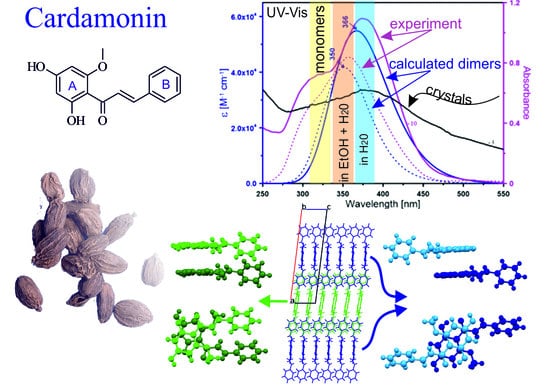
Structure and Physical Properties of Cardamonin: A Spectroscopic and Computational Approach
Abstract
1. Introduction
2. Results and Discussion
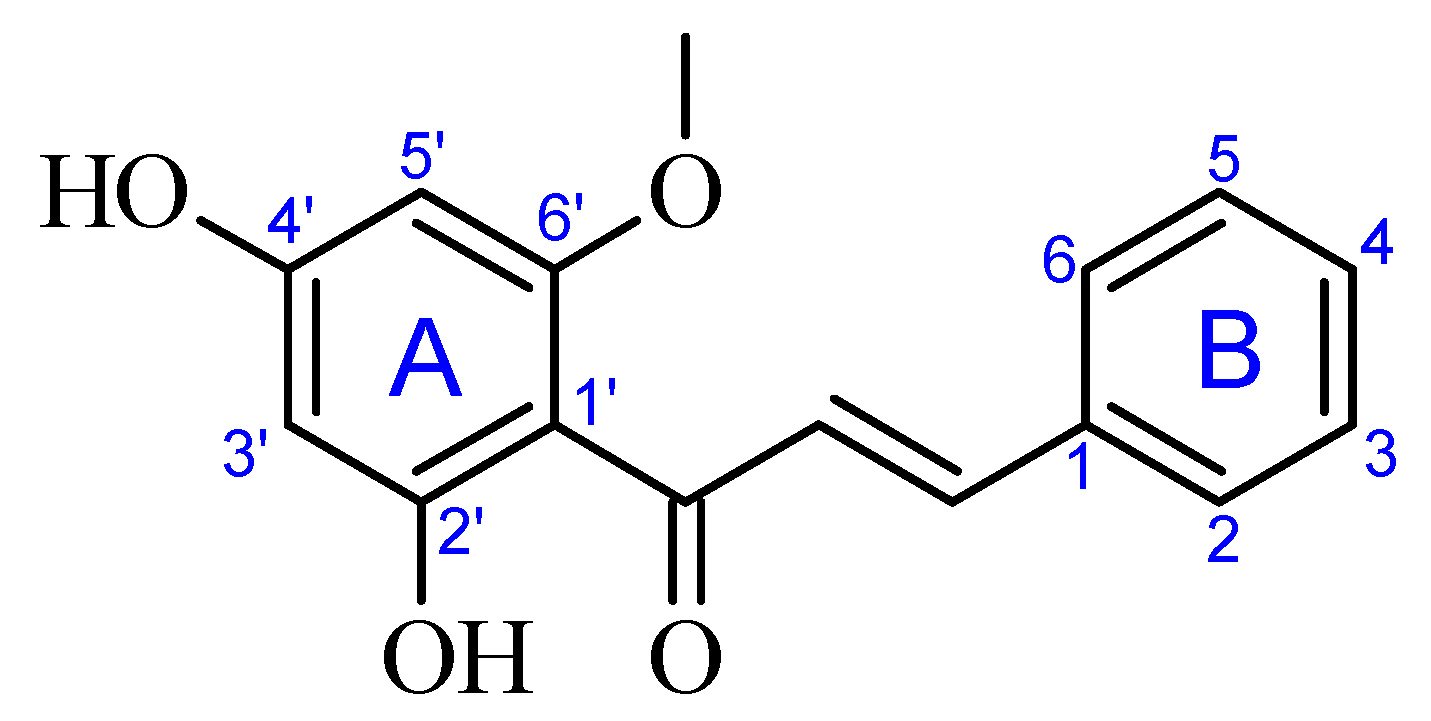
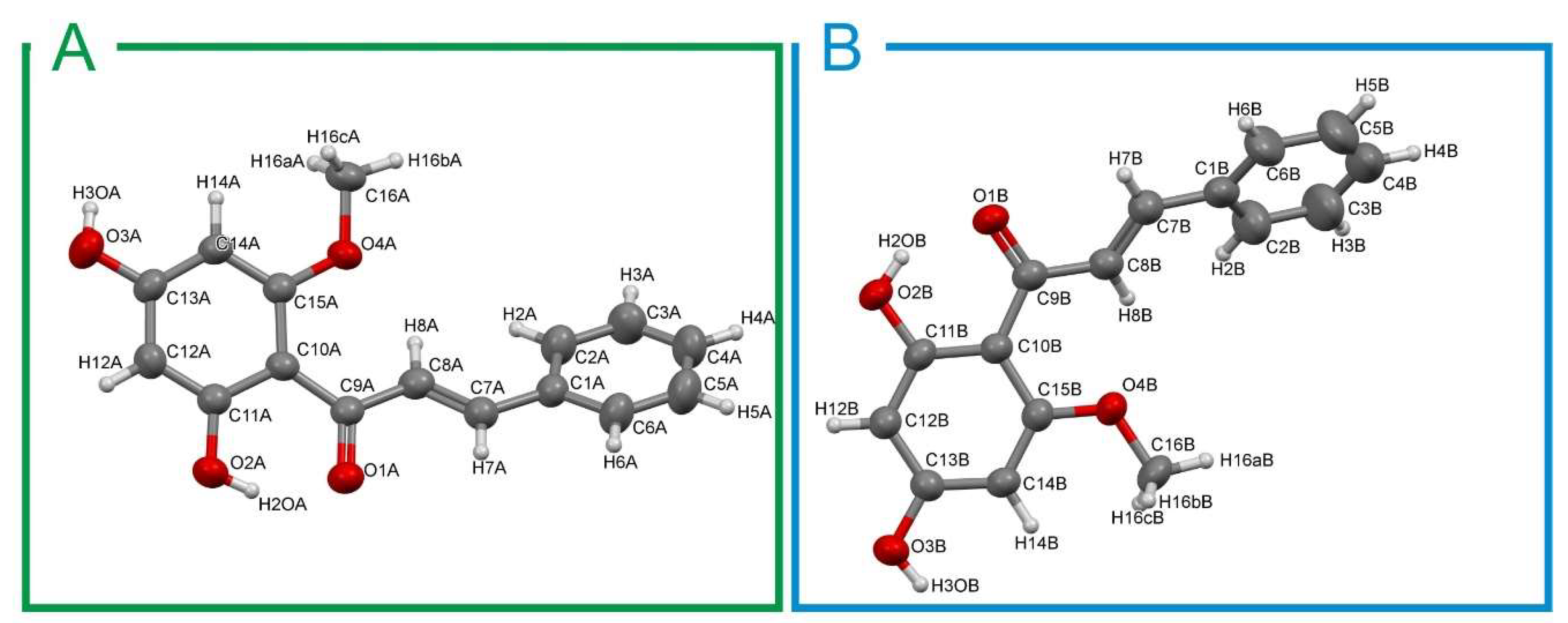
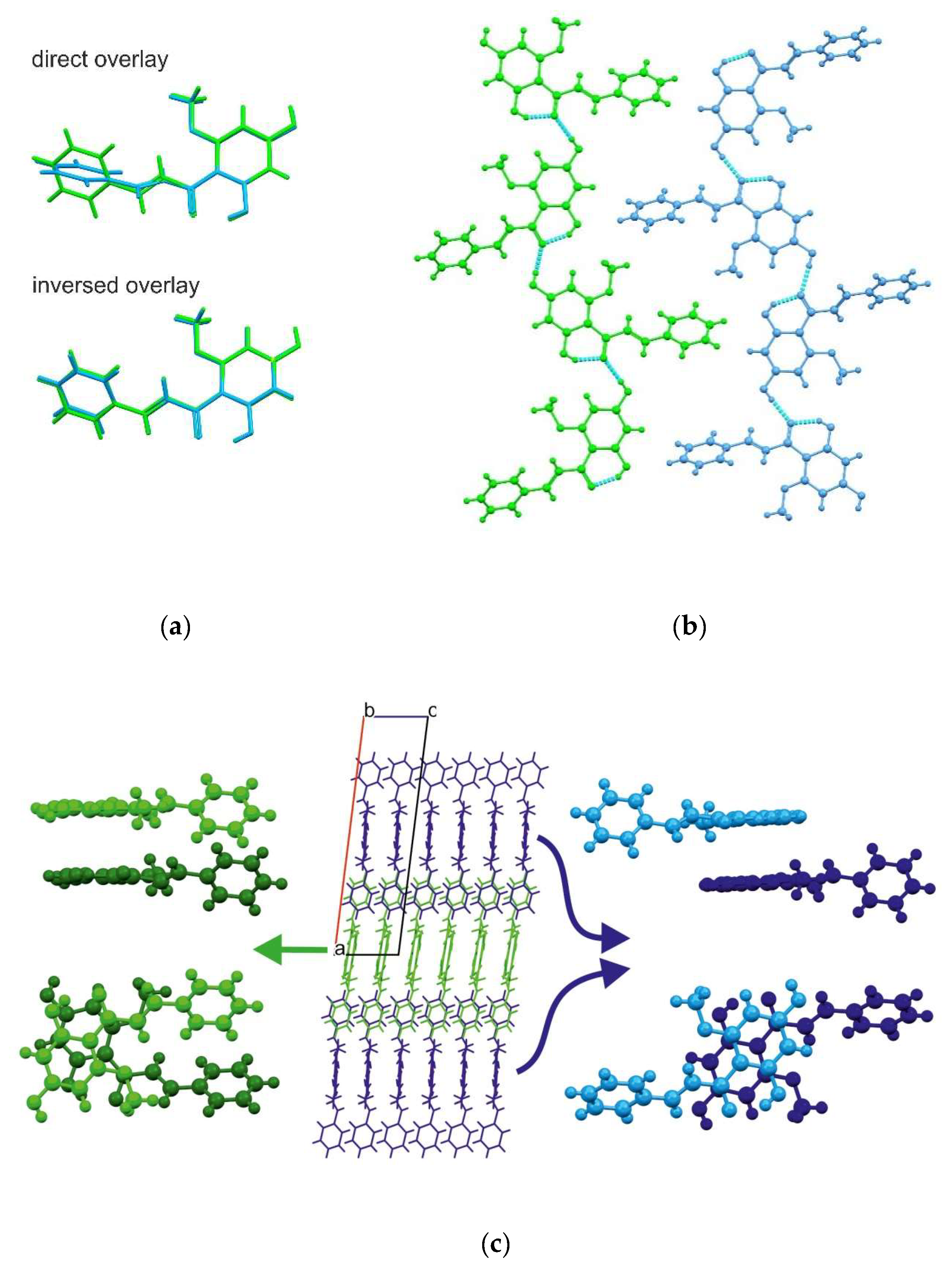
2.1. Crystal Structure Description
2.2. Theoretical Calculations
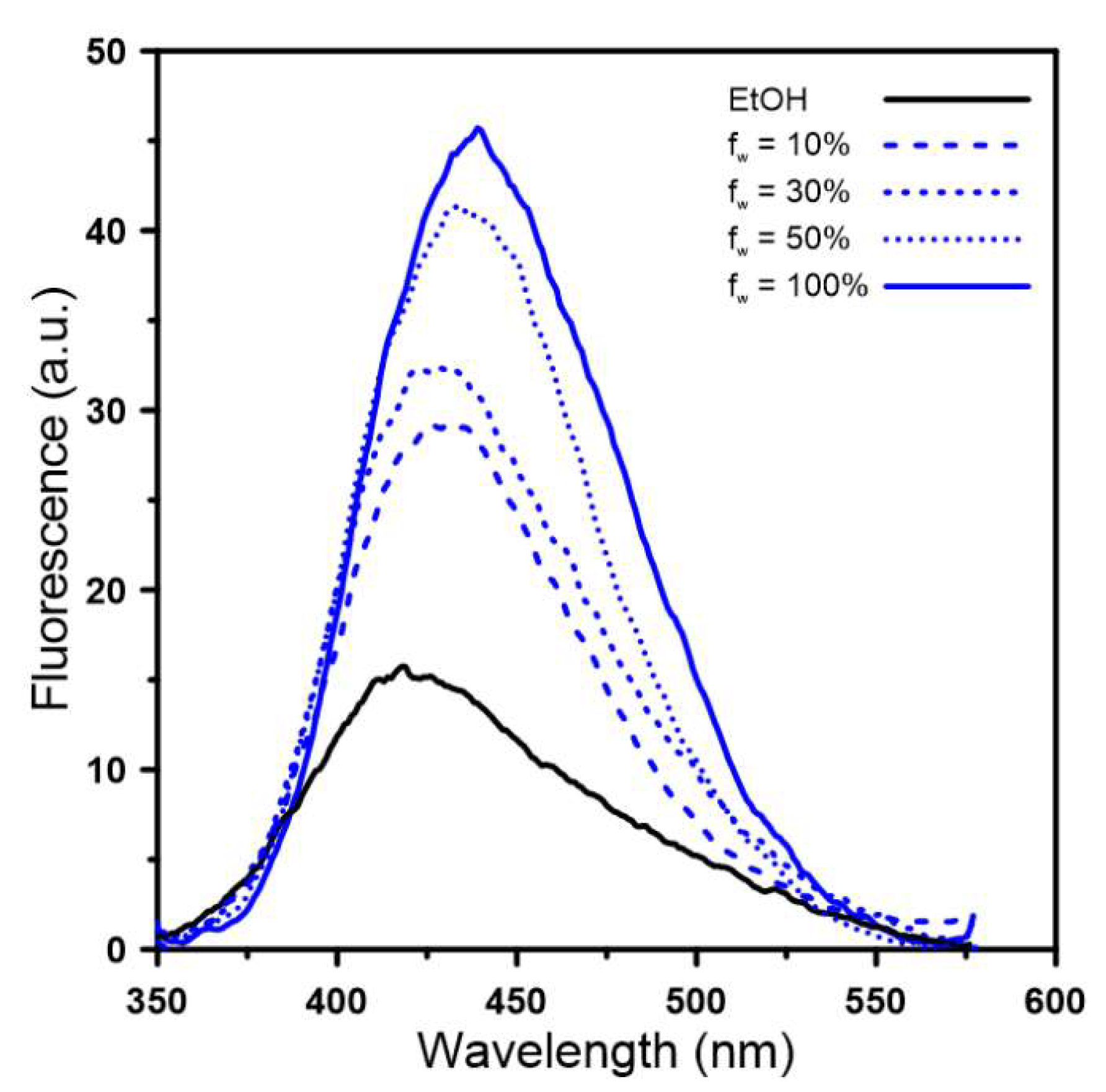
2.3. Spectral Properties
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. UV-Vis Spectroscopy
3.2.2. Steady-State Fluorescence Spectroscopy
3.2.3. Single Crystal X-ray Diffraction
3.2.4. Gaussian16 Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tuan, H.N.; Minh, B.H.; Tran, P.T.; Lee, J.H.; Oanh, H.V.; Ngo, Q.M.T.; Nguyen, Y.N.; Lien, P.T.K.; Tran, M.H. The Effects of 2′,4′-Dihydroxy-6′-methoxy-3′,5′- dimethylchalcone from Cleistocalyx operculatus Buds on Human Pancreatic Cancer Cell Lines. Molecules 2019, 24, 2538. [Google Scholar] [CrossRef] [PubMed]
- Abu Bakar, A.; Akhtar, M.N.; Ali, N.M.; Yeap, S.K.; Quah, C.K.; Loh, W.S.; Alitheen, N.B.; Zareen, S.; Ul-Haq, Z.; Shah, S.A.A. Design, synthesis and docking studies of flavokawain B type chalcones and their cytotoxic effects on MCF-7 and MDA-MB-231 cell lines. Molecules 2018, 23, 616. [Google Scholar] [CrossRef] [PubMed]
- Budziak, I.; Arczewska, M.; Kaminski, D.M. Formation of prenylated chalcone xanthohumol cocrystals: Single crystal X-ray diffraction, vibrational spectroscopic study coupled with multivariate analysis. Molecules 2019, 24, 4245. [Google Scholar] [CrossRef] [PubMed]
- Vogel, S.; Ohmayer, S.; Brunner, G.; Heilmann, J. Natural and non-natural prenylated chalcones: Synthesis, cytotoxicity and anti-oxidative activity. Bioorgan. Med. Chem. 2008, 16, 4286–4293. [Google Scholar] [CrossRef]
- Gaonkar, S.L.; Vignesh, U.N. Synthesis and pharmacological properties of chalcones: A review. Res. Chem. Intermediat. 2017, 43, 6043–6077. [Google Scholar] [CrossRef]
- Carvalho, P.S., Jr.; Custodio, J.M.; Vaz, W.F.; Cirilo, C.C.; Cidade, A.F.; Aquino, G.L.; Campos, D.M.; Cravo, P.; Coelho, C.J.; Oliveira, S.S.; et al. Conformation analysis of a novel fluorinated chalcone. J. Mol. Model. 2017, 23, 97. [Google Scholar] [CrossRef]
- Ekbote, A.; Patil, P.S.; Maidur, S.R.; Chia, T.S.; Quah, C.K. Structural, third-order optical nonlinearities and figures of merit of(E)-1-(3-substituted phenyl)-3-(4-fluorophenyl) prop-2-en-1-oneunder CW regime: New chalcone derivatives for optical limiting applications. Dyes Pigm. 2017, 139, 720–729. [Google Scholar] [CrossRef]
- Jaiswal, S.; Sharma, A.; Shukla, M.; Lal, J. Gender-related pharmacokinetics and bioavailability of a novel anticancer chalcone, cardamonin, in rats determined by liquid chromatography tandem mass spectrometry. J. Chromatogr. B 2015, 986, 23–30. [Google Scholar] [CrossRef]
- Zhang, J.W.; Sikka, S.; Siveen, K.S.; Lee, J.H.; Um, J.Y.; Kumar, A.P.; Chinnathambi, A.; Alharbi, S.A.; Basappa; Rangappa, K.S.; et al. Cardamonin represses proliferation, invasion, and causes apoptosis through the modulation of signal transducer and activator of transcription 3 pathway in prostate cancer. Apoptosis 2017, 22, 158–168. [Google Scholar] [CrossRef]
- Shrivastava, S.; Jeengar, M.K.; Thummuri, D.; Koval, A.; Katanaev, V.L.; Marepally, S.; Naidu, V.G.M. Cardamonin, a chalcone, inhibits human triple negative breast cancer cell invasiveness by downregulation of Wnt/-beta-catenin signaling cascades and reversal of epithelial-mesenchymal transition. Biofactors 2017, 43, 152–169. [Google Scholar] [CrossRef]
- James, S.; Aparna, J.S.; Paul, A.M.; Lankadasari, M.B.; Mohammed, S.; Binu, V.S.; Santhoshkumar, T.R.; Reshmi, G.; Harikumar, K.B. Cardamonin inhibits colonic neoplasia through modulation of MicroRNA expression. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Park, S.; Gwak, J.; Han, S.J.; Oh, S. Cardamonin suppresses the proliferation of colon cancer cells by promoting beta-catenin degradation. Biol. Pharm. Bull. 2013, 36, 1040–1044. [Google Scholar] [CrossRef]
- Ping, C.P.; Mohamad, T.A.S.T.; Akhtar, M.N.; Perimal, E.K.; Akira, A.; Ali, D.A.I.; Sulaiman, M.R. Antinociceptive effects of cardamonin in mice: Possible involvement of TRPV1, glutamate, and opioid receptors. Molecules 2018, 23, 2237. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.J.; Hou, Y.N.; Yao, J.; Fang, J.G. Activation of Nrf2-driven antioxidant enzymes by cardamonin confers neuroprotection of PC12 cells against oxidative damage. Food Funct. 2017, 8, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Gong, X. The conformational, electronic and spectral properties of chalcones: A density functional theory study. J. Mol. Struct. 2009, 901, 226–231. [Google Scholar] [CrossRef]
- Arczewska, M.; Kaminski, D.M.; Gieroba, B.; Gagos, M. Acid-base properties of xanthohumol: A computational and experimental investigation. J. Nat. Prod. 2017, 80, 3195–3203. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Wu, J.; Liu, X.; Zhao, B.; Wang, Z. Study on structural and spectral properties of isobavachalcone and 4-hydroxyderricin by computational method. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 126, 254–259. [Google Scholar] [CrossRef]
- Memon, A.H.; Ismail, Z.; Aisha, A.F.; Al-Suede, F.S.; Hamil, M.S.; Hashim, S.; Saeed, M.A.; Laghari, M.; Abdul Majid, A.M. Isolation, characterization, crystal Structure elucidation, and anticancer study of dimethyl cardamonin, isolated from Syzygium campanulatum Korth. Evid. Based Complement. Alternat. Med. 2014, 2014, 470179. [Google Scholar] [CrossRef]
- Karabacak, M.; Kose, E.; Atac, A.; Asiri, A.M.; Kurt, M. Monomeric and dimeric structures analysis and spectroscopic characterization of 3,5-difluorophenylboronic acid with experimental (FT-IR, FT-Raman, H-1 and C-13 NMR, UV) techniques and quantum chemical calculations. J. Mol. Struct. 2014, 1058, 79–96. [Google Scholar] [CrossRef]
- Olsztynska-Janus, S.; Szymborska, K.; Komorowska, M.; Lipinski, J. Conformational changes of L-phenylalanine - Near infrared-induced mechanism of dimerization: B3LYP studies. J. Mol. Struct. 2009, 911, 1–7. [Google Scholar] [CrossRef]
- Shukla, S.; Srivastava, A.; Srivastava, K.; Tandon, P.; Jamalis, J.; Singh, R.B. Non-covalent interactions and spectroscopic study of chalcone derivative 1-(4-chlorophenyl)-3-(5-methylfuran-2-yl) prop-2-en-1-one. J. Mol. Struct. 2020, 1201. [Google Scholar] [CrossRef]
- Custodio, J.M.F.; Guimaraes-Neto, J.J.A.; Awad, R.; Queiroz, J.E.; Verde, G.M.V.; Mottin, M.; Neves, B.J.; Andrade, C.H.; Aquino, G.L.B.; Valverde, C.; et al. Molecular modelling and optical properties of a novel fluorinated chalcone. Arab. J. Chem. 2020, 13, 3362–3371. [Google Scholar] [CrossRef]
- Zhuang, C.; Zhang, W.; Sheng, C.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef] [PubMed]
- Aljancic, I.S.; Vuckovic, I.; Jadranin, M.; Pesic, M.; Dordevic, I.; Podolski-Renic, A.; Stojkovic, S.; Menkovic, N.; Vajs, V.E.; Milosavljevic, S.M. Two structurally distinct chalcone dimers from Helichrysum zivojinii and their activities in cancer cell lines. Phytochemistry 2014, 98, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Menezes, C.J.M.D.S.; Diederich, M.F. Natural dimers of coumarin, chalcones, and resveratrol and the link between structure and pharmacology. Eur. J. Med. Chem. 2019, 182, 111637. [Google Scholar] [CrossRef]
- Tauc, J. The Optical Properties of Solids; Academic Press: New York, NY, USA, 1966. [Google Scholar]
- Zhou, Z.X.; Parr, R.G. Activation hardness—New index for describing the orientation of electrophilic aromatic-substitution. J. Am. Chem. Soc. 1990, 112, 5720–5724. [Google Scholar] [CrossRef]
- Kaminski, D.M.; Gaweda, K.; Arczewska, M.; Senczyna, B.; Gagos, M. A kinetic study of xanthohumol cyclization to isoxanthohumol - A role of water. J. Mol. Struct. 2017, 1139, 10–16. [Google Scholar] [CrossRef]
- Maidur, S.R.; Jahagirdar, J.R.; Patil, P.S.; Chia, T.S.; Quah, C.K. Structural characterizations, Hirshfeld surface analyses, and third order nonlinear optical properties of two novel chalcone derivatives. Opt. Mater. 2018, 75, 580–594. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Gao, J.; Lu, R.; Liu, F. Adjustment of the solid fluorescence of a chalcone derivative through controlling steric hindrance. RSC Adv. 2017, 7, 46354–46357. [Google Scholar] [CrossRef]
- Karuppusamy, A.; Vandana, T.; Kannan, P. Pyrene based chalcone materials as solid state luminogens with aggregation-induced enhanced emission properties. J. Photochem. Photobiol. A. 2017, 345, 11–20. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, L.; Luo, H.; Wang, H.; Yang, D.; He, F. Flavanone-based fluorophores with aggregation-induced emission enhancement characteristics for mitochondria-imaging and zebrafish-imaging. Molecules 2020, 25, 3298. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Liu, B. Aggregation-induced emission: Recent advances in materials and biomedical applications. Angew. Chem. Int. Ed. 2020, 59, 9868–9886. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, F.; Zhang, H.; Xie, Z.; Xie, W.; Xu, H.; Li, B.; Shen, F.; Ye, L.; Hanif, M.; et al. Tight intermolecular packing through supramolecular interactions in crystals of cyano substituted oligo (para-phenylenevinylene): A key factor for aggregation-induced emission. ChemComm 2007, 23, 1–3. [Google Scholar]
- Cai, M.; Gao, Z.; Zhou, X.; Wang, X.; Chen, S.; Zhao, Y.; Qian, Y.; Shi, N.; Mi, B.; Xie, L.; et al. A small change in molecular structure, a big difference in the AIEE mechanism. Phys. Chem. Chem. Phys. 2012, 14, 5289–5296. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Adamo, C.; Jacquemin, D. The calculations of excited-state properties with Time-Dependent Density Functional Theory. Chem. Soc. Rev. 2013, 42, 845–856. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
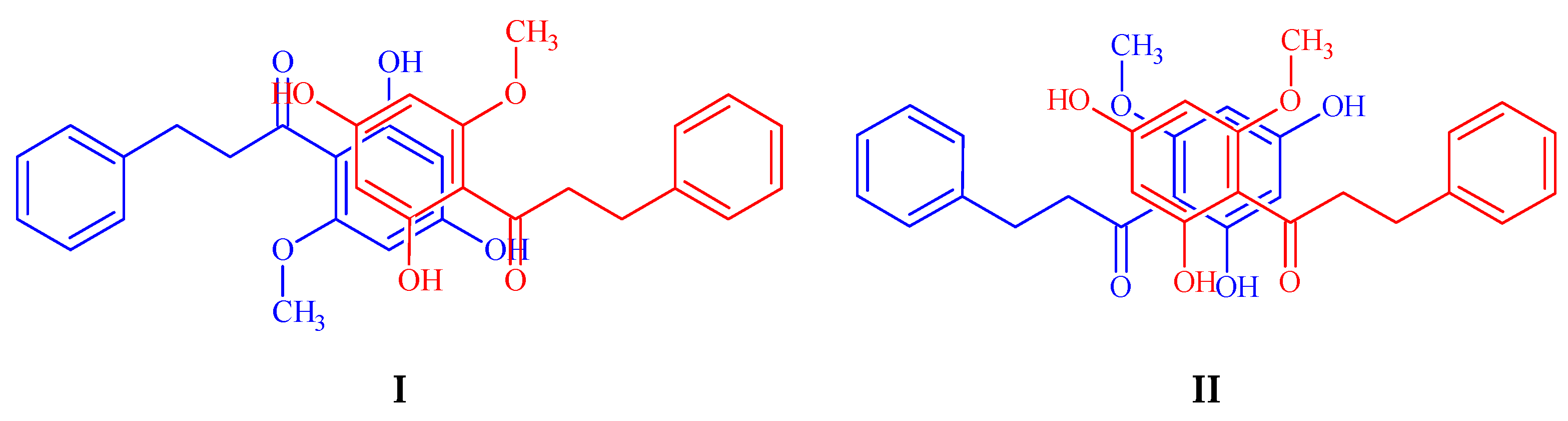
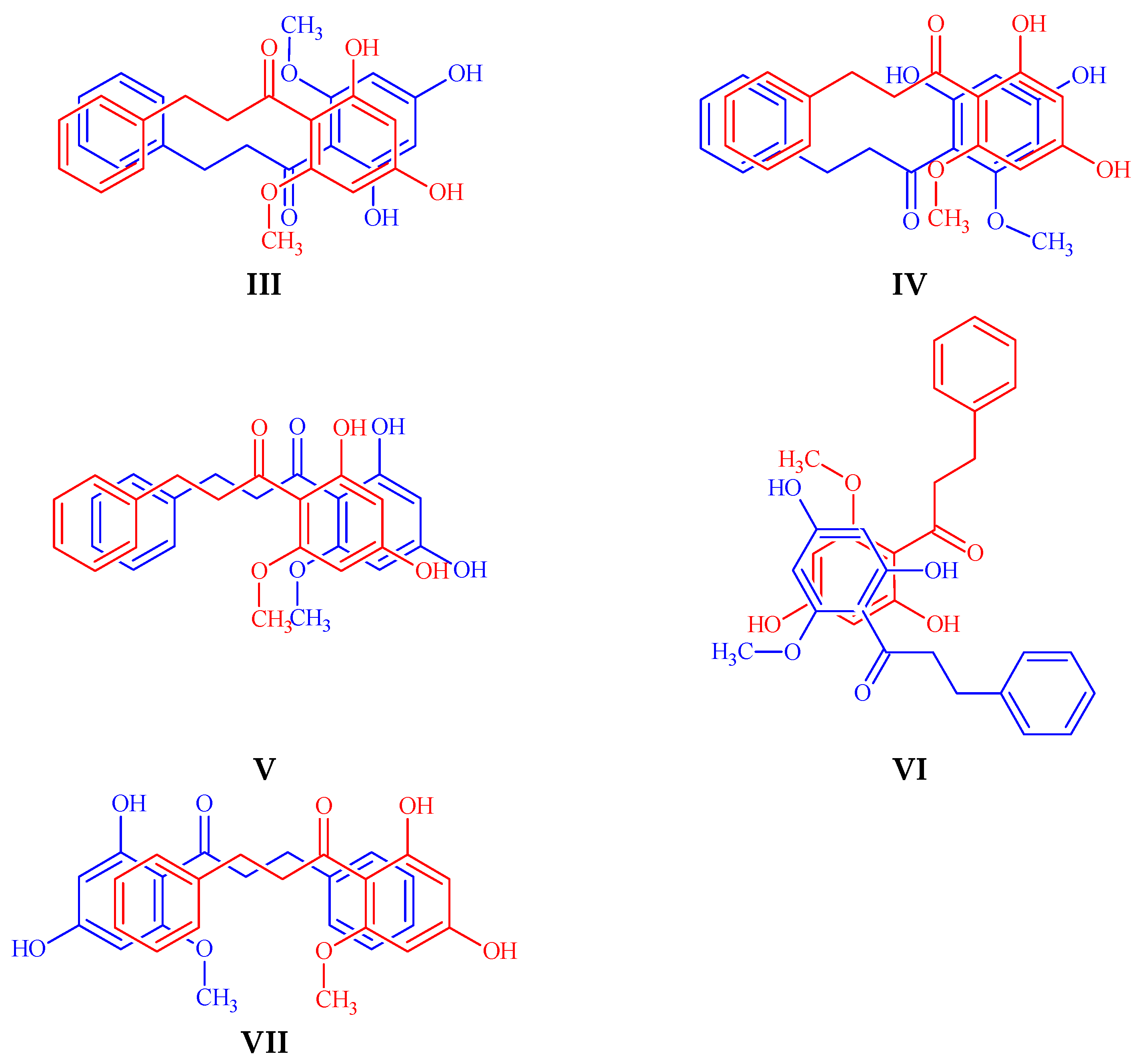

| Molecular Formula | C20H20O3 |
|---|---|
| Temperature (K) | 293(2) |
| Crystal system | monoclinic |
| Space group | P21/c |
| a (Å) | 25.7114(6) |
| b (Å) | 15.3506(3) |
| c (Å) | 6.8717(2) |
| α (°) | 90 |
| β (°) | 97.058(3) |
| γ (°) | 90 |
| V (Å3) | 2691.60(12) |
| Z | 8 |
| Calculated density (g cm−3) | 1.3339 |
| Absorption coefficient (mm−1) | 0.792 |
| F(000) | 1140 |
| Completeness | 99% |
| θ range for data collection (°) | 4.499–68.185 |
| Index ranges | −26 ≤ h ≤ 30 |
| −17 ≤ k ≤ 18 | |
| −7 ≤ l ≤ 8 | |
| Reflections collected/unique | 25,350/4917 |
| (Rint = 0.0355) | |
| Observed/restraints/parameters | 2073/0/368 |
| Goodness of fit on F2 | 1.0594 |
| Final R indices (I > 2sigma(I)) | R1 = 0.0678 |
| wR2 = 0.2274 | |
| R indices (all data) | R1 = 0.0839 |
| wR2 = 0.2409 | |
| Largest diff. peak and hole (e Å−3) | 0.3/−0.3 |
| CCDC Number | 2014912 |
| D–H···A | D–H (Å) | H···A (Å) | Angle (degree) | Symmetry |
|---|---|---|---|---|
| O1A–H2OA···O2A | 0.82 | 1.761 | 148.3 | x, y, z |
| O1A–H3OA···O3A | 0.82 | 1.907 | 173.9 | 2 − x, −1/2 + y, 1.5 − z |
| O1B···H2OB–O2B | 0.82 | 1.774 | 146.7 | x, y, z |
| O3B–H3OB···O3B | 0.82 | 1.898 | 174.7 | 1 − x, −1/2 + y, 1.5−z |
| Dimer | Energy (a.u.) |
|---|---|
| II | 0 |
| III | 8.56 |
| I | 26.85 |
| VII | 28.88 |
| V | 31.98 |
| IV | 72.07 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budziak, I.; Arczewska, M.; Kamiński, D.M. Structure and Physical Properties of Cardamonin: A Spectroscopic and Computational Approach. Molecules 2020, 25, 4070. https://doi.org/10.3390/molecules25184070
Budziak I, Arczewska M, Kamiński DM. Structure and Physical Properties of Cardamonin: A Spectroscopic and Computational Approach. Molecules. 2020; 25(18):4070. https://doi.org/10.3390/molecules25184070
Chicago/Turabian StyleBudziak, Iwona, Marta Arczewska, and Daniel M. Kamiński. 2020. "Structure and Physical Properties of Cardamonin: A Spectroscopic and Computational Approach" Molecules 25, no. 18: 4070. https://doi.org/10.3390/molecules25184070
APA StyleBudziak, I., Arczewska, M., & Kamiński, D. M. (2020). Structure and Physical Properties of Cardamonin: A Spectroscopic and Computational Approach. Molecules, 25(18), 4070. https://doi.org/10.3390/molecules25184070