1. Introduction
Steel slag is mainly composed of impurities in steelmaking charge, slagging materials (limestone, iron ore, dolomite, etc.), eroded furnace linings and oxides of many elements formed by oxidation in the furnace charge [
1,
2]. The output of steel slag accounts for about 15–20% of the total steel output [
3]. Mass production and accumulation of steel slag not only occupy a large area of valuable land resources but also cause some pollution and damage to the environment [
4]. The main mineral phase of steel slag is similar to cement, so it has certain potential hydraulic properties and is a potential resource [
2,
5]. However, the utilization rate of steel slag in China is relatively small at present, not more than 40% [
6]. The large-scale and high value-added application of steel slag is limited by the inherent factors of the properties of steel slag: first of all, the composition fluctuation of steel slag brings some difficulties to the stability control when it is used as a raw material for production [
7]; secondly, when steel slag is used as a raw material for building materials, because of its high volume expansion, grinding energy consumption and the hidden danger of poor stability, it cannot wholly replace cement [
4,
8,
9]. Improving the utilization rate of steel slag is an effective way to alleviate the load of steel slag on the land and environment, turn waste into treasure and produce certain economic and social benefits.
Steel slag has many applications, such as producing fertilizers, improving soil, treating sewage or as a sinter flux [
7,
10,
11], and it is mainly used as an aggregate or as a substitute for cement in the construction field. Steel slag has certain cementitious activity and is often used as a mineral admixture to replace part of cement. S. Kourounis et al. [
12] studied the effect of steel slag replacing part of cement on the properties and hydration of cementitious materials, and it was concluded that steel slag composite cement can reach the strength classes 42.5 and 32.5 of EN 197-1 and steel slag cement had excellent physical properties. Shekhar Saxena et al. [
13] used steel slag aggregate to replace the traditional basalt aggregate to prepare concrete, which had a denser microstructure and stronger durability. The compressive strength and flexural strength increased by 33% and 9.8%, respectively, and the elastic modulus increased by 22%. Hisham Qasrawi et al. [
14] used steel slag with high stability to replace part of ordinary river sand as an aggregate to prepare concrete. When the steel slag replaced was 15–30%, the 28d compressive strength of concrete increased by 1.1–1.3 times, and the tensile strength was increased by 1.4–2.4 times when the substitution amount was 30–50%. However, the concrete performance would be greatly reduced when the steel slag aggregate was too large. Nicola Faraone et al. [
15] found that the larger the particle size of steel slag sand within a certain range, the greater the compressive strength of the test block, and increasing the particle size of steel slag sand could improve the mechanical properties of the mortar test block prepared by steel slag sand. The use of steel slag as an aggregate in pavement could improve its bending resistance, wear resistance and durability, and improve the overall performance of the pavement. The research on steel slag is mainly focused on making a cementitious material as an admixture or fine aggregate, but research on using steel slag as a cementitious material and fine aggregate in concrete is rarely involved.
Using steel slag as a cementing material and fine aggregate in concrete is feasible. Steel slag powder contains f-CaO, active RO phase and other components, which will produce volume expansion in the process of a hydration reaction [
9,
16,
17,
18], thus compensating the shrinkage of concrete and reducing or even replacing the use of an expansion agent in concrete [
19]. Besides, steel slag sand has weak activity, and the interfacial reaction may occur in the alkaline environment, which may improve the interfacial connection properties. Moreover, compared with river sand, steel slag sand is rough and porous and can produce a “pin effect” [
20], further improving the bond strength with cementing materials.
Based on the large amount of steel slag heaped and discarded, and the practical problems of the large-scale and high value-added application of steel slag, it is of great significance to study using steel slag as a cementitious material and fine aggregate. In this paper, the hydration process of alkali-activated materials prepared by steel slag as a cementitious material and fine aggregate was investigated. The phase composition and micro-morphology of hydration products were analyzed by XRD, NMR and SEM, and then the hydration development process was discussed, revealing the corresponding relationship between microstructure and macroscopic properties. It would provide a theoretical basis for the large-scale and high value-added utilization of steel slag.
3. Experimental Materials and Test
3.1. Raw Material
Steel slag (basic oxygen steel slag) was produced by Wuhan Iron and Steel Group Co., Ltd. (Wuhan, China), the steel slag was broken, then the large particles were used as steel slag sand, and the small particles were made into steel slag powder (SS) by drying and grinding. Granulated blast furnace slag powder (BFS) was created by WISCO Green Metallurgical Slag Co., Ltd. (Wuhan, China), and the expansion agent (EA) came from Tianjin Baoming Company (Tianjin, China). The chemical composition of all raw powder is shown in
Table 4. The screening of steel slag sand and river sand is shown in
Table 5. The alkali activator is composed of 1 wt.% NaOH, 1 wt.% Na
2SO
4 and 4 wt.% sodium silicate.
3.2. Sample Preparation
The water/binder ratio is controlled to 0.3 by adding 0.3 wt.% water reducing agent during the preparation of paste samples, and the total amount of cementitious material is 500 kg/m
3. The mix proportion of cementitious materials is shown in
Table 6.
According to the mix proportion in
Table 3, the powder raw materials were mixed evenly, then the liquid raw materials (activator, water reducer, retarder and water) were added, fully stirred to form the slurry, and then cast to prepare the paste sample. The aggregate river sand (1500 kg/m
3) or steel slag sand (1500 kg/m
3) was added into the slurry prepared by the mix proportion of sample 2, mixed evenly and then cast to form mortar samples. Both the paste samples and mortar samples were molded with a 40 × 40 × 40 cm mold, and then demolded after 24 h. All samples were cured at 25 ± 2 °C and 95% humidity for different times. Mortar samples were prepared according to <GB/T 25181-2010> [
35].
3.3. Test and Characterization
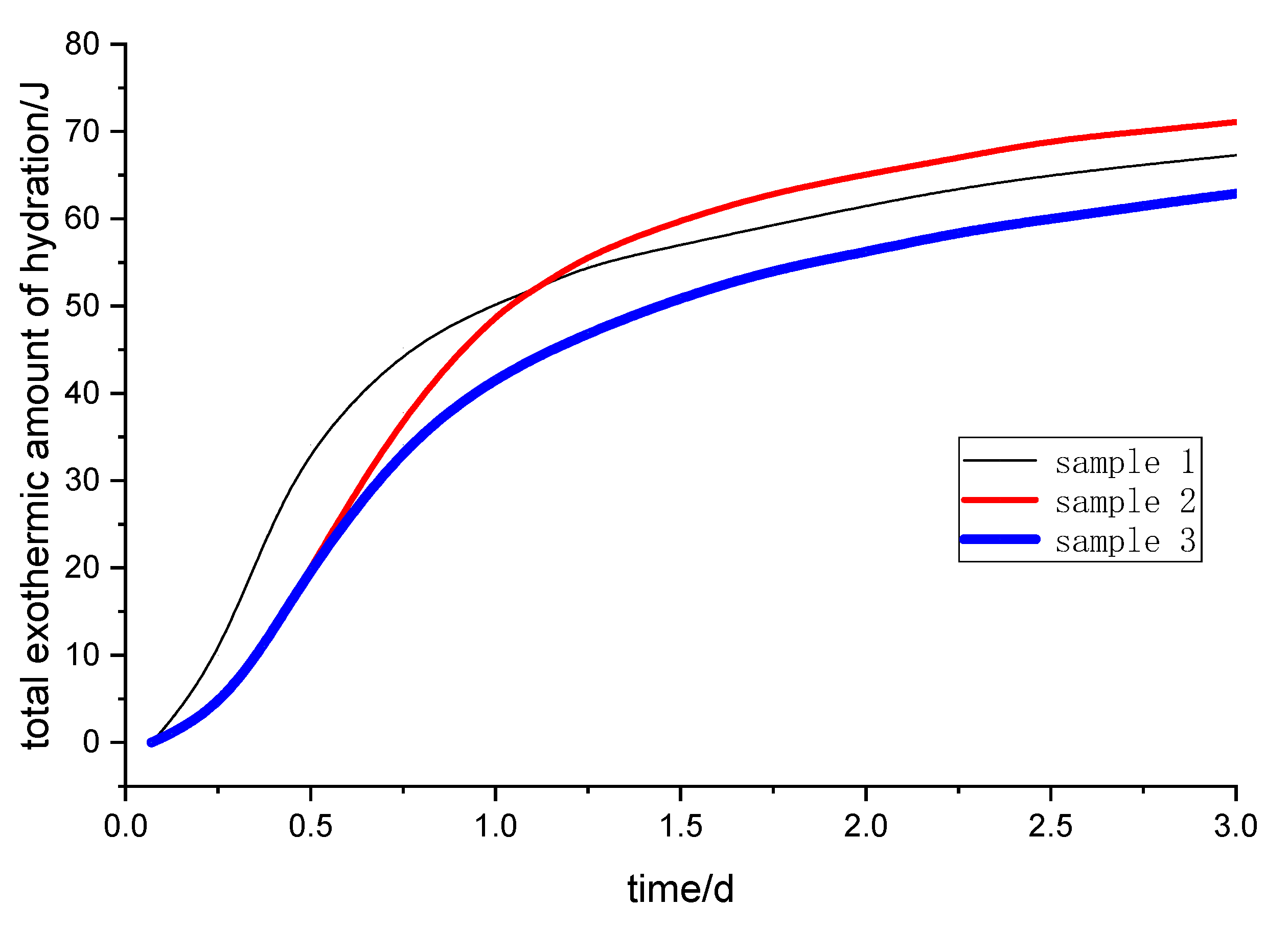
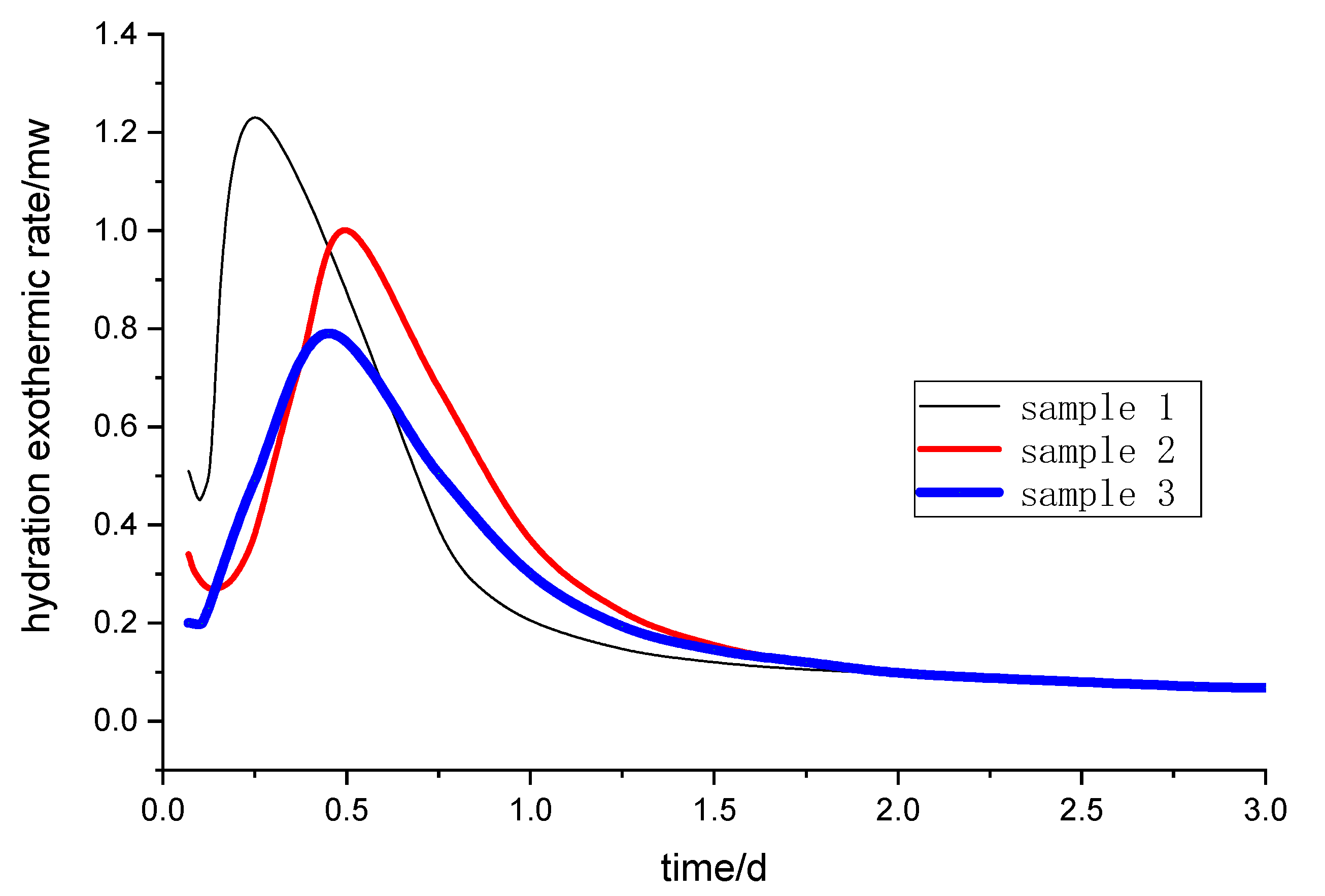
The exothermic processes of the steel slag-granulated blast furnace slag powder system were measured by a hydration microcalorimeter (TAM Air, TA Instruments, New Castle, NY, USA) at 20 °C. The steel slag powder was mixed with other raw materials to prepare a paste, then the resulting paste was injected into an ampoule that was then put into a calorimeter. The heat flow record time is 3 days.
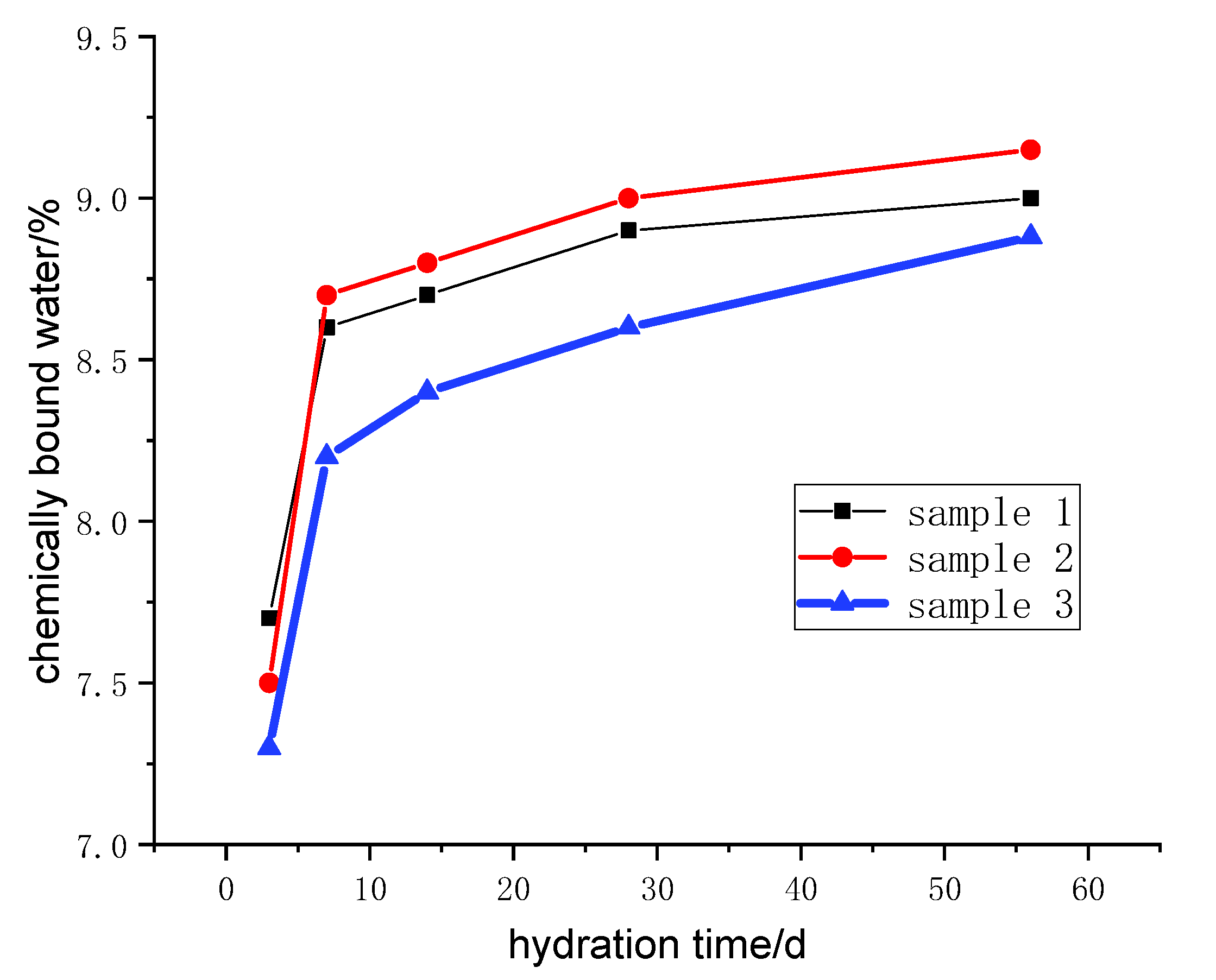
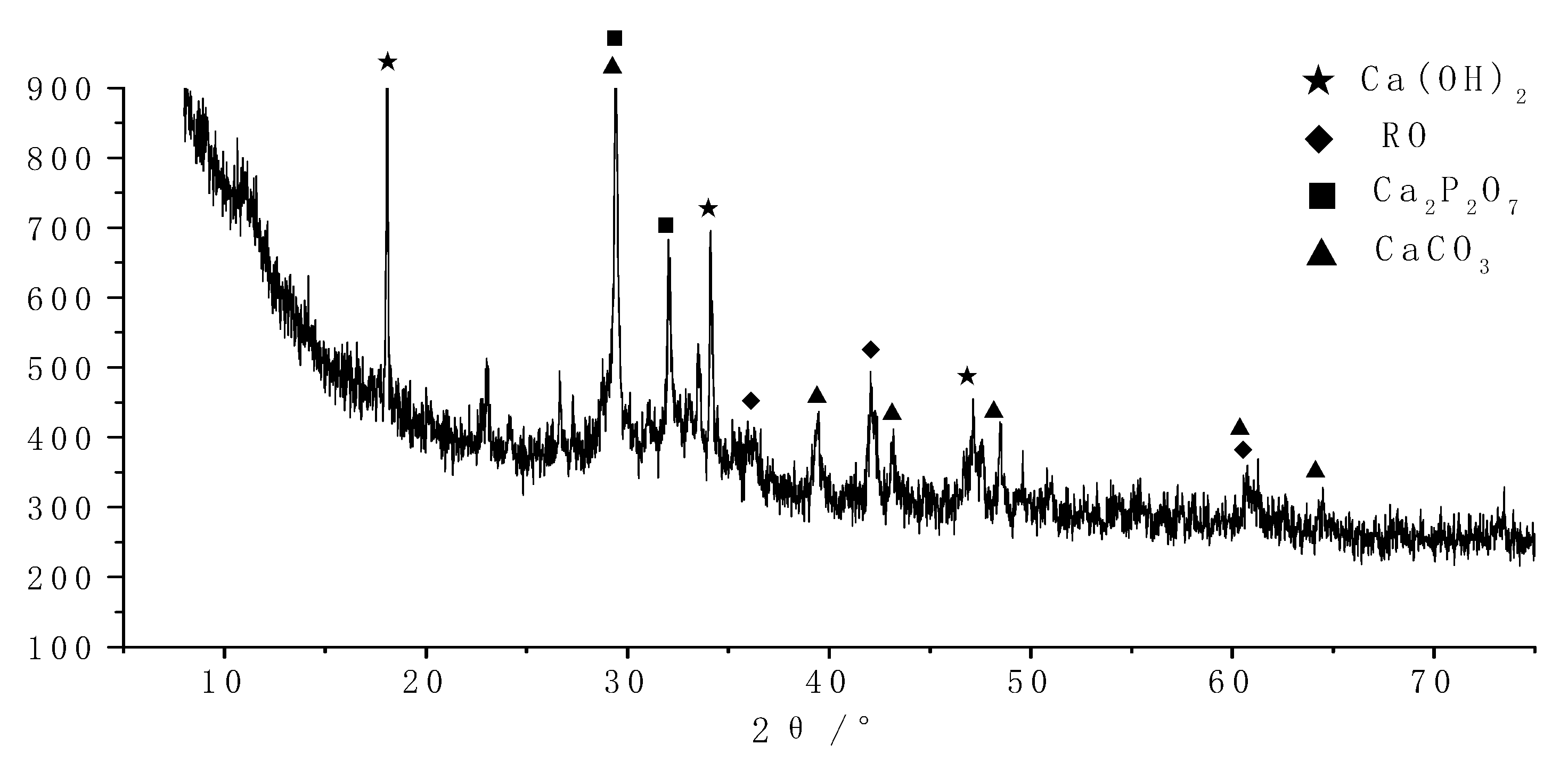
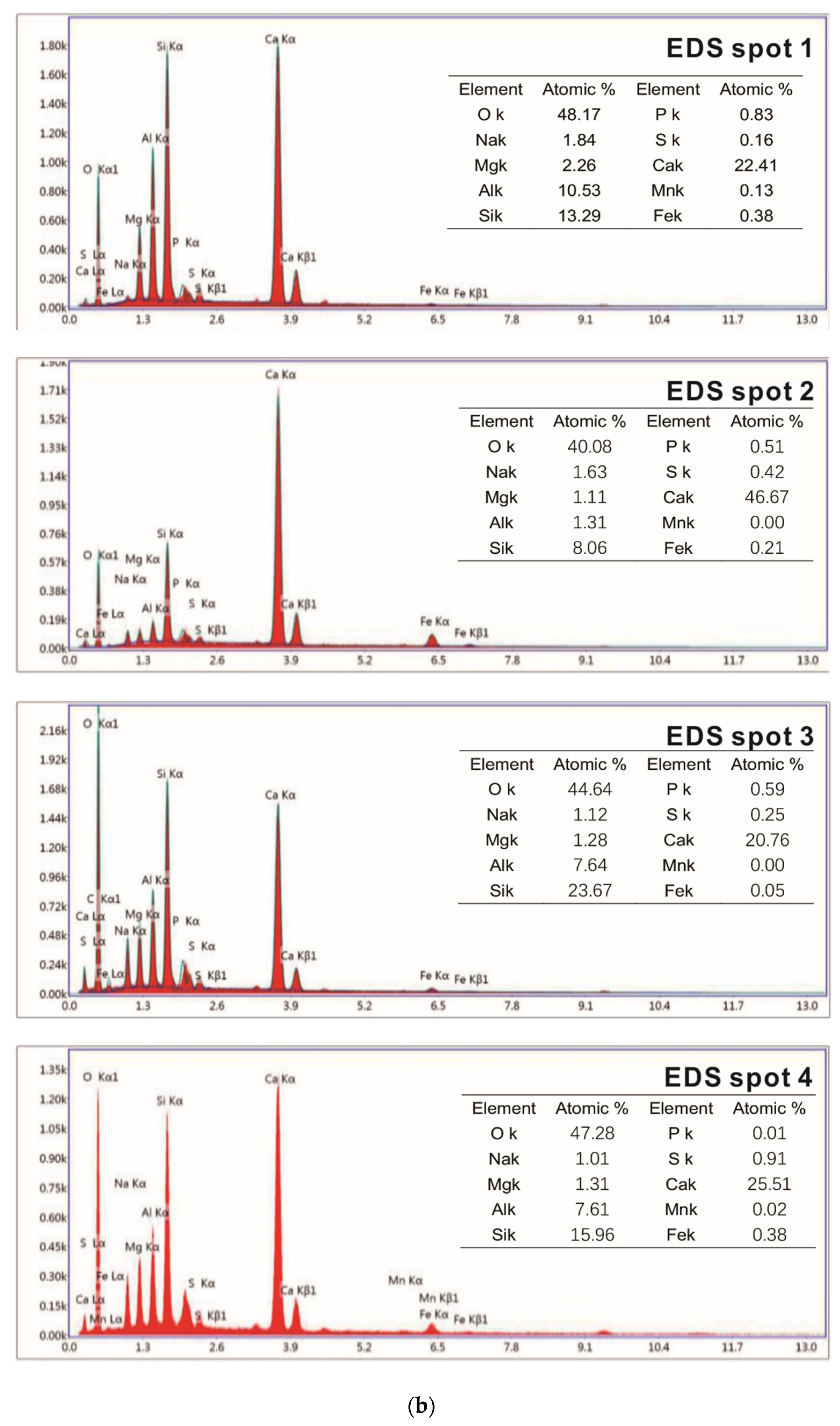
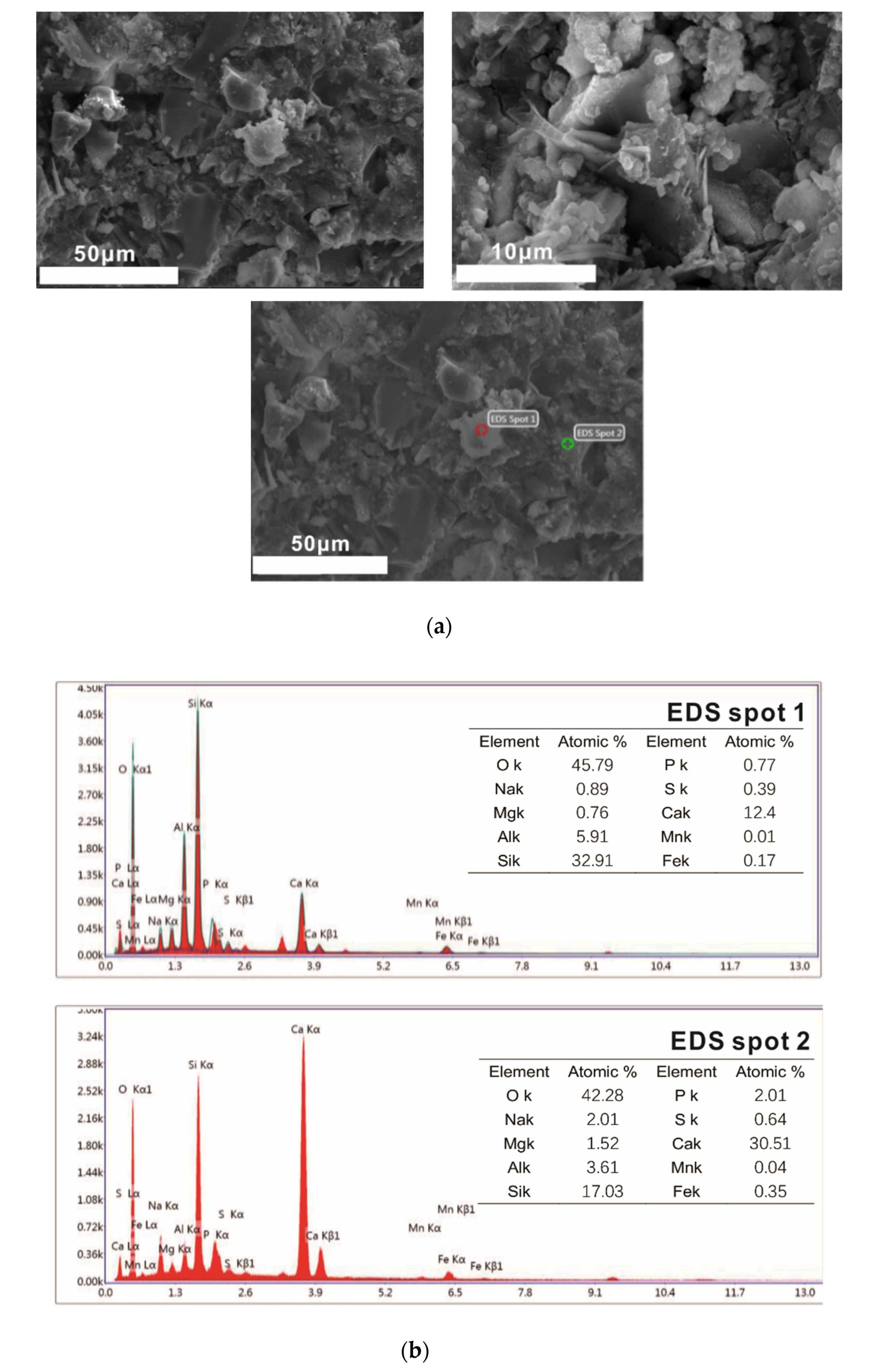
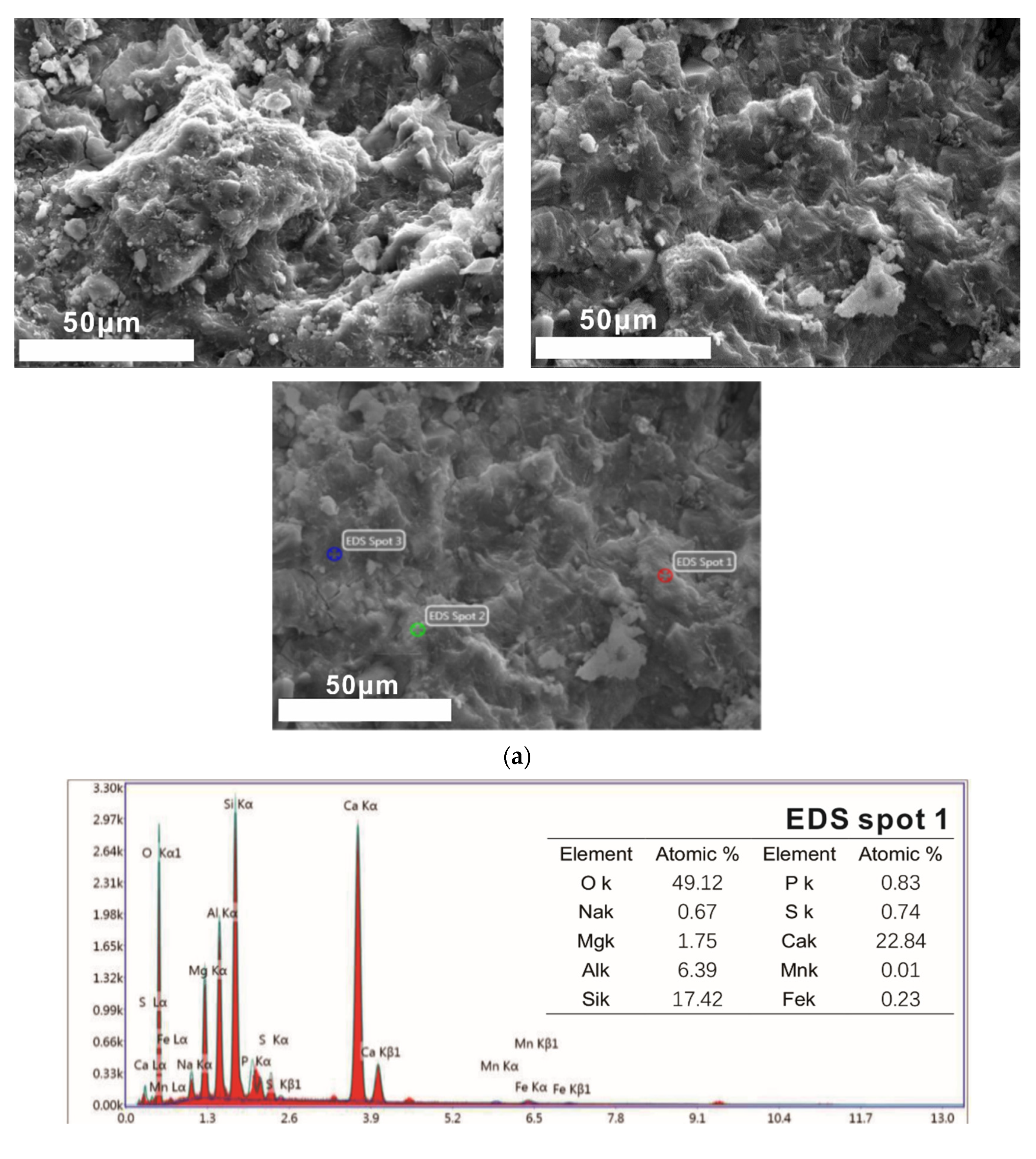
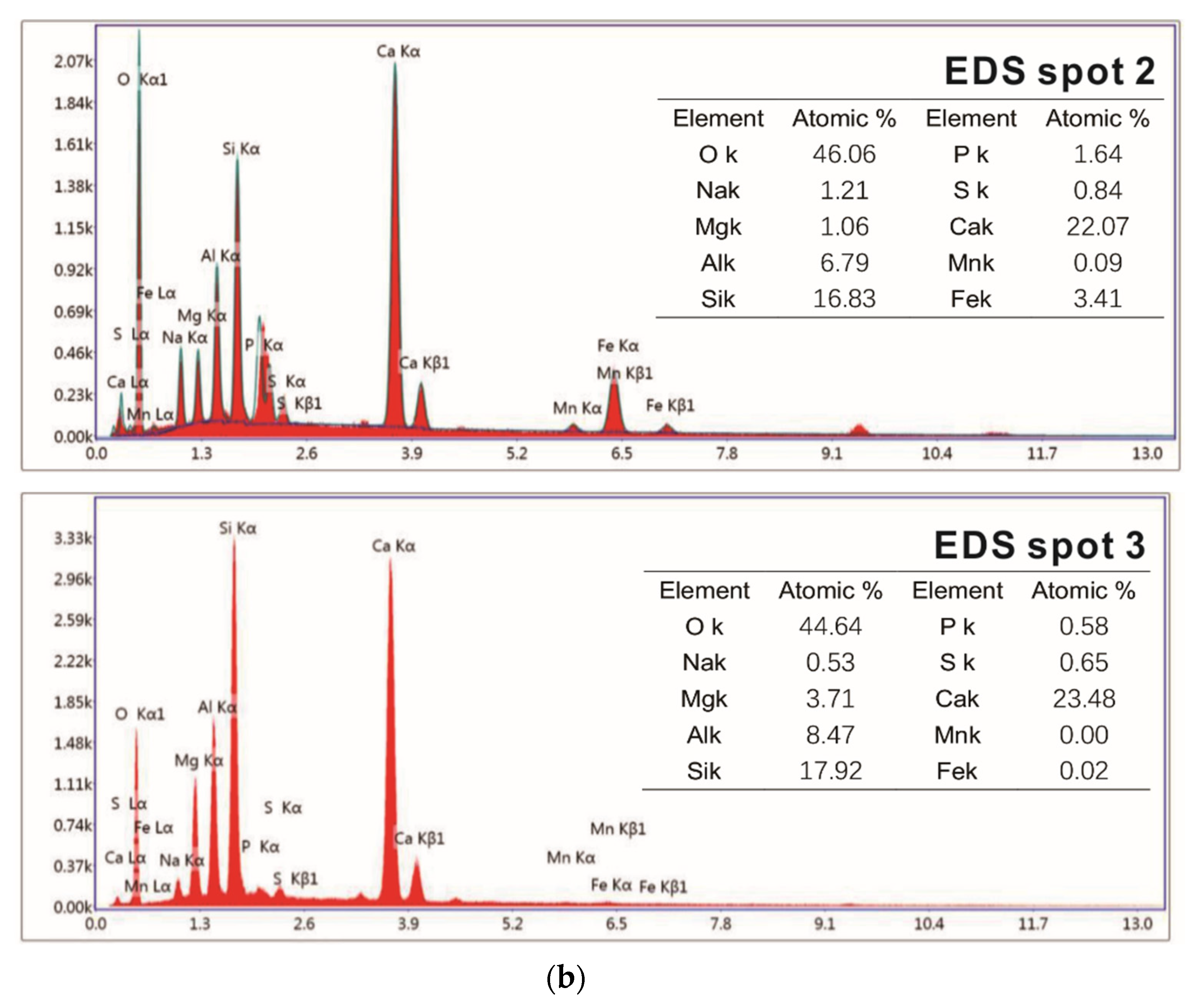
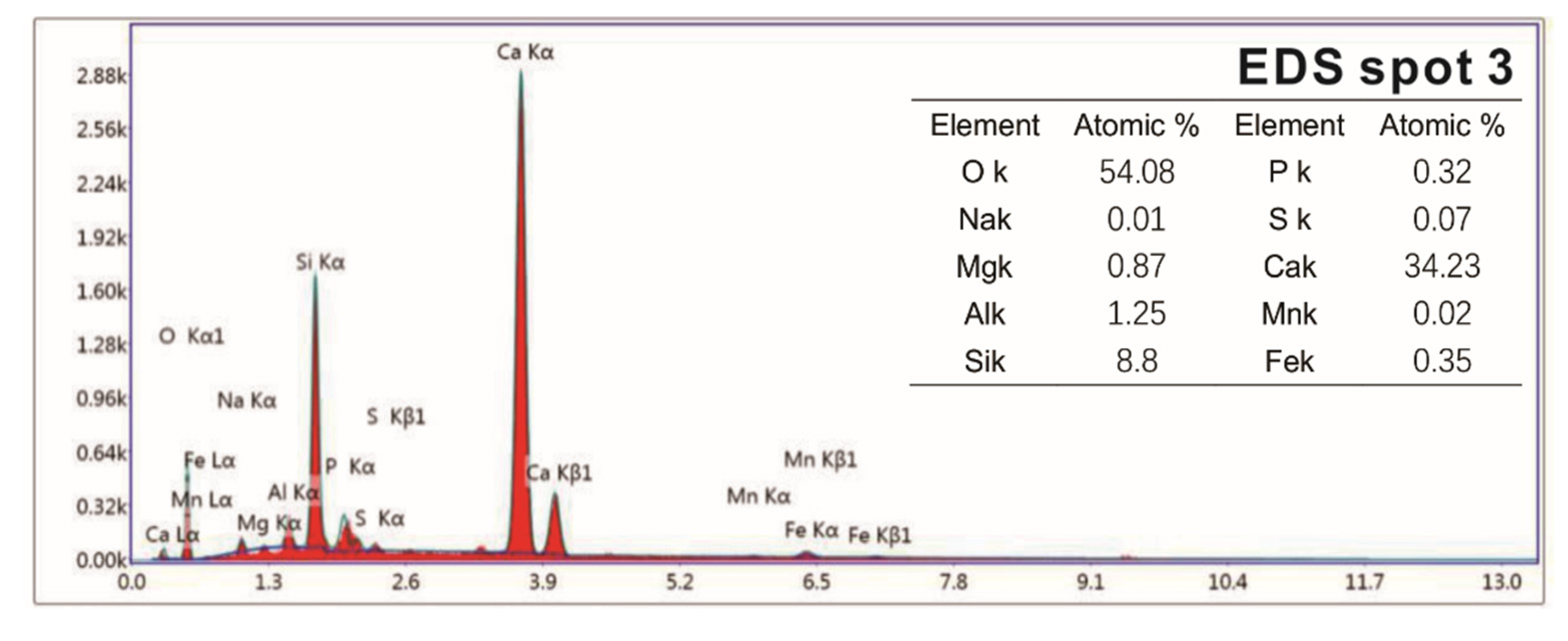
Samples of different reaction ages were broken into pieces and placed in alcohol to stop hydration. Then, the phases’ composition of hydration products was investigated by an X-ray diffraction (XRD) diagram (D8 ADVANCE, Bruker, Karlsruhe, German), with continuous scanning, the 2θ range of 5° to 75°, a scanning rate of 5°/min and a step width of 0.0195°. The internal structure of the hydration products of the system that reacted for 3 days and 28 days was tested by a nuclear magnetic resonance (NMR) instrument (AVANGE III HD400MHz, Bruker, Karlsruhe, German). The morphology structure and micro-elements of hydration products were observed by scanning electron microscope (SEM) patterns (ULTRAPLUS-43-13, Carl Zeiss, Jena, German) and accompanying energy disperse spectroscopy (EDS) patterns (X-max 50, Oxford Instruments, Oxfordshire, UK).
The content of the test and characterization in this study is shown in
Table 7.
4. Conclusions
In this paper, the hydration process of alkali-activated materials prepared by steel slag as a cementing material and fine aggregate was studied. The phase composition and micro-morphology of the hydration products were analyzed. The development process of hydration and the corresponding relationship between microstructure and macro-properties were discussed. Based on the above analysis and discussion, the following conclusions are drawn:
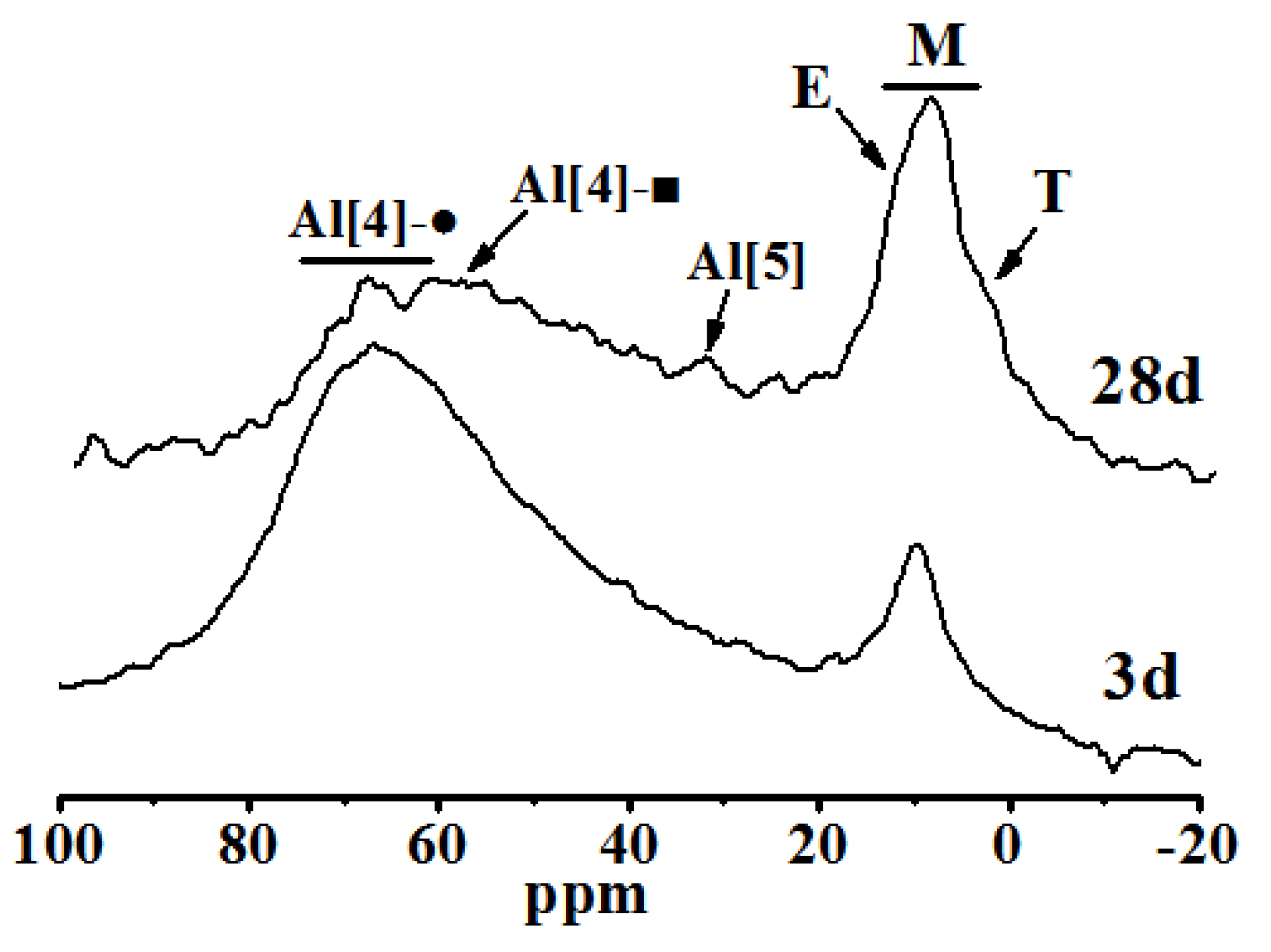
(1) The retarder has a certain retarding effect on the alkali-activated steel slag and blast furnace slag powder cementitious system, and the main hydration products are crystalline Ca(OH)2 and amorphous-phase C-A-S-H gel.
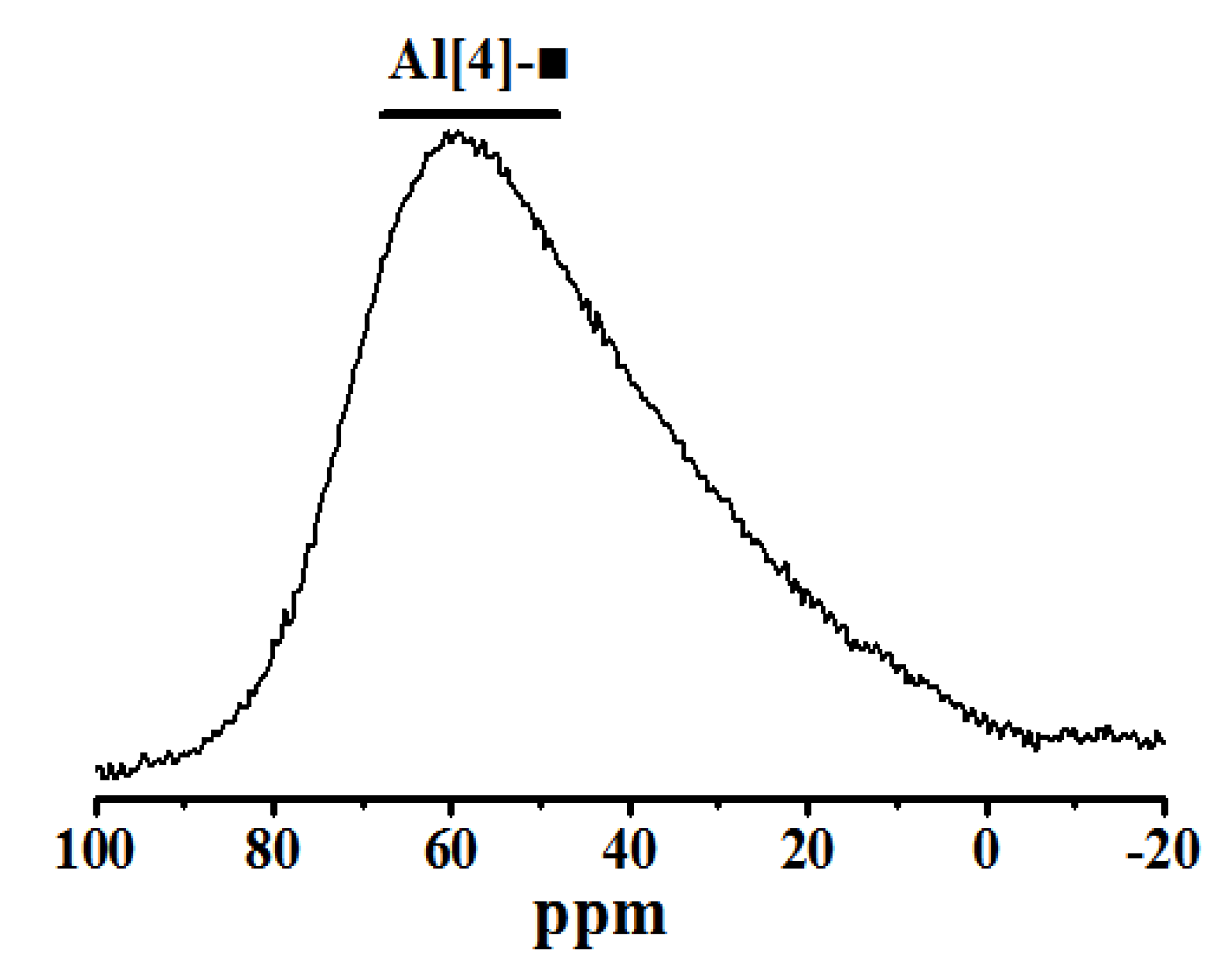
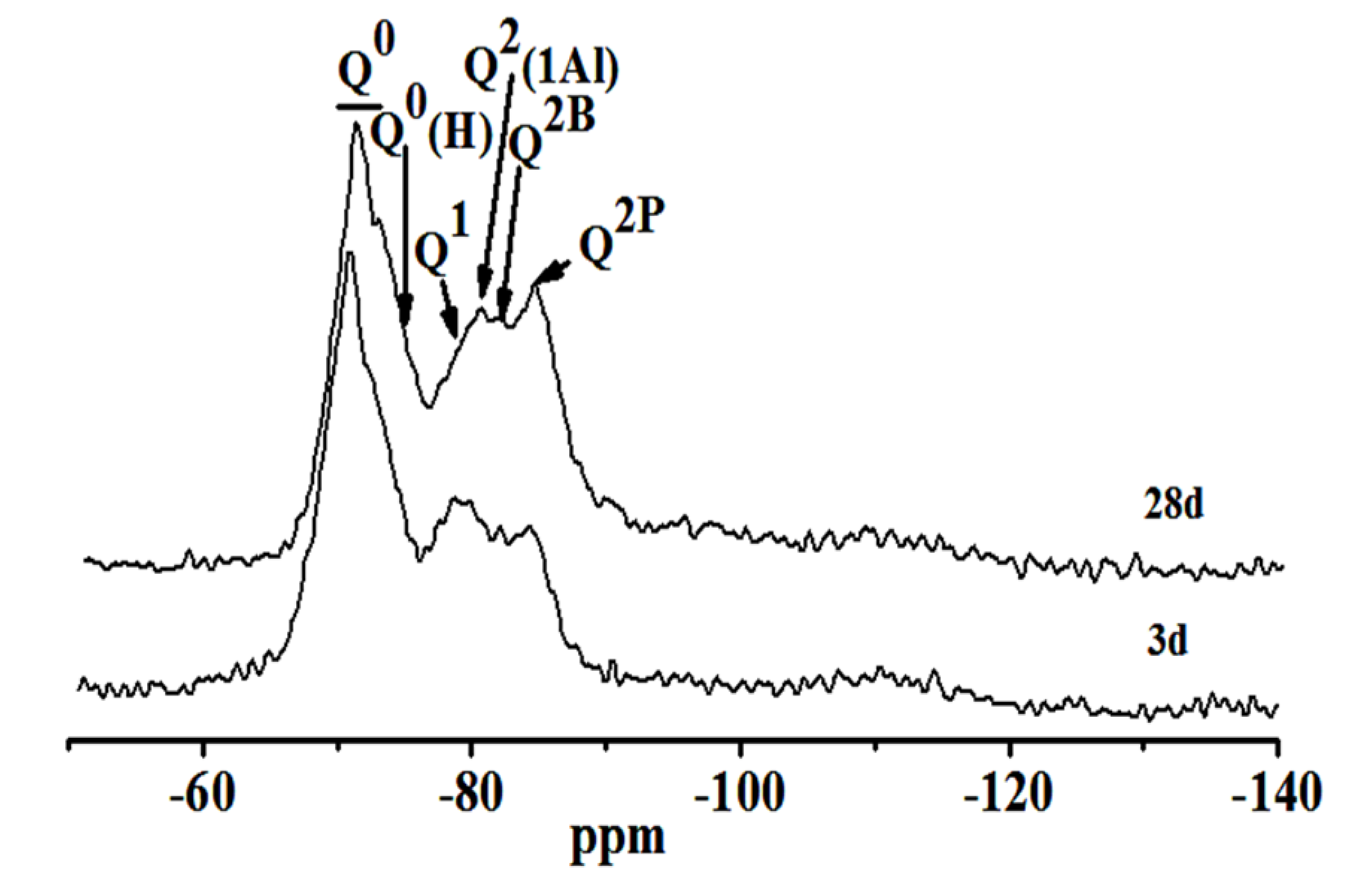
(2) With the extension of the curing age, the amount of C-S-H (C-A-S-H) gel and the average molecular chain length increase, so the degree of polymerization increases. Al[4]/Si decreases, so the degree of Si in C-S-H replaced by Al3+ reduces, while C/S increases first and then decreases with the progress of hydration. Further, the structure of cement paste is compact.
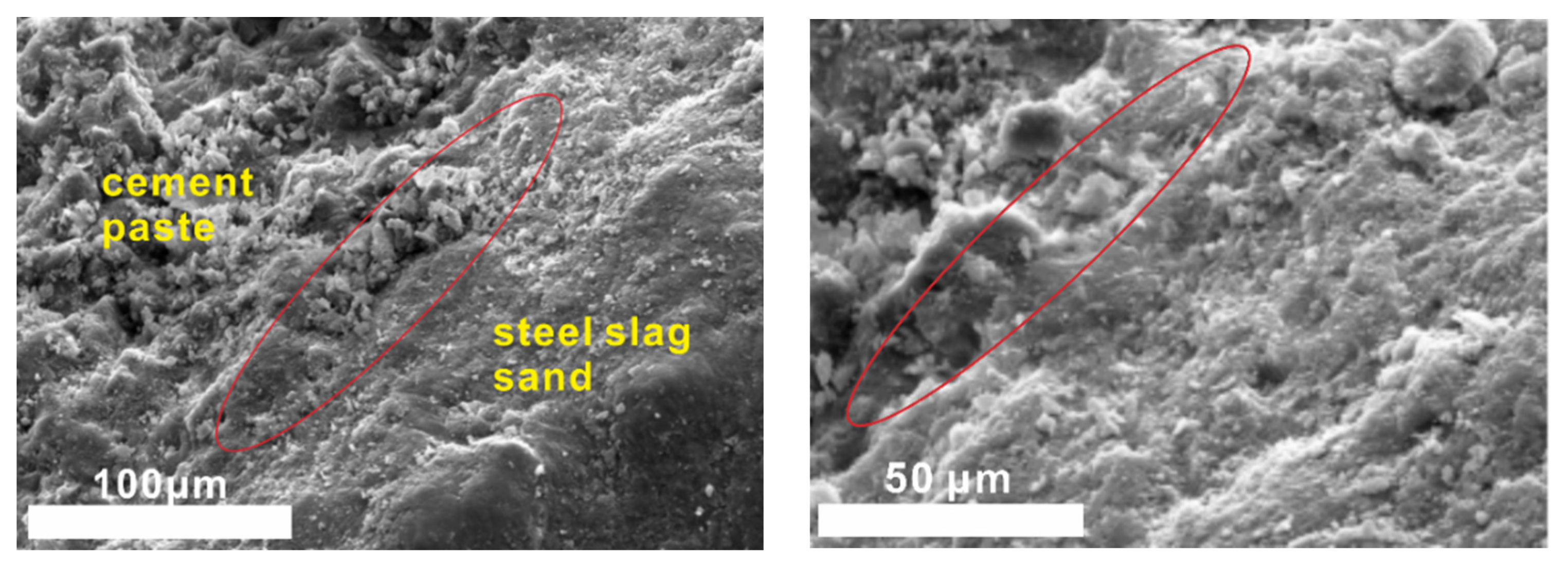
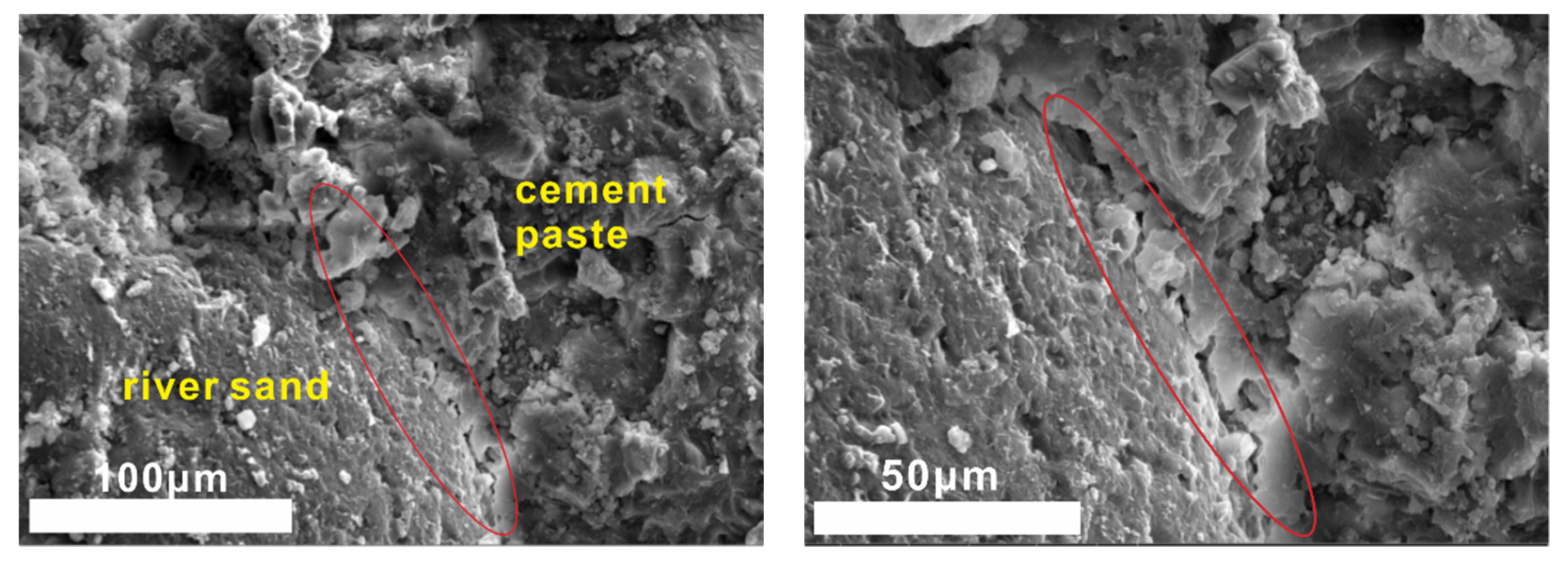
(3) The interface between steel slag sand and cement paste is denser than that of river sand, since the surface of steel slag sand is partially hydrated to form C-A-S-H gel and Ca(OH)2. As a result, the compressive strength of concrete prepared by steel slag sand is higher than that of river sand at the same mix ratio.