Geopolymer Carbon-Based for Ultra-Wideband Absorbent Applications
Abstract
1. Introduction
2. Results
2.1. Surfactant Impact
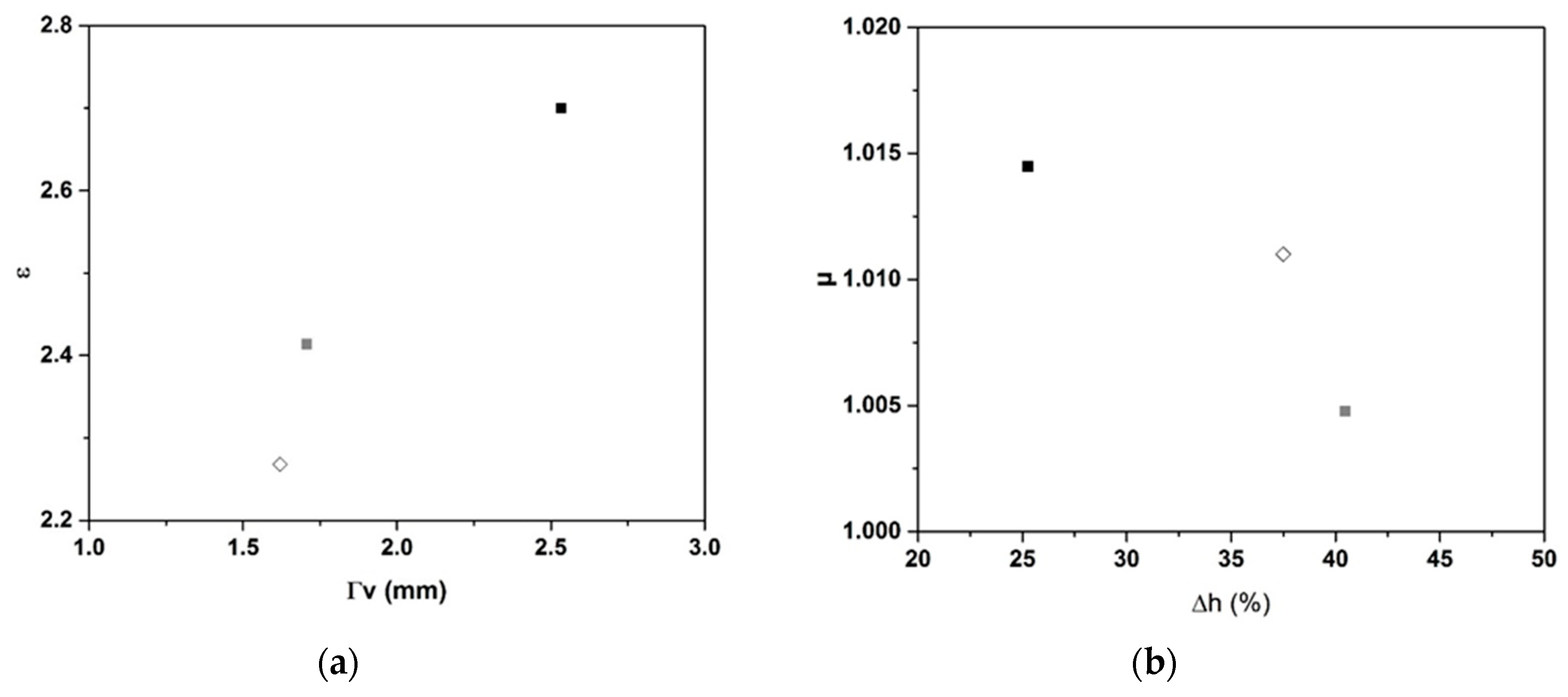
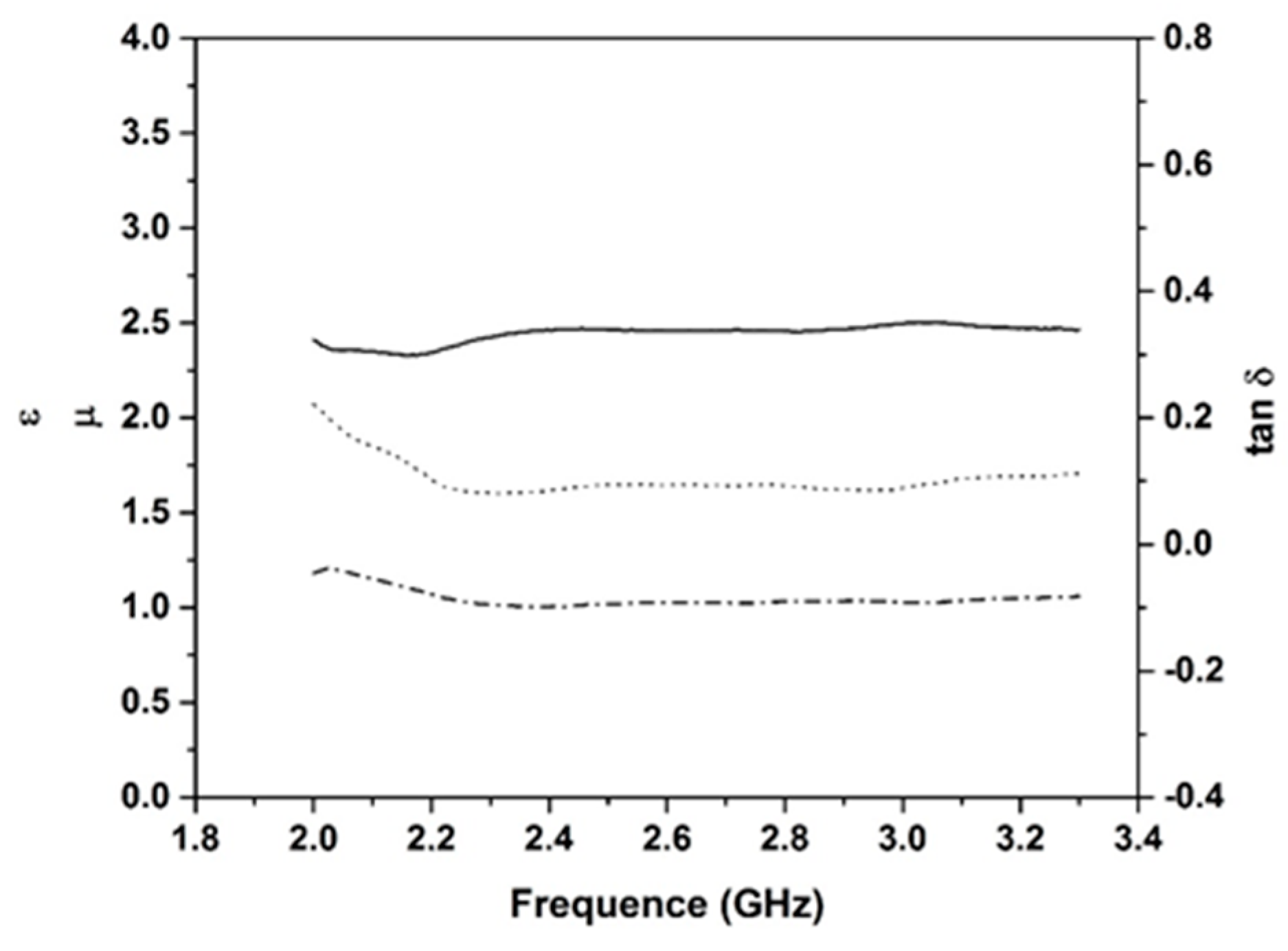
2.2. Dielectric Properties
3. Discussion
4. Materials and Methods
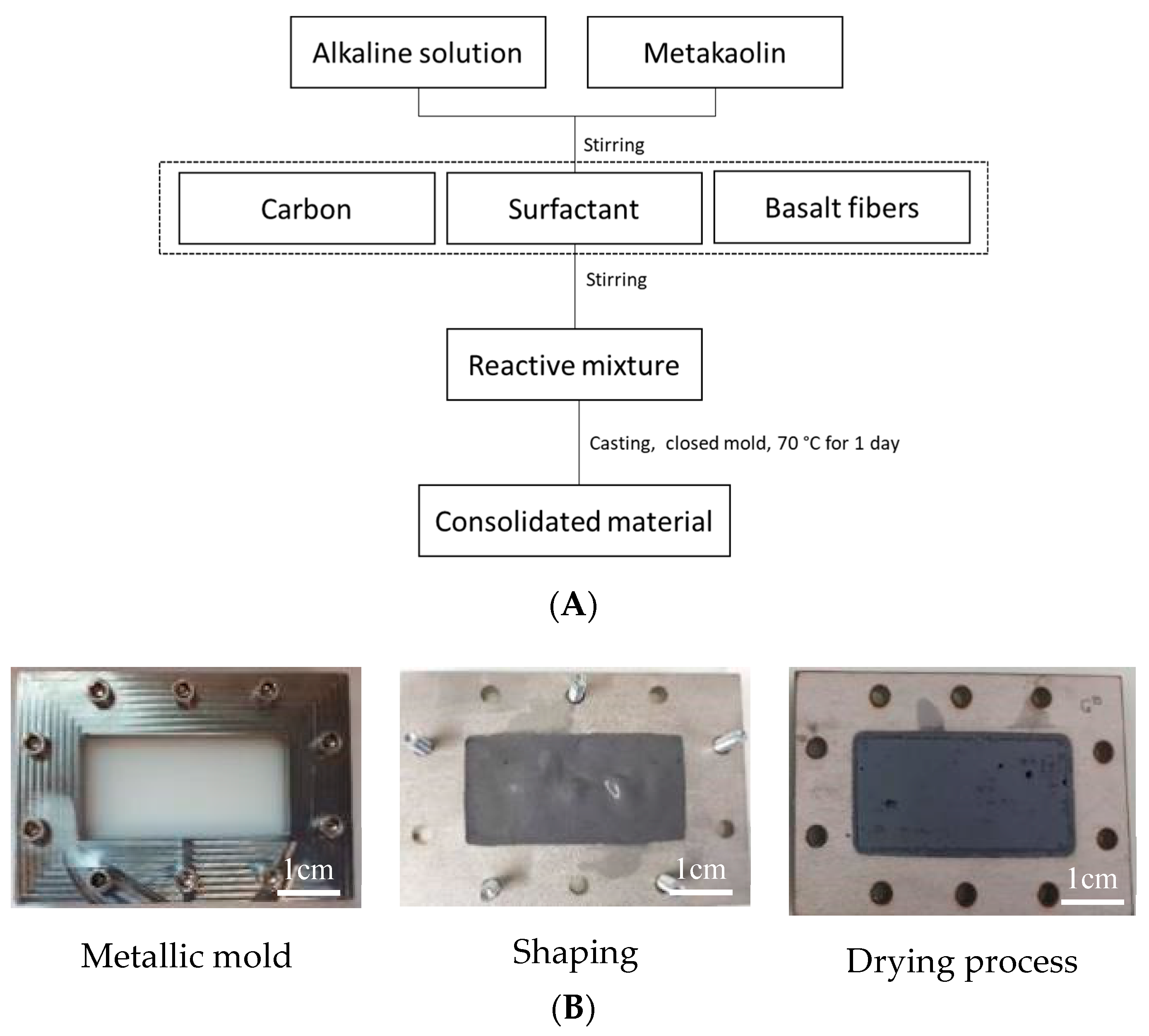
4.1. Materials and Synthesis
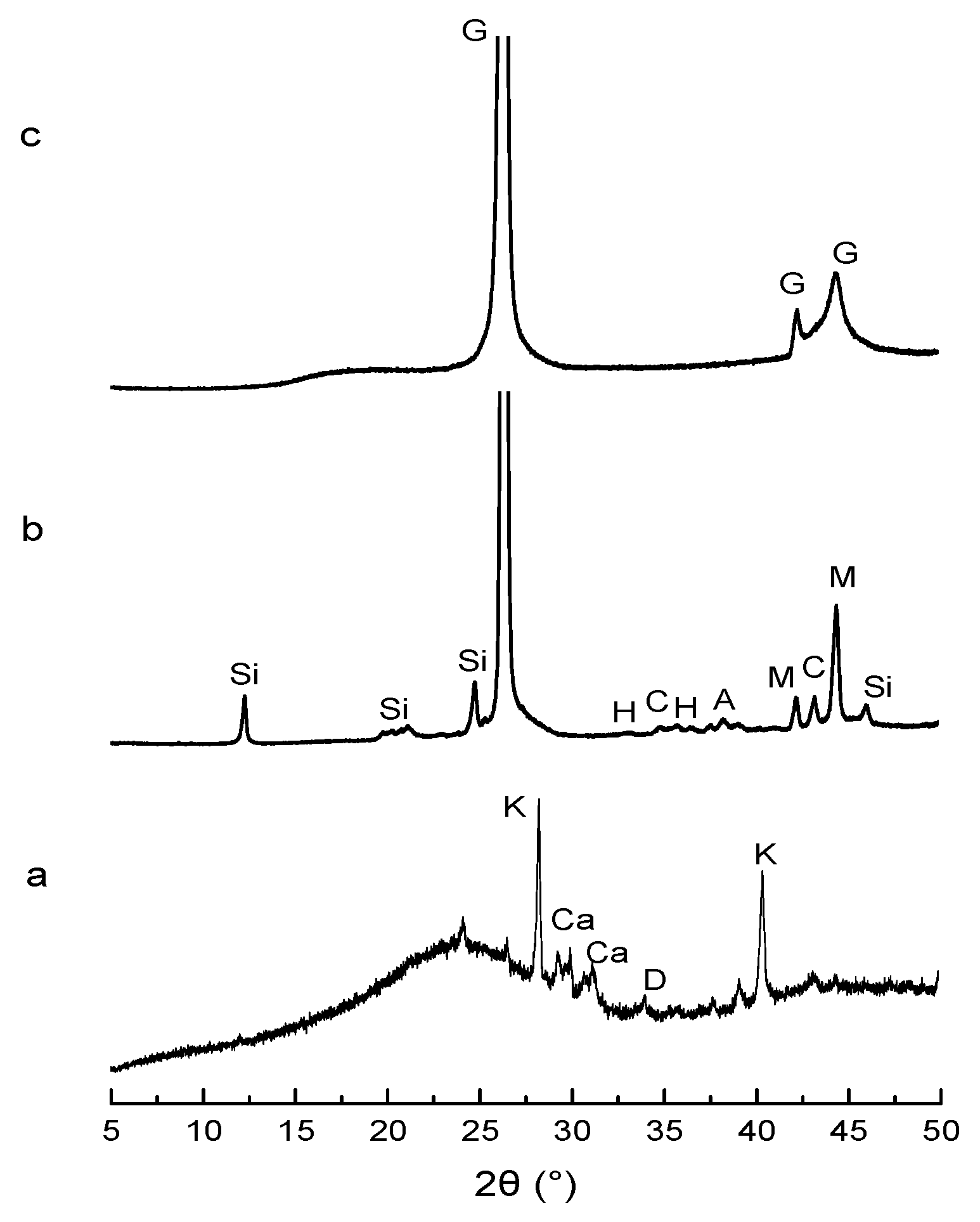
4.2. Technical Characterization
5. Conclusions
- Surfactant addition induces the volume expansion owing to a change in interfacial strength. However, the nonionic surfactant was preferred over the anionic surfactant, thanks to its performance on the volume expansion at lower concentrations.
- Dielectric investigations reveal an increase of permittivity with increasing carbon content, for example, ε = 2.27 and tan δ value of 0.19.
- The addition of magnetite reveals only a minor impact on the samples magnetic properties.
- An increase is observed with increasing humidity, but a reversible behavior is observed when the humidity is decreased.
Author Contributions
Funding
Conflicts of Interest
References
- Abbas, S.M.; Chandra, M.; Verma, A.; Chatterjee, R.; Goel, T.C. Complex permittivity and microwave absorption properties of a composite dielectric absorber. Compos. Part A Appl. Sci. Manuf. 2006, 37, 2148–2154. [Google Scholar] [CrossRef]
- Kanapitsas, A.; Pissis, P.; Karabanova, L.; Sergeeva, L.; Apekis, L. Broadband dielectric relaxation spectroscopy in interpenetrating polymer networks of polyurethane-copolymer of butyl methacrylate and dimethacrylate triethylene glycol. Polym. Gels Netw. 1998, 6, 83–102. [Google Scholar] [CrossRef]
- Kumar, R.S.; Narayanan, T.N.; Reena, M.; Anantharaman, M.; Srivastava, A.; Vajtai, R.; Ajayan, P.M. Ni filled flexible multi-walled carbon nanotube-polystyrene composite films as efficient microwave absorbers. Appl. Phys. Lett. 2011, 99, 113–116. [Google Scholar]
- Nornikman, H.; Soh, P.J.; Azremi, A.A.H.; Anuar, M. Performance simulation of pyramidal and wedge microwave absorbers. In Proceedings of the Third Asia International Conference on Modelling and Simulation, AMS’09 (IEEE, 2009), Bandung, Bali, Indonesia, 25–29 May 2009; pp. 649–654. [Google Scholar]
- Emerson, W. Electromagnetic wave absorbers and anechoic chambers through the years. IEEE Trans. Antennas Propag. 1973, 21, 484–490. [Google Scholar] [CrossRef]
- Simmons, A.; Emerson, W. An anechoic chamber making use of a new broadband absorbing material, 1958. IRE Int. Conv. Rec. 1966, 1, 34–41. [Google Scholar] [CrossRef]
- Feng, Y.B.; Qiu, T.; Shen, C.Y. Absorbing properties and structural design of microwave absorbers based on carbonyl iron and barium ferrite. J. Magn. Magn. Mater. 2007, 318, 8–13. [Google Scholar] [CrossRef]
- Shin, J.Y.; Oh, J.H. The microwave absorbing phenomena of ferrite microwave absorber. IEEE Trans. Magn. 1993, 29, 3437–3439. [Google Scholar] [CrossRef]
- Qin, F.; Brosseau, C. A review and analysis of microwave absorption in polymer composites filled with carbonaceous particles. J. Appl. Phys. 2012, 111, 061301. [Google Scholar] [CrossRef]
- Huang, S.; Zhou, W.; Luo, F.; Ping, W.; Dongmei, Z. Mechanical and dielectric properties of short carbon fiber reinforced Al2O3, composites with MgO additive. J. Ceram. Int. 2014, 40, 2785–2791. [Google Scholar] [CrossRef]
- Godlewska, P.; Schmidt, H.P.; Ok, Y.S.; Oleszczuk, P. Biochar for composting improvement and contaminants reduction. A review. Bioresour. Technol. 2017, 246, 193–202. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Applications, 4th ed.; Institute Géopolymère: Saint-Quentin, France, 2015. [Google Scholar]
- Prud’homme, E.; Michaud, P.; Joussein, E.; Clacens, J.M.; Rossignol, S. Role of alkaline cations and water content on geomaterial foams: Monitoring during formation. J. Non Cryst Solids 2011, 357, 1270–1278. [Google Scholar] [CrossRef]
- Gharzouni, A.; Joussein, E.; Samet, B.; Baklouti, S.; Rossignol, S. Effect of the reactivity of alkaline solution and metakaolin on geopolymer formation. J. Non Cryst. Solids. 2015, 410, 127–134. [Google Scholar] [CrossRef]
- Israelachvili, J. Intermolecular and Surface Forces; Academic Press: New York, NY, USA, 1991. [Google Scholar]
- Cherifa, Z. Micellisation d’un Système Modèle Tensioactif Mixte Cationique/Non Ionique. Ph.D. Thesis, University of Oran, Oran, Algeria, 6 April 2008. [Google Scholar]
- Ping, J.; Zhang, L.; Tang, D.; Longjie, L.; Jijiang, G.; Guicai, Z.; Haihua, P. Effect of nano-SiO2 and surfactants on the oil-water interfacial properties. Colloid Polym. Sci. 2019, 297, 903–915. [Google Scholar]
- Sett, S.; Karakashev, S.I.; Smoukov, S.K.; Yarin, A.L. Ion-specific effects in foams. Adv. Colloid Interface Sci. 2015, 225, 98–113. [Google Scholar] [CrossRef]
- Petkova, B.; Tcholakova, S.; Chenkova, M.; Golemanov, K.; Denkov, N.; Thorley, D.; Stoyanov, S. Foamability of aqueous solutions: Role of surfactant type and concentration. Adv. Colloid Interface Sci. 2020, 276, 102084. [Google Scholar] [CrossRef]
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena, 4th ed.; Surfactant Research Institute Brooklyn College, The City University of New York: New York, NY, USA, 2012. [Google Scholar]
- Texter, J. Reactions and Synthesis in Surfactant Systems; Strider Research Corporation Rochester: New York, NY, USA, 2001. [Google Scholar]
- Schick, M.J.; Manning, D.J. Micelle formation in mixtures of nonionic and anionic detergents. J. Am. Oil Chem. Soc. 1966, 43, 133–136. [Google Scholar] [CrossRef]
- Lu, Y.; Qin, B. Experimental investigation of closed porosity of inorganic solidified foam designed to prevent coal fires. Hindawi Publ. Corp. Adv. Mater. Sci. Eng. 2015. [Google Scholar] [CrossRef]
- Zhou, W.; Yan, W.; Li, N.; Li, Y.; Dai, Y.; Han, B.; Wei, Y. Preparation and characterization of mullite foam ceramics with porous struts from white clay and industrial alumina. Ceram. Int. 2018, 44, 22950–22956. [Google Scholar] [CrossRef]
- Fernandez, E.; Benito, J.M.; Pazos, C.; Coca, J. Ceramic membrane ultrafiltration of anionic and nonionic surfactant solutions. J. Membr. Sci. 2005, 246, 1–6. [Google Scholar] [CrossRef]
- Cilla, M.S.; Colombo, P.; Morelli, M.R. Geopolymer foams by gel casting. Ceram. Int. 2014, 40, 5723–5730. [Google Scholar] [CrossRef]
- ImageJ Is a Java-Based Image Processing Program Developed at the National Institutes of Health and the Laboratory for Optical and Computational Instrumentation. Available online: https://imagej.nih.gov/ij (accessed on 16 June 2020).
- Korat, L.; Ducman, V. The influence of the stabilizing agent SDS on porosity development in alkali-activated fly-ash based foams. Cem. Concr. Compos. 2017, 80, 168–174. [Google Scholar] [CrossRef]
- Oliveira, C.; Rodrigues, R.T.; Rubio, J. Nucleation, growth and coalescence phenomena of air bubbles on quartz particles in different aqueous solutions bubble science. Eng. Technol. 2014, 5, 15–24. [Google Scholar]
- Findenegg, G.H.; Pasucha, B.; Strunk, H. Adsorption of non-ionic surfactants from aqueous solutions on graphite: Adsorption isotherms and calorimetric enthalpies of displacement for C8E4 and related compounds. Colloids Surf. 1989, 37, 223–233. [Google Scholar] [CrossRef]
- Esumi, K.; Ueno, M. Structure-Performance Relationships in Surfactants; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Farges, R.; Gharzouni, A.; Ravier, B.; Jeulin, P.; Rossignol, S. Insulating foams and dense geopolymers from biochar by-products. J. Ceram. Sci. Technol. 2018, 9, 193–200. [Google Scholar]
- Calberg, C.; Blacherd, S.; Gubbels, F.; Brouersd, F.; Deltour, R.; Jérôme, R. Electrical and dielectric properties of carbon black filled co-continuous two-phase polymer blends. J. Phys. D Appl. Phys. 1999, 32, 1517–1525. [Google Scholar] [CrossRef]
- Huanqin, Z.; Zhu, J.; Seow, Y.; Cheng, Y.; Zhichuan, J.X.; Guangbin, J. Green synthesis of hierarchically porous carbons with tunable dielectric response for microwave absorption. Ceram. Int. 2020, 46, 15447–15455. [Google Scholar]
- Landolt, H.; Börnstein, R.; Landolt, H. Numerical Data and Functional Relationships in Science and Technology; Springer: Berlin, Germany, 1986. [Google Scholar]
- Nees, P.; Sreedevi, C.P.; Umadevi, K.S.; Sikha, S.K.; Kizhakooden, J.; Jolly, A.; Joseph, V.P. Humidity sensitive flexible microwave absorbing sheet using polyaniline–polytetrafluoroethylene Composite. Arab. J. Sci. Eng. 2019, 44, 553–560. [Google Scholar]
- Vlasceanu, I.N.; Gharzouni, A.; Tantot, O.; Lalande, M.; Elissalde, C.; Rossignol, S. Geopolymer as dielectric materials for ultra-wideband antenna applications: Impact of magnetite addition and humidity. Open Ceram. 2020, 2, 100013. [Google Scholar] [CrossRef]
- Zhang, Y.; He, P.; Yuan, J.; Yang, C.; Jia, D.; Zhou, Y. Effects of graphite on the mechanical and microwave absorption properties of geopolymer based composites. Ceram. Int. 2017, 43, 2325–2332. [Google Scholar] [CrossRef]
- Hotta, M.; Hayashi, M.; Nagata, K. High temperature measurement of complex permittivity and permeability of Fe3O4 powders in the frequency range of 0.2 to 13.5 GHz. ISIJ Int. 2011, 51, 491–497. [Google Scholar] [CrossRef]
- Hotta, M.; Hayashi, M.; Nishikata, A.; Nagata, K. Complex permittivity and permeability of SiO2 and Fe3O4 powders in microwave frequency range between 0.2 and 13.5 GHz. ISIJ Int. 2009, 49, 1443–1448. [Google Scholar] [CrossRef]
- Cui, X.M.; Zheng, G.J.; Han, Y.C.; Su, F.; Zhou, J. A study on electrical conductivity of chemosynthetic Al2O3–2SiO2 geoploymer materials. J. Power Source 2008, 184, 652–656. [Google Scholar] [CrossRef]
- Cerovic, D.; Asanovic, K.A.; Maletic, S.B.; Dojcilovic, J.R. Comparative study of the electrical and structural properties of woven fabrics. Composites Part B 2013, 49, 65–70. [Google Scholar] [CrossRef]
- Li, Z.; Jin, Z.; Shao, S.; Zhao, T.; Wang, P. Influence of moisture content on electromagnetic response of concrete studied using a homemade apparatus. Sensors 2019, 19, 4637. [Google Scholar] [CrossRef] [PubMed]
- Malkawi, A.B.; Al-Mattarneh, H.; Achara, B.E.; Muhammed, B.S.; Nuruddin, M.F. Dielectric properties for characterization of fly ash-based geopolymerbinders. Constr. Build Mater. 2018, 189, 19–32. [Google Scholar] [CrossRef]
- Ahmad, Z. Polymer Dielectric Materials; IntechOpen: London, UK, 2012. [Google Scholar]
- He, P.; Jia, L.; Ma, G.; Wang, R.; Yuan, J.; Duan, X.; Yang, Z.; Jia, D. Effects of fiber contents on the mechanical and microwave absorbent properties of carbon fiber felt reinforced geopolymer composites. Ceram. Int. 2018, 44, 10726–10734. [Google Scholar] [CrossRef]
- Han, K.H.; Talyzin, A.; Dzwilewski, A.; Makarova, T.L.; Höhne, R.; Esquinazi, P.; Spemann, D.; Dubrovinsky, L.S. Magnetic properties of carbon phases synthesized using high pressure-high temperature treatment. Phys. Rev. B 2005, 72, 224424. [Google Scholar] [CrossRef]
- Gopalakrishnan, R.; Barathan, S.; Govindarajan, D. Magnetic Susceptibility Measurements on Fly Ash Admixtured Cement Hydrated with Groundwater and Seawater. J. Mater. Sci. 2012, 2, 32–36. [Google Scholar] [CrossRef]
- Jiles, D.C.; Owen, C.V.; Spitzig, W.A. Magnetic properties of porous iron compacts. NDT E Int. 1987, 6, 119–127. [Google Scholar] [CrossRef]
- Cadle, R.D. Particle Size Theory and Industrial Applications; Reinhold Publishing Corp.: New York, NY, USA, 1965. [Google Scholar]
- Allen, T. Particle Size Measurement, 3rd ed.; Springer: Berlin, Germany, 1981. [Google Scholar]
- Henon, J. Elaboration de matériaux poreux géopolymères à porosité multi-échelle et contrôlée. Ph.D. Thesis, University of Limoges, Limoges, France, 11 July 2012. [Google Scholar]
- Nicolson, A.M.; Ross, G.F. Measurement of the intrinsic properties of materials by time domain techniques. IEEE Trans. Instrum. Meas. 1970, 19, 377–382. [Google Scholar] [CrossRef]
- Weir, W.B. Automatic measurement of complex dielectric constant and permeability at microwave frequencies. Proc. IEEE 1974, 62, 33–36. [Google Scholar] [CrossRef]
- Lemaire, D.; Cros, D.; Jallageas, H.; Guillon, P. Material characterisation from-160/spl deg/C up to 800/spl deg/C in centimeter and millimeter wavelength frequency band. In Proceedings of the 20th Biennial Conference on Precision Electromagnetic Measurements, Braunschweig, Germany, 17–21 June 1996; pp. 72–73. [Google Scholar]
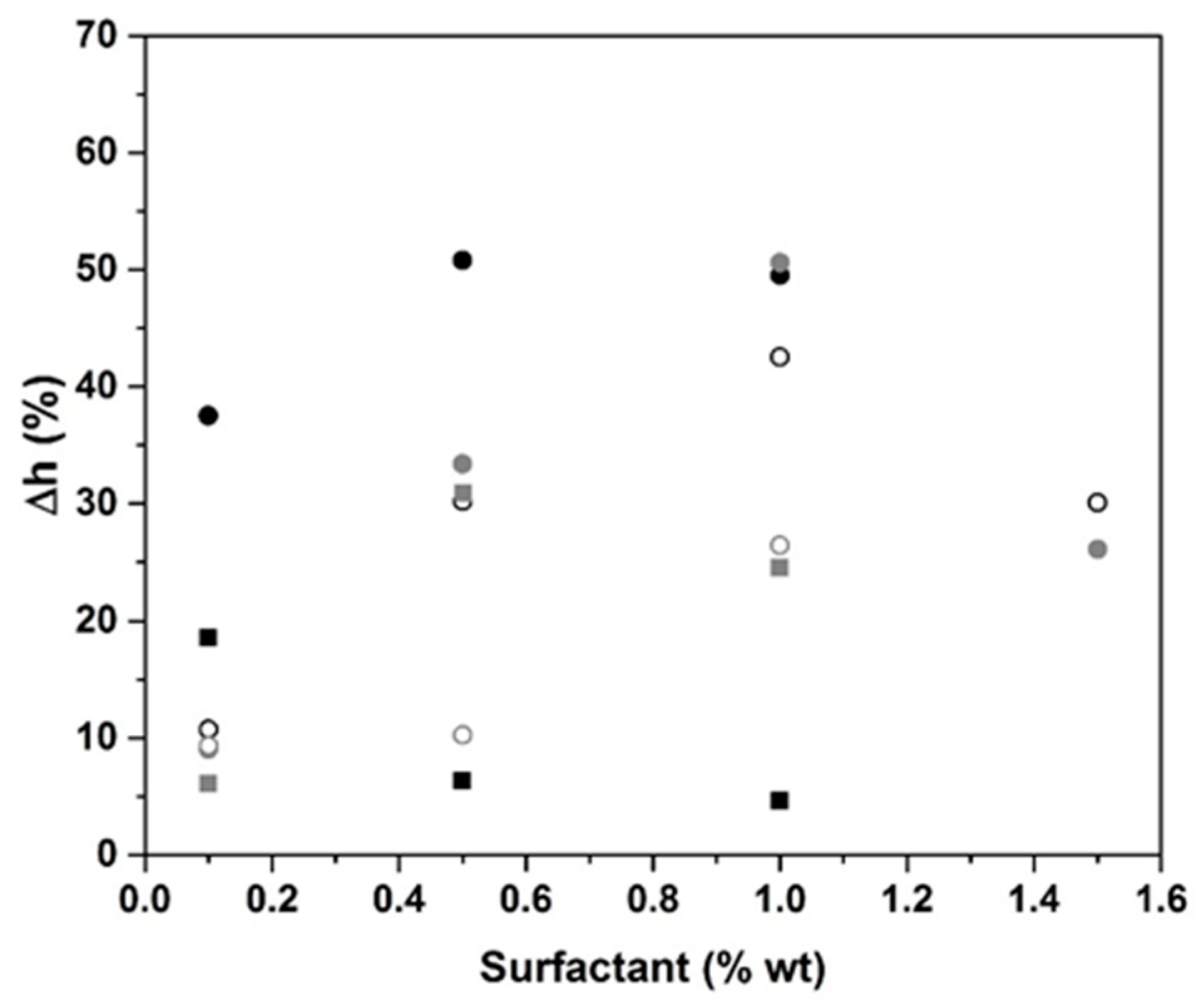
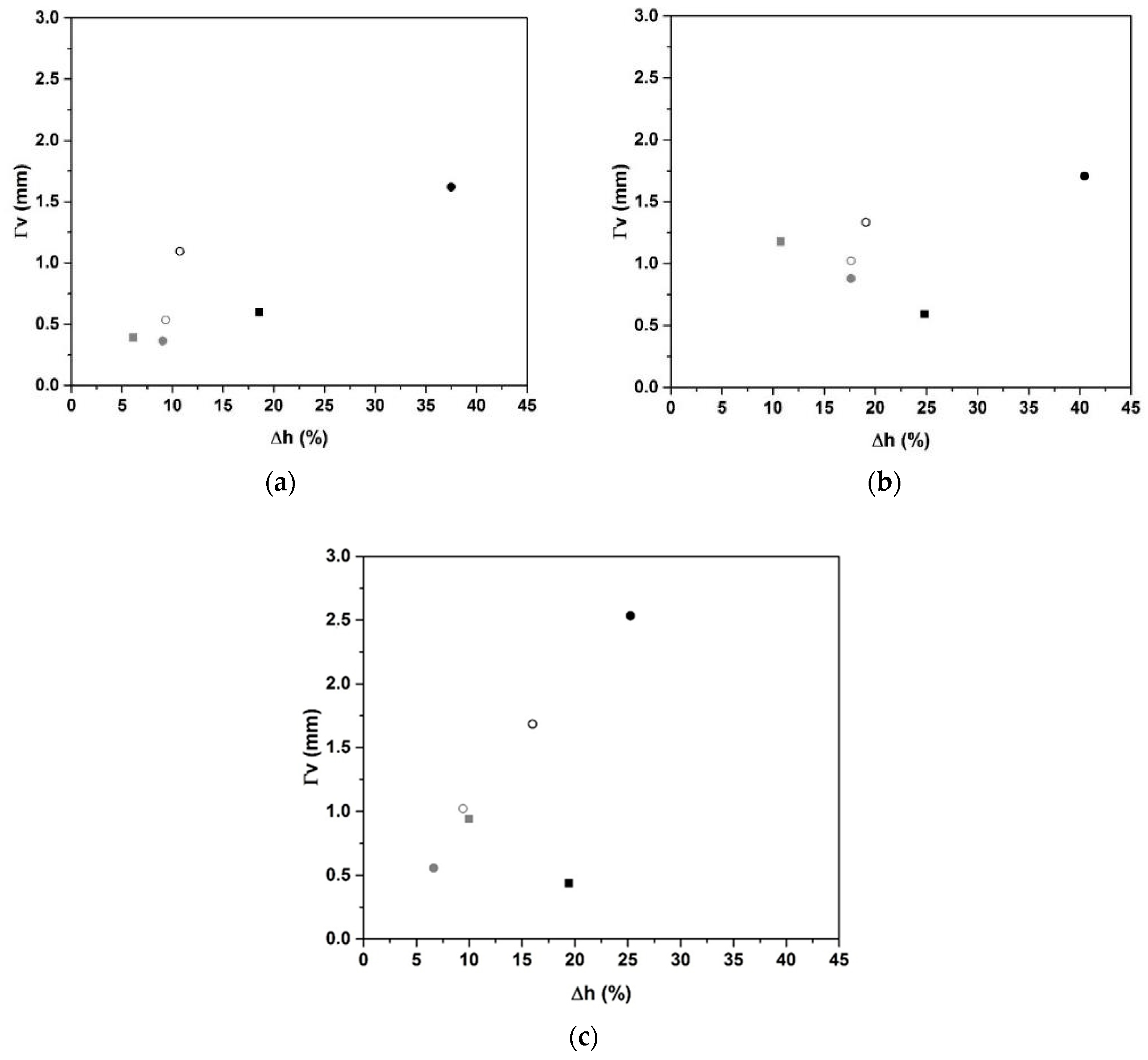


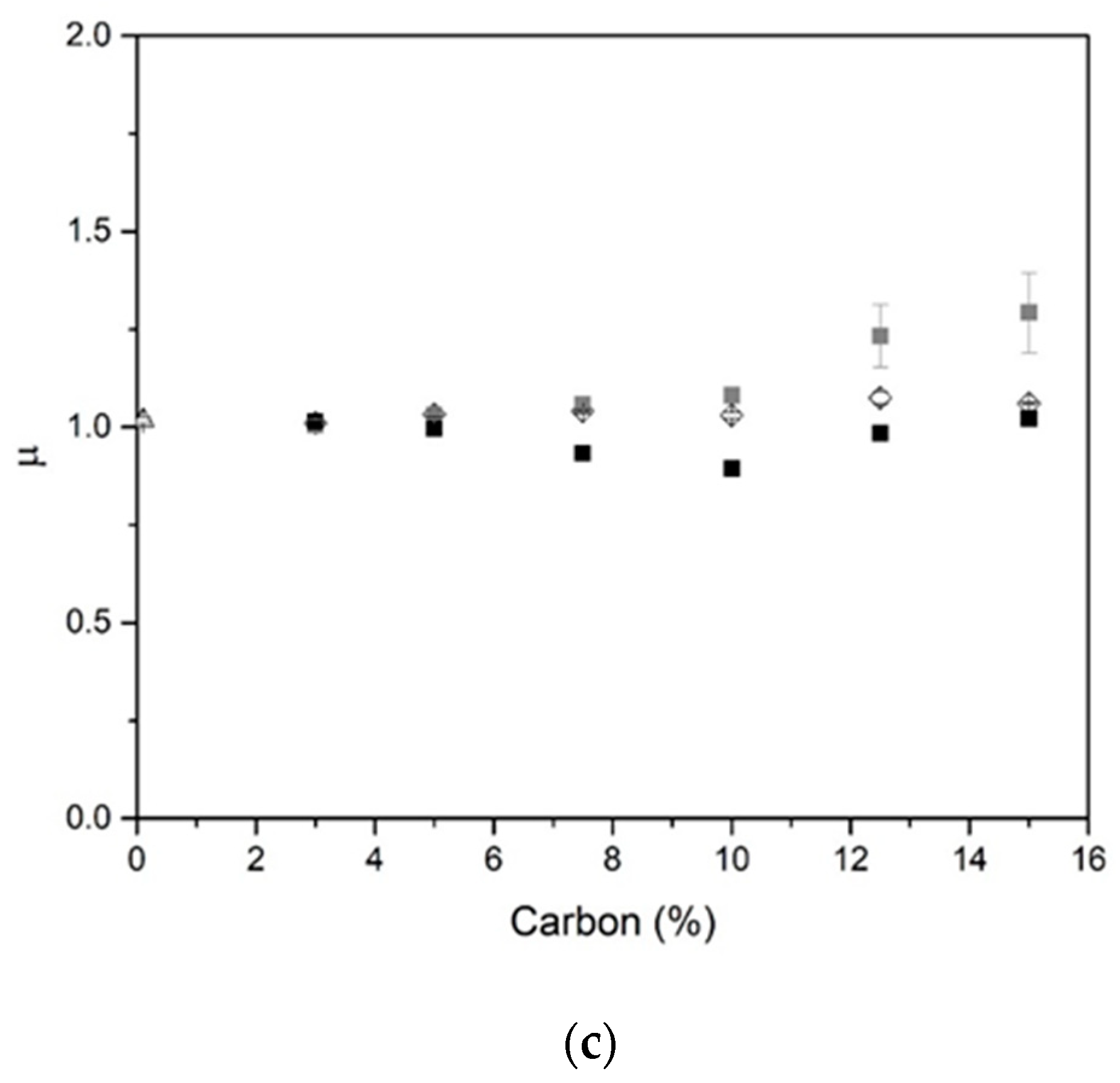
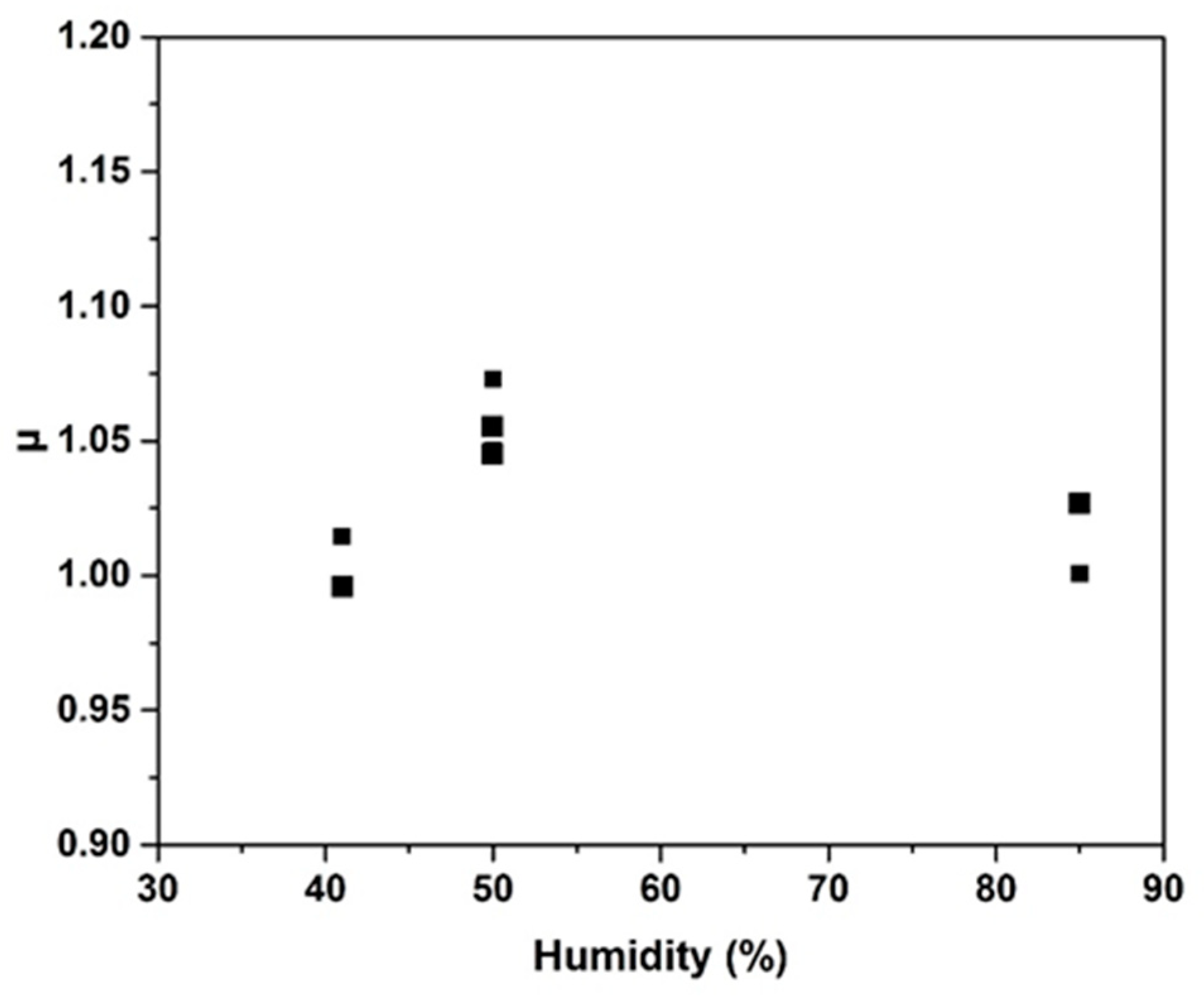

| Type of Surfactant | Percentage of Surfactant | Volume Expansion (%) | Pore Size (mm) | ||||
|---|---|---|---|---|---|---|---|
| Biochar | Graphite 75% | Graphite 99% | Biochar | Graphite 75% | Graphite 99% | ||
| BG | 0.1 | 38 | 40 | 25 | 1.6 | 1.7 | 2.5 |
| CG | 0.1 | 9 | 18 | 7 | 0.4 | 0.9 | 0.6 |
| APG | 0.1 | 11 | 19 | 16 | 1.0 | 1.3 | 1.7 |
| LQ | 0.1 | 9 | 18 | 9 | 0.5 | 1.0 | 1.0 |
| Tego | 0.1 | 19 | 25 | 19 | 0.6 | 0.6 | 0.4 |
| H66 | 0.1 | 6 | 11 | 10 | 0.4 | 1.2 | 0.9 |
| Name of Surfactant | Internal Morphology | ||
|---|---|---|---|
| Biochar | Graphite 75 | Graphite 99 | |
| BG |  |  |  |
| CG |  |  |  |
| Tego |  |  |  |
| H66 |  |  |  |
| Type of Carbon | Humidity (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 50 (during 12 days) | 85 (4 days) | 50 (3 days) | |||||||
| ε | tan δ | µ | ε | tan δ | µ | ε | tan δ | µ | |
| Biochar | 2.7 | 0.13 | 1.04 | 4.27 | 0.37 | 1.04 | 2.75 | 0.16 | 1.09 |
| Graphite 75 | 2.47 | 0.09 | 1.00 | 3.82 | 0.28 | 0.98 | 2.50 | 0.11 | 1.03 |
| Graphite 99 | 2.87 | 0.12 | 1.01 | 4.76 | 0.35 | 1.00 | 2.95 | 0.15 | 1.07 |
| Carbon Type | Supplier | Carbon (%) | Particle Size (µm) | Impurities (%) |
|---|---|---|---|---|
| Biochar | Maillot | 81 | 4–119 | 19 |
| Graphite 75 | Alfa Aesar | 75 | 45 | 25 |
| Graphite 99 | 99 | 45 | 1 |
| Name of Surfactant | Supplier | Type | pH | Density (g/cm3) | CMC (ppm at 25 °C) |
|---|---|---|---|---|---|
| TRITON™ BG-10 (BG) | Dow | nonionic | 7.6 | 1.152 | 1591 |
| TRITON™ CG-110 (CG) | nonionic | 5.7 | 1.150 | 1748 | |
| CAFLON APG C6 SMP (APG) | Univar B.V. | nonionic | 7–9 | 1.150–1.170 | * |
| SPAN™ 80-LQ-(RB) (LQ) | Croda | nonionic | * | * | * |
| TEGO® Dispers 653 (Tego) | Evonik | anionic | 8–9 | 1.075 | * |
| TRITON™ H-66 (H66) | Dow | anionic | 8.4 | 1.249 | * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlasceanu, I.N.; Gharzouni, A.; Tantot, O.; Martinod, E.; Rossignol, S. Geopolymer Carbon-Based for Ultra-Wideband Absorbent Applications. Molecules 2020, 25, 4218. https://doi.org/10.3390/molecules25184218
Vlasceanu IN, Gharzouni A, Tantot O, Martinod E, Rossignol S. Geopolymer Carbon-Based for Ultra-Wideband Absorbent Applications. Molecules. 2020; 25(18):4218. https://doi.org/10.3390/molecules25184218
Chicago/Turabian StyleVlasceanu, Ioana Nicoleta, Ameni Gharzouni, Olivier Tantot, Edson Martinod, and Sylvie Rossignol. 2020. "Geopolymer Carbon-Based for Ultra-Wideband Absorbent Applications" Molecules 25, no. 18: 4218. https://doi.org/10.3390/molecules25184218
APA StyleVlasceanu, I. N., Gharzouni, A., Tantot, O., Martinod, E., & Rossignol, S. (2020). Geopolymer Carbon-Based for Ultra-Wideband Absorbent Applications. Molecules, 25(18), 4218. https://doi.org/10.3390/molecules25184218







