Abstract
In a series of anti-inflammatory screenings of lauraceous plants, the methanolic extract of the leaves of Machilus japonica var. kusanoi (Hayata) J.C. Liao showed potent inhibition on both superoxide anion generation and elastase release in human neutrophils. Bioassay-guided fractionation of the leaves of M. japonica var. kusanoi led to the isolation of twenty compounds, including six new butanolides, machinolides A–F (1–6), and fourteen known compounds (7–20). Their structures were characterized by 1D and 2D NMR, UV, IR, CD, and MS data. The absolute configuration of the new compounds were unambiguously confirmed by single-crystal X-ray diffraction analyses (1, 2, and 3) and Mosher’s method (4, 5, and 6). In addition, lignans, (+)-eudesmin (11), (+)-methylpiperitol (12), (+)-pinoresinol (13), and (+)-galbelgin (16) exhibited inhibitory effects on N-formyl-methionyl-leucyl-phenylalanine/cytochalasin B (fMLP/CB)-induced superoxide anion generation in human neutrophils with IC50 values of 8.71 ± 0.74 μM, 2.23 ± 0.92 μM, 6.81 ± 1.07 μM, and 7.15 ± 2.26 μM, respectively. The results revealed the anti-inflammatory potentials of Formosan Machilus japonica var. kusanoi.
1. Introduction
Neutrophils play an important role in the human body against infections [1]. In response to immune stimulation, activated neutrophils generate a series of cytotoxic substances, such as the superoxide anion (O2•−), a precursor of other ROS, granule proteases, and bioactive lipids. The superoxide anion is known to cause damage to cells and tissues, stimulate macrophages, and trigger a cascade of inflammatory pathways [2]. Neutrophil elastase is one of the serine proteases stored in large amounts in neutrophil granules and is involved in the nonoxidative pathway of the intracellular and extracellular immune response [3]. Neutrophil elastase is stimulated by neutrophils and causes the destruction of tissue in chronic inflammatory disease [2]. Besides, the persistent overexpression of neutrophils is involved in various conditions, such as rheumatoid arthritis, asthma, psoriasis, and ischemic heart disease.
Lauraceous plants are a dominant family in South and East Asia, consisting of aromatic trees and shrubs. They stand out, resulting in its economic benefits and diverse bioactivities. A previous investigation showed that some lauraceous plants exhibit bioactivities, such as cytotoxicity, anti-tuberculosis, anti-inflammatory, and antiplatelet activities [4]. Recently, we completed the anti-inflammatory screening of 174 methanolic extracts from 60 Taiwanese lauraceous plants. Among the screening results, the methanolic extract of the leaves of Machilus japonica var. kusanoi showed potent anti-inflammatory activity on both superoxide anion generation and elastase release in human neutrophils.
The Machilus genus comprises about 100 species with accepted names, mainly distributed in East Asia [5]. Previous studies of Machilus species identified various classes of chemical constituents, such as lignans, flavonoids, and terpenoids [4]. M. japonica var. kusanoi is a large evergreen tree endemic to Taiwan and is distributed in broad-leaved forests from lowlands up to 1400 m throughout the island [5]. Few investigations of M. japonica var. kusanoi have been published before. Only ten compounds were isolated from this plant [6,7,8], and only antimicrobial along with anti-α-glucosidase activity of this plant have been found previously [8,9]. Based on anti-inflammatory screening results and the rare investigation of the leaves from M. japonica var. kusanoi, the aims of this study are the isolation of components from the leaves of M. japonica var. kusanoi and the evaluation of their anti-inflammatory effects.
2. Results
After anti-inflammatory assay-guided fractionation of the leaves of M. japonica var. kusanoi, we successfully isolated six new butanolides (1−6) (Figure 1) and 14 known compounds (7–20) (Supplementary Materials, Figure S1). The phytochemical spectra of compounds 1 to 6 are available in the Supplementary Materials, Figures S2–S57. In particular, Mosher’s method and X-ray crystallographic analysis were applied to determine the absolute configuration of the new compounds. Moreover, anti-inflammatory effects of isolates on neutrophil pro-inflammatory responses were evaluated by the suppression of N-formyl-methionyl-leucyl-phenylalanine/cytochalasin B (fMLP/CB)-induced superoxide anion (O2•−) generation and elastase release. The structure identification of the new compounds and anti-inflammatory activity results are illustrated below.
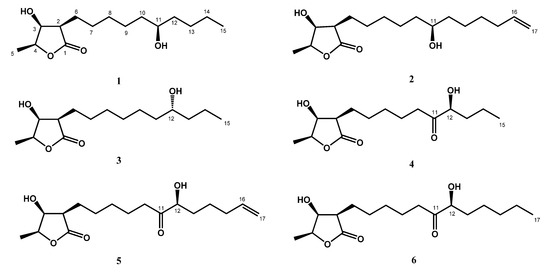
Figure 1.
Structures of new compounds 1–6.
Compound 1 was obtained as colorless needles. Its molecular formula was determined as C15H28O4 from high-resolution electrospray ionization mass spectroscopy (HRESIMS) data (m/z 295.18923 [M + Na]+ (calcd. for 295.18853)), implying two degrees of unsaturation. The infrared spectroscopy (IR) spectrum showed typical absorptions of C=O (1736 cm−1) for γ-lactone and hydroxy groups (3433 cm−1). The 1H-NMR spectrum of 1 displayed signals of three oxymethines at δH 3.59 (1H, m, H-11), 4.45 (1H, qd, J = 6.6, 3.2 Hz, H-4), and 4.31 (1H, dd, J = 4.8, 3.2 Hz, H-3), two methyl groups including one doublet methyl group at δH 1.43 (3H, d, J = 6.6 Hz, H-5) and one triplet methyl group at δH 0.91 (3H, t, J = 7.0 Hz, H-15), and alkyl side chains at δH 1.66 (1H, m, H-6b), δH 1.82 (1H, m, H-6a), and δH 1.26~1.47 (14H, m, H-7~H-10, H-12~H-14) (Table 1). The γ-lactone was confirmed by IR spectrum, the 1H-1H correlation spectroscopy (COSY) correlations between H-2/H-3/H-4/H-5 and the heteronuclear multiple bond correlation (HMBC) between H-2/C-1 (δ 177.5), C-3 (δ 71.2), H-3/C-1, and H-4/C-3 (Figure 2). The doublet methyl group (C-5) was connected to C-4, based on the COSY correlations between H-5/H-4, and HMBC correlations between H-5/C-3, C-4 (δ 78.8) (Figure 2). The HMBC showed correlations H-6/C-2 (δ 47.6), C-3, C-7 (δ 27.5), and C-8 (δ 29.4), which supported that the alkyl chain was located at C-2 (Figure 2). The key correlations in the nuclear Overhauser enhancement spectroscopy (NOESY) spectrum (H-2 showed correlation with H-3, H-4, and no correlation with H-5; H-3 showed correlation with H-4 and no correlation with H-5) confirmed that H-2, H-3, and H-4 were in the same phase (Figure 3). However, a remaining hydroxy group (δC 71.9) was located at a position of the alkyl chain which cannot be determined by NMR spectrum. Finally, the location of the remaining hydroxy group and the absolute configuration of 1 was further confirmed by single-crystal X-ray diffraction (Figure 4). The results proved that the stereochemistry of 1 should be shown as 2R,3S,4S,11R-form in the Oak Ridge thermal ellipsoid plot program (ORTEP) diagram. Thus, compound 1 was elucidated and named machinolide A.

Table 1.
1H and 13C-NMR data of machinolides A–C (1–3).
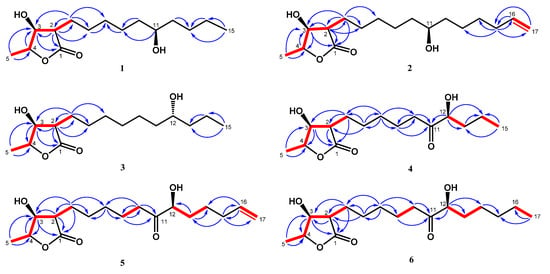
Figure 2.
Key 1H-1H COSY (━) and HMBC (H→C) correlations of machinolides A–F (1–6).
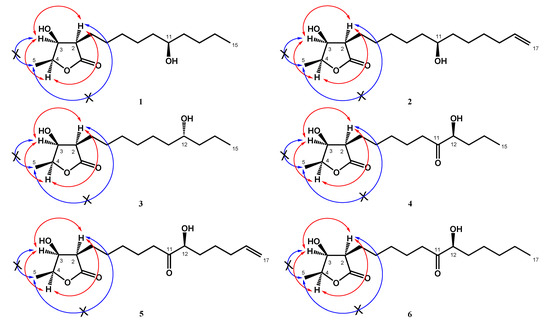
Figure 3.
NOESY (H↔H) correlations of machinolides A–F (1–6).
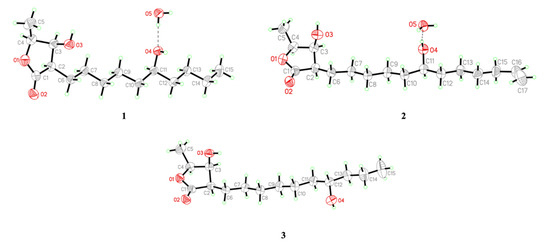
Figure 4.
Perspective drawing of X-ray structures of machinolides A–C (1–3).
Compound 2 was isolated as colorless needles. Its molecular formula was established as C17H30O4 by HREIMS data (m/z 321.20370 [M + Na]+ (calcd. for 321.20363)). The 1H-NMR spectrum of 2 was similar to that of 1, except for the presence of a terminal double bond at δH 5.81 (1H, ddt, J = 17.1, 10.2, 6.6 Hz, H-16), 4.94 (1H, ddt, J = 10.2, 3.3, 1.5 Hz, H-17b), and 5.00 (1H, ddt, J = 17.1, 3.3, 1.5 Hz, H-17a) in 2 (Table 1). Comparison of 13C-NMR spectrum of 2 and 1 also supported the presence of a terminal double bond [δC 138.9 (C-16), 114.4 (C-17)] in 2. The HMBC correlations between H-15/C-16, C-17 and H-16, H-17/C-15 (δ 33.7) were further confirmed that the terminal double bond was located at C-16 and C-17 (Figure 2). The NOESY correlations of 2 were similar to those of 1, indicating that H-2, H-3 and H-4 were in the same phase in 2 (Figure 3). The absolute configuration of 2 was confirmed by single-crystal X-ray diffraction and assigned as 2R,3S,4S,11S-form (Figure 4). According to the above data, the structure of 2 was determined and named machinolide B.
Compound 3 was yielded as colorless needles and assigned the molecular formula C15H28O4 through analysis of its HRESIMS data (m/z 273.20656 [M + H]+ (calcd. for 273.20658). All the spectra of 3 were similar to those of 1. However, electron ionization mass spectra (EIMS) showed the different fragments between 3 (m/z 215 (56), 186 (37)) and 1 (m/z 229 (39), 200 (24)), which suggests that the position of the hydroxy group in the alkyl chain was different. The hydroxy group of 3 was located at C-12 and the absolute configuration of 3 was assigned as 2R,3S,4S,12R-form, which were both determined by single-crystal X-ray diffraction (Figure 4). Therefore, compound 3 was named machinolide C, and its structure was further confirmed by COSY and HMBC experiments (Figure 2).
Compound 4 was obtained as a colorless oil. The ESIMS analysis of 4 showed the [M+H]+ ion at m/z 287, in agreement with the molecular formula of C15H26O5, as confirmed by HRESIMS. Compound 4 had similar IR and 1H-NMR spectra to those of 3, except for the presence of a ketone group at C-11 (δ 212.6) in the 13C-NMR spectrum (Table 2). The HMBC correlation between H-9, H-10, H-12/C-11, and H-12/C-11, C-13, C-14 supported the position of the ketone group and hydroxy group at C-11 and C-12, respectively (Figure 2). The planar structure of 4 was decided. The CD spectrum of 4 showed a negative cotton effect at 219.5 nm, which was similar to malleastrumolide A [10]. Thus, the absolute configuration of C-2 was determined as R-form. The NOESY correlations between H-2/H-3, H-2/H-4, and H-3/H-4 confirmed that H-2, H-3, and H-4 were in the same phase (Figure 3). Hence, the absolute configuration of 4 was determined as 2R,3S,4S-form. Based on the 13C-NMR-based empirical rules, the chemical shifts of C-3 and C-4 in 4 were similar to those of 2R,3S,4S-form compounds in the literature [11]. According to these two pieces of evidence, the absolute configuration of C-2, C-3, and C-4 in 4 was established to be 2R,3S,4S-form. The absolute configuration of C-12 was determined by Mosher’s method [12]. Based on the Δδ values of the (S)-MTPA and (R)-MTPA esters in chloroform-d1, the absolute configuration of C-12 was established as S-form (Figure 5). Accordingly, the absolute configuration of 4 was defined as 2R,3S,4S,12S. The structure of 4 was confirmed and named machinolide D.

Table 2.
1H and 13C-NMR data of machinolides D–F (4–6).
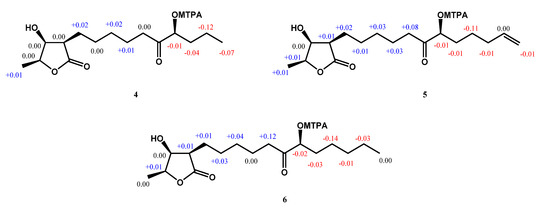
Figure 5.
Results with the modified Mosher’s method (ΔδS–R) of machinolides D–F (4–6).
Compound 5 was isolated as a colorless oil. The ESIMS (m/z 313 [M + H]+) and HRESIMS (m/z 335.18295 [M + Na]+ (calcd. for 335.18290)) data were used to establish the molecular formula of compound 5 as C17H28O5. The 1H-NMR spectrum of 5 was similar to that of 4, except for the presence of a terminal double bond at δH 5.78 (1H, ddt, J = 17.2, 10.4, 6.8 Hz, H-16), 4.98 (1H, m, H-17b), and 5.03 (1H, m, H-17a) in 4 (Table 2). The HMBC correlation between H-15/C-16, C-17, H-16/C-15, and the COSY correlation between H-16/H-17 supports the presence of a terminal double bond (Figure 2). The CD spectrum (a negative cotton effect at 217.5 nm) and NOESY correlation (Figure 3) of 5 were also similar to 4. Moreover, in accordance with the 13C-NMR-based empirical rules [11], the chemical shifts of C-3 and C-4 in 5 were similar to those of 2R,3S,4S-form compounds in the previous data [11], showing that the absolute configuration of 6 was 2R,3S,4S-form. The absolute configuration of C-12 in 5 was established as S-form by Mosher’s method (Figure 5). On the basis of the above results, the structure and absolute configuration of 5 were determined and named machinolide E.
Compound 6 was purified as a colorless oil. Its molecular formula of C17H30O5, two protons more than 5, was determined by EIMS (m/z 315 [M + H]+) and HRESIMS m/z 337.19849 [M + Na]+ (calcd. for 337.19855). The difference between 6 and 5 is that the terminal double bond in 5 is replaced by the ethyl group in 6. The HMBC correlations between H-17/C-15, C-16, and the COSY correlation between H-16/H-17 (Figure 2) also supported the presence of the ethyl group. The absolute configuration of 6 was elucidated as 2R,3S,4S,12S-form by the CD spectrum, NOESY correlation, and Mosher’s method. As determined by the above observations, the structure of 6 was elucidated as a new compound and named machinolide F.
By comparison of the experiments and reported spectroscopic data ([α]D, UV, IR, NMR, and MS), known compounds were identified as one apocarotenoid: blumenol A (7) [13], one benzenoid: amisbenzoic acid (8) [14], one chlorophyll: pheophytin a (9) [15], one coumarin: isofraxidin (10) [16], six lignans: (+)-eudesmin (11) [17], (+)-methylpiperitol (12) [18], (+)-pinoresinol (13) [19], (+)-syringaresinol (14) [20], (2S,5S)-diveratryl-(3R,4S)-dimethyltetrahydrofuran (15) [21], and (+)-galbelgin (16) [22], three sesquiterpenoids: β-eudesmol (17) [23], caryophyllene oxide (18), and clovane-2α,9β-diol (19) [24], and one steroid: β-sitosterol (20) [25].
In this study, eight isolates present in sufficient amounts (1, 2, 3, 6, 11–13, and 16) were evaluated for an inhibitory effect on fMLP/CB-induced superoxide anion (O2•−) generation and elastase release (Table 3). (+)-Eudesmin (11), (+)-methylpiperitol (12), (+)-pinoresinol (13), and (+)-galbelgin (16) displayed inhibitory activity on superoxide anions in fMLP/CB-stimulated human neutrophils with IC50 values of 8.71 ± 0.74 μM, 2.23 ± 0.92 μM, 6.81 ± 1.07 μM, and 7.15 ± 2.26 μM, respectively. LY294002 (Sigma-Aldrich), a potent phosphatidylinositol 3-kinase (PI3K) inhibitor, was used as a positive control to inhibit O2•− generation and elastase release, with IC50 values of 2.17 ± 0.53, and 6.38 ± 1.72 μM, respectively.

Table 3.
Effect of compounds on superoxide anion generation and elastase release in fMLP/CB-stimulated human neutrophils.
3. Discussion
Inflammation is triggered by infection or tissue injury. In our series of anti-inflammatory screenings of lauraceous plants, the leaves of M. japonica var. kusanoi stand out as a research candidate. Focusing on the anti-inflammatory activity results in this paper, the lignans, (+)-eudesmin (11), (+)-methylpiperitol (12), (+)-pinoresinol (13), and (+)-galbelgin (16) exhibited inhibitory activities on superoxide anion generation. (+)-Methylpiperitol (12) showed better anti-inflammatory activity than (+)-eudesmin (11), suggesting the methylenedioxy group may enhance the anti-inflammatory activity. (+)-Methylpiperitol (12) exhibited similar anti-inflammatory activity as (+)-pinoresinol (13), indicating the replacement of the methoxy group may not influence anti-inflammatory activity. The results suggested that the furofuran-type lignan containing a methylenedioxy group showed the best anti-inflammatory activity in this study. More importantly, this is the first report on the anti-inflammatory activity of M. japonica var. kusanoi.
Butanolides (γ-butyrolactones) are four-carbon heterocyclic lactone ring structures reported from some specific families (Myristicaceae [26], Meliaceae [10], Actinomycetes [27,28,29,30,31]), especially in Lauraceae plants (Machilus sp. [32,33,34], Lindera sp. [35,36], Litsea sp. [37], Cinnamomum sp. [38,39,40], Persea sp. [41]). The characteristic butanolides in Lauraceae plants contain an alkyl side chain group at C-2, a hydroxy group at C-3, and one methyl group at C-4, with or without a double bond between C-2/C-3 and C-2/C-6. In this report, six new compounds, machinolides A–F (1–6), were butanolide compounds without a double bond between C-2/C-3 or C-2/C-6. This type of butanolide has not been isolated from Machilus before, which might improve our understanding of secondary metabolites from Machilus species. The chemical results can contribute to the chemotaxonomy of Machilus species.
Although the potency of the lignans exhibiting anti-inflammatory activity in this study was similar to bioactive lignans described in the literature [42], it is worth noting that most of the lignans with anti-inflammatory activity in this study have not been reported previously. Besides, there are no anti-inflammatory medicines act via inhibiting superoxide anion and neutrophil elastase. The research shows some lead compounds and will help develop novel anti-inflammatory drugs.
4. Materials and Methods
4.1. General Experiment Procedures
Optical rotations were measured on a Jasco P-2000 polarimeter (Jasco, Kyoto, Japan), and IR spectra (ATR) were acquired with a Jasco FT/IR-4600 spectrometer. We recorded 1D (1H, 13C, DEPT) and 2D (COSY, NOESY, HSQC, HMBC) NMR spectra on a Varian Germini-2000 spectrometer (Varian, Inc. Vacuum Technologies, Lexington, MA, USA) operated at 200 (1H) and 50 MHz (13C), a Varian Unityplus-400 spectrometer (Varian, Inc. Vacuum Technologies, Lexington, MA, USA) operated at 400 (1H) and 100 MHz (13C), a Varian Mercuryplus-400 spectrometer (Varian, Inc. Vacuum Technologies, Lexington, MA, USA) operated at 400 (1H) and 100 MHz (13C), and a Varian VNMRS-600 spectrometer (Varian, Inc. Vacuum Technologies, Lexington, MA, USA) operated at 600 (1H) and 150 MHz (13C). Low-resolution mass spectra were obtained with POLARIS Q Thermo Finnigan (Thermo Fisher Scientific, Chicago, IL, USA), Waters ZQ 4000 (Waters, Milford, MA, USA), and VG Quattro GC/MS/MS/DS (Waters, Milford, MA, USA) mass spectrometers. EIMS were taken on a JEOL JMS-700 mass spectrometer (JEOL, Tokyo, Japan). HRESIMS were recorded on a Bruker APEX II mass spectrometer (Bruker, Karlsruhe, Germany) and VARIAN 901-MS (Varian, CA, USA). Silica gel (70–230 and 230–400 mesh; Silicycle, QC, Canada) was used for column chromatography (CC), and silica gel 60 F254 (Merck, Darmstadt, Germany) and RP-18 F254S (Merck, Darmstadt, Germany) were used for thin layer chromatography (TLC) and preparative TLC, respectively, visualized with a Ce2(SO4)3 aqueous solution. Further purification was performed by medium-performance liquid chromatography (MPLC; ceramic pump: VSP-3050; EYELA, Kyoto, Japan).
4.2. Plant Material
The leaves of Machilus japonica var. kusanoi (Hayata) J.C. Liao were collected in March 2018 in Mudan Township, Pingtung County, Taiwan, and identified by I.-S.C. A voucher specimen (Chen 5480) was deposited with the herbarium of the College of Pharmacy, Kaohsiung Medical University, Kaohsiung, Taiwan.
4.3. Extraction and Isolation
Dried leaves (5.8 kg) of M. japonica var. kusanoi were extracted at room temperature with methanol (MeOH) (30 L) three times to yield a MeOH extract (730 g). The MeOH extract was suspended in water and partitioned with ethyl acetate (EtOAc) to give a water layer (265.4 g), EtOAc layer (390 g), and precipitate (72 g). The EtOAc layer (390 g) was taken and 100 g were subjected to column chromatography (silica gel; n-hexane/EtOAc 100/0 to 0/100 EtOAc, then washed with 100% acetone and 100% methanol) to yield six fractions (Fr. 1–6). Fr. 3 (15.1 g) was subjected to open column (silica gel; n-hexane/acetone 6/1 to 2/1, column size: 3 × 70 cm) to yield 13 fractions (Fr.3-1–3-13). Fr. 3-9 was subjected to MPLC (RP-18; water/methanol 1:1; column size: 1.5 × 30 cm) to give seven fractions (Fr. 3-9-1–3-9-7). Fr. 3-9-1 was subjected to MPLC (silica gel; n-hexane/CH2Cl2/EtOAc 2/2/1 to 1/1/1; column size: 1 × 30 cm) to afford 14 fractions (Fr. 3-9-1-1–3-9-1-14) and compound 7 (30.5 mg). Fr. 3-9-1-11 was subjected to MPLC (silica gel; n-hexane/CH2Cl2/methanol 15/20/1; column size: 1 × 30 cm) to produce compound 6 (14.0 mg). Fr. 3-9-2 was subjected to MPLC (silica gel; n-hexane/CH2Cl2/EtOAc 2/2/1; column size: 1 × 30 cm) to obtain 10 fractions (Fr. 3-9-2-1–3-9-2-10). Fr. 3-9-2-9 was subjected to MPLC (silica gel; n-hexane/CH2Cl2/EtOAc 2/1/1; column size: 1 × 30 cm) to afford compound 11 (10.7 mg). Fr. 3-9-3 was subjected to MPLC (silica gel; n-hexane/acetone 2/1; column size: 1 × 30 cm) to furnish compound 19 (1.0 mg). Fr. 3-10 was separated with Sephadex LH-20 (column size: 3 × 70 cm) and eluted with methanol to provide seven fractions (3-10-1–3-10-7). Fr. 3-10-2 was subjected to MPLC (silica gel; H2O/methanol 1/1 to 2/3; column size: 1.5 × 30 cm) to gain 14 fractions (Fr. 3-10-2-1–3-10-2-14). Fr. 3-10-2-4 was subjected to MPLC (silica gel; CH2Cl2/EtOAc 3/1; column size: 1 × 30 cm) to obtain compound 4 (5.3 mg). Fr. 3-10-2-7 was subjected to MPLC (silica gel; CH2Cl2/EtOAc 3/1; column size: 1 × 30 cm) to produce compound 5 (0.9 mg). Fr. 3-10-2-13 was subjected to MPLC (silica gel; CH2Cl2/EtOAc 4/1; column size: 1 × 30 cm) to yield compound 2 (2.3 mg). Fr. 3-10-2-15 was subjected to MPLC (silica gel; CH2Cl2/acetone 15/1; column size: 1 × 30 cm) to afford five fractions (Fr. 3-10-2-15-1–3-10-2-15-5). Fr. 3-10-2-15-3 was subjected to MPLC (silica gel; CH2Cl2/EtOAc 3/1; column size: 1 × 30 cm) to give compounds 3 (2.8 mg) and 1 (5.9 mg). Fr. 3-10-4 was subjected to MPLC (silica gel; H2O/methanol 2/3; column size: 1 × 30 cm) to produce 11 fractions (Fr. 3-10-4-1–3-10-4-11). Fr. 3-10-4-2 was subjected to MPLC (silica gel; n-hexane/CH2Cl2/EtOAc 2/2/1; column size: 1 × 30 cm) to furnish compound 8 (0.5 mg). Fr. 3-10-5 was subjected to MPLC (RP-18; water/methanol 2/1 to 1/1; column size: 1 × 30 cm) to give compound 10 (0.3 mg). Fr. 3-7 was subjected to MPLC (silica gel; n-hexane/EtOAc 3/1 to 3/2; column size: 1.5 × 30 cm) to give five fractions (Fr. 3-7-1–3-7-5). Fr. 3-7-3 was subjected to MPLC (RP-18; water/methanol 1/1 to 1/3; column size: 1.5 × 30 cm) to provide nine fractions (Fr. 3-7-3-1–3-7-3-9). Fr. 3-7-3-5 was subjected to MPLC (silica gel; n-hexane/CH2Cl2/EtOAc 4/2/1; column size: 1 × 30 cm) to afford 10 fractions (Fr. 3-7-3-5-1–3-7-3-5-10). Fr. 3-7-3-5-4 was subjected to MPLC (silica gel; n-hexane/CH2Cl2/EtOAc 1/3/0.3; column size: 1 × 30 cm) to give compound 12 (4.6 mg). Fr. 3-7-3-7 was subjected to MPLC (silica gel; n-hexane/CH2Cl2/EtOAc 4/2/1; column size: 1 × 30 cm) to produce nine fractions (Fr. 3-7-3-7-1–3-7-3-7-9). Fr. 3-7-3-7-2 was subjected to MPLC (silica gel; n-hexane/CH2Cl2/EtOAc 6/2/1; column size: 1 × 30 cm) to obtain compounds 15 (0.2 mg) and 16 (2.5 mg). Fr. 3-7-3-7-6 was subjected to MPLC (RP-18; water/methanol 1/3; column size: 1 × 30 cm) to give compound 17 (2.9 mg). Fr. 3-11 was separated with Sephadex LH-20 (column size: 3 × 70 cm) and eluted with methanol to provide 11 fractions (3-11-1–3-11-11). Fr. 3-11-7 was subjected to MPLC (RP-18; water/acetone 3/2; column size: 1 × 30 cm) to give compound 13 (0.5 mg). Fr. 2 was subjected to column chromatography (silica gel; n-hexane/CH2Cl2/acetone 17/1/1 to 10/1/1) to yield ten fractions (Fr. 2-1–2-10). Fr. 2-4 was subjected to MPLC (silica gel; n-hexane/acetone 40/1 to 20/1; column size: 2 × 30 cm) to yield nine fractions (Fr. 2-4-1–2-4-9). Fr. 2-4-3 was subjected to MPLC (RP-18; water/acetone 1/5; column size: 1.5 × 30 cm) to produce nine fractions (Fr. 2-4-3-1–2-4-3-9). Fr. 2-4-3-3 was subjected to MPLC (silica gel; n-hexane/acetone 40/0.5; column size: 1 × 30 cm) to afford eight fractions (Fr. 2-4-3-3-1–2-4-3-3-8). Fr. 2-4-3-3-3 was subjected to HPLC to obtain two fractions (Fr. 2-4-3-3-3-1–2-4-3-3-3-2). Fr. 2-4-3-3-3-2 was further separated with prep. RP-18 TLC (water/acetonitrile = 1/10) to give compound 18 (1.9 mg). Fr. 2-7 was subjected to column chromatography (silica gel; n-hexane/CH2Cl2/acetone 20/4/1 to 12/4/1) to produce eight fractions (Fr. 2-7-1–2-7-8). Fr. 2-7-2 was subjected to MPLC (silica gel; n-hexane/CH2Cl2/acetone 12/4/1; column size: 2 × 30 cm) to give compound 20 (1.6 g). Fr. 2-8 was subjected to column chromatography (silica gel; n-hexane/CH2Cl2/acetone 16/16/1 to 8/16/1) to afford 14 fractions (Fr. 2-8-1–2-8-14). Fr. 2-8-7 was subjected to MPLC (silica gel; n-hexane/acetone 6/1; column size: 1.5 × 30 cm) to produce seven fractions (Fr. 2-8-7-1–2-8-7-7). Fr. 2-8-7-5 was subjected to MPLC (silica gel; n-hexane/CH2Cl2/acetone 16/16/1; column size: 1 × 30 cm) to produce compound 9 (15.2 mg). Fr. 4 was subjected to column chromatography (silica gel; n-hexane/acetone 5/1 to 3/1) to yield six fractions (Fr. 4-1–4-6). Fr. 4-5 was separated with Sephadex LH-20 (column size: 3 × 70 cm) and eluted with methanol to provide six fractions (4-5-1–4-5-6). Fr. 4-5-4 was subjected to MPLC (RP-18; water/methanol 1/1; column size: 1.5 × 30 cm) to produce six fractions (Fr. 4-5-4-1–4-5-4-6). Fr. 4-5-4-1 was subjected to MPLC (RP-18; water/methanol 1/1; column size: 1 × 30 cm) to afford four fractions (Fr. 4-5-4-1-1–4-5-4-1-4). Fr. 4-5-4-1-1 was subjected to MPLC (silica gel; n-hexane/CH2Cl2/acetone 2/2/1; column size: 1 × 30 cm) to give compound 14 (1.6 mg). (Supplementary Materials, Figure S58)
4.3.1. Machinolide A (1)
Colorless needles; −29.6 (c 0.30, MeOH); IR νmax (ATR): 3433 (OH), 1736 (γ-lactone) cm−1; 1H-NMR and 13C-NMR (Table 1); ESIMS m/z 273 [M + H]+; EIMS m/z (rel. int.): 254 ([M − H2O]+, 3), 215 (56), 186 (37), 129 (99), 57 (100); HRESIMS m/z 295.18923 [M + Na]+ (calcd. for C15H28NaO4, 295.18853).
4.3.2. Machinolide B (2)
Colorless needles; −42.1 (c 0.092, MeOH); IR νmax (ATR): 3436 (OH), 1737 (γ-lactone) cm−1; 1H-NMR and 13C-NMR (Table 1); ESIMS m/z 299 [M + H]+; HRESIMS m/z 321.20370 [M + Na]+ (calcd. for C17H30NaO4, 321.20363).
4.3.3. Machinolide C (3)
Colorless needles; −42.0 (c 0.14, MeOH); IR νmax (ATR): 3321 (OH), 1743 (γ-lactone) nm; 1H-NMR and 13C-NMR (Table 2); ESIMS m/z 273 [M + H]+; EIMS m/z (rel. int.): 254 ([M − H2O]+, 3), 229 (39), 200 (24), 129 (80), 57 (100); HRESIMS m/z 273.20656 [M + H]+ (calcd. for C15H29O4, 273.20658).
4.3.4. Machinolide D (4)
Colorless oil; −42.5 (c 0.25, MeOH); IR νmax (ATR): 3437 (OH), 1752 (γ-lactone), 1708 (C=O) cm−1; CD λext (MeOH) (Δε): 280 (+15.43), 219.5 (−54.44) nm; 1H-NMR and 13C-NMR (Table 3); ESIMS m/z 287 [M + H]+; HRESIMS m/z 309.16726 [M + Na]+ (calcd. for C15H26NaO5, 309.16725).
4.3.5. Machinolide E (5)
Colorless oil; −35.4 (c 0.145, MeOH); IR νmax (ATR): 3445 (OH), 1748 (γ-lactone), 1713 (C=O) cm−1; CD λext (MeOH) (Δε): 280.5 (+17.23), 217.5 (−52.40) nm; 1H-NMR and 13C-NMR (Table 3); ESIMS m/z 313 [M + H]+; HRESIMS m/z 335.18295 [M + Na]+ (calcd. for C17H28NaO5, 335.18290).
4.3.6. Machinolide F (6)
Colorless oil; −39.4 (c 0.85, MeOH); IR νmax (ATR): 3440 (OH), 1751 (γ-lactone), 1705 (C=O) cm−1; CD λext (MeOH) (Δε): 280.5 (+17.34), 217.5 (−52.74) nm; 1H-NMR and 13C-NMR (Table 3); ESIMS m/z 315 [M + H]+; HRESIMS m/z 337.19849 [M + Na]+ (calcd. for C17H30NaO5, 337.19855).
4.4. X-Ray Crystallographic Data for Machinolide A (1), Machinolide B (2), and Machinolide C (3)
The absolute configurations of 1, 2, and 3 were determined from data collected on a Bruker D8 VENTURE single-crystal XRD equipped with Oxford Cryostream 800+. Crystallographic data for 1: C15H30O5, M = 290.39, size 0.220 × 0.097 × 0.057 mm3, orthorhombic, space group P212121, a = 4.72807(10) Å, b = 12.9141(3) Å, c = 27.8178(6) Å, α = β = γ = 90°, V = 1698.53(6) Å3, T = 200(2) K, Z = 4, dcalcd = 1.136 Mg/m3, λ(Cu Kα) = 1.54178 Å, F(000) = 640, reflections collected/independent reflections 9080/3467 [R(int) = 0.0307], final R indices R1 = 0.0331, wR2 = 0.0928, GOF on F2 = 1.046, absolute structure parameter = −0.02(7).
Crystallographic data for 2: C17H32O5, M = 316.42, size 0.397 × 0.052 × 0.036 mm3, orthorhombic, space group P212121, a = 4.76120(10) Å, b = 12.8614(4) Å, c = 30.6325(9) Å, α = β = γ = 90°, V = 1875.80(9) Å3, Z = 4, dcalcd = 1.120 Mg/m3, λ(Cu Kα) = 1.54178 Å, F(000) = 696, reflections collected/independent reflections 10397/3812 [R(int) = 0.0366], final R indices R1 = 0.0568, wR2 = 0.1537, GOF on F2 = 1.036, absolute structure parameter = −0.01 (14).
Crystallographic data for 3: C15H28O4, M = 272.37, size 0.392 × 0.089 × 0.014 mm3, orthorhombic, space group P212121, a = 4.75710(10) Å, b = 9.7931(2) Å, c = 35.3857(8) Å, α = β = γ = 90°, V = 1648.50(6) Å3, T = 200(2) K, Z = 4, dcalcd = 1.097 Mg/m3, λ(Cu Kα) = 1.54178 Å, F(000) = 600, reflections collected/independent reflections 15562/3377 [R(int) = 0.0525], final R indices R1 = 0.0431, wR2 = 0.1152, GOF on F2 = 1.037, absolute structure parameter = 0.02(10).
4.5. Preparation of (S)-MTPA and (R)-MTPA Esters of 4a, 4b, 5a, 5b, 6a, and 6b from 4, 5, and 6
Compound 4 (1.0 mg, 3.5 µmol) and pyridine-d5 (10.9 µL, 135.4 µmol) was transferred to a vial. The contents of the vial were dissolved in chloroform-d1 (1090 µL, [4] = 3.5 mM). R-(−)-MPTA-Cl (10.9 µL, 58.3 µmol) was added to the vial, the vial was capped and the contents were stirred at room temperature (2–4 h). The (S)-MTPA ester (4a) was purified by prep. TLC plate (n-hexane/EtOAc = 1/1), and its 1H-NMR spectra were obtained. The (R)-MTPA ester (4b) was prepared with (S)-MTPA chloride in the same manner. The same method was used to prepare the (S)- and (R)-MTPA esters of 5a, 5b, 6a, and 6b (Supplementary Materials, Figures S59–S64).
4.6. Superoxide Anion and Elastase Release Assays
The ability of the test compounds to modulate superoxide anion generation and elastase release by neutrophils was evaluated according to the studies published by co-author Professor Tsong-Long Hwang [2,43]. The superoxide generation assay was based on the reduction of ferricytochrome c by superoxide dismutase (SOD). Elastase substrate (methoxysuccinyl-Ala-Ala-Pro-Val-p-nitroanilide, 100 μM; Merck) was used to detect elastase release. Elastase level was detected at OD405 nm using a spectrophotometer. PI3K inhibitor LY29002 served as a positive control for the neutrophil assays. All assays were repeated at least three times. Results are presented as mean ± standard error of the mean (SEM). The Student’s t-test was used to compare the test compound with a DMSO (0.1%) control. A probability of less than 0.05 was considered significant.
5. Conclusions
Six new butanolides, machinolides A–F (1–6), together with 14 known compounds, were obtained from the leaves of M. japonica var. kusanoi. The absolute configurations of these new compounds were assigned by their CD spectrum, single-crystal X-ray diffraction analyses, and Mosher’s method. Hence, absolute configurations of all new compounds were determined as 2R,3S,4S-form in a furan ring, and the chiral center in the side chain group was R-form in 1 and 3, and S-form in 2, 4, 5, and 6. Besides, butanolides and lignans were major skeletons in this study. Bioactivity results indicated that lignans could reduce superoxide anion generation in fMLP/CB-stimulated human neutrophils, and the anti-inflammatory activities of those compounds were as potent as compounds in the literature [42]. Furthermore, the structure-activity relationship (SAR) discussion of anti-inflammatory activity compounds indicated that furofuran lignan with methylenedioxy was the most active structure. To our knowledge, this is the first report on anti-inflammatory activity from the leaves of M. japonica var. kusanoi and the results are helpful to patients with inflammation-related disease.
Supplementary Materials
Figure S1: Structures of known compounds 7–20, Figures S2–S57: The phytochemical spectra of compounds 1–6. Figure S58: Extraction and isolation of the leaves from M. japonica var. kusanoi, Figures S59–S64: The phytochemical spectra of compounds 4a, 4b, 5a, 5b, 6a, and 6b. Table S1: Inhibitory effects of crude extracts from the leaves of M. japonica var. kusanoi on superoxide anion generation and elastase release in fMLP/CB-induced human neutrophils.
Author Contributions
Conceptualization, H.-S.C.; Methodology, H.-S.C., C.-H.L., and T.-L.H.; Formal Analysis, H.-S.C. and T.-L.H.; Investigation, S.-L.L.; Resources, H.-S.C.; Data Curation, H.-S.C., S.-S.Y., and T.-L.H.; Writing—Original Draft Preparation, S.-L.L. and H.-C.W.; Writing—Review and Editing, H.-S.C. and T.-L.H.; Visualization, H.-S.C.; Supervision, H.-S.C.; Project Administration, H.-S.C.; Funding Acquisition, H.-S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Ministry of Science and Technology, R.O.C. (MOST 109-2628-B-037-015), and a Kaohsiung Medical University grant (KMU-TC108A03-8 and KMU-TC108A03-9).
Acknowledgments
We thank the Center for Research Resources and Development of Kaohsiung Medical University for providing a nuclear magnetic resonance (NMR) spectrometer, and also senior technician Chyi-Jia Wang for measuring the 2D NMR data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chiang, C.-C.; Cheng, W.-J.; Korinek, M.; Lin, C.-Y.; Hwang, T.-L. Neutrophils in Psoriasis. Front. Immunol. 2019, 10, 2376. [Google Scholar] [CrossRef]
- Hwang, T.-L.; Su, Y.-C.; Chang, H.-L.; Leu, Y.-L.; Chung, P.-J.; Kuo, L.-M.; Chang, Y.-J. Suppression of superoxide anion and elastase release by C18 unsaturated fatty acids in human neutrophils. J. Lipid Res. 2009, 50, 1395–1408. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, B.; Horwitz, M.; Jenne, D.E.; Gauthier, F. Neutrophil Elastase, Proteinase 3, and Cathepsin G as Therapeutic Targets in Human Diseases. Pharmacol. Rev. 2010, 62, 726–759. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-S.; Chen, I.-S. Chemical constituents and bioactivity of Formosan lauraceous plants. J. Food Drug Anal. 2016, 24, 247–263. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.C. Lauraceae in Flora of Taiwanm, 2nd ed.; Editorial Committee of the Flora of Taiwan: Taipei, Taiwan, 1996; Volume 2, pp. 1433–1499. [Google Scholar]
- Masao, T.; Yang, T.H.; Teh, L.S. Studies on the alkaloids of Formosan Lauraceous plants. I. Alkaloids of Machilus kusanoi Hayata. (1). The isolation of L-(-)-N-norarmepavine. Yakugaku Zasshi 1963, 83, 15–18. [Google Scholar] [CrossRef][Green Version]
- Teh, L.S. Studies on the alkaloids of Formosan Louraceous plants. II. Alkaloids of Machilus Kusanoi Hayata. (2). The isolation of dl-coclaurine. Yakugaku Zasshi 1963, 83, 19–21. [Google Scholar] [CrossRef]
- Lee, S.-S.; Lin, Y.-S.; Chen, C.-K. Three Adducts of Butenolide and Apigenin Glycoside from the Leaves of Machilus Japonica. J. Nat. Prod. 2009, 72, 1249–1252. [Google Scholar] [CrossRef]
- Ho, C.-L.; Hsu, K.-P.; Tseng, Y.-H.; Wang, E.I.-C.; Liao, P.-C.; Chou, J.-C.; Lin, C.-N.; Su, Y.-C. Composition and antimicrobial activities of the leaf essential oil of Machilus kusanoi from Taiwan. Nat. Prod. Commun. 2011, 6. [Google Scholar] [CrossRef]
- Du, Y.; Abedi, A.K.; Valenciano, A.L.; Fernaández-Murga, M.L.; Cassera, M.B.; Rasamison, V.E.; Applequist, W.L.; Miller, J.S.; Kingston, D.G.I. Isolation of the New Antiplasmodial Butanolide, Malleastrumolide A, from Malleastrum sp. (Meliaceae) from Madagascar. Chem. Biodivers. 2017, 14, e1700331. [Google Scholar] [CrossRef]
- Lorenzo, M.; Brito, I.; Cueto, M.; D’Croz, L.; Darias, J. 13C NMR-Based Empirical Rules to Determine the Configuration of Fatty Acid Butanolides. Novel γ-Dilactones from Pterogorgia spp. Org. Lett. 2006, 8, 5001–5004. [Google Scholar] [CrossRef]
- Hoye, T.R.; Jeffrey, C.S.; Shao, F. Mosher ester analysis for the determination of absolute configuration of stereogenic (chiral) carbinol carbons. Nat. Protoc. 2007, 2, 2451–2458. [Google Scholar] [CrossRef]
- Pardede, A.; Adfa, M.; Kusnanda, A.J.; Ninomiya, M.; Koketsu, M. Flavonoid rutinosides from Cinnamomum parthenoxylon leaves and their hepatoprotective and antioxidant activity. Med. Chem. Res. 2017, 26, 2074–2079. [Google Scholar] [CrossRef]
- Chang, C.-W.; Chang, H.-S.; Cheng, M.-J.; Peng, C.-F.; Chen, I.-S. Identification of Five New Minor Constituents from the Whole Plant of Amischotolype hispida. Helvetica Chim. Acta 2015, 98, 347–358. [Google Scholar] [CrossRef]
- Liu, C.-M.; Kao, C.-L.; Wu, H.-M.; Li, W.-J.; Huang, C.-T.; Li, H.-T.; Chen, C.-Y. Antioxidant and Anticancer Aporphine Alkaloids from the Leaves of Nelumbo nucifera Gaertn. cv. Rosa-plena. Molecules 2014, 19, 17829–17838. [Google Scholar] [CrossRef]
- Wu, M.-D.; Cheng, M.-J.; Lin, R.-J.; Chan, H.-Y.; Hsieh, S.-Y.; Chang, H.-S.; Lin, C.-L.; Chen, J.-J. Chemical Constituents of the Fungus Biscogniauxia cylindrospora. Chem. Nat. Compd. 2019, 55, 924–926. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Geng, Z.; Guo, S.; Cao, J.; Zhang, Z.; Pang, X.; Chen, Z.; Du, S.S.; Deng, Z. Antifeedant Activities of Lignans from Stem Bark of Zanthoxylum armatum DC. against Tribolium castaneum. Molecules 2018, 23, 617. [Google Scholar] [CrossRef]
- Holzbach, J.C.; Lopes, L.M.X. Aristolactams and Alkamides of Aristolochia gigantea. Molecules 2010, 15, 9462–9472. [Google Scholar] [CrossRef]
- Lee, S.Y.; Woo, K.W.; Kim, C.S.; Lee, D.U.; Lee, K.R. New Lignans from the Aerial Parts of Rudbeckia laciniata. Helvetica Chim. Acta 2013, 96, 320–325. [Google Scholar] [CrossRef]
- Sribuhom, T.; Sriphana, U.; Thongsri, Y.; Yenjai, C. Chemical constituents from the stems of Alyxia schlechteri. Phytochem. Lett. 2015, 11, 80–84. [Google Scholar] [CrossRef]
- Park, C.H.; Kim, K.H.; Lee, I.K.; Lee, S.Y.; Choi, S.U.; Lee, J.H.; Lee, K.R. Phenolic constituents of Acorus gramineus. Arch. Pharmacal Res. 2011, 34, 1289–1296. [Google Scholar] [CrossRef]
- Rye, C.E.; Barker, D. Asymmetric Synthesis of (+)-Galbelgin, (−)-Kadangustin J, (−)-Cyclogalgravin and (−)-Pycnanthulignenes A and B, Three Structurally Distinct Lignan Classes, Using a Common Chiral Precursor. J. Org. Chem. 2011, 76, 6636–6648. [Google Scholar] [CrossRef] [PubMed]
- You, C.-X.; Yang, K.; Wang, C.-F.; Zhang, W.; Wang, Y.; Han, J.; Fan, L.; Du, S.S.; Geng, Z.; Deng, Z. Cytotoxic Compounds Isolated from Murraya tetramera Huang. Molecules 2014, 19, 13225–13234. [Google Scholar] [CrossRef] [PubMed]
- Collado, I.G.; Hanson, J.R.; Macías-Sánchez, A.J.; Mobbs, D. The Biotransformation of Some Clovanes by Botrytis cinerea. J. Nat. Prod. 1998, 61, 1348–1351. [Google Scholar] [CrossRef] [PubMed]
- Ashour, A.; Amer, M.; Marzouk, A.M.; Shimizu, K.; Kondo, R.; El-Sharkawy, S. Corncobs as a Potential Source of Functional Chemicals. Molecules 2013, 18, 13823–13830. [Google Scholar] [CrossRef]
- Lopes, N.P.; Silva, D.H.S.; Kato, M.J.; Yoshida, M. Butanolides as a common feature of Iryanthera lancifolia and Virola surinamensis. Phytochemistry 1998, 49, 1405–1410. [Google Scholar] [CrossRef]
- Franco, C.M.M.; Borde, U.P.; Vijayakumar, E.K.S.; Chatterjee, S.; Blumbach, J.; Ganguli, B.N. Butalactin, a new butanolide antibiotic. Taxonomy, fermentation, isolation and biological activity. J. Antibiot. 1991, 44, 225–231. [Google Scholar] [CrossRef]
- Nihira, T.; Shimizu, Y.; Kim, H.S.; Yamada, Y. Structure-activity relationships of virginiae butanolide C, an inducer of virginiamycin production in Streptomyces virginiae. J. Antibiot. 1988, 41, 1828–1837. [Google Scholar] [CrossRef]
- Kim, H.S.; Tada, H.; Nihira, T.; Yamada, Y. Purification and characterization of virginiae butanolide C-binding protein, a possible pleiotropic signal-transducer in Streptomyces virginiae. J. Antibiot. 1990, 43, 692–706. [Google Scholar] [CrossRef]
- Hoshino, S.; Wakimoto, T.; Onaka, H.; Abe, I. Chojalactones A–C, Cytotoxic Butanolides Isolated from Streptomyces sp. Cultivated with Mycolic Acid Containing Bacterium. Org. Lett. 2015, 17, 1501–1504. [Google Scholar] [CrossRef]
- Li, F.; Chen, D.; Lu, S.; Yang, G.; Zhang, X.; Chen, Z.; Fan, S.; Wu, S.-H.; He, J. Anti-Influenza A Viral Butenolide from Streptomyces sp. Smu03 Inhabiting the Intestine of Elephas maximus. Viruses 2018, 10, 356. [Google Scholar] [CrossRef]
- Cheng, M.-J.; Tsai, I.-L.; Lee, S.-J.; Jayaprakasam, B.; Chen, I.-S. Steryl epoxide, secobutanolide and butanolides from the stem wood of Machilus zuihoensis. Phytochemical 2005, 66, 1180–1185. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-T.; Lin, S.; Gan, M.; Liu, B.; Zi, J.; Song, W.-X.; Zhang, Y.-L.; Fan, X.-N.; Liu, Y.; Tan, W.; et al. Butanolide derivatives from the bark of Machilus yaoshansis. J. Asian Nat. Prod. Res. 2012, 14, 713–720. [Google Scholar] [CrossRef]
- Kim, W.; Lyu, H.-N.; Kwon, H.-S.; Kim, Y.S.; Lee, K.-H.; Kim, -Y.; Chakraborty, G.; Choi, K.Y.; Yoon, H.S.; Kim, K.-T. Obtusilactone B from Machilus Thunbergii Targets Barrier-to-Autointegration Factor to Treat Cancer. Mol. Pharmacol. 2012, 83, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-P.; Huang, G.-J.; Huang, H.-C.; Chen, Y.-C.; Chang, C.-I.; Wang, S.-Y.; Chen, I.-S.; Tseng, Y.-H.; Chien, S.-C.; Kuo, Y.-H. A New Butanolide Compound from the Aerial Part of Lindera akoensis with Anti-inflammatory Activity. Molecules 2012, 17, 6585–6592. [Google Scholar] [CrossRef] [PubMed]
- Tsai, I.-L.; Hung, C.-H.; Duh, C.-Y.; Chen, I.-S. Cytotoxic Butanolides and Secobutanolides from the Stem Wood of Formosan Lindera communis. Planta Medica 2002, 68, 142–145. [Google Scholar] [CrossRef]
- Cheng, M.-J.; Wang, T.-A.; Lee, S.-J.; Chen, I.-S. A new butanolide and a new secobutanolide from Litsea lii var. nunkao-tahangensis. Nat. Prod. Res. 2010, 24, 647–656. [Google Scholar] [CrossRef]
- Shen, K.-H.; Lin, E.-S.; Kuo, P.-L.; Chen, C.-Y.; Hsu, Y.-L. Isolinderanolide B, a Butanolide Extracted From the Stems of Cinnamomum subavenium, Inhibits Proliferation of T24 Human Bladder Cancer Cells by Blocking Cell Cycle Progression and Inducing Apoptosis. Integr. Cancer Ther. 2011, 10, 350–358. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Wang, H.-M.; Wu, T.-W.; Chen, Y.-J.; Shieh, J.-J.; Lin, J.-H.; Ho, T.-F.; Luo, R.-J.; Chen, C.-Y.; Chang, C.-C. Subamolide B Isolated from Medicinal Plant Cinnamomum subavenium Induces Cytotoxicity in Human Cutaneous Squamous Cell Carcinoma Cells through Mitochondrial and CHOP-Dependent Cell Death Pathways. Evid. Based Complement Alternat. Med. 2013, 2013, 1–13. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Hsu, Y.-L.; Chen, Y.-Y.; Hung, J.-Y.; Huang, M.-S.; Kuo, P.-L. Isokotomolide A, a new butanolide extracted from the leaves of Cinnamomum kotoense, arrests cell cycle progression and induces apoptosis through the induction of p53/p21 and the initiation of mitochondrial system in human non-small cell lung cancer A549 cells. Eur. J. Pharmacol. 2007, 574, 94–102. [Google Scholar] [CrossRef]
- Le Dang, Q.; Kwon, H.R.; Choi, Y.H.; Choi, G.J.; Jang, K.S.; Park, M.S.; Lim, C.H.; Ngoc, L.H.; Kim, J.-C. Nematicidal activity against Bursaphelenchus xylophilus of isoobtusilactone A isolated from Persea americana. Nematology 2010, 12, 247–253. [Google Scholar] [CrossRef]
- Kuo, P.; Hung, H.-Y.; Nian, C.-W.; Hwang, T.-L.; Cheng, J.-C.; Kuo, D.-H.; Lee, E.-J.; Tai, S.-H.; Wu, T.-S. Chemical Constituents and Anti-inflammatory Principles from the Fruits of Forsythia suspensa. J. Nat. Prod. 2017, 80, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.-L.; Li, G.-L.; Lan, Y.-H.; Chia, Y.-C.; Hsieh, P.-W.; Wu, Y.-H.; Wu, Y.-C. Potent inhibition of superoxide anion production in activated human neutrophils by isopedicin, a bioactive component of the Chinese medicinal herb Fissistigma oldhamii. Free. Radic. Boil. Med. 2009, 46, 520–528. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of all compounds are available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).