Recent Advances in Affinity MOF-Based Sorbents with Sample Preparation Purposes
Abstract
1. Introduction
2. Applications of Affinity-Based MOF Materials in Sample Treatment
2.1. Bioaffinity-Based MOFs
2.2. Immuno- and Mimetic-Based MOFs
2.3. Miscellaneous Affinity Ligand-Based MOFs
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MOF | Metal-organic framework |
| Bio-MOFs | Biological metal-organic frameworks |
| MS | Mass detector |
| SPE | Solid-phase extraction |
| MIPs | Molecular imprinted polymers |
| NPs | Nanoparticles |
| PDA | Polydopamine |
| LOD | Limit of detection |
| MALDI | Matrix-assisted laser desorption/ionization |
| TOF | Time of flight |
| HPLC | High-performance chromatography |
| RSD | Relative standard deviation |
| GSH | Glutathione |
| Ee | Enantiomeric excess |
| FLD | Fluorescence detection |
| ZIF | Zeolitic imidazolate framework |
| CS | Chitosan |
| CD | cyclodextrin |
| PCBs | Polychlorinated biphenyls |
| Ade | Adenine |
| SPME | Solid-phase micro-extraction |
| PAHs | Polycyclic aromatic hydrocarbons |
| FID | Flame ionization detector |
References
- Yaghi, O.M.; Li, H. Hydrothermal Synthesis of a Metal-Organic Framework Containing Large Rectangular Channels. J. Am. Chem. Soc. 1995, 117, 10401–10402. [Google Scholar] [CrossRef]
- Férey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Gangu, K.K.; Maddila, S.; Mukkamala, S.B.; Jonnalagadda, S.B. A review on contemporary Metal-Organic Framework materials. Inorganica Chim. Acta 2016, 446, 61–74. [Google Scholar] [CrossRef]
- Li, X.; Ma, W.; Li, H.; Bai, Y.; Liu, H. Metal-organic frameworks as advanced sorbents in sample preparation for small organic analytes. Coord. Chem. Rev. 2019, 397, 1–13. [Google Scholar] [CrossRef]
- Lee, J.H.; Jeoung, S.; Chung, Y.G.; Moon, H.R. Elucidation of flexible metal-organic frameworks: Research progresses and recent developments. Coord. Chem. Rev. 2019, 389, 161–188. [Google Scholar] [CrossRef]
- Wang, Q.; Astruc, D. State of the Art and Prospects in Metal-Organic Framework (MOF)-Based and MOF-Derived Nanocatalysis. Chem. Rev. 2020, 120, 1438–1511. [Google Scholar] [CrossRef]
- Aceituno Melgar, V.M.; Kim, J.; Othman, M.R. Zeolitic imidazolate framework membranes for gas separation: A review of synthesis methods and gas separation performance. J. Ind. Eng. Chem. 2015, 28, 1–15. [Google Scholar] [CrossRef]
- Dhaka, S.; Kumar, R.; Deep, A.; Kurade, M.B.; Ji, S.W.; Jeon, B.H. Metal–organic frameworks (MOFs) for the removal of emerging contaminants from aquatic environments. Coord. Chem. Rev. 2019, 380, 330–352. [Google Scholar] [CrossRef]
- Zhang, S.; Pei, X.; Gao, H.; Chen, S.; Wang, J. Metal-organic framework-based nanomaterials for biomedical applications. Chin. Chem. Lett. 2019, 31, 1060–1070. [Google Scholar] [CrossRef]
- Gu, Z.Y.; Yang, C.X.; Chang, N.; Yan, X.P. Metal-organic frameworks for analytical chemistry: From sample collection to chromatographic separation. Acc. Chem. Res. 2012, 45, 734–745. [Google Scholar] [CrossRef]
- Pacheco-Fernández, I.; González-Hernández, P.; Pasán, J.; Ayala, J.H.; Pino, V. The Rise of Metal-Organic Frameworks in Analytical Chemistry. In Handbook of Smart Materials in Analytical Chemistry; De la Guardia, M., Esteve-Turrillas, F.A., Eds.; Wiley: Chichester, UK, 2019; pp. 463–502. [Google Scholar]
- Stylianou, K.C.; Gómez, L.; Imaz, I.; Verdugo-Escamilla, C.; Ribas, X.; Maspoch, D. Engineering Homochiral Metal-Organic Frameworks by Spatially Separating 1D Chiral Metal-Peptide Ladders: Tuning the Pore Size for Enantioselective Adsorption. Chem.-A Eur. J. 2015, 21, 9964–9969. [Google Scholar] [CrossRef]
- Shen, K.; Zhang, M.; Zheng, H. Critical factors influencing the structures and properties of metal-organic frameworks. CrystEngComm 2015, 17, 981–991. [Google Scholar] [CrossRef]
- Imaz, I.; Rubio-Martínez, M.; An, J.; Solé-Font, I.; Rosi, N.L.; Maspoch, D. Metal-biomolecule frameworks (MBioFs). Chem. Commun. 2011, 47, 7287–7302. [Google Scholar] [CrossRef]
- Cai, H.; Huang, Y.L.; Li, D. Biological metal–organic frameworks: Structures, host–guest chemistry and bio-applications. Coord. Chem. Rev. 2019, 378, 207–221. [Google Scholar] [CrossRef]
- Azzouz, A.; Kailasa, S.K.; Lee, S.S.; Rascón, A.J.; Ballesteros, E.; Zhang, M.; Kim, K.H. Review of nanomaterials as sorbents in solid-phase extraction for environmental samples. TrAC-Trends Anal. Chem. 2018, 108, 347–369. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Yang, L.; Wang, Z.; Liu, H. Recent advances in applications of metal–organic frameworks for sample preparation in pharmaceutical analysis. Coord. Chem. Rev. 2020, 411, 213235. [Google Scholar] [CrossRef]
- Ma, W.; Li, X.; Bai, Y.; Liu, H. Applications of metal-organic frameworks as advanced sorbents in biomacromolecules sample preparation. TrAC-Trends Anal. Chem. 2018, 109, 154–162. [Google Scholar] [CrossRef]
- Faraji, M.; Yamini, Y.; Gholami, M. Recent Advances and Trends in Applications of Solid-Phase Extraction Techniques in Food and Environmental Analysis; Springer: Berlin/Heidelberg, Germany, 2019; Volume 82. [Google Scholar]
- Wang, Y.; Rui, M.; Lu, G. Recent applications of metal–organic frameworks in sample pretreatment. J. Sep. Sci. 2018, 41, 180–194. [Google Scholar] [CrossRef]
- Chisvert, A.; Cárdenas, S.; Lucena, R. Dispersive micro-solid phase extraction. TrAC-Trends Anal. Chem. 2019, 112, 226–233. [Google Scholar] [CrossRef]
- Anderson, S.L.; Stylianou, K.C. Biologically derived metal organic frameworks. Coord. Chem. Rev. 2017, 349, 102–128. [Google Scholar] [CrossRef]
- Li, M.Y.; Wang, F.; Gu, Z.G.; Zhang, J. Synthesis of homochiral zeolitic metal-organic frameworks with amino acid and tetrazolates for chiral recognition. RSC Adv. 2017, 7, 4872–4875. [Google Scholar] [CrossRef]
- Chan, J.Y.; Zhang, H.; Nolvachai, Y.; Hu, Y.; Zhu, H.; Forsyth, M.; Gu, Q.; Hoke, D.E.; Zhang, X.; Marriot, P.J.; et al. Incorporation of Homochirality into a Zeolitic Imidazolate Framework Membrane for Efficient Chiral Separation. Angew. Chemie 2018, 130, 17376–17380. [Google Scholar] [CrossRef]
- Mon, M.; Ferrando-Soria, J.; Grancha, T.; Fortea-Pérez, F.R.; Gascon, J.; Leyva-Pérez, A.; Armentano, D.; Pardo, E. Selective Gold Recovery and Catalysis in a Highly Flexible Methionine-Decorated Metal-Organic Framework. J. Am. Chem. Soc. 2016, 138, 7864–7867. [Google Scholar] [CrossRef] [PubMed]
- Mon, M.; Qu, X.; Ferrando-Soria, J.; Pellicer-Carreño, I.; Sepúlveda-Escribano, A.; Ramos-Fernandez, E.V.; Jansen, J.C.; Armentano, D.; Pardo, E. Fine-tuning of the confined space in microporous metal-organic frameworks for efficient mercury removal. J. Mater. Chem. A 2017, 5, 20120–20125. [Google Scholar] [CrossRef]
- Ma, W.; Xu, L.; Li, X.; Shen, S.; Wu, M.; Bai, Y.; Liu, H. Cysteine-Functionalized Metal-Organic Framework: Facile Synthesis and High Efficient Enrichment of N-Linked Glycopeptides in Cell Lysate. ACS Appl. Mater. Interfaces 2017, 9, 19562–19568. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Yang, M.; Jiang, P.; Lan, F.; Wu, Y. Multi-affinity sites of magnetic guanidyl-functionalized metal-organic framework nanospheres for efficient enrichment of global phosphopeptides. Nanoscale 2018, 10, 8391–8396. [Google Scholar] [CrossRef]
- Pérez-Cejuela, H.M.; Mon, M.; Ferrando-Soria, J.; Pardo, E.; Armentano, D.; Simó-Alfonso, E.F.; Herrero-Martínez, J.M. Bio-metal-organic frameworks for molecular recognition and sorbent extraction of hydrophilic vitamins followed by their determination using HPLC-UV. Microchim. Acta 2020, 187. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Q.; Deng, C. L-cysteine-modified metal-organic frameworks as multifunctional probes for efficient identification of N-linked glycopeptides and phosphopeptides in human crystalline lens. Anal. Chim. Acta 2019, 1061, 110–121. [Google Scholar] [CrossRef]
- Fan, L.; Deng, M.; Lin, C.; Xu, C.; Liu, Y.; Shi, Z.; Wang, Y.; Xu, Z.; Li, L.; He, M. A multifunctional composite Fe3O4/MOF/l-cysteine for removal, magnetic solid phase extraction and fluorescence sensing of Cd(II). RSC Adv. 2018, 8, 10561–10572. [Google Scholar] [CrossRef]
- Sattar, T.; Athar, M. Hydrothermal Synthesis and Characterization of Copper Glycinate (Bio-MOF-29) and Its in Vitro Drugs Adsorption Studies. Open J. Inorg. Chem. 2017, 07, 17–27. [Google Scholar] [CrossRef]
- Liu, M.; Wang, J.; Yang, Q.; Hu, N.; Zhang, W.; Zhu, W.; Wang, R.; Suo, Y.; Wang, J. Patulin removal from apple juice using a novel cysteine-functionalized metal-organic framework adsorbent. Food Chem. 2019, 270, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wright, P.A. Opening the Door to Peptide-Based Porous Solids. Nature 2010, 329, 1025–1026. [Google Scholar] [CrossRef] [PubMed]
- Raboe, J.; Yue, Y.-F.; Chong, S.Y.; Stylianou, K.C.; Bacsa, J.; Bradshaw, D.; Darling, G.R.; Berry, N.G.; Khimyak, Y.Z.; Ganin, A.Y.; et al. An Adaptable Peptide-Based Porous Material. Science 2010, 329, 1053–1057. [Google Scholar] [CrossRef] [PubMed]
- Lillo, V.; Galán-Mascarós, J.R. Transition metal complexes with oligopeptides: Single crystals and crystal structures. Dalt. Trans. 2014, 43, 9821–9833. [Google Scholar] [CrossRef] [PubMed]
- Katsoulidis, A.P.; Park, K.S.; Antypov, D.; Martí-Gastaldo, C.; Miller, G.J.; Warren, J.E.; Robertson, C.M.; Blanc, F.; Darling, G.R.; Berry, N.G.; et al. Guest-Adaptable and Water-Stable Peptide-Based Porous Materials by Imidazolate Side Chain Control. Angew. Chemie 2014, 126, 197–202. [Google Scholar] [CrossRef]
- Martí-Gastaldo, C.; Antypov, D.; Warren, J.E.; Briggs, M.E.; Chater, P.A.; Wiper, P.V.; Miller, G.J.; Khimyak, Y.Z.; Darling, G.R.; Berry, N.G.; et al. Side-chain control of porosity closure in single-and multiple-peptide-based porous materials by cooperative folding. Nat. Chem. 2014, 6, 343–351. [Google Scholar] [CrossRef]
- Liu, Q.; Deng, C.H.; Sun, N. Hydrophilic tripeptide-functionalized magnetic metal-organic frameworks for the highly efficient enrichment of N-linked glycopeptides. Nanoscale 2018, 10, 12149–12155. [Google Scholar] [CrossRef]
- Navarro-Sánchez, J.; Argente-García, A.I.; Moliner-Martínez, Y.; Roca-Sanjuán, D.; Antypov, D.; Campíns-Falcó, P.; Rosseinsky, M.J.; Martí-Gastaldo, C. Peptide Metal-Organic Frameworks for Enantioselective Separation of Chiral Drugs. J. Am. Chem. Soc. 2017, 139, 4294–4297. [Google Scholar] [CrossRef]
- Zhuang, J.; Young, A.P.; Tsung, C.K. Integration of Biomolecules with Metal–Organic Frameworks. Small 2017, 13, 1–14. [Google Scholar] [CrossRef]
- Chen, G.; Huang, S.; Kou, X.; Wei, S.; Huang, S.; Jiang, S.; Shen, J.; Zhu, F.; Ouyang, G. A Convenient and Versatile Amino-Acid-Boosted Biomimetic Strategy for the Nondestructive Encapsulation of Biomacromolecules within Metal–Organic Frameworks. Angew. Chemie-Int. Ed. 2019, 58, 1463–1467. [Google Scholar] [CrossRef]
- Hoop, M.; Walde, C.F.; Riccò, R.; Mushtaq, F.; Terzopoulou, A.; Chen, X.Z.; deMello, A.J.; Doonan, C.J.; Falcaro, P.; Nelson, B.J.; et al. Biocompatibility characteristics of the metal organic framework ZIF-8 for therapeutical applications. Appl. Mater. Today 2018, 11, 13–21. [Google Scholar] [CrossRef]
- Liu, G.; Xu, Y.; Han, Y.; Wu, J.; Xu, J.; Meng, H.; Zhang, X. Immobilization of lysozyme proteins on a hierarchical zeolitic imidazolate framework (ZIF-8). Dalt. Trans. 2017, 46, 2114–2121. [Google Scholar] [CrossRef] [PubMed]
- Gkaniatsou, E.; Sicard, C.; Ricoux, R.; Mahy, J.P.; Steunou, N.; Serre, C. Metal-organic frameworks: A novel host platform for enzymatic catalysis and detection. Mater. Horizons 2017, 4, 55–63. [Google Scholar] [CrossRef]
- Feng, D.; Liu, T.F.; Su, J.; Bosch, M.; Wei, Z.; Wan, W.; Yuan, D.; Chen, Y.P.; Wang, X.; Wang, K.; et al. Stable metal-organic frameworks containing single-molecule traps for enzyme encapsulation. Nat. Commun. 2015, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xue, S.; Lin, Y.; Luo, J.; Kong, L. Immobilization of porcine pancreatic lipase onto a metal-organic framework, PPL@MOF: A new platform for efficient ligand discovery from natural herbs. Anal. Chim. Acta 2019, 1099, 94–102. [Google Scholar] [CrossRef]
- Nadar, S.S.; Vaidya, L.; Maurya, S.; Rathod, V.K. Polysaccharide based metal organic frameworks (polysaccharide–MOF): A review. Coord. Chem. Rev. 2019, 396, 1–21. [Google Scholar] [CrossRef]
- Ma, W.; Xu, L.; Li, Z.; Sun, Y.; Bai, Y.; Liu, H. Post-synthetic modification of an amino-functionalized metal-organic framework for highly efficient enrichment of N-linked glycopeptides. Nanoscale 2016, 8, 10908–10912. [Google Scholar] [CrossRef]
- Giménez, R.E.; Piccinini, E.; Azzaroni, O.; Rafti, M. Lectin-Recognizable MOF Glyconanoparticles: Supramolecular Glycosylation of ZIF-8 Nanocrystals by Sugar-Based Surfactants. ACS Omega 2019, 4, 842–848. [Google Scholar] [CrossRef]
- Wisser, D.; Wisser, F.M.; Raschke, S.; Klein, N.; Leistner, M.; Grothe, J.; Brunner, E.; Kaskel, S. Biological chitin-MOF composites with hierarchical pore systems for air-filtration applications. Angew. Chemie-Int. Ed. 2015, 54, 12588–12591. [Google Scholar] [CrossRef]
- Rajkumar, T.; Kukkar, D.; Kim, K.H.; Sohn, J.R.; Deep, A. Cyclodextrin-metal–organic framework (CD-MOF): From synthesis to applications. J. Ind. Eng. Chem. 2019, 72, 50–66. [Google Scholar] [CrossRef]
- Liu, G.; Li, L.; Xu, D.; Huang, X.; Xu, X.; Zheng, S.; Zhang, Y.; Lin, H. Metal–organic framework preparation using magnetic graphene oxide–β-cyclodextrin for neonicotinoid pesticide adsorption and removal. Carbohydr. Polym. 2017, 175, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, N.; Chen, T.; Ma, Y.; Li, Q. A green cyclodextrin metal-organic framework as solid-phase extraction medium for enrichment of sulfonamides before their HPLC determination. Microchem. J. 2018, 138, 401–407. [Google Scholar] [CrossRef]
- Pichon, V. Aptamer-based and immunosorbents. In Solid-Phase Extraction; Poole, C., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 151–183. [Google Scholar]
- Turiel, E.; Esteban, A.M. Molecularly imprinted polymers. In Solid-Phase Extraction; Poole, C., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 215–233. [Google Scholar]
- Liu, X.; Yue, T.; Qi, K.; Qiu, Y.; Guo, X. Porous graphene based electrochemical immunosensor using Cu3(BTC)2 metal-organic framework as nonenzymatic label. Talanta 2020, 217, 121042. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, S.K.; Bhardwaj, N.; Mohanta, G.C.; Kumar, P.; Sharma, A.L.; Kim, K.H.; Deep, A. Immunosensing of Atrazine with Antibody-Functionalized Cu-MOF Conducting Thin Films. ACS Appl. Mater. Interfaces 2015, 7, 26124–26130. [Google Scholar] [CrossRef]
- Yim, C.; Lee, H.; Lee, S.; Jeon, S. One-step immobilization of antibodies on ZIF-8/Fe3O4 hybrid nanoparticles for the immunoassay of Staphylococcus aureus. RSC Adv. 2017, 7, 1418–1422. [Google Scholar] [CrossRef]
- Xu, D.; Ge, K.; Chen, Y.; Qi, S.; Tian, Y.; Wang, S.; Qiu, J.; Wang, X.; Dong, Q.; Liu, Q. Cobalt-Iron mixed-metal-organic framework (Co3Fe-MMOF) as peroxidase mimic for highly sensitive enzyme-linked immunosorbent assay (ELISA) detection of Aeromonas hydrophila. Microchem. J. 2020, 154, 104591. [Google Scholar] [CrossRef]
- Sharma, T.K.; Bruno, J.G.; Dhiman, A. ABCs of DNA aptamer and related assay development. Biotechnol. Adv. 2017, 35, 275–301. [Google Scholar] [CrossRef]
- Lin, S.; Gan, N.; Zhang, J.; Qiao, L.; Chen, Y.; Cao, Y. Aptamer-functionalized stir bar sorptive extraction coupled with gas chromatography-mass spectrometry for selective enrichment and determination of polychlorinated biphenyls in fish samples. Talanta 2016, 149, 266–274. [Google Scholar] [CrossRef]
- Lin, S.; Gan, N.; Cao, Y.; Chen, Y.; Jiang, Q. Selective dispersive solid phase extraction-chromatography tandem mass spectrometry based on aptamer-functionalized UiO-66-NH2 for determination of polychlorinated biphenyls. J. Chromatogr. A 2016, 1446, 34–40. [Google Scholar] [CrossRef]
- Jiang, D.; Hu, T.; Zheng, H.; Xu, G.; Jia, Q. Aptamer-Functionalized Magnetic Conjugated Organic Framework for Selective Extraction of Traces of Hydroxylated Polychlorinated Biphenyls in Human Serum. Chem.-A Eur. J. 2018, 24, 10390–10396. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, Y.; Zhi, Y.; Wang, X.; Wu, Y.; Zheng, Y. Aptamer-modified magnetic metal-organic framework MIL-101 for highly efficient and selective enrichment of ochratoxin A. J. Sep. Sci. 2019, 42, 716–724. [Google Scholar] [CrossRef]
- Lu, Z.; Jiang, Y.; Wang, P.; Xiong, W.; Qi, B.; Zhang, Y.; Xiang, D.; Zhai, K. Bimetallic organic framework-based aptamer sensors: A new platform for fluorescence detection of chloramphenicol. Anal. Bioanal. Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.H.; Zhang, R.R.; Mu, L.N.; Huang, Y.P.; Liu, Z.S. Fabrication of core-shell sol-gel hybrid molecularly imprinted polymer based on metal–organic framework. Eur. Polym. J. 2019, 121, 1–8. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, X.; Li, Y.; Guo, H.; Lu, W.; Nie, L. Synthesis of a Molecularly Imprinted Polymer on NH2-MIL-101(Cr) for Specific Recognition of Diclofenac Sodium. J. Nanosci. Nanotechnol. 2019, 20, 1807–1813. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; He, J.; Huang, Z.; Li, H.; Zhang, Y.; Wang, H.; Rui, C.; Li, Y.; You, L.; Li, K.; et al. An amino-functionalized zirconium-based metal-organic framework of type UiO-66-NH2 covered with a molecularly imprinted polymer as a sorbent for the extraction of aflatoxins AFB1, AFB2, AFG1 and AFG2 from grain. Microchim. Acta 2020, 187, 32. [Google Scholar] [CrossRef]
- Ma, N.; Feng, C.; Qu, P.; Wang, G.; Liu, J.; Liu, J.X.; Wang, J.P. Determination of Tetracyclines in Chicken by Dispersive Solid Phase Microextraction Based on Metal-Organic Frameworks/Molecularly Imprinted Nano-polymer and Ultra Performance Liquid Chromatography. Food Anal. Methods 2020, 13, 1211–1219. [Google Scholar] [CrossRef]
- Qian, K.; Deng, Q.; Fang, G.; Wang, J.; Pan, M.; Wang, S.; Pu, Y. Metal-organic frameworks supported surface-imprinted nanoparticles for the sensitive detection of metolcarb. Biosens. Bioelectron. 2016, 79, 359–363. [Google Scholar] [CrossRef]
- Fu, H.R.; Zhang, J. Structural transformation and hysteretic sorption of light hydrocarbons in a flexible Zn-pyrazole-adenine framework. Chem.-A Eur. J. 2015, 21, 5700–5703. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Jhung, S.H. Adsorptive removal of wide range of pharmaceuticals and personal care products from water using bio-MOF-1 derived porous carbon. Microporous Mesoporous Mater. 2018, 270, 102–108. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, Y.; Zheng, J.; Xu, J.; Jiang, R.; Shen, Y.; Jiang, J.; Zhu, F.; Su, C.; Ouyang, G. Isoreticular bio-MOF 100-102 coated solid-phase microextraction fibers for fast and sensitive determination of organic pollutants by the pore structure dominated mechanism. Analyst 2015, 140, 4384–4387. [Google Scholar] [CrossRef]
- Zhang, S.; Yao, W.; Fu, D.; Zhang, C.; Zhao, H. Fabrication of magnetic zinc adeninate metal–organic frameworks for the extraction of benzodiazepines from urine and wastewater. J. Sep. Sci. 2018, 41, 1864–1870. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.H.; Yu, J.; Fu, Y.Y.; Zhou, P.X. In situ hydrothermal growth of a dual-ligand metal-organic framework film on a stainless steel fiber for solid-phase microextraction of polycyclic aromatic hydrocarbons in environmental water samples. RSC Adv. 2016, 6, 14042–14048. [Google Scholar] [CrossRef]
- Zhou, J.; Liang, Y.; He, X.; Chen, L.; Zhang, Y. Dual-Functionalized Magnetic Metal-Organic Framework for Highly Specific Enrichment of Phosphopeptides. ACS Sustain. Chem. Eng. 2017, 5, 11413–11421. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, S.; Xu, J.; Yin, L.; Sun, F.; Zhou, N.; Ouyang, G. Fabrication of 8-aminocaprylic acid doped UIO-66 as sensitive solid-phase microextraction fiber for nitrosamines. Talanta 2018, 178, 629–635. [Google Scholar] [CrossRef]
- Su, H.; Wang, Z.; Jia, Y.; Deng, L.; Chen, X.; Zhao, R.; Chan, T.W.D. A cadmium(II)-based metal-organic framework material for the dispersive solid-phase extraction of polybrominated diphenyl ethers in environmental water samples. J. Chromatogr. A 2015, 1422, 334–339. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Khan, M.A.; Siddiqui, M.R.; Alothman, Z.A. Development of citric anhydride anchored mesoporous MOF through post synthesis modification to sequester potentially toxic lead (II) from water. Microporous Mesoporous Mater. 2018, 261, 198–206. [Google Scholar] [CrossRef]
- Li, S.; Jia, M.; Guo, H.; Hou, X. Development and application of metal organic framework/chitosan foams based on ultrasound-assisted solid-phase extraction coupling to UPLC-MS/MS for the determination of five parabens in water. Anal. Bioanal. Chem. 2018, 410, 6619–6632. [Google Scholar] [CrossRef]
- Luo, Z.; Chen, H.; Wu, S.; Yang, C.; Cheng, J. Enhanced removal of bisphenol A from aqueous solution by aluminum-based MOF/sodium alginate-chitosan composite beads. Chemosphere 2019, 237, 124493. [Google Scholar] [CrossRef]
- El-Shahat, M.; Abdelhamid, A.E.; Abdelhameed, R.M. Capture of iodide from wastewater by effective adsorptive membrane synthesized from MIL-125-NH2 and cross-linked chitosan. Carbohydr. Polym. 2020, 231, 115742. [Google Scholar] [CrossRef]
- Amiri, A.; Tayebee, R.; Abdar, A.; Narenji Sani, F. Synthesis of a zinc-based metal-organic framework with histamine as an organic linker for the dispersive solid-phase extraction of organophosphorus pesticides in water and fruit juice samples. J. Chromatogr. A 2019, 1597, 39–45. [Google Scholar] [CrossRef]
- Yang, X.; Xia, Y. Urea-modified metal-organic framework of type MIL-101(Cr) for the preconcentration of phosphorylated peptides. Microchim. Acta 2016, 183, 2235–2240. [Google Scholar] [CrossRef]




| Amino Acid Ligand | Analytes | Sample | Method | Remarkable Features | Ref. |
|---|---|---|---|---|---|
| Alanine, serine and valine | Carvone (terpenoid) | Ethanol | s-SPE-CD | E.E. (18.6–43.2%) | [23] |
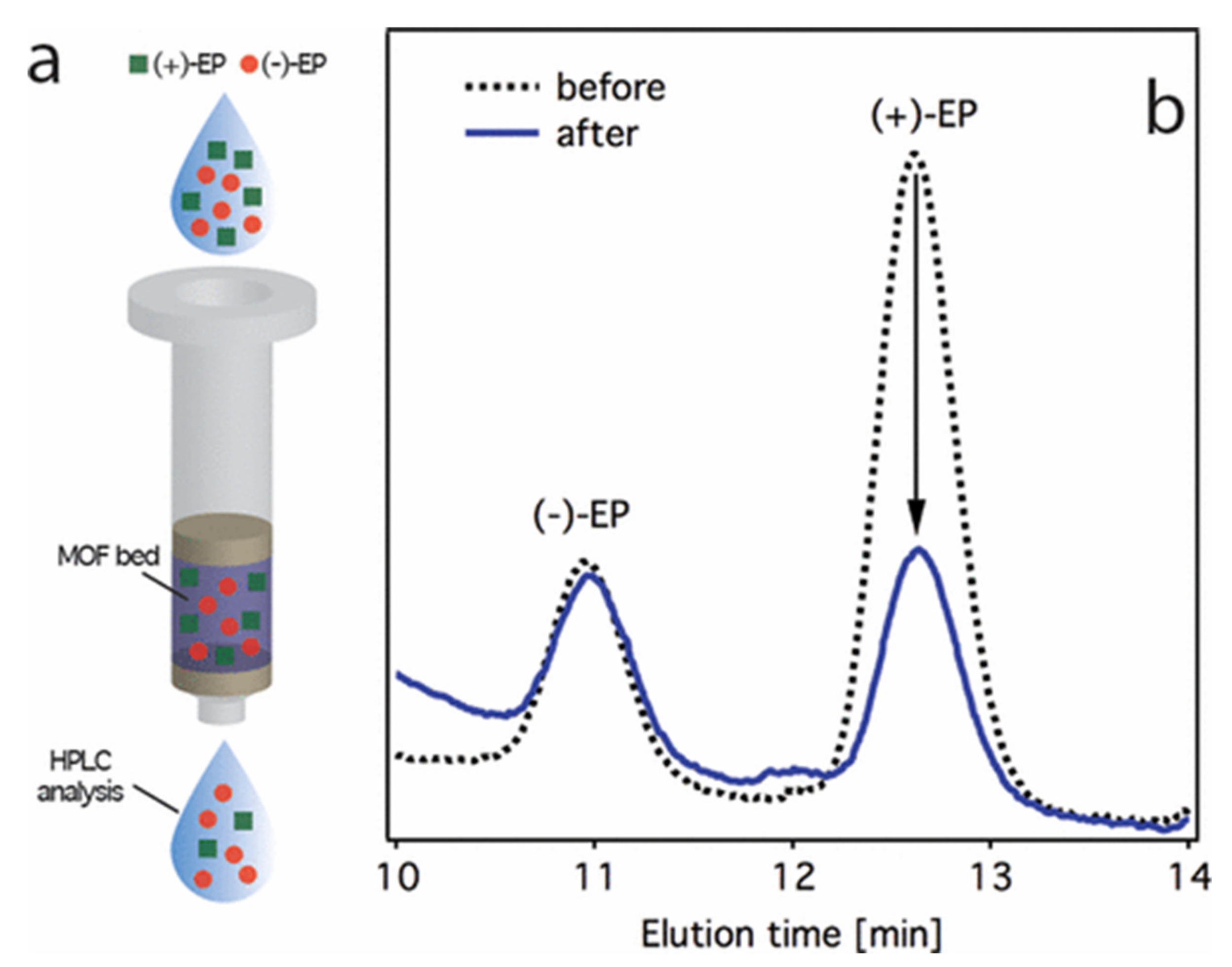
| Histidine | racemic 1-phenylethanol | Gas | GC | E.E. (76% with R form); good reproducibility | [24] |
| Methionine | Au(I) and Au(III) | Water | d-SPE-ICP-AES and SEM | R. (~100%);%); Q./T.A. (300/3 h, 600/1 h mg g−1) a | [25] |
| Methionine | Hg(II) | Tap water | d-SPE-ICP-MS | R. (99.95%); Q. (284 mg g−1); T.A. <15 min | [26] |
| Cysteine | N-Linked Glycopeptides | HeLa cell lysate | d-SPE-MALDI-TOF MS | R. (~80%); Q. (150 mg g−1); LOD (1 fmol); A.T. (5 min); selectivity (1:50) | [27] |
| Arginine | Phosphopeptides | Tryptic digest of rat brain lysate | m-SPE-MALDI-TOF MS | LOD (10 fmol); Selectivity (1:1000) | [28] |
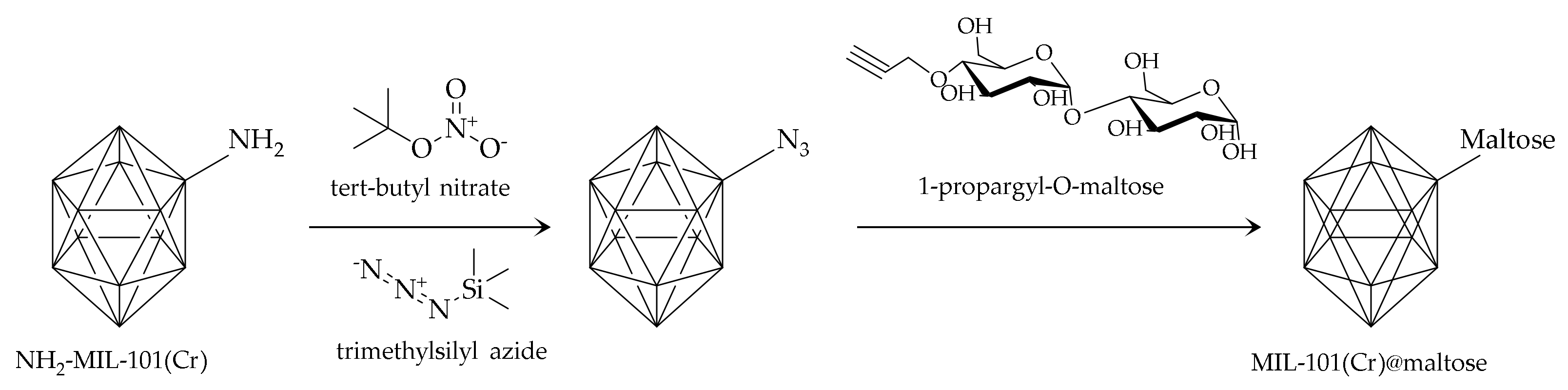
| Serine | B-vitamins | Juices and E.D. | SPE-HPLC-UV | 25 mg of sorbent; 5 reuses; Q. (50 mg g−1); LOD (0.4–1.12 μg L−1); R. (75–123%) | [29] |
| Cysteine | Glycopeptides and phosphopeptides | H.C.L.P. | MSPE-MALDI-TOF MS | LOD (0.1 fmol mL−1); 5 reuses; high selectivity (1:100) | [30] |
| Cysteine | Cd(II) | Wastewater | m-SPE-ICP-AES UAE-FLD | R.E. (~100); 5 reuses; 50 mg of sorbent; A.T. (10 min); Q. (248.24 mg g−1) | [31] |
| Glycine | Drugs | Water | d-SPE-HPLC (TGA and powder XRD patterns, before and after) | Q. (30–180 mg g−1) | [32] |
| Cysteine | Patulin (toxin) | Apple juice | s-SPE-HPLC-UV | 50 mg of sorbent, Q. (4.38 mg g−1) A.T. (3 h), non-toxic at <10 mg L−1 | [33] |
| Affinity Based MOF Sorbent | Analytes | Matrix | LOD | Remarkable Features | Reference |
|---|---|---|---|---|---|
| Ab@ZIF-8/Fe3O4 | Staphylococcus Aureus | Milk | 300 cfu mL−1 | Selective in the presence of other bacteria | [59] |
| Co3Fe-MMOF@PDA@Ab | Aeromonas Hydrophila | Water | 17 cfu mL−1 | R. (60–70%), Selective | [60] |
| MOF-5-Apt | PCBs | Fish | 0.003–0.004 ng mL−1 | R. (89–97%); E.F. (1930–2304); RSD. (<5%) | [62] |
| Fe3O4@PDA@UiO-66-Apt | PCBs | Soil | 0.010–0.015 ng mL−1 | R. (89–95%); Q. (195–218 ng mg−1); 60 reuses | [63] |
| Cu/UiO-66@Apt | PCBs | Serum | 2.1 pg mL−1 | R. (88–102%); Q. (37.17 μg g−1); 10 reuses; A.T. (30 min); RSD. (<4%); Selective | [64] |
| Apt-MMIL-101 | OTA | Corn and peanut | 0.067 ng L−1 | R. (83–108%); Q. (553 pmol mg−1); 12 reuses; E.F. (167); RSD (12%); A.T. (40 min); D.T. (30 min) | [65] |
| Cu/UiO-66@Apt | CAP | Fish | 0.09 nmol L−1 | R. (97–104%); A.T. (40 min); Selective | [66] |
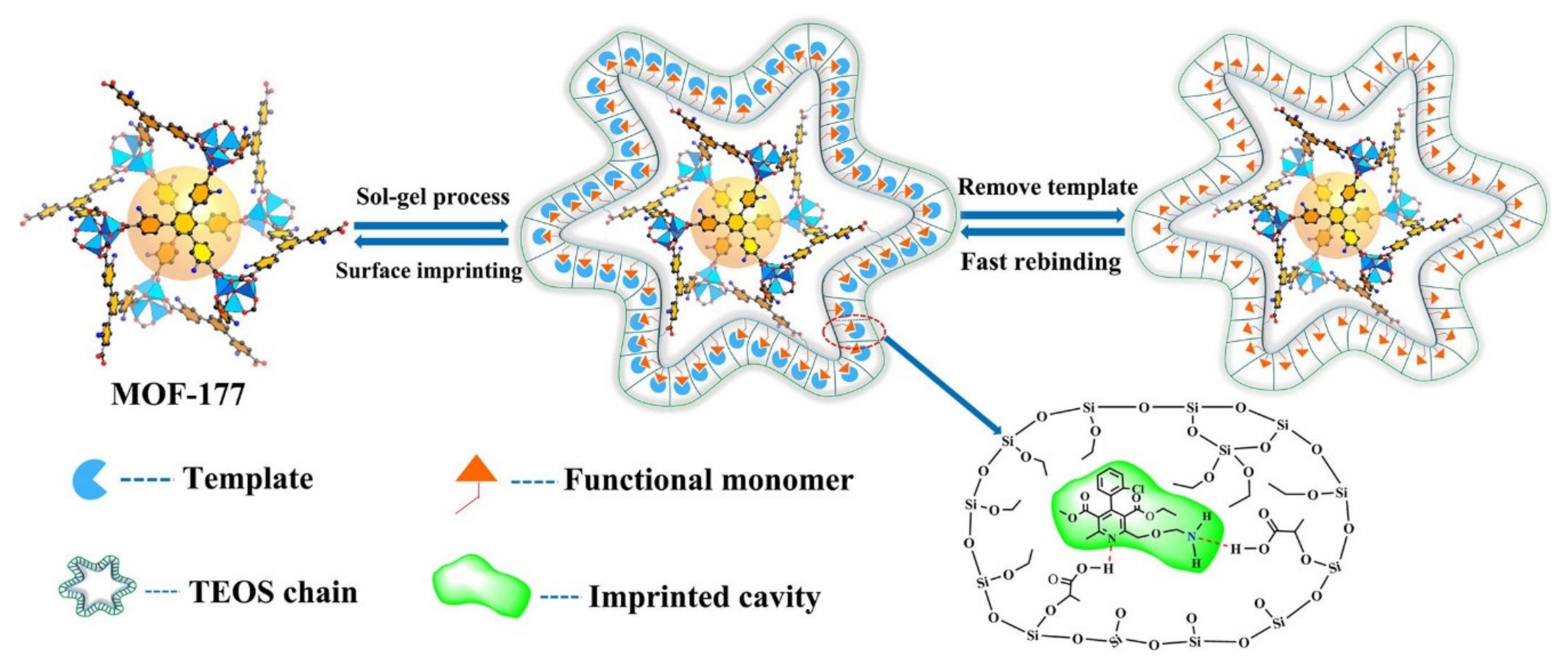
| MOF-177@MIPs | S-amlodipine | – | – | Q. (536 mg g−1); I.F. (5.79) | [67] |
| NH2-MIL-101@MIP | DS | Urine | – | R. (88–102%); Q. (15.78 mg g−1); RSD. (7%); A.T. (20 min); Selective | [68] |
| UiO-66-NH2@MIP | AT (B1, B2, G1, G2) | Grain | 90–130 ng kg−1 | R. (74–99%); Q. (4–10 mg g−1); RSD. (<6%); Selective | [69] |
| UiO-66-NH2@MIP | TCs | Chicken muscle | 0.2–0.6 ng g−1 | R. (70–95%); Q. (2200–3000 mg g−1); RSD. (<12%); A.T. (15 min); E.F. (18–37) | [70] |
| MIL-101@MIP | Metolcarb | Pear juice | 0.0689 mg L−1 | R. (74–96%); Q. (1.32 mg g−1); Selective; RSD. (<4.2%) | [71] |
| Biomolecule Ligand | Method | Analytes | Sample | R. (%)/LOD | Remarkable Features | Ref. |
|---|---|---|---|---|---|---|
| Adenine | d-SPE-UV | PCPs | Water | –/– | A.T. (12 h); 3 mg sorbent; 4 reuses | [73] |
| Adenine | SPME-GC-FID | PAHs and OCPs | Water | 80–111/2.2–2.3 ng L−1 | A.T. (40 min); D. T. (300 s) | [74] |
| Adenine | m-SPE-LC-MS | Benzodiazepines | Urine and water | 80–95/0.7–2.4 ng L−1 | A.T. (40 min); D.T. (15 min); E.F. (37–102); 10 reuses | [75] |
| Adenine | SPME-GC-FID | PAHs | Water | 80–115/20–5600 ng L−1 | A.T. (30 min) D.T. (5 min) | [76] |
| ATP | d-SPE-MALDI-TOF | Phosphopeptides | Serum and milk | 85/5 fmol | Q. (100 mg g−1); 3 reuses; A.T. (30 min) D.T. (15 min) Low RSD; Selective (1:1000) | [77] |
| 8-aminocaprylic acid | SPME-GC-MS | Nitrosamines | Latex globes | 85–113/2.6–6.1 ng L−1 | A.T: (30 min); RSD (<12%) | [78] |
| Lactate | d-SPE-NCI-GC-MS | PBDEs | Environmental water | 84–102/0.08–0.15 ng L−1 | RSD (<12%) | [79] |
| Citric anhydride | d-SPE-AAS | Pb(II) | Water | 80/– | Q. (390 mg g−1); A.T. (120 min) | [80] |
| Chitosan | d-SPE-UPLC-MS/MS | Parabens | Water | 79–102/90–450 ng L−1 | A.T. (20 min); D.T. (15 min); 10 reuses; RSD (<7.4%) | [81] |
| Sodium Alginate/Chitosan | d-SPE-HPLC-UV | Bisphenol A | Wastewater | –/– | Q. (101–137 mg g−1); A.T. (18 h) | [82] |
| Chitosan | d-SPE-UV | Iodine | Wastewater | 96/– | Q. (400 mg g−1); A.T. (90 min); 5 reuses | [83] |
| Histamine | d-SPE-GC-FID | OPPs | Water and juice | 92–100/30–210 ng L−1 | E.F. (803–914); 8 reuses | [84] |
| Urea | SPE MALDI-TOF-MS | Phosphopeptides | Nonfat milk | –/10−10M | Selectivity (1:200); 5 reuses | [85] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Cejuela, H.M.; Herrero-Martínez, J.M.; Simó-Alfonso, E.F. Recent Advances in Affinity MOF-Based Sorbents with Sample Preparation Purposes. Molecules 2020, 25, 4216. https://doi.org/10.3390/molecules25184216
Pérez-Cejuela HM, Herrero-Martínez JM, Simó-Alfonso EF. Recent Advances in Affinity MOF-Based Sorbents with Sample Preparation Purposes. Molecules. 2020; 25(18):4216. https://doi.org/10.3390/molecules25184216
Chicago/Turabian StylePérez-Cejuela, Héctor Martínez, José Manuel Herrero-Martínez, and Ernesto F. Simó-Alfonso. 2020. "Recent Advances in Affinity MOF-Based Sorbents with Sample Preparation Purposes" Molecules 25, no. 18: 4216. https://doi.org/10.3390/molecules25184216
APA StylePérez-Cejuela, H. M., Herrero-Martínez, J. M., & Simó-Alfonso, E. F. (2020). Recent Advances in Affinity MOF-Based Sorbents with Sample Preparation Purposes. Molecules, 25(18), 4216. https://doi.org/10.3390/molecules25184216