0 | 70 | 245 | 362 | 357 | 44 | 310 | 311 | 300 | 291 | 292 | 289 | |
| 47 | 12.6.2017 | 2 | 84 | 259 | 360 | 345 | 44 | 300 | 292 | 287 | 285 | 273 | 265 | |
| 50 | 12.6.2017 | 2 | 108 | 233 | 317 | 327 | 47 | 272 | 287 | 251 | 249 | 232 | 219 | |
| 44 | 26.6.2017 | 4 | 100 | 270 | 392 | 399 | 43 | 350 | 342 | 324 | 326 | 313 | 305 | |
| 49 | 26.6.2017 | 4 | 95 | 255 | 360 | 364 | 49 | 303 | 310 | 281 | 284 | 254 | 256 | |
| 45 | 22.8.2017 | 12 | 94 | 260 | 326 | 326 | 47 | 284 | 290 | 274 | 267 | 290 | 246 | |
| 46 | 22.8.2017 | 12 | 58 | 225 | 304 | 300 | 48 | 256 | 239 | 225 | 219 | 206 | 199 | |
| 42 | 13.11.2017 | 24 | 89 | 262 | 359 | 358 | 46 | 329 | 318 | 316 | 305 | 290 | 284 | |
| 43 | 13.11.2017 | 24 | 93 | 252 | 285 | 278 | 48 | 271 | 260 | 246 | 242 | 226 | 224 | |
| Storage Sample | Sampling Date | Storage Time | Tree Age | Tree Height | D1.3 m | Log Length | Log Diameter | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Butt End | Middle | Top | ||||||||||||
| Number | Weeks | Years | dm | mm | mm | dm | mm | mm | mm | mm | mm | mm | ||
| Winter Storage | 22 | 7.2.2017 | 0 | 69 | 114 | 121 | 124 | 46 | 133 | 143 | 113 | 113 | 98 | 97 |
| 23 | 7.2.2017 | 0 | 65 | 124 | 127 | 131 | 50 | 136 | 146 | 119 | 125 | 102 | 103 | |
| 8 | 21.2.2017 | 2 | 103 | 114 | 132 | 125 | 48 | 152 | 145 | 122 | 120 | 97 | 97 | |
| 29 | 21.2.2017 | 2 | 38 | 129 | 151 | 145 | 50 | 153 | 160 | 145 | 136 | 110 | 110 | |
| 10 | 7.3.2017 | 4 | 86 | 142 | 155 | 153 | 50 | 177 | 175 | 149 | 152 | 130 | 125 | |
| 16 | 7.3.2017 | 4 | 82 | 125 | 126 | 129 | 51 | 150 | 150 | 120 | 122 | 103 | 103 | |
| 18 | 2.5.2017 | 12 | 81 | 121 | 129 | 121 | 51 | 180 | 157 | 120 | 115 | 100 | 109 | |
| 25 | 2.5.2017 | 12 | 50 * | 130 | 130 | 137 | 53 | 153 | 155 | 130 | 129 | 104 | 108 | |
| 4 | 25.7.2017 | 24 | 87 | 143 | 139 | 140 | 48 | 150 | 153 | 133 | 131 | 118 | 118 | |
| 27 | 25.7.2017 | 24 | 49 | 114 | 136 | 137 | 51 | 143 | 144 | 126 | 129 | 105 | 101 | |
| Summer Storage | 54 | 30.5.2017 | 0 | 90 | 117 | 147 | 134 | 51 | 165 | 195 | 134 | 137 | 106 | 109 |
| 56 | 30.5.2017 | 0 | 87 | 112 | 119 | 131 | 52 | 141 | 145 | 121 | 120 | 93 | 92 | |
| 58 | 12.6.2017 | 2 | 62 | 121 | 125 | 119 | 51 | 143 | 140 | 113 | 108 | 90 | 91 | |
| 59 | 12.6.2017 | 2 | 55 | 100 | 124 | 124 | 52 | 144 | 143 | 112 | 109 | 75 | 79 | |
| 55 | 26.6.2017 | 4 | 85 | 130 | 157 | 155 | 55 | 184 | 184 | 154 | 143 | 126 | 121 | |
| 61 | 26.6.2017 | 4 | 70 | 128 | 145 | 137 | 53 | 132 | 136 | 119 | 120 | 98 | 96 | |
| 53 | 22.8.2017 | 12 | 70 | 127 | 164 | 162 | 51 | 187 | 199 | 159 | 157 | 135 | 136 | |
| 60 | 22.8.2017 | 12 | 56 | 127 | 147 | 144 | 47 | 186 | 176 | 139 | 143 | 124 | 124 | |
| 52 | 13.11.2017 | 24 | 79 | 134 | 158 | 170 | 51 | 202 | 193 | 157 | 150 | 132 | 135 | |
| 57 | 13.11.2017 | 24 | 98 | 153 | 145 | 144 | 51 | 165 | 163 | 140 | 130 | 124 | 115 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jyske, T.; Brännström, H.; Sarjala, T.; Hellström, J.; Halmemies, E.; Raitanen, J.-E.; Kaseva, J.; Lagerquist, L.; Eklund, P.; Nurmi, J. Fate of Antioxidative Compounds within Bark during Storage: A Case of Norway Spruce Logs. Molecules 2020, 25, 4228. https://doi.org/10.3390/molecules25184228
Jyske T, Brännström H, Sarjala T, Hellström J, Halmemies E, Raitanen J-E, Kaseva J, Lagerquist L, Eklund P, Nurmi J. Fate of Antioxidative Compounds within Bark during Storage: A Case of Norway Spruce Logs. Molecules. 2020; 25(18):4228. https://doi.org/10.3390/molecules25184228
Chicago/Turabian StyleJyske, Tuula, Hanna Brännström, Tytti Sarjala, Jarkko Hellström, Eelis Halmemies, Jan-Erik Raitanen, Janne Kaseva, Lucas Lagerquist, Patrik Eklund, and Juha Nurmi. 2020. "Fate of Antioxidative Compounds within Bark during Storage: A Case of Norway Spruce Logs" Molecules 25, no. 18: 4228. https://doi.org/10.3390/molecules25184228
APA StyleJyske, T., Brännström, H., Sarjala, T., Hellström, J., Halmemies, E., Raitanen, J.-E., Kaseva, J., Lagerquist, L., Eklund, P., & Nurmi, J. (2020). Fate of Antioxidative Compounds within Bark during Storage: A Case of Norway Spruce Logs. Molecules, 25(18), 4228. https://doi.org/10.3390/molecules25184228






