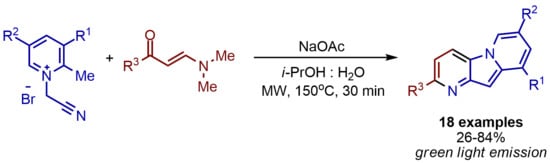
Microwave-Assisted Synthesis of Fluorescent Pyrido[2,3-b]indolizines from Alkylpyridinium Salts and Enaminones
Abstract
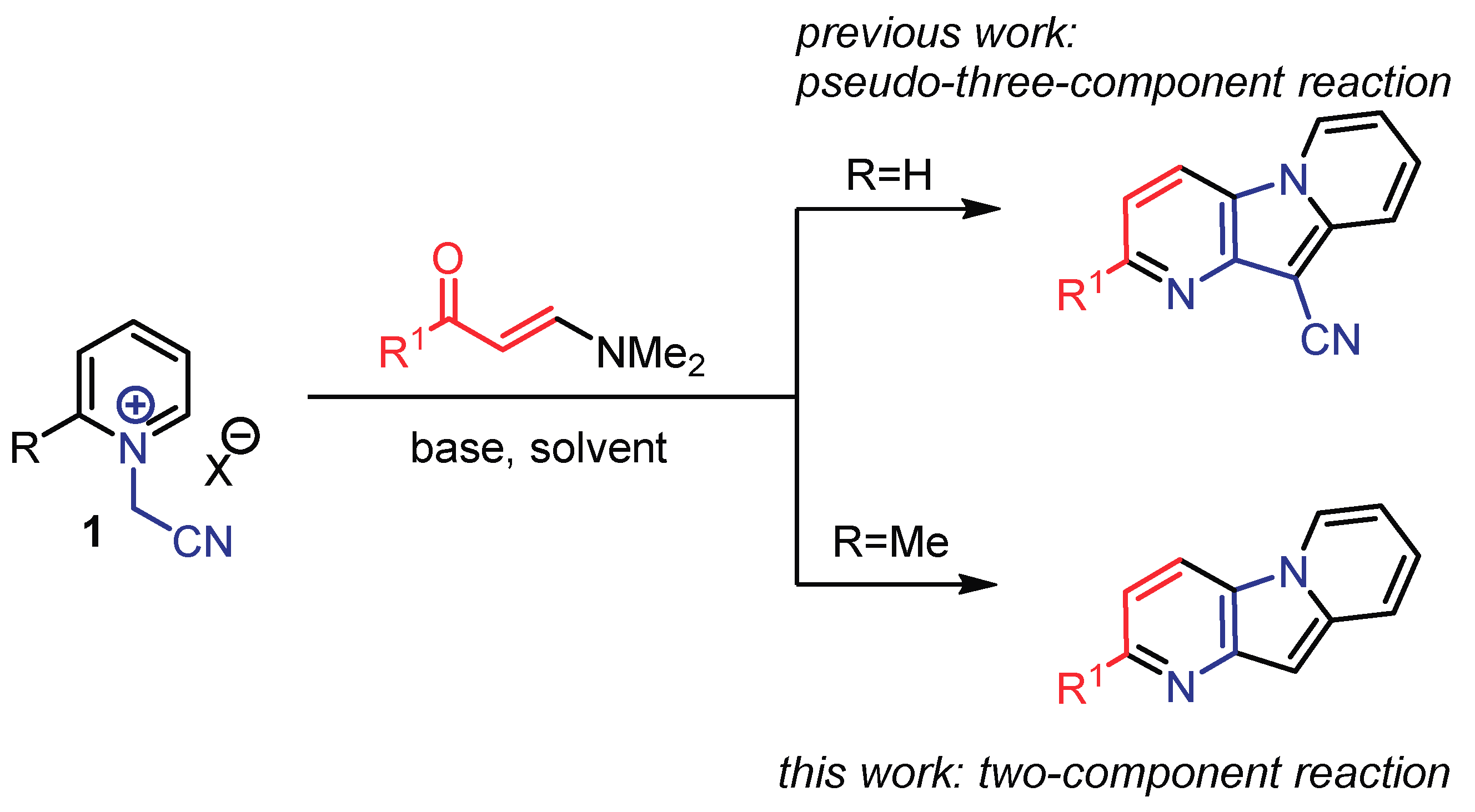
1. Introduction
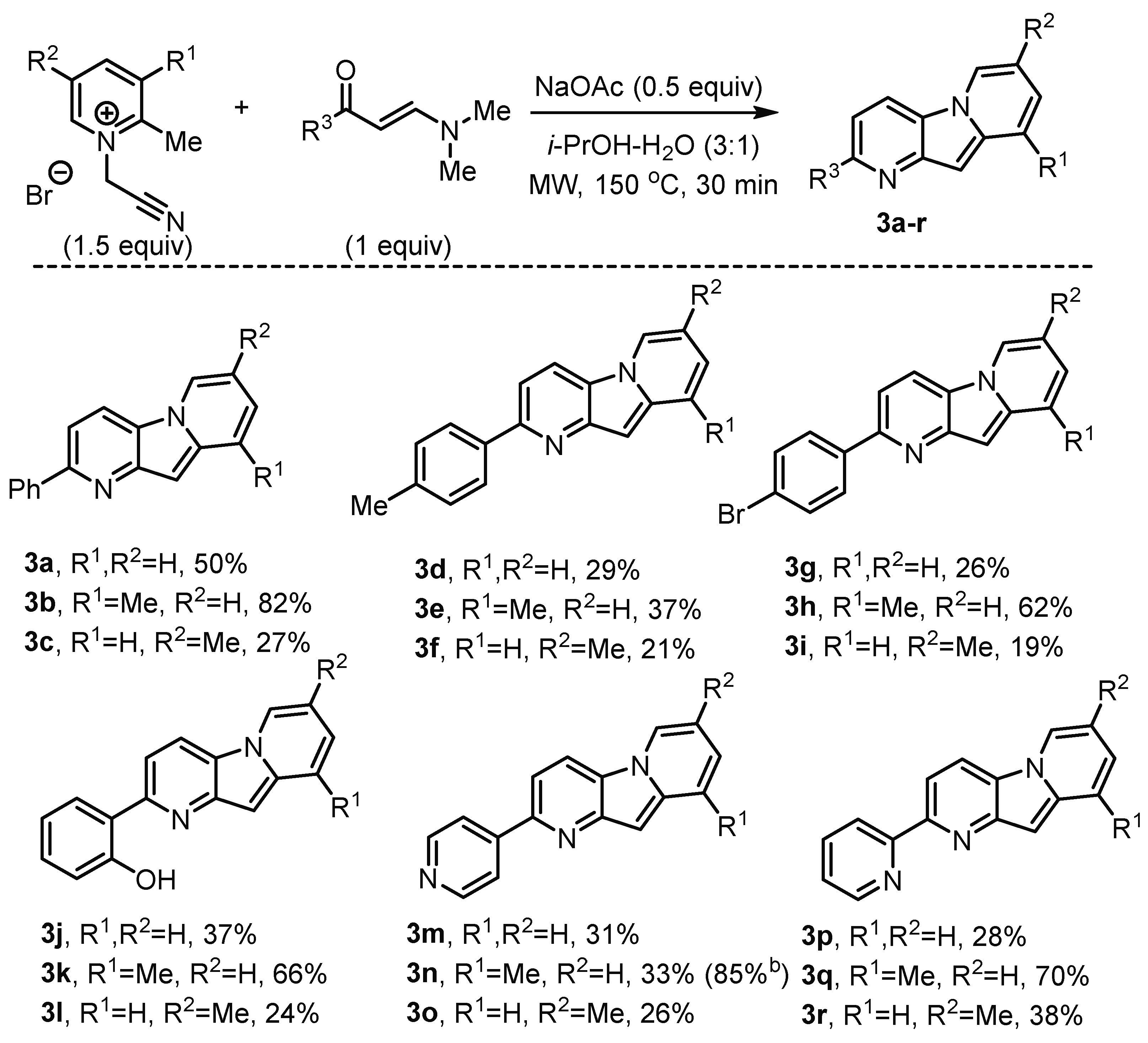
2. Results
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Synthesis of Salts 1a–c
3.3. General Procedure for the Synthesis of Enaminones 2
3.4. General Procedure for the Synthesis of Compounds 3a–r
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kappert, F.; Sreeramulu, S.; Jonker, H.R.A.; Richter, C.; Rogov, V.V.; Proschak, E.; Hargittay, B.; Saxena, K.; Schwalbe, H. Structural characterization of the interaction of the fibroblast growth factor receptor with a small molecule allosteric inhibitor. Chemistry 2018, 24, 7861–7865. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Yang, H.; Zhu, T.; Yu, L.; Chen, J.; Gu, L.; Huang, Z.; An, L. Synthesis, cytotoxicity and structure-activity relationship of indolizinoquinolinedione derivatives as DNA topoisomerase IB catalytic inhibitors. Eur. J. Med. Chem. 2018, 152, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, E.H.; Kim, J.; Kim, S.H.; Kim, I. Biological evaluation of indolizine-chalcone hybrids as new anticancer agents. Eur. J. Med. Chem. 2018, 144, 435–443. [Google Scholar] [CrossRef]
- Tatipamula, V.B.; Kolli, M.K.; Lagu, S.B.; Paidi, K.R.; Rsddy, R.; Yejella, R.P. Novel indolizine derivatives lowers blood glucose levels in streptozotocin-induced diabetic rats: A histopathological approach. Pharm. Rep. 2019, 71, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.H.; Jung, Y.; Kim, S.H.; Kim, I. Synthesis, characterization and biological evaluation of anti-cancer indolizine derivatives via inhibiting β-catenin activity and activating p53. Bioorg. Med. Chem. Lett. 2016, 26, 110–113. [Google Scholar] [CrossRef]
- Sardaru, M.C.; Craciun, A.M.; Al Matarneh, C.M.; Sandu, I.A.; Amarandi, R.M.; Popovici, L.; Ciobanu, C.I.; Peptanariu, D.; Pinteala, M.; Mangalagiu, I.I.; et al. Cytotoxic substituted indolizines as new colchicine site tubulin polymerisation inhibitors. J. Enzym. Inhib. Med. Chem. 2020, 35, 1581–1595. [Google Scholar] [CrossRef]
- Albaladejo, M.J.; González-Soria, M.J.; Alonso, F. Metal-free remote-site C-H alkenylation: Regio- and diastereoselective synthesis of solvatochromic dyes. Green Chem. 2018, 20, 701–712. [Google Scholar] [CrossRef]
- Gayton, J.; Autry, S.A.; Meador, W.; Parkin, S.R.; Hill, G.A.; Hammer, N.I.; Delcamp, J.H. Indolizine-Cyanine dyes: Near infrared emissive cyanine dyes with increased Stokes shifts. J. Org. Chem. 2019, 84, 687–697. [Google Scholar] [CrossRef]
- Cheema, H.; Baumann, A.; Loya, E.K.; Brogdon, P.; McNamara, L.E.; Carpenter, C.A.; Hammer, N.I.; Mathew, S.; Risko, C.; Delcamp, J.H. Near-Infrared-Absorbing indolizine-porphyrin push-pull dye for dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2019, 11, 16474–16489. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, Y.; Tse, A.K.W.; Zhou, X.; Chen, Y.; Tse, Y.C.; Wong, K.M.C.; Ho, C.Y. Synthesis and study of Au(iii)-indolizine derivatives: Turn-on luminescence by photo-induced controlled release. Chem. Commun. 2019, 55, 4471–4474. [Google Scholar] [CrossRef]
- Lee, Y.; Cho, W.; Sung, J.; Kim, E.; Park, S.B. Monochromophoric design strategy for tetrazine-based colorful bioorthogonal probes with a single fluorescent core skeleton. J. Am. Chem. Soc. 2018, 140, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.; Liu, A.; Shen, S.; Cao, X.; Li, F.; Ge, Y. An indolizine-rhodamine based FRET fluorescence sensor for highly sensitive and selective detection of Hg2+ in living cells. RSC Adv. 2017, 7, 40829–40833. [Google Scholar] [CrossRef]
- Bayazit, M.K.; Pålsson, L.O.; Coleman, K.S. Sensing properties of light-emitting single walled carbon nanotubes prepared via click chemistry of ylides bound to the nanotube surface. RSC Adv. 2015, 5, 36865–36873. [Google Scholar] [CrossRef]
- Airinei, A.; Tigoianu, R.; Danac, R.; Al Matarneh, C.M.; Isac, D.L. Steady state and time resolved fluorescence studies of new indolizine derivatives with phenanthroline skeleton. J. Lumin. 2018, 199, 6–12. [Google Scholar] [CrossRef]
- Kucukdisli, M.; Opatz, T. A modular synthesis of polysubstituted indolizines. Eur. J. Org. Chem. 2012, 2012, 4555–4564. [Google Scholar] [CrossRef]
- Cai, Q.; Zhu, Y.P.; Gao, Y.; Sun, J.J.; Wu, A.X. A direct method for the synthesis of indolizine derivatives from easily available aromatic ketones, pyridines, and acrylonitrile derivatives. Can. J. Chem. 2013, 91, 414–419. [Google Scholar] [CrossRef]
- Wang, W.; Han, J.; Sun, J.; Liu, Y. CuBr-Catalyzed aerobic decarboxylative cycloaddition for the synthesis of indolizines under solvent-free conditions. J. Org. Chem. 2017, 82, 2835–2842. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, X.; He, C.; Duan, C. Aminonaphthalimide-based imidazolium podands for turn-on fluo. Org. Biomol. Chem. 2010, 8, 2873–3084. [Google Scholar] [CrossRef]
- Bonte, S.; Ghinea, I.O.; Dinica, R.; Baussanne, I.; Demeunynck, M. Investigation of the pyridinium ylide-alkyne cycloaddition as a fluorogenic coupling reaction. Molecules 2016, 21, 332. [Google Scholar] [CrossRef]
- Yavari, I.; Ghafouri, K.; Naeimabadi, M.; Halvagar, M.R. A synthesis of functionalized 2-Indolizin-3-yl-1,3-benzothiazoles from 1-(1,3-Benzothiazol-2-ylmethyl)pyridinium Iodide and Acetylenic Esters. Synlett 2018, 29, 243–245. [Google Scholar] [CrossRef]
- Yavari, I.; Sheykhahmadi, J.; Naeimabadi, M.; Halvagar, M.R. Iodine-mediated sp3 C–H functionalization of methyl ketones: A one-pot synthesis of functionalized indolizines via the 1,3-dipolar cycloaddition reaction between pyridinium ylides and ynones. Mol. Divers. 2017, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Douglas, T.; Pordea, A.; Dowden, J. Iron-Catalyzed indolizine synthesis from pyridines, diazo compounds, and alkynes. Org. Lett. 2017, 19, 6396–6399. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Zhang, M.; Yu, S.; Ju, K.; Wang, C.; He, X. New route synthesis of indolizines via 1,3-dipolar cycloaddition of pyridiniums and alkynes. Tetrahedron Lett. 2009, 50, 6981–6984. [Google Scholar] [CrossRef]
- Fu, Q.; Yan, C.G. Molecular diversity of cycloaddition reactions of the functionalized pyridinium salts with 3-phenacylideneoxindoles. Tetrahedron 2013, 69, 5841–5849. [Google Scholar] [CrossRef]
- Zheng, P.; Li, C.; Mou, C.; Pan, D.; Wu, S.; Xue, W.; Jin, Z.; Chi, Y.R. Efficient access to 2-Pyrones via carbene-catalyzed oxidative [3+3] reactions between enals and nitrogen Ylides. Asian J. Org. Chem. 2019, 8, 1067–1070. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Duan, W.D.; Chen, J.; Hu, Y. Base-Promoted cascade reactions of 3-(1-Alkynyl)chromones with pyridinium Ylides to Chromeno[2,3-D]azepine derivatives. J. Org. Chem. 2019, 84, 4467–4472. [Google Scholar] [CrossRef]
- Kucukdisli, M.; Opatz, T. Two-step synthesis of 2-aminoindolizines from 2-alkylpyridines. Eur. J. Org. Chem. 2014, 2014, 5836–5844. [Google Scholar] [CrossRef]
- Storozhenko, O.A.; Festa, A.A.; Ndoutoume, D.R.B.; Aksenov, A.V.; Varlamov, A.V.; Voskressensky, L.G. Mn-mediated sequential three-component domino Knoevenagel/cyclization/Michael addition/oxidative cyclization reaction towards annulated imidazo[1,2-a]pyridines. Beilstein J. Org. Chem. 2018, 14, 3078–3087. [Google Scholar] [CrossRef]
- Voskressensky, L.G.; Storozhenko, O.A.; Festa, A.A.; Novikov, R.A.; Varlamov, A.V. Synthesis of Chromenoimidazoles, annulated with an azaindole moiety, through a base-promoted domino reaction of cyano methyl quaternary salts. Synthesis 2017, 49, 2753–2760. [Google Scholar]
- Voskressensky, L.G.; Sokolova, E.A.; Festa, A.A.; Varlamov, A.V. A novel domino condensation-intramolecular nucleophilic cyclization approach towards annulated thiochromenes. Tetrahedron Lett. 2013, 54, 5172–5173. [Google Scholar] [CrossRef]
- Sokolova, E.A.; Festa, A.A.; Golantsov, N.E.; Lukonina, N.S.; Ioffe, I.N.; Varlamov, A.V.; Voskressensky, L.G. Highly fluorescent pyrido[2,3-b]indolizine-10-carbonitriles through pseudo three-component reactions of N-(Cyanomethyl)pyridinium salts. Eur. J. Org. Chem. 2019, 2019, 6770–6775. [Google Scholar] [CrossRef]
- Kro, R.; Mostafavi, M.; Lampre, I. Preferential solvation of coumarin 153. The role of hydrogen bonding. J. Phys. Chem. A 2002, 106, 1708–1713. [Google Scholar]
- Shen, Y.M.; Grampp, G.; Leesakul, N.; Hu, H.W.; Xu, J.H. Synthesis and emitting properties of the blue-light fluorophores indolizino[3,4,5-ab]isoindole derivatives. Eur. J. Org. Chem. 2007, 2007, 3718–3726. [Google Scholar] [CrossRef]
- Park, S.; Kwon, D.I.; Lee, J.; Kim, I. When indolizine meets quinoline: Diversity-oriented synthesis of new polyheterocycles and their optical properties. ACS Comb. Sci. 2015, 17, 459–469. [Google Scholar] [CrossRef]
- Singh, D.K.; Kim, S.; Lee, J.H.; Lee, N.K.; Kim, J.; Lee, J.; Kim, I. 6-(Hetero)arylindolizino[1,2-c]quinolines as highly fluorescent chemical space: Synthesis and photophysical properties. J. Heterocycl. Chem. 2020, 57, 3018–3028. [Google Scholar] [CrossRef]
- Sung, J.; Lee, Y.; Cha, J.H.; Park, S.B.; Kim, E. Development of fluorescent mitochondria probe based on 1,2-dihydropyrrolo[3,4-b]indolizine-3-one. Dyes Pigments 2017, 145, 461–468. [Google Scholar] [CrossRef]
- Al-Zaydi, K.M.; Borik, R.M. Microwave assisted condensation reactions of 2-aryl hydrazonopropanals with nucleophilic reagents and dimethyl acetylenedicarboxylate. Molecules 2007, 12, 2061–2079. [Google Scholar] [CrossRef]
Sample Availability: Not available. |



| Entry a | Base | 1a: 2a: Base | Yield, % b |
|---|---|---|---|
| 1 | NaOAc | 3: 1: 5 | 34 |
| 2 | 3: 1: 1 | 40 | |
| 3 | 3: 1: 0.5 | 46 | |
| 4 | 3: 1: 0.1 | 12 | |
| 5 | 1.5: 1: 0.5 | 50 | |
| 6 | 1: 1: 0.5 | 44 | |
| 7 | Et3N | 1.5: 1: 0.5 | 31 |
| 8 | DIPEA | 1.5: 1: 0.5 | 25 |
| 9 | NH4OAc | 1.5: 1: 0.5 | 34 |
| 10 | K2CO3 | 1.5: 1: 0.5 | 5 |
| 11 | Cs2CO3 | 1.5: 1: 0.5 | 21 |
| 12 c | NaOAc | 1.5: 1: 0.5 | 47 |
| 13 d | NaOAc | 1.5: 1: 0.5 | 33 |
| 14 e | NaOAc | 1.5: 1: 0.5 | 37 |
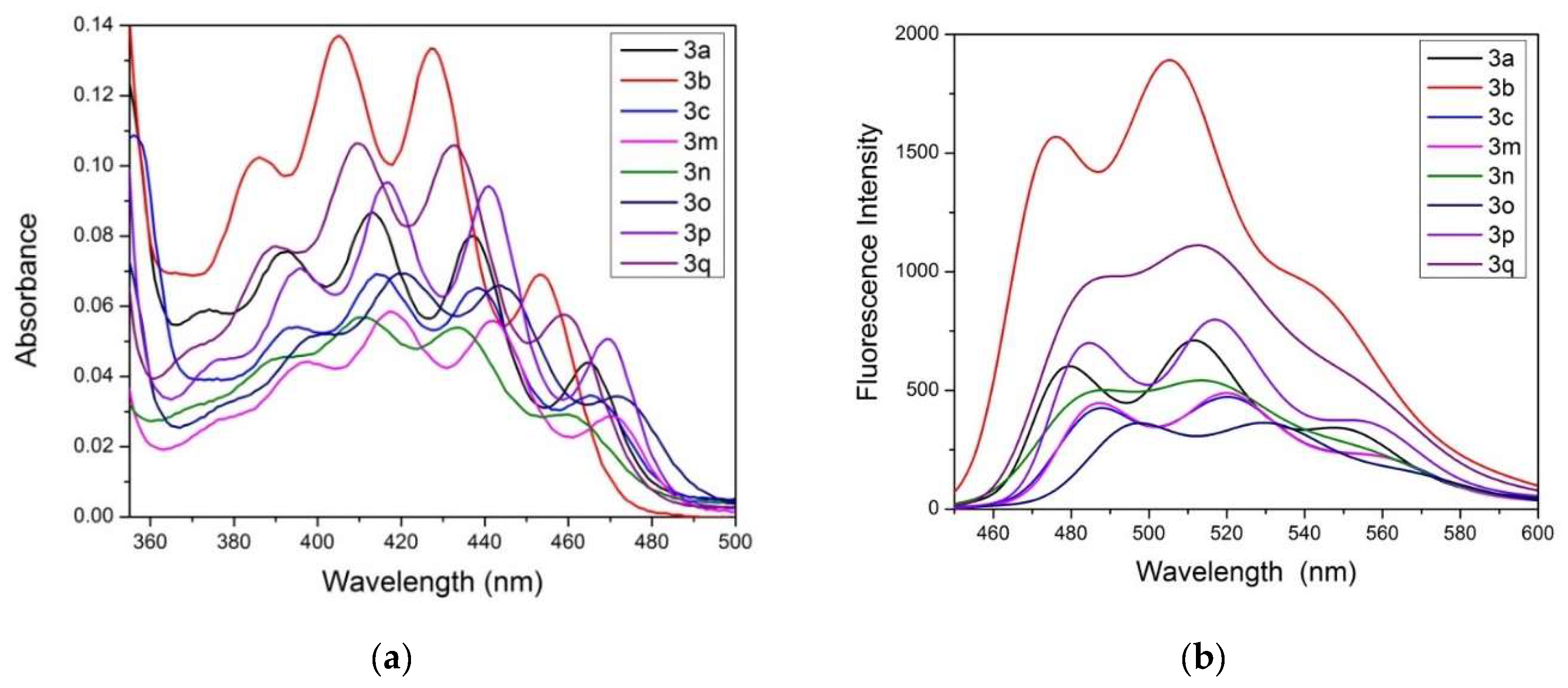
| Compound | Abs [a] | ε [b] | Emission [a] | FQY [c] | Stokes Shift |
|---|---|---|---|---|---|
| [nm] | [(M cm)−1 (109)] | [nm] | [%] | [cm−1] | |
| 3a | 413 | 1.652 | 511 | 77 | 4643 |
| 3b | 404 | 1.616 | 505 | 82 | 4950 |
| 3c | 414 | 1.656 | 520 | 64 | 4923 |
| 3m | 418 | 1.672 | 519 | 57 | 4655 |
| 3n | 410 | 1.640 | 513 | 63 | 4897 |
| 3o | 420 | 1.680 | 528 | 55 | 4870 |
| 3p | 416 | 1.664 | 516 | 64 | 4658 |
| 3q | 409 | 1.636 | 512 | 77 | 4918 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokolova, E.A.; Festa, A.A.; Subramani, K.; Rybakov, V.B.; Varlamov, A.V.; Voskressensky, L.G.; Van der Eycken, E.V. Microwave-Assisted Synthesis of Fluorescent Pyrido[2,3-b]indolizines from Alkylpyridinium Salts and Enaminones. Molecules 2020, 25, 4059. https://doi.org/10.3390/molecules25184059
Sokolova EA, Festa AA, Subramani K, Rybakov VB, Varlamov AV, Voskressensky LG, Van der Eycken EV. Microwave-Assisted Synthesis of Fluorescent Pyrido[2,3-b]indolizines from Alkylpyridinium Salts and Enaminones. Molecules. 2020; 25(18):4059. https://doi.org/10.3390/molecules25184059
Chicago/Turabian StyleSokolova, Ekaterina A., Alexey A. Festa, Karthikeyan Subramani, Victor B. Rybakov, Alexey V. Varlamov, Leonid G. Voskressensky, and Erik V. Van der Eycken. 2020. "Microwave-Assisted Synthesis of Fluorescent Pyrido[2,3-b]indolizines from Alkylpyridinium Salts and Enaminones" Molecules 25, no. 18: 4059. https://doi.org/10.3390/molecules25184059
APA StyleSokolova, E. A., Festa, A. A., Subramani, K., Rybakov, V. B., Varlamov, A. V., Voskressensky, L. G., & Van der Eycken, E. V. (2020). Microwave-Assisted Synthesis of Fluorescent Pyrido[2,3-b]indolizines from Alkylpyridinium Salts and Enaminones. Molecules, 25(18), 4059. https://doi.org/10.3390/molecules25184059