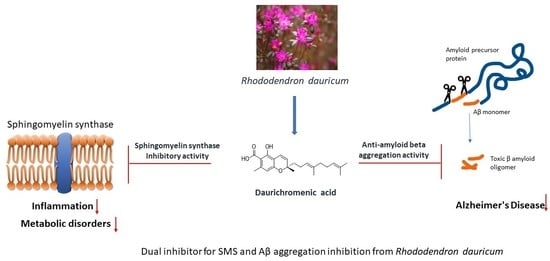
Daurichromenic Acid from the Chinese Traditional Medicinal Plant Rhododendron dauricum Inhibits Sphingomyelin Synthase and Aβ Aggregation
Abstract
1. Introduction
2. Results and Discussion
2.1. Preparation of Daurichromenic Acid (1) and Its Derivatives (2–7)
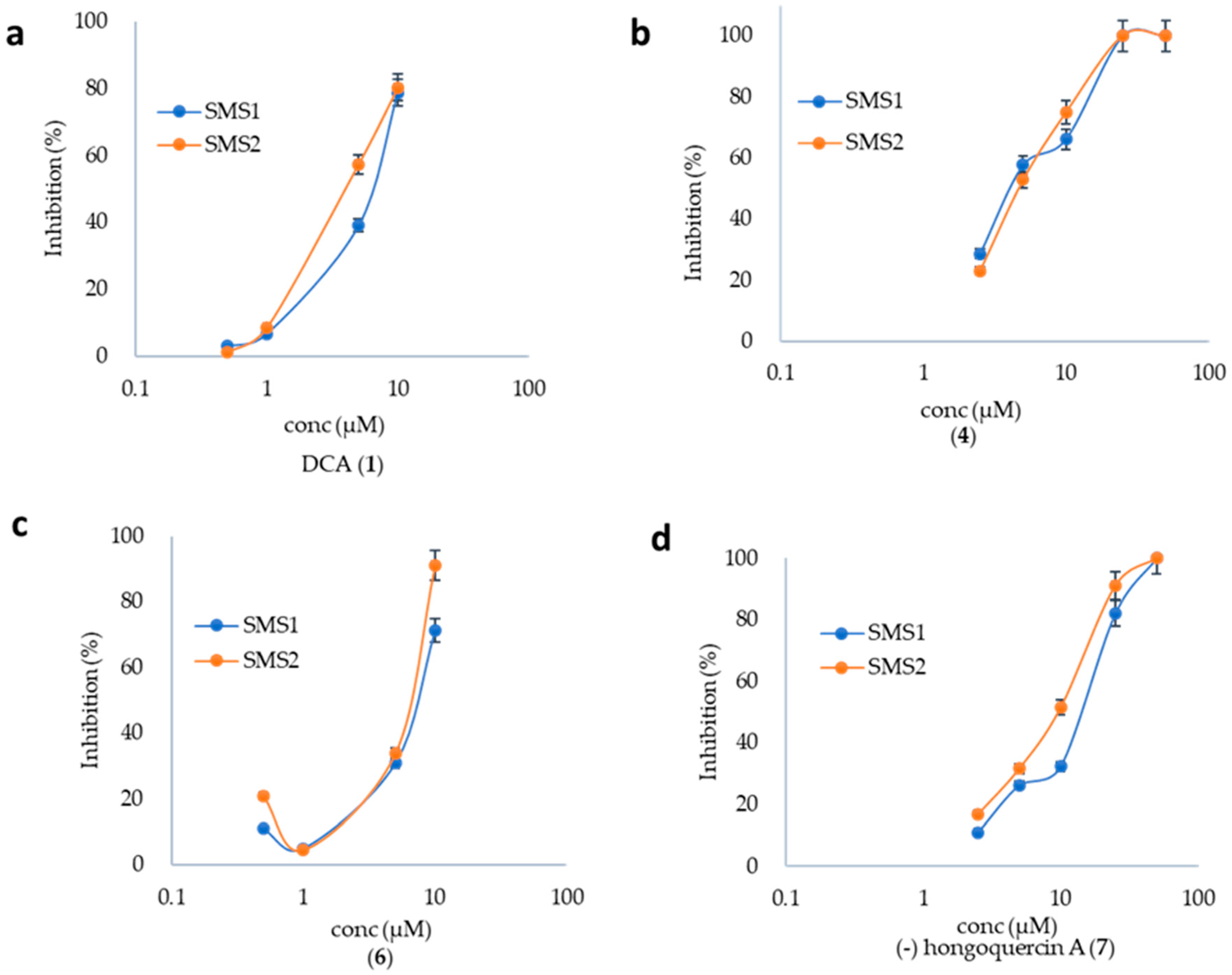
2.2. SMS Inhibition by DCA and Its Derivatives
2.3. Inhibition of Aβ Aggregation by DCA and Its Derivatives
2.4. DCA and Compound (4) Inhibit Both SMS and Aβ Aggregation
3. Materials and Methods
3.1. Extraction and Isolation of Active Compounds from Rhododendron dauricum
3.2. Synthesis of DCA Derivatives
3.2.1. Methyl Ester of DCA (2)
3.2.2. Methyl(S,E)-2-(4,8-dimethylnona-3,7-dien-1-yl)-5-methoxy-2,7-dimethyl-2H-chromene-6 carboxylate (3)
3.2.3. (S,E)-2-(4,8-Dimethylnona-3,7-dien-1-yl)-5-methoxy-2,7-dimethyl-2H-chromene-6-carboxylic acid (4)
3.2.4. (−)-(5R,8S,10R)-9,15-Didehydro hongoquercin A methyl ester (5)
3.2.5. (−)-(5R,8S,10R)-9,15-Didehydro hongoquercin A (6)
3.2.6. (−) Hongoquercin A (7)
3.3. SMS Assay
3.4. Aβ Inhibition Aggregation Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cao, Y.; Chu, Q.; Ye, J. Chromatographic and electrophoretic methods for pharmaceutically active compounds in Rhododendron dauricum. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 812, 231–240. [Google Scholar] [CrossRef]
- Taura, F.; Iijima, M.; Lee, J.B.; Hashimoto, T.; Asakawa, Y.; Kurosaki, F. Daurichromenic acid-producing oxidocyclase in the young leaves of Rhododendron dauricum. Nat. Prod. Commun. 2014, 9, 1329–1332. [Google Scholar] [CrossRef] [PubMed]
- Saeki, H.; Hara, R.; Takahashi, H.; Iijima, M.; Munakata, R.; Kenmoku, H.; Fuku, K.; Sekihara, A.; Yasuno, Y.; Shinada, T.; et al. An aromatic farnesyltransferase functions in biosynthesis of the anti-HIV meroterpenoid daurichromenic acid. Plant Physiol. 2018, 178, 535–551. [Google Scholar] [CrossRef]
- Iwata, N.; Wang, N.; Yao, X.; Kitanaka, S. Structures and histamine release inhibitory effects of prenylated orcinol derivatives from Rhododendron dauricum. J. Nat. Prod. 2004, 67, 1106–1109. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H. Discovery and development of natural product-derived chemotherapeutic agents based on a medicinal chemistry approach. J. Nat. Prod. 2010, 73, 500–516. [Google Scholar] [CrossRef]
- Taura, F.; Iijima, M.; Kurosaki, F. Daurichromenic acid and grifolic acid: Phytotoxic meroterpenoids that induce cell death in cell culture of their producer Rhododendron dauricum. Plant Signal. Behav. 2018, 13, e1422463. [Google Scholar] [CrossRef]
- Tafesse, F.G.; Huitema, K.; Hermansson, M.; Van Der Poel, S.; Van Den Dikkenberg, J.; Uphoff, A.; Somerharju, P.; Holthuis, J.C.M. Both sphingomyelin synthases SMS1 and SMS2 are required for sphingomyelin homeostasis and growth in human HeLa cells. J. Biol. Chem. 2007, 282, 17537–17547. [Google Scholar] [CrossRef]
- Mitsutake, S.; Zama, K.; Yokota, H.; Yoshida, T.; Tanaka, M.; Mitsui, M.; Ikawa, M.; Okabe, M.; Tanaka, Y.; Yamashita, T.; et al. Dynamic modification of sphingomyelin in lipid microdomains controls development of obesity, fatty liver, and type 2 diabetes. J. Biol. Chem. 2011, 286, 28544–28555. [Google Scholar] [CrossRef]
- Hanamatsu, H.; Ohnishi, S.; Sakai, S.; Yuyama, K.; Mitsutake, S.; Takeda, H.; Hashino, S.; Igarashi, Y. Altered levels of serum sphingomyelin and ceramide containing distinct acyl chains in young obese adults. Nutr. Diabetes 2014, 4, e141. [Google Scholar] [CrossRef]
- Kim, Y.J.; Greimel, P.; Hirabayashi, Y. GPRC5B-mediated sphingomyelin synthase 2 phosphorylation plays a critical role in insulin resistance. iScience 2018, 8, 250–266. [Google Scholar] [CrossRef]
- Yuyama, K.; Mitsutake, S.; Igarashi, Y. Pathological roles of ceramide and its metabolites in metabolic syndrome and Alzheimer’s disease. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2014, 1841, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, T.; Hashizume, C.; Taniguchi, M.; Furumoto, H.; Han, J.; Gao, R.; Kinami, S.; Kosaka, T.; Okazaki, T. Sphingomyelin synthase 2 deficiency inhibits the induction of murine colitis-associated colon cancer. FASEB J. 2017, 9, 3816–3830. [Google Scholar] [CrossRef]
- Swamy, M.M.M.; Murai, Y.; Ohno, Y.; Jojima, K.; Kihara, A.; Mitsutake, S.; Igarashi, Y.; Yu, J.; Yao, M.; Suga, Y.; et al. Structure-inspired design of a sphingolipid mimic sphingosine-1-phosphate receptor agonist from a naturally occurring sphingomyelin synthase inhibitor. Chem. Commun. 2018, 54, 12758–12761. [Google Scholar] [CrossRef] [PubMed]
- Othman, M.A.; Yuyama, K.; Murai, Y.; Igarashi, Y.; Mikami, D.; Sivasothy, Y.; Awang, K.; Monde, K. Malabaricone C as natural sphingomyelin synthase inhibitor against diet-induced obesity and its lipid metabolism in mice. ACS Med. Chem. Lett. 2019, 10, 1154–1158. [Google Scholar] [CrossRef]
- Jalencas, X.; Mestres, J. On the Origins of Drug Polypharmacology. Med. Chem. Commun. 2013, 4, 80–87. [Google Scholar] [CrossRef]
- Paolini, G.V.; Shapland, R.H.B.; van Hoorn, W.P.; Mason, J.S.; Hopkins, A.L. Global mapping of pharmacological space. Nat. Biotechnol. 2006, 24, 805–815. [Google Scholar] [CrossRef]
- Prati, F.; Uliassi, E.; Bolognesi, M.L. Two diseases, one approach: Multitarget drug discovery in Alzheimer’s and neglected tropical diseases. MedChemComm 2014, 5, 853–861. [Google Scholar] [CrossRef]
- Ren, B.; Liu, Y.; Zhang, Y.; Cai, Y.; Gong, X.; Chang, Y.; Xu, L.; Zheng, J. Genistein: A dual inhibitor of both amyloid β and human islet amylin peptides. ACS Chem. Neurosci. 2018, 9, 1215–1224. [Google Scholar] [CrossRef]
- Ono, K.; Yamada, M. Antioxidant compounds have potent anti-fibrillogenic and fibril-destabilizing effects for alpha-synuclein fibrils in vitro. J. Neurochem. 2006, 97, 105–115. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.; Lee, W.Y.W.; Or, P.M.Y.; Wan, D.C.C.; Kwan, Y.W.; Yeung, J.H.K. Miltirone is a dual inhibitor of P-glycoprotein and cell growth in Doxorubicin-resistant HepG2 cells. J. Nat. Prod. 2015, 78, 2266–2275. [Google Scholar] [CrossRef]
- Janefjord, E.; Mååg, J.L.V.; Harvey, B.S.; Smid, S.D. Cannabinoid effects on β amyloid fibril and aggregate formation, neuronal and microglial-activated neurotoxicity in vitro. Cell. Mol. Neurobiol. 2014, 34, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Mándi, A.; Swamy, M.M.M.; Taniguchi, T.; Anetai, M.; Monde, K. Reducing molecular flexibility by cyclization for elucidation of absolute configuration by CD calculations: Daurichromenic acid. Chirality 2016, 28, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Roll, D.M.; Manning, J.K.; Carter, G.T. Hongoquercins A and B, new sesquiterpenoid antibiotics: Isolation, structure elucidation, and antibacterial activity. J. Antibiot. 1998, 51, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Abbanat, D.A.; Singh, M.P.; Greenstein, M. Hongoquercins, new antibacterial agents from the fungus LL-23G227: Fermentation and biological activity. J. Antibiot. 1998, 51, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Tsujimori, H.; Bando, M.; Mori, K. Synthesis and absolute configuration of hongoquercin a, an antibacterial sesquiterpene-substituted orsellinic acid isolated as a fungal metabolite. Eur. J. Org. Chem. 2000, 2020, 297–302. [Google Scholar] [CrossRef]
- Giorgetti, S.; Greco, C.; Tortora, P.; Aprile, F.A. Targeting amyloid aggregation: An overview of strategies and mechanisms. Int. J. Mol. Sci. 2018, 19, 2677. [Google Scholar] [CrossRef]
- Ishigaki, Y.; Tanaka, H.; Akama, H.; Ogara, T.; Uwai, K. A microliter-scale high-throughput screening system with quantum-dot nanoprobes for amyloid-β aggregation inhibitors. PLoS ONE 2013, 8, e72992. [Google Scholar] [CrossRef]
- Tokuraku, K.; Ikezu, T. Imaging of amyloid β aggregation using a novel quantum dot nanoprobe and its advanced applications. In Bio-Nanoimaging: Protein Misfolding and Aggregation; Academic Press: Cambridge, MA, USA, 2014; pp. 121–131. [Google Scholar]
- Akiba, M.; Kinoshita, K.; Kino, Y.; Sato, J.I.; Koyama, K. Isolation of three new meroterpenoids and seven known compounds from Albatrellus yasudae and their Aβ-aggregation inhibitory activity. Bioorg. Med. Chem. Lett. 2020, 30, 126808. [Google Scholar] [CrossRef]
- Sasaki, R.; Tainaka, R.; Ando, Y.; Hashi, Y.; Deepak, H.V.; Suga, Y.; Murai, Y.; Anetai, M.; Monde, K.; Ohta, K.; et al. An automated microliter-scale high-throughput screening system (MSHTS) for real-time monitoring of protein aggregation using quantum-dot nanoprobes. Sci. Rep. 2019, 9, 2587. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

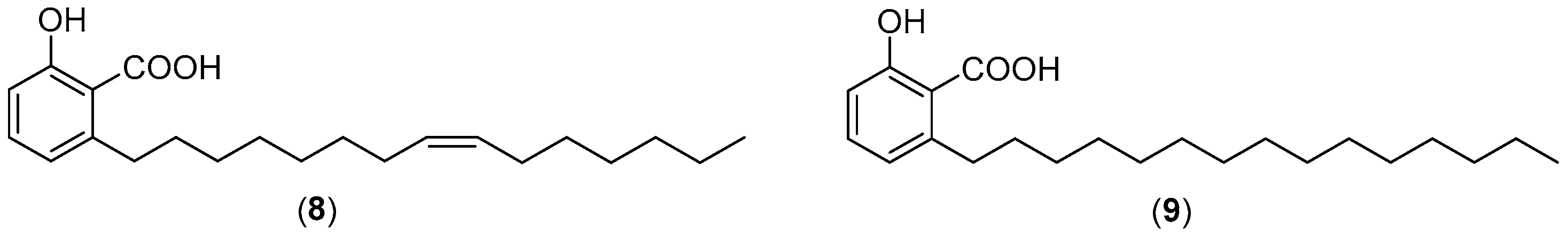

| Compounds | SMS Assay (SMS1) | SMS Assay (SMS2) | MHSTS Assay |
|---|---|---|---|
| (IC50 in µM) | (IC50 in µM) | (EC50 in µM) | |
| 1 | 7 | 4 | 57 |
| 4 | 17 | 10 | 74 |
| 7 | 4 | 5 | 238 |
| 8 | 1.5 | 1.5 | 149 |
| 9 | 2 | 2 | 296 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deepak, H.V.; Swamy, M.M.M.; Murai, Y.; Suga, Y.; Anetai, M.; Yo, T.; Kuragano, M.; Uwai, K.; Tokuraku, K.; Monde, K. Daurichromenic Acid from the Chinese Traditional Medicinal Plant Rhododendron dauricum Inhibits Sphingomyelin Synthase and Aβ Aggregation. Molecules 2020, 25, 4077. https://doi.org/10.3390/molecules25184077
Deepak HV, Swamy MMM, Murai Y, Suga Y, Anetai M, Yo T, Kuragano M, Uwai K, Tokuraku K, Monde K. Daurichromenic Acid from the Chinese Traditional Medicinal Plant Rhododendron dauricum Inhibits Sphingomyelin Synthase and Aβ Aggregation. Molecules. 2020; 25(18):4077. https://doi.org/10.3390/molecules25184077
Chicago/Turabian StyleDeepak, Hadya Virupaksha, Mahadeva M. M. Swamy, Yuta Murai, Yoshiko Suga, Masaki Anetai, Takuro Yo, Masahiro Kuragano, Koji Uwai, Kiyotaka Tokuraku, and Kenji Monde. 2020. "Daurichromenic Acid from the Chinese Traditional Medicinal Plant Rhododendron dauricum Inhibits Sphingomyelin Synthase and Aβ Aggregation" Molecules 25, no. 18: 4077. https://doi.org/10.3390/molecules25184077
APA StyleDeepak, H. V., Swamy, M. M. M., Murai, Y., Suga, Y., Anetai, M., Yo, T., Kuragano, M., Uwai, K., Tokuraku, K., & Monde, K. (2020). Daurichromenic Acid from the Chinese Traditional Medicinal Plant Rhododendron dauricum Inhibits Sphingomyelin Synthase and Aβ Aggregation. Molecules, 25(18), 4077. https://doi.org/10.3390/molecules25184077