Luminescent Sensor Based on Ln(III) Ternary Complexes for NAD(P)H Detection
Abstract
1. Introduction
2. Results and Discussion
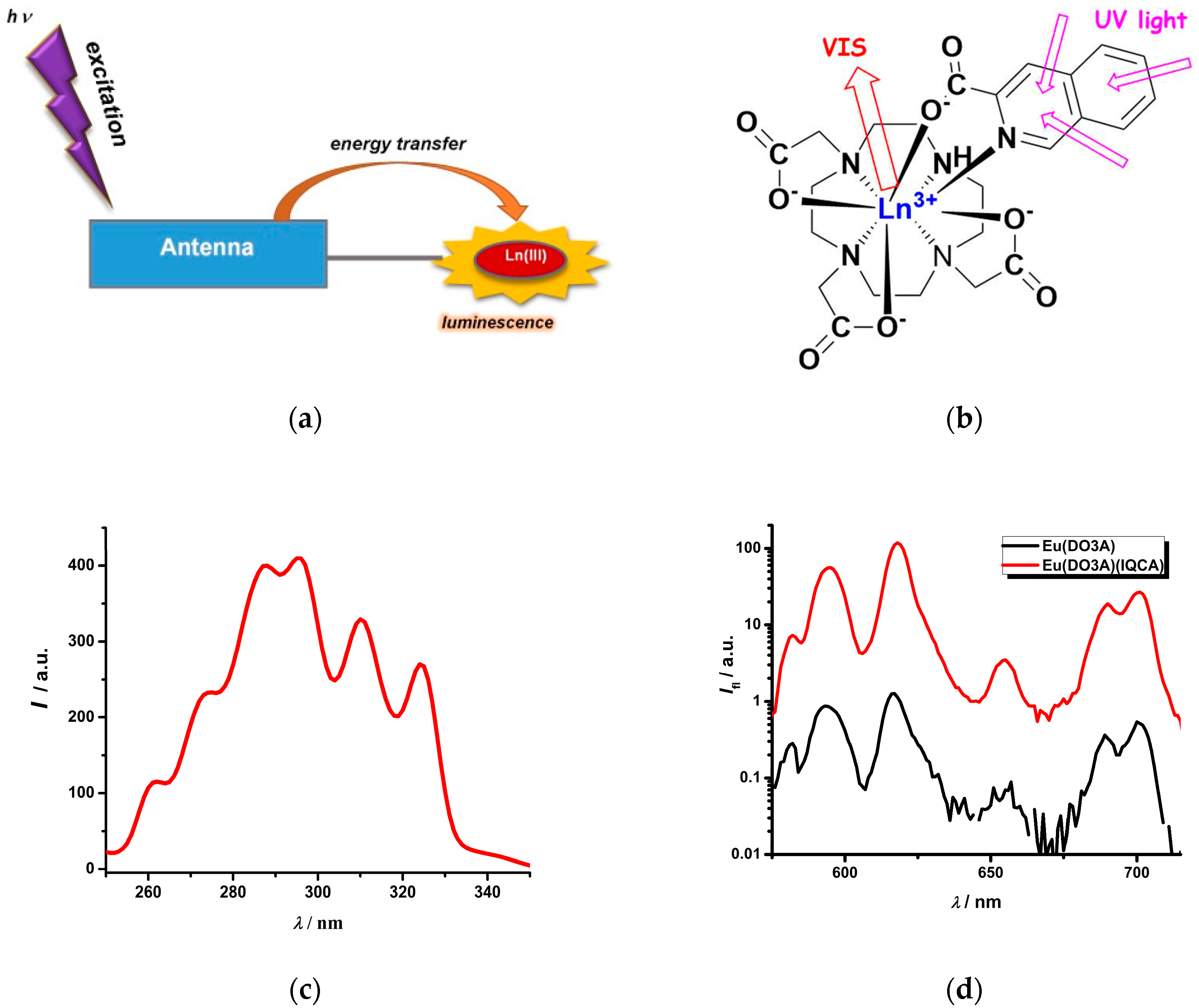
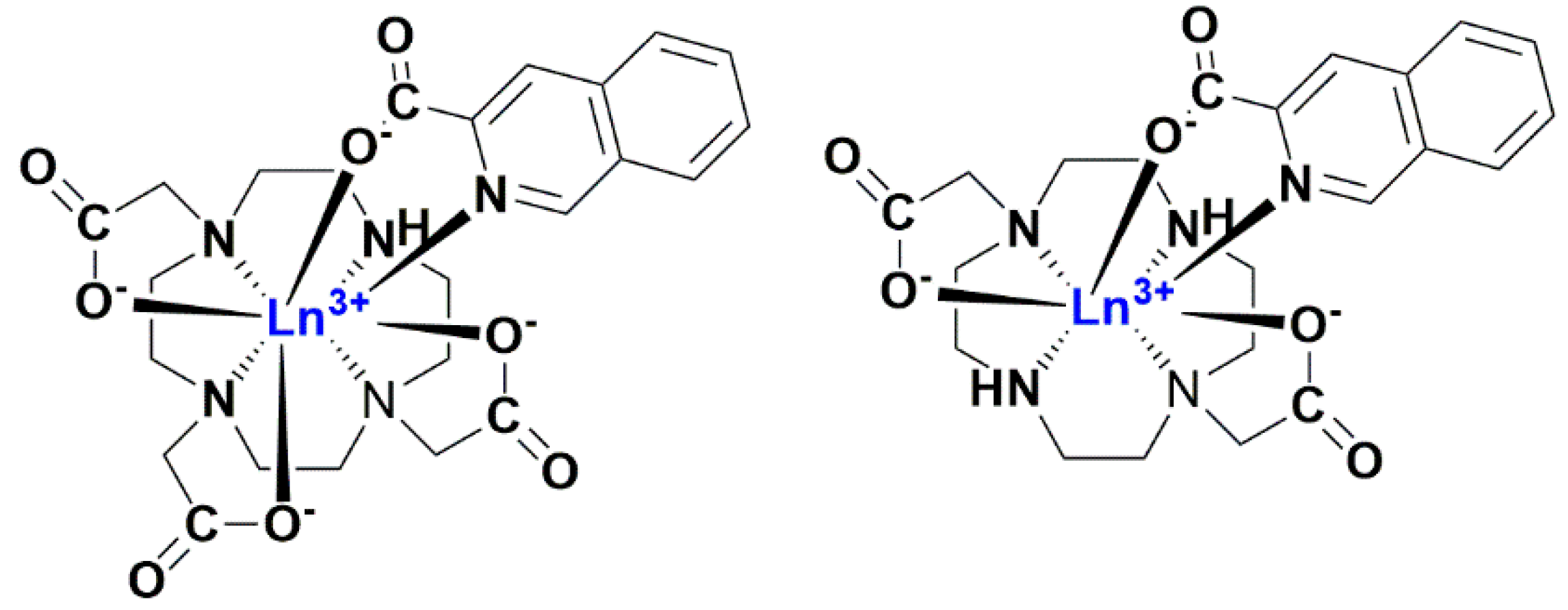
2.1. Ln(III) Complexes with an Antenna Ligand
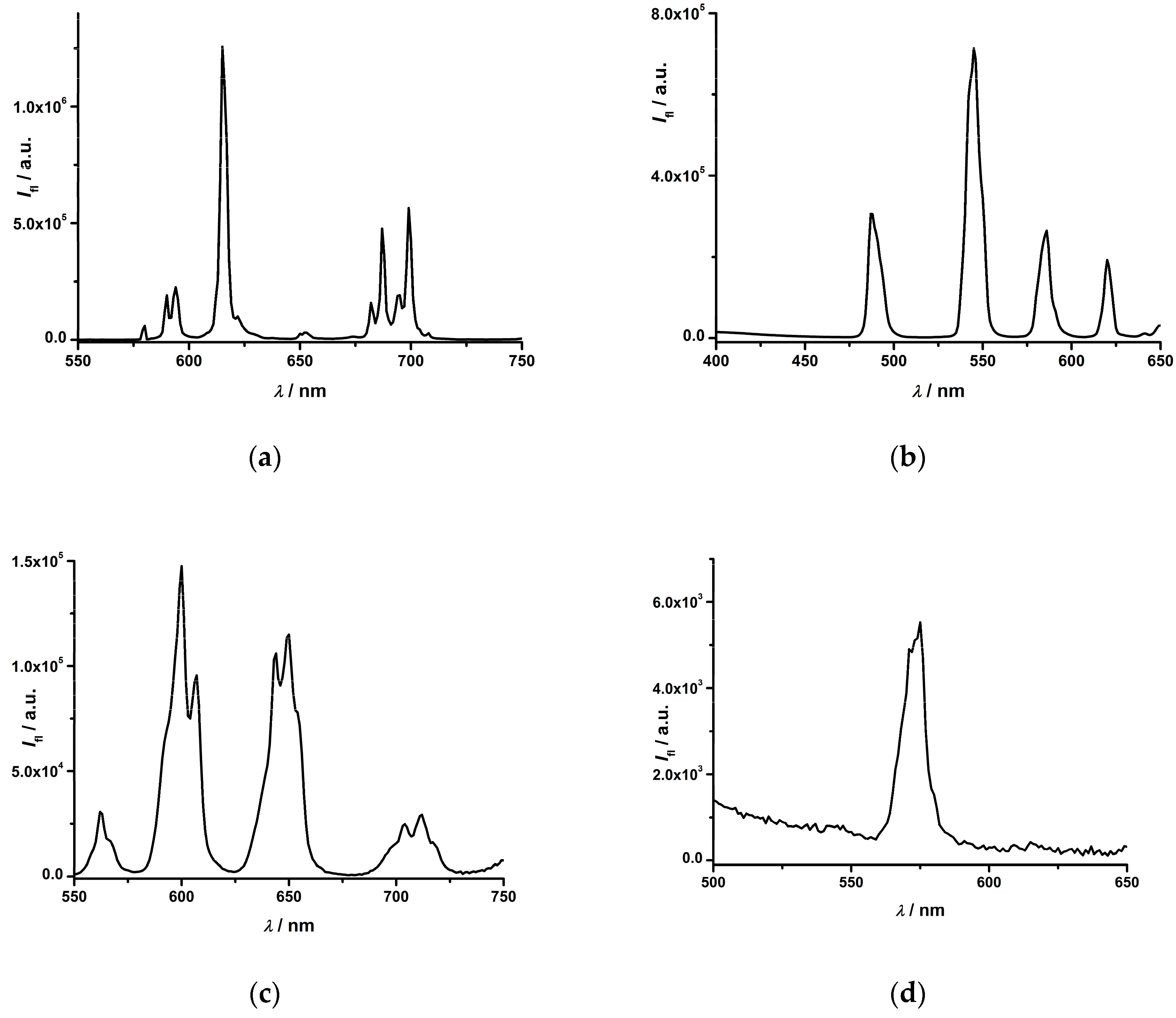
2.2. Photophysical Study of Ln(III) Ternary Complexes in the Presence of the NADPH Ligand
2.3. Photophysical Study of the Eu(III) Complex in the Presence of an NADH/NAD+ Pair
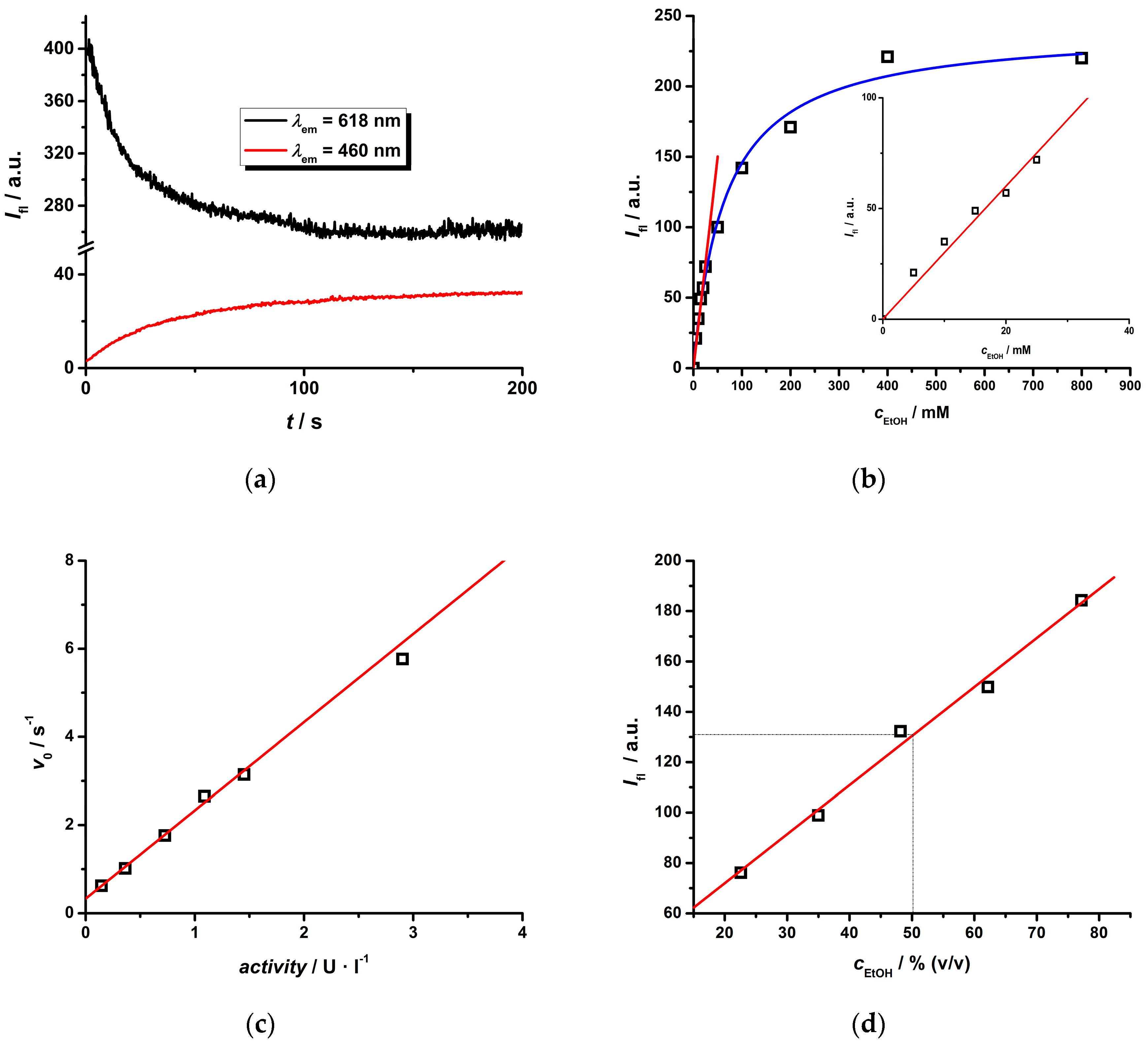
2.4. Cascade Enzymatic Reaction
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
The Shielding Effect of NAD(P)H on Luminescence of Ln(III) Ternary Complexes
≈ ln(10) × k × φNAD(P)H × I0 × ε NAD(P)H × l × c NAD(P)H ≈ k′NAD(P)H × cNAD(P)H
≈ ln(10) × k × φ LnL × I0 × ε LnL × l × c LnL ≈ k″ × εLnL × cLnL
k × φLnL × I0 × (10−ln(10) × ε NAD(P)H × l × cNAD(P)H − 10−ln(10) × ε LnL × l × cLnL)
≈ ln(10) × l × (ε LnL × c LnL − ε NAD(P)H × c NAD(P)H)
References
- Bűnzli, J.C.; Piguet, C. Taking advantage of luminescent lanthanide ions. Chem. Soc. Rev. 2005, 34, 1048–1077. [Google Scholar] [CrossRef]
- Bűnzli, J.C. Lanthanide Luminescence for Biomedical Analyses and Imaging. Chem. Rev. 2010, 110, 2729–2755. [Google Scholar] [CrossRef]
- Bűnzli, J.C.; Eliseeva, S.V. Lanthanide NIR luminescence for telecommunications, bioanalyses and solar energy conversion. J. Rare Earths 2010, 28, 824–832. [Google Scholar] [CrossRef]
- Bűnzli, J.C. On the design of highly luminescent lanthanide complexes. Coord. Chem. Rev. 2015, 293–294, 19–47. [Google Scholar] [CrossRef]
- Sigel, A.S.H.; Pyle, A.M.; Sigel, A.; Sigel, H. The Lanthanides and Their Interrelations with Biosystems. In Metal Ions in Biological Systems; Sigel, A., Sigel, H., Eds.; Marcel Dekker: New York, NY, USA, 2003; Volume 40. [Google Scholar]
- Shuvaev, S.; Starck, M.; Parker, D. Responsive, water-soluble Europium(III) luminescent probes. Chem. Eur. J. 2017, 23, 9974–9989. [Google Scholar] [CrossRef]
- New, E.; Parker, D.; Smith, D.G.; Walton, J.W. Development of responsive lanthanide probes for cellular applications. Curr. Opin. Chem. Biol. 2010, 14, 238–246. [Google Scholar] [CrossRef]
- Montgomery, C.P.; Murray, B.S.; New, E.J.; Pal, R.; Parker, D. Cell-Penetrating Metal Complex Optical Probes: Targeted and Responsive Systems Based on Lanthanide Luminescence. Acc. Chem. Res. 2009, 42, 925–937. [Google Scholar] [CrossRef]
- Mathieu, E.; Sipos, A.; Demeyere, E.; Phipps, D.; Sakaveli, D.; Borbas, K.E. Lanthanide-based tools for the investigation of cellular environments. Chem. Commun. 2018, 54, 10021–10035. [Google Scholar] [CrossRef]
- Thibon, A.; Pierre, V.C. Principles of responsive lanthanide-based luminescent probes for cellular imaging. Anal. Bioanal. Chem. 2009, 394, 107–120. [Google Scholar] [CrossRef]
- Hefern, M.C.; Matosziuk, L.M.; Meade, T.J. Lanthanide probes for bioresponsive imaging. Chem. Rev. 2014, 114, 4496–4539. [Google Scholar] [CrossRef]
- Hewit, S.H.; Butler, S.J. Application of lanthanide luminescence in probing enzyme activity. Chem. Commun. 2018, 54, 6635–6647. [Google Scholar] [CrossRef]
- Zwier, J.M.; Bazin, H.; Lamarque, L.; Mathis, G. Luminescent lanthanide cryptates: From the bench to the bedside. Inorg. Chem. 2014, 53, 1854–1866. [Google Scholar] [CrossRef]
- Kovacs, D.; Lu, X.; Meszaros, L.S.; Ott, M.; Andres, J.; Borbas, K.E. Photophysics of Coumarin and Carbostyril-Sensitized Luminescent Lanthanide Complexes: Implications for Complex Design in Multiplex Detection. J. Am. Chem. Soc. 2017, 139, 5756–5767. [Google Scholar] [CrossRef]
- Pershagen, E.; Borbas, K.E. Multiplex Detection of Enzymatic Activity with Responsive Lanthanide-Based Luminescent Probes. Angew. Chem. Int. Ed. 2015, 54, 1787–1790. [Google Scholar] [CrossRef]
- Pershagen, E.; Nordholm, J.; Borbas, K.E. Luminescent Lanthanide Complexes with Analyte-Triggered Antenna Formation. J. Am. Chem. Soc. 2012, 134, 9832–9835. [Google Scholar] [CrossRef]
- Gunnlaugsson, T.; Leonard, J.P. Responsive lanthanide luminescent cyclen complexes: From switching/sensing to supramolecular architectures. Chem. Commun. 2005, 3114–3131. [Google Scholar] [CrossRef]
- Stasiuk, G.J.; Long, N.J. The ubiquitous DOTA and its derivatives: The impact of 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid on biomedical imaging. Chem. Commun. 2013, 49, 2732–2746. [Google Scholar] [CrossRef]
- Brücher, E.; Baranyai, Z.; Tircsó, G. The future of biomedical imaging: Synthesis and chemical properties of the DTPA and DOTA derivative ligands and their complexes. In Biomedical Imaging: The Chemistry of Labels, Probes, and Contrast Agents; Braddock, M., Ed.; Royal Society of Chemistry: Cambridge, UK, 2012; Chapter 5.2; pp. 208–260. [Google Scholar]
- Táborský, P.; Svobodová, I.; Lubal, P.; Hnatejko, Z.; Lis, S.; Hermann, P. Formation and dissociation kinetics of Eu(III) complexes with H5do3ap and similar dota-like ligands. Polyhedron 2007, 26, 4119–4130. [Google Scholar] [CrossRef]
- Mamedov, I.; Táborský, P.; Lubal, P.; Laurent, S.; Elst, L.V.; Mayer, H.A.; Logothetis, N.K.; Angelovski, G. Relaxometric, Thermodynamic and Kinetic Studies of Lanthanide(III) Complexes of DO3A-Based Propylphosphonates. Eur. J. Inorg. Chem. 2009, 3298–3306. [Google Scholar] [CrossRef]
- Campello, M.P.C.; Lacerda, S.; Santos, I.C.; Pereira, G.A.; Geraldes, C.F.G.C.; Kotek, J.; Hermann, P.; Vaněk, J.; Lubal, P.; Kubíček, V.; et al. Lanthanide(III) Complexes of 4,10-Bis(phosphonomethyl)-1,4,7,10-tetraazacyclododecane-1,7-diacetic acid (trans-H6do2a2p) in Solution and in the Solid State: Structural Studies Along the Series. Chem. Eur. J. 2010, 16, 8446–8465. [Google Scholar] [CrossRef]
- Vaněk, J.; Lubal, P.; Ševčíková, R.; Polášek, M.; Hermann, P. Mono(pyridine-N-oxide) analog of DOTA as a suitable organic reagent for a sensitive and selective fluorimetric determination of Ln(III) ions. J. Lumin. 2012, 132, 2030–2035. [Google Scholar] [CrossRef]
- Smrčka, F.; Lubal, P. The time-resolved fluorescence study of kinetics and thermodynamics of Eu(III) and Tb(III) complexes with the DO2A macrocyclic ligand. New J. Chem. 2018, 42, 7993–8000. [Google Scholar] [CrossRef]
- Försterová, M.; Jandurová, Z.; Marques, F.; Gano, L.; Lubal, P.; Vaněk, J.; Hermann, P.; Santos, I. Chemical and biological evaluation of 153Sm and 166Ho complexes of 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrakis(methylphosphonic acid monoethylester) (H4dotpOEt). J. Inorg. Biochem. 2008, 102, 1531–1540. [Google Scholar] [CrossRef]
- Merbach, A.E.; Helm, L.; Tóth, E. The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging; Willey: Hoboken, NJ, USA, 2013. [Google Scholar]
- Hermann, P.; Kotek, J.; Kubíček, V.; Lukeš, I. Gadolinium(III) complexes as MRI contrast agents: Ligand design and properties of the complexes. Dalton Trans. 2008, 23, 3027–3047. [Google Scholar] [CrossRef]
- Vaněk, J.; Lubal, P.; Hermann, P.; Anzenbacher, P., Jr. Luminescent Sensor for Carbonate Ion Based on Lanthanide(III) Complexes of 1,4,7,10-Tetraazacyclododecane-1,4,7-Triacetic Acid (DO3A). J. Fluoresc. 2013, 23, 57–69. [Google Scholar] [CrossRef]
- Vaněk, J.; Smrčka, F.; Lubal, P.; Třísková, I.; Trnková, L. Dual carbonate sensor based on Eu(III) complex of DO3A ligand. Mon. Chem. 2016, 147, 925–934. [Google Scholar] [CrossRef]
- Smrčka, F.; Lubal, P.; Šídlo, M. The urea biosensor based on luminescence of Eu(III) ternary complex of DO3A ligand. Mon. Chem. 2017, 148, 1945–1952. [Google Scholar] [CrossRef]
- Stryer, L. Biochemistry; Freeman: New York, NY, USA, 1988. [Google Scholar]
- Banerjee, R. Redox Biochemistry; Willey: Hoboken, NJ, USA, 2008. [Google Scholar]
- Blinova, K.; Carroll, S.; Bose, S.; Smirnov, A.V.; Harvey, J.J.; Knutson, J.R.; Balaban, R.S. Distribution of mitochondrial NADH fluorescence lifetimes: Steady-state kinetics of matrix NADH interactions. Biochemistry 2005, 44, 2585–2594. [Google Scholar] [CrossRef]
- Blacker, T.S.; Berecz, T.; Duchen, M.R.; Szabadkai, G. Assessment of Cellular Redox State Using NAD(P)H Fluorescence Intensity and Lifetime. Bio-Protoc. 2017, 7, e2105. [Google Scholar] [CrossRef]
- Sharma, A.; Quantrill, N.S.M. Measurement of ethanol using fluorescence quenching. Spectrochim. Acta A 1994, 50, 1161–1177. [Google Scholar] [CrossRef]
- Lee, S.-L.; Shih, H.-T.; Chi, Y.-C.; Li, Y.-P.; Yin, S.-J. Oxidation of methanol, ethylene glycol, and isopropanol with human alcohol dehydrogenases and the inhibition by ethanol and 4-methylpyrazole. Chem-Biol. Interact. 2011, 191, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Arnold, M.A. Fluorescence quenching of thionine by reduced nicotinamide adenine-dinucleotide. Spectrochim. Acta A 1992, 48, 647–651. [Google Scholar] [CrossRef]
- Sharma, A. Photolytic oxidation of reduced nicotinamide adenine-dinucleotide. Spectrochim. Acta A 1992, 48, 893–897. [Google Scholar] [CrossRef]
- Kudoa, H.; Sawai, M.; Suzuki, Y.; Wang, X.; Gessei, T.; Takahashi, D.; Arakawa, T.; Mitsubayashi, K. Fiber-optic bio-sniffer (biochemical gas sensor) for high-selective monitoring of ethanol vapor using 335nm UV-LED. Sens. Actuators B 2010, 147, 676–680. [Google Scholar] [CrossRef]
- Iitani, K.; Chien, P.-J.; Suzuki, T.; Toma, K.; Arakawa, T.; Iwasaki, Y.; Mitsubayashi, K. Improved Sensitivity of Acetaldehyde Biosensor by Detecting ADH Reverse Reaction-Mediated NADH Fluoro-Quenching for Wine Evaluation. ACS Sens. 2017, 2, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Thungon, P.D.; Kakoti, A.; Ngashangva, L.; Goswami, P. Advances in developing rapid, reliable and portable detection systems for alcohol. Biosens. Bioelectr. 2017, 97, 83–99. [Google Scholar] [CrossRef]
- Martins, A.F.; Eliseeva, S.V.; Carvalho, H.F.; Teixeira, J.M.C.; Paula, C.T.B.; Hermann, P.; Platas-Iglesias, C.; Petoud, S.; Tóth, E.; Geraldes, C.F.G.C. A Bis(pyridine N-oxide) Analogue of DOTA: Relaxometric Properties of the Gd-III Complex and Efficient Sensitization of Visible and NIR-Emitting Lanthanide(III) Cations Including Pr(III) and Ho(III). Chem. Eur. J. 2014, 20, 14834–14845. [Google Scholar] [CrossRef]
- Hashami, Z.; Martins, A.F.; Funk, A.M.; Jordan, V.C.; Petoud, S.; Eliseeva, S.V.; Kovacs, Z. Lanthanide DO3A-Tropolone Complexes: Efficient Dual MR/NIR Imaging Probes in Aqueous Medium. Eur. J. Inorg. Chem. 2017, 4965–4968. [Google Scholar] [CrossRef]
- Peterson, K.L.; Margherio, M.J.; Doan, P.; Wilke, K.T.; Pierre, V.C. Basis for Sensitive and Selective Time-Delayed Luminescence of Hydroxyl Radical by Lanthanide Complexes. Inorg. Chem. 2013, 52, 9390–9398. [Google Scholar] [CrossRef]
- Supkowski, R.M.; de Horrocks, W.W., Jr. Lanthanide Ions of Electron Transfer in Proteins. In Metal Ions in Biological Systems; Sigel, A., Sigel, H., Eds.; Marcel Dekker: New York, NY, USA, 2003; Volume 40. [Google Scholar]
- Krausková, L.; Procházková, J.; Klásková, M.; Filipová, L.; Chaloupková, R.; Malý, S.; Damborský, J.; Heger, D. Suppression of protein inactivation during freezing by minimizing pH changes using ionic cryoprotectants. Int. J. Pharm. 2016, 509, 41–49. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

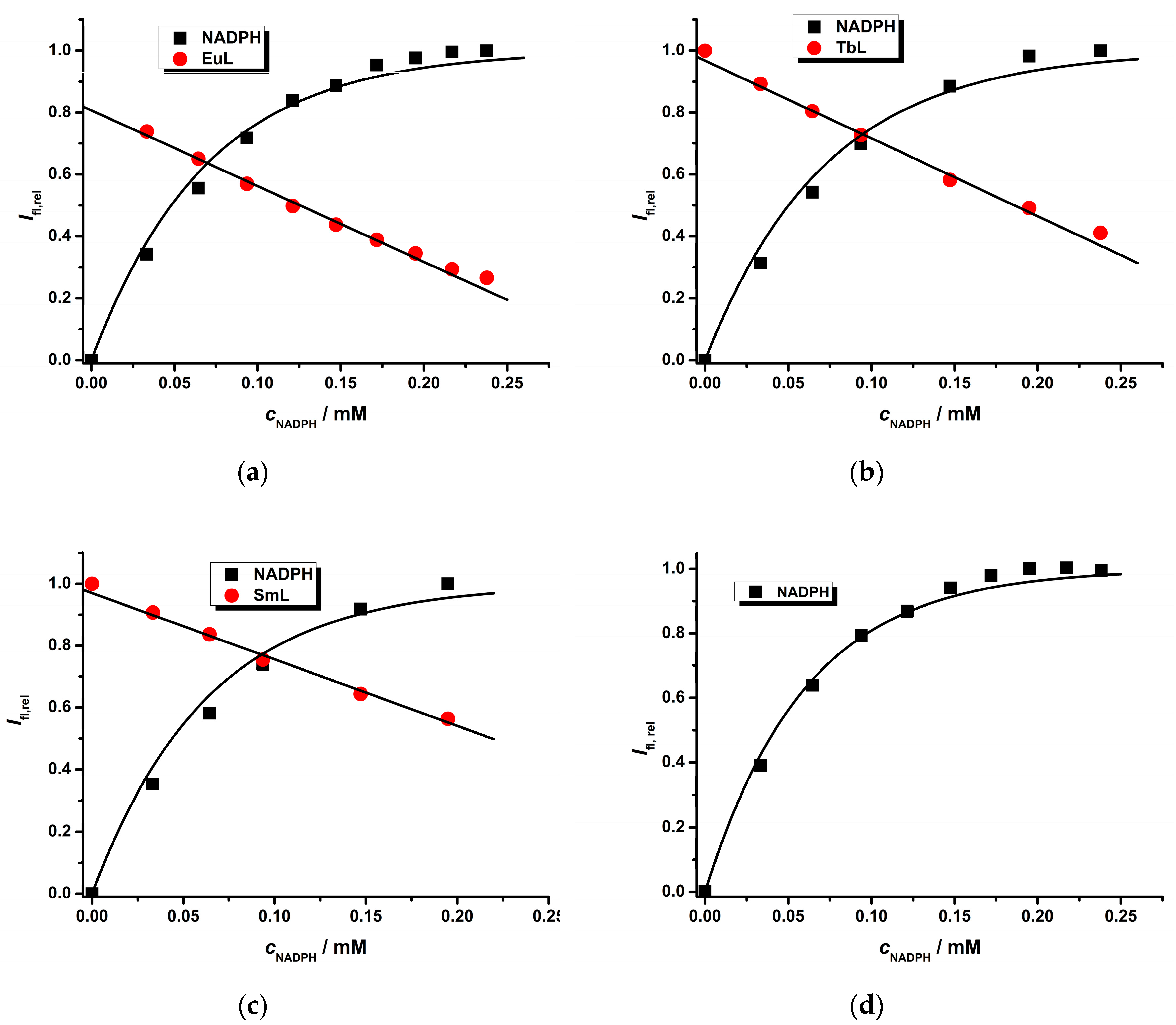
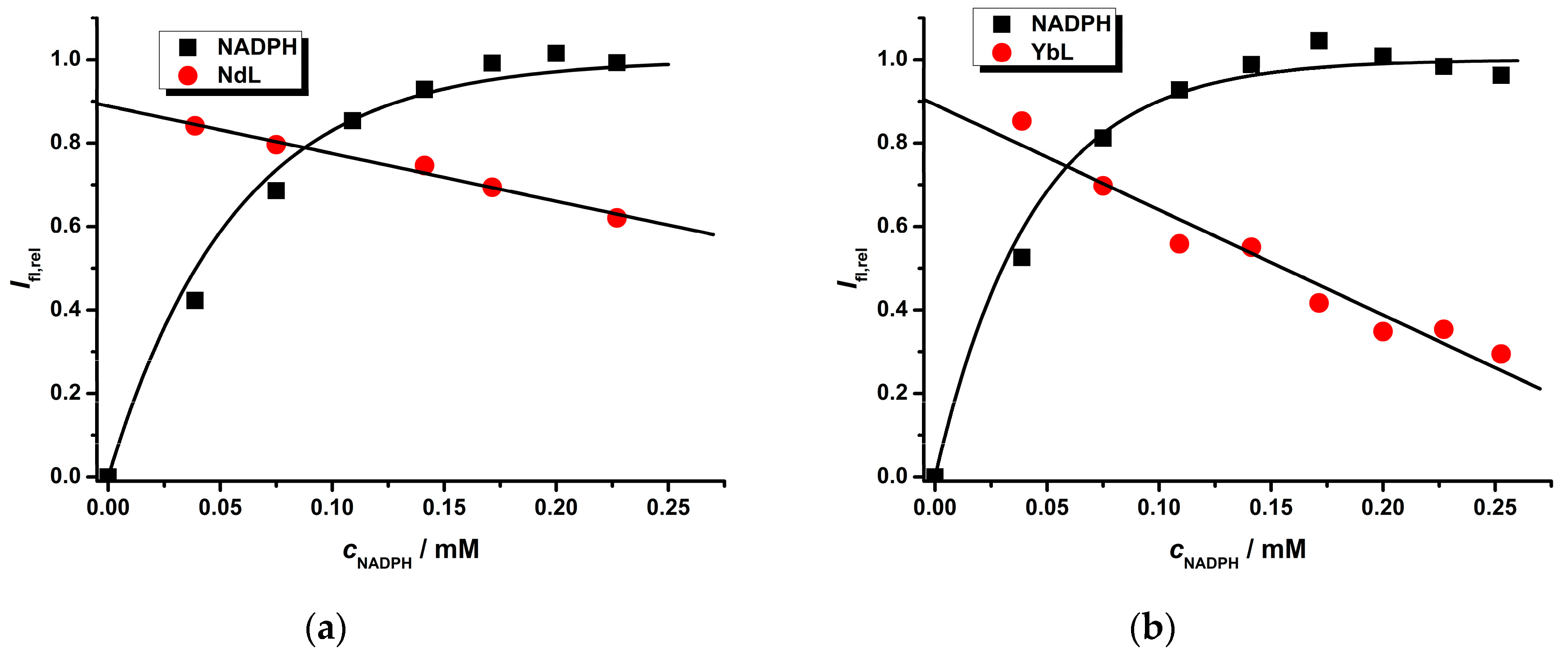

| Ln(III) Complex | NADPH a k′/mM−1 | LnL b KQ/mM−1 | LnL c | Ifl,reld | |
|---|---|---|---|---|---|
| bslope/mM−1 | aintercept | ||||
| --- | 7.1 ± 0.1 | --- | --- | --- | --- |
| Eu(III) | 6.3 ± 0.2 | 9.5 ± 0.6 | 2.45 ± 0.08 | 0.80 ± 0.01 | 1.000 |
| Tb(III) | 6.0 ± 0.3 | 5.8 ± 0.3 | 2.5 ± 0.1 | 0.97 ± 0.01 | 1.042 |
| Sm(III) | 6.9 ± 0.3 | 4.2 ± 0.1 | 2.2 ± 0.1 | 0.97 ± 0.01 | 0.136 |
| Nd(III) | 7.7 ± 0.3 | 2.2 ± 0.3 | 1.14 ± 0.08 | 0.89 ± 0.01 | 0.008 |
| Yb(III) | 10.1 ± 0.4 | 10.2 ± 0.8 | 2.5 ± 0.2 | 0.89 ± 0.04 | 0.025 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smrčka, F.; Lubal, P. Luminescent Sensor Based on Ln(III) Ternary Complexes for NAD(P)H Detection. Molecules 2020, 25, 4164. https://doi.org/10.3390/molecules25184164
Smrčka F, Lubal P. Luminescent Sensor Based on Ln(III) Ternary Complexes for NAD(P)H Detection. Molecules. 2020; 25(18):4164. https://doi.org/10.3390/molecules25184164
Chicago/Turabian StyleSmrčka, Filip, and Přemysl Lubal. 2020. "Luminescent Sensor Based on Ln(III) Ternary Complexes for NAD(P)H Detection" Molecules 25, no. 18: 4164. https://doi.org/10.3390/molecules25184164
APA StyleSmrčka, F., & Lubal, P. (2020). Luminescent Sensor Based on Ln(III) Ternary Complexes for NAD(P)H Detection. Molecules, 25(18), 4164. https://doi.org/10.3390/molecules25184164






