A Quantitative Phytochemical Comparison of Olive Leaf Extracts on the Australian Market
Abstract
1. Introduction
2. Results
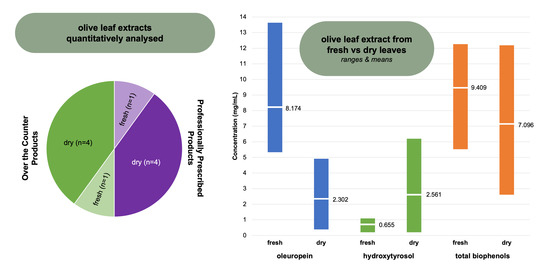
2.1. Oleuropein, Hydroxytyrosol, and Total Biophenols—Comparison between Product Categories
2.2. Oleuropein, Hydroxytyrosol, and Total Biophenols—Comparison between Extracts Prepared from Fresh Leaf or Dry Leaf
2.3. Oleacein and Oleocanthal—Comparison between Product Categories
2.4. Maslinic Acid and Oleanolic Acid—Comparison between Product Categories
2.5. Label Declarations of Oleuropein and Hydroxytyrosol and Quantity Per Daily Maximum Dosage
3. Discussion
4. Materials and Methods
4.1. Total Biophenols and Hydroxytyrosol Content
4.2. Oleuropein
- A1 = area of oleuropein
- mL = sample weight (g)
- V = final volume (mL)
- C = calibration curve gradient
4.3. Maslinic and Oleanolic Acid
- AMA = area of maslinic acid
- AOA = area of oleanolic acid
- CCMA = calibration curve gradient of maslinic acid standard
- CCOA = calibration curve gradient of oleanolic acid standard
- V = final volume (mL)
- W = sample weight (g)
4.4. Density
Author Contributions
Funding
Conflicts of Interest
References
- Hanbury, D. On the febrifuge properties of the olive (Olea europaea, L). Pharm. J. 1854, 13, 353–354. [Google Scholar]
- Barbaro, B.; Toietta, G.; Maggio, R.; Arciello, M.; Tarocchi, M.; Galli, A.; Balsano, C. Effects of the olive-derived polyphenol oleuropein on human health. Int. J. Mol. Sci. 2014, 15, 18508–18524. [Google Scholar] [CrossRef] [PubMed]
- Vogel, P.; Machado, I.K.; Garavaglia, J.; Zani, V.T.; de Souza, D.; Dal Bosco, S.M. Beneficios polifenoles hoja de olivo (Olea europaea L) para la salud humana. Nutr. Hosp. 2015, 31, 1427–1433. [Google Scholar] [CrossRef]
- Ahamad, J.; Toufeeq, I.; Khan, M.A.; Ameen, M.S.M.; Anwer, E.T.; Uthirapathy, S.; Mir, S.R.; Ahmad, J. Oleuropein: A natural antioxidant molecule in the treatment of metabolic syndrome. Phyther. Res. 2019, 33, 3112–3128. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, M.; Kiani, A.K.; Paolacci, S.; Manara, E.; Kurti, D.; Dhuli, K.; Bushati, V.; Miertus, J.; Pangallo, D.; Baglivo, M.; et al. Hydroxytyrosol: A natural compound with promising pharmacological activities. J. Biotechnol. 2020, 309, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Susalit, E.; Agus, N.; Effendi, I.; Tjandrawinata, R.R.; Nofiarny, D.; Perrinjaquet-Moccetti, T.; Verbruggen, M. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: Comparison with Captopril. Phytomedicine 2011, 18, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Lockyer, S.; Rowland, I.; Spencer, J.P.E.; Yaqoob, P.; Stonehouse, W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: A randomised controlled trial. Eur. J. Nutr. 2017, 56, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- de Bock, M.; Derraik, J.G.B.; Brennan, C.M.; Biggs, J.B.; Morgan, P.E.; Hodgkinson, S.C.; Hofman, P.L.; Cutfield, W.S. Olive (Olea europaea L.) leaf polyphenols improve insulin sensitivity in middle-aged overweight men: A randomized, placebo-controlled, crossover trial. PLoS ONE 2013, 8, e57622. [Google Scholar] [CrossRef] [PubMed]
- Somerville, V.; Moore, R.; Braakhuis, A. The Effect of Olive Leaf Extract on Upper Respiratory Illness in High School Athletes: A Randomised Control Trial. Nutrients 2019, 11, 358. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.M. The effect of olive leaf extract in decreasing the expression of two pro-inflammatory cytokines in patients receiving chemotherapy for cancer. A randomized clinical trial. Saudi Dent. J. 2013, 25, 141–147. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency Committee on Herbal Medicinal Products (HMPC). Assessment Report on Olea europaea L., folium; EMA: London, UK, 2017. [Google Scholar]
- Therapeutic Goods Administration, Australian Department of Health. Evidence Guidelines—Guidelines on the Evidence Required to Support Indications for Listed Complementary Medicines Version 3.0. Available online: https://www.tga.gov.au/sites/default/files/evidence-guidelines.pdf (accessed on 13 April 2019).
- Bauer, R. Quality Criteria and Standardization of Phytopharmaceuticals: Can Acceptable Drug Standards be Achieved? Drug Inf. J. 1998, 32, 101–110. [Google Scholar] [CrossRef]
- Wurglics, M.; Westerhoff, K.; Kaunzinger, A.; Wilke, A.; Baumeister, A.; Dressman, J.; Schubert-Zsilavecz, M. Comparison of German St. John’s Wort Products According to Hyperforin and Total Hypericin Content. J. Am. Pharm. Assoc. 2001, 41, 560–566. [Google Scholar] [CrossRef]
- Ghanbari, R.; Anwar, F.; Alkharfy, K.M.; Gilani, A.-H.; Saari, N. Valuable nutrients and functional bioactives in different parts of olive (Olea europaea L.)—A review. Int. J. Mol. Sci. 2012, 13, 3291–3340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wen, M.; Zhou, P.; Tian, M.; Zhou, J.; Zhang, L. Analysis of chemical composition in Chinese olive leaf tea by UHPLC-DAD-Q-TOF-MS/MS and GC-MS and its lipid-lowering effects on the obese mice induced by high-fat diet. Food Res. Int. 2020, 128, 108785. [Google Scholar] [CrossRef] [PubMed]
- Ghomari, O.; Merzouki, M.; Benlemlih, M. Optimization of bioconversion of oleuropein, of olive leaf extract, to hydroxytyrosol by Nakazawaea molendini-olei using HPLC-UV and a method of experimental design. J. Microbiol. Methods 2020, 106010. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
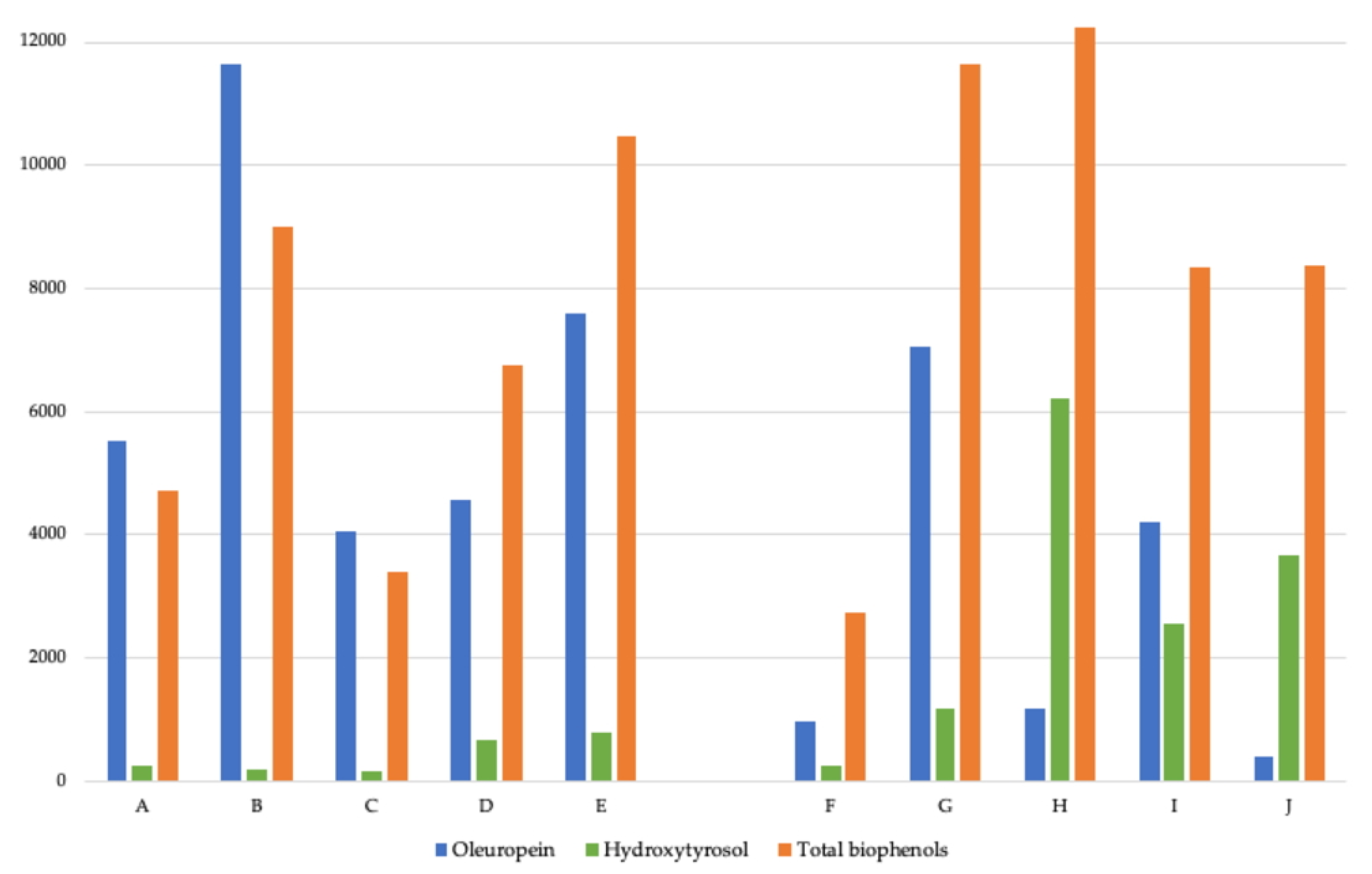

| Sample | A | B | C | D | E | F | G | H | I | J |
|---|---|---|---|---|---|---|---|---|---|---|
| Density (g/mL) | 1.170 | 1.170 | 1.210 | 1.170 | 1.170 | 0.952 | 0.929 | 0.997 | 0.987 | 0.996 |
| Oleuropein (ppm) | 5526.42 | 11653.72 | 4066.34 | 4560.88 | 7594.15 | 951.49 | 7050.89 | 1157.95 | 4203.78 | 383.60 |
| Oleuropein (mg/mL) | 6.466 | 13.635 | 4.920 | 5.336 | 8.885 | 0.906 | 6.550 | 1.154 | 4.149 | 0.382 |
| Hydroxytyrosol (ppm) | 241.20 | 174.30 | 163.10 | 667.40 | 781.90 | 236.90 | 1176.40 | 6215.90 | 2564.30 | 3667.50 |
| Hydroxytyrosol (mg/mL) | 0.282 | 0.204 | 0.197 | 0.781 | 0.915 | 0.226 | 1.093 | 6.197 | 2.531 | 3.653 |
| Oleacein (ppm) | 39.70 | 112.50 | 126.10 | 916.80 | 1773.30 | 103.60 | 254.50 | 305.80 | 245.20 | 168.60 |
| Oleacein (mg/mL) | 0.046 | 0.132 | 0.153 | 1.073 | 2.075 | 0.099 | 0.236 | 0.305 | 0.242 | 0.168 |
| Oleocanthal (ppm) | 0 | 0 | 0 | 41.70 | 59.70 | 0 | 0 | 0 | 0 | 0 |
| Oleocanthal (mg/mL) | 0 | 0 | 0 | 0.049 | 0.070 | 0 | 0 | 0 | 0 | 0 |
| Total biophenols (ppm) | 4719.40 | 8997.50 | 3380.70 | 6762.40 | 10481.40 | 2744.60 | 11647.10 | 12229.00 | 8352.90 | 8374.80 |
| Total biophenols (mg/mL) | 5.522 | 10.527 | 4.091 | 7.912 | 12.263 | 2.613 | 10.820 | 12.192 | 8.244 | 8.341 |
| Maslinic acid (ppm) | 0 | 7.45 | 9.51 | 66.98 | 144.21 | 274.82 | 813.96 | 131.93 | 21.26 | 28.98 |
| Maslinic acid (mg/mL) | 0 | 0.009 | 0.012 | 0.078 | 0.169 | 0.262 | 0.756 | 0.132 | 0.021 | 0.029 |
| Oleanolic acid (ppm) | 0 | 8.88 | 0 | 326.37 | 584.31 | 352.56 | 560.12 | 228.29 | 37.71 | 41.55 |
| Oleanolic acid (mg/mL) | 0 | 0.010 | 0 | 0.382 | 0.684 | 0.336 | 0.520 | 0.228 | 0.037 | 0.041 |
| Sample | Summary of Label Declaration | DER * | Maximum Adult Daily Dosage (mL) | Oleuropein Per Maximum Dosage (mg) | Hydroxytyrosol Per Maximum Dosage (mg) |
|---|---|---|---|---|---|
| A | Each 15 mL contains leaf extract equiv approx 12 g fresh leaf, standardized to contain 66 mg oleuropein and 0.75 mg hydroxytyrosol | 1:1.25 (fresh) | 15 | 96.989 | 4.233 |
| B | Each 10 mL contains extract equiv 8.5 g fresh leaf, standardized to contain 136 mg oleuropein | 1:1.18 (fresh) | 10 | 136.349 | 2.039 |
| C | Each 5 mL contains extract equiv dry leaf 5 g, standardized oleuropein 22 mg | 1:1 (dry) | 10 | 49.203 | 1.974 |
| D | Each 15 mL contains extract equiv 15 g fresh leaf, standardized to 75 mg oleuropein | 1:1 (fresh) | 15 | 80.043 | 11.713 |
| E | Each 15 mL contains extract equiv 15 g fresh leaf, standardized to 110 mg oleuropein | 1:1 (fresh) | 15 | 133.277 | 13.722 |
| F | n/a | 1:2 (dry) | 12 | 10.870 | 2.706 |
| G | n/a | 1:2 (fresh) | 7 | 45.852 | 7.650 |
| H | n/a | 1:1 (dry) | 3 | 3.463 | 18.592 |
| I | Each 1 mL contains equiv 500 mg dry leaf | 1:2 (dry) | 7 | 29.044 | 17.717 |
| J | Each 1 mL contains equiv 500 mg dry leaf | 1:2 (dry) | 7 | 2.674 | 25.570 |
| Sample Code | Product Name | Batch | Expiry Date | Fresh or Dry Leaf | Product Category * | ARTG Identifier ** |
|---|---|---|---|---|---|---|
| A | Comvita Medi Olive 66 | 15446 | Sep 2019 | Fresh | OTC | 252958 |
| B | Comvita Cardiovascular Support Medi Olive 136 | 15479 | 28 Sep 2019 | Fresh | OTC | 291745 |
| C | Healthy Care Olive Leaf Extract | 699984 | Aug 2020 | Dry | OTC | 162092 |
| D | Wellgrove Immune Support | OLE230218 (I) | 31 Aug 2020 | Fresh | OTC | 312527 |
| E | Wellgrove Heart Health | OLE60.8022 | 31 Jul 2020 | Fresh | OTC | 312833 |
| F | Pharmaceutical Plant Company (dry) | 130005 | Jan 2020 | Dry | PROF | n/a |
| G | Pharmaceutical Plant Company (fresh) | 4512 | Mar 2023 | Fresh | PROF | n/a |
| H | Herbal Extract Company | 280.140H7 | Aug 2020 | Dry | PROF | n/a |
| I | MediHerb | 22760 | Nov 2021 | Dry | PROF | n/a |
| J | Optimal Rx | 14273 | 9 Feb 2021 | Dry | PROF | n/a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breakspear, I.; Guillaume, C. A Quantitative Phytochemical Comparison of Olive Leaf Extracts on the Australian Market. Molecules 2020, 25, 4099. https://doi.org/10.3390/molecules25184099
Breakspear I, Guillaume C. A Quantitative Phytochemical Comparison of Olive Leaf Extracts on the Australian Market. Molecules. 2020; 25(18):4099. https://doi.org/10.3390/molecules25184099
Chicago/Turabian StyleBreakspear, Ian, and Claudia Guillaume. 2020. "A Quantitative Phytochemical Comparison of Olive Leaf Extracts on the Australian Market" Molecules 25, no. 18: 4099. https://doi.org/10.3390/molecules25184099
APA StyleBreakspear, I., & Guillaume, C. (2020). A Quantitative Phytochemical Comparison of Olive Leaf Extracts on the Australian Market. Molecules, 25(18), 4099. https://doi.org/10.3390/molecules25184099