Preparation of Continuous Highly Hydrophobic Pure Silica ITQ-29 Zeolite Layers on Alumina Supports
Abstract
1. Introduction
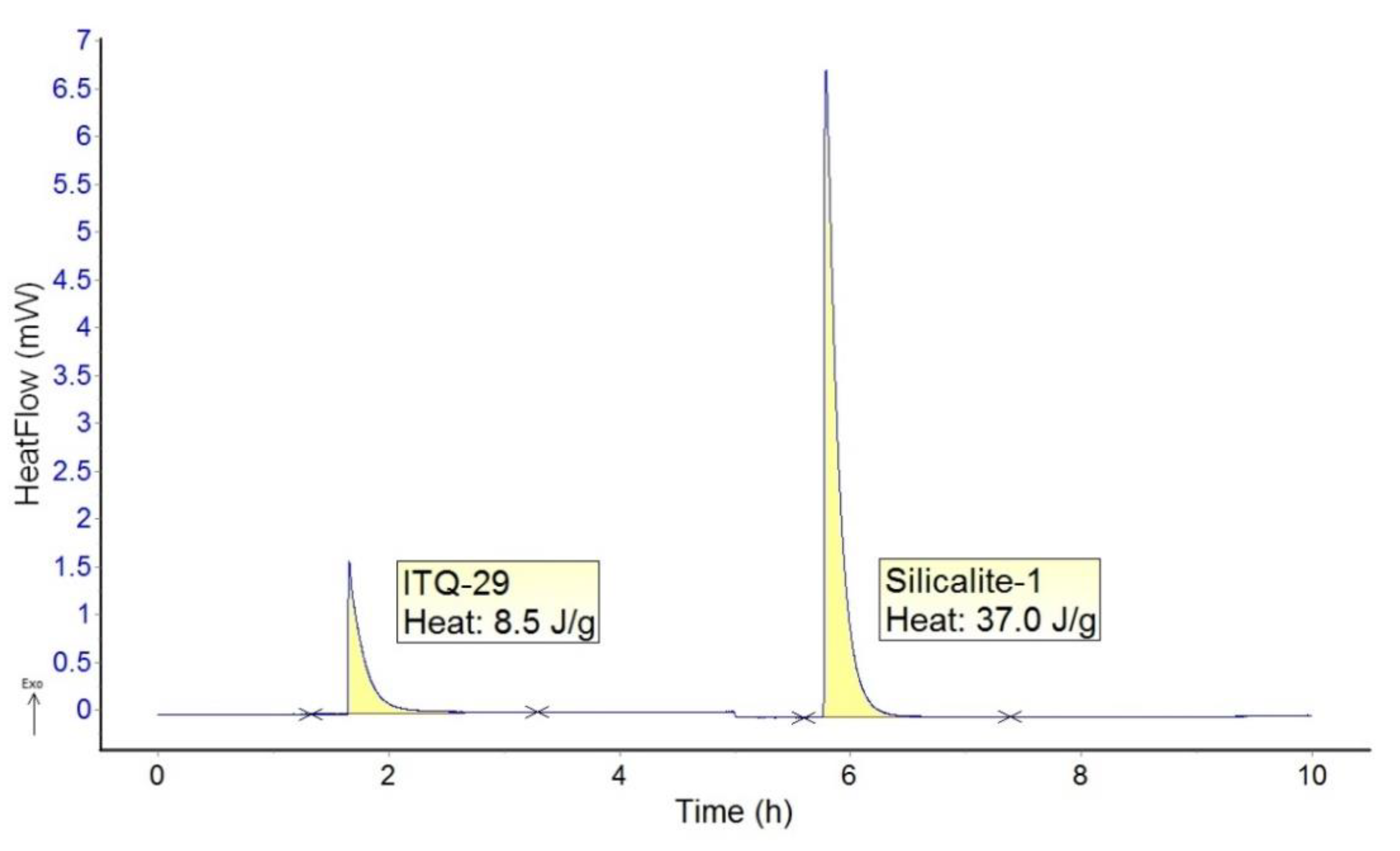
2. Results and Discussion
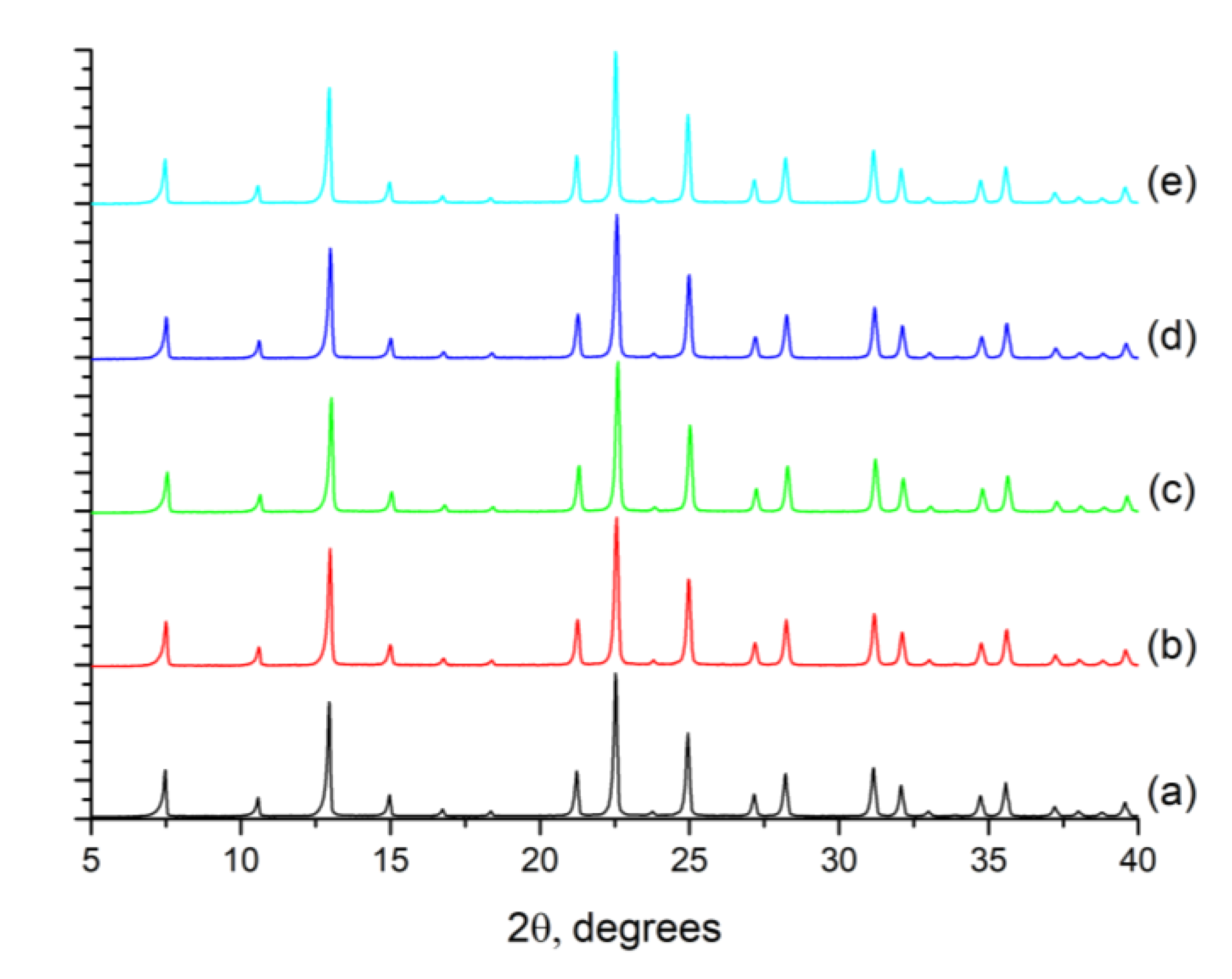
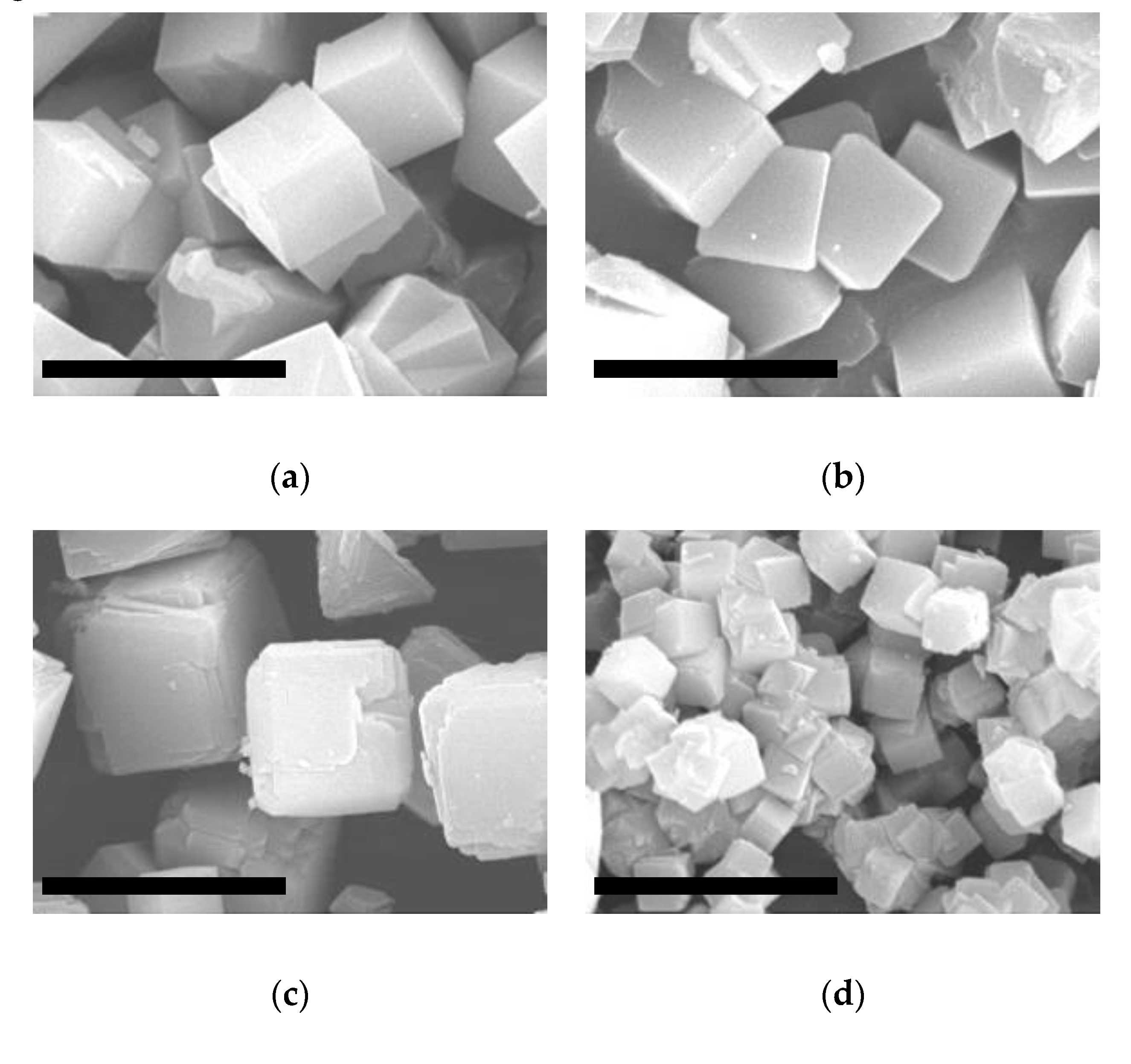
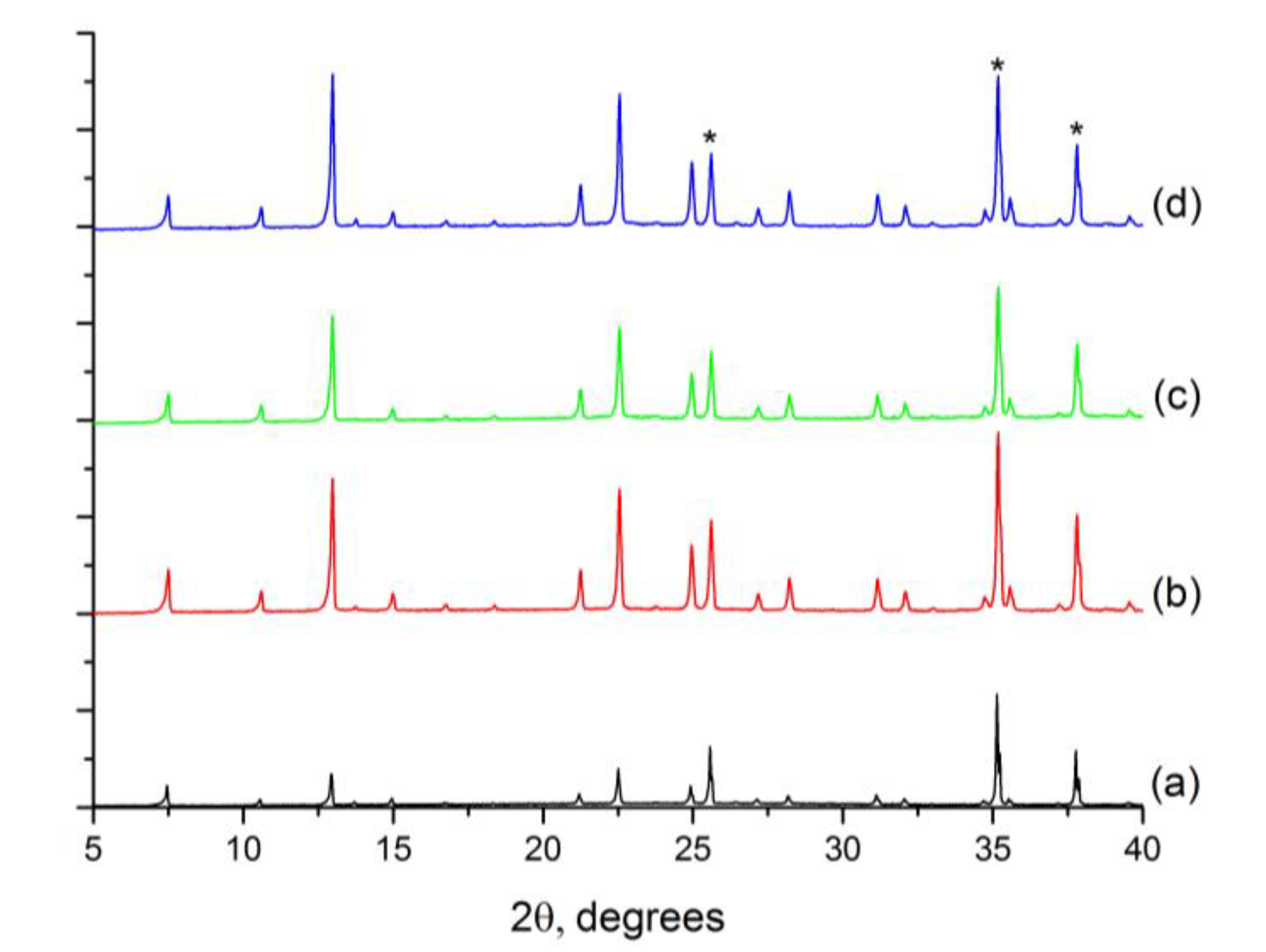
2.1. ITQ-29 Seeds
2.2. ITQ-29 Zeolite on Porous Alumina Supports
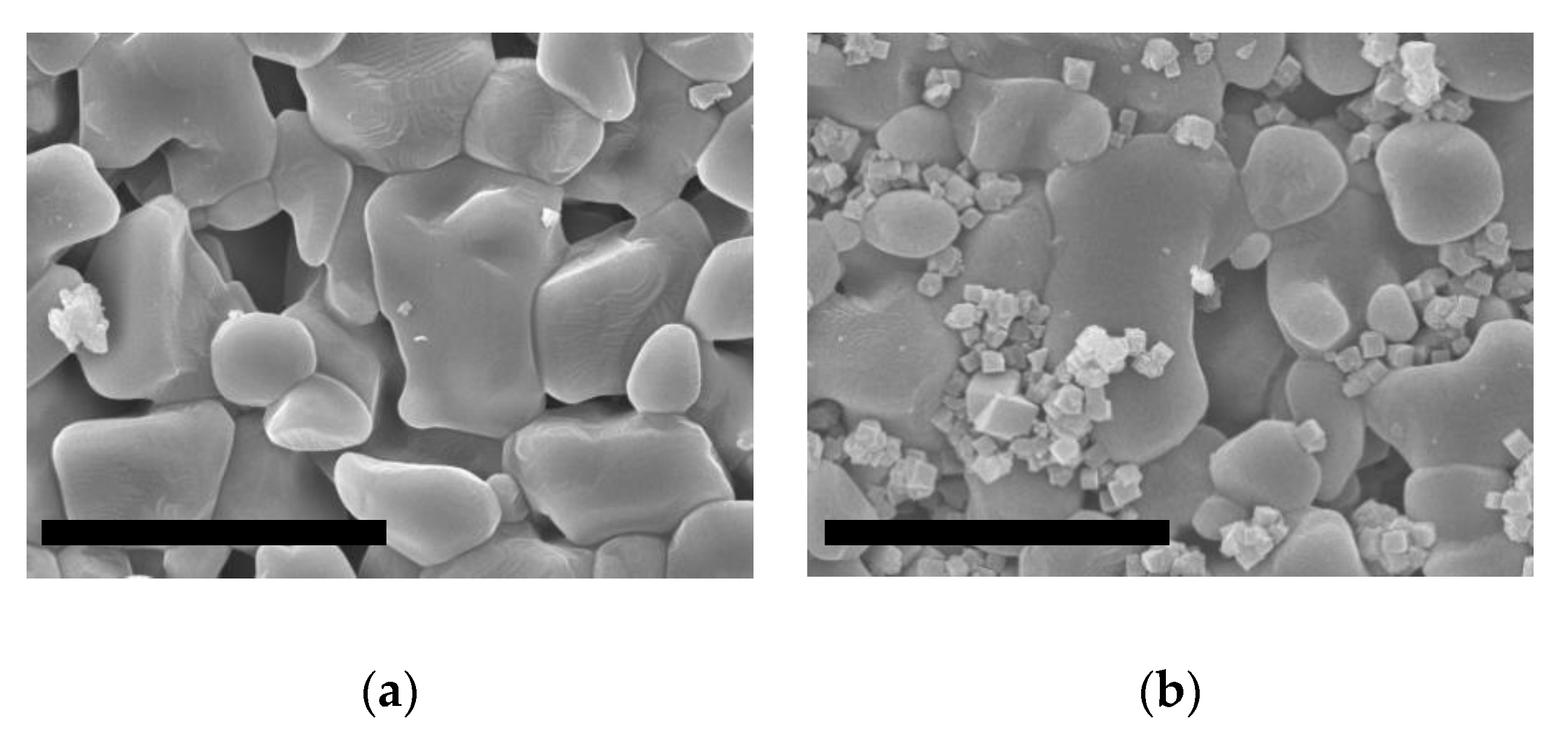
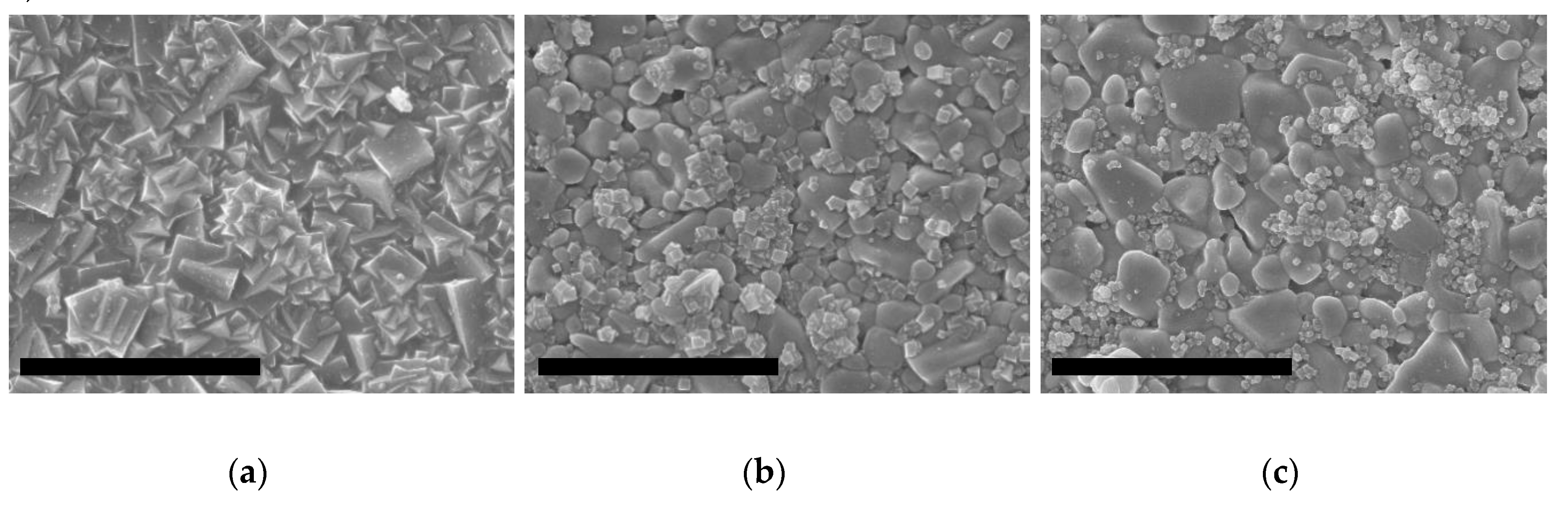
2.2.1. Seeding of the Supports
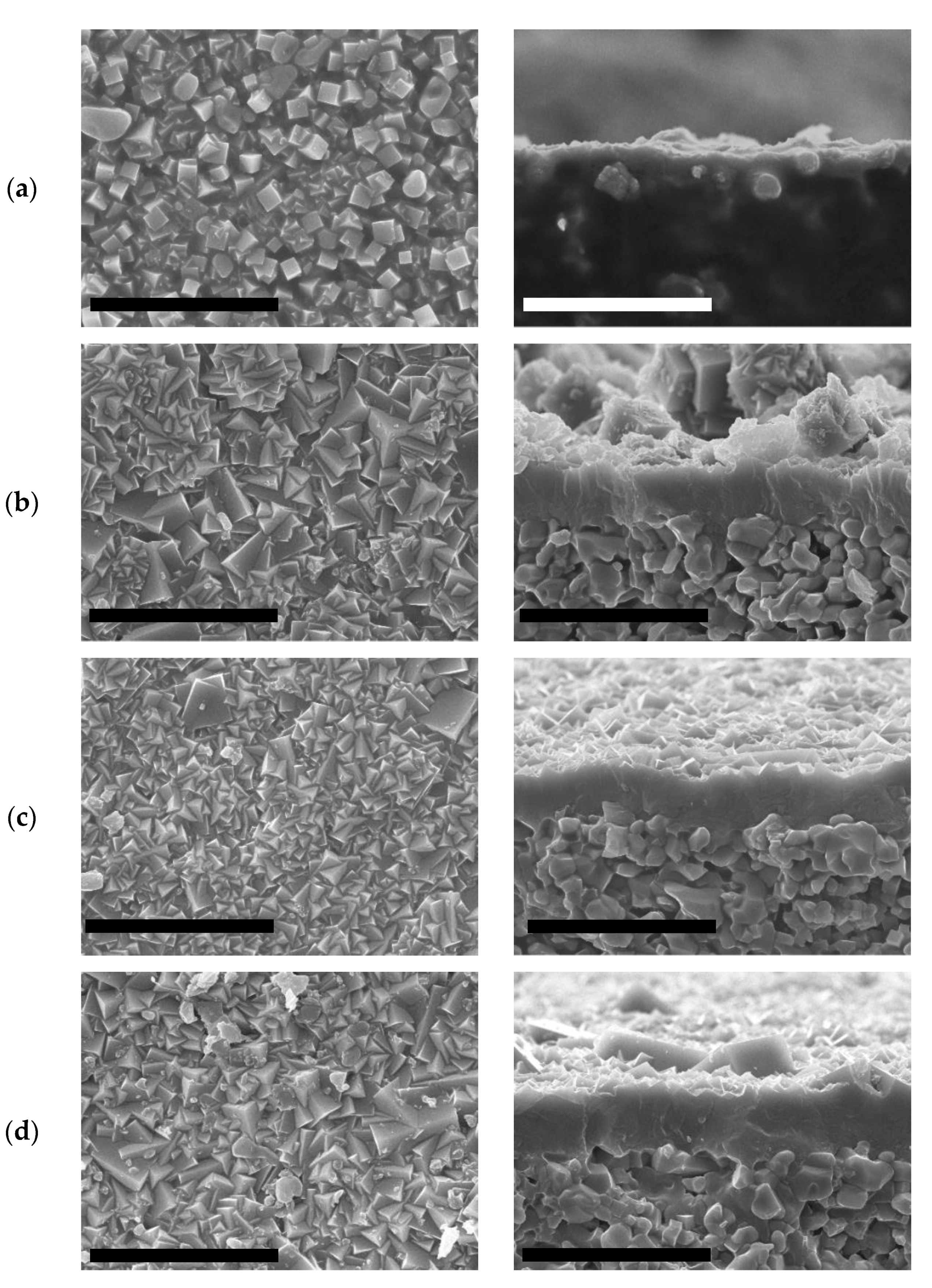
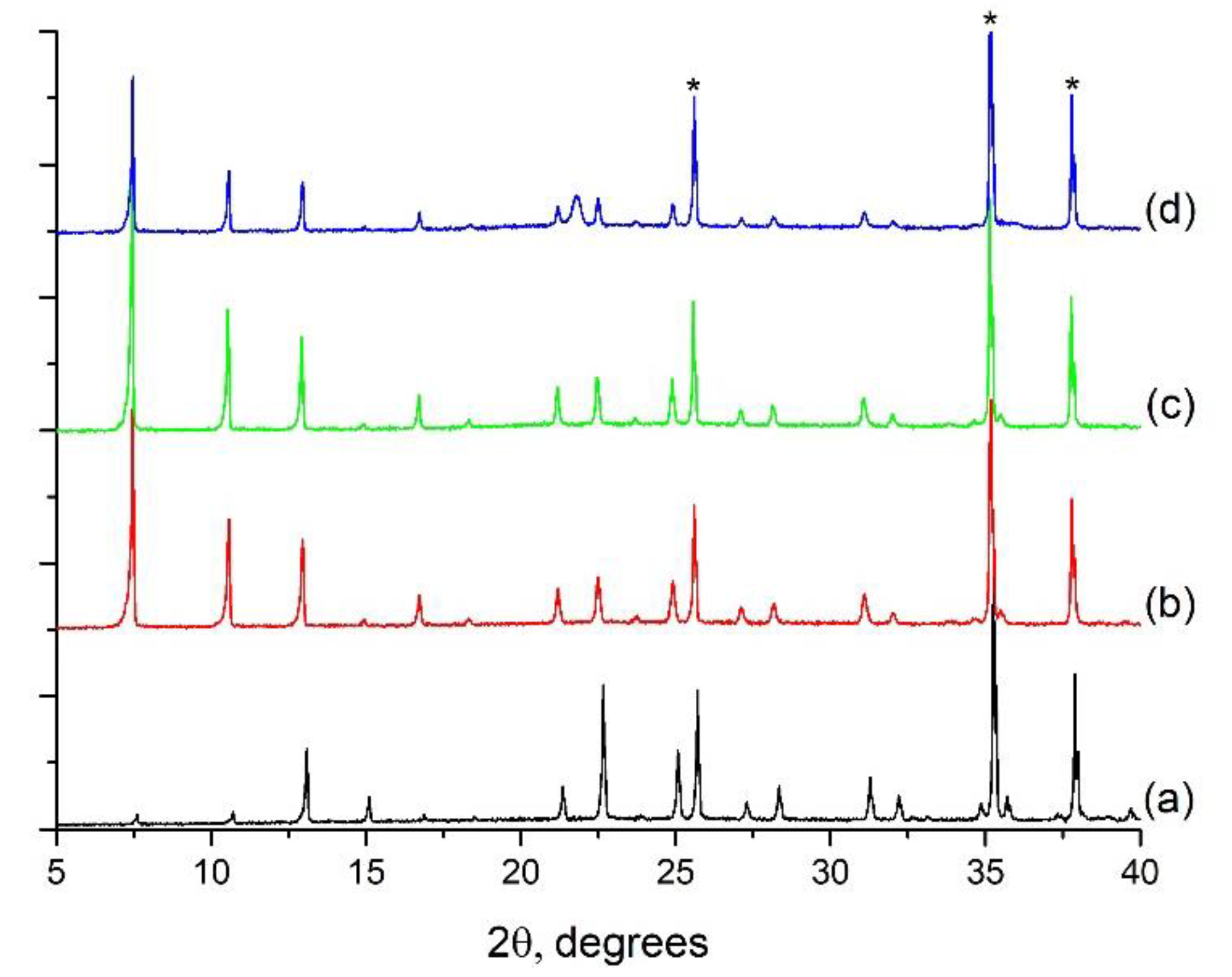
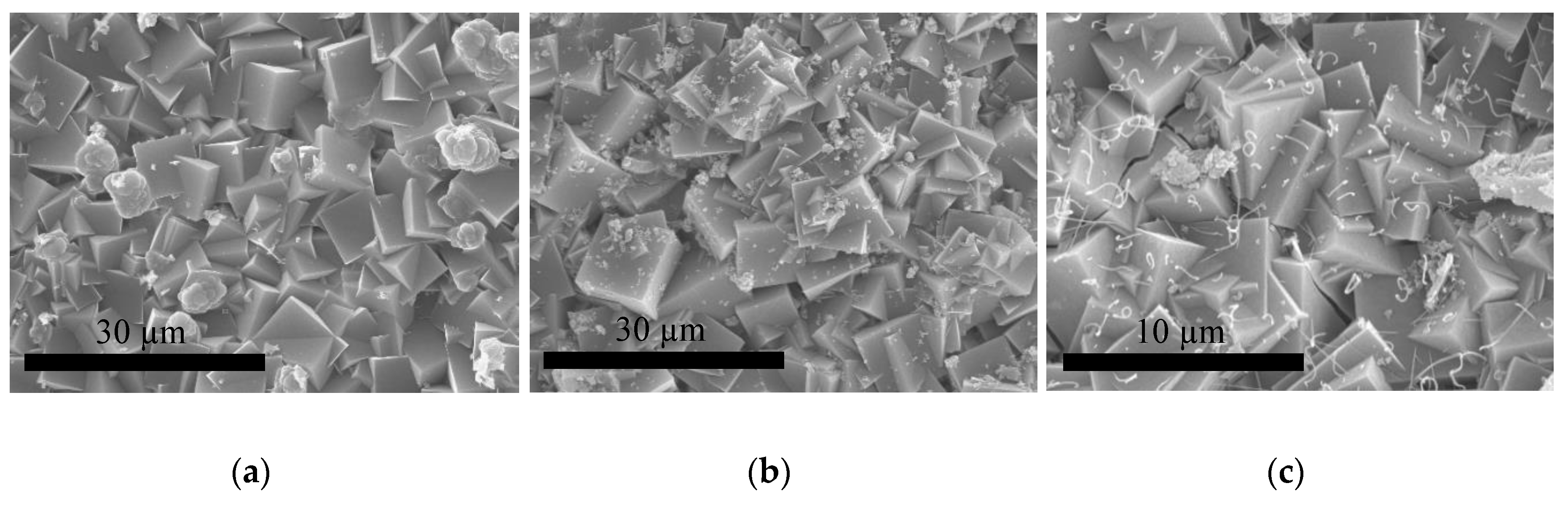
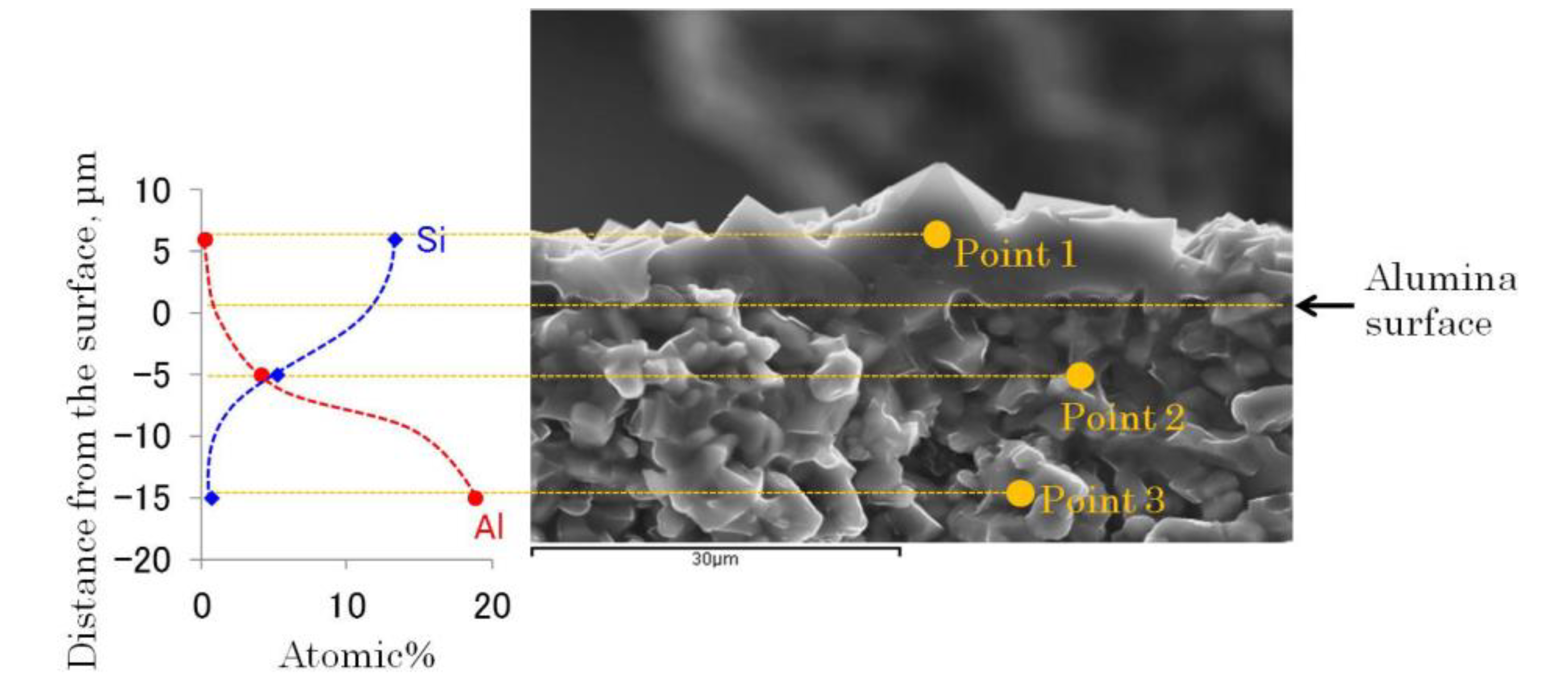
2.2.2. Growth of ITQ-29 Zeolite on Alumina Supports
3. Materials and Methods
3.1. Preparation of ITQ-29 Seeds
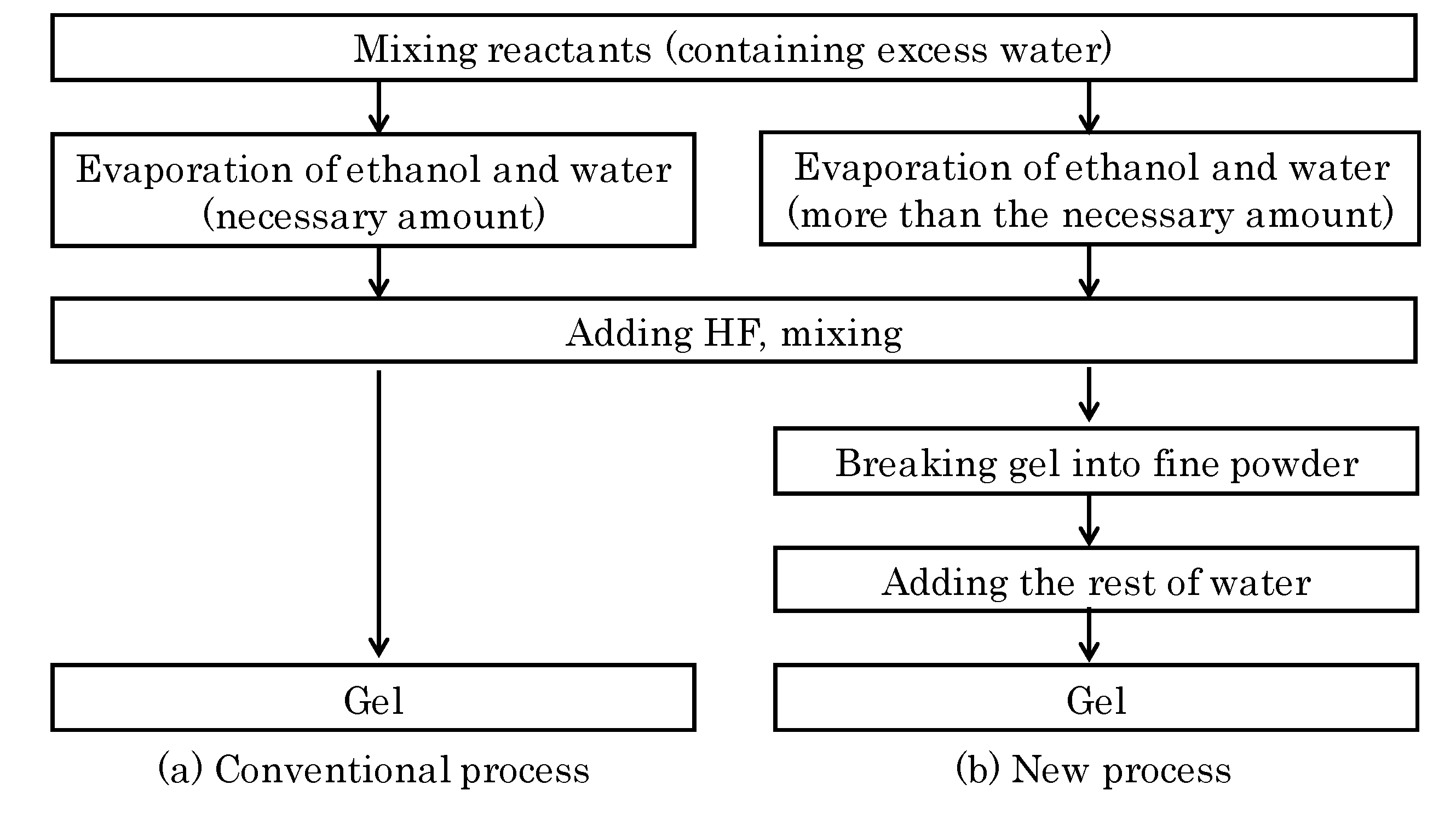
3.2. Preparation of ITQ-29 Zeolite on Alumina Supports
3.3. Characterization Tecniques
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mascal, M. Chemicals from biobutanol: Technologies and markets. Biofuels Bioprod. Biorefin. 2012, 6, 483–493. [Google Scholar] [CrossRef]
- Ndaba, B.; Chiyanzu, I.; Marx, S. N-Butanol derived from biochemical and chemical routes: A review. Biotechnol. Rep. 2015, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.J.; Ramaswamy, S.; Liu, Y. Separation and purification of biobutanol during bioconversion of biomass. Sep. Purif. Technol. 2014, 132, 513–540. [Google Scholar] [CrossRef]
- Barton, W.E.; Daugulis, A.J. Evaluation of solvents for extractive butanol fermentation with Clostridium acetobutylicum and the use of poly(propylene glycol) 1200. Appl. Microbiol. Biotechnol. 1992, 36, 632–639. [Google Scholar] [CrossRef]
- Raganati, F.; Procentese, A.; Olivieri, G.; Russo, M.E.; Salatino, P.; Marzocchella, A. Bio-butanol recovery by adsorption/desorption processes. Sep. Purif. Technol. 2020, 235, 116145. [Google Scholar] [CrossRef]
- Xue, C.; Zhao, J.B.; Chen, L.J.; Bai, F.W.; Yang, S.T.; Sun, J.X. Integrated butanol recovery for an advanced biofuel: Current state and prospects. Appl. Microbiol. Biotechnol. 2014, 98, 3463–3474. [Google Scholar] [CrossRef]
- Sadrimajd, P.; Rene, E.R.; Lens, P.N.L. Adsorptive recovery of alcohols from a model syngas fermentation broth. Fuel 2019, 254, 115590. [Google Scholar] [CrossRef]
- Qureshi, N.; Hughes, S.; Maddox, I.S.; Cotta, M.A. Energy-efficient recovery of butanol from model solutions and fermentation broth by adsorption. Bioprocess. Biosyst. Eng. 2005, 27, 215–222. [Google Scholar] [CrossRef]
- Milestone, N.B.; Bibby, D.M. Concentration of alcohols by adsorption on silicalite. J. Chem. Tech. Biotechnol. 1981, 31, 732–736. [Google Scholar] [CrossRef]
- Yang, R.T. Adsorbents Fundamentals and Applications; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Available online: http://www.iza-structure.org/databases/ (accessed on 31 July 2020).
- Weckhuysen, B.M.; Yu, J. Recent advances in zeolite chemistry and catalysis. Chem. Soc. Rev. 2015, 44, 7022–7024. [Google Scholar] [CrossRef]
- Dusselier, M.; Davis, M.E. Small-Pore Zeolites: Synthesis and Catalysis. Chem Rev. 2018, 118, 5265–5329. [Google Scholar] [CrossRef] [PubMed]
- Rangnekar, N.; Mittal, N.; Elyassi, B.; Caro, J.; Tsapatsis, M. Zeolite membranes—A review and comparison with MOFs. Chem. Soc. Rev. 2015, 44, 7128–7154. [Google Scholar] [CrossRef] [PubMed]
- Korelskiy, D.; Leppäjärvi, T.; Zhou, H.; Grahn, M.; Tanskanen, J.; Hedlund, J. High flux MFI membranes for pervaporation. J. Membr. Sci. 2013, 427, 381–389. [Google Scholar] [CrossRef]
- Negishi, H.; Sakaki, K.; Ikegami, T. Silicalite pervaporation membrane exhibiting a separation factor of over 400 for butanol. Chem. Lett. 2010, 39, 1312–1314. [Google Scholar] [CrossRef]
- Ueno, K.; Negishi, H.; Okuno, T.; Tawarayama, H.; Ishikawa, S.; Miyamoto, M.; Uemiya, S.; Oumi, Y. Effects of seed crystal type on the growth and microstructures of silicalite-1 membranes on tubular silica supports via gel-free steam-assisted conversion. Microporous Mesoporous Mater. 2019, 289, 109645. [Google Scholar] [CrossRef]
- Elyassi, B.; Jeon, M.Y.; Tsapatsis, M.; Narasimharao, K.; Basahel, S.N.; Al Thabaiti, S. Ethanol/water mixture pervaporation performance of b-oriented silicalite-1 membranes made by gel-free secondary growth. Aiche. J. 2016, 62, 556–563. [Google Scholar] [CrossRef]
- Lan, J.; Saulat, H.; Wu, H.; Li, L.; Yang, J.; Lu, J.; Zhang, Y. Manipulation on microstructure of MFI membranes by binary structure directing agents. Microporous Mesoporous Mater. 2020, 299, 110128. [Google Scholar] [CrossRef]
- Ueno, K.; Yamada, S.; Negishi, H.; Okuno, T.; Tawarayama, H.; Ishikawa, S.; Miyamoto, M.; Uemiya, S.; Oumi, Y. Fabrication of pure-silica *BEA-type zeolite membranes on tubular silica supports coated with dilute synthesis gel via steam-assisted conversion. Sep. Purif. Technol. 2020, 247, 116934. [Google Scholar] [CrossRef]
- Kida, K.; Maeta, Y.; Yogo, K. Pure silica CHA-type zeolite membranes for dry and humidified CO2/CH4 mixtures separation. Sep. Purif. Technol 2018, 197, 116–121. [Google Scholar] [CrossRef]
- Imasaka, S.; Nakai, A.; Araki, S.; Yamamoto, H. Synthesis and gas permeation of STT-type zeolite membranes. J. Jpn. Pet. Inst. 2018, 61, 263–271. [Google Scholar] [CrossRef]
- Reed, T.B.; Breck, D.W. Crystalline zeolites. II. Crystal structures of synthetic zeolite, type A. J. Am. Chem. Soc. 1956, 78, 5972–5977. [Google Scholar] [CrossRef]
- Corma, A.; Rey, F.; Rius, J.; Sabater, M.J.; Valencia, S. Supramolecular self-assembled molecules as organic directing agent for synthesis of zeolites. Nature 2004, 431, 287–290. [Google Scholar] [CrossRef] [PubMed]
- García, E.J.; Pérez-Pellitero, J.; Pirngruber, G.D.; Jallut, C.; Palomino, M.; Rey, F.; Valencia, S. Tuning the Adsorption Properties of Zeolites as Adsorbents for CO2 Separation: Best Compromise between the Working Capacity and Selectivity. Ind. Eng. Chem. Res. 2014, 53, 9860–9874. [Google Scholar] [CrossRef]
- Palomino, M.; Corma, A.; Rey, F.; Valencia, S. New Insights on CO2−Methane Separation Using LTA Zeolites with Different Si/Al Ratios and a First Comparison with MOFs. Langmuir 2010, 26, 1910–1917. [Google Scholar] [CrossRef]
- Van der Perre, S.; Gelin, P.; Claessens, B.; Martin-Calvo, A.; Cousin Saint Remi, J.; Duerinck, T.; Baron, G.V.; Palomino, M.; Sánchez, L.Y.; Valencia, S.; et al. Intensified Biobutanol Recovery by using Zeolites with Complementary Selectivity. ChemSusChem 2017, 10, 2968–2977. [Google Scholar] [CrossRef]
- Serrano, D.P.; Calleja, G.; Botas, J.A.; Gutiérrez, F.J. Characterization of adsorptive and hydrophobic properties of silicalite-1, ZSM-5, TS-1 and Beta zeolites by TPD techniques. Sep. Purif. Technol. 2007, 54, 1–9. [Google Scholar] [CrossRef]
- Zhang, K.; Lively, R.P.; Noel, J.D.; Dose, M.E.; McCool, B.A.; Chance, R.R.; Koros, W.J. Adsorption of Water and Ethanol in MFI-Type Zeolites. Langmuir 2012, 28, 8664–8673. [Google Scholar] [CrossRef]
- Demontis, P.; Stara, G.; Suffritti, G.B. Behavior of Water in the Hydrophobic Zeolite Silicalite at Different Temperatures. A Molecular Dynamics Study. J. Phys. Chem. B 2003, 107, 4426–4436. [Google Scholar] [CrossRef]
- Tiscornia, I.; Valencia, S.; Corma, A.; Téllez, C.; Coronas, J.; Santamaría, J. Preparation of ITQ-29 (Al-free zeolite A) membranes. Microporous Mesoporous Mater. 2008, 110, 303–309. [Google Scholar] [CrossRef]
- Hunt, H.K.; Lew, C.M.; Sun, M.; Yan, Y.; Davis, M.E. Pure-silica zeolite thin films by vapor phase transport of fluoride for low-k applications. Microporous Mesoporous Mater. 2010, 128, 12–18. [Google Scholar] [CrossRef]
- Fernández-Barquín, A.; Casado-Coterillo, C.; Palomino, M.; Valencia, S.; Irabien, A. Permselectivity improvement in membranes for CO2/N2 separation. Separ. Purif. Technol. 2016, 157, 102–111. [Google Scholar] [CrossRef]
- Casado-Coterillo, C.; Fernández-Barquín, A.; Valencia, S.; Irabien, A. Estimating CO2/N2 permselectivity through Si/Al = 5 small-pore zeolites/PTMSP mixed matrix membranes: Influence of temperature and topology. Membranes 2018, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Barquín, A.; Casado-Coterillo, C.; Palomino, M.; Valencia, S.; Irabien, A. LTA/Poly(1-trimethylsilyl-1-propyne) mixed-matrix membranes for high-temperature CO2/N2 separation. Chem. Eng. Technol. 2015, 38, 658–666. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B. Practical Aspects of Powder Diffraction Data Analysis. Stud. Surf. Sci. Catal. 1994, 85, 391–428. [Google Scholar] [CrossRef]
- McCusker, L.B.; Zou, X. Diffraction methods for zeolite structural characterization. In Verified Syntheses of Zeolitic Materials; Mintova, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 78–83. Available online: http://www.iza-online.org/synthesis/VS_3rdEd.pdf (accessed on 14 July 2020).
- White, J.; Dutta, P.K.; Shqau, K.; Verweij, H. Synthesis of zeolite L membranes with sub-micron to micron thicknesses. Microporous Mesoporous Mater. 2008, 115, 389–398. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, J.H.; Lee, Y.J.; Jeong, N.C.; Yoon, K.B. Manual Assembly of Microcrystal Monolayers on Substrates. Angew. Chem. Int. Ed. 2007, 46, 3087–3090. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palomino, M.; Ono, H.; Valencia, S.; Corma, A. Preparation of Continuous Highly Hydrophobic Pure Silica ITQ-29 Zeolite Layers on Alumina Supports. Molecules 2020, 25, 4150. https://doi.org/10.3390/molecules25184150
Palomino M, Ono H, Valencia S, Corma A. Preparation of Continuous Highly Hydrophobic Pure Silica ITQ-29 Zeolite Layers on Alumina Supports. Molecules. 2020; 25(18):4150. https://doi.org/10.3390/molecules25184150
Chicago/Turabian StylePalomino, Miguel, Hideki Ono, Susana Valencia, and Avelino Corma. 2020. "Preparation of Continuous Highly Hydrophobic Pure Silica ITQ-29 Zeolite Layers on Alumina Supports" Molecules 25, no. 18: 4150. https://doi.org/10.3390/molecules25184150
APA StylePalomino, M., Ono, H., Valencia, S., & Corma, A. (2020). Preparation of Continuous Highly Hydrophobic Pure Silica ITQ-29 Zeolite Layers on Alumina Supports. Molecules, 25(18), 4150. https://doi.org/10.3390/molecules25184150







