Recent Emergence of Rhenium(I) Tricarbonyl Complexes as Photosensitisers for Cancer Therapy
Abstract
1. Introduction
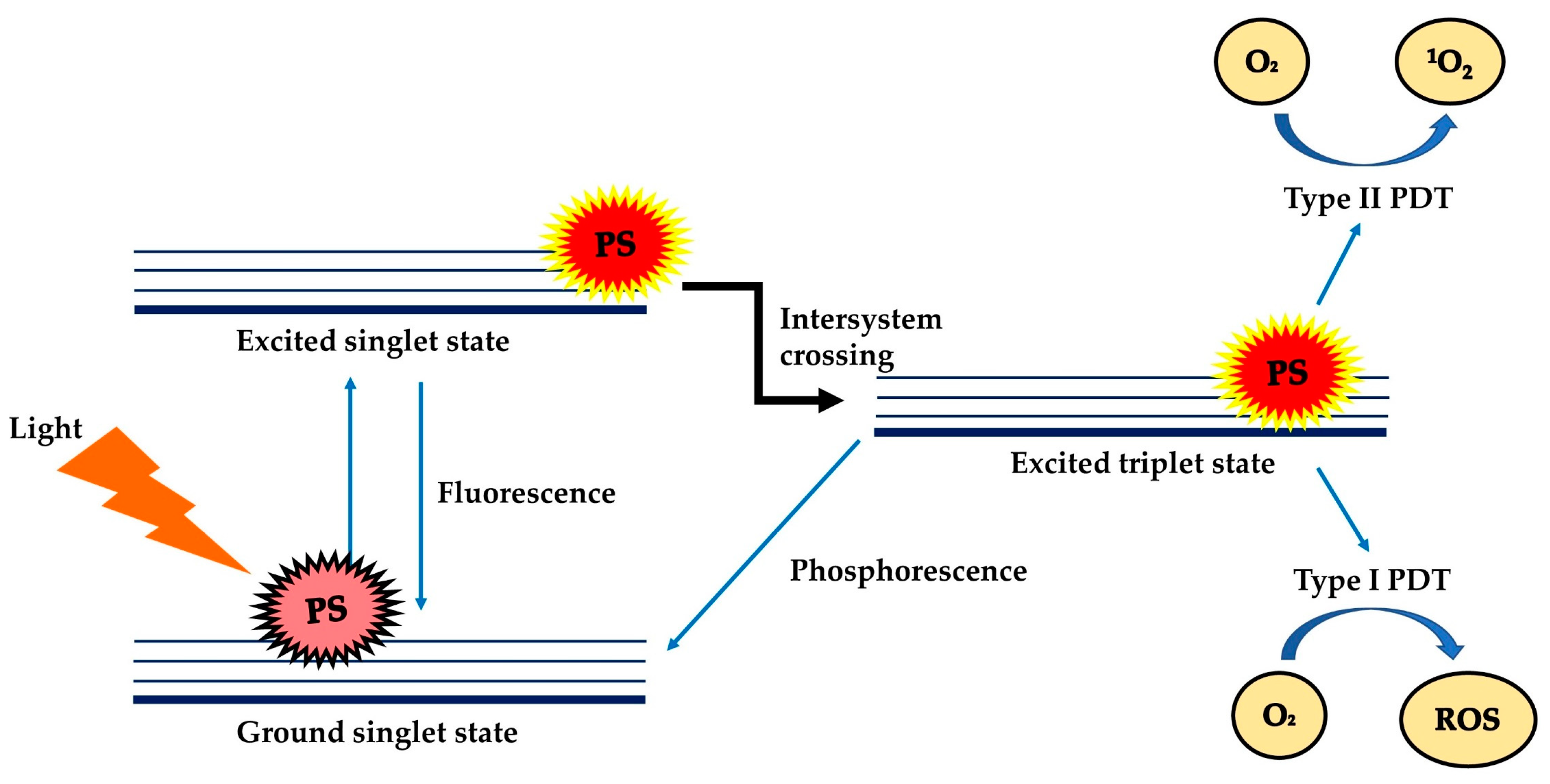
2. Photosensitisers for Photodynamic Therapy—A Brief History
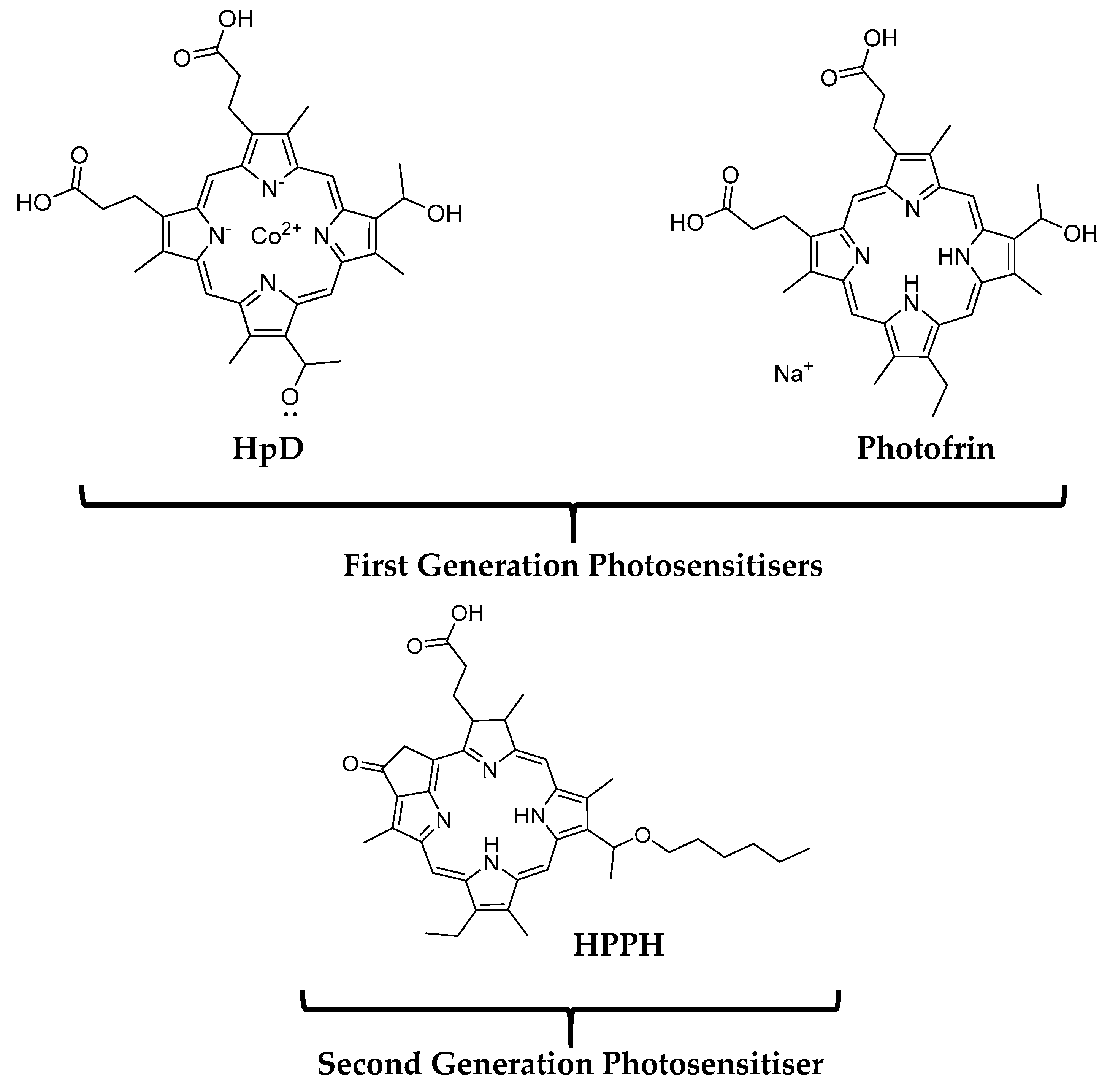
2.1. First and Second Generation Photosensitisers
2.2. Third Generation Photosensitisers
2.3. Characteristics of an Ideal Class of Photosensitisers for PDT
3. Rhenium(I) Tricarbonyl Complexes: General Overview
3.1. Background of Rhenium (Re)
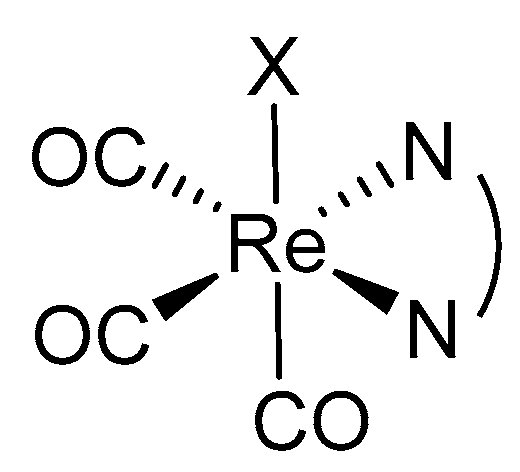
3.2. Re(I) Complexes: Brief History and Suitability as a PDT PS
4. Phototoxic Effect of Rhenium(I) Tricarbonyl Complexes
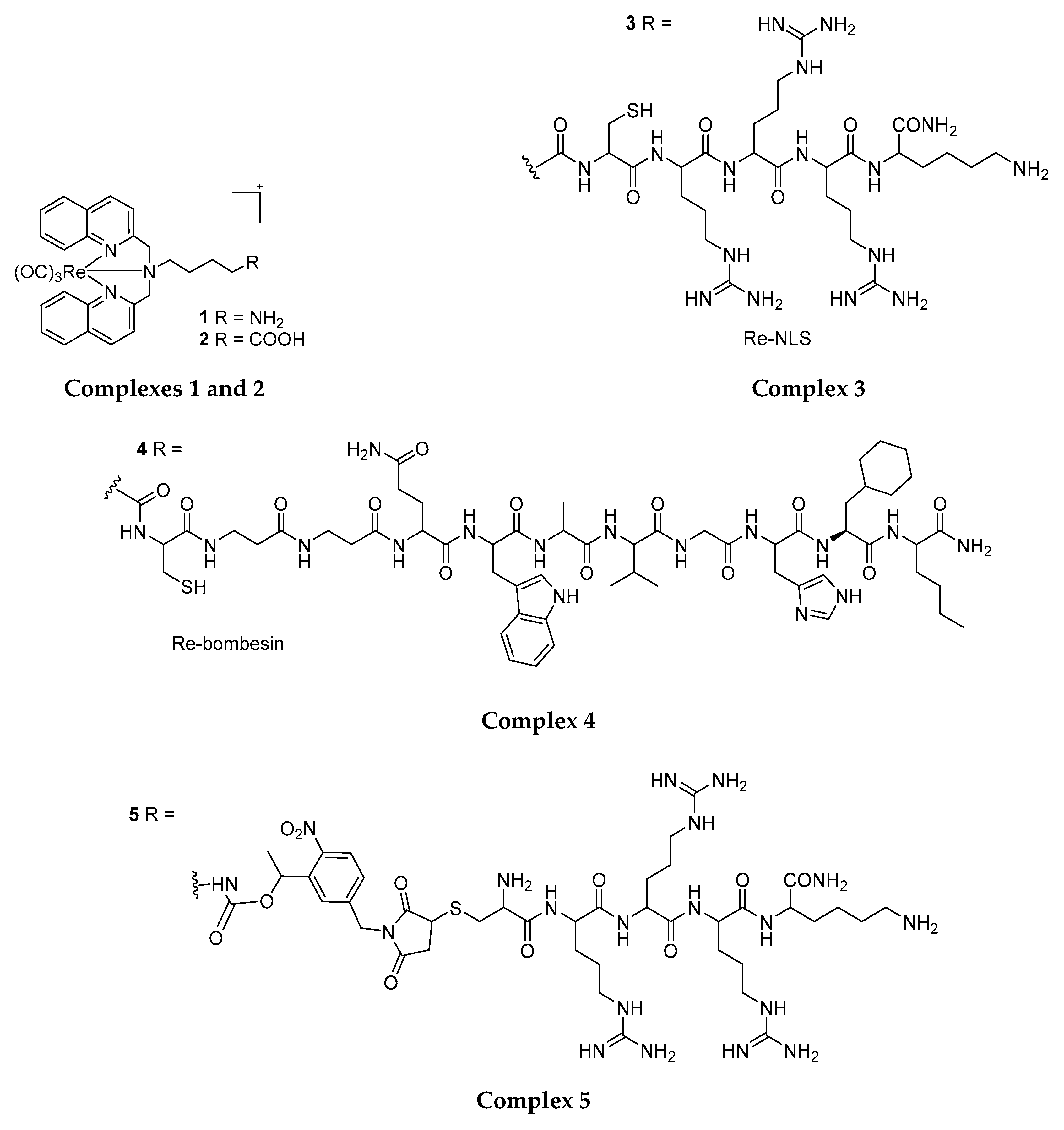
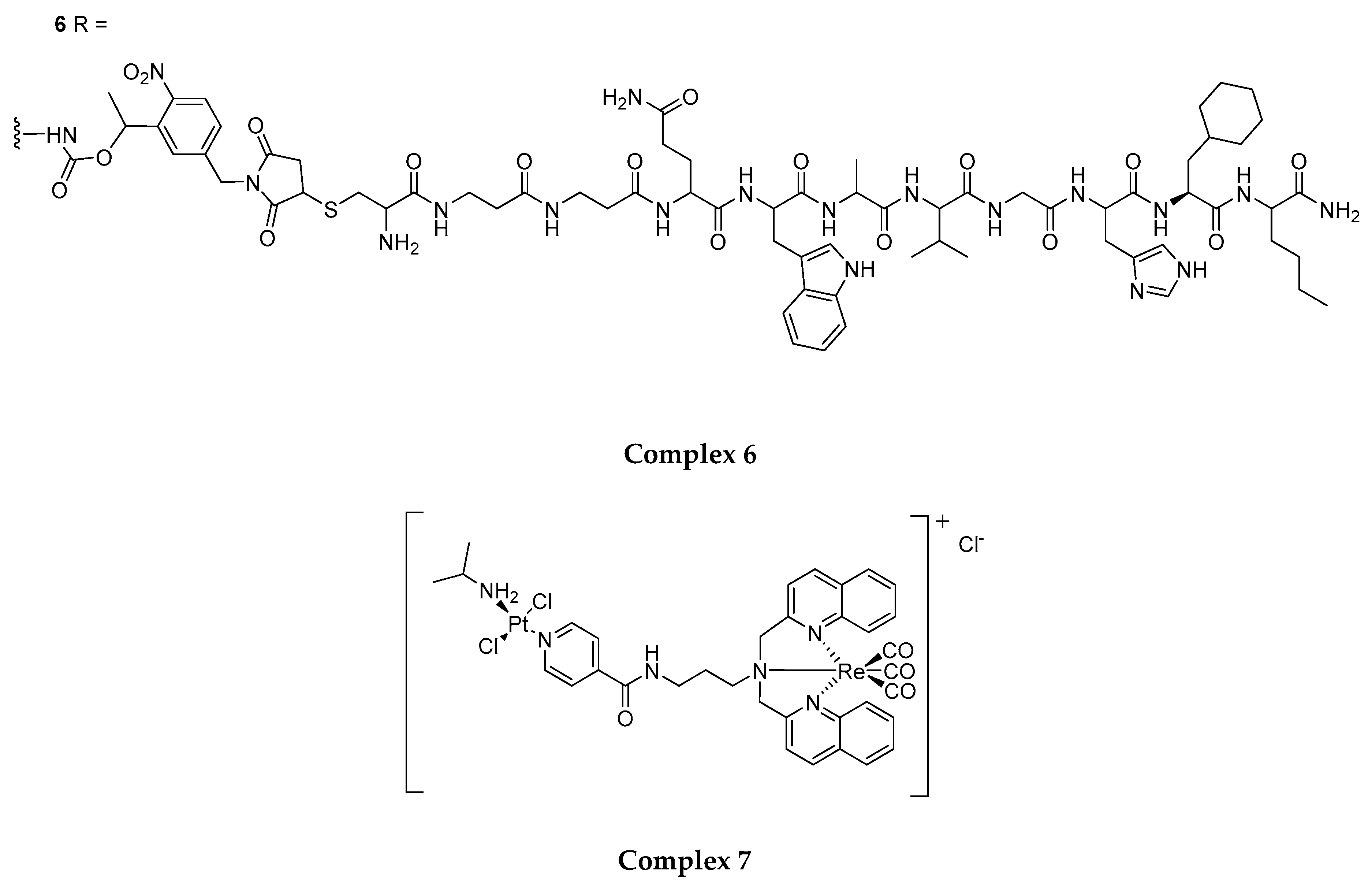
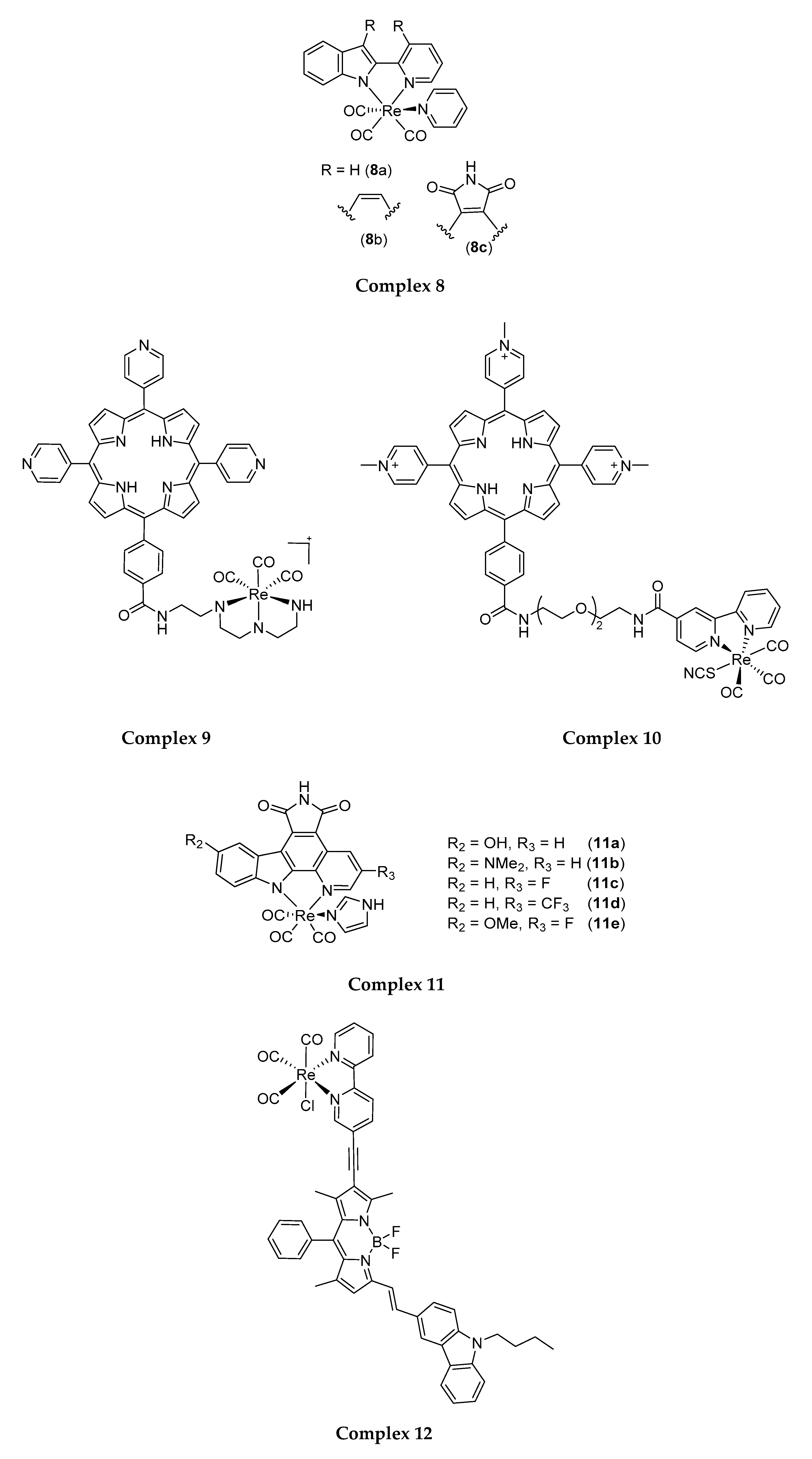
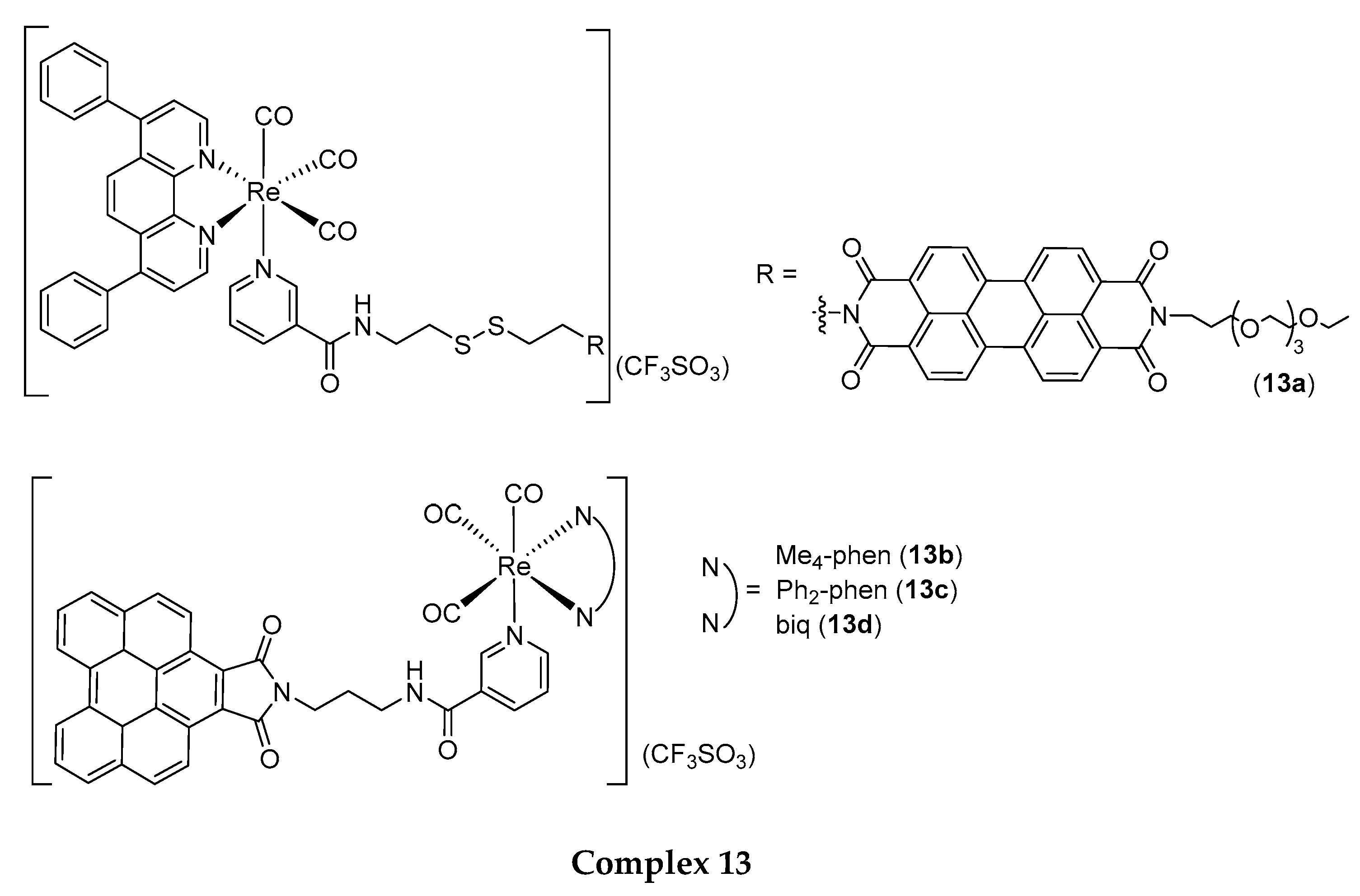
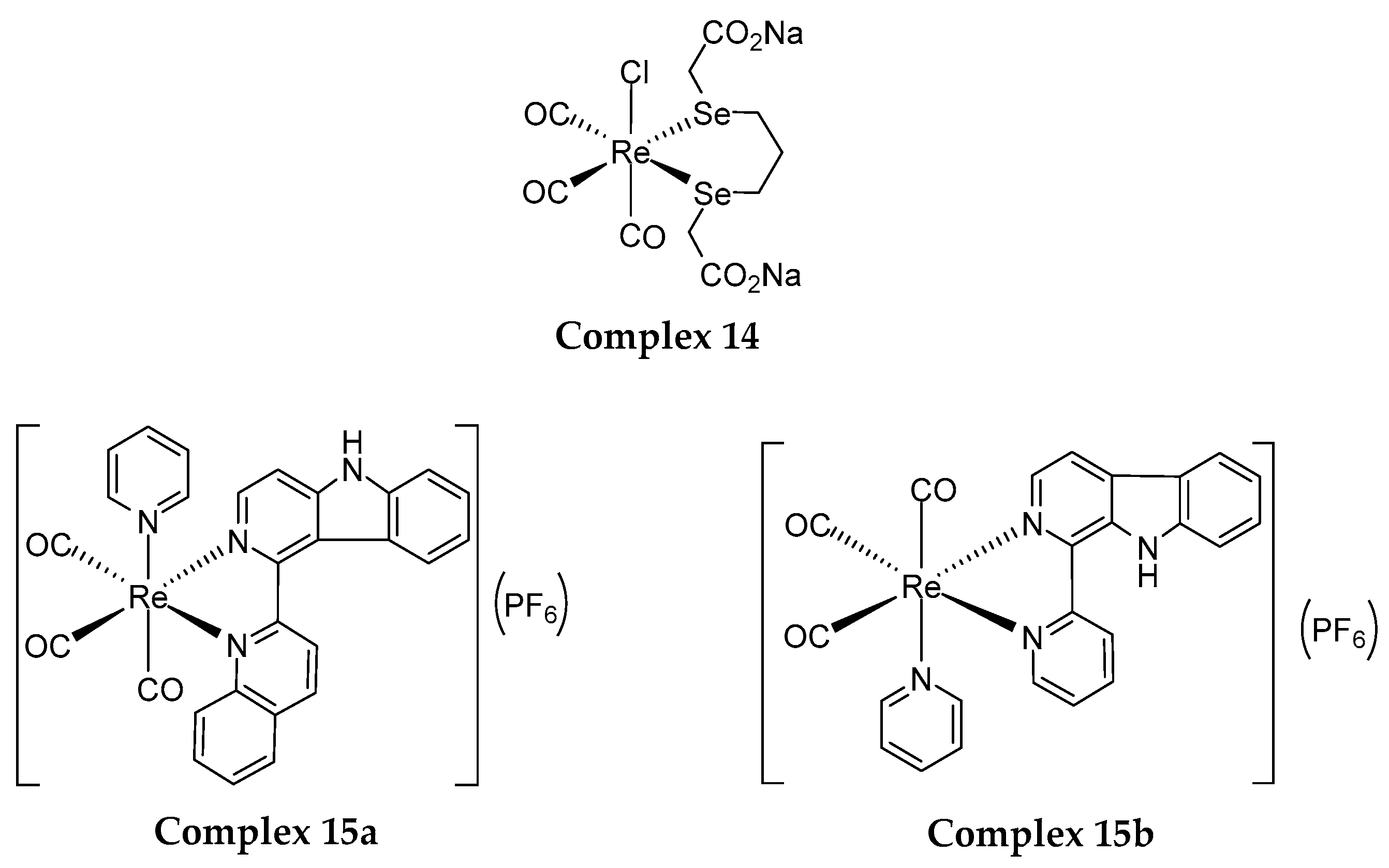
4.1. Rhenium(I) Tricarbonyl Complexes in PDT: Modifications and Advancements
4.2. Structural Modification for the Enhancement of Re(I) Complexes’ Phototoxicity, Tissue Selectivity and Photo-Stability
4.3. Structural Modification for the Improvement of Re(I) Complexes’ Photo-Absorption Profiles
5. Re(I) Tricarbonyl Complexes: In Vivo Studies
6. Future Perspective—How to Improve Anticancer Activity of Re(I) Tricarbonyl Complexes in PDT?
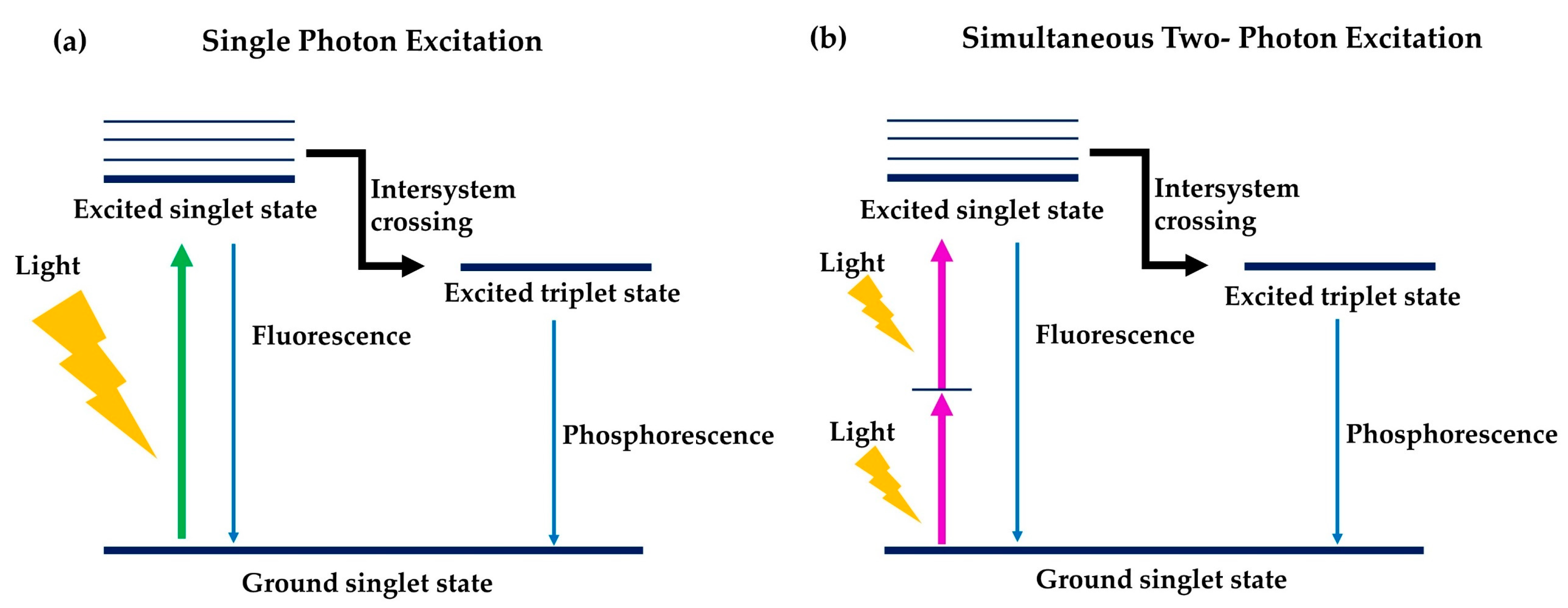
6.1. Two-Photon Photodynamic Therapy
6.2. Anticancer Combinatorial Therapy
6.3. Advancement in Nanoparticles with Photosensitisers Utilising Drug Combination Study
6.4. Photothermal Therapy
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cancer. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 24 November 2019).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA A Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Cheng, Z.; Al Zaki, A.; Hui, J.Z.; Muzykantov, V.R.; Tsourkas, A. Multifunctional nanoparticles: Cost versus benefit of adding targeting and imaging capabilities. Science 2012, 338, 903–910. [Google Scholar] [CrossRef]
- Qin, S.Y.; Zhang, A.Q.; Cheng, S.X.; Rong, L.; Zhang, X.Z. Drug self-delivery systems for cancer therapy. Biomaterials 2017, 112, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Strobel, O.; Neoptolemos, J.; Jaeger, D.; Buechler, M.W. Optimizing the outcomes of pancreatic cancer surgery. Nat. Rev. Clin. Oncol. 2019, 16, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, W.; Werner, J.; Jäger, D.; Debus, J.; Büchler, M.W. Improvement of surgical results for pancreatic cancer. Lancet Oncol. 2013, 14, e476–e485. [Google Scholar] [CrossRef]
- Auperin, A.; Le Pechoux, C.; Rolland, E.; Curran, W.J.; Furuse, K.; Fournel, P.; Belderbos, J.; Clamon, G.; Ulutin, H.C.; Paulus, R.; et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J. Clin. Oncol. 2010, 28, 2181–2190. [Google Scholar] [CrossRef]
- Crabtree, T.D.; Denlinger, C.E.; Meyers, B.F.; El Naqa, I.; Zoole, J.; Krupnick, A.S.; Kreisel, D.; Patterson, G.A.; Bradley, J.D. Stereotactic body radiation therapy versus surgical resection for stage I non–small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2010, 140, 377–386. [Google Scholar] [CrossRef]
- Grills, I.S.; Mangona, V.S.; Welsh, R.; Chmielewski, G.; McInerney, E.; Martin, S.; Wloch, J.; Ye, H.; Kestin, L.L. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non–small-cell lung cancer. J. Clin. Oncol. 2010, 28, 928–935. [Google Scholar] [CrossRef]
- Onishi, H.; Shirato, H.; Nagata, Y.; Hiraoka, M.; Fujino, M.; Gomi, K.; Karasawa, K.; Hayakawa, K.; Niibe, Y.; Takai, Y.; et al. Stereotactic body radiotherapy (SBRT) for operable stage I non–small-cell lung cancer: Can SBRT be comparable to surgery? Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1352–1358. [Google Scholar] [CrossRef]
- Sun, C.; Ansari, D.; Andersson, R.; Wu, D. Does gemcitabine-based combination therapy improve the prognosis of unresectable pancreatic cancer? World J. Gastroenterol. 2012, 18, 4944. [Google Scholar] [CrossRef]
- Chiriva-Internati, M.; Bot, A. A new era in cancer immunotherapy: Discovering novel targets and reprogramming the immune system. Int. Rev. Immunol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Cancer immunotherapy, part 3: Challenges and future trends. Pharm. Ther. 2017, 42, 514–521. [Google Scholar]
- Suda, K.; Mitsudomi, T. Successes and limitations of targeted cancer therapy in lung cancer. Prog. Tumor Res. 2014, 41, 62–77. [Google Scholar] [PubMed]
- Bhuvaneswari, R.; Gan, Y.Y.; Soo, K.C.; Olivo, M. The effect of photodynamic therapy on tumor angiogenesis. Cell. Mol. Life Sci. 2009, 66, 2275–2283. [Google Scholar] [CrossRef]
- Oleinick, N.L.; Evans, H.H. The photobiology of photodynamic therapy: Cellular targets and mechanisms. Radiat. Res. 1998, 150, S146–S156. [Google Scholar] [CrossRef]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef]
- Bonnett, R. Photosensitizers of the porphyrin and phthalocyanine series for photodynamic therapy. Chem. Soc. Rev. 1995, 24, 19–33. [Google Scholar] [CrossRef]
- Leonidova, A.; Pierroz, V.; Rubbiani, R.; Lan, Y.; Schmitz, A.G.; Kaech, A.; Sigel, R.K.O.; Ferrari, S.; Gasser, G. Photo-induced uncaging of a specific Re(I) organometallic complex in living cells. Chem. Sci. 2014, 5, 4044–4056. [Google Scholar] [CrossRef]
- Zuluaga, M.; Lange, N. Combination of photodynamic therapy with anti-cancer agents. Curr. Med. Chem. 2008, 15, 1655–1673. [Google Scholar] [CrossRef]
- Robertson, C.A.; Evans, D.H.; Abrahamse, H. Photodynamic therapy (PDT): A short review on cellular mechanisms and cancer research applications for PDT. J. Photochem. Photobiol. B Biol. 2009, 96, 1–8. [Google Scholar] [CrossRef]
- Bown, S.G.; Rogowska, A.Z.; Whitelaw, D.E.; Lees, W.R.; Lovat, L.B.; Ripley, P.; Jones, L.; Wyld, P.; Gillams, A.; Hatfield, A.W.R. Photodynamic therapy for cancer of the pancreas. Gut 2002, 50, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Alexiades-Armenakas, M. Laser-mediated photodynamic therapy. Clin. Dermatol. 2006, 24, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.C.; Patterson, M.S.; Lilge, L. Implicit and Explicit Dosimetry in Photodynamic Therapy: A New Paradigm; Springer: London, UK, 1997. [Google Scholar]
- Robinson, D.J.; de Bruijn, H.S.; van der Veen, N.; Stringer, M.R.; Brown, S.B.; Star, W.M. Fluorescence photobleaching of ALA-induced protoporphyrin IX during photodynamic therapy of normal hairless mouse skin: The effect of light dose and irradiance and the resulting biological effect. Photochem. Photobiol. 1998, 67, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Boere, I.A.; Robinson, D.J.; de Bruijn, H.S.; van den Boogert, J.; Tilanus, H.W.; Sterenborg, H.J.C.M.; De Bruin, R.W.F. Monitoring in situ dosimetry and protoporphyrin IX fluorescence photobleaching in the normal rat esophagus during 5-aminolevulinic acid photodynamic therapy. Photochem. Photobiol. 2003, 78, 271. [Google Scholar] [CrossRef]
- Ackroyd, R.; Kelty, C.; Brown, N.; Reed, M. The history of photodetection and photodynamic therapy. Photochem. Photobiol. 2001, 74, 656. [Google Scholar] [CrossRef]
- Allison, R.R.; Sibata, C.H. Oncologic photodynamic therapy photosensitizers: A clinical review. Photodiagnosis Photodyn. Ther. 2010, 7, 61–75. [Google Scholar] [CrossRef]
- Wu, L.; Murphy, R.P. Photodynamic therapy: A new approach to the treatment of choroidal neovascularization secondary to age-related macular degeneration. Curr. Opin. Ophthalmol. 1999, 10, 217–220. [Google Scholar] [CrossRef]
- Brown, J.M. Tumor hypoxia in cancer therapy. Methods Enzymol. 2007, 435, 295–321. [Google Scholar]
- Bozzini, G.; Colin, P.; Betrouni, N.; Maurage, C.A.; Leroy, X.; Simonin, S.; Martin-Schmitt, C.; Villers, A.; Mordon, S. Efficiency of 5-ALA mediated photodynamic therapy on hypoxic prostate cancer: A preclinical study on the dunning R3327-AT2 rat tumor model. Photodiagnosis Photodyn. Ther. 2013, 10, 296–303. [Google Scholar] [CrossRef]
- Nowak-Stepniowska, A.; Pergoł, P.; Padzik-Graczyk, A. Photodynamic method of cancer diagnosis and therapy--mechanisms and applications. Postep. Biochem. 2013, 59, 53–63. [Google Scholar]
- Juzeniene, A.; Moan, J. The history of PDT in Norway. part one: Identification of basic mechanisms of general PDT. Photodiagn. Photodyn. Ther. 2007, 4, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Luksiene, Z. Photodynamic therapy: Mechanism of action and ways to improve the efficiency of treatment. Medicina (Kaunaslithuania) 2003, 39, 1137–1150. [Google Scholar]
- Leonidova, A.; Pierroz, V.; Rubbiani, R.; Heier, J.; Ferrari, S.; Gasser, G. Towards cancer cell-specific phototoxic organometallic rhenium(I) complexes. Dalton Trans. 2014, 43, 4287–4294. [Google Scholar] [CrossRef] [PubMed]
- Kitanovic, I.; Can, S.; Alborzinia, H.; Kitanovic, A.; Pierroz, V.; Leonidova, A.; Pinto, A.; Spingler, B.; Ferrari, S.; Molteni, R.; et al. A deadly organometallic luminescent probe: Anticancer activity of a Re(I) bisquinoline complex. Chem.—A Eur. J. 2014, 20, 2496–2507. [Google Scholar] [CrossRef]
- Kimura, M.; Miyajima, K.; Kojika, M.; Kono, T.; Kato, H. Photodynamic therapy (PDT) with chemotherapy for advanced lung cancer with airway stenosis. Int. J. Mol. Sci. 2015, 16, 25466–25475. [Google Scholar] [CrossRef]
- Du, K.; Mick, R.; Busch, T.; Zhu, T.; Finlay, J.; Yu, G.; Yodh, A.G.; Malkowicz, S.B.; Smith, D.; Whittington, R.; et al. Preliminary results of interstitial motexafin lutetium-mediated PDT for prostate cancer. Lasers Surg. Med. Off. J. Am. Soc. Laser Med. Surg. 2006, 38, 427–434. [Google Scholar] [CrossRef]
- Triesscheijn, M.; Ruevekamp, M.; Aalders, M.; Baas, P.; Stewart, F.A. Outcome of mTHPC mediated photodynamic therapy is primarily determined by the vascular response. Photochem. Photobiol. 2005, 81, 1161–1167. [Google Scholar] [CrossRef]
- Brown, S.B.; Brown, E.A.; Walker, I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004, 5, 497–508. [Google Scholar] [CrossRef]
- Berenbaum, M.; Bonnett, R.; Chevretton, E.; Akande-Adebakin, S.; Ruston, M. Selectivity ofmeso-tetra (hydroxyphenyl) porphyrins and chlorins and of photofrin II in causing photodamage in tumour, skin, muscle and bladder. The comcept of cost-benefit in analysing the results. Lasers Med. Sci. 1993, 8, 235–243. [Google Scholar] [CrossRef]
- Breskey, J.D.; Lacey, S.E.; Vesper, B.J.; Paradise, W.A.; Radosevich, J.A.; Colvard, M.D. Photodynamic therapy: Occupational hazards and preventative recommendations for clinical administration by healthcare providers. Photomed. Laser Surg. 2013, 31, 398–407. [Google Scholar] [CrossRef]
- Reynolds, T. Photodynamic therapy expands its horizons. J. Natl. Cancer Inst. 1997. [Google Scholar] [CrossRef] [PubMed]
- Bellnier, D.A.; Greco, W.R.; Nava, H.; Loewen, G.M.; Oseroff, A.R.; Dougherty, T.J. Mild skin photosensitivity in cancer patients following injection of photochlor (2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a; HPPH) for photodynamic therapy. Cancer Chemother. Pharmacol. 2006, 57, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Bellnier, D.A.; Greco, W.R.; Loewen, G.M.; Nava, H.; Oseroff, A.R.; Pandey, R.K.; Tsuchida, T.; Dougherty, T.J. Population pharmacokinetics of the photodynamic therapy agent 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a in cancer patients. Cancer Res. 2003, 63, 1806–1813. [Google Scholar] [PubMed]
- Mody, T.D. Pharmaceutical development and medical applications of porphyrin-type macrocycles. J. Porphyr. Phthalocyanines 2000, 4, 362–367. [Google Scholar] [CrossRef]
- Hillemanns, P.; Petry, K.; Soergel, P.; Collinet, P.; Ardaens, K.; Gallwas, J.; Luyten, A.; Dannecker, C. Efficacy and safety of hexaminolevulinate photodynamic therapy in patients with low-grade cervical intraepithelial neoplasia. Lasers Surg. Med. 2014, 46, 456–461. [Google Scholar] [CrossRef]
- O’Connor, A.E.; Gallagher, W.M.; Byrne, A.T. Porphyrin and nonporphyrin photosensitizers in oncology: Preclinical and clinical advances in photodynamic therapy. Photochem. Photobiol. 2009, 85, 1053–1074. [Google Scholar] [CrossRef]
- Zeitouni, N.C.; Oseroff, A.; Najarian, D.J. Photodynamic therapy. In Skin Cancer Management; Springer: New York, NY, USA, 2009; pp. 41–56. [Google Scholar]
- Arnold, R. Dyes and pigments: New research; Nova Science: Hauppauge, NY, USA, 2009. [Google Scholar]
- Ashur, I.; Goldschmidt, R.; Pinkas, I.; Salomon, Y.; Szewczyk, G.; Sarna, T.; Scherz, A. Photocatalytic generation of oxygen radicals by the water-soluble bacteriochlorophyll derivative WST11, noncovalently bound to serum albumin. J. Phys. Chem. A 2009, 113, 8027–8037. [Google Scholar] [CrossRef]
- Sickenberg, M.; Schmidt-Erfurth, U.; Miller, J.W.; Pournaras, C.J.; Zografos, L.; Piguet, B.; Donati, G.; Laqua, H.; Barbazetto, I.; Gragoudas, E.S.; et al. A preliminary study of photodynamic therapy using verteporfin for choroidal neovascularization in pathologic myopia, ocular histoplasmosis syndrome, angioid streaks, and idiopathic causes. Arch. Ophthalmol. 2000, 118, 327–336. [Google Scholar] [CrossRef]
- Mellish, K.J.; Brown, S.B. Verteporfin: A milestone in opthalmology and photodynamic therapy. Expert Opin. Pharmacother. 2001, 2, 351–361. [Google Scholar] [CrossRef]
- Gold, M.H. History of Photodynamic Therapy; Springer: New York, NY, USA, 2011; pp. 1–4. [Google Scholar]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Baas, P.; van Mansom, I.; van Tinteren, H.; Stewart, F.A.; van Zandwijk, N. Effect of N-acetylcysteïne on photofrin-induced skin photosensitivity in patients. Lasers Surg. Med. 1995, 16, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.K.; Fong, L.S.; Zhang, Y. Nanoparticles in photodynamic therapy: An emerging paradigm. Adv. Drug Deliv. Rev. 2008, 60, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, C.; Figueiró Longo, J.P.; Azevedo, R.B.; Zhang, H.; Muehlmann, L.A. An updated overview on the development of new photosensitizers for anticancer photodynamic therapy. Acta Pharm. Sin. B 2018, 8, 137–146. [Google Scholar] [CrossRef]
- Kessel, D.; Thompson, P. Purification and analysis of hematoporphyrin and hematoporphyrin derivative by gel exclusion and reverse-phase chromatography. Photochem. Photobiol. 1987, 46, 1023–1025. [Google Scholar] [CrossRef]
- Yoon, I.; Li, J.Z.; Shim, Y.K. Advance in photosensitizers and light delivery for photodynamic therapy. Clin. Endosc. 2013, 46, 7–23. [Google Scholar] [CrossRef]
- Josefsen, L.B.; Boyle, R.W. Photodynamic therapy: Novel third-generation photosensitizers one step closer? Br. J. Pharmacol. 2008, 154, 1–3. [Google Scholar] [CrossRef]
- Kataoka, H.; Nishie, H.; Hayashi, N.; Tanaka, M.; Nomoto, A.; Yano, S.; Joh, T. New photodynamic therapy with next-generation photosensitizers. Ann. Transl. Med. 2017, 5, 183. [Google Scholar] [CrossRef]
- Savellano, M.D.; Hasan, T. Targeting cells that overexpress the epidermal growth factor receptor with polyethylene glycolated BPD verteporfin photosensitizer immunoconjugates. Photochem. Photobiol. 2003, 77, 431. [Google Scholar] [CrossRef]
- Lee, H.M.; Jeong, Y.; Kim, D.H.; Kwak, T.W.; Chung, C.; Kim, C.H.; Kang, D.H. Ursodeoxycholic acid-conjugated chitosan for photodynamic treatment of HuCC-T1 human cholangiocarcinoma cells. Int. J. Pharm. 2013, 454, 74–81. [Google Scholar] [CrossRef]
- Tarragó-Trani, M.T.; Jiang, S.; Harich, K.C.; Storrie, B. Shiga-like toxin subunit B (SLTB)-enhanced delivery of chlorin e6 (Ce6) improves cell killing. Photochem. Photobiol. 2006, 82, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Gariépy, J. The use of shiga-like toxin 1 in cancer therapy. Crit. Rev. Oncol. 2001, 39, 99–106. [Google Scholar] [CrossRef]
- Mari, C.; Pierroz, V.; Ferrari, S.; Gasser, G. Combination of Ru(II) complexes and light: New frontiers in cancer therapy. Chem. Sci. 2015, 6, 2660–2686. [Google Scholar] [CrossRef] [PubMed]
- Jakubaszek, M.; Goud, B.; Ferrari, S.; Gasser, G. Mechanisms of action of Ru(II) polypyridyl complexes in living cells upon light irradiation. Chem. Commun. 2018, 54, 13040–13059. [Google Scholar] [CrossRef]
- McKenzie, L.K.; Bryant, H.E.; Weinstein, J.A. Transition metal complexes as photosensitisers in one-and two-photon photodynamic therapy. Coord. Chem. Rev. 2019, 379, 2–29. [Google Scholar] [CrossRef]
- Zeng, L.; Gupta, P.; Chen, Y.; Wang, E.; Ji, L.; Chao, H.; Chen, Z.S. The development of anticancer ruthenium(II) complexes: From single molecule compounds to nanomaterials. Chem. Soc. Rev. 2017, 46, 5771–5804. [Google Scholar] [CrossRef]
- Soliman, N.; McKenzie, L.K.; Karges, J.; Bertrand, E.; Tharaud, M.; Jakubaszek, M.; Guérineau, V.; Goud, B.; Hollenstein, M.; Gasser, G.; et al. Ruthenium-initiated polymerization of lactide: A route to remarkable cellular uptake for photodynamic therapy of cancer. Chem. Sci. 2020, 11, 2657–2663. [Google Scholar] [CrossRef]
- Knoll, J.D.; Turro, C. Control and utilization of ruthenium and rhodium metal complex excited states for photoactivated cancer therapy. Coord. Chem. Rev. 2015, 282, 110–126. [Google Scholar] [CrossRef]
- Monro, S.; Colon, K.L.; Yin, H.; Roque, I.I.I.J.; Konda, P.; Gujar, S.; Thummel, R.P.; Lilge, L.; Cameron, C.G.; McFarland, S.A. Transition metal complexes and photodynamic therapy from a tumor-centered approach: Challenges, opportunities, and highlights from the development of TLD1433. Chem. Rev. 2018, 119, 797–828. [Google Scholar] [CrossRef]
- McFarland, S.A.; Mandel, A.; Dumoulin-White, R.; Gasser, G. Metal-based photosensitizers for photodynamic therapy: The future of multimodal oncology? Curr. Opin. Chem. Biol. 2020, 56, 23–27. [Google Scholar] [CrossRef]
- Brandis, A.S.; Salomon, Y.; Scherz, A. Chlorophyll sensitizers in photodynamic therapy. In Chlorophylls and Bacteriochlorophylls; Advances in Photosynthesis and Respiration; Springer: Dordrecht, The Netherlands; pp. 461–483.
- Sternberg, E.D.; Dolphin, D.; Brückner, C. Porphyrin-based photosensitizers for use in photodynamic therapy. Tetrahedron 1998, 54, 4151–4202. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one—Photosensitizers, photochemistry and cellular localization. Photodiagn. Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef]
- Celli, J.P.; Spring, B.Q.; Rizvi, I.; Evans, C.L.; Samkoe, K.S.; Verma, S.; Pogue, B.W.; Hasan, T. Imaging and photodynamic therapy: Mechanisms, monitoring, and optimization. Chem. Rev. 2010, 110, 2795–2838. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Bleam, W.F. Soil and Environmental Chemistry; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Greenwood. Chemistry of Elements. Sykepleien 1968, 55, 412. [Google Scholar]
- Emsley, J. Nature’s Building Blocks: Everything You Need to Know about the Elements; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Machlan, L.A.; Gramlich, J.W.; Powell, L.J.; Lamhert, G.M. Absolute isotopic abundance ratio and atomic weight of a reference sample of gallium. J. Res. Natl. Bur. Stand. 1986, 91, 323–331. [Google Scholar] [CrossRef]
- Brodzinski, R.L.; Conway, D.C. Decay of rhenium-187. Phys. Rev. 1965, 138, B1368. [Google Scholar] [CrossRef]
- Phillips, W.T.; Goins, B.; Bao, A.; Vargas, D.; Guttierez, J.E.; Trevino, A.; Miller, J.R.; Henry, J.; Zuniga, R.; Vecil, G.; et al. Rhenium-186 liposomes as convection-enhanced nanoparticle brachytherapy for treatment of glioblastoma. Neuro-oncology 2012, 14, 416–425. [Google Scholar] [CrossRef]
- Garcia, C.V.; Parrilha, G.L.; Rodrigues, B.L.; Teixeira, S.F.; De Azevedo, R.A.; Ferreira, A.K.; Beraldo, H. Tricarbonylrhenium(I) complexes with 2-acetylpyridine-derived hydrazones are cytotoxic to NCI-H460 human large cell lung cancer. New J. Chem. 2016, 40, 7379–7387. [Google Scholar] [CrossRef]
- Jeong, J.M.; Knapp, F.F. Use of the oak ridge national laboratory tungsten-188/rhenium-188 generator for preparation of the rhenium-188 HDD/lipiodol complex for trans-arterial liver cancer therapy. Semin. Nucl. Med. 2008, 38, S19–S29. [Google Scholar] [CrossRef]
- Haase, A.A.; Bauer, E.B.; Kühn, F.E.; Crans, D.C. Speciation and toxicity of rhenium salts, organometallics and coordination complexes. Coord. Chem. Rev. 2019, 394, 135–161. [Google Scholar] [CrossRef]
- Bauer, E.B.; Haase, A.A.; Reich, R.M.; Crans, D.C.; Kühn, F.E. Organometallic and coordination rhenium compounds and their potential in cancer therapy. Coord. Chem. Rev. 2019, 393, 79–117. [Google Scholar] [CrossRef]
- Find Trials. Available online: https://clinicaltrials.gov/ (accessed on 20 April 2020).
- Maximum Tolerated Dose, Safety, and Efficacy of Rhenium Nanoliposomes in Recurrent Glioma—Full Text View. Available online: https://clinicaltrials.gov/ (accessed on 21 April 2020).
- Treatment of Non-responding to Conventional Therapy Inoperable Liver Cancers by in situ introduction of imdendrim—Full Text View. Available online: https://clinicaltrials.gov/ (accessed on 21 April 2020).
- Lipiodol as an Imaging Biomarker in Patients with Primary and Metastatic Liver Cancer—Full Text View. Available online: https://clinicaltrials.gov/ (accessed on 21 April 2020).
- Hieber, W.; Fuchs, H. About metal carbonyls. XXXIX. amine substituted rhenium carbonyls. J. Inorg. Gen. Chem. 1941, 248, 269–275. [Google Scholar]
- Pearson, R.G. Hard and soft acids and bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Zhao, Q.; Huang, C.; Li, F. Phosphorescent heavy-metal complexes for bioimaging. Chem. Soc. Rev. 2011, 40, 2508–2524. [Google Scholar] [CrossRef]
- Fernández-Moreira, V.; Thorp-Greenwood, F.L.; Coogan, M.P. Application of d6 transition metal complexes in fluorescence cell imaging. Chem. Commun. 2010, 46, 186–202. [Google Scholar] [CrossRef]
- Konkankit, C.C.; Marker, S.C.; Knopf, K.M.; Wilson, J.J. Anticancer activity of complexes of the third-row transition metals, rhenium, osmium, and iridium. Dalton Trans. 2018, 47, 9934–9974. [Google Scholar] [CrossRef]
- Amoroso, A.J.; Coogan, M.P.; Dunne, J.E.; Fernández-Moreira, V.; Hess, J.B.; Hayes, A.J.; Lloyd, D.; Millet, C.; Pope, S.J.A.; Williams, C. Rhenium fac tricarbonyl bisimine complexes: Biologically useful fluorochromes for cell imaging applications. Chem. Commun. 2007, 3066–3068. [Google Scholar] [CrossRef]
- Joshi, T.; Gasser, G. Towards tris(diimine)–ruthenium(II) and bis(quinoline)–Re(I)(CO)3 complexes as photoactivated anticancer drug candidates. Synlett 2015, 26, 275–284. [Google Scholar] [CrossRef]
- Leonidova, A.; Gasser, G. Underestimated potential of organometallic rhenium complexes as anticancer agents. Acs Chem. Biol. 2014, 9, 2180–2193. [Google Scholar] [CrossRef]
- Quental, L.; Raposinho, P.; Mendes, F.; Santos, I.; Navarro-Ranninger, C.; Alvarez-Valdes, A.; Huang, H.; Chao, H.; Rubbiani, R.; Gasser, G.; et al. Combining imaging and anticancer properties with new heterobimetallic Pt(II)/M(I) (M = Re, 99mTc) complexes. Dalton Trans. 2017, 46, 14523–14536. [Google Scholar] [CrossRef] [PubMed]
- Mari, C.; Pierroz, V.; Rubbiani, R.; Patra, M.; Hess, J.; Spingler, B.; Oehninger, L.; Schur, J.; Ott, L.; Salassa, L.; et al. DNA intercalating Ru(II) polypyridyl complexes as effective photosensitizers in photodynamic therapy. Chem.—A Eur. J. 2014, 20, 14421–14436. [Google Scholar] [CrossRef]
- Kastl, A.; Dieckmann, S.; Wähler, K.; Völker, T.; Kastl, L.; Merkel, A.L.; Vultur, A.; Shannan, B.; Harms, K.; Ocker, M.; et al. Rhenium complexes with visible-light-induced anticancer activity. ChemMedChem 2013, 8, 924–927. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [PubMed]
- Gianferrara, T.; Spagnul, C.; Alberto, R.; Gasser, G.; Ferrari, S.; Pierroz, V.; Bergamo, A.; Alessio, E. Towards matched pairs of porphyrin-ReI/99mTc I conjugates that combine photodynamic activity with fluorescence and radio imaging. ChemMedChem 2014, 9, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Kraljić, I.; Mohsni, S.E. A new method for the detection of singlet oxygen in aqueous solutions. Photochem. Photobiol. 1978, 28, 577–581. [Google Scholar] [CrossRef]
- Wähler, K.; Ludewig, A.; Szabo, P.; Harms, K.; Meggers, E. Rhenium complexes with red-light-induced anticancer activity. Eur. J. Inorg. Chem. 2014, 2014, 807–811. [Google Scholar] [CrossRef]
- Niu, L.Y.; Guan, Y.S.; Chen, Y.Z.; Wu, L.Z.; Tung, C.H.; Yang, Q.Z. A turn-on fluorescent sensor for the discrimination of cystein from homocystein and glutathione. Chem. Commun. 2013, 49, 1294–1296. [Google Scholar] [CrossRef]
- Lu, H.; Mac, K.J.; Yang, Y.; Shen, Z. Structural modification strategies for the rational design of red/NIR region BODIPYs. Chem. Soc. Rev. 2014, 43, 4778–4823. [Google Scholar] [CrossRef]
- Yu, X.; Jia, X.; Yang, X.; Liu, W.; Qin, W. Synthesis and photochemical properties of BODIPY-functionalized silica nanoparticles for imaging Cu2+ in living cells. Rsc Adv. 2014, 4, 23571–23579. [Google Scholar] [CrossRef]
- Zhong, F.; Yuan, X.; Zhao, J.; Wang, Q. Visible light-harvesting tricarbonyl Re(I) complex: Synthesis and application in intracellular photodynamic effect and luminescence imaging. Sci. China Chem. 2016, 59, 70–77. [Google Scholar] [CrossRef]
- Zhao, L.; Odaka, H.; Ono, H.; Kajimoto, S.; Hatanaka, K.; Hobley, J.; Fukumura, H. Dynamics of Re(2,2′-bipyridine)(CO)3C1 MLCT formation and decay after picosecond pulsed X-ray excitation and femtosecond UV excitation. Photochem. Photobiol. Sci. 2005, 4, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Yip, A.M.; Shum, J.; Liu, H.; Zhou, H.; Jia, M.; Niu, N.; Li, Y.; Yu, C.; Lo, K.K.W. Luminescent rhenium(I)–polypyridine complexes appended with a perylene diimide or benzoperylene monoimide moiety: Photophysics, intracellular sensing, and photocytotoxic activity. Chem. –A Eur. J. 2019, 25, 8970–8974. [Google Scholar] [CrossRef]
- Collery, P.; Veena, V.; Harikrishnan, A.; Desmaele, D. The rhenium(I)-diselenoether anticancer drug targets ROS, TGF-β1, VEGF-A, and IGF-1 in an in vitro experimental model of triple-negative breast cancers. Investig. New Drugs 2019, 37, 973–983. [Google Scholar] [CrossRef]
- Kermagoret, A.; Morgant, G.; d’Angelo, J.; Tomas, A.; Roussel, P.; Bastian, G.; Collery, P.; Desmaële, D. Synthesis, structural characterization and biological activity against several human tumor cell lines of four rhenium(I) diseleno-ethers complexes: Re (CO)3Cl (PhSe (CH2) 2SePh), Re (CO)3Cl (PhSe (CH2) 3SePh), Re(CO)3Cl (HO2C–CH2Se (CH2) 2SeCH2–CO2H) and Re (CO)3Cl (HO2C–CH2Se (CH2)3SeCH2–CO2H). Polyhedron 2011, 30, 347–353. [Google Scholar]
- Collery, P.; Santoni, F.; Mohsen, A.; Mignard, C.; Desmaele, D. Negative impact of total body irradiation on the antitumor activity of rhenium-(I)-diselenoether. Anticancer Res. 2016, 36, 5813–5819. [Google Scholar] [CrossRef][Green Version]
- Capper, M.S.; Rehkämper, M.; Packman, H. Rhenium based complexes and in vivo testing: A brief history. ChemBioChem 2020. [Google Scholar] [CrossRef]
- He, L.; Pan, Z.; Qin, W.; Li, Y.; Tan, C.; Mao, Z. Impairment of the autophagy-related lysosomal degradation pathway by an anticancer rhenium(I) complex. Dalton Trans. 2019, 48, 4398–4404. [Google Scholar] [CrossRef]
- Knopf, K.M.; Murphy, B.L.; Macmillan, S.N.; Baskin, J.M.; Barr, M.P.; Boros, E.; Wilson, J.J. In vitro anticancer activity and in vivo biodistribution of rhenium(I) tricarbonyl aqua complexes. J. Am. Chem. Soc. 2017, 139, 14302–14314. [Google Scholar] [CrossRef]
- Collery, P.; Mohsen, A.; Kermagoret, A.; Corre, S.; Bastian, G.; Tomas, A.; Wei, M.; Santoni, F.; Guerra, N.; Desmaële, D.; et al. Antitumor activity of a rhenium(I)-diselenoether complex in experimental models of human breast cancer. Investig. New Drugs 2015, 33, 848–860. [Google Scholar] [CrossRef]
- Collery, P.; Santoni, F.; Ciccolini, J.; Tran, T.N.N.; Mohsen, A.; Desmaele, D. Dose effect of rhenium(I)-diselenoether as anticancer drug in resistant breast tumor-bearing mice after repeated administrations. Anticancer Res. 2016, 36, 6051–6057. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Heinemann, F.; Karges, J.; Gasser, G. Critical overview of the use of Ru(II) polypyridyl complexes as photosensitizers in one-photon and two-photon photodynamic therapy. Acc. Chem. Res. 2017, 50, 2727–2736. [Google Scholar] [CrossRef]
- Shen, Y.; Shuhendler, A.J.; Ye, D.; Xu, J.; Chen, H. Two-photon excitation nanoparticles for photodynamic therapy. Chem. Soc. Rev. 2016, 45, 6725–6741. [Google Scholar] [CrossRef]
- Sun, B.; Wang, L.; Li, Q.; He, P.; Liu, H.; Wang, H.; Yang, Y.; Li, J. Bis (pyrene)-doped cationic dipeptide nanoparticles for two-photon-activated photodynamic therapy. Biomacromolecules 2017, 18, 3506–3513. [Google Scholar] [CrossRef]
- Baggaley, E.; Weinstein, J.A.; Williams, J.G. Lighting the way to see inside the live cell with luminescent transition metal complexes. Coord. Chem. Rev. 2012, 256, 1762–1785. [Google Scholar] [CrossRef]
- Bolze, F.; Jenni, S.; Sour, A.; Heitz, V. Molecular photosensitisers for two-photon photodynamic therapy. Chem. Commun. 2017, 53, 12857–12877. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Kobuke, Y. Recent advances in two-photon photodynamic therapy. Anti-Cancer Agents Med. Chem. 2008, 8, 269–279. [Google Scholar] [CrossRef]
- Sun, J.; Xin, Q.; Yang, Y.; Shah, H.; Cao, H.; Qi, Y.; Gong, J.R.; Li, J.B. Nitrogen-doped graphene quantum dots coupled with photosensitizers for one-/two-photon activated photodynamic therapy based on a FRET mechanism. Chem. Commun. 2018, 54, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, H.; Han, M.; Sun, B.; Li, J. Multilayer microcapsules for FRET analysis and two-photon-activated photodynamic therapy. Angew. Chem. Int. Ed. 2016, 55, 13538–13543. [Google Scholar] [CrossRef]
- Liu, H.; Yang, Y.; Wang, A.; Han, M.; Cui, W.; Li, J. Hyperbranched polyglycerol-doped mesoporous silica nanoparticles for one-and two-photon activated photodynamic therapy. Adv. Funct. Mater. 2016, 26, 2561–2570. [Google Scholar] [CrossRef]
- Ogawa, K.; Hasegawa, H.; Inaba, Y.; Kobuke, Y.; Inouye, H.; Kanemitsu, Y.; Kohno, E.; Hirano, T.; Ogura, S.I.; Okura, I. Water-soluble bis (imidazolylporphyrin) self-assemblies with large two-photon absorption cross sections as potential agents for photodynamic therapy. J. Med. Chem. 2006, 49, 2276–2283. [Google Scholar]
- Dahlstedt, E.; Collins, H.A.; Balaz, M.; Kuimova, M.K.; Khurana, M.; Wilson, B.C.; Philips, D.; Anderson, H.L. One-and two-photon activated phototoxicity of conjugated porphyrin dimers with high two-photon absorption cross sections. Org. Biomol. Chem. 2009, 7, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Collins, H.A.; Khurana, M.; Moriyama, E.H.; Mariampillai, A.; Dahlstedt, E.; Balaz, M.; Kuimova, M.K.; Drobizhev, M.; Yang, V.X.D.; Philips, D.; et al. Blood-vessel closure using photosensitizers engineered for two-photon excitation. Nat. Photonics 2008, 2, 420–424. [Google Scholar] [CrossRef]
- Kuimova, M.K.; Collins, H.A.; Balaz, M.; Dahlstedt, E.; Levitt, J.A.; Sergent, N.; Suhling, K.; Drobizhev, M.; Makarov, N.S.; Rebane, A.; et al. Photophysical properties and intracellular imaging of water-soluble porphyrin dimers for two-photon excited photodynamic therapy. Org. Biomol. Chem. 2009, 7, 889–896. [Google Scholar] [CrossRef]
- Kargesa, J.; Kuangb, S.; Maschiettoc, F.; Blacqued, O.; Ciofinic, I.; Chao, H.; Gasser, G. Ruthenium complexes for 1-and 2-photon photodynamic therapy: From in silico prediction to in vivo applications. Chemrxiv 2020. [Google Scholar] [CrossRef]
- Sherlock, B.; Warren, S.C.; Alexandrov, Y.; Yu, F.; Stone, J.; Knight, J.; Neil, M.A.A.; Paterson, C.; French, P.M.W.; Dunsby, C. In vivo multiphoton microscopy using a handheld scanner with lateral and axial motion compensation. J. Biophotonics 2018, 11, e201700131. [Google Scholar] [CrossRef] [PubMed]
- Qiu, K.; Wen, Y.; Ouyang, C.; Liao, X.; Liu, C.; Rees, T.W.; Zhang, Q.; Ji, L.; Chao, H. The stepwise photodamage of organelles by two-photon luminescent ruthenium (ii) photosensitizers. Chem. Commun. 2019, 55, 11235–11238. [Google Scholar] [CrossRef] [PubMed]
- Hess, J.; Huang, H.; Kaiser, A.; Pierroz, V.; Blacque, O.; Chao, H.; Gasser, G. Evaluation of the medicinal potential of two ruthenium(II) polypyridine complexes as one-and two-photon photodynamic yherapy photosensitizers. Chem. –A Eur. J. 2017, 23, 9888–9896. [Google Scholar] [CrossRef]
- Huang, H.; Yu, B.; Zhang, P.; Huang, J.; Chen, Y.; Gasser, G.; Ji, L.; Chao, H. Highly charged ruthenium(II) polypyridyl complexes as lysosome localized photosensitizers for two-photon photodynamic therapy. Angew. Chem. Int. Ed. 2015, 54, 14049–14052. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Castellano, F.N.; Gryczynski, I.; Gryczynski, Z.; Dattelbaum, J.D. Two-photon excitation of rhenium metal–ligand complexes. J. Photochem. Photobiol. A 1999, 122, 95–101. [Google Scholar] [CrossRef]
- Ferri, E.; Donghi, D.; Panigati, M.; Prencipe, G.; D’Alfonso, L.; Zanoni, I.; Baldoli, C.; Maiorana, S.; D’Alfonso, G.; Licandro, E. Luminescent conjugates between dinuclear rhenium (I) complexes and peptide nucleic acids (PNA) for cell imaging and DNA targeting. Chem. Commun. 2010, 46, 6255–6257. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Law, W.; Aalinkeel, R.; Yu, Y.; Nair, B.; Wu, J.; Mahajan, S.; Reynolds, J.L.; Li, Y.; Lai, C.K.; et al. Biodegradable cationic polymeric nanocapsules for overcoming multidrug resistance and enabling drug–gene co-delivery to cancer cells. Nanoscale 2014, 6, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Mansour, H.M.; Zhang, Y.; Deng, X.; Chen, Y.; Wang, J.; Pan, Y.; Zhao, J. Reversion of multidrug resistance by co-encapsulation of doxorubicin and curcumin in chitosan/poly (butyl cyanoacrylate) nanoparticles. Int. J. Pharm. 2012, 426, 193–201. [Google Scholar] [CrossRef]
- Vivek, R.; Thangam, R.; NipunBabu, V.; Rejeeth, C.; Sivasubramanian, S.; Gunasekaran, P.; Muthuchelian, K.; Kannan, S. Multifunctional HER2-antibody conjugated polymeric nanocarrier-based drug delivery system for multi-drug-resistant breast cancer therapy. Acs Appl. Mater. Interfaces 2014, 6, 6469–6480. [Google Scholar] [CrossRef]
- Duan, X.; Xiao, J.; Yin, Q.; Zhang, Z.; Yu, H.; Mao, S.; Li, Y. Smart pH-sensitive and temporal-controlled polymeric micelles for effective combination therapy of doxorubicin and disulfiram. Acs Nano 2013, 7, 5858–5869. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Lavasanifar, A. Traceable multifunctional micellar nanocarriers for cancer-targeted co-delivery of MDR-1 siRNA and doxorubicin. Acs Nano 2011, 5, 5202–5213. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, J.; Ye, Z.; Li, J.; Wang, A.; Hu, J.; Bai, S.; Yin, J. Self-assembly of photosensitive and chemotherapeutic drugs for combined photodynamic-chemo cancer therapy with real-time tracing property. Colloids Surf. A: Physicochem. Eng. Asp. 2019, 574, 44–51. [Google Scholar] [CrossRef]
- Doustvandi, M.A.; Mohammadnejad, F.; Mansoori, B.; Tajalli, H.; Mohammadi, A.; Mokhtarzadeh, A.; Baghbani, E.; Khaze, V.; Hajiasgharzadeh, K.; Moghaddam, M.M.; et al. Photodynamic therapy using zinc phthalocyanine with low dose of diode laser combined with doxorubicin is a synergistic combination therapy for human SK-MEL-3 melanoma cells. Photodiagnosis Photodyn. Ther. 2019, 28, 88–97. [Google Scholar] [CrossRef]
- Leijen, S.; Burgers, S.A.; Baas, P.; Pluim, D.; Tibben, M.; van Werkhoven, E.; Alessio, E.; Sava, G.; Beijnen, J.H.; Schellens, J.H.M. Phase I/II study with ruthenium compound NAMI-A and gemcitabine in patients with non-small cell lung cancer after first line therapy. Investig. New Drugs 2015, 33, 201–214. [Google Scholar] [CrossRef]
- Mignani, S.; Bryszewska, M.; Klajnert-Maculewicz, B.; Zablocka, M.; Majoral, J. Advances in combination therapies based on nanoparticles for efficacious cancer treatment: An analytical report. Biomacromolecules 2015, 16, 1–27. [Google Scholar] [CrossRef]
- Hu, C.J.; Zhang, L. Therapeutic nanoparticles to combat cancer drug resistance. Curr. Drug Metab. 2009, 10, 836–841. [Google Scholar] [CrossRef]
- Yuan, Y.G.; Peng, Q.L.; Gurunathan, S. Silver nanoparticles enhance the apoptotic potential of gemcitabine in human ovarian cancer cells: Combination therapy for effective cancer treatment. Int. J. Nanomed. 2017, 12, 6487–6502. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu, E.; Cagel, M.; Lagomarsino, E.; Moretton, M.; Chiappetta, D.A. Paclitaxel: What has been done and the challenges remain ahead. Int. J. Pharm. 2017, 526, 474–495. [Google Scholar] [CrossRef] [PubMed]
- Kundranda, M.N.; Niu, J. Albumin-bound paclitaxel in solid tumors: Clinical development and future directions. Drug Des. Dev. Ther. 2015, 9, 3767–3777. [Google Scholar] [CrossRef] [PubMed]
- Gaio, E.; Guerrini, A.; Ballestri, M.; Varchi, G.; Ferroni, C.; Martella, E.; Columbaro, M.; Moret, F.; Reddi, E. Keratin nanoparticles co-delivering docetaxel and chlorin e6 promote synergic interaction between chemo-and photo-dynamic therapies. J. Photochem. Photobiol. B Biol. 2019, 199, 111598. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, M.A. Plasmonic photo-thermal therapy (PPTT). Alex. J. Med. 2011, 47, 1–9. [Google Scholar] [CrossRef]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2008, 23, 217. [Google Scholar] [CrossRef]
- Spyratou, E.; Makropoulou, M.; Efstathopoulos, E.P.; Georgakilas, A.G.; Sihver, L. Recent advances in cancer therapy based on dual mode gold nanoparticles. Cancers 2017, 9, 173. [Google Scholar] [CrossRef]
- Mouratidis, P.X.; Rivens, I.; ter Haar, G. A study of thermal dose-induced autophagy, apoptosis and necroptosis in colon cancer cells. Int. J. Hyperth. 2015, 31, 476–488. [Google Scholar] [CrossRef]
- Cherukuri, P.; Glazer, E.S.; Curley, S.A. Targeted hyperthermia using metal nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 339–345. [Google Scholar] [CrossRef]
- Saw, W.S.; Ujihara, M.; Chong, W.Y.; Voon, S.H.; Imae, T.; Kiew, L.V.; Lee, H.B.; Sim, K.S.; Chung, L.Y. Size-dependent effect of cystine/citric acid-capped confeito-like gold nanoparticles on cellular uptake and photothermal cancer therapy. Colloids Surf. B Biointerfaces 2018, 161, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhu, X.; Cao, C.; Sun, J.; Liu, J. Transferrin modified ruthenium nanoparticles with good biocompatibility for photothermal tumor therapy. J. Colloid Interface Sci. 2018, 511, 325–334. [Google Scholar] [CrossRef] [PubMed]
| Photosensitisers | Application | References |
|---|---|---|
| NPe6 (Talaporfin sodium) | Non-small cell lung carcinoma | [37] |
| Motexafin lutetium | Prostate cancer | [38] |
| Temoporfin | Head, neck, prostate and pancreatic cancers | [39,40,41] |
| Porfimer sodium | Obstructive oesophageal, lung, bladder and cervical cancers | [28,42,43] |
| 2-(1-Hexyloxyethyl)-2-devinyl pyropheophorbide-a | Head, lung and neck cancers, basal cell carcinoma | [44,45,46] |
| Hexaminolevulinate | Bladder cancer | [47] |
| Methyl aminolevulinate | Basal cell carcinoma | [40,48,49] |
| Aluminium phthalocyanine tetrasulfonate | Lung, breast, skin and stomach cancers | [50] |
| Padeliporfin | Early-stage of prostate cancer | [51] |
| Verteporfin | Basal cell carcinoma | [52,53] |
| Photodynamic Therapy | |||
| One-Photon Photodynamic Therapy | Two-Photon Photodynamic Therapy | ||
| Similarities | |||
| The general mechanism is the same, with the presence of light and oxygen, the photosensitisers are excited to its excited triplet state which leads to the production of reactive oxygen species (ROS) and thus, causing cell death. | |||
| Differences | |||
| One-photon photosensitiser is used | Photosensitisers | Two-photon photosensitiser is used | |
| 600–800 nm | Ideal range of wavelength (nm) of the photosensitisers | Wide range, not fixed, can go as low as 300 nm | |
| A laser within the UV-visible range | Activation of photosensitisers | Two low energy photons of near-infrared region of light absorbed simultaneously | |
| Less | Precision of cancer treatment | Higher | |
| Shallower | Depth of tissue penetration | Deeper | |
| - | Determination of ability of photosensitiser to absorb 2 photons simultaneously | Quantified by two-photon cross-sections, δ, which is expressed in Goeppert-Mayer (GM), best to > 50 GM | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liew, H.S.; Mai, C.-W.; Zulkefeli, M.; Madheswaran, T.; Kiew, L.V.; Delsuc, N.; Low, M.L. Recent Emergence of Rhenium(I) Tricarbonyl Complexes as Photosensitisers for Cancer Therapy. Molecules 2020, 25, 4176. https://doi.org/10.3390/molecules25184176
Liew HS, Mai C-W, Zulkefeli M, Madheswaran T, Kiew LV, Delsuc N, Low ML. Recent Emergence of Rhenium(I) Tricarbonyl Complexes as Photosensitisers for Cancer Therapy. Molecules. 2020; 25(18):4176. https://doi.org/10.3390/molecules25184176
Chicago/Turabian StyleLiew, Hui Shan, Chun-Wai Mai, Mohd Zulkefeli, Thiagarajan Madheswaran, Lik Voon Kiew, Nicolas Delsuc, and May Lee Low. 2020. "Recent Emergence of Rhenium(I) Tricarbonyl Complexes as Photosensitisers for Cancer Therapy" Molecules 25, no. 18: 4176. https://doi.org/10.3390/molecules25184176
APA StyleLiew, H. S., Mai, C.-W., Zulkefeli, M., Madheswaran, T., Kiew, L. V., Delsuc, N., & Low, M. L. (2020). Recent Emergence of Rhenium(I) Tricarbonyl Complexes as Photosensitisers for Cancer Therapy. Molecules, 25(18), 4176. https://doi.org/10.3390/molecules25184176






