Biased Opioid Antagonists as Modulators of Opioid Dependence: Opportunities to Improve Pain Therapy and Opioid Use Management
Abstract
:1. Introduction
2. Evidence for Multiple Receptor Conformations with Distinct Signaling Pathways, and the Potential of Biased Agonists
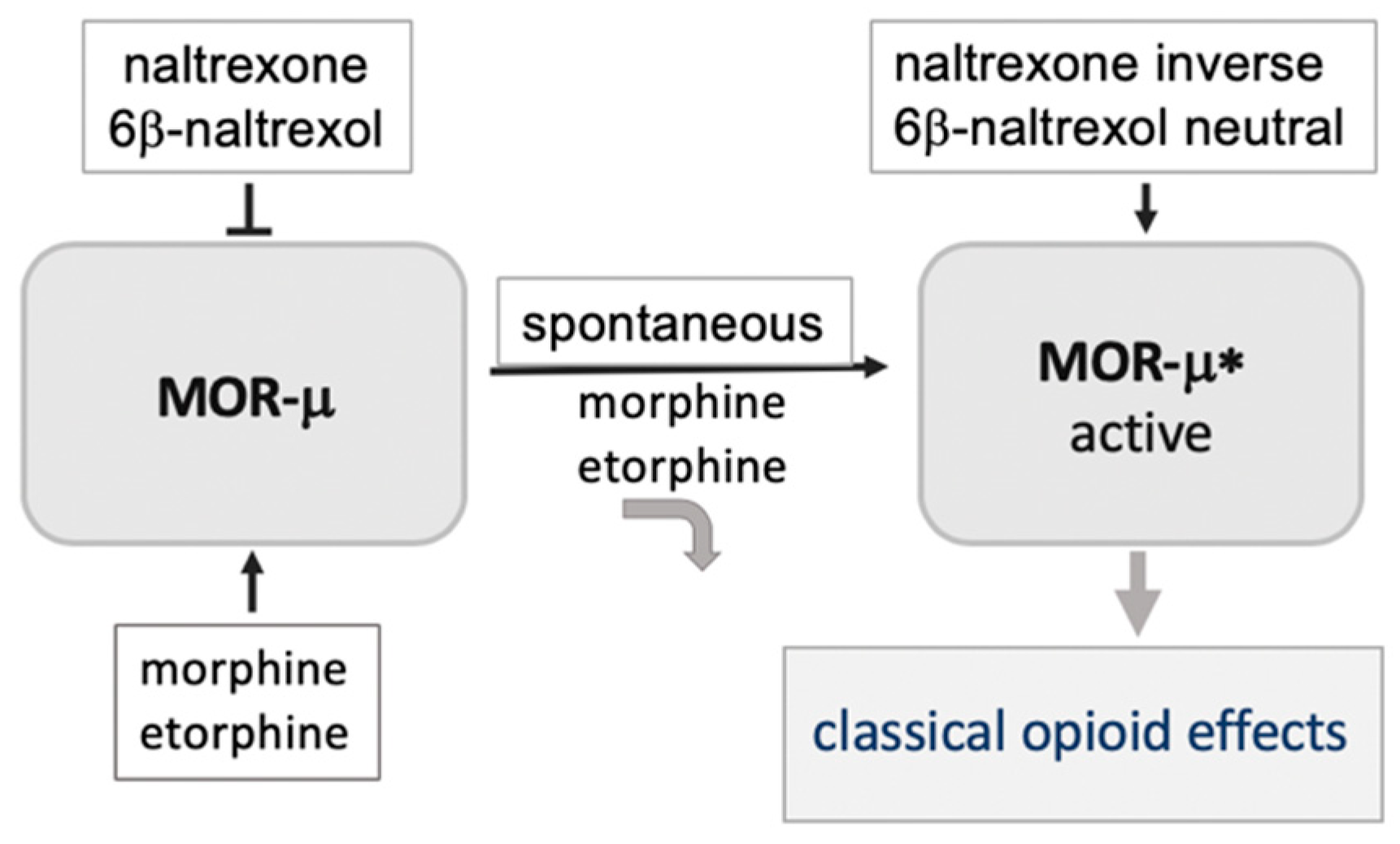
3. A Basally Active Receptor Mediating MOR Signaling (MOR-μ*)
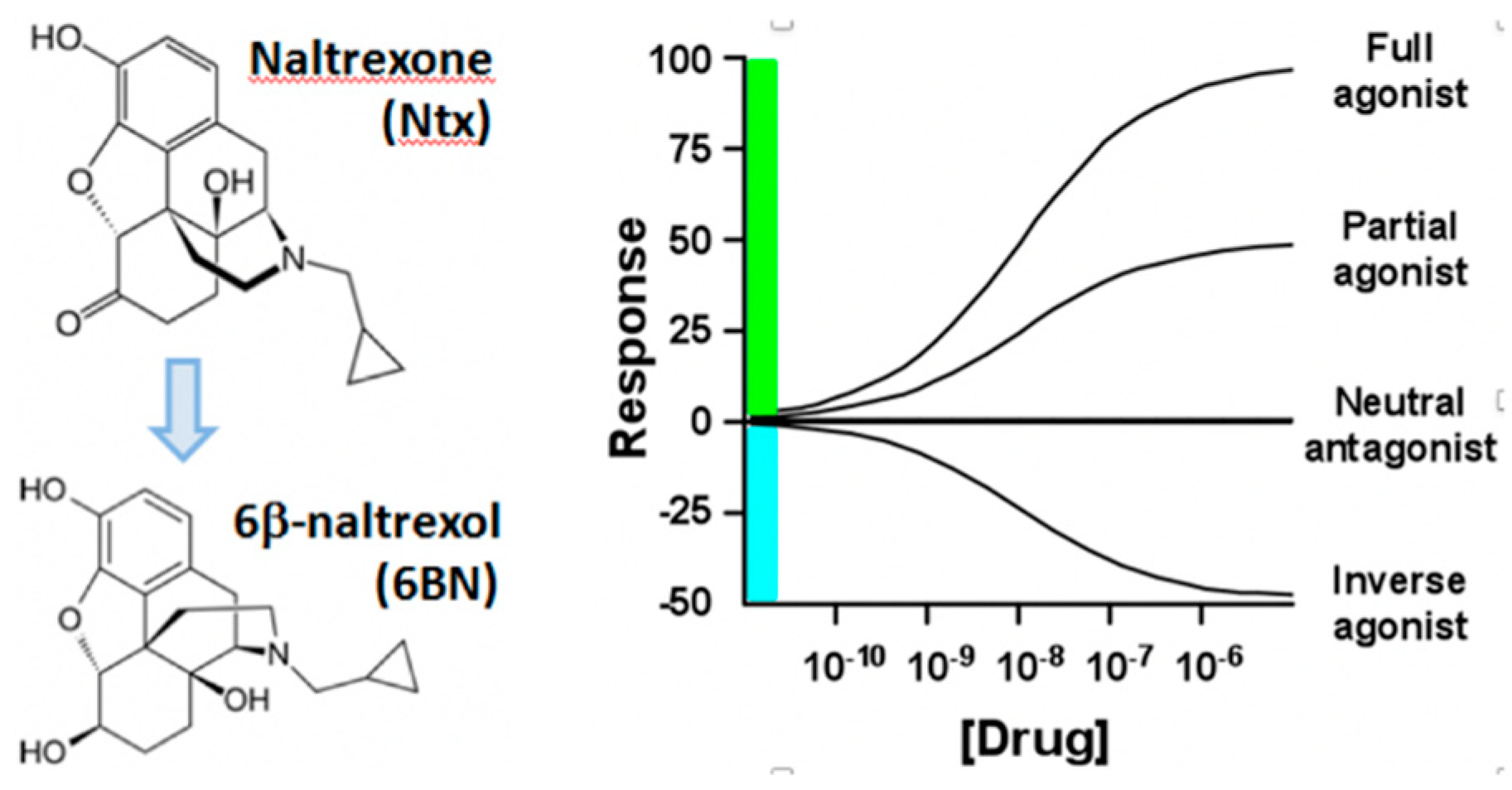
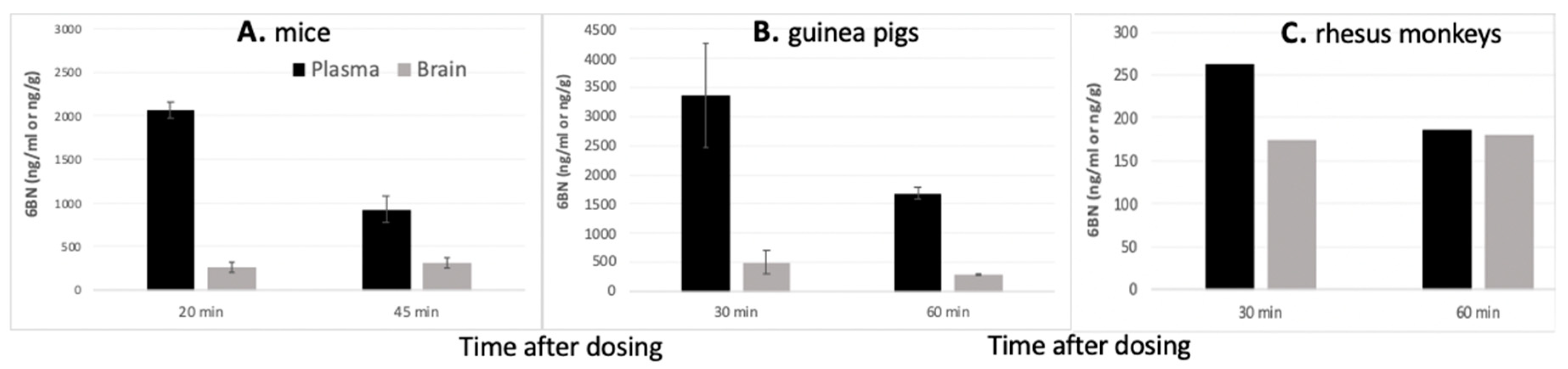
4. Peripherally Active μ Opioid Receptor Antagonists (PAMORA) and 6β-Naltrexol
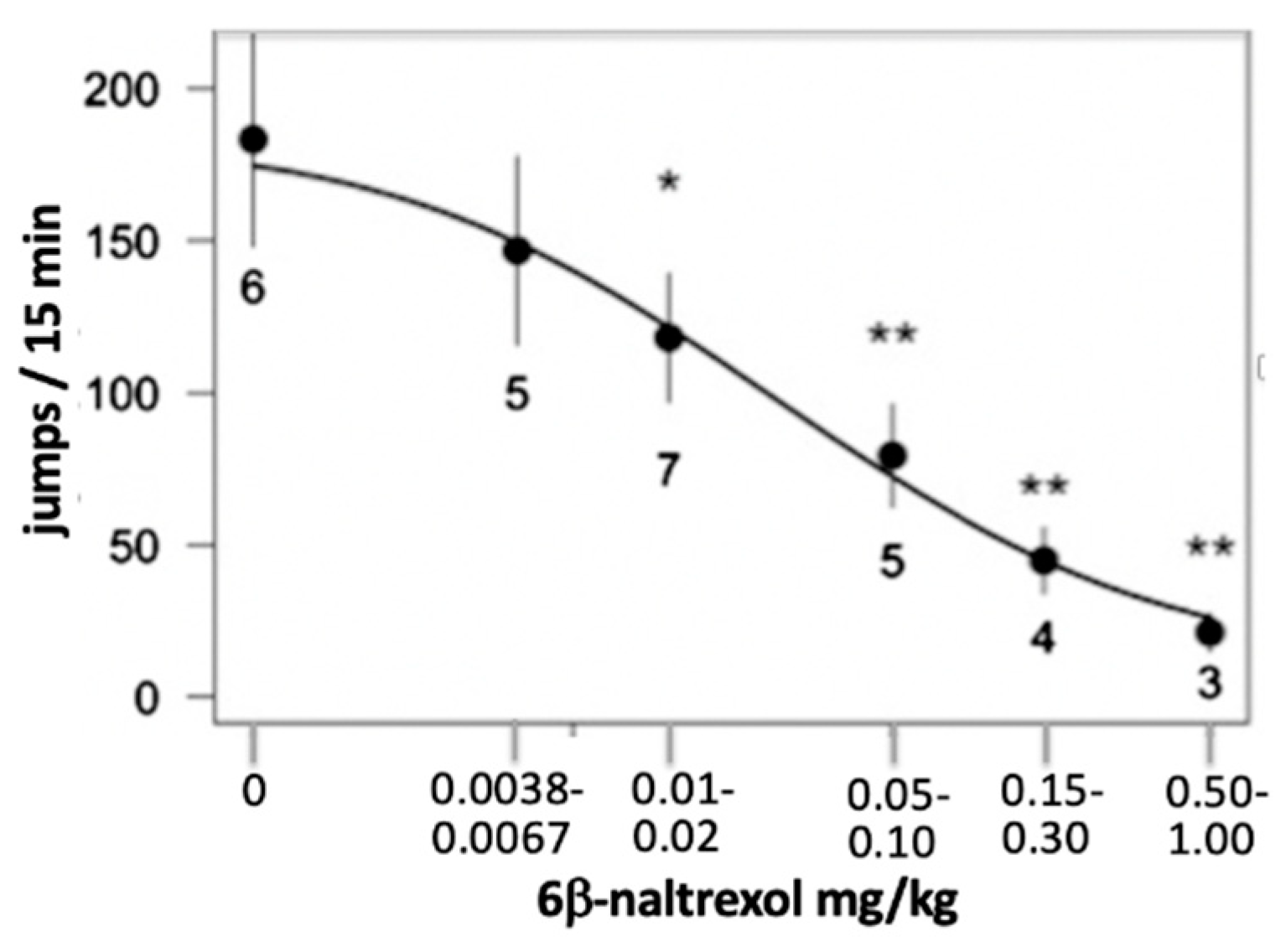
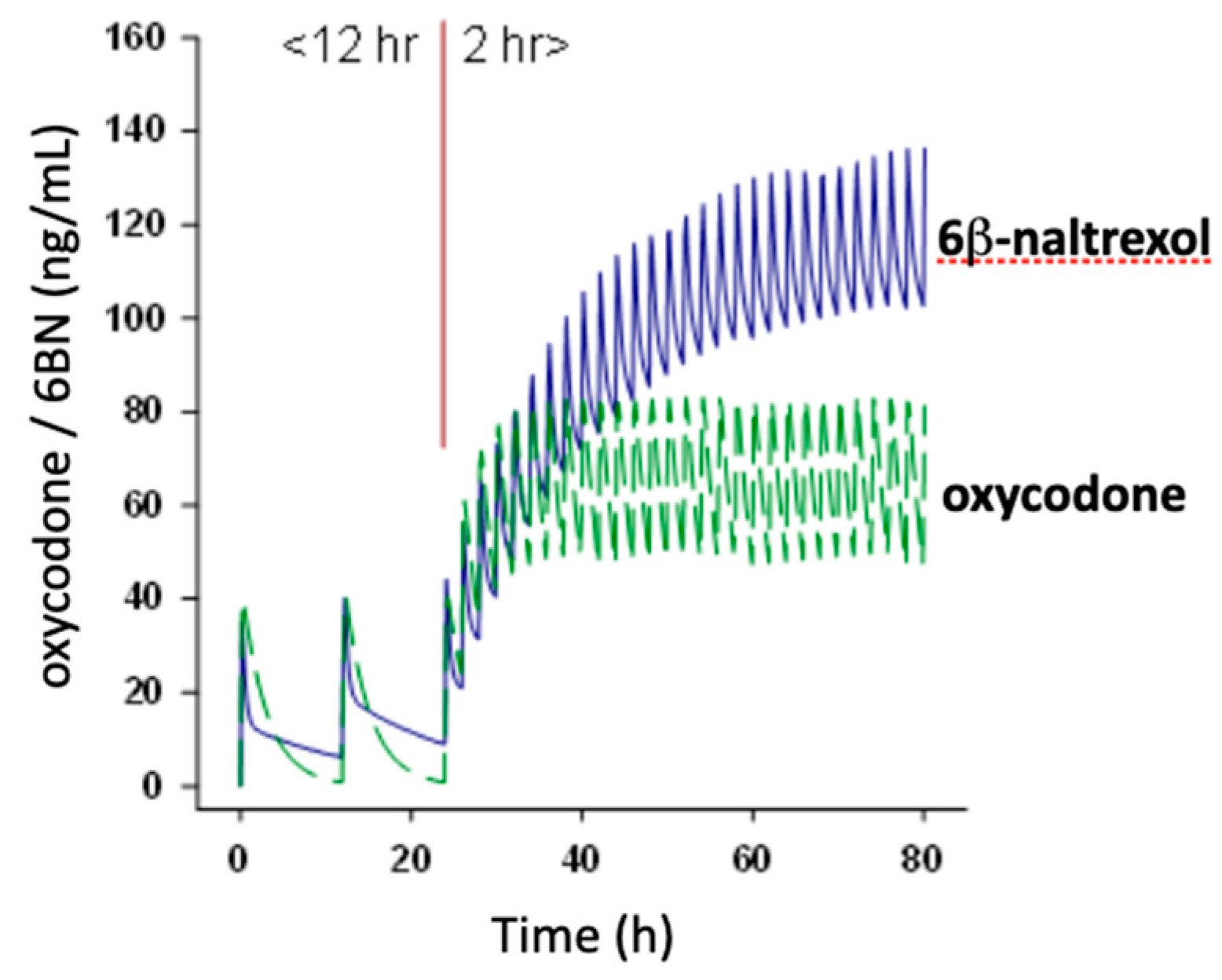
5. 6BN Prevents Development of Opioid Dependence with High Potency
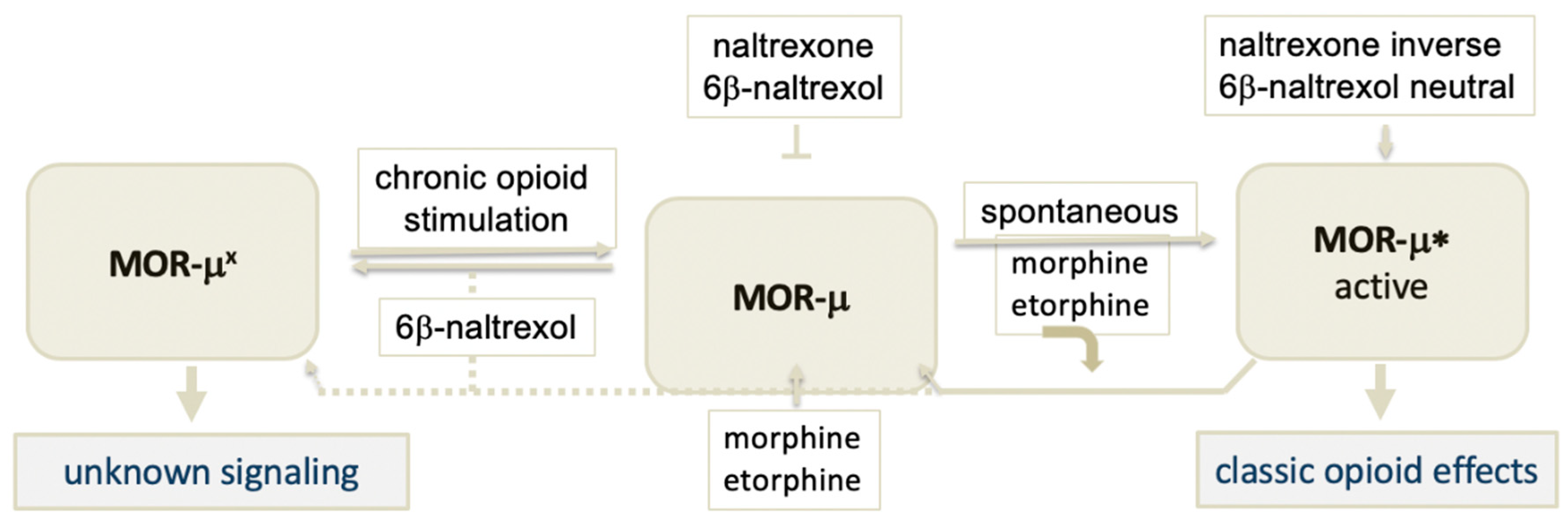
6. Hypothesis: A Novel MOR Receptor Model Relevant to Opioid Dependence Invoking a Site with High Affinity to 6BN
7. Potential Clinical Applications
8. Biased Antagonism at GPCRs and Future Studies
9. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Valentino, R.J.; Volkow, N.D. Untangling the complexity of opioid receptor function. Neuropsychopharm 2018, 43, 2514–2520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, Y.; Filizola, M. Opioid receptors: Structural and mechanistic insights into pharmacology and signaling. Eur. J. Pharmacol. 2015, 763, 206–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burford, N.T.; Traynor, J.R.; Alt, A. Positive allosteric modulators of the μ-opioid receptor: A novel approach for future pain medications. Br. J. Pharmacol. 2015, 172, 277–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Y.; Duc, N.M.; Rasmussen, S.G.F.; Hilger, D.; Kubiak, X.; Wang, L.; Bohon, J.; Kim, H.R.; Wegrecki, M.; Asuru, A.; et al. Assembly of a GPCR-G Protein Complex. Cell 2019, 177, 1232–1242. [Google Scholar] [CrossRef] [PubMed]
- Park, P.S. Ensemble of G protein-coupled receptor active states. Curr. Med. Chem. 2012, 19, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Hauser, A.S.; Attwood, M.M.; Rask-Andersen, M.; Schiöth, H.B.; Gloriam, D.E. Trends in GPCR drug discovery: New agents, targets and indications. Nat. Rev. Drug Discov. 2017, 6, 829–842. [Google Scholar] [CrossRef] [PubMed]
- Seyedabadi, M.; Ghahremani, M.H.; Albert, P.R. Biased signaling of G protein coupled receptors (GPCRs): Molecular determinants of GPCR/transducer selectivity and therapeutic potential. Pharmacol. Ther. 2019, 200, 148–178. [Google Scholar] [CrossRef] [PubMed]
- Wootten, D.; Christopoulos, A.; Marti-Solano, M.; Babu, M.M.; Sexton, P.M. Mechanisms of signalling and biased agonism in G protein-coupled receptors. Nat. Rev. Mol. Cell Biol. 2018, 19, 638–653. [Google Scholar] [CrossRef] [PubMed]
- Schmid, C.L.; Kennedy, N.M.; Ross, N.C.; Lovell, K.M.; Yue, Z.; Morgenweck, J.; Cameron, M.D.; Bannister, T.D.; Bohn, L.M. Bias factor and therapeutic window correlate to predict safer opioid analgesics. Cell 2017, 171, 1165–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Violin, J.D.; Crombie, A.L.; Soergel, D.G.; Lark, M.W. Biased ligands at G-protein-coupled receptors: Promise and progress. Trends Pharmacol. Sci. 2014, 35, 308–316. [Google Scholar] [CrossRef]
- Chan, H.C.S.; McCarthy, D.; Li, J.; Palczewski, K.; Yuan, S. Designing safer analgesics via μ-opioid receptor pathways. Trends Pharmacol. Sci. 2017, 38, 1016–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grim, T.W.; Schmid, C.L.; Stahl, E.L.; Pantouli, P.; Ho, J.-H.; Acevedo-Canabal, A.; Kennedy, N.M.; Cameron, M.D.; Bannister, T.D.; Bohn, L.M. A G protein signaling-biased agonist at the μ-opioid receptor reverses morphine tolerance while preventing morphine withdrawal. Neuropsychopharm 2019, 45, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Jeske, N.A. Dynamic Opioid Receptor Regulation in the Periphery. Mol. Pharmacol. 2019, 95, 463–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corder, G.; Tawfik, V.L.; Wang, D.; Sypek, E.I.; Low, S.A.; Dickinson, J.R.; Sotoudeh, C.; Clark, J.D.; Barres, B.A.; Bohlen, C.J.; et al. Loss of μ opioid receptor signaling in nociceptors, but not microglia, abrogates morphine tolerance without disrupting analgesia. Nat. Med. 2017, 23, 164–173. [Google Scholar]
- Streicher, J.M.; Bilsky, E.J. Peripherally acting mu-opioid receptor antagonists for the treatment of opioid-related side effects: Mechanism of action and clinical implications. J. Pharm. Pract. 2017, 31, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, L.C.; Chavera, T.S.; Jamshidi, R.J.; Berg, K.A.; Clarke, W.P. Constitutive Desensitization of Opioid Receptors in Peripheral Sensory Neurons. J. Pharmacol. Exp. Ther. 2016, 359, 411–419. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Bilsky, E.J.; Porreca, F.; Sadée, W. Constitutive μ Receptor Activation as a Regulatory Mechanism Underlying Narcotic Tolerance and Dependence. Life Sci. 1994, 54, PL339–PL350. [Google Scholar] [CrossRef]
- Sadee, W.; Wang, D.; Bilsky, E.J. Basal opioid receptor activity, neutral antagonists, and therapeutic opportunities. Life Sci. 2005, 76, 1427–1437. [Google Scholar] [CrossRef]
- Wang, D.; Raehal, K.M.; Lin, E.T.; Lowery, J.J.; Kieffer, B.L.; Bilsky, E.J.; Sadée, W. Basal signaling mu opioid receptor in mouse brain: Role in narcotic dependence. J. Pharm. Exp. Ther. 2004, 308, 512–520. [Google Scholar] [CrossRef]
- Perry, D.C.; Rosenbaum, J.S.; Kurowski, M.; Sadée, W. 3H-Etorphine Receptor Binding In Vivo: Small Fractional Occupancy Elicits Analgesia. Molec. Pharmacol. 1982, 21, 272–279. [Google Scholar]
- Hoare, S.R.J.; Pierre, N.; Moya, A.G.; Larson, B. Kinetic operational models of agonism for G-protein-coupled receptors. J. Theor. Biol. 2018, 446, 168–204. [Google Scholar] [CrossRef] [PubMed]
- Corder, G.; Doolen, S.; Donahue, R.R.; Winter, M.K.; Jutras, B.K.L.; He, Y.; Hu, X.; Wieskopf, J.S.; Mogil, J.S.; Storm, D.R.; et al. Constitutive μ-opioid receptor activity leads to long-term endogenous analgesia and dependence. Science 2013, 341, 1394–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco, C.; Volkow, N.D. Management of opioid use disorder in the USA: Present status and future directions. Lancet 2019, 393, 1760–1772. [Google Scholar] [CrossRef] [PubMed]
- Wachman, E.M.; Saia, K.; Miller, M.; Valle, E.; Shrestha, H.; Carter, G.; Werler, M.; Jones, H. Naltrexone Treatment for Pregnant Women with Opioid Use Disorder Compared with Matched Buprenorphine Control Subjects. Clin. Ther. 2019, 41, 1681–1689. [Google Scholar] [CrossRef]
- Dunbar, J.L.; Turncliff, R.Z.; Dong, Q.; Silverman, B.L.; Ehrich, E.W.; Lasseter, K.C. Single-and multiple-dose pharmacokinetics of long-acting injectable naltrexone. Alcoholism Clin. Exper. Res. 2006, 30, 480–490. [Google Scholar] [CrossRef]
- Porter, S.J.; Somogyi, A.A.; White, J.M. In vivo and in vitro potency studies of 6β-naltrexol, the major human metabolite of naltrexone. Addict. Biol. 2002, 7, 219–225. [Google Scholar] [CrossRef]
- Raehal, K.M.; Lowery, J.J.; Bhamidipati, C.M.; Paolino, R.M.; Blair, J.R.; Wang, D.; Sadée, W.; Bilsky, E.J. In vivo characterization of 6β-naltrexol, an opioid ligand with less inverse agonist activity compared with naltrexone and naloxone in opioid-dependent mice. J. Pharmacol. Exp. Ther. 2005, 313, 1150–1162. [Google Scholar] [CrossRef] [Green Version]
- Sirohi, S.; Dighe, S.V.; Madia, P.A.; Yoburn, B.C. The relative potency of inverse opioid agonists and a neutral opioid antagonist in precipitated withdrawal and antagonism of analgesia and toxicity. J. Pharmacol. Exp. Ther. 2009, 330, 513–519. [Google Scholar] [CrossRef] [Green Version]
- Yancey-Wrona, J.E.; Raymond, T.J.; Mercer, H.K.; Sadee, W.; Bilsky, E.J. 6β-Naltrexol preferentially antagonizes opioid effects on gastrointestinal transit compared to antinociception in mice. Life Sci. 2009, 85, 413–420. [Google Scholar] [CrossRef]
- Ko, M.C.; Divin, M.F.; Lee, H.; Woods, J.H.; Traynor, J.R. Differential in Vivo Potencies of Naltrexone and 6β-Naltrexol in the Monkey. J. Pharmacol. Exp. Ther. 2006, 316, 772–779. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Raehal, K.M.; Bilsky, E.J.; Sadee, W. Inverse agonists and neutral antagonists at μ opioid receptor (MOR): Possible role of basal receptor signaling in narcotic dependence. J. Neurochem. 2001, 77, 1590–1600. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Sun, X.; Sadee, W. Different effects of opioid antagonists on mu, delta, and kappa opioid receptors with and without agonist pretreatment. J. Pharmacol. Exp. Ther. 2007, 321, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, A.J.; Brown, A.L.; Oldmeadow, C.; Harris, A.; Gill, A.; Sadler, C.; Ribbons, K.; Attia, J.; Barker, D.; Ghijben, P.; et al. Effectiveness and cost-effectiveness of unsupervised buprenorphine-naloxone for the treatment of heroin dependence in a randomized waitlist-controlled trial. Drug Alcohol Depend. 2017, 174, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Rosado, J.; Walsh, S.L.; Bigelow, G.E.; Strain, E.C. Sublingual buprenorphine/naloxone precipitated withdrawal in subjects maintained on 100 mg of daily methadone. Drug Alcohol Depend. 2007, 90, 261–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowen, A.; Lewis, J.W.; MacFarlane, I.R. Agonist and antagonist properties of buprenorphine, a new antinociceptive agent. Brit. J. Pharmacol. 1977, 60, 537–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzschentke, T.M. Reassessment of buprenorphine in conditioned place preference: Temporal and pharmacological considerations. Psychopharmacology 2004, 172, 58–67. [Google Scholar] [CrossRef]
- Fürst, S.; Zádori, Z.S.; Zádor, F.; Király, K.; Balogh, M.; László, S.B.; Hutka, B.; Mohammadzadeh, A.; Calabrese, C.; Galambos, A.R.; et al. On the Role of Peripheral Sensory and Gut Mu Opioid Receptors: Peripheral Analgesia and Tolerance. Molecules 2020, 25, 2473. [Google Scholar] [CrossRef]
- Song, X.; Wang, D.; Qu, X.; Dong, N.; Teng, S. A meta-analysis of naldemedine for the treatment of opioid-induced constipation. Expert Rev. Clin. Pharmacol. 2019, 12, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Kalvass, J.C.; Olson, E.R.; Cassidy, M.P.; Selley, D.E.; Pollack, G.M. Pharmacokinetics and pharmacodynamics of seven opioids in P-glycoprotein-competent mice: Assessment of unbound brain EC50,u and correlation of in vitro, preclinical, and clinical data. J. Pharmacol. Exp. Ther. 2007, 323, 346–355. [Google Scholar] [CrossRef]
- Farid, W.O.; Dunlop, S.A.; Tait, R.J.; Hulse, G.K. The effects of maternally administered methadone, buprenorphine and naltrexone on offspring: Review of human and animal data. Curr. Neuropharmacol. 2008, 6, 125–150. [Google Scholar] [CrossRef] [Green Version]
- Yancey-Wrona, J.; Dallaire, B.; Bilsky, E.; Bath, B.; Burkart, J.; Wenster, L.; Magiera, D.; Yang, X.; Phelps, M.; Sadee, W. 6β-naltrexol, a peripherally selective opioid antagonist that inhibits morphine- induced slowing of gastrointestinal transit: An exploratory study. Pain Medicine 2011, 12, 1727–1737. [Google Scholar] [CrossRef] [PubMed]
- Oberdick, J.; Ling, Y.; Phelps, M.A.; Yudovich, M.S.; Schilling, K.; Sadee, W. Preferential delivery of an opioid antagonist to the fetal brain in pregnant mice. J. Pharmacol. Exp. Ther. 2016, 358, 22–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safa, A.; Lau, A.R.; Aten, S.; Schilling, K.; Bales, K.L.; Miller, V.; Fitzgerald, J.; Chen, M.; Hill, K.; Dzwigalski, K.; et al. Pharmacological prevention of neonatal opioid withdrawal in a pregnant guinea pig model. bioRxiv 2020. [Google Scholar] [CrossRef]
- Walwyn, W.M.; Chen, C.; Kim, H.; Minasyan, A.; Ennes, H.S.; McRoberts, J.A.; Marvizon, J.C.G. Sustained suppression of hyperalgesia during latent sensitization by mu-, delta-, and kappa-opioid receptors and 2A-adrenergic receptors: Role of constitutive activity. Neurobiol. Dis. 2016, 36, 204–221. [Google Scholar]
- Perry, D.C.; Mullis, K.B.; Øie, S.; Sadée, W. Opiate Antagonist Receptor Binding In Vivo: Evidence for a New Receptor Binding Model. Brain Research 1980, 199, 49–61. [Google Scholar] [CrossRef]
- Araldi, D.; Ferrari, L.F.; Levine, J.D. Hyperalgesic priming (type II) induced by repeated opioid exposure: Maintenance mechanisms. Pain 2017, 158, 1204–1216. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Sun, X.; Bohn, L.M.; Sadée, W. Opioid receptor homo- and hetero-dimerization in living cells by quantitative bioluminescence resonance energy transfer. Molec. Pharmacol. 2005, 67, 2173–2184. [Google Scholar] [CrossRef]
- Gupta, A.; Décaillot, F.M.; Devi, L.A. Targeting opioid receptor heterodimers: Strategies for screening and drug development. AAPS J. 2006, 8, E153–E159. [Google Scholar] [CrossRef] [PubMed]
- Charles, A.C.; Mostovskaya, N.; Asas, K.; Evans, C.J.; Dankovich, M.L.; Hales, T.G. Coexpression of delta-opioid receptors with mu receptors in GH3 cells changes the functional response to micro agonists from inhibitory to excitatory. Mol. Pharmacol. 2003, 63, 89–95. [Google Scholar] [CrossRef]
- Scherer, P.C.; Zaccor, N.W.; Neumann, N.M.; Vasavda, C.; Barrow, R.; Ewald, A.J.; Rao, F.; Sumner, C.J.; Snyder, S.H. TRPV1 is a physiological regulator of μ-opioid receptors. Proc. Natl. Acad. Sci. USA 2017, 114, 13561–13566. [Google Scholar] [CrossRef] [Green Version]
- Quillan, J.M.; Carlson, K.W.; Song, C.; Wang, D.; Sadée, W. Differential Effects of μ Opioid Receptor (MOR) Ligands on Ca2+ Signaling. J. Pharmacol. Exp. Ther. 2002, 302, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Grevel, J.; Sadée, W. An Opiate Binding Site in Rat Brain is Highly Selective for 4,5-Epoxymorphinans. Science 1983, 221, 1198–1201. [Google Scholar] [CrossRef] [PubMed]
- AIKO Biotechnology. A Phase-I, Two-Stage, Double-Blind, Placebo-Controlled, Pharmacokinetic and Pharmacodynamic Trial of Low Doses of Intravenous 6β-Naltrexol (AIKO-150) in Opioid-Dependent Subjects. ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT00829777?term=AIKO&rank=1 (accessed on 31 August 2020).
- Kocherlakota, P. Neonatal abstinence syndrome. Pediatrics 2004, 134, e547–e561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, H.E.; Kaltenbach, K.; Heil, S.H.; Stine, S.M.; Coyle, M.G.; Arria, A.M.; O’Grady, K.E.; Selby, P.; Martin, P.R.; Fischer, G. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N. Engl. J. Med. 2010, 363, 2320–2331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, M.C.; Crowley, M.; Wexelblatt, S.; Ford, S.; Kuhnell, P.; Kaplan, H.C.; McClead, R.; Macaluso, M.; Lannon, C. Ohio perinatal quality collaborative improves care of neonatal narcotic abstinence syndrome. Pediatrics 2018, 141, e20170900. [Google Scholar] [CrossRef] [Green Version]
- Conradt, E.; Crowell, S.E.; Lester, B.M. Early life stress and environmental influences on the neurodevelopment of children with prenatal opioid exposure. Neurobiol. Stress 2018, 9, 48–54. [Google Scholar] [CrossRef]
- Arlettaz, R.; Kashiwagi, M.; Das-Kundu, S.; Fauchere, J.C.; Lang, A.; Bucher, H.U. Methadone maintenance program in pregnancy in a swiss perinatal center (II): Neonatal outcome and social resources. Acta Obstet. Gynecol. Scand. 2005, 84, 145–150. [Google Scholar] [CrossRef]
- Leri, F.; Burns, L.H. Ultra-low-dose naltrexone reduces the rewarding potency of oxycodone and relapse vulnerability in rats. Pharmacol. Biochem. Behav. 2005, 82, 252–262. [Google Scholar] [CrossRef]
- Tompkins, D.A.; Lanier, R.K.; Harrison, J.A.; Strain, E.C.; Bigelow, G.E. Human abuse liability assessment of oxycodone combined with ultra-low-dose naltrexone. Psychopharmacology 2010, 210, 471–480. [Google Scholar] [CrossRef] [Green Version]
- Stott, L.A.; Hall, D.A.; Holliday, N.D. Unravelling intrinsic efficacy and ligand bias at G protein coupled receptors: A practical guide to assessing functional data. Biochem. Pharmacol. 2016, 101, 1–12. [Google Scholar] [CrossRef]
- Grozdanovic, M.; Laffey, K.G.; Abdelkarim, H.; Hitchinson, B.; Harijith, A.; Moon, H.G.; Park, G.Y.; Rousslang, L.K.; Masterson, J.C.; Furuta, G.T.; et al. Novel peptide nanoparticle-biased antagonist of CCR3 blocks eosinophil recruitment and airway hyperresponsiveness. J. Allergy Clin. Immunol. 2019, 143, 669.e12–680.e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desimine, V.L.; McCrink, K.A.; Parker, B.M.; Wertz, S.L.; Maning, J.; Lymperopoulos, A. Biased agonism/antagonism of cardiovascular GPCRs for heart failure therapy. Int. Rev. Cell Mol. Biol. 2018, 339, 41–61. [Google Scholar] [PubMed]
- Gomes, I.; Sierra, S.; Lueptow, L.; Gupta, A.; Gouty, S.; Margolis, E.B.; Cox, B.M.; Devi, L.A. Biased signaling by endogenous opioid peptides. Proc. Natl. Acad. Sci. USA 2020, 117, 11820–11828. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.L.; Lane, J.R.; Coudrat, T.; Sexton, P.M.; Christopoulos, A.; Canals, M. Biased agonism of endogenous opioid peptides at the μ-opioid receptor. Mol. Pharmacol. 2015, 88, 335–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Species | Test | Agonist (Dose, Route) | Antagonist ID50, or pA2, KI Binding (Rote) | Ref. | |
|---|---|---|---|---|---|
| Naltrexone | 6β-Naltrexol | ||||
| mouse | hotplate | morphine (30 mg/kg, i.p.) | 0.007 mg/kg (i.p.) | 1.3 mg/kg (i.p.) | [26] |
| mouse | withdrawal jumping | morphine (73 mg pellet, s.c., 3d) | 0.09 mg/kg (i.p.) | 6.9 mg/kg (i.p.) | [27] |
| mouse | tail-flick | hydrocodone (3.2 mg/kg, i.v.) | 0.53 mg/kg (p.o.) * | 2.4 mg/kg (p.o.) * | [31] |
| rhesus monkey | tail-withdrawal | alfentanil (0.01–5 mg/kg, s.c.) | pA2 8.5 * (0.0032–0.32 mg/kg, s.c.) | pA2 6.5* (0.32–3.2 mg/kg, s.c.) | [29] |
| rhesus monkey | precipitated withdrawal | morphine (6.4 mg/kg, i.m. for 3d) (respiratory functions) | 0.004 mg/kg (i.m. for 3d) | 0.33 mg/kg (i.m. for 3d) | [29] |
| rhesus monkey | MOR binding, | 3H-DAMGO (1 nM, in vitro) 3H-diprenorphine (0.2 nM) | 0.31 nM, Ki = 1.7 nM | Ki = 0.74 nM KI = 3.2 nM | [29] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadee, W.; Oberdick, J.; Wang, Z. Biased Opioid Antagonists as Modulators of Opioid Dependence: Opportunities to Improve Pain Therapy and Opioid Use Management. Molecules 2020, 25, 4163. https://doi.org/10.3390/molecules25184163
Sadee W, Oberdick J, Wang Z. Biased Opioid Antagonists as Modulators of Opioid Dependence: Opportunities to Improve Pain Therapy and Opioid Use Management. Molecules. 2020; 25(18):4163. https://doi.org/10.3390/molecules25184163
Chicago/Turabian StyleSadee, Wolfgang, John Oberdick, and Zaijie Wang. 2020. "Biased Opioid Antagonists as Modulators of Opioid Dependence: Opportunities to Improve Pain Therapy and Opioid Use Management" Molecules 25, no. 18: 4163. https://doi.org/10.3390/molecules25184163
APA StyleSadee, W., Oberdick, J., & Wang, Z. (2020). Biased Opioid Antagonists as Modulators of Opioid Dependence: Opportunities to Improve Pain Therapy and Opioid Use Management. Molecules, 25(18), 4163. https://doi.org/10.3390/molecules25184163







