Diels–Alder Cycloaddition to the Bay Region of Perylene and Its Derivatives as an Attractive Strategy for PAH Core Expansion: Theoretical and Practical Aspects
Abstract
1. Introduction
2. Cycloaddition to Unsubstituted Perylene
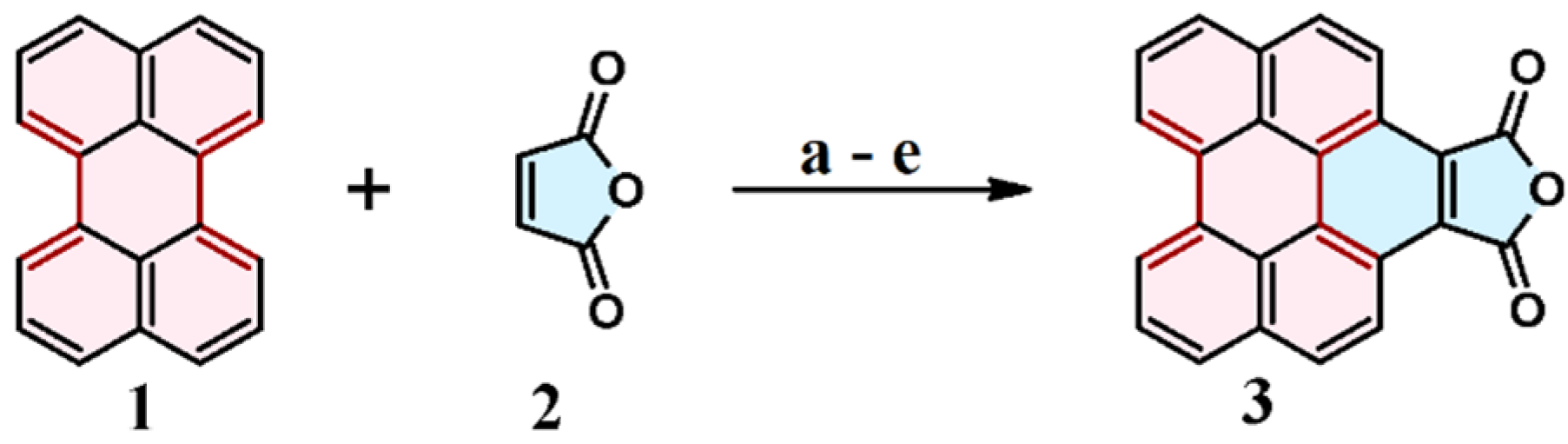
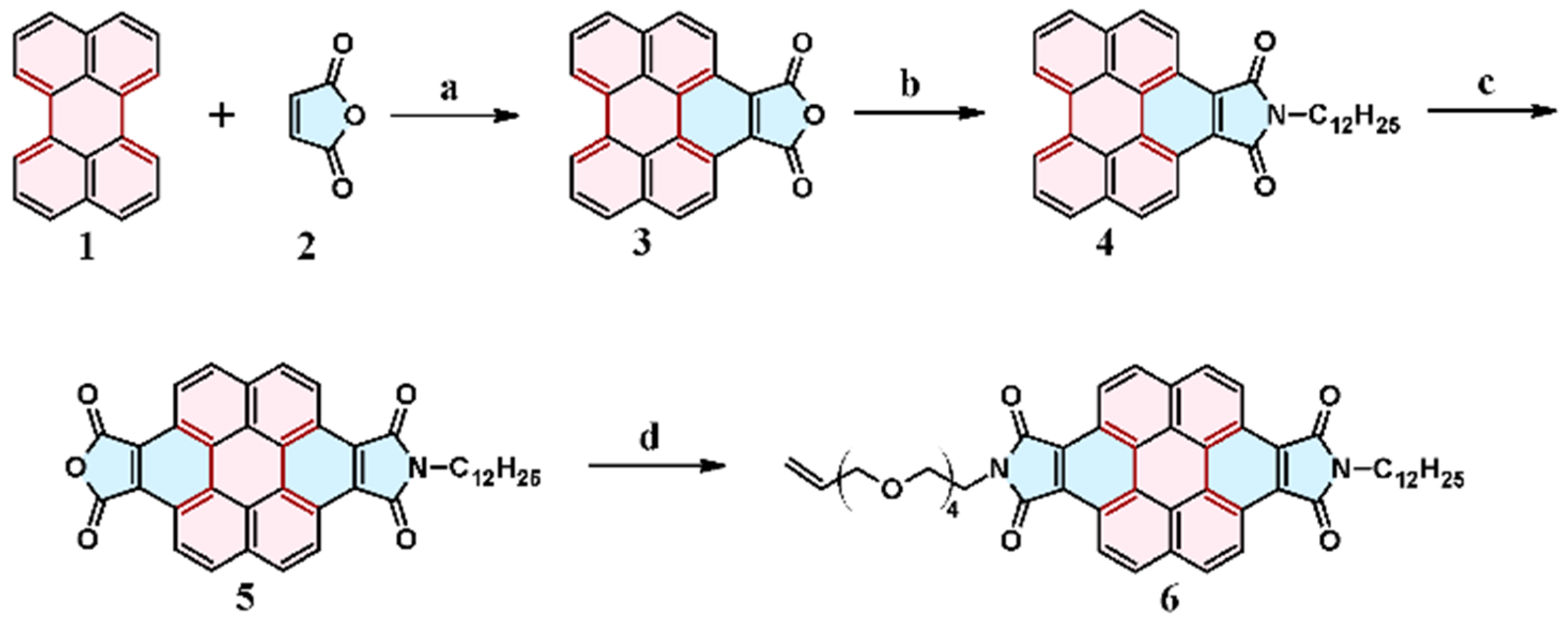
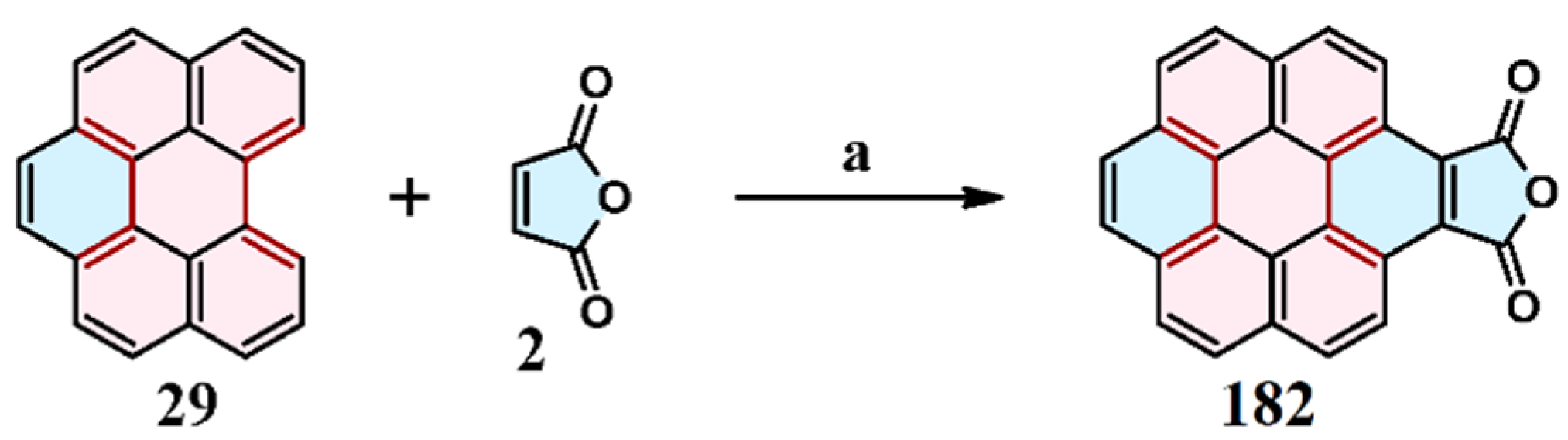
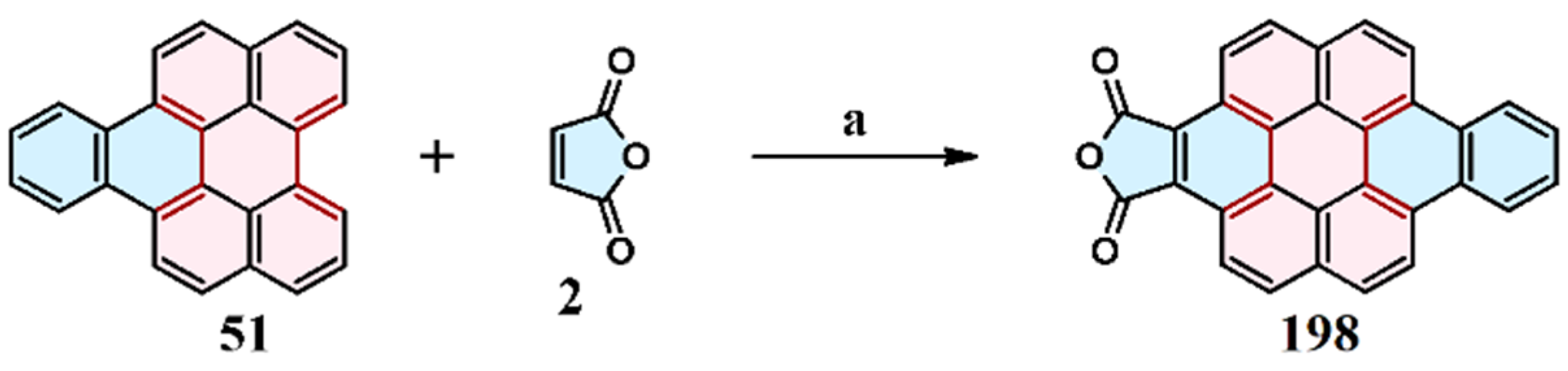
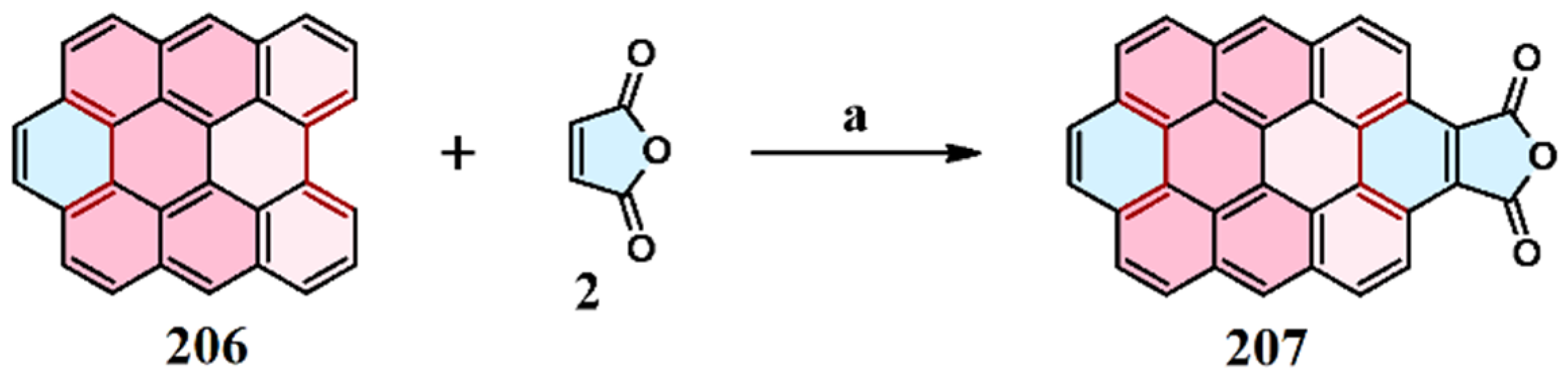
2.1. Cycloaddition of Maleic Anhydride
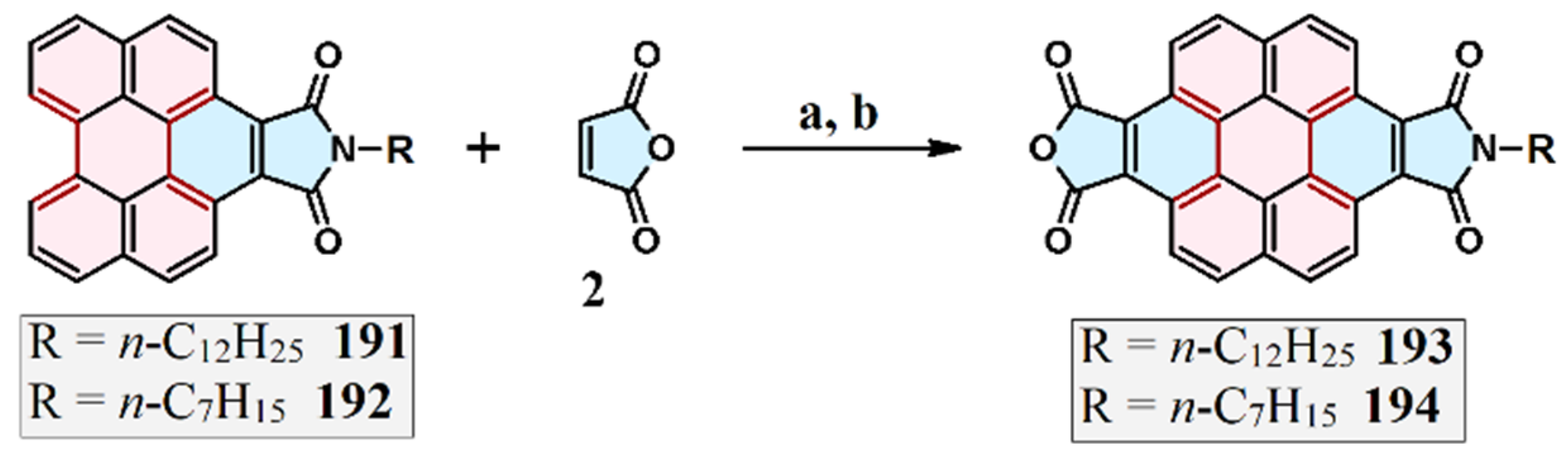
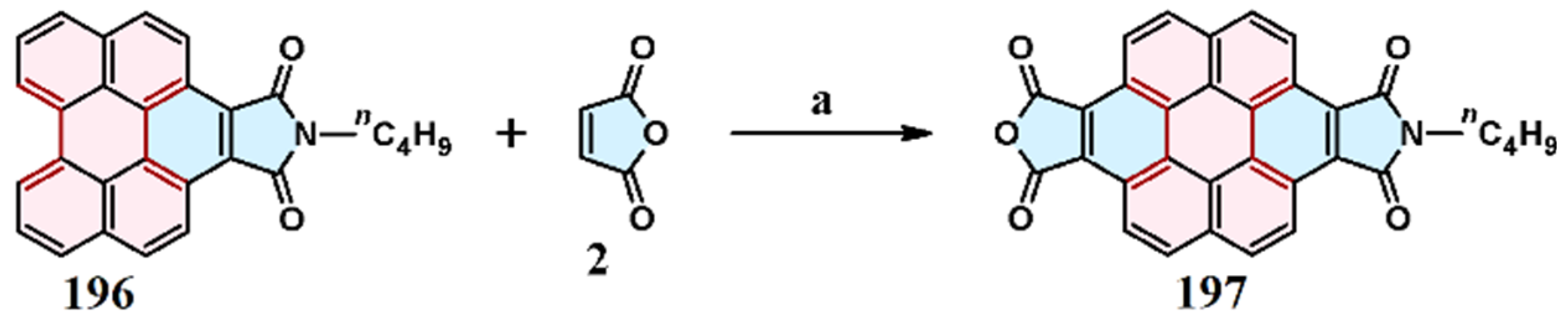
2.2. Cycloaddition of N-Substituted Maleimides
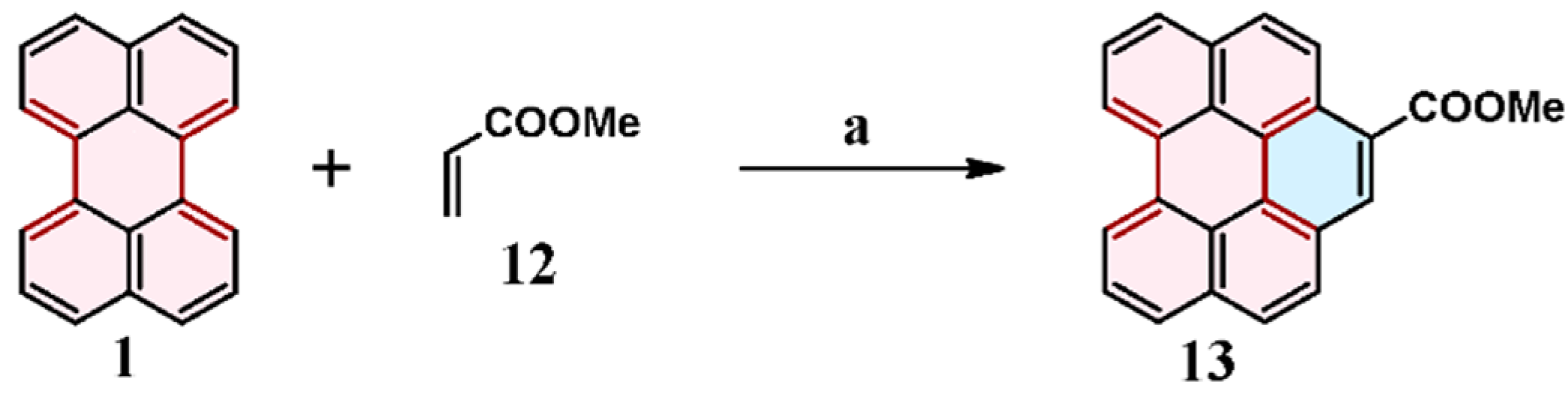
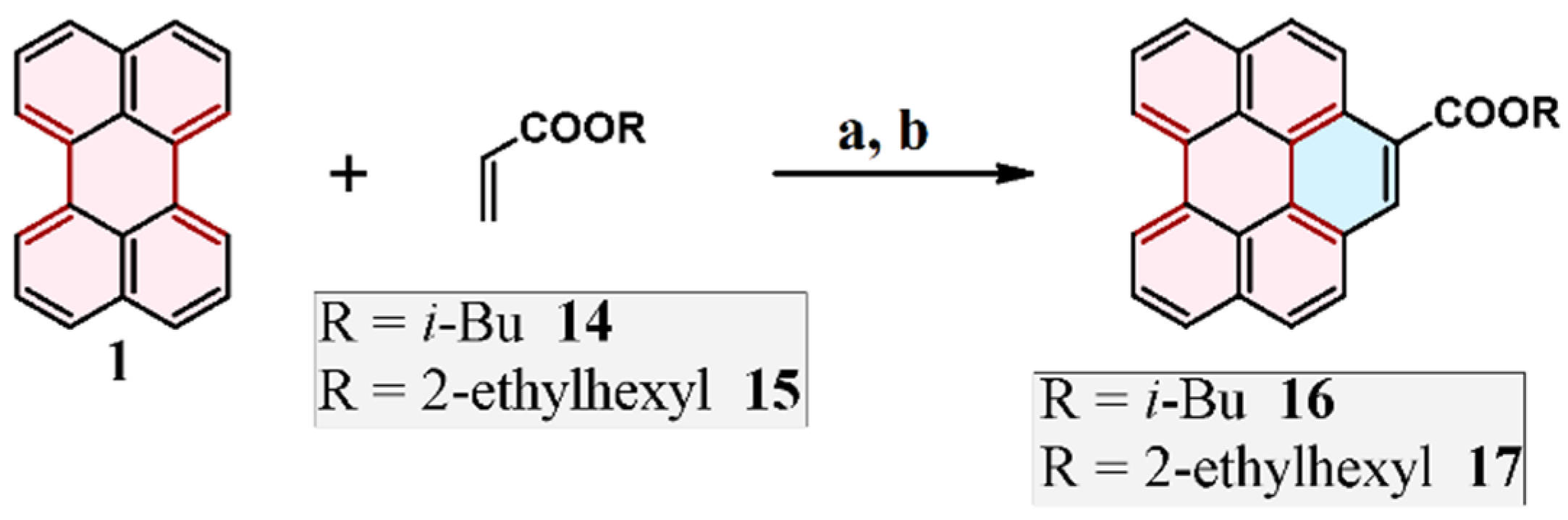
2.3. Cycloaddition of Alkyl Acrylates
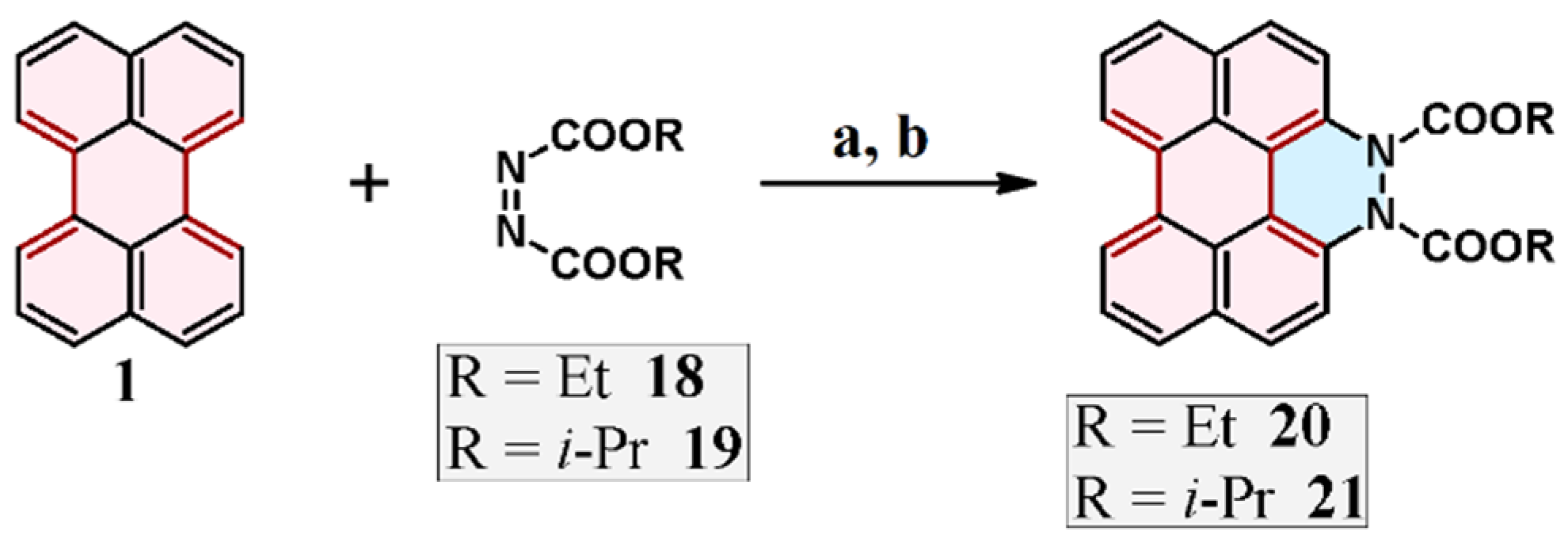
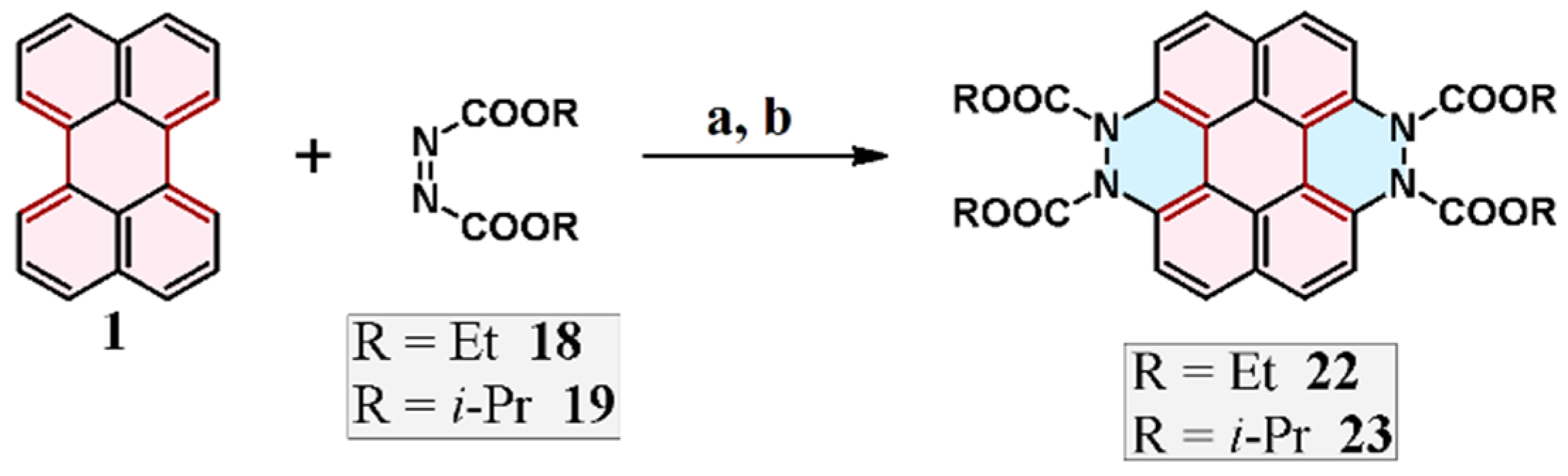
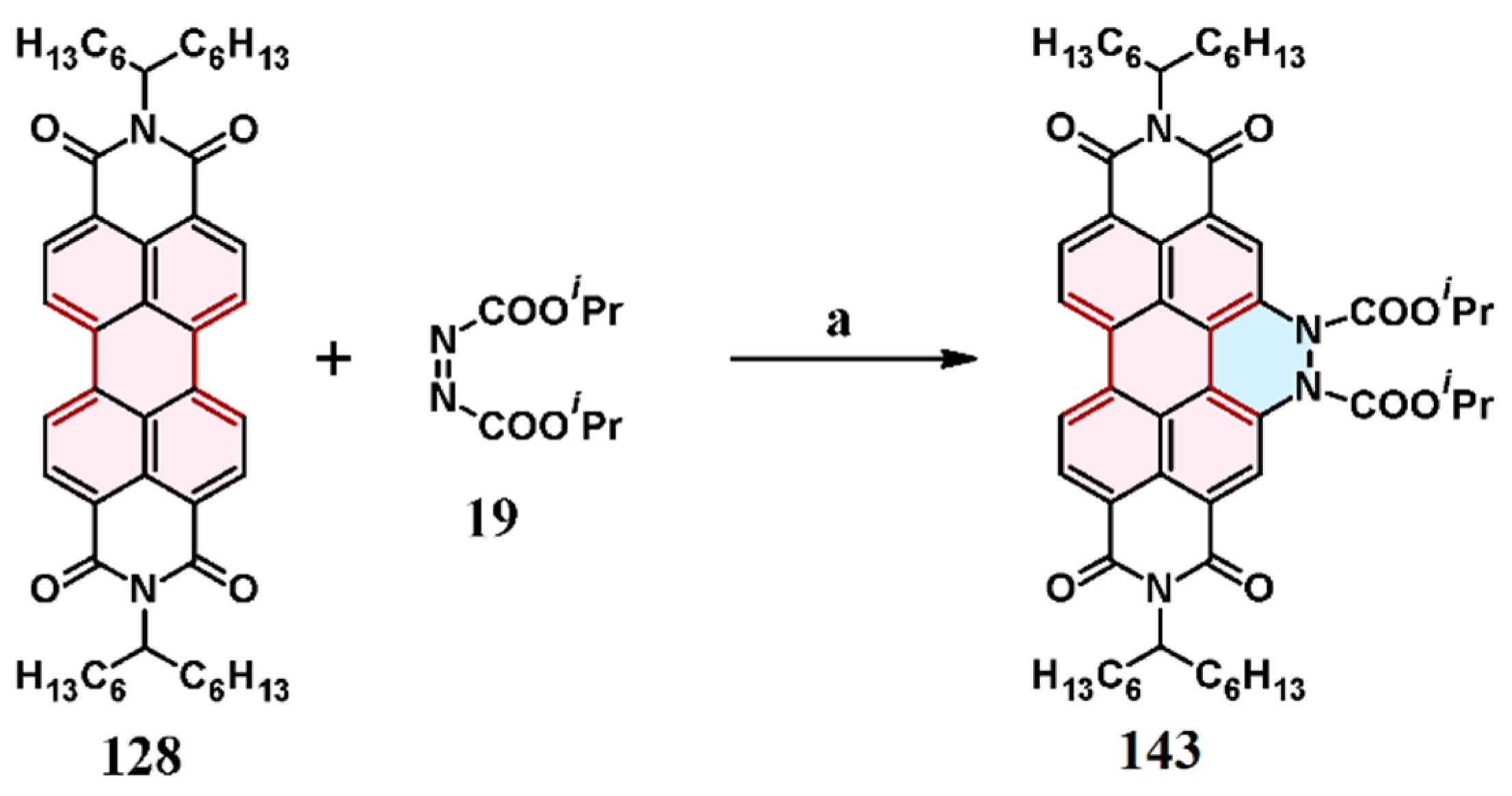
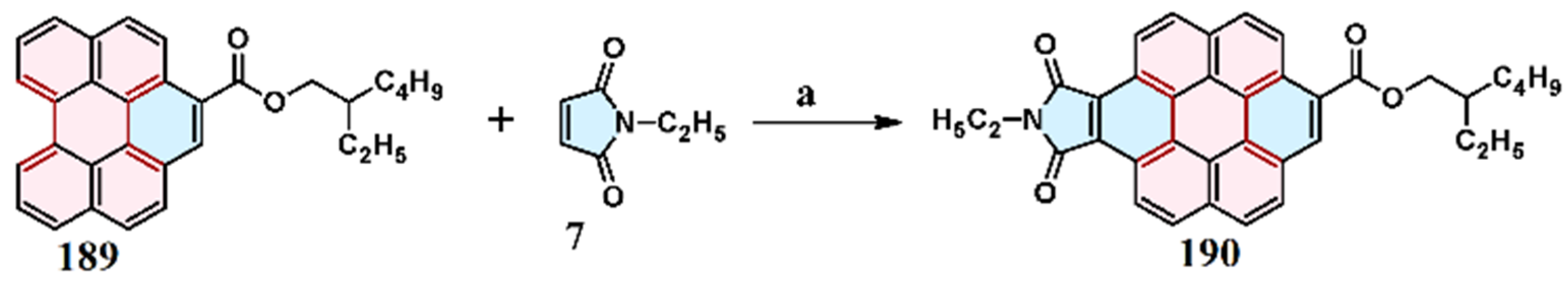
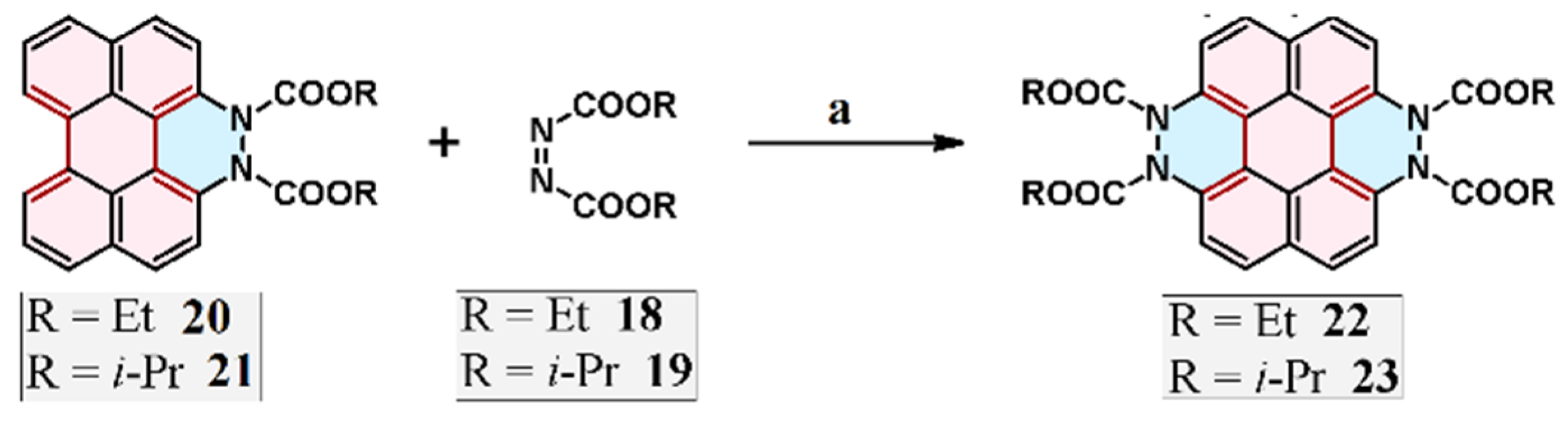
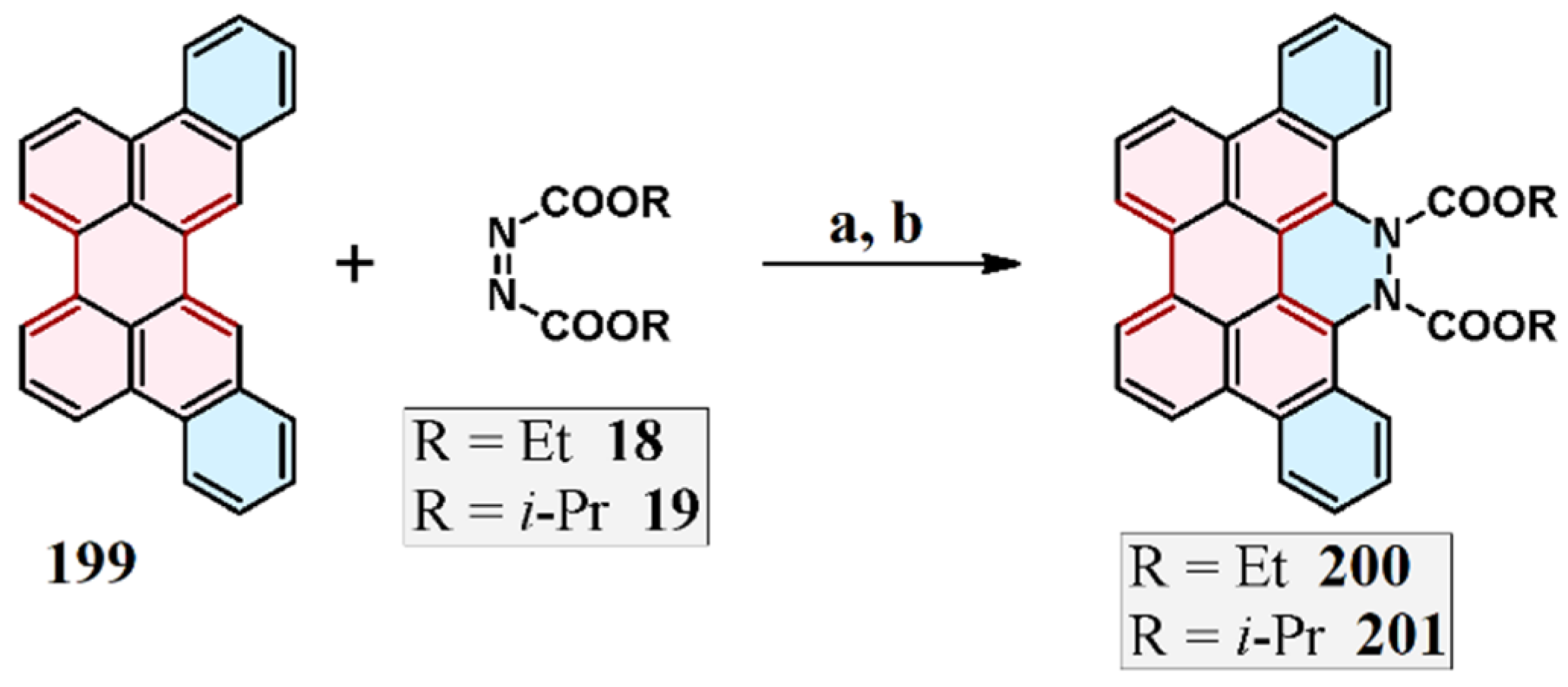
2.4. Cycloaddition of Dialkyl Diazenedicarboxylate
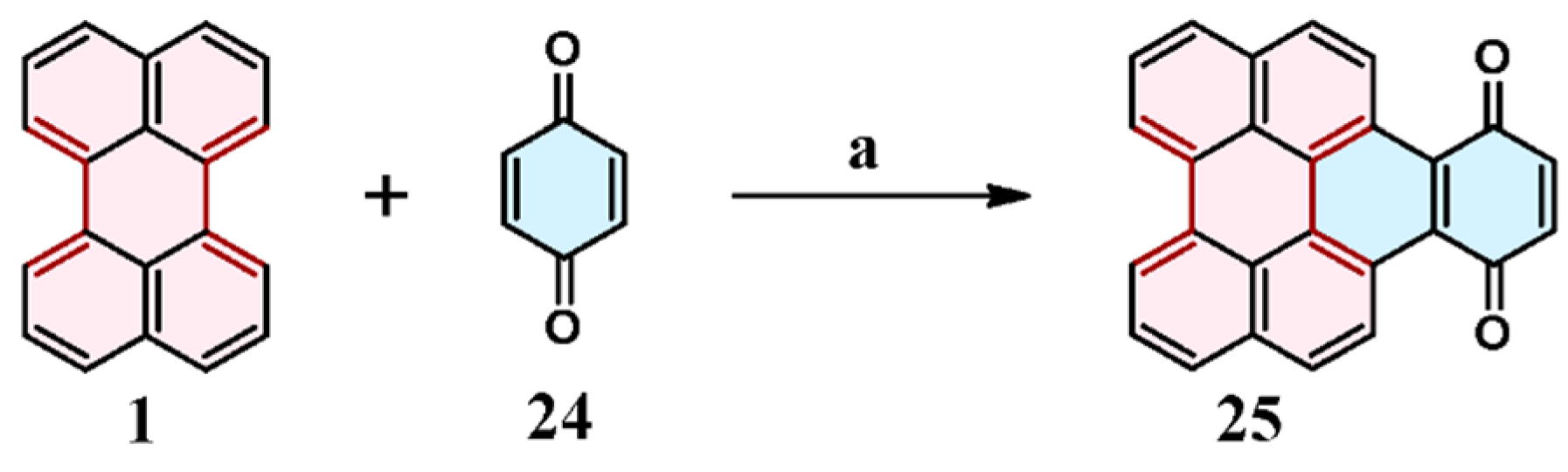
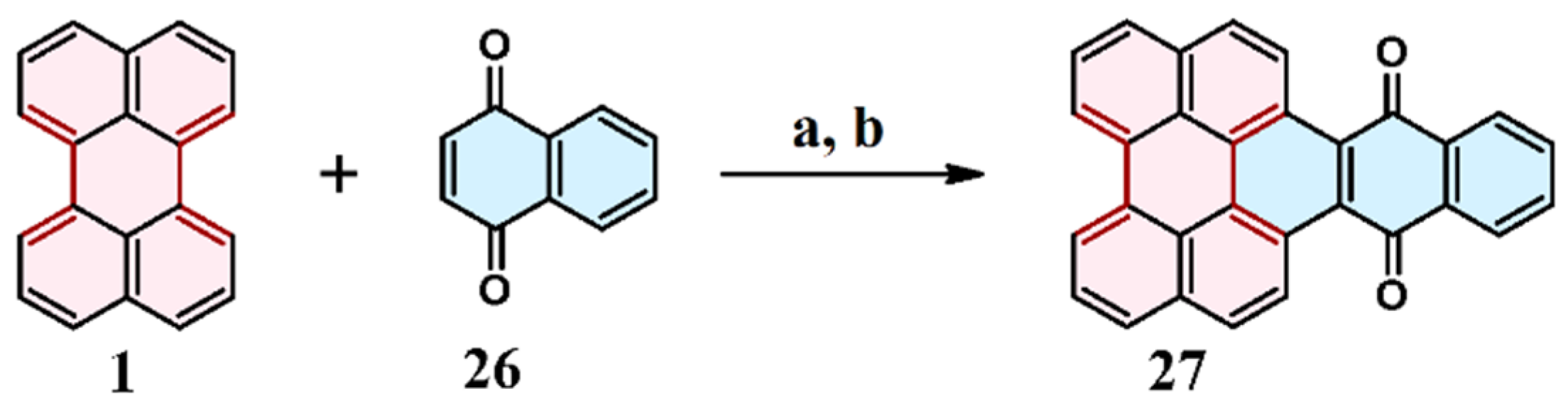
2.5. Cycloaddition of Benzo- and Naphthoquinones
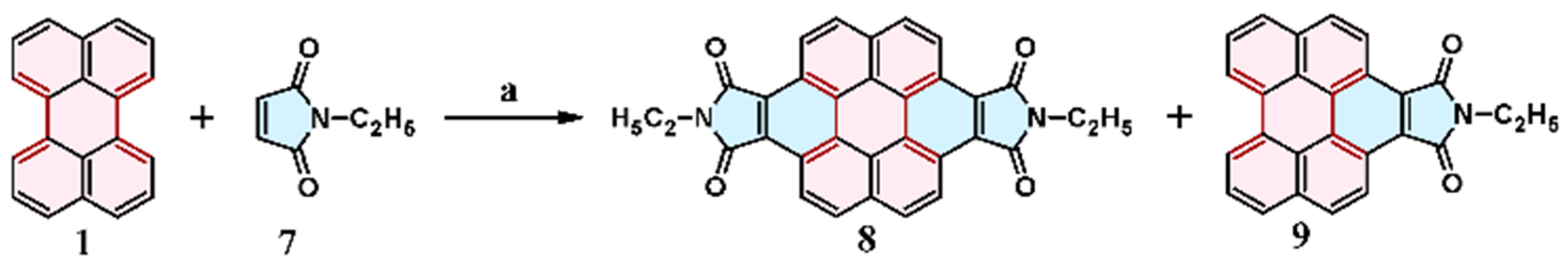
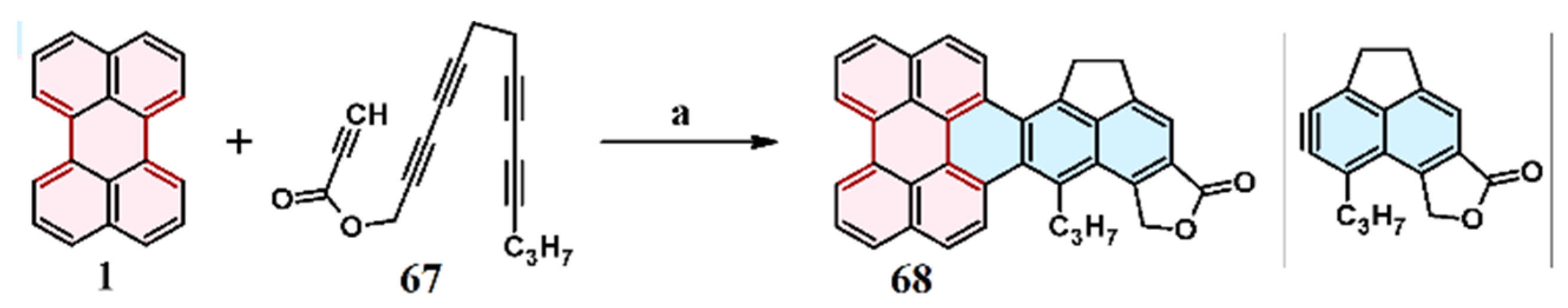
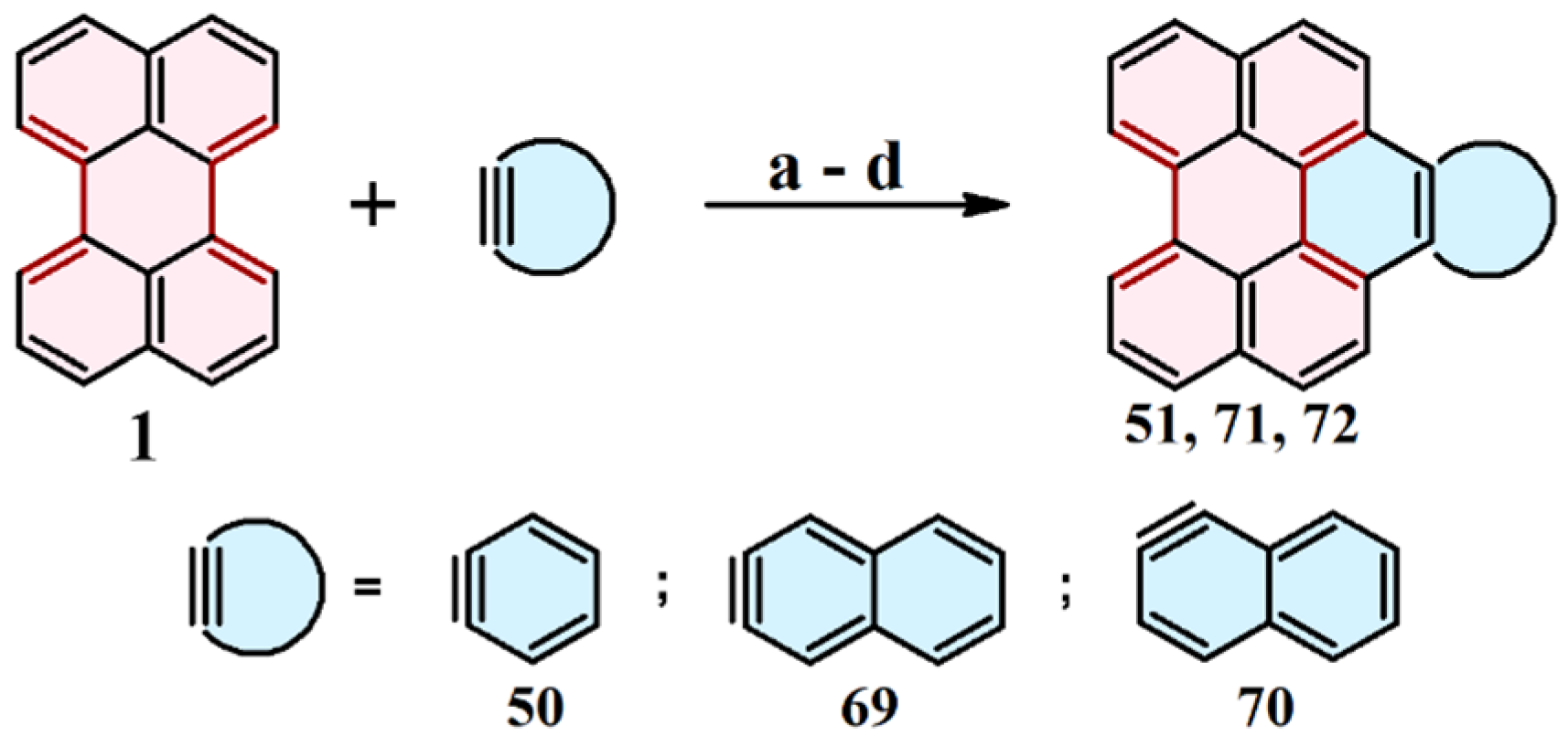
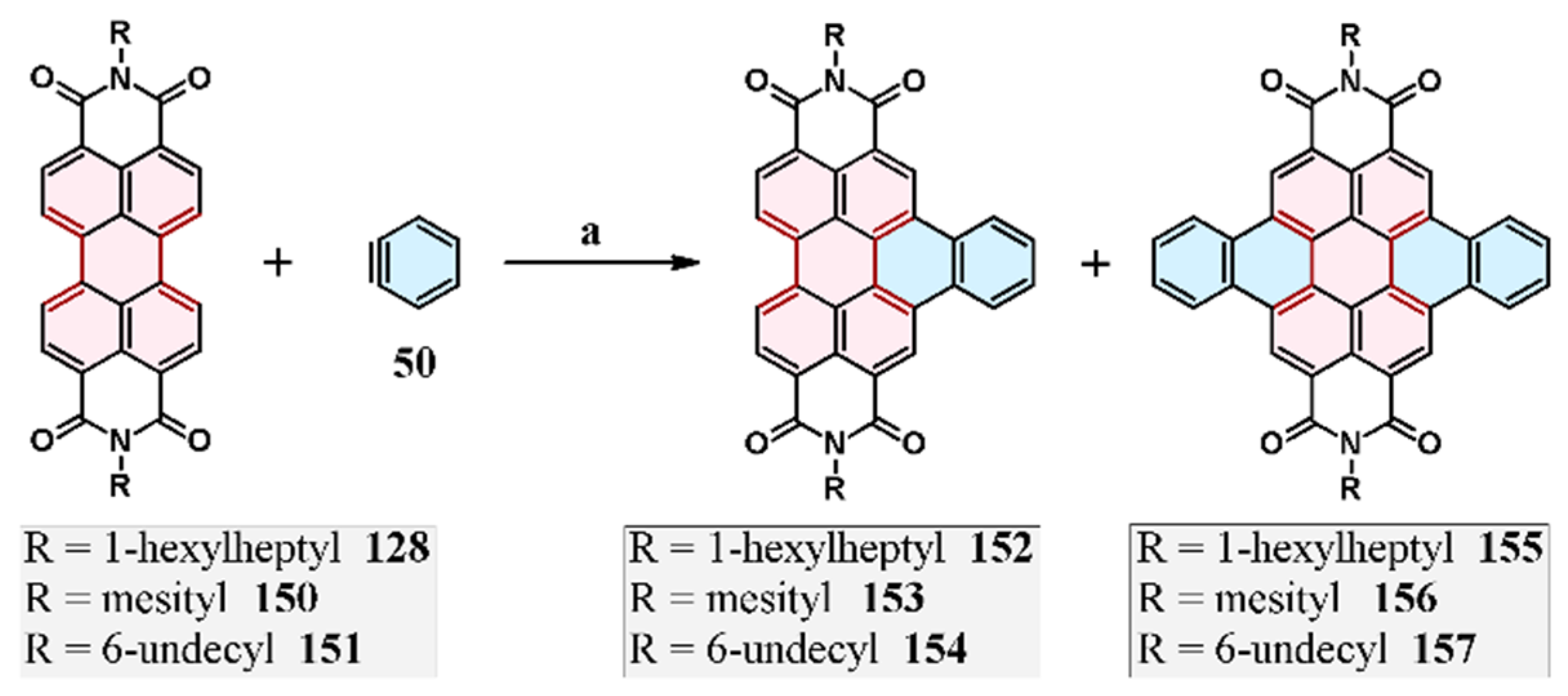
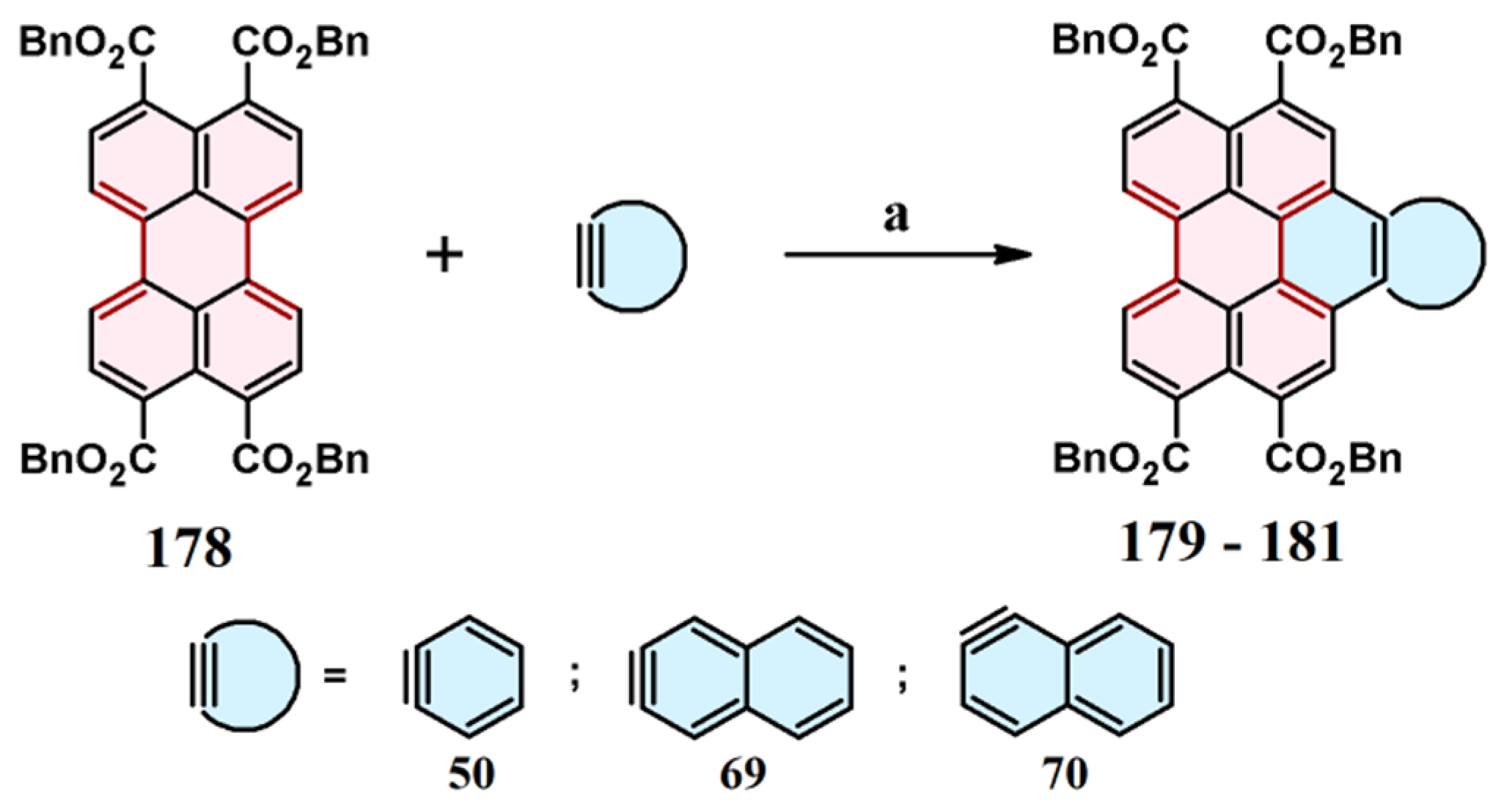
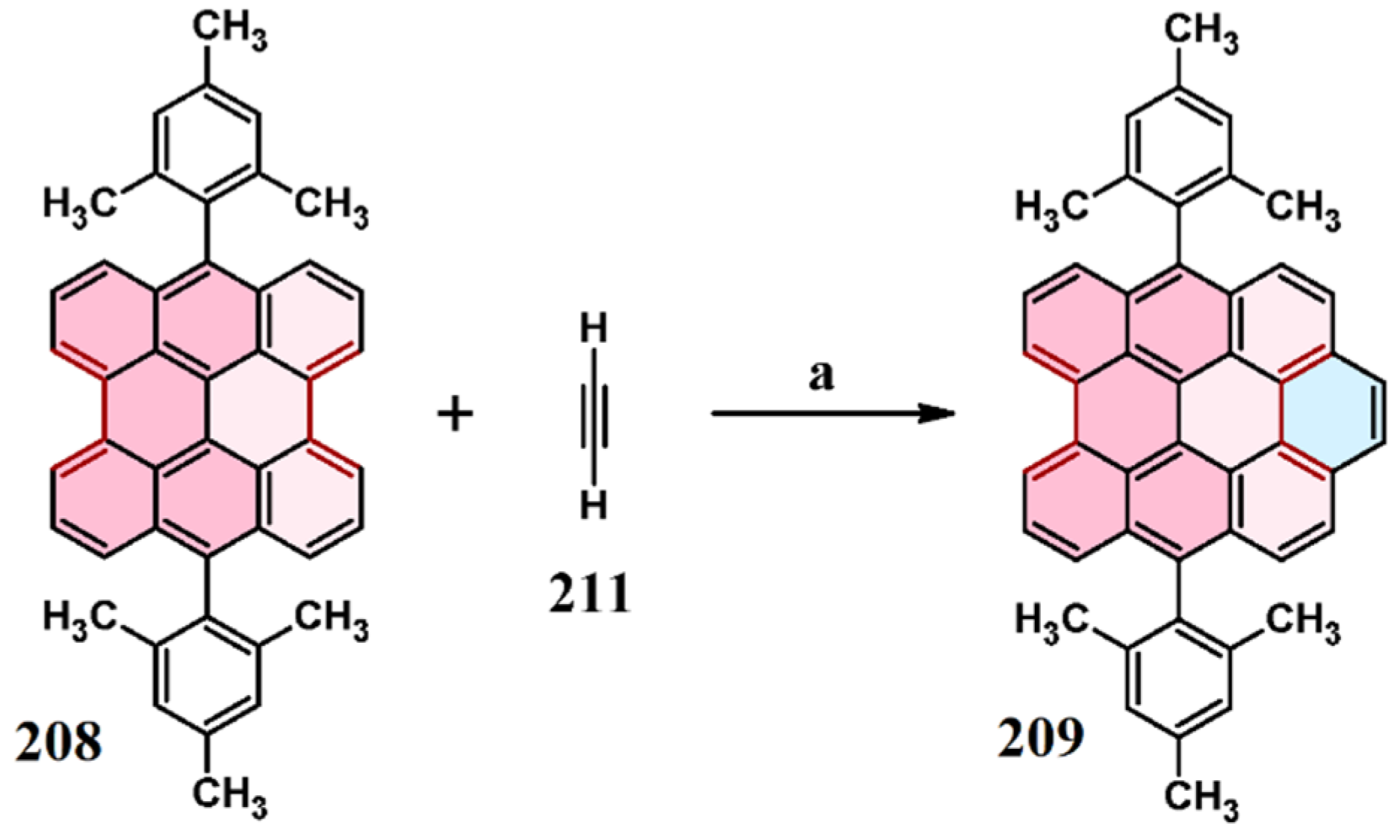
2.6. Cycloaddition of Acetylene
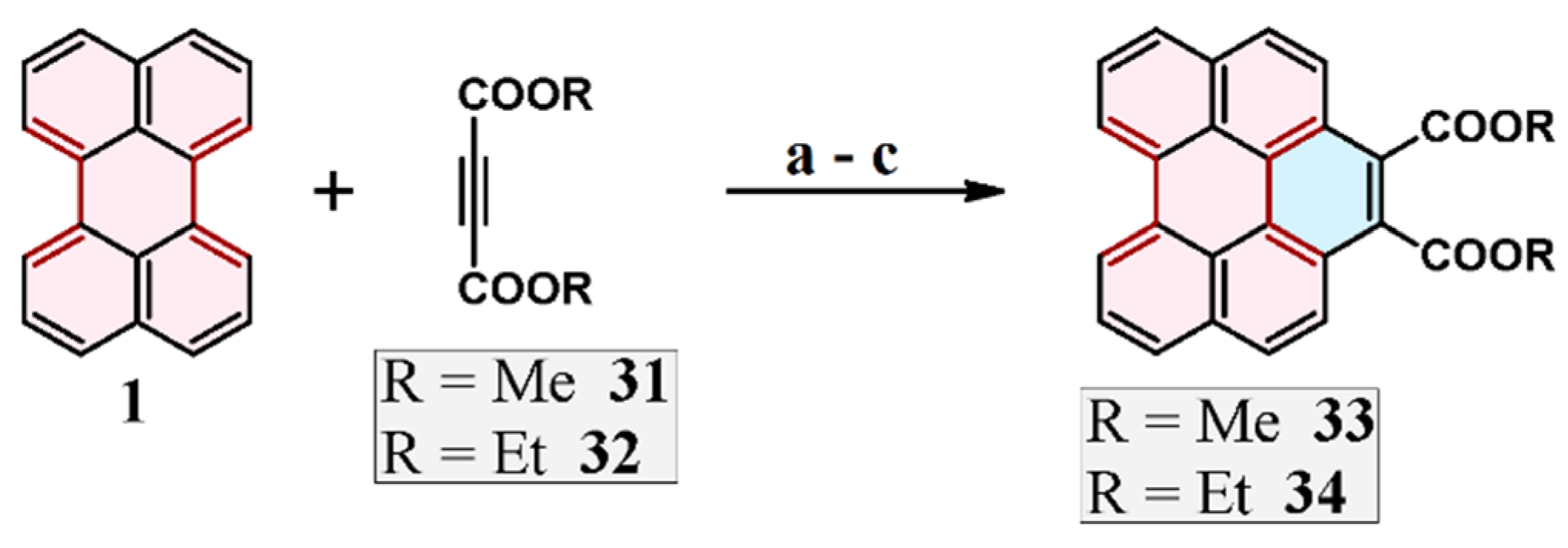
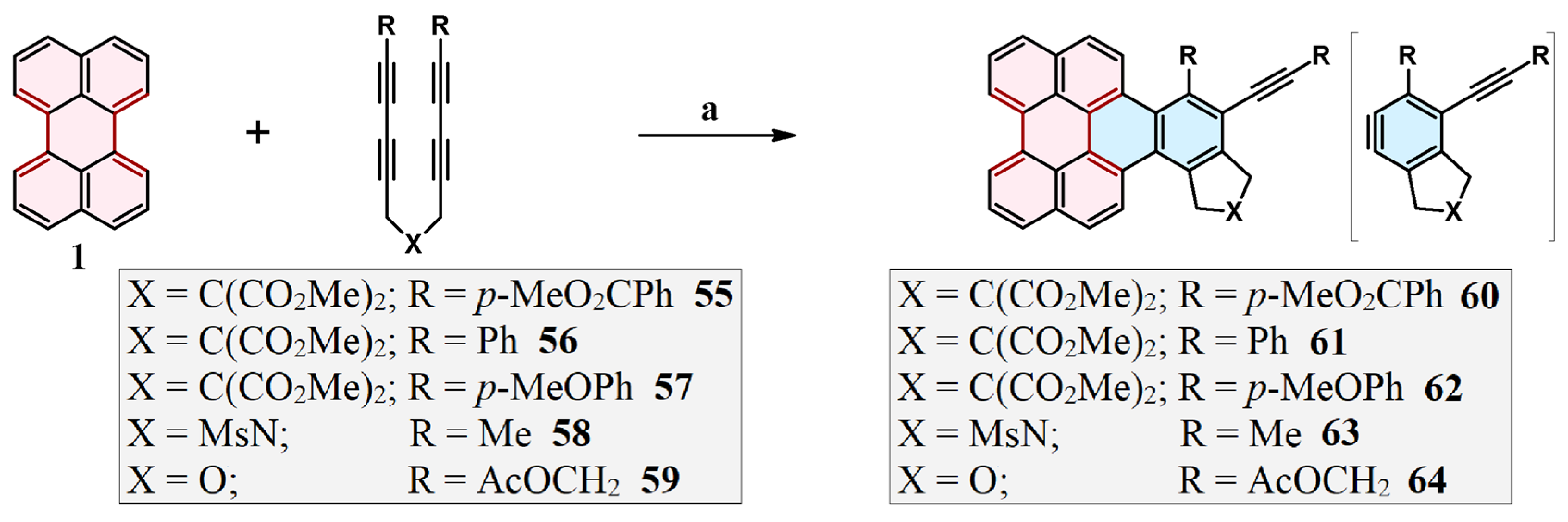
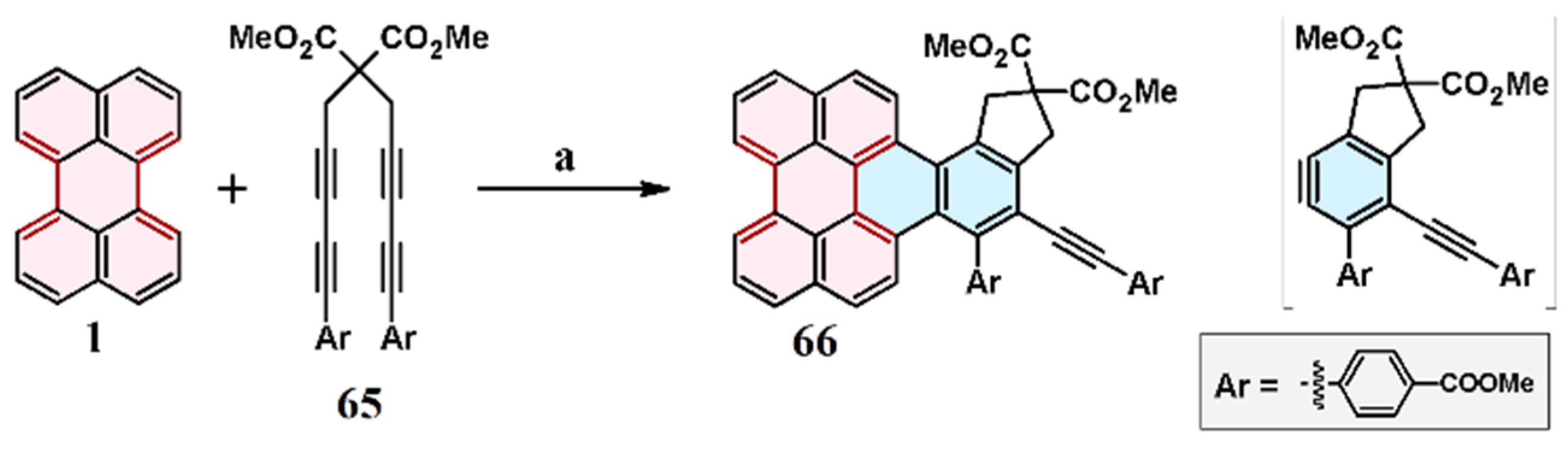
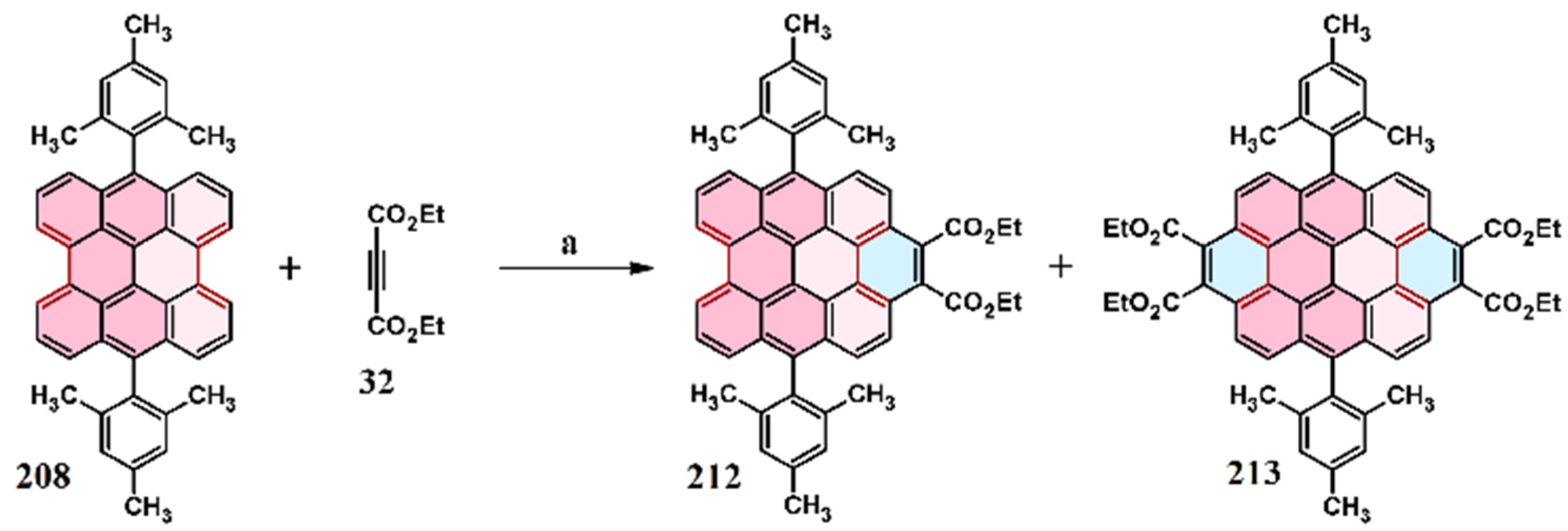
2.7. Cycloaddition of Acetylenedicarboxylates
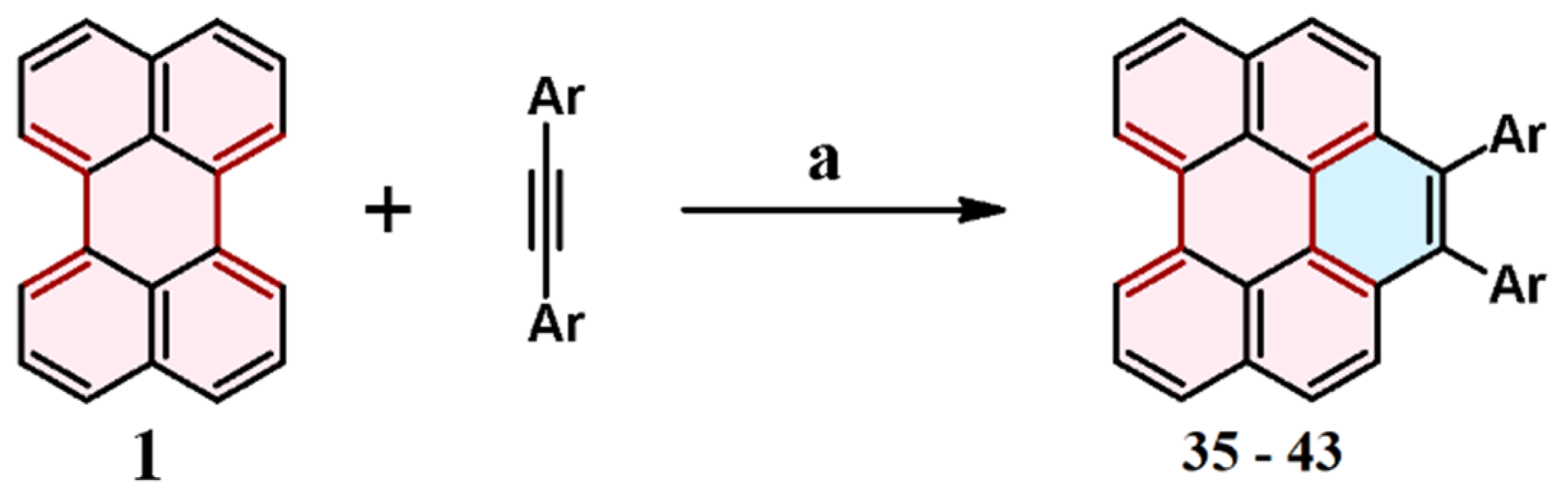
2.8. Cycloaddition of Diarylacetylenes
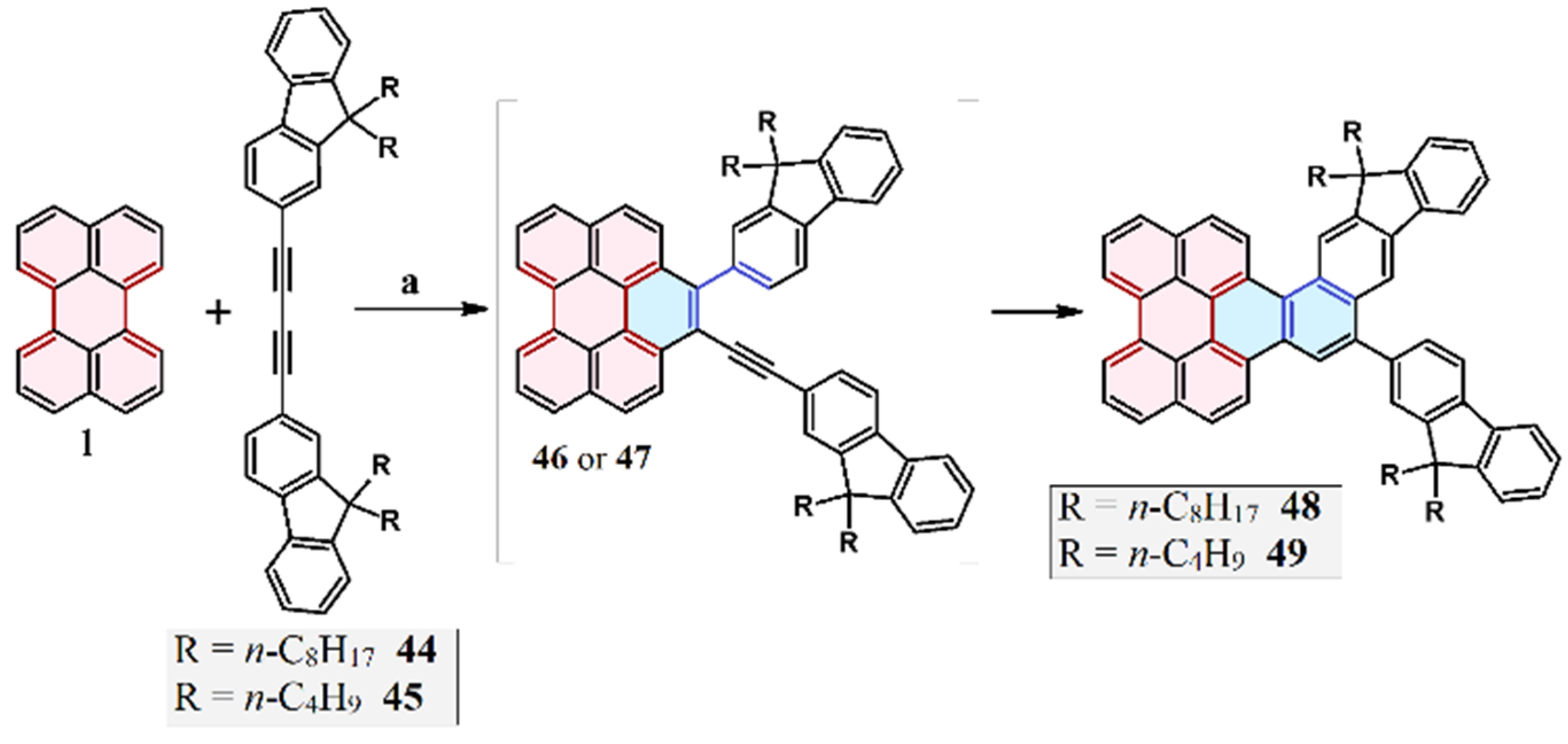
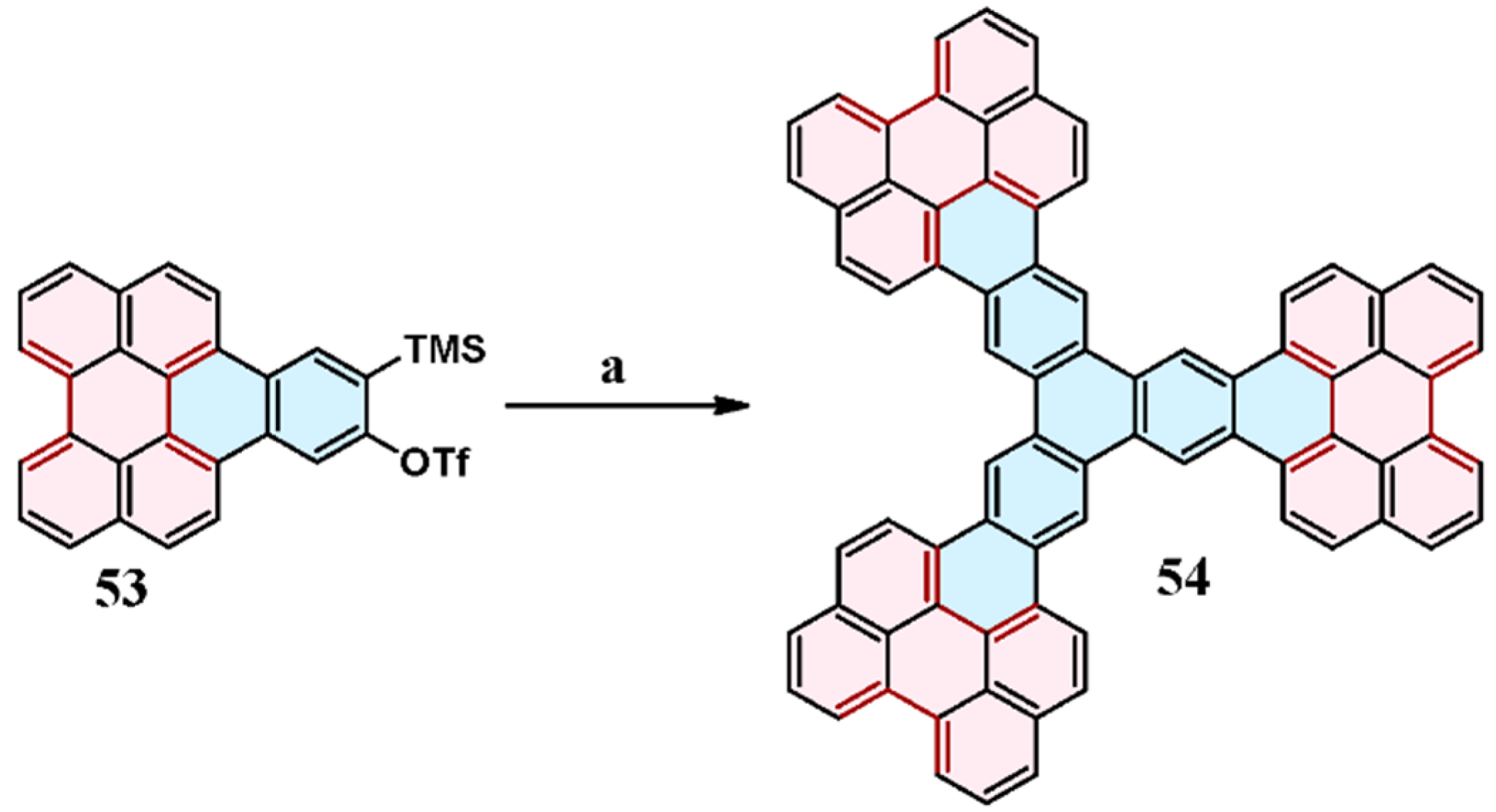
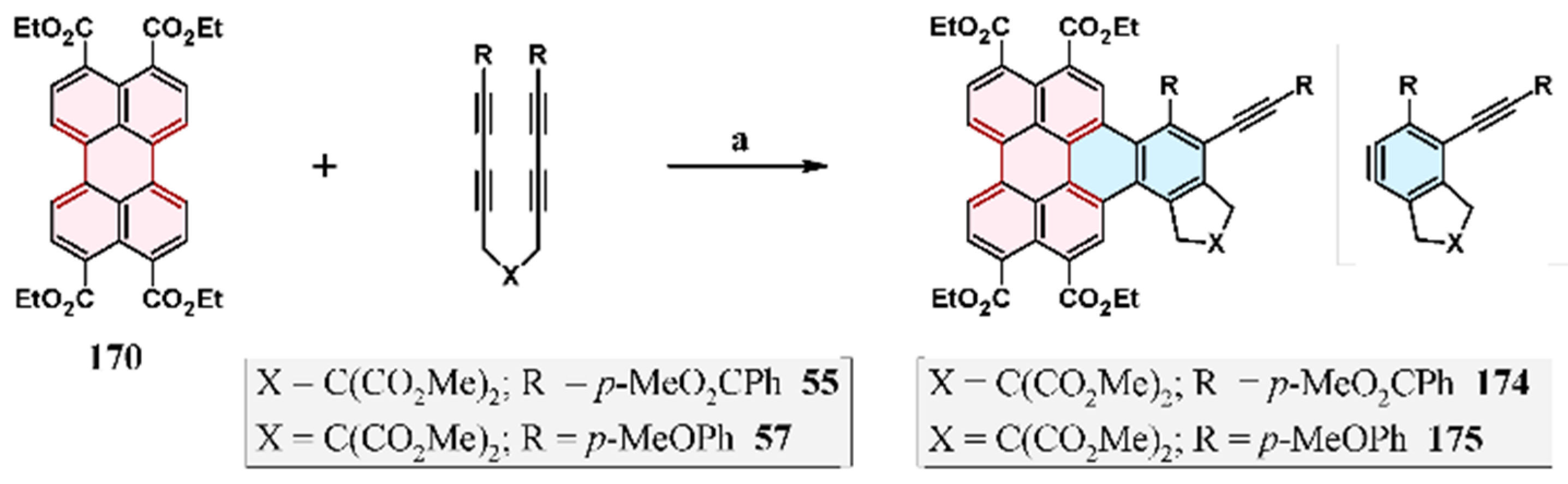
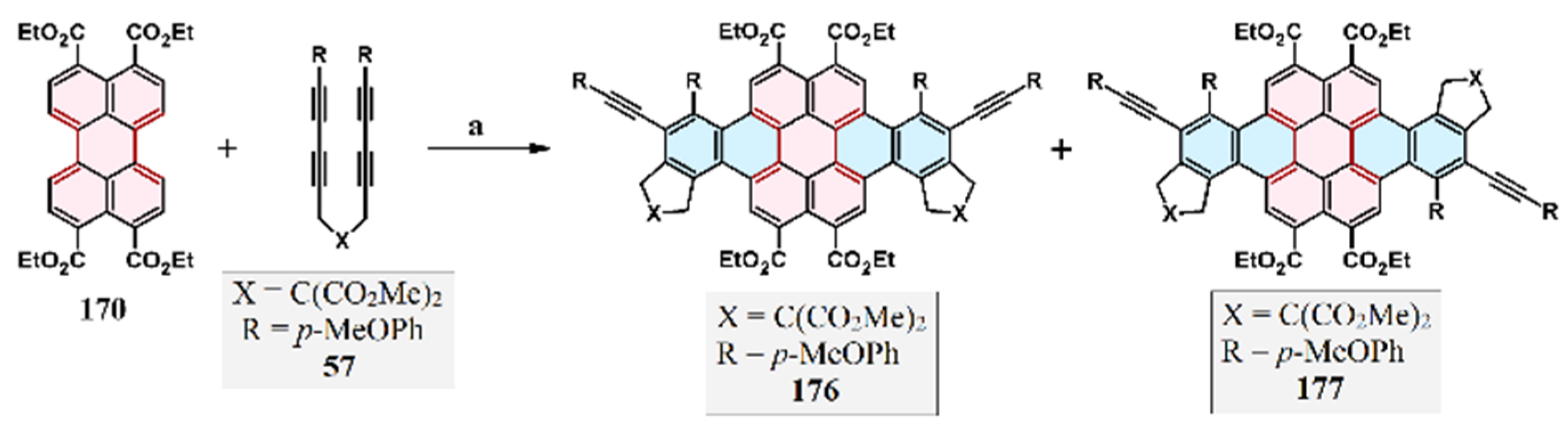
2.9. Reaction of 1,4-Diaryl-1,3-Butadiynes with Perylene: Domino-Type Cycloaddition–Cycloaromatization
2.10. Cycloaddition of Benzyne and Naphthynes
3. Cycloaddition to Alkyl Perylenes
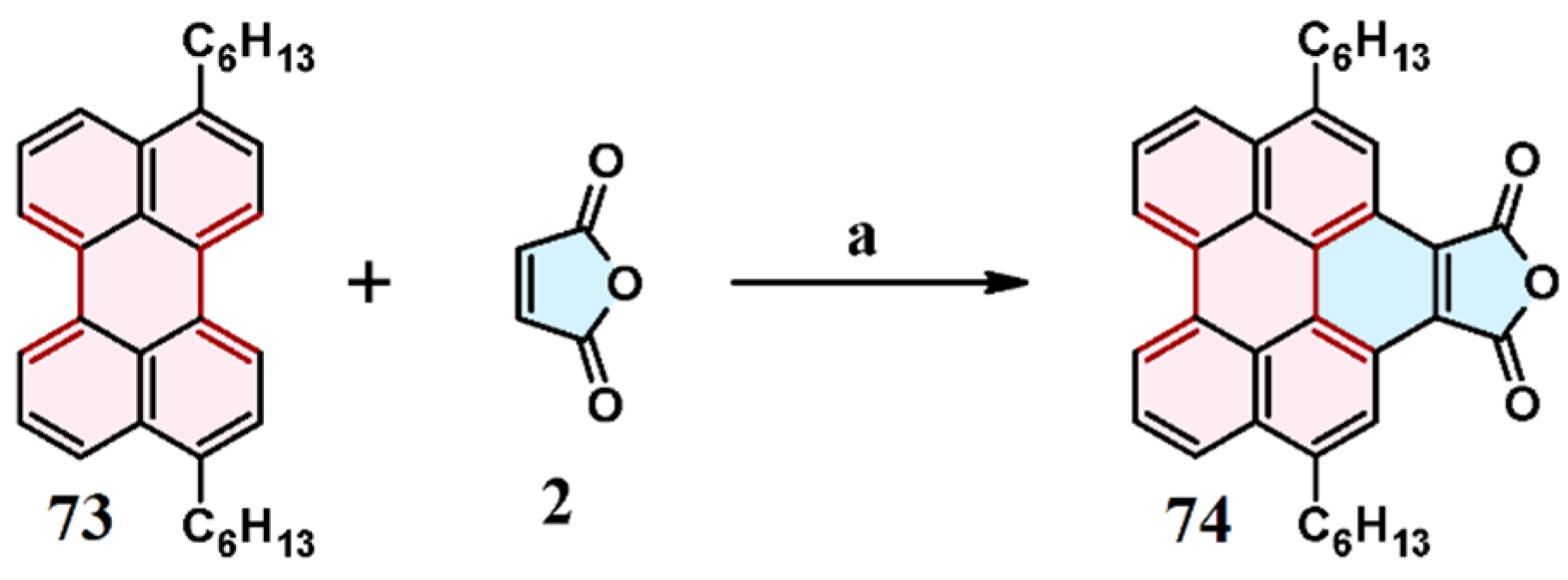
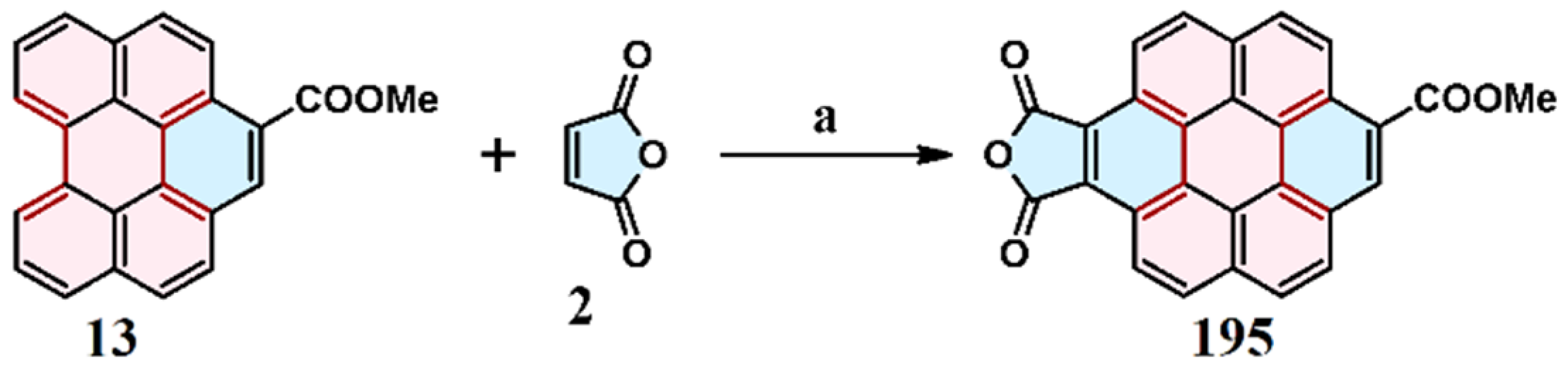
3.1. Cycloaddition of Maleic Anhydride
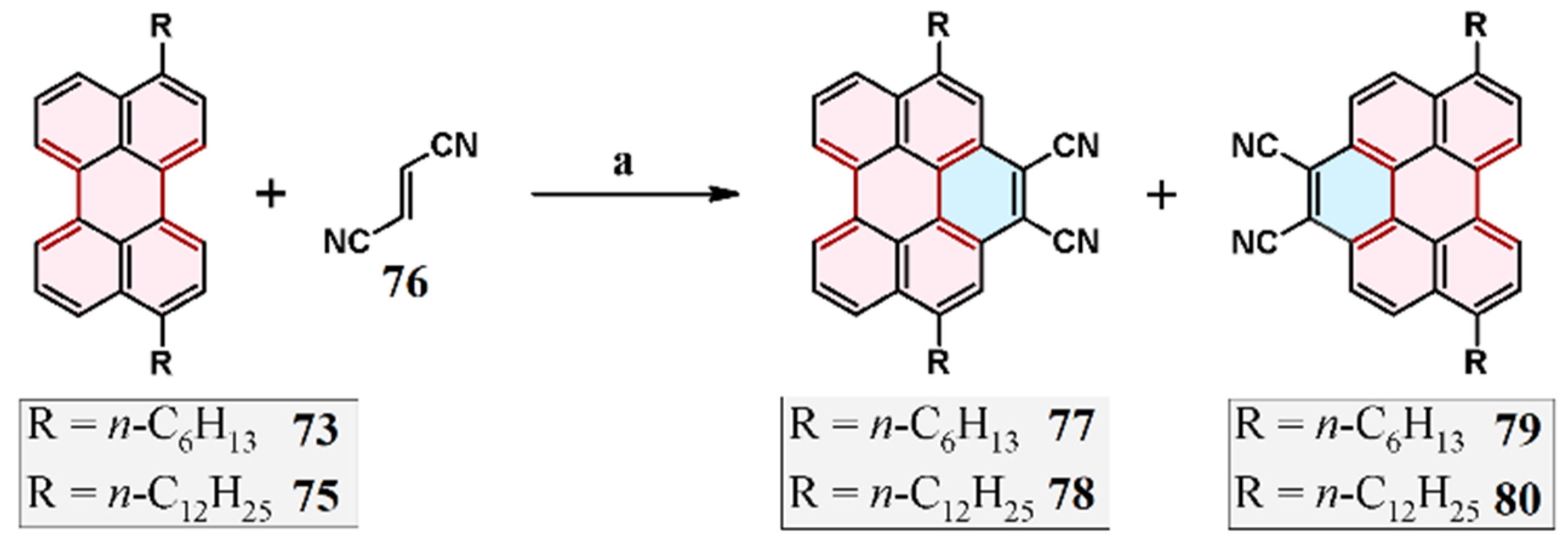
3.2. Cycloaddition of Fumarodinitrile to 3,10-di-(N-Hexyl)- and 3,10-di-(N-Dodecyl)Perylene
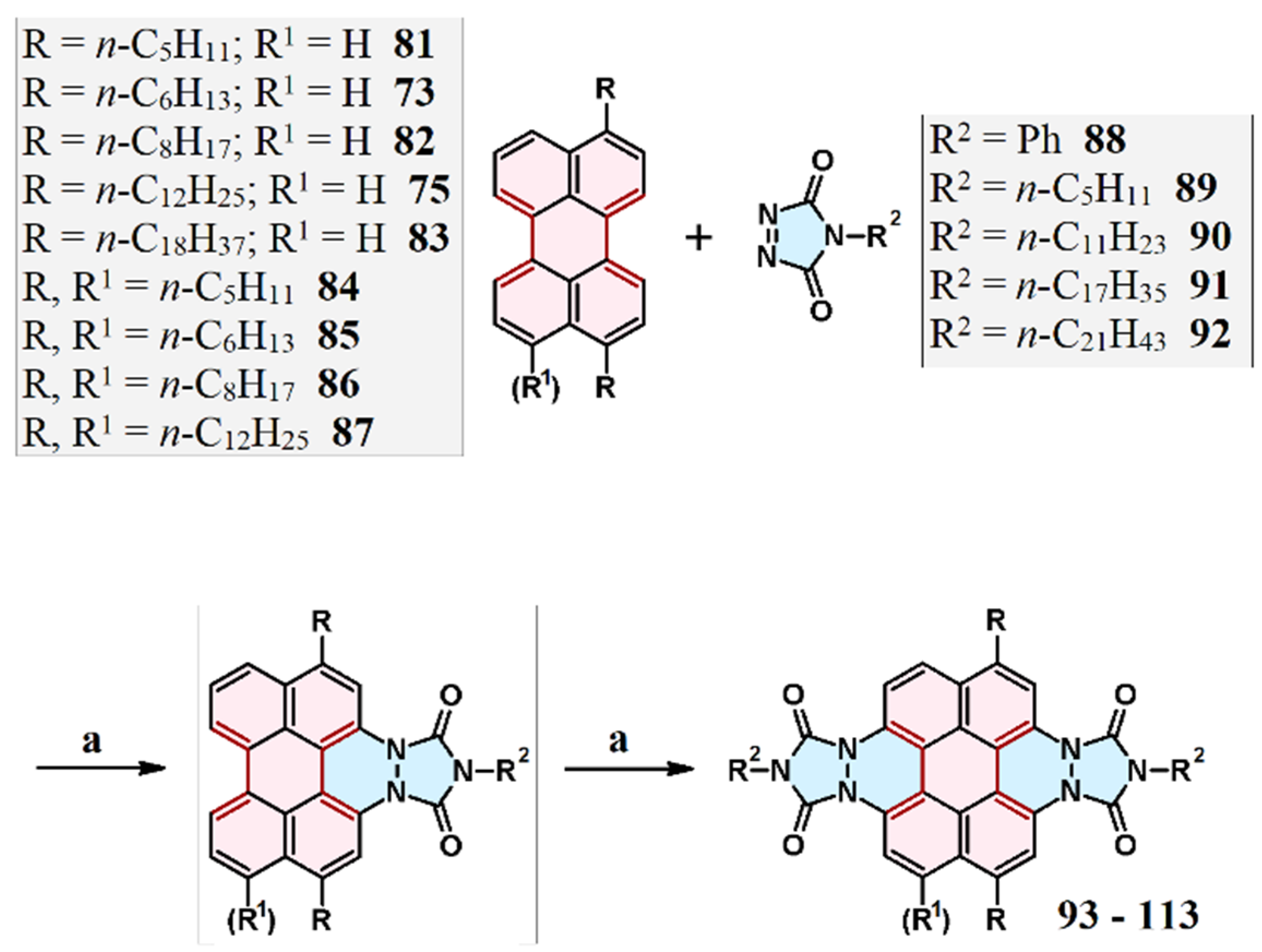
3.3. Cycloaddition of Triazolinedione
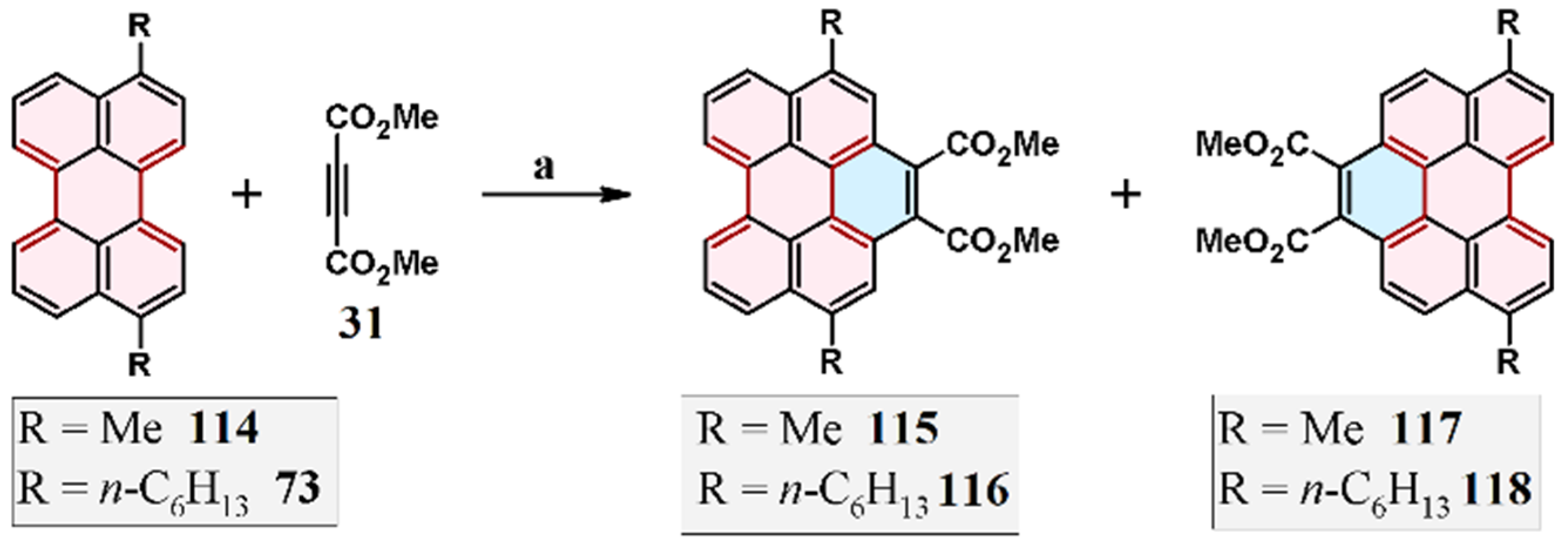
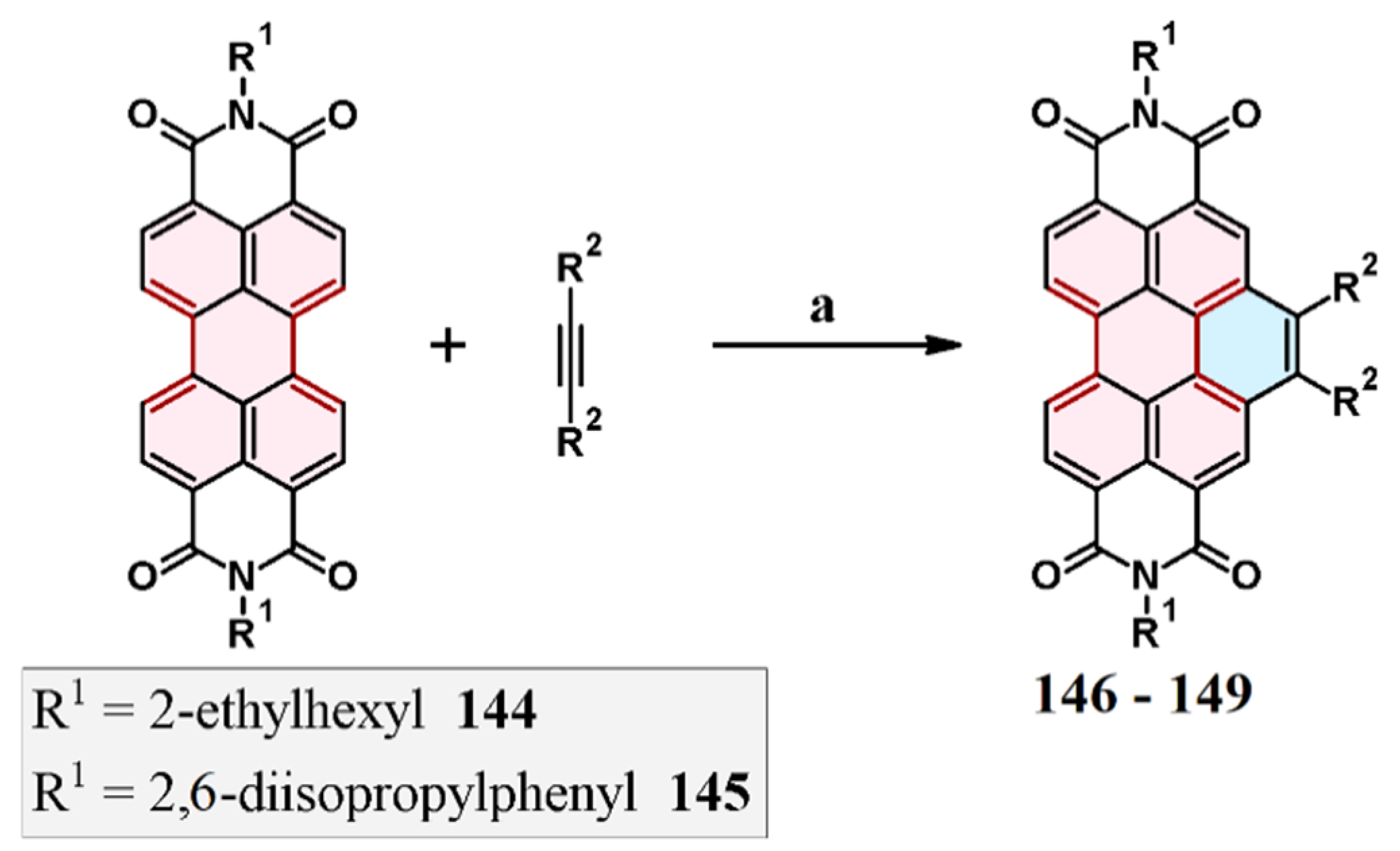
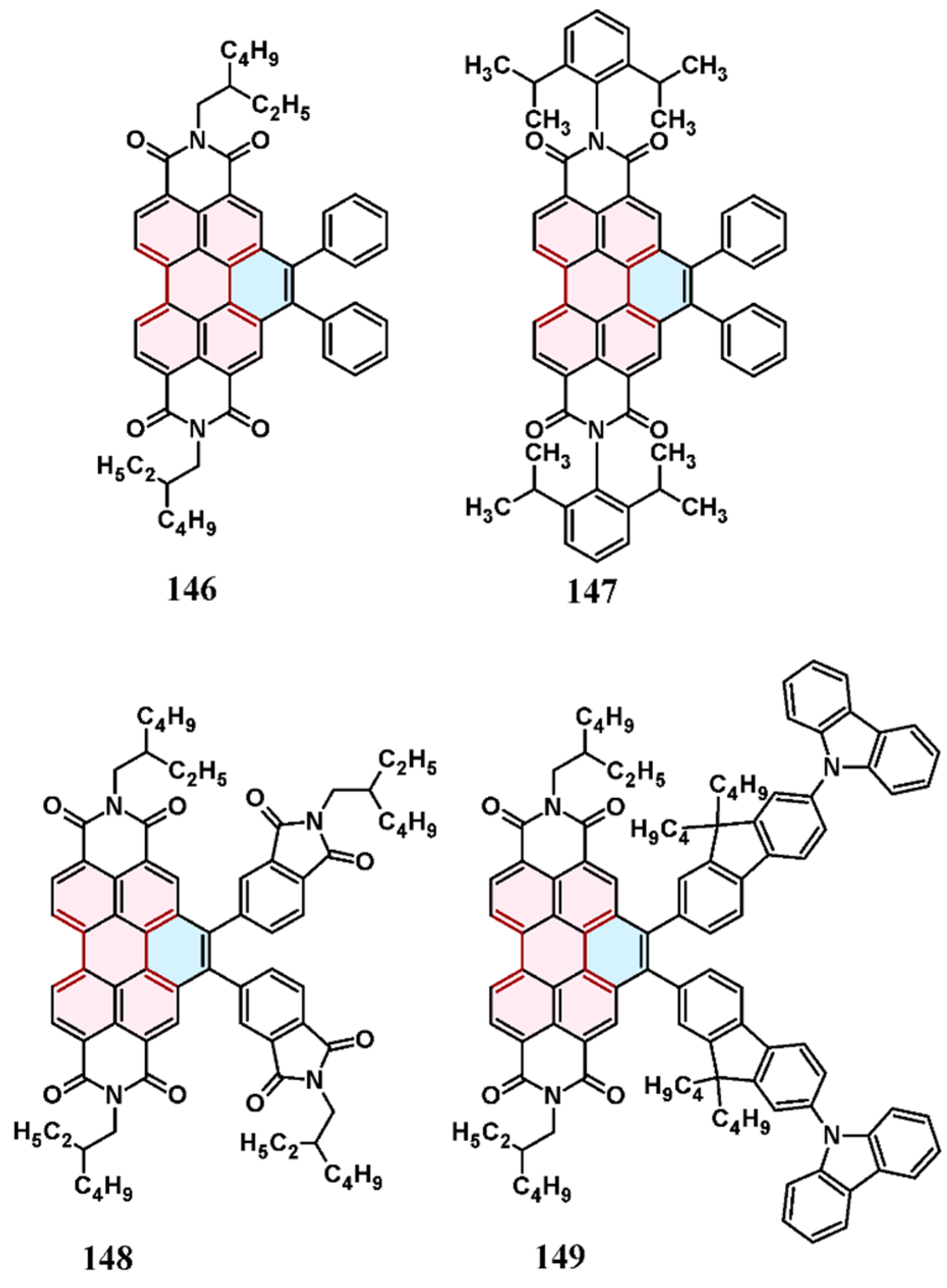
3.4. Cycloaddition of Dialkyl Acetylenedicarboxylates
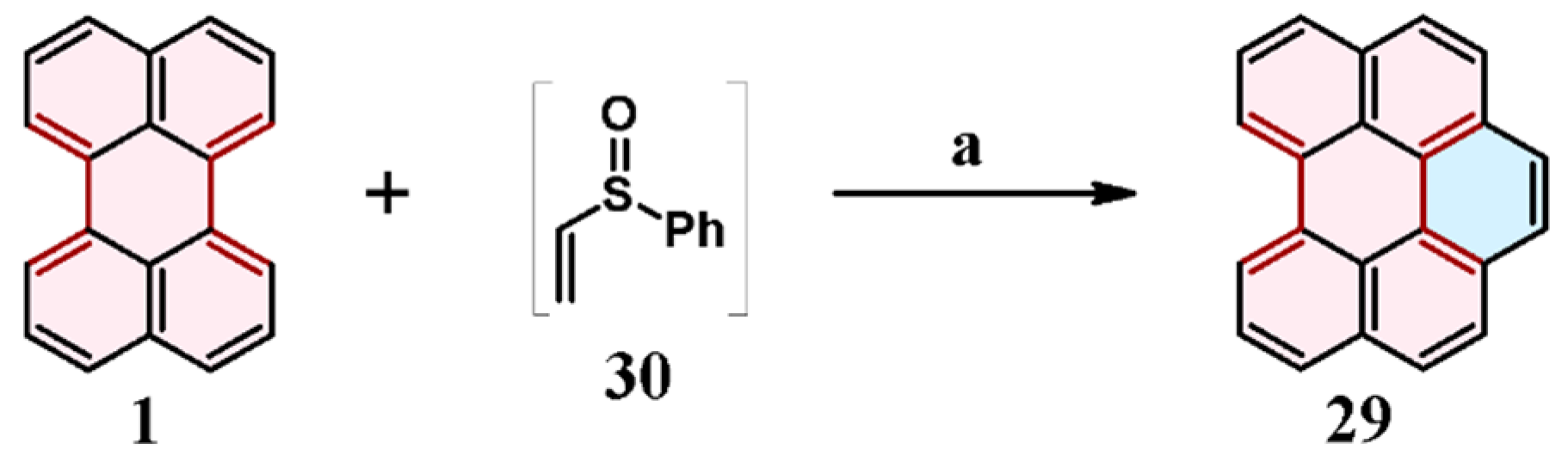
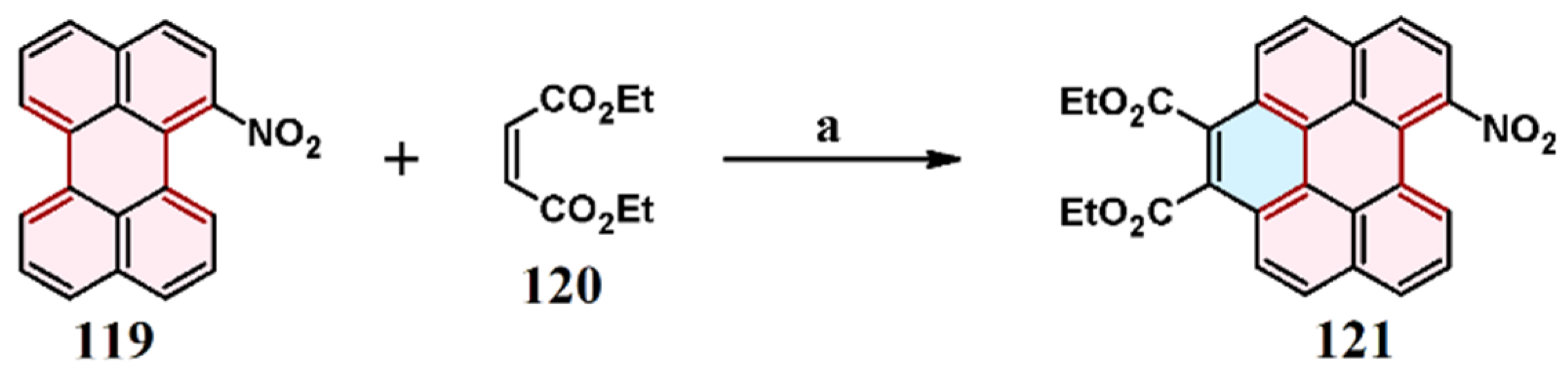
4. Cycloaddition to Nitroperylene
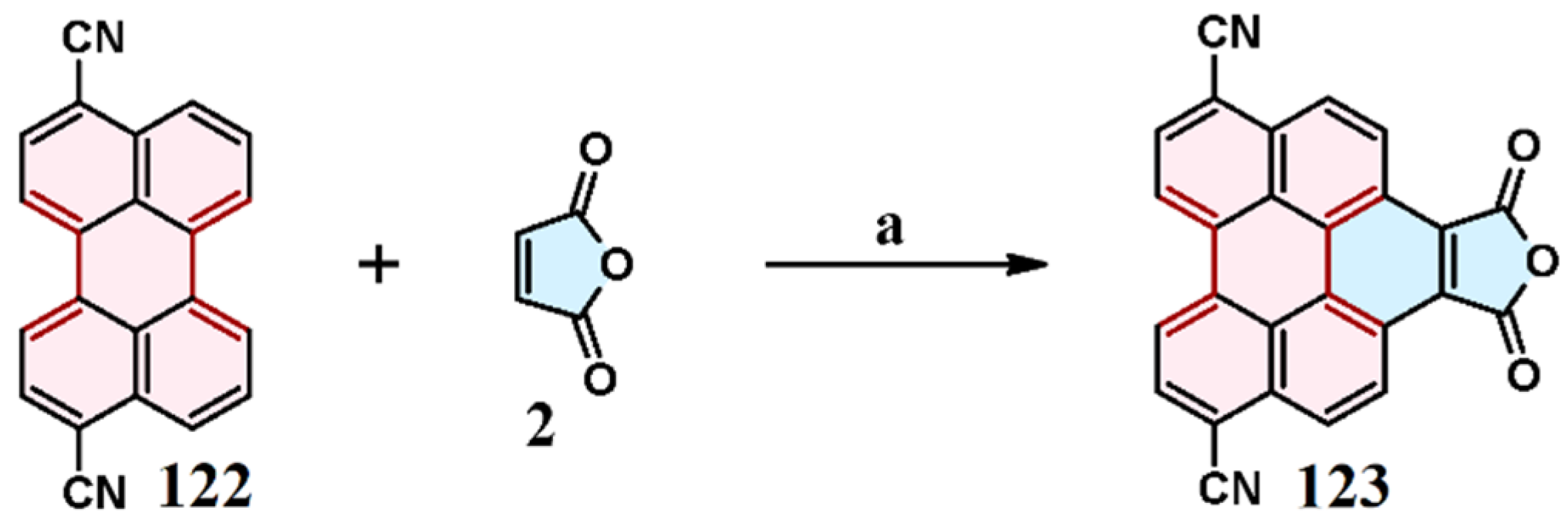
5. Cycloaddition to Dicyanoperylene
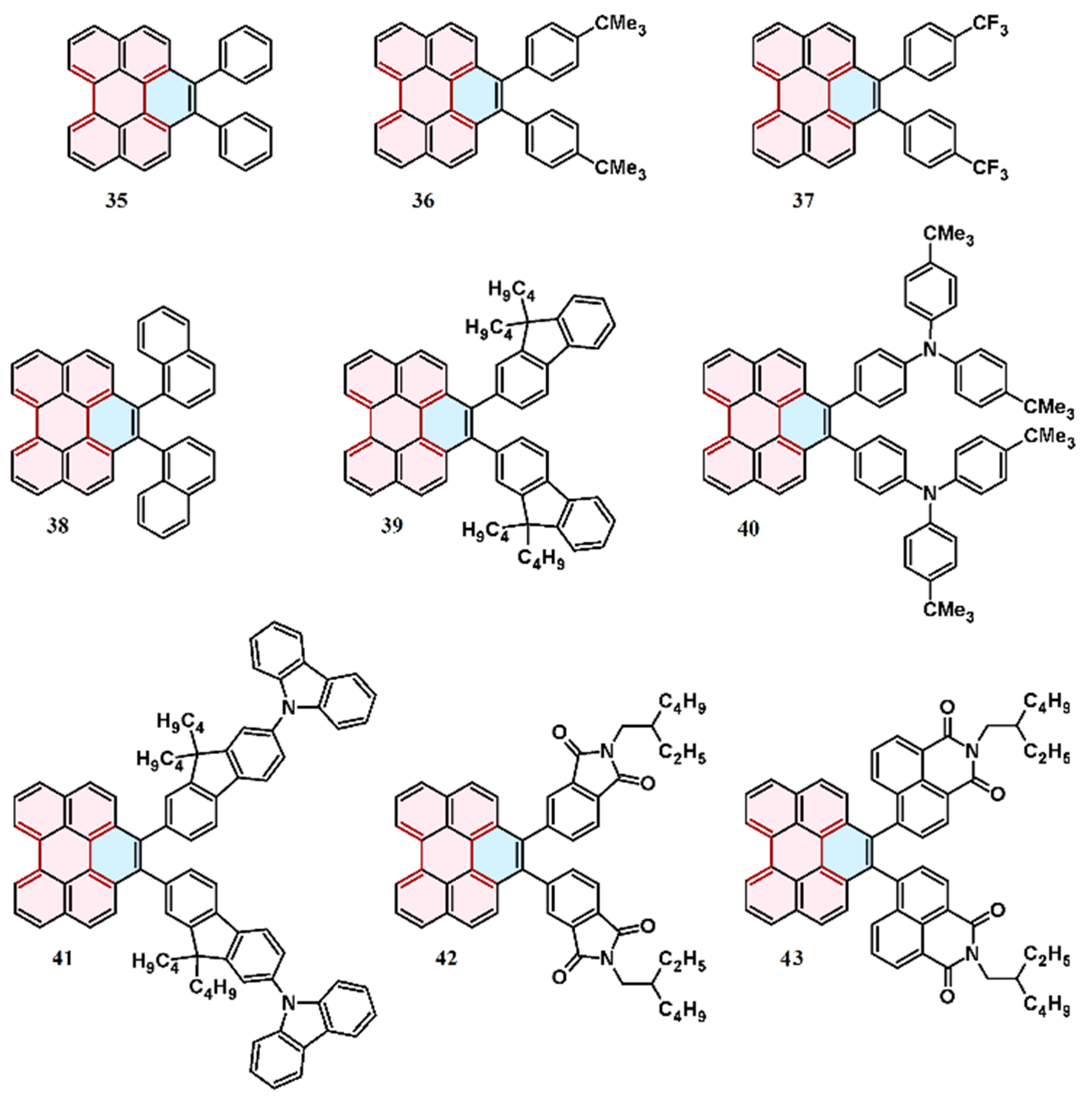
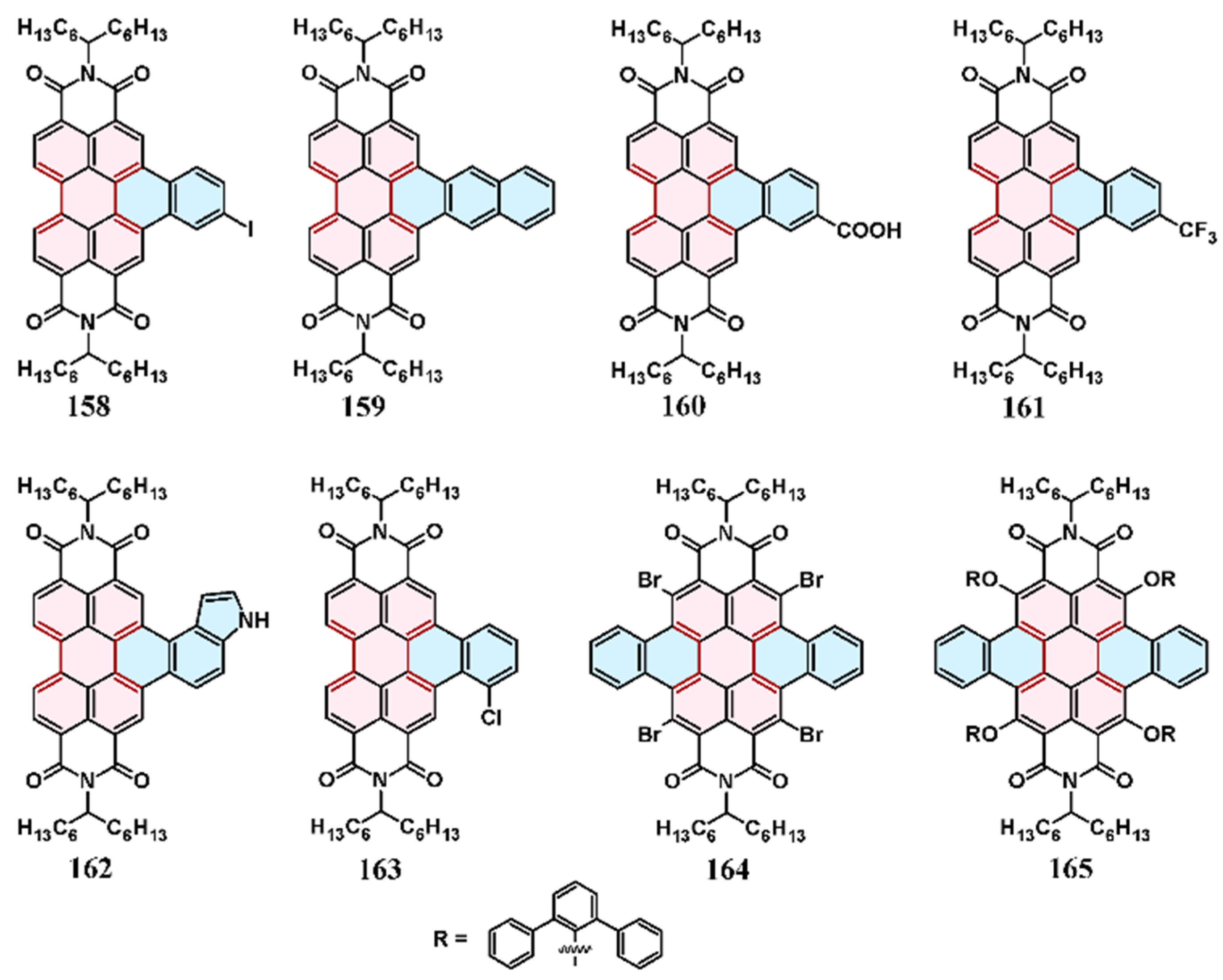
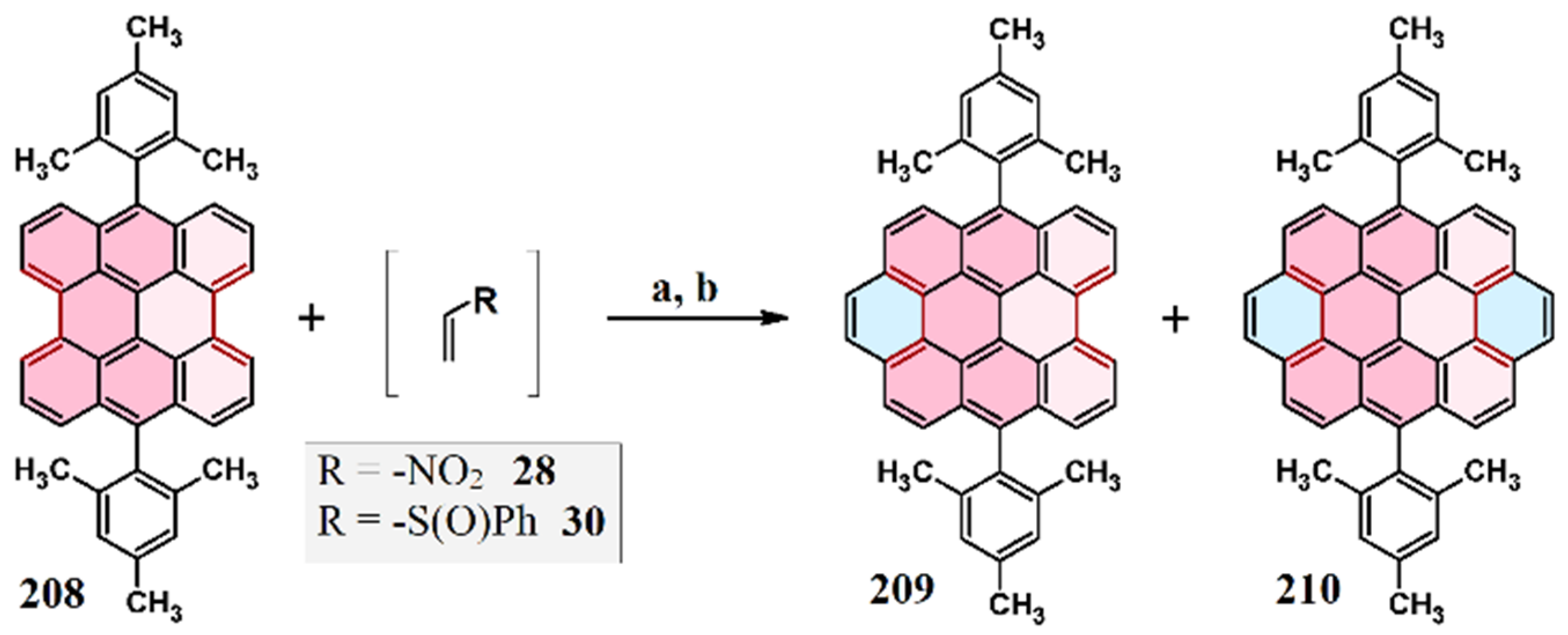
6. Cycloaddition to Aryl(diaryl)perylenes
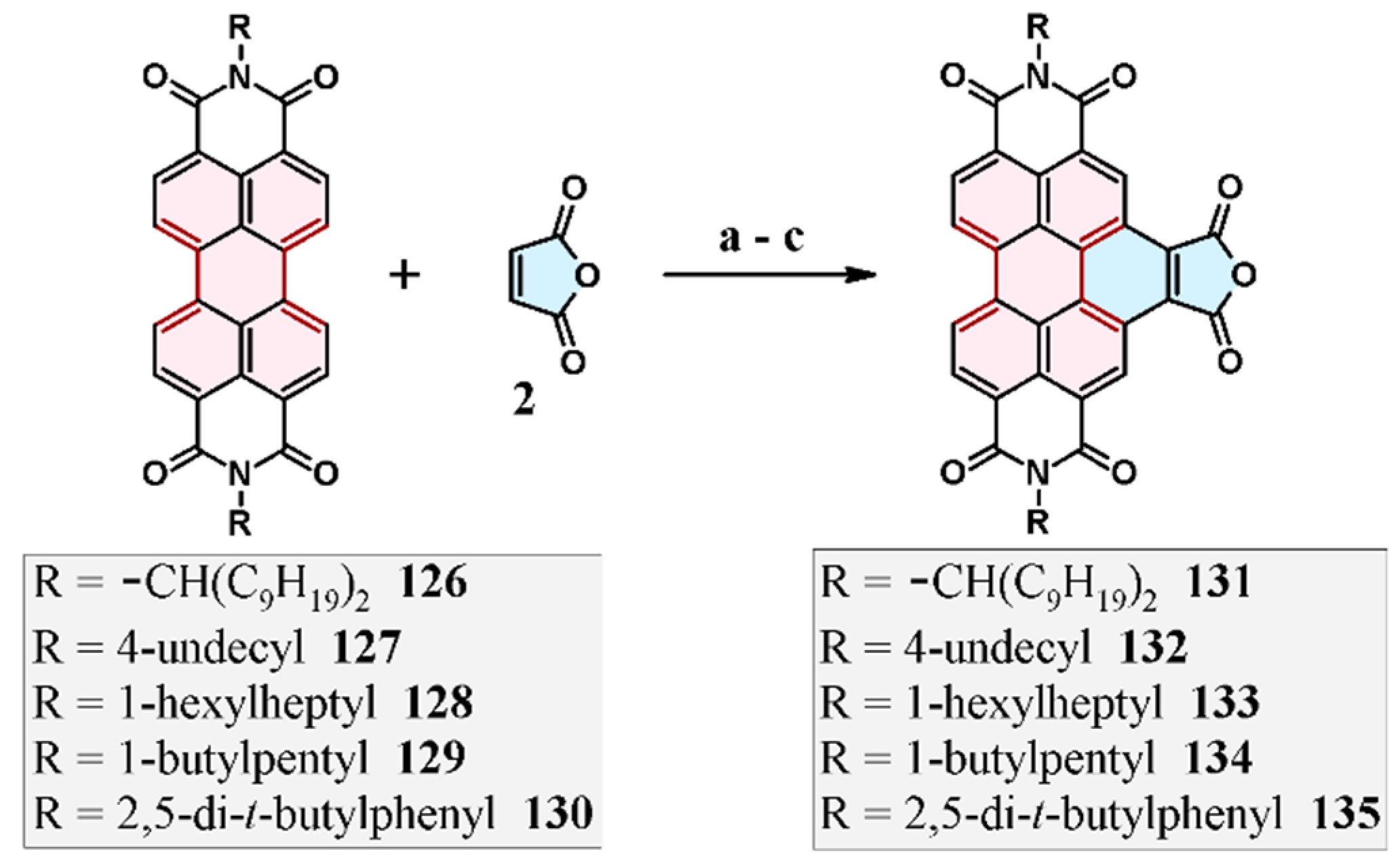
7. Cycloaddition to Perylene Bisimides and Their Derivatives
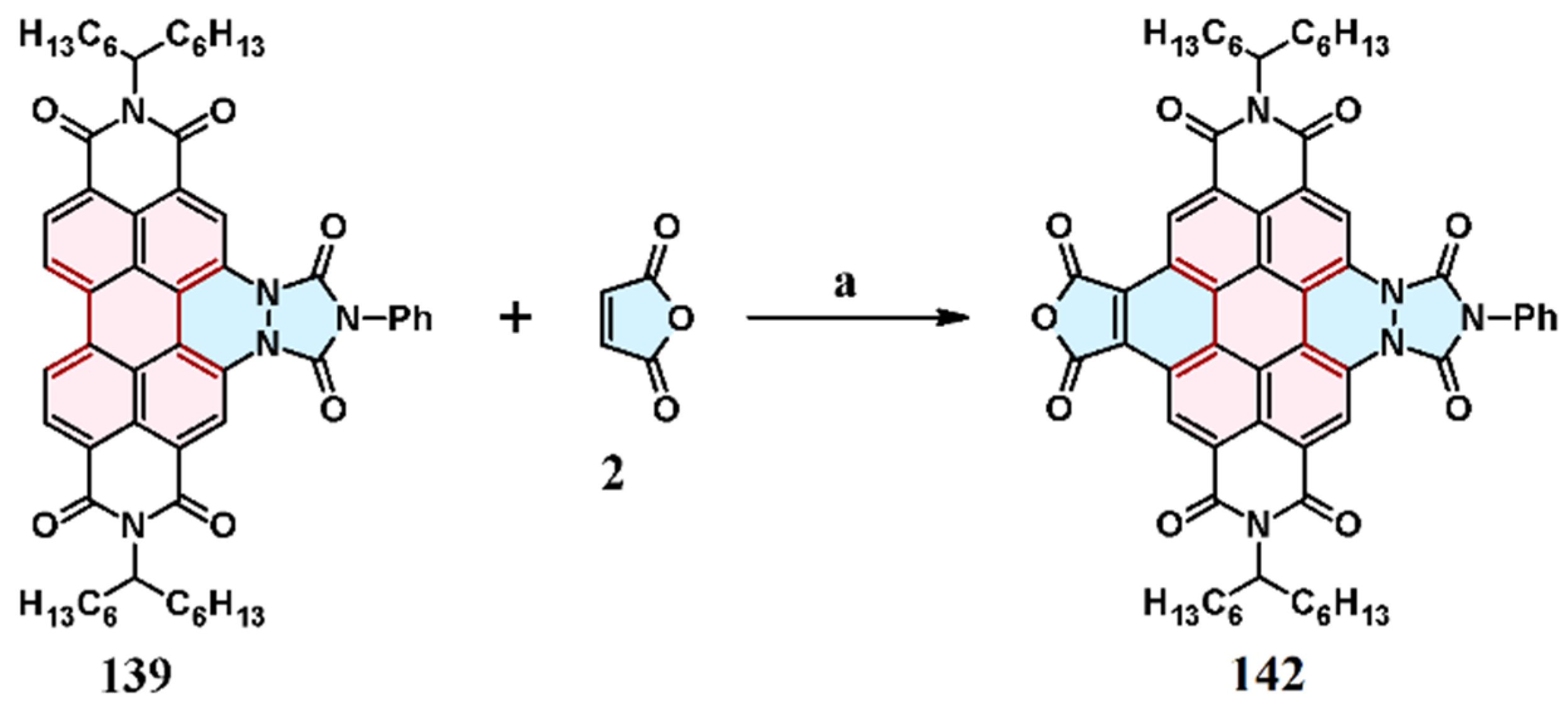
7.1. Cycloaddition of Maleic Anhydride
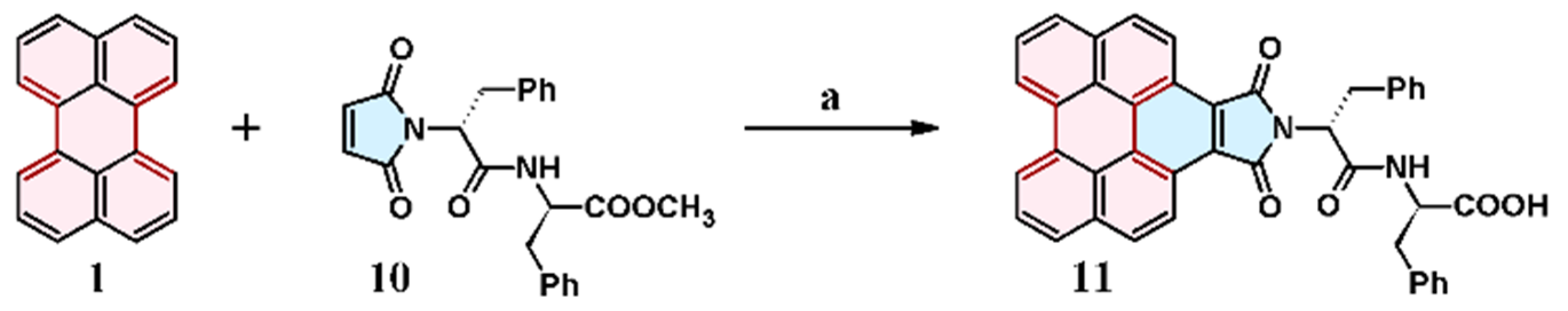
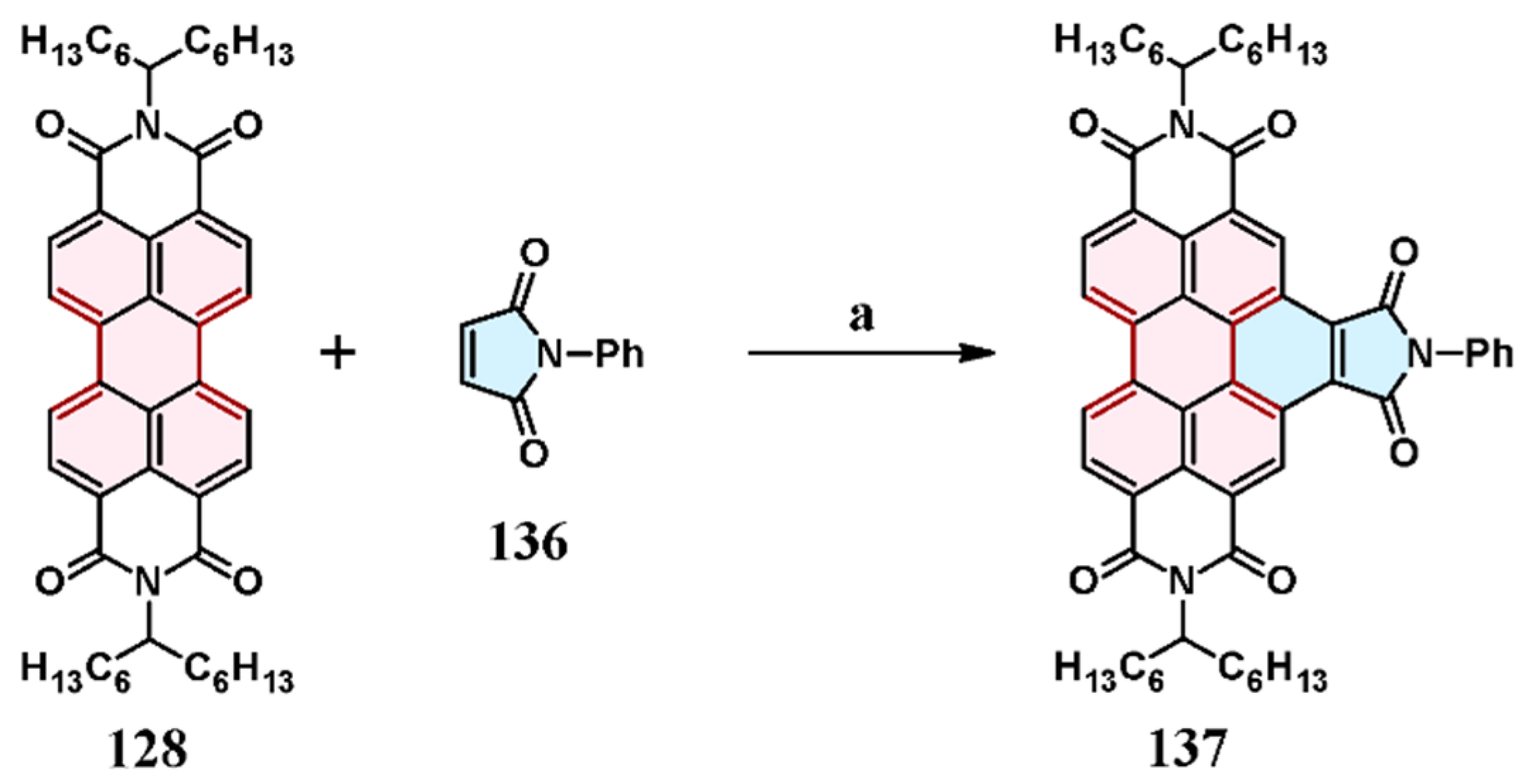
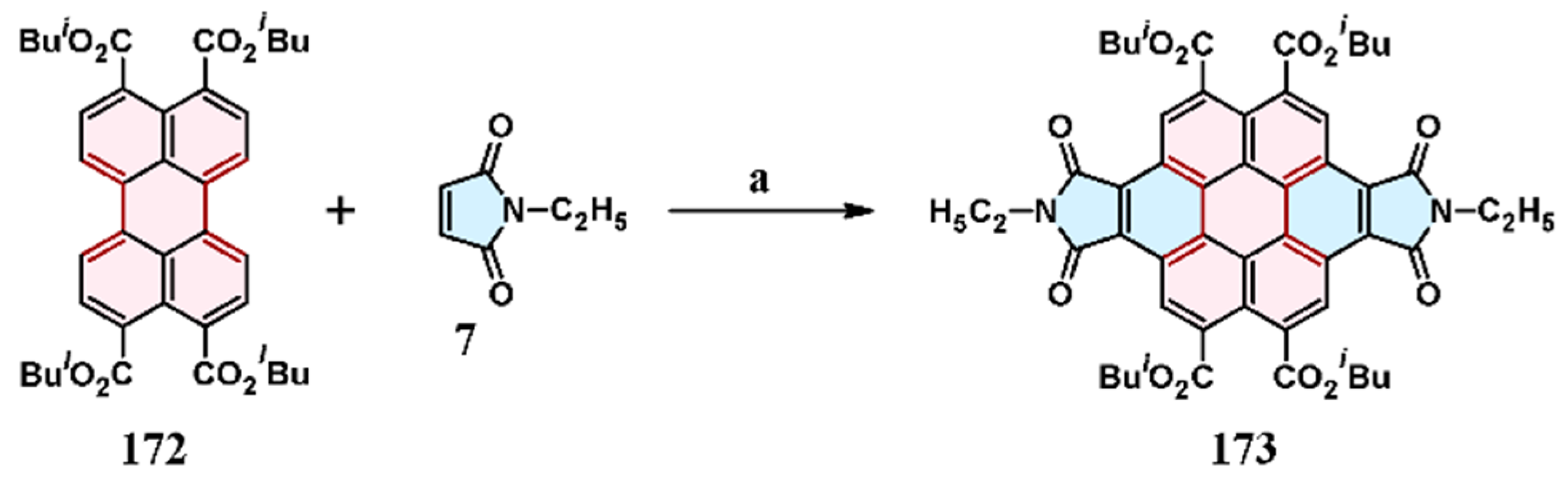
7.2. Cycloaddition of N-Phenyl Maleimide
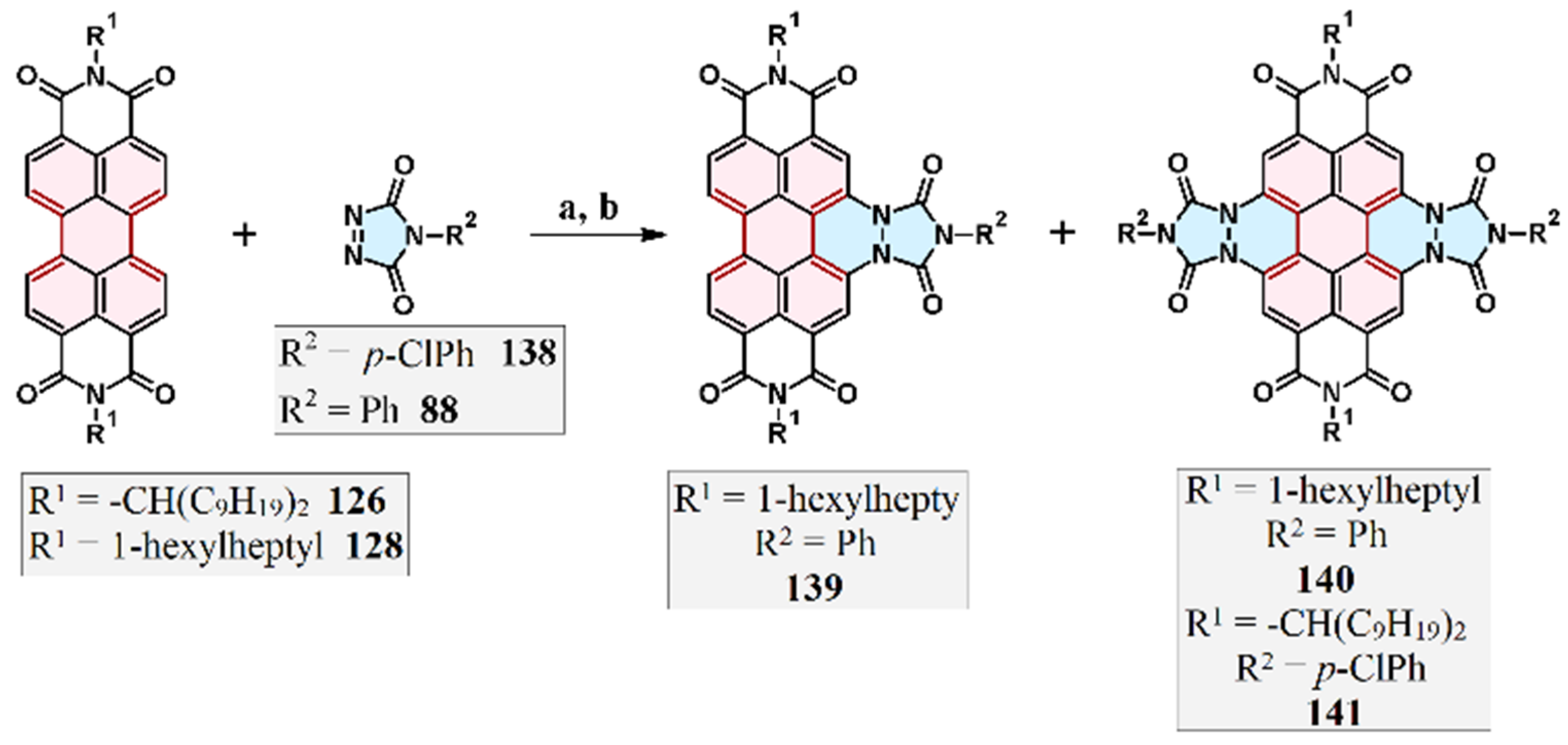
7.3. Cycloaddition of 1,2,4-Triazol-3,5-Diones to Perylene Bisimides
7.4. Cycloaddition of Azodicarboxylate
7.5. Cycloaddition of Diarylacetylenes
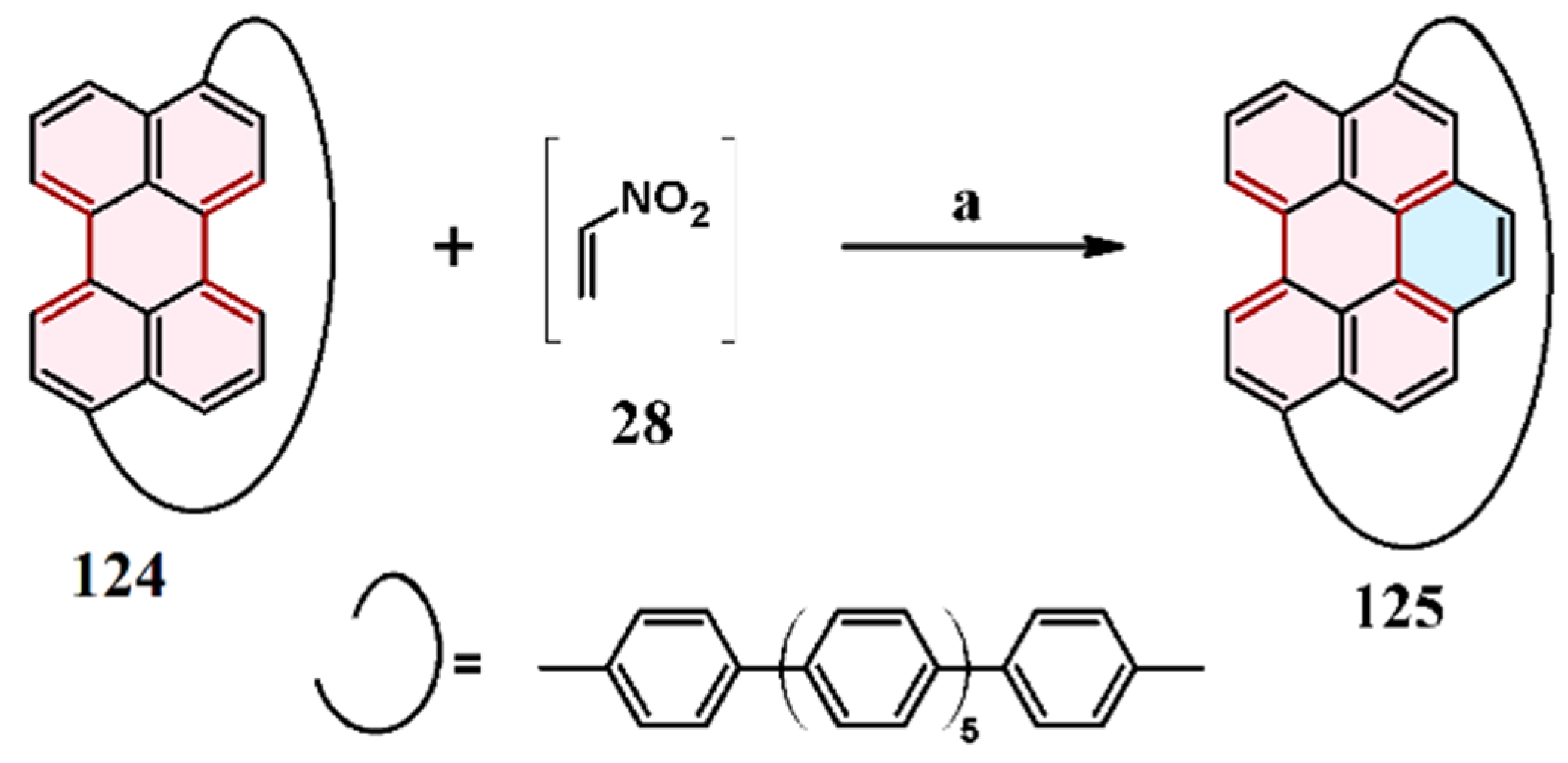
7.6. Cycloaddition of Arynes
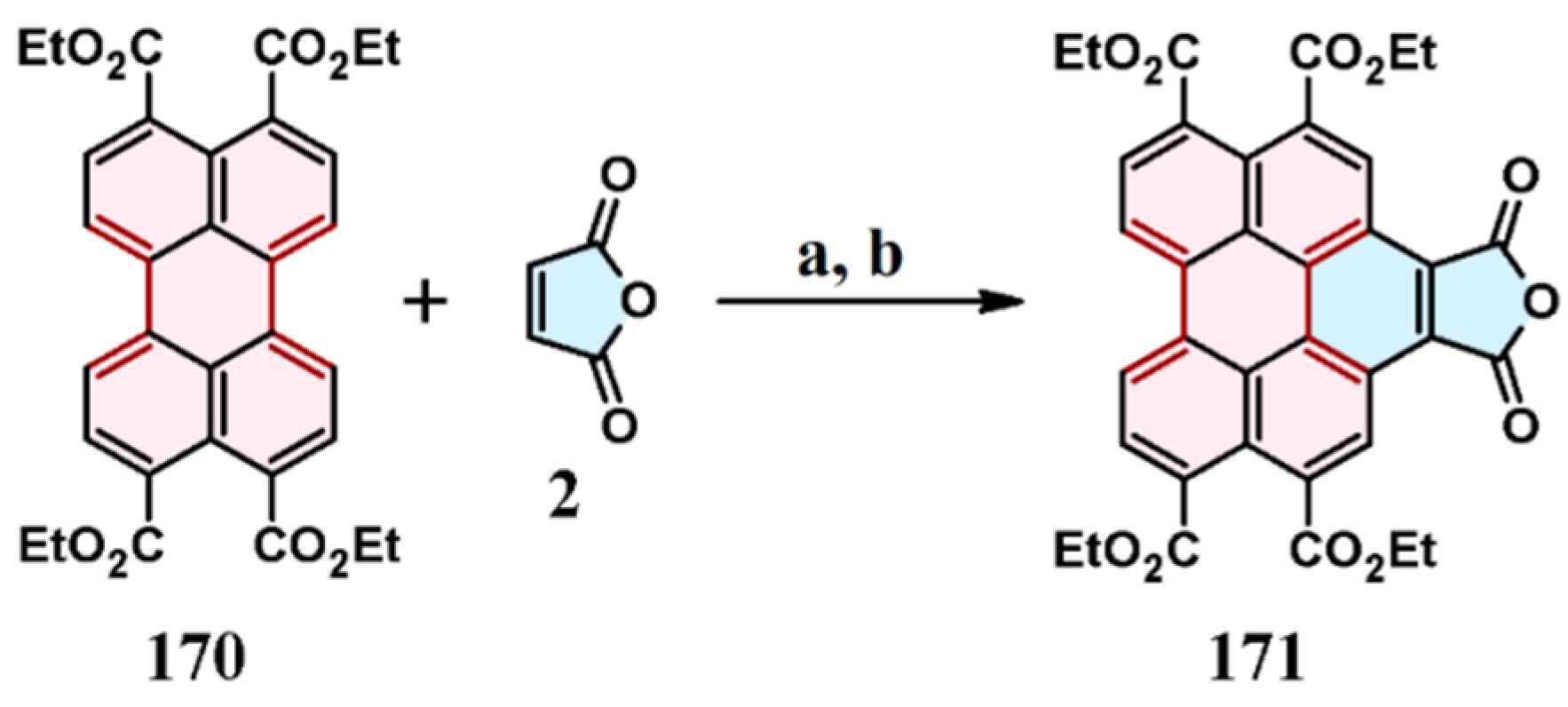
8. Cycloaddition to Perylenetetracarboxylic Acid Tetraesters
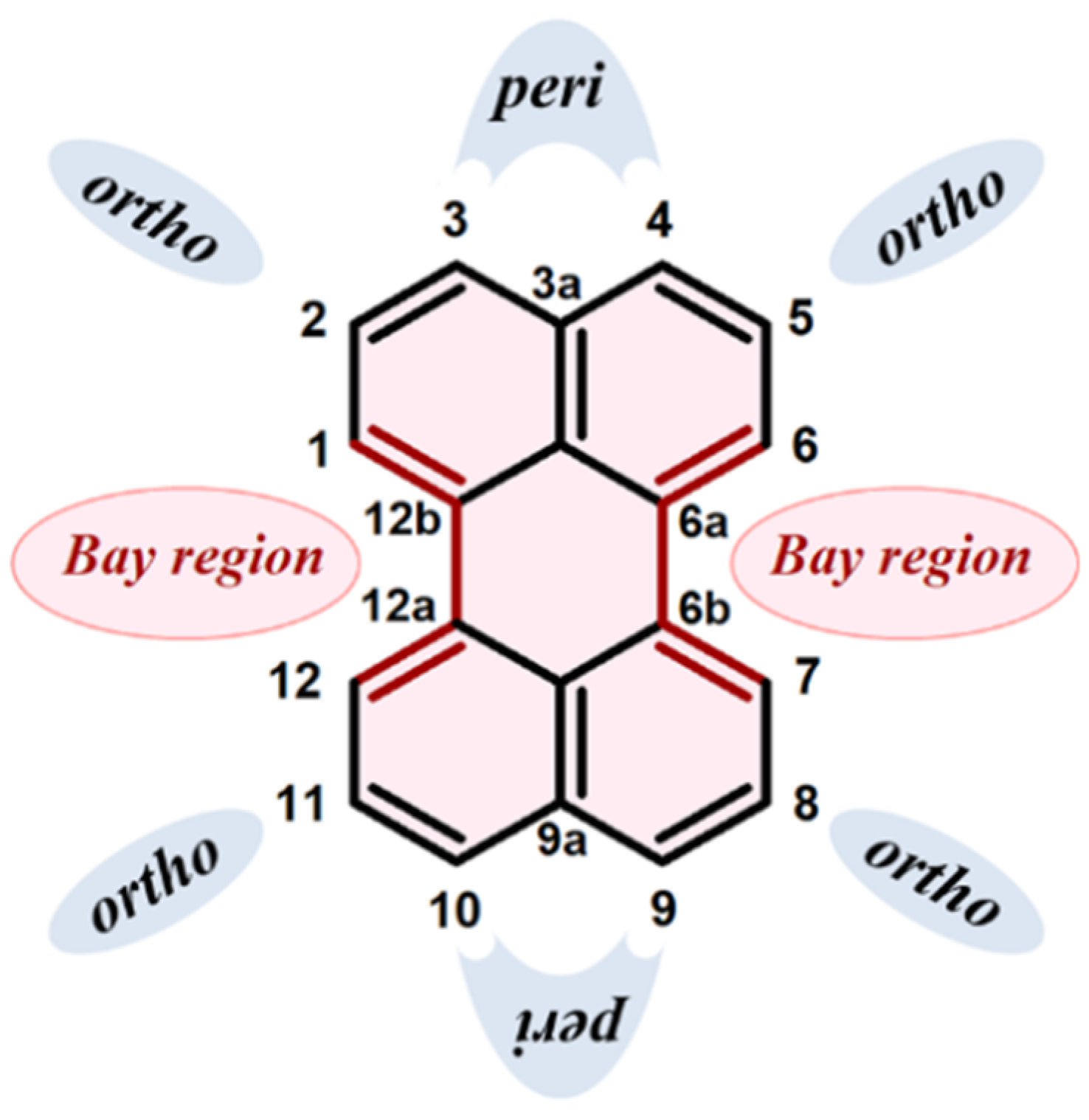
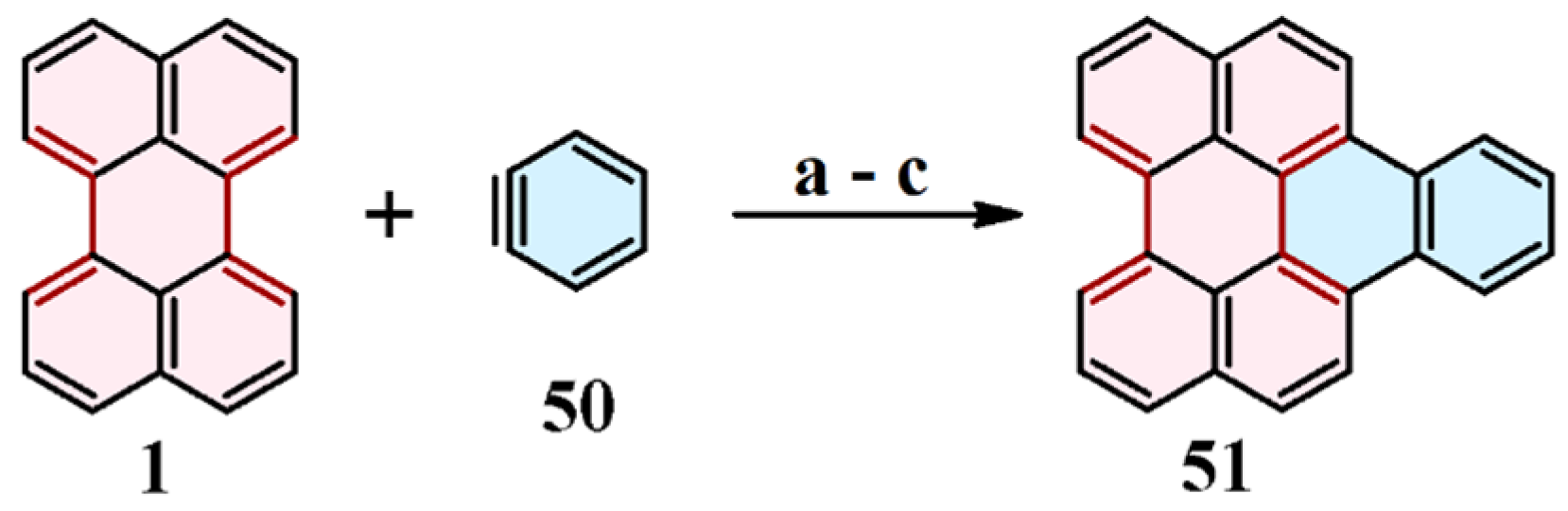
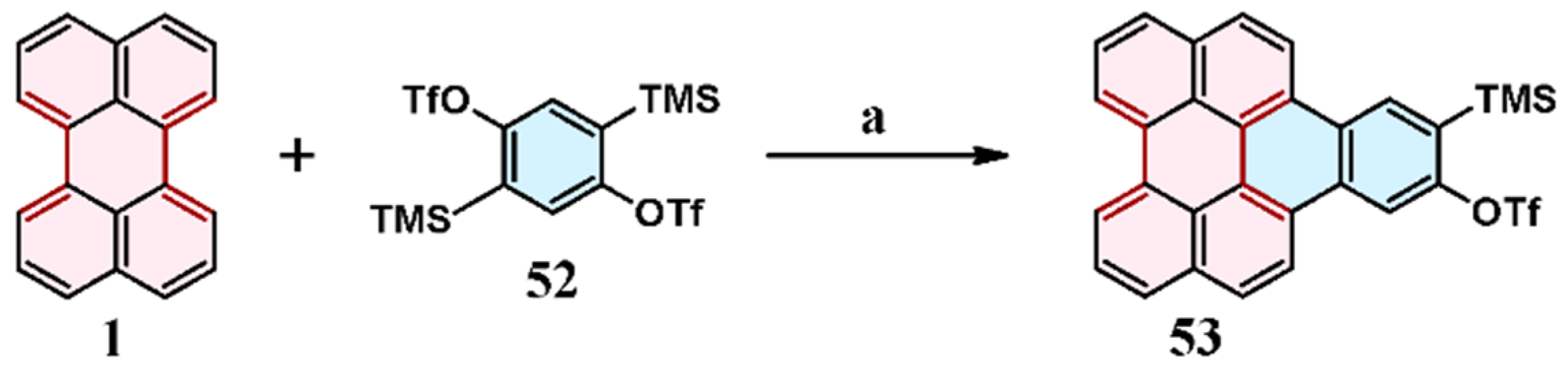
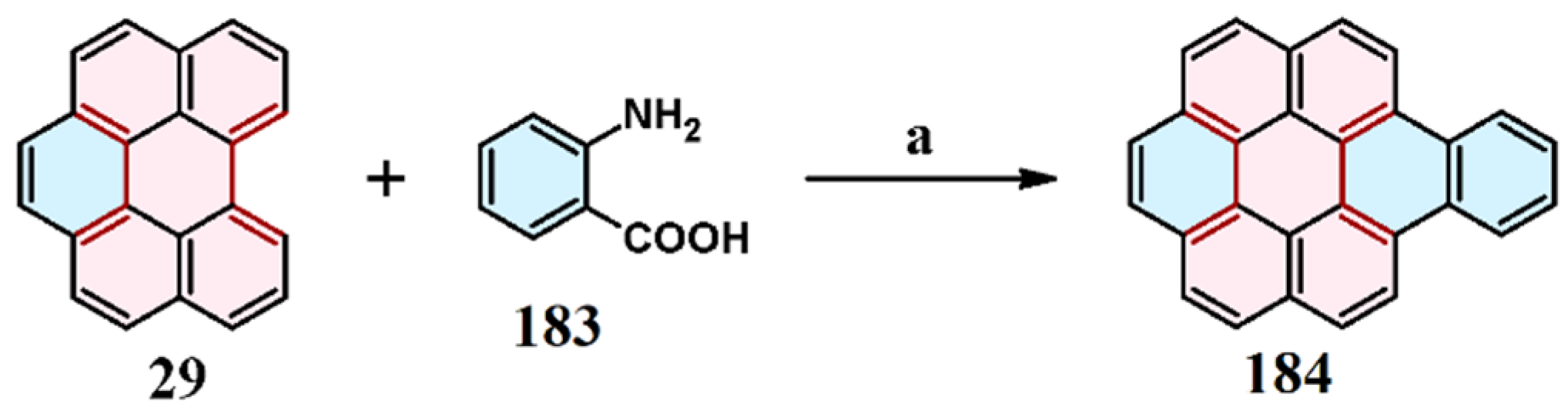
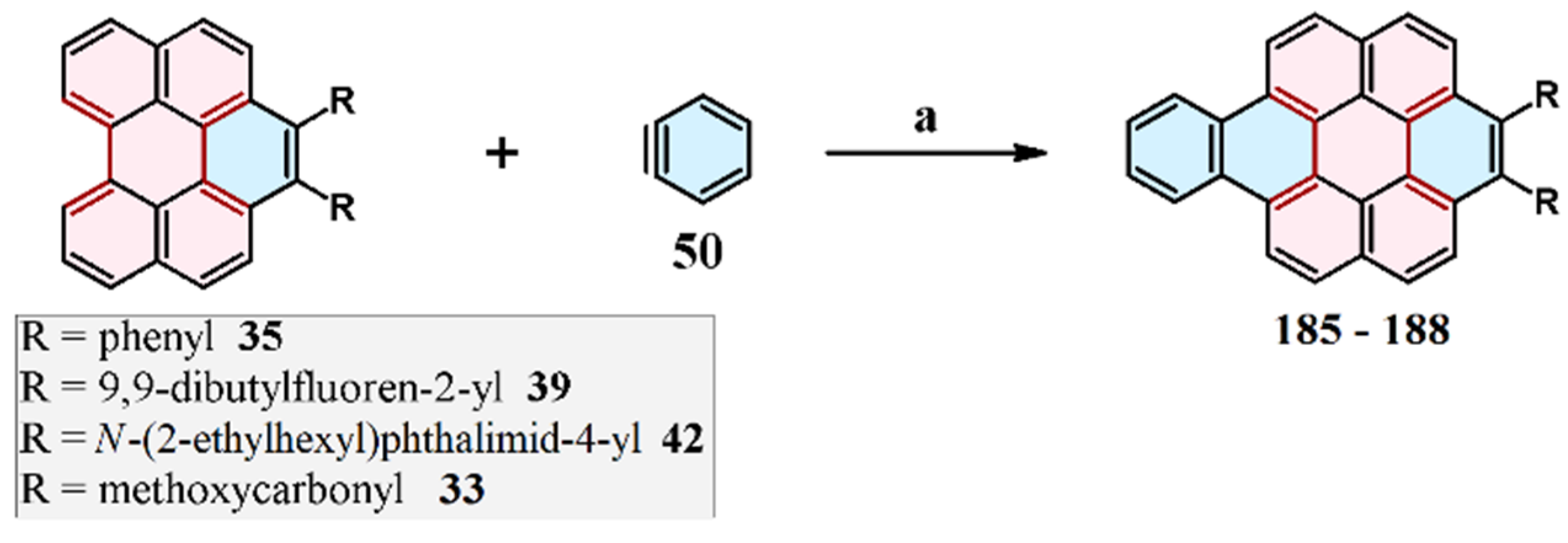
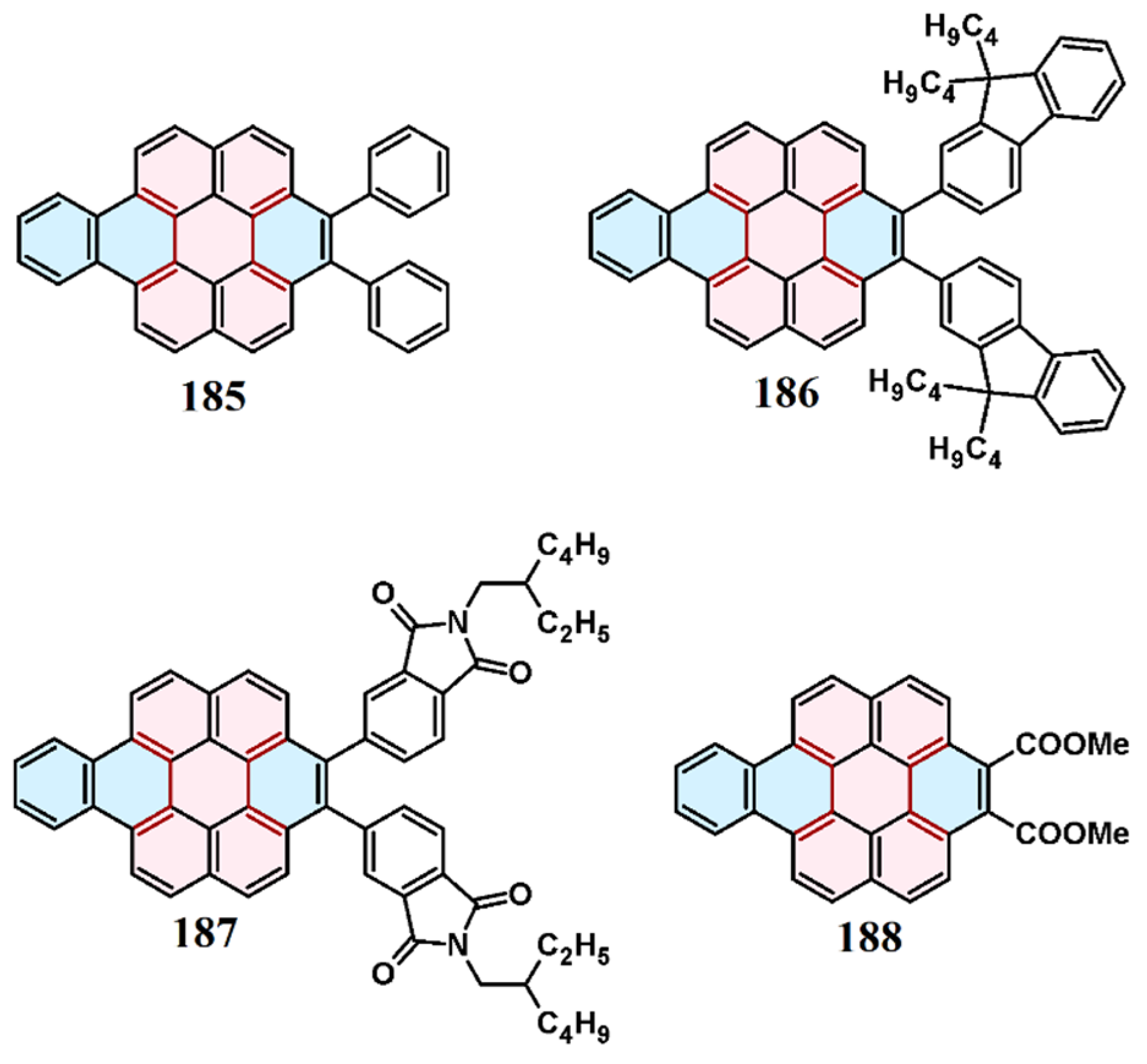
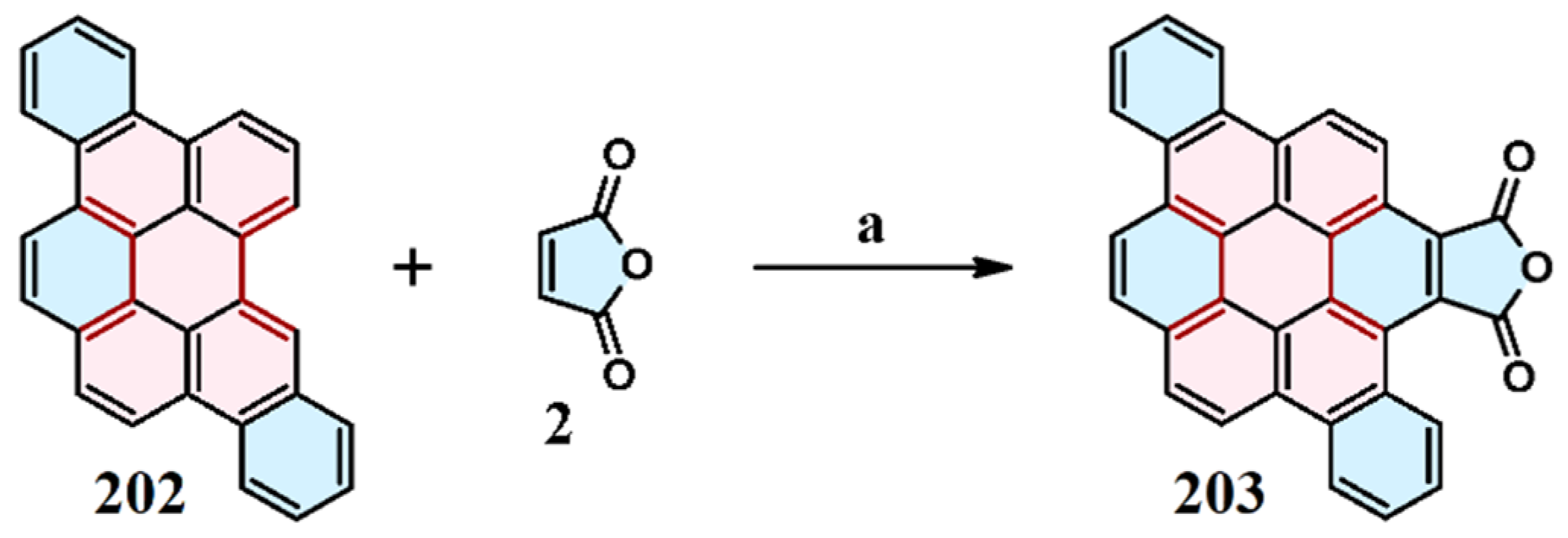
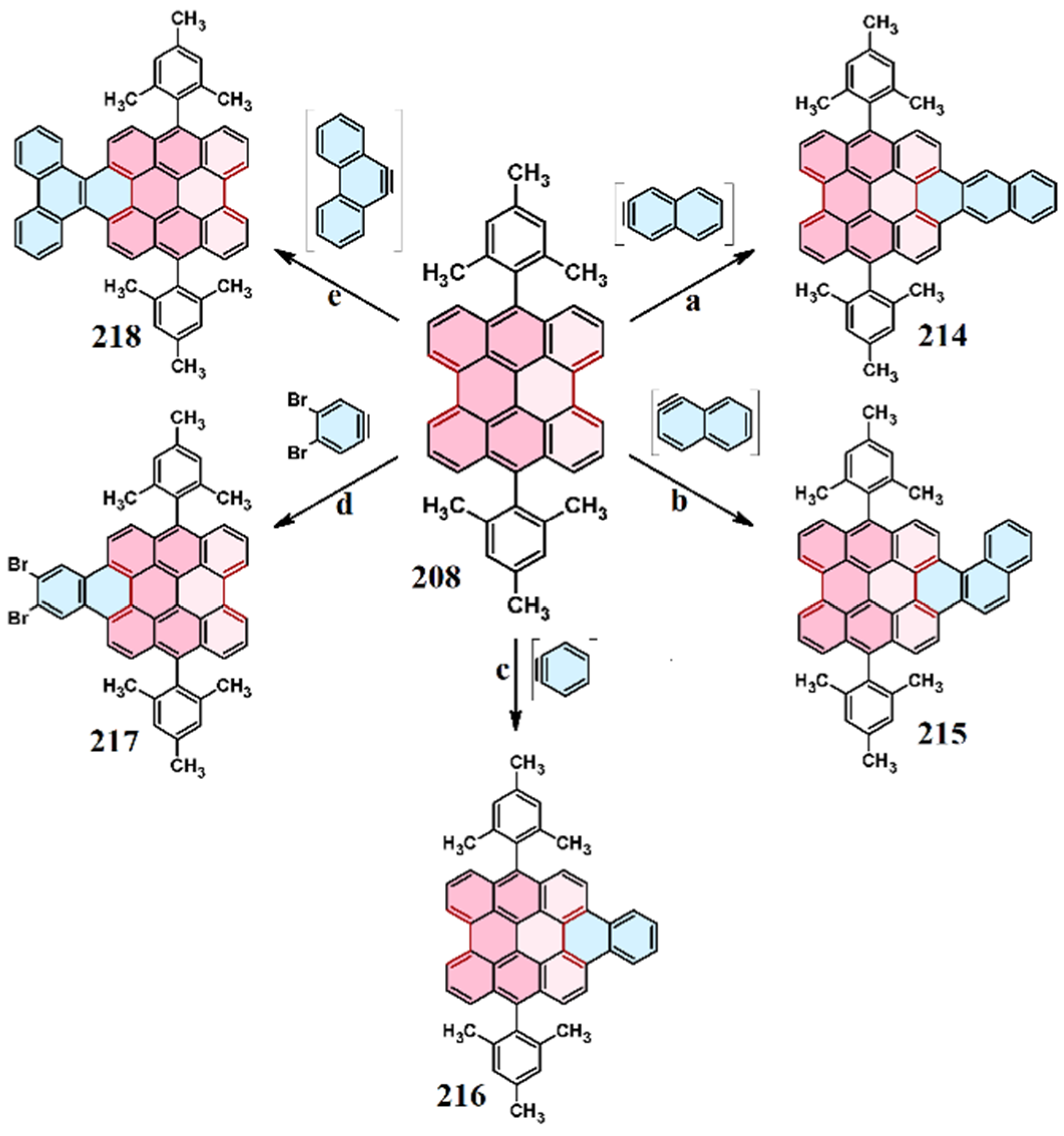
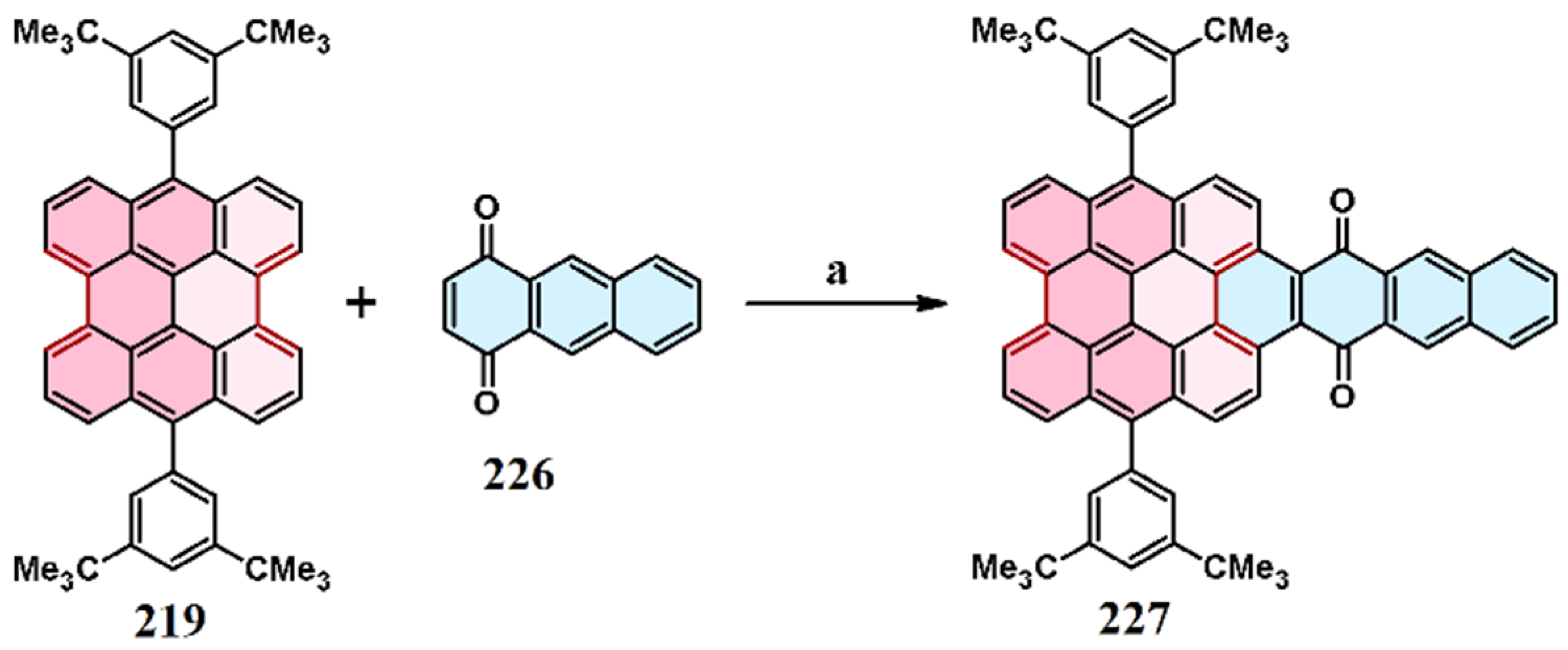
9. Cycloaddition to Benzo[ghi]perylene and Its Derivatives Bay Region
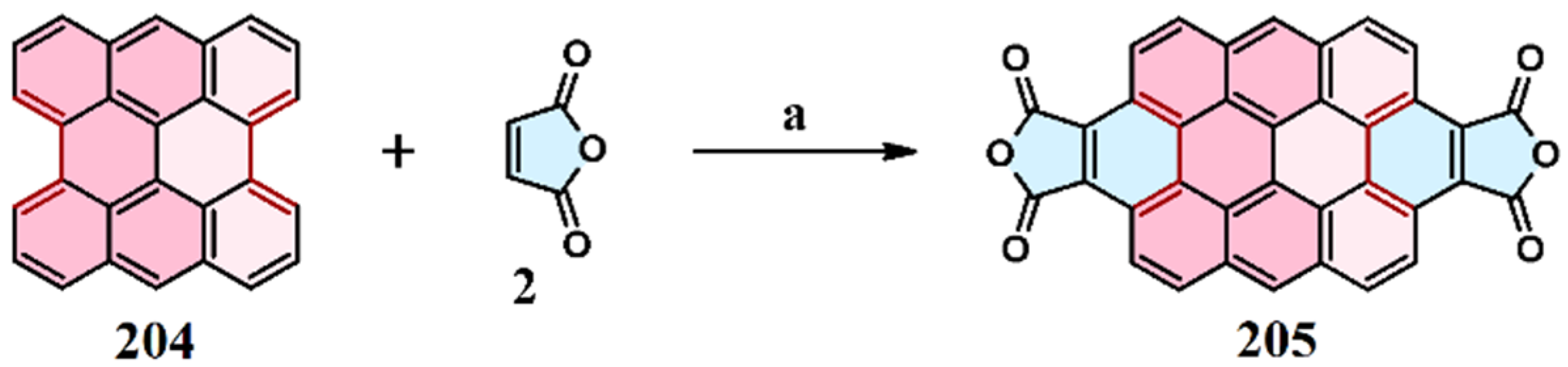
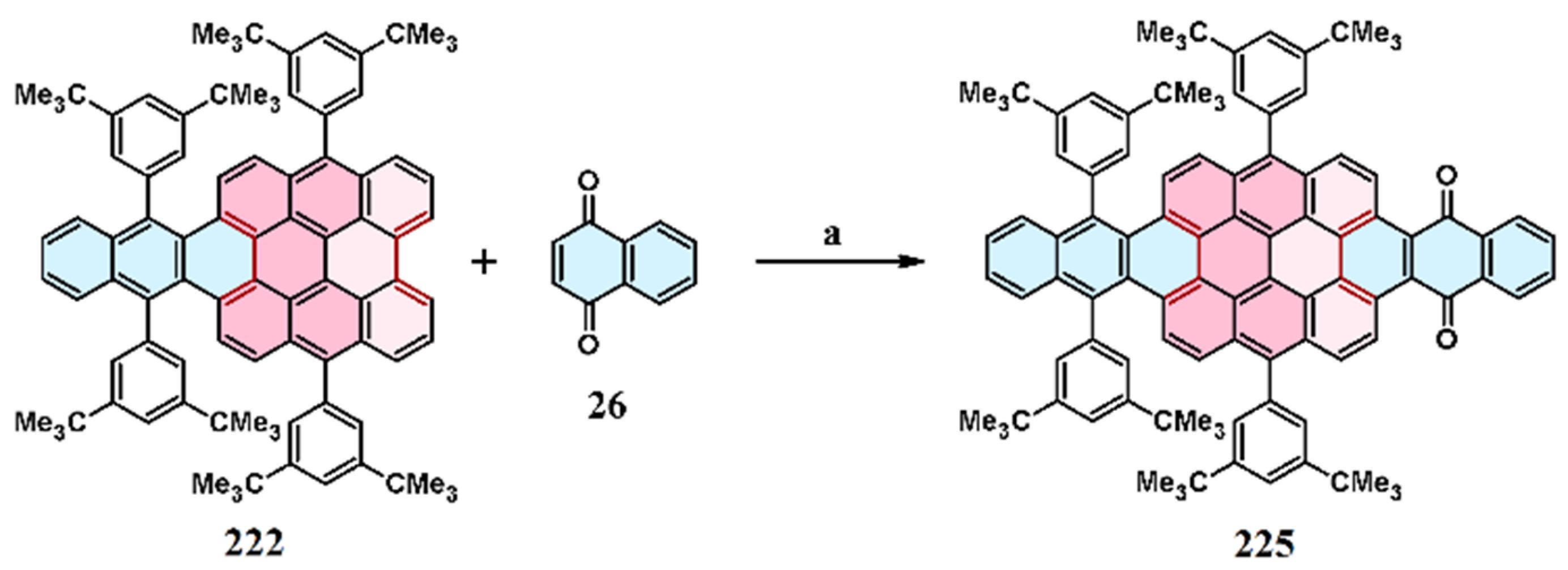
10. Cycloaddition to Tribenzoperylene
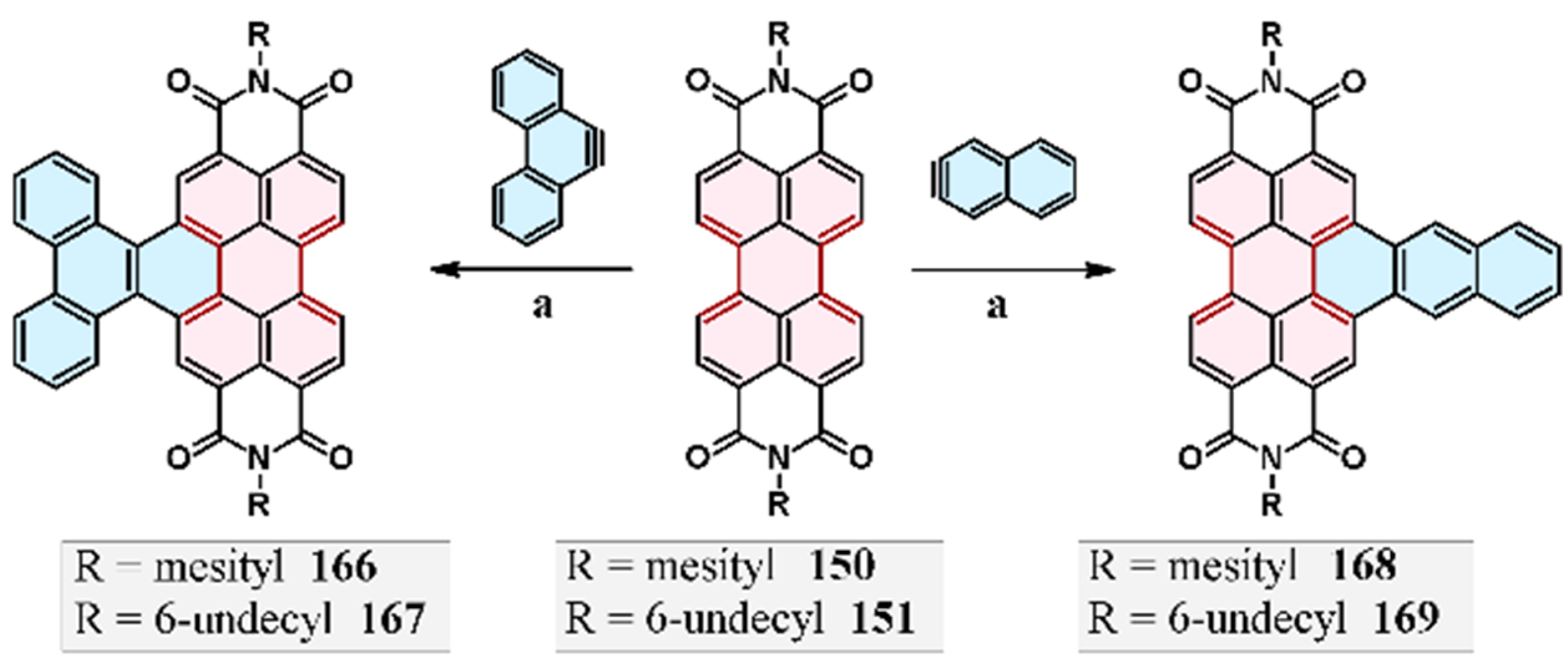
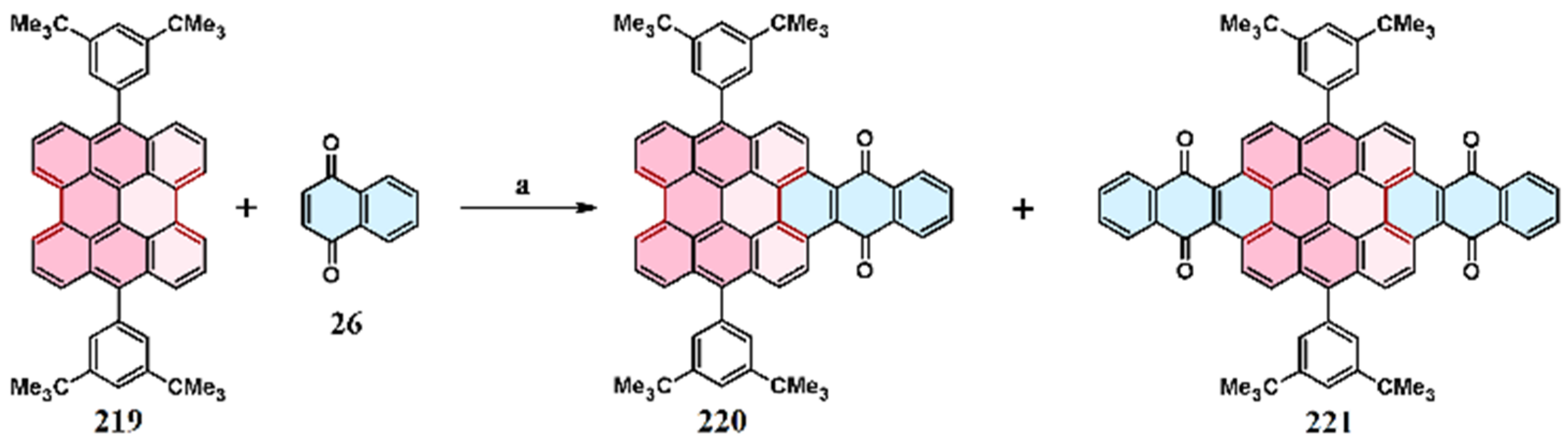
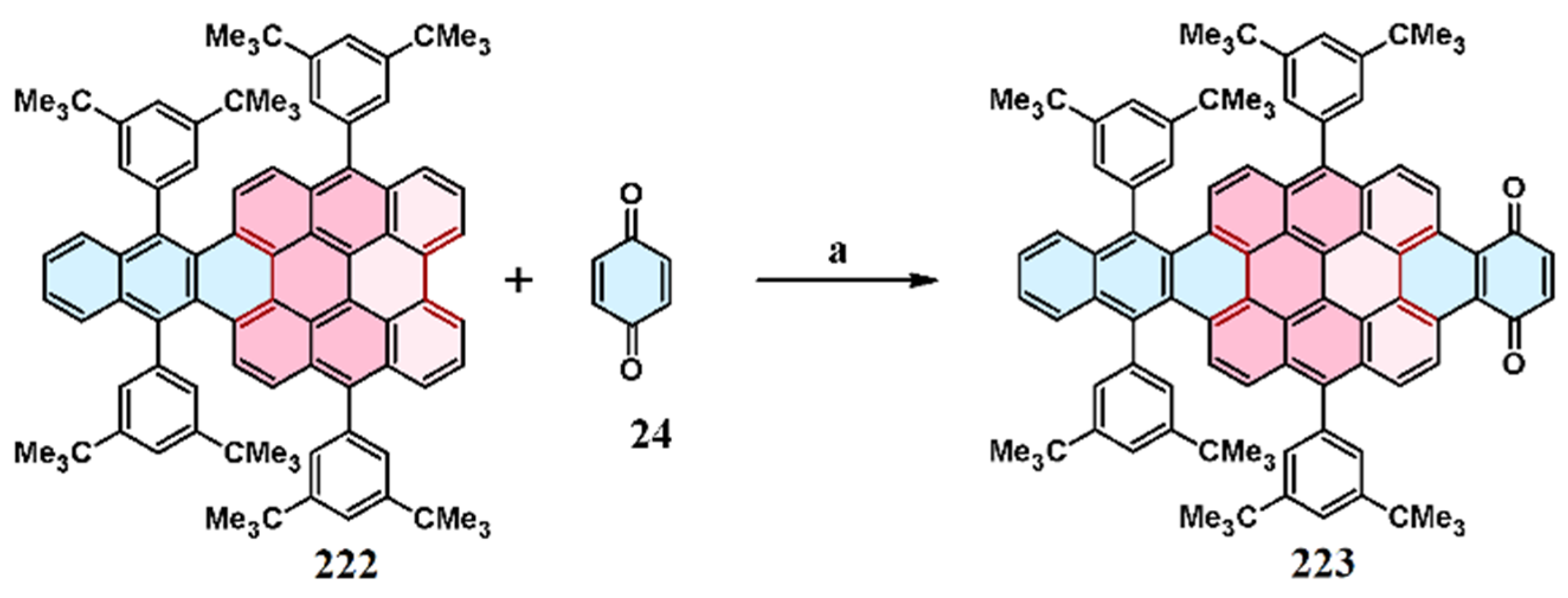
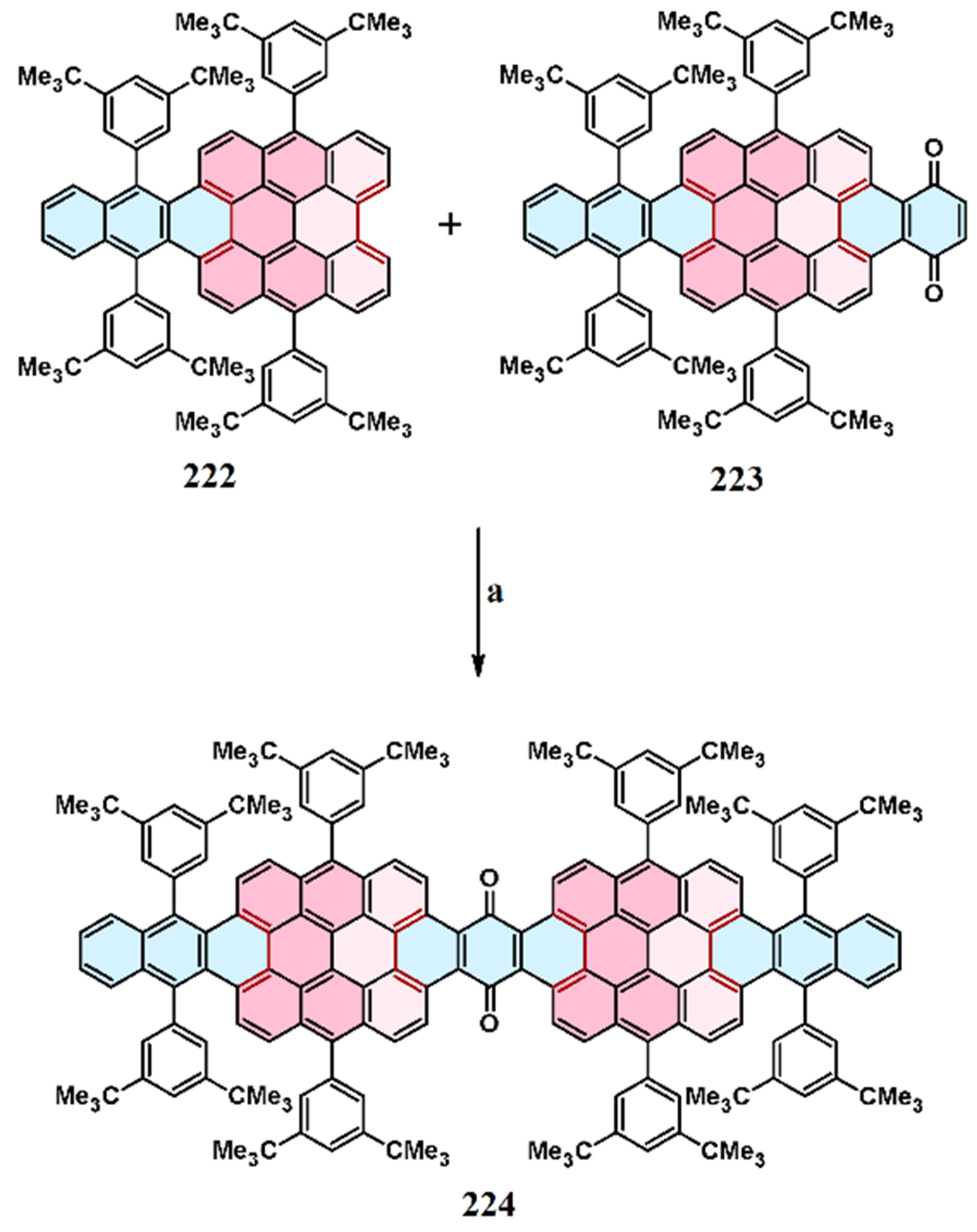
11. Cycloaddition to Bisanthene and Its Derivatives
11.1. Cycloaddition of Maleic Anhydride
11.2. Cycloaddition of Acetylene
11.3. Cycloaddition of Acetylenedicarboxylate to Di-Mesityl-Bisanthene
11.4. Cycloaddition of Arynes
11.5. Cycloaddition of Benzo- and Naphthoquinones
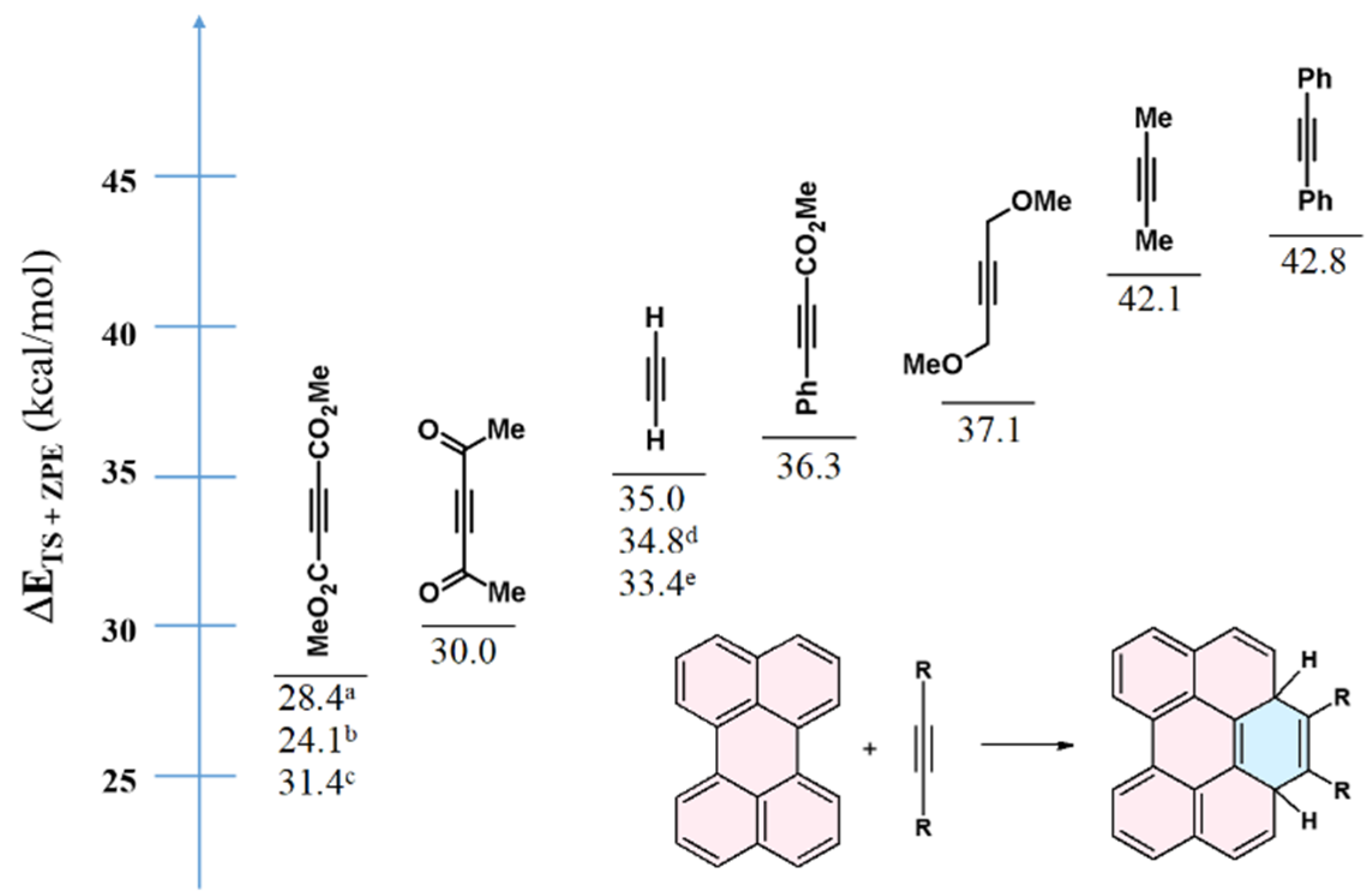
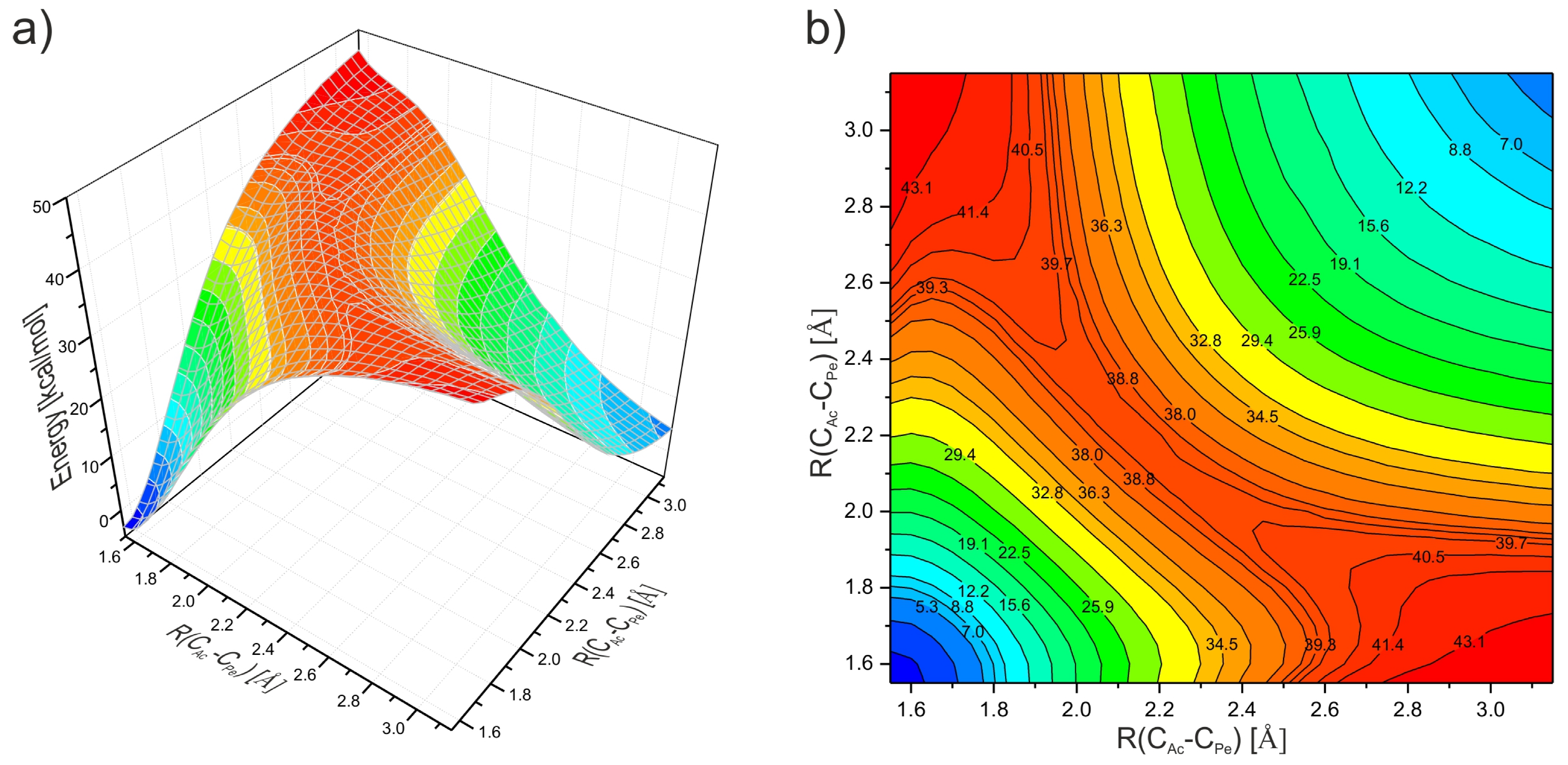
12. Computational Investigations of the Diels–Alder Cycloaddition to Perylene and Its Analogs’ Bay Regions
13. Importance of Cycloaddition to the Bay Regions of Perylene and Its Derivatives for Chemistry and Technology: Current Status and Perspectives
14. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sun, Z.; Ye, Q.; Chi, C.; Wu, J. Low band gap polycyclic hydrocarbons: From closed-shell near infrared dyes andsemiconductors to open-shell radicals. Chem. Soc. Rev. 2012, 41, 7857–7889. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, M.; Pschirer, N.G.; Baumgarten, M.; Müllen, K. Polyphenylene-Based materials for organic photovoltaics. Chem. Rev. 2010, 110, 6817–6855. [Google Scholar] [CrossRef] [PubMed]
- Fermi, A.; Orfanos, I.; Avramopoulos, A.; De Leo, F.; Demitri, N.; Bergamini, G.; Ceroni, P.; Papadopoulos, M.G.; Couris, S.; Bonifazi, D. Tailoring colors by O-annulation of polycyclic aromatic hydrocarbons. Chem. Eur. J. 2017, 23, 2353–2378. [Google Scholar]
- Wu, J.; Pisula, W.; Müllen, K. Graphenes as potential material for electronics. Chem. Rev. 2007, 107, 718–747. [Google Scholar] [CrossRef]
- Bendikov, M.; Wudl, F. Tetrathiafulvalenes, oligoacenenes, and their buckminsterfullerene derivatives: The brick and mortar of organic electronics. Chem. Rev. 2004, 104, 4891–4946. [Google Scholar] [CrossRef]
- Houk, K.N.; Lee, P.S.; Nendel, M. Polyacene and cyclacene geometries and electronic structures: Bond equalization, vanishing band gaps, and triplet ground states contrast with polyacetylene. J. Org. Chem. 2001, 66, 5517–5521. [Google Scholar] [CrossRef]
- Purushothaman, B.; Bruzek, M.; Parkin, S.R.; Miller, A.-F.; Anthony, J.E. Synthesis and structural characterization of crystalline nonacenes. Angew. Chem. Int. Ed. 2011, 50, 7013–7017. [Google Scholar] [CrossRef]
- Dong, D.; Fang, D.; Li, H.; Zhu, C.; Zhao, X.; Li, J.; Jin, L.; Xie, L.; Chen, L.; Zhao, J.; et al. C−H direct arylated 6h -indolo[2,3-b]quinoxaline derivative as a thickness-dependenthole-injection layer. Chem. Asian J. 2017, 12, 920–926. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, J.; Yin, Z.; Duong, H.M.; Qiao, F.; Boey, F.; Hu, X.; Zhang, H.; Wudl, F. Synthesis, characterization, and physical properties of a conjugated heteroacene: 2-methyl-1,4,6,7,8,9-hexaphenylbenz(g)isoquinolin-3(2H)-one (BIQ). Chem. Asian J. 2011, 6, 856–862. [Google Scholar] [CrossRef]
- Li, G.; Wu, Y.; Gao, J.; Wang, C.; Li, J.; Zhang, H.; Zhao, Y.; Zhao, Y.; Zhang, Q. Synthesis and physical properties of four hexazapentacene derivatives. J. Am. Chem. Soc. 2012, 134, 20298–20301. [Google Scholar] [CrossRef]
- Zhao, J.; Li, R.; Ai, W.; Dong, D.; Li, J.; Chen, L.; Xie, L.; Yu, T.; Huang, W. Pi-Extended diindole-fused azapentacenone: Synthesis, characterization, and photophysical and lithium-storage properties. Chem. Asian J. 2016, 11, 1382–1387. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Duong, H.M.; Liu, Y.; Shi, W.; Ji, L.; Li, G.; Li, S.; Liu, X.-W.; Ma, J.; Wudl, F.; et al. Synthesis and structure characterization of a stable nonatwistacene. Angew. Chem. Int. Ed. 2012, 51, 6094–6098. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.Y.; Zhou, F.; Gao, J.; Li, G.; Wang, C.; Xu, Q.-F.; Zhang, Q.; Lu, J.-M. Synthesis, characterization, and nonvolatile ternary memory behavior of a larger heteroacene with nine linearly fused rings and two different heteroatoms. J. Am. Chem. Soc. 2013, 135, 14086–14089. [Google Scholar] [CrossRef] [PubMed]
- Bunz, U.H.F. The larger linear N-heteroacenes. Acc. Chem. Res. 2015, 48, 1676–1686. [Google Scholar] [CrossRef] [PubMed]
- Weil, T.; Vosch, T.; Hofkens, J.; Peneva, K.; Müllen, K. The rylene colorant family—Tailored nanoemitters for photonics research and applications. Angew. Chem. Int. Ed. 2010, 49, 9068–9093. [Google Scholar] [CrossRef]
- Huang, C.; Barlow, S.; Marder, S.R. Perylene-3,4,9,10-tetracarboxylic acid diimides: Synthesis, physical properties, and use in organic electronics. J. Org. Chem. 2011, 76, 2386–2407. [Google Scholar] [CrossRef]
- Li, C.; Wonneberger, H. Perylene imides for organic photovoltaics: Yesterday, today, and tomorrow. Adv. Mater. 2012, 24, 613–636. [Google Scholar] [CrossRef]
- Kozma, E.; Catellani, M. Perylene diimides based materials for organic solar cells. Dyes Pigments 2013, 98, 160–179. [Google Scholar] [CrossRef]
- Lin, Y.; Zhan, X. Oligomer molecules for efficient organic photovoltaics. Acc. Chem. Res. 2016, 49, 175–183. [Google Scholar] [CrossRef]
- Zhan, X.; Facchetti, A.; Barlow, S.; Marks, T.J.; Ratner, M.A.; Wasielewski, M.R.; Marder, S.R. Rylene and related diimides for organic electronics. Adv. Mater. 2011, 23, 268–284. [Google Scholar] [CrossRef]
- Debije, M.G.; Chen, Z.; Piris, J.; Neder, R.B.; Watson, M.M.; Müllen, K.; Würthner, F. Dramatic increase in charge carrier lifetime in a liquid crystalline perylene bisimide derivative upon bay substitution with chlorine. J. Mater. Chem. 2005, 15, 1270–1276. [Google Scholar] [CrossRef]
- Herbst, W.; Hunger, K. Perylene and perinone pigments. In Industrial Organic Pigments: Production, Properties, Applications, 2nd ed.; Wiley: New York, NY, USA, 2006; pp. 467–475. [Google Scholar]
- Markiewicz, J.T.; Wudl, F. Perylene, oligorylenes, and aza-analogs. ACS Appl. Mater. Interfaces 2015, 7, 28063–28085. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Zu, Y.; Herrmann, A.; Geerts, Y.; Müllen, K.; Bard, A.J. Electrochemistry, spectroscopy and electrogenerated chemiluminescence of perylene, terrylene, and quaterrylene diimides in aprotic solution. J. Am. Chem. Soc. 1999, 121, 3513–3520. [Google Scholar] [CrossRef]
- Quante, H.; Müllen, K. Quaterrylenebis (dicarboximides). Angew. Chem. Int. Ed. Engl. 1995, 34, 1323–1325. [Google Scholar] [CrossRef]
- Cotlet, M.; Vosch, T.; Habuchi, S.; Weil, T.; Müllen, K.; Hofkens, J.; De Schryver, F. Probing intramolecular förster resonance energy transfer in a naphthaleneimide–peryleneimide–terrylenediimide-based dendrimer by ensemble and single-molecule fluorescence spectroscopy. J. Am. Chem. Soc. 2005, 127, 9760–9768. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, U.; Zajączkowski, W.; Chen, L.; Bouillière, F.; Wang, D.; Koynov, K.; Pisula, W.; Müllen, K.; Wennemers, H. Hierarchical supramolecular assembly of sterically demanding π-systems byconjugation with oligoprolines. Angew. Chem. Int. Ed. 2014, 53, 12537–12541. [Google Scholar]
- Rocard, L.; Berezin, A.; De Leo, F.; Bonifazi, D. Templated chromophore assembly by dynamic covalent bonds. Angew. Chem. Int. Ed. 2015, 54, 15739–15743. [Google Scholar] [CrossRef]
- Zhylitskaya, H.; Cybińska, J.; Chmielewski, P.; Lis, T.; Stępień, M. Bandgap engineering in π-extended pyrroles. A modular approach to electron-deficient chromophores with multi-redox activity. J. Am. Chem. Soc. 2016, 138, 11390–11398. [Google Scholar] [CrossRef]
- Maggini, L.; Bonifazi, D. Hierarchised luminescent organic architectures: Design, synthesis, self-assembly, selforganisation and functions. Chem. Soc. Rev. 2012, 41, 211–241. [Google Scholar] [CrossRef]
- Ajayaghosh, A.; Praveen, V.K.; Vijayakumar, C. Organogels as scaffolds for excitation energy transfer and light harvesting. Chem. Soc. Rev. 2008, 37, 109–122. [Google Scholar] [CrossRef]
- Babu, S.S.; Aimi, J.; Ozawa, H.; Shirahata, N.; Saeki, A.; Seki, S.; Ajayaghosh, A.; Möhwald, H.; Nakanishi, T. Solvent-Free luminescent organic liquids. Angew. Chem. Int. Ed. 2012, 51, 3391–3395. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, R.; Míšek, J.; Sakai, N.; Matile, S. Supramolecular n/p-heterojunction photosystems with oriented multicolored antiparallel redox gradients (OMARG-SHJs). Chem. Soc. Rev. 2010, 39, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Müllen, K.; Rabe, J.P. Nanographenes as active components of single-molecule electronics and how ascanning tunneling microscope puts them to work. Acc. Chem. Res. 2008, 41, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Dai, L. Functionalization of graphene for efficient energy conversion and storage. Acc. Chem. Res. 2013, 46, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Stępień, M.; Gońka, E.; Żyła, M.; Sprutta, N. Heterocyclic nanographenes and other polycyclic heteroaromatic compounds: Synthetic routes, properties, and applications. Chem. Rev. 2017, 117, 3479–3716. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Ozaki, K.; Itami, K. Annulative π-extension (APEX): Rapid access to fused arenes, heteroarenes, and nanographenes. Angew. Chem. Int. Ed. 2017, 56, 11144–11164. [Google Scholar] [CrossRef]
- Hasobe, T. Photo- and electro-functional self-assembled architectures of porphyrins. Phys. Chem. Chem. Phys. 2012, 14, 15975–15987. [Google Scholar] [CrossRef]
- Hasobe, T. Supramolecular nanoarchitectures for light energy conversion. Phys. Chem. Chem. Phys. 2010, 12, 44–57. [Google Scholar] [CrossRef]
- Nakano, M.; Mori, H.; Shinamura, S.; Takimiya, K. Naphtho[2,3-b:6,7-b′]dichalcogenophenes: Syntheses, characterizations, and chalcogeneatom effects on organic field-effect transistor and organic photovoltaic devices. Chem. Mater. 2012, 24, 190–198. [Google Scholar] [CrossRef]
- Wang, X.; Zhi, L.; Tsao, N.; Tomović, Ż.; Li, J.; Müllen, K. Transparent carbon films as electrodes in organic solar cells. Angew. Chem. Int. Ed. 2008, 47, 2990–2992. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, J.; Chow, P.C.Y.; Jiang, K.; Zhang, J.; Zhu, Z.; Zhang, J.; Huang, F.; Yan, H. Nonfullerene acceptor molecules for bulk heterojunction organic solar cells. Chem. Rev. 2018, 118, 3447–3507. [Google Scholar] [CrossRef] [PubMed]
- Sawabe, K.; Imakawa, M.; Nakano, M.; Yamao, T.; Hotta, S.; Iwasa, Y.; Takenobu, T. Current-Confinement structure and extremely high current density in organic light-emitting transistors. Adv. Mater. 2012, 24, 6141–6146. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Liu, B.; Png, R.Q.; Zhang, K.; Lim, K.A.; Luo, J.; Shao, J.; Ho, P.K.H.; Chi, C.; Wu, J. New discotic mesogens based on triphenylene-fused triazatruxenes: Synthesis, physical properties, and self-assembly. Chem. Mater. 2010, 22, 435–449. [Google Scholar] [CrossRef]
- Más-Montoya, M.; Ortiz, R.P.; Curiel, D.; Espinosa, A.; Allain, M.; Facchetti, A.; Marks, T.J. Isomeric carbazolocarbazoles: Synthesis, characterization and comparative study inorganic field effect transistors. J. Mater. Chem. C 2013, 1, 1959–1969. [Google Scholar] [CrossRef]
- Dadvand, A.; Moiseev, A.G.; Sawabe, K.; Sun, W.-H.; Djukic, B.; Chung, I.; Takenobu, T. Maximizing field-effect mobility and solid-state luminescence in organic semiconductors. Angew. Chem. Int. Ed. 2012, 51, 3837–3841. [Google Scholar] [CrossRef]
- Verma, S.; Manna, M.K.; Pandey, S.K.; Das, A.K.; Mukherjee, S. Benzo[ghi]perylene monoimide based photosensitive lamellar Cd-doped ZnO nanohybrids. RSC Adv. 2014, 4, 62603–62614. [Google Scholar] [CrossRef]
- Manna, M.K.; Aaryashree Verma, S.; Mukherjee, S.; Das, A.K. Lamellar peptide–cadmium-doped zinc oxide nanohybrids that emit white ligh. ChemPlusChem 2016, 81, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Zollinger, H. Colorants derived from perylene and other polycyclic aromatic compounds. In Color Chemistry, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2001; pp. 298–306. [Google Scholar]
- Zhang, B.; Soleimaninejad, H.; Jones, D.J.; White, J.M.; Ghiggino, K.P.; Smith, T.A.; Wong, W.W.H. Highly fluorescent molecularly insulated perylene diimides: Effect of concentration on photophysical properties. Chem. Mater. 2017, 29, 8395–8403. [Google Scholar] [CrossRef]
- Meng, D.; Sun, D.; Zhong, C.; Liu, T.; Fan, B.; Huo, L.; Li, Y.; Jiang, W.; Choi, H.; Kim, T.; et al. High-Performance solution-processed non-fullerene organic solar cells based on selenophene-containing perylene bisimide acceptor. J. Am. Chem. Soc. 2016, 138, 375–380. [Google Scholar] [CrossRef]
- Li, G.; Zhao, Y.; Li, J.; Cao, J.; Zhu, J.; Sun, X.W.; Zhang, Q. Synthesis, characterization, physical properties, and OLED application of single BN fused perylene diimide. J. Org. Chem. 2015, 80, 196–203. [Google Scholar] [CrossRef]
- Endres, A.H.; Schaffroth, M.; Paulus, F.; Reiss, H.; Wadepohl, H.; Rominger, F.; Kramer, R.; Bunz, U.H.F. Coronene-Containing N-heteroarenes: 13 rings in a row. J. Am. Chem. Soc. 2016, 138, 1792–1795. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Gao, J.; Ma, T.; Wang, M.; Zhang, A.; Shi, Z.; Wei, Y. Facile synthesis and replacement reactions of mono-substituted perylene bisimide dyes. Dyes Pigments 2012, 95, 450–454. [Google Scholar] [CrossRef]
- Wang, R.; Shi, Z.; Zhang, C.; Zhang, A.; Chen, J.; Guo, W.; Sun, Z. Facile synthesis and controllable bromination of asymmetrical intermediates of perylenemonoanhydride/monoimide diester. Dyes Pigments 2013, 98, 450–458. [Google Scholar] [CrossRef]
- Ma, Y.; Shi, Z.; Zhang, A.; Li, J.; Wei, X.; Jiang, T.; Li, Y.; Wang, X. Self-Assembly, optical and electrical properties of five membered O- or S-heterocyclic annulated perylene diimides. Dyes Pigments 2016, 135, 41–48. [Google Scholar] [CrossRef]
- Fan, Q.; Cheng, K.; Yang, Z.; Zhang, R.; Yang, M.; Hu, X.; Ma, X.; Bu, L.; Lu, X.; Xiong, X.; et al. Perylene-diimide-based nanoparticles as highly efficient photoacoustic agents for deep brain tumor imaging in living mice. Adv. Mater. 2015, 27, 843–847. [Google Scholar] [CrossRef]
- Chen, W.; Yang, X.; Long, G.; Wan, X.; Chen, Y.; Zhang, Q. A perylene diimide (PDI)-based small molecule with tetrahedral configuration as a nonfullerene acceptor for organic solar cells. J. Mater. Chem. C 2015, 3, 4698–4705. [Google Scholar] [CrossRef]
- Würther, F.; Saha-Mӧller, C.R.; Fimmel, B.; Leowanawat, P.; Schmidt, D. Perylene bisimide dye assemblies as archetype functional supramolecular materials. Chem. Rev. 2016, 116, 962–1052. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.; Kwok, R.T.; Lam, J.W.; Tang, B.Z. Aggregation-Induced emission: Together we shine, united we soar. Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef]
- Helin, W.; Lingcheng, C.; Zhenbo, Z.; Yi, X. Aryl-Bisalkynyl bridged perylene diimides dimers: Efficient synthesis, properties andimproved electron mobilities. Dyes Pigments 2017, 144, 184–189. [Google Scholar]
- Sun, J.; Wang, M.; Xu, P.; Zhang, S.; Shi, Z. Synthesis of water-soluble perylene dicarboximide derivatives containing pyridineoxide groups. Synth. Commun. 2012, 42, 1472–1479. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, T.; Zeng, W.; Xie, D.; Luo, Z.; Sun, Y.; Yang, C. Thienobenzene-fused perylene bisimide as a non-fullerene acceptor for organic solar cellswith a high open-circuit voltage and power conversion efficiency. Mater. Chem. Front. 2017, 1, 749–756. [Google Scholar] [CrossRef]
- Gong, Y.; Chang, K.; Chen, C.; Han, M.; Zhan, X.; Min, J.; Jiao, X.; Li, Q.; Li, Z. Pyrene-Fused PDI based ternary solar cells: High power conversion efficiency over 10%, and improved device thermal stability. Mater. Chem. Front. 2019, 3, 93–102. [Google Scholar] [CrossRef]
- McAfee, S.M.; Dayneko, S.V.; Hendsbee, A.D.; Josse, P.; Blanchard, P.; Cabanetos, C.; Welch, G.C. Applying direct heteroarylation synthesis to evaluate organic dyes as the core component in PDI-based molecular materials for fullerene-free organic solar cells. J. Mater. Chem. A 2017, 5, 11623–11633. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, T.; Cheng, W.; Wu, K.; Xie, D.; Huo, L.; Sun, Y.; Yang, C. A three-dimensional thiophene-annulated perylene bisimide as a fullerene-free acceptor fora high performance polymer solar cell with the highest PCE of 8.28% and a VOC over 1.0 V. J. Mater. Chem. C 2018, 6, 1136–1142. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.; Zeng, W.; Xia, S.; Zhou, F.; Chen, H.; He, F.; Yang, C. Regulating the optoelectronic properties of small molecule donors with multiple alternative electron-donor and acceptor units for organic solar cells. J. Mater. Chem. A 2018, 6, 8101–8108. [Google Scholar] [CrossRef]
- Lin, K.; Wang, S.; Wang, Z.; Yin, Q.; Liu, X.; Jia, J.; Jia, X.; Luo, P.; Jiang, X.; Duan, C.; et al. Electron acceptors with a truxene core and perylene diimide branches for organic solar cells: The effect of ring-fusion. Front. Chem. 2018, 6, 328. [Google Scholar] [CrossRef] [PubMed]
- Ostroverkhova, O. Nitroxide-Mediated radical polymerizations. In Handbook of Organic Materials for Electronic and Photonic Devices, 2nd ed.; Elsevier: Cambridge, MA, USA, 2018; pp. 81–83. [Google Scholar]
- Nowak-Król, A.; Würthner, F. Progress in the synthesis of perylene bisimide dyes. Org. Chem. Front. 2019, 6, 1272–1318. [Google Scholar] [CrossRef]
- Bressan, G.; Green, D.; Chan, Y.; Bulman Page, P.C.; Jones, G.A.; Meech, S.R.; Heisler, I.A. One- to two-exciton transitions in perylene bisimide dimer revealed by two dimensional electronic spectroscopy. J. Phys. Chem. A 2019, 123, 1594–1601. [Google Scholar] [CrossRef]
- Avellanal-Zaballa, E.; Durán-Sampedro, G.; Prieto-Castañeda, A.; Agarrabeitia, A.R.; García-Moreno, I.; López-Arbeloa, I.; Bañuelos, J.; Ortiz, M.J. Rational molecular design enhancing the photonic performance of red-emitting perylene bisimide dyes. Phys. Chem. Chem. Phys. 2017, 19, 13210–13218. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, X.; Wang, Y.; Li, Z. Supramolecular self-assembly of perylene bisimide derivatives assisted by various groups. Langmuir 2019, 35, 342–358. [Google Scholar] [CrossRef]
- Khan, Q.U.; Tian, G.; Bao, L.; Qi, S.; Wu, D. Highly uniform supramolecular nano-films derived from carbazole-containing perylene diimide via surface-supported self-assembly and their electrically bistable memory behavior. New J. Chem. 2018, 42, 11506–11515. [Google Scholar] [CrossRef]
- Yamauchi, M.; Masuo, S. Colloidal quantum dot arrangement assisted by perylene bisimide self-assembly. Chem. Eur. J. 2019, 25, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Slattum, P.; Wang, C.; Zang, L. Self-Assembly of perylene imide molecules into 1D nanostructures: Methods, morphologies, and applications. Chem. Rev. 2015, 115, 11967–11998. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Lee, H.W.; Mannsfeld, S.; Stoltenberg, R.M.; Jung, E.; Jin, Y.W.; Kim, J.M.; Yoo, J.B.; Bao, Z. Solution-Processed, high-performance n-channel organic microwire transistors. Proc. Natl. Acad. Sci. USA 2009, 106, 6065–60670. [Google Scholar]
- Würther, F. Perylene bisimide dyes as versatile building blocks for functional supramolecular Architectures. Chem. Commun. 2004, 35, 1564–1579. [Google Scholar] [CrossRef]
- Jones, B.A.; Ahrens, M.J.; Yoon, M.H.; Facchetti, A.; Marks, T.J.; Wasilewski, M.R. High-Mobility air-stable n-type semiconductors with processing versatility: Dicyanoperylene-3,4:9,10-bis(dicarboximides). Angew. Chem. Int. Ed. 2004, 43, 6363–6366. [Google Scholar] [CrossRef]
- Wen, Y.G.; Liu, Y.Q. Recent progress in n-channel organic thin-film transistors. Adv. Mater. 2010, 22, 1331–1345. [Google Scholar] [CrossRef]
- Mende-Schmidt, L.; Fechtenkötter, A.; Müllen, K.; Moons, E.; Friend, R.H.; MacKenzie, J.D. Self-Organized discotic liquid crystals for high-efficiency organic photovoltaics. Science 2001, 293, 1119–1122. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, Y.; Wang, J.; Hou, J.; Li, Y.; Zhu, D.; Zhan, X. A Star-shaped perylene diimide electron acceptor for high-performance organic solar cells. Adv. Mater. 2014, 26, 5137–5142. [Google Scholar] [CrossRef]
- Zhan, C.; Yao, J. More than conformational “twisting” or “coplanarity”: Molecular strategies for designing high-efficiency nonfullerene organic solar cells. Chem. Mater. 2016, 28, 1948–1964. [Google Scholar] [CrossRef]
- Chen, N.; Lu, J.; Wang, D.; Zheng, C.; Wu, H.; Zhang, H.; Gao, D. A double-cable poly(fluorene-alt-thiophene) with bay-substituted perylenediimide pendants: An efficient interfacial material in bulk-heterojunction solar cells. Macromolecules 2018, 51, 80–90. [Google Scholar] [CrossRef]
- Matussek, M.; Filapek, M.; Gancarz, P.; Krompiec, S.; Małecki, J.G.; Kotowicz, S.; Siwy, M.; Maćkowski, S.; Chrobok, A.; Schab-Balcerzak, E.; et al. Synthesis and photophysical properties of new perylene bisimide derivatives for application as emitting materials in OLEDs. Dyes Pigments 2018, 159, 590–599. [Google Scholar] [CrossRef]
- Meng, X.; Zhu, W.; Tian, H. Perylene bisimide derivatives for organic light-emitting diodes. In Introduction to Organic Electronic and Optoelectronic Materials and Devices, 2nd ed.; Sun, S.-S., Dalton, L.R., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 150–151. [Google Scholar]
- Mako, T.L.; Racicot, J.M.; Levine, M. Supramolecular luminescent sensors. Chem. Rev. 2019, 119, 322–477. [Google Scholar] [CrossRef]
- Shoyama, K.; Mahl, M.; Seifert, S.; Würthner, F. A general synthetic route to polycyclic aromatic dicarboximides by palladiumcatalyzed annulation reaction. J. Org. Chem. 2018, 83, 5339–5346. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.E.; Facchetti, A.; Heeney, M.; Marder, S.R.; Zhan, X. n-Type organic semiconductors in organic electronics. Adv. Mater. 2010, 22, 3876–3892. [Google Scholar] [CrossRef]
- Jung, B.J.; Tremblay, N.J.; Yeh, M.L.; Katz, H.E. Molecular design and synthetic approaches to electron-transporting organic transistorsemiconductors. Chem. Mater. 2011, 23, 568–582. [Google Scholar] [CrossRef]
- Sonawane, S.L.; Asha, S.K. Fluorescent cross-linked polystyrene perylenebisimide/oligo(p-phenylenevinylene)microbeads with controlled particle size, tunable colors, and high solid state emission. ACS Appl. Mater. Interfaces 2013, 5, 12205–12214. [Google Scholar] [CrossRef]
- Sun, J.P.; Hendsbee, A.D.; Dobson, A.J.; Welch, G.C.; Hill, I.G. Perylene diimide based all small-molecule organic solar cells: Impact of branched-alkylside chains on solubility, photophysics, self-assembly, and photovoltaic parameters. Org. Electron. 2016, 35, 151–157. [Google Scholar] [CrossRef]
- McAfee, S.M.; Topple, J.M.; Hill, I.G.; Welch, G.C. Key components to the recent performance increases of solution processed non-fullerenesmall molecule acceptors. J. Mater. Chem. A 2015, 3, 16393–16408. [Google Scholar] [CrossRef]
- Nielsen, C.B.; Holliday, S.; Chen, H.Y.; Cryer, S.J.; McCulloch, I. Non-Fullerene electron acceptors for use in organic solar cells. Acc. Chem. Res. 2015, 48, 2803–2812. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, J.; Zhang, Z.-G.; Bai, H.; Li, Y.; Zhu, D.; Zhan, X. An electronacceptor challenging fullerenes for efficient polymer solar cells. Adv. Mater. 2015, 27, 1170–1174. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhao, F.; He, Q.; Huo, L.; Wu, Y.; Parker, T.C.; Ma, W.; Sun, Y.; Wang, C.; Zhu, D.; et al. High-Performance electron acceptor with thienyl side chains for organic photovoltaics. J. Am. Chem. Soc. 2016, 138, 4955–4961. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; He, Q.; Zhao, F.; Huo, L.; Mai, J.; Lu, X.; Su, C.-J.; Li, T.; Wang, J.; Zhu, J.; et al. A facile planar fused-ring electron acceptor for as-cast polymer solar cells with 8.71% efficiency. J. Am. Chem. Soc. 2016, 138, 2973–2976. [Google Scholar] [CrossRef]
- Schmidt, C.D.; Lang, N.; Jux, N.; Hirsch, A. A facile route to water-soluble coronenes and benzo[ghi]perylenes. Chem. Eur. J. 2011, 17, 5289–5299. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.; Philipp, M.; Waigel, W.; Schmidt, D.; Würthner, F. Library of azabenz-annulated core-extended perylene derivatives with diverse substitution patterns and tunable electronic and optical properties. J. Org. Chem. 2016, 81, 8394–8405. [Google Scholar] [CrossRef] [PubMed]
- Vollbrecht, J.; Wiebeler, C.; Neuba, A.; Bock, H.; Schumacher, S.; Kitzerow, H. Bay-Extended distorted perylene esters showing visible luminescence after ultraviolet excitation: Photophysical and electrochemical analysis. J. Phys. Chem. C 2016, 120, 7839–7848. [Google Scholar] [CrossRef]
- Wang, R.; Li, G.; Zhang, A.; Wang, W.; Cui, G.; Zhao, J.; Shi, Z.; Tang, B. Efficient energy-level modification of novel pyran-annulated perylene diimides for photocatalytic water splitting. Chem. Commun. 2017, 53, 6918–6921. [Google Scholar] [CrossRef]
- Gupta, R.K.; Dey, A.; Singh, A.; Iyer, P.K.; Sudhakar, A.A. Heteroatom bay-annulated perylene bisimides: New materials for organic field effect transistors. ACS Appl. Electron. Mater. 2019, 1, 1378–1386. [Google Scholar] [CrossRef]
- Zang, Y.; Li, C.Z.; Chueh, C.C.; Williams, S.T.; Jiang, W.; Wang, Z.-H.; Yu, J.S.; Jen, A.K.Y. Integrated molecular, interfacial, and device engineering towards high-performance non-fullerene based organic solar cells. Adv. Mater. 2014, 26, 5708–5714. [Google Scholar] [CrossRef]
- Liu, T.; Ge, Y.; Sun, B.; Fowler, B.; Li, H.; Nuckolls, C.; Xiao, S. Synthesis, regioselective bromination, and functionalization of coronene tetracarboxydiimide. J. Org. Chem. 2019, 84, 2713–2720. [Google Scholar] [CrossRef]
- Hendsbee, A.D.; Sun, J.P.; Law, W.K.; Yan, H.; Hill, I.G.; Spasyuk, D.M.; Welch, G.C. Synthesis, self-assembly, and solar cell performance of N-annulated perylene non-fullerene acceptors. Chem. Mater. 2016, 28, 7098–7109. [Google Scholar] [CrossRef]
- Ball, M.L.; Zhang, B.; Xu, Q.; Paley, D.W.; Ritter, V.C.; Ng, F.; Steigerwald, M.L.; Nuckolls, C. Influence of molecular conformation on electron transport in giant, conjugated macrocycles. J. Am. Chem. Soc. 2018, 140, 10135–10139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; Huang, J.; Hu, H.; Zhang, G.; Ma, T.; Chow, P.C.Y.; Ade, H.; Pan, D.; Yan, H. Ring-Fusion of perylenediimide acceptor enabling efficient nonfullerene organic solar cells with a small voltage loss. J. Am. Chem. Soc. 2017, 139, 16092–16095. [Google Scholar] [CrossRef] [PubMed]
- Sekida, S.; Kameyama, T.; Koga, T.; Hadano, S.; Watanabe, S.; Niko, Y. Highly lipophilic and solid emissive N-annulated perylene bisimide synthesis for facile preparation of bright and far-red excimer fluorescent nano-emulsions with large Stokes shift. J. Photochem. Photobiol. A 2018, 364, 16–21. [Google Scholar] [CrossRef]
- Zhan, C.; Jiang, Y.-Y.; Yang, M.-Y.; Lu, L.-H.; Xiao, S.Q. Synthesis and optoelectronic properties of a novel molecular semiconductor of dithieno[5,6-b:11,12-b’]coronene-2,3,8,9-tetracarboxylic tetraester. Chin. Chem. Lett. 2014, 25, 65–68. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.; Li, M.; Chen, C.; Xu, S.; Tang, X.; Chen, L.; Chen, R.; Huang, W. Synthesis and application of perylene-embedded benzoazoles for small-molecule organic solar cells. Org. Lett. 2018, 20, 6376–6379. [Google Scholar] [CrossRef]
- Gupta, R.K.; Ulla, H.; Satyanarayan, M.N.; Sudhakar, A.A. A perylene-tiazine-based star-shaped green light emitter for organic light emitting diodes. Eur. J. Org. Chem. 2018, 2018, 1608–1613. [Google Scholar] [CrossRef]
- Yoshida, M.; Sakai, H.; Ohkubo, K.; Fukuzumi, S.; Hasobe, T. Inter- and intramolecular electron-transfer reduction properties of coronenediimides derivatives via photoinduced processes. J. Phys. Chem. C 2017, 122, 13333–13346. [Google Scholar] [CrossRef]
- Gupta, R.K.; Pathak, S.K.; Pradhan, B.; Gupta, M.; Pal, S.K.; Sudhakar, A.A. Bay-Annulated perylene tetraesters: A new class of discotic liquid crystals. ChemPhysChem 2016, 17, 859–872. [Google Scholar] [CrossRef]
- Liu, X.; Chen, M.; Xiao, C.; Xue, N.; Zhang, L. Soluble twisted diarenoperylenes: Synthesis, characterization, and device performance. Org. Lett. 2018, 20, 4512–4515. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Schneider, J.A.; Wei, Z.; Lai, W.-Y.; Huang, W.; Wudl, F.; Zheng, Y. Catalyst-Free one-step synthesis of ortho-tetraaryl perylene diimides for efficient OPV non-fullerene acceptors. J. Mater. Chem. C 2017, 5, 2781–2785. [Google Scholar] [CrossRef]
- Welsh, T.A.; Laventure, A.; Alahmadi, A.F.; Zhang, G.; Baumgartner, T.; Zou, Y.; Jäkle, F.; Welch, G.C. Borane incorporation in a non-fullerene acceptor to tune steric and electronic properties and improve organic solar cell performance. ACS Appl. Energy Mater. 2019, 2, 1229–1240. [Google Scholar] [CrossRef]
- Li, L.; Hong, Y.-J.; Chen, D.-Y.; Lin, M.J. A laterally extended perylene hexacarboxylate via Diels-Alder reaction for high-performance organic lithium-ion batteries. Electrochim. Acta 2017, 254, 255–261. [Google Scholar] [CrossRef]
- Schulze, M.; Steffen, A.; Würthner, F. Near-IR phosphorescent ruthenium(II) and iridium(III) perylene bisimide metal complexes. Angew. Chem. Int. Ed. 2015, 54, 1570–1573. [Google Scholar] [CrossRef]
- Liu, M.; Yang, J.; Lang, C.; Zhang, Y.; Zhou, E.; Liu, Z.; Guo, F.; Zhao, L. Fused perylene diimide-based polymeric acceptors for efficient all-polymer solar cells. Macromolecules 2017, 50, 7559–7566. [Google Scholar] [CrossRef]
- Zhao, D.; Wu, Q.; Cai, Z.; Zheng, T.; Chen, W.; Lu, J.; Yu, L. Electron acceptors based on α-substituted perylene diimide (PDI) for organic solar cells. Chem. Mater. 2016, 28, 1139–1146. [Google Scholar] [CrossRef]
- Takahashi, M.; Asaba, K.; Lua, T.T.; Inuzuka, T.; Uemura, N.; Sakamoto, M.; Sengoku, T.; Yoda, H. Controllable monobromination of perylene ring system: Synthesis of bay-functionalized perylene dyes. J. Org. Chem. 2018, 83, 624–631. [Google Scholar] [CrossRef]
- Kaufmann, C.; Bialas, D.; Stolte, M.; Würther, F. Discrete π-stacks of perylene bisimide dyes within folda-dimers: Insight into long- and short-range exciton coupling. J. Am. Chem. Soc. 2018, 140, 9986–9995. [Google Scholar] [CrossRef]
- Regar, R.; Mishra, R.; Mondal, P.K.; Sankar, J. Metal-Free annulation at the ortho- and bay-positions of perylene bisimide leading to lateral π-extension with strong NIR absorption. J. Org. Chem. 2018, 83, 9547–9552. [Google Scholar] [CrossRef]
- Mao, W.; Zhang, J.; Li, X.; Li, C.; Tian, H. Regioisomerically pure multiaryl coronene derivatives: Highly efficient synthesis via bay-extended perylene tetrabutylester. Chem. Commun. 2017, 53, 5052–5065. [Google Scholar] [CrossRef]
- Meng, D.; Liu, G.; Xiao, C.; Shi, Y.; Zhang, L.; Jiang, L.; Baldridge, K.K.; Li, Y.; Siegel, J.S.; Wang, Z. Corannurylene pentapetalae. J. Am. Chem. Soc. 2019, 141, 5402–5408. [Google Scholar] [CrossRef]
- Rao, K.V.; George, S.J. Synthesis and controllable self-assembly of a novel coronene bisimide amphiphile. Org. Lett. 2010, 12, 2656–2659. [Google Scholar] [CrossRef]
- Kelber, J.; Achard, M.F.; Durola, F.; Bock, H. Distorted arene core allows room-temperature columnar liquid-crystal glass with minimal side chains. Angew. Chem. Int. Ed. 2012, 124, 5200–5203. [Google Scholar] [CrossRef]
- Vollbrecht, J.; Bock, H.; Wiebeler, C.; Schumacher, S.; Kitzerow, H. Polycyclic aromatic hydrocarbons obtained by lateral core extension of mesogenic perylenes: Absorption and optoelectronic properties. Chem. Eur. J. 2014, 20, 12026–12031. [Google Scholar] [CrossRef]
- Schuster, N.J.; Paley, D.W.; Jockusch, S.; Ng, F.; Steigerwald, M.R.; Nuckolls, C. Electron delocalization in perylene diimide helicenes. Angew. Chem. Int. Ed. 2016, 55, 13519–13523. [Google Scholar] [CrossRef]
- Eversloh, C.L.; Li, C.; Müllen, K. Core-Extended perylene tetracarboxdiimides: The homologous series of coronene tetracarboxdiimides. Org. Lett. 2011, 13, 4148–4150. [Google Scholar] [CrossRef]
- Ito, H.; Segawa, Y.; Murakami, K.; Itami, K. Polycyclic arene synthesis by annulative π-extension. J. Am. Chem. Soc. 2019, 141, 3–10. [Google Scholar] [CrossRef]
- Nakamuro, T.; Kumazawa, K.; Ito, H.; Itami, K. Bay-region-selective annulative π-extension (APEX) of perylene diimides with arynes. Synlett 2019, 30, 423–428. [Google Scholar] [CrossRef]
- Zink-Lorre, N.; Doncel-Giménez, A.; Font-Sanchis, E.; Calbo, J.; Sastre-Santos, Á.; Ortí, E.; Fernández-Lázaro, F. Diels-Alder reaction on perylenediimides: Synthesis and theoretical study of core-expanded diimides. Org. Chem. Front. 2019, 6, 2860–2871. [Google Scholar] [CrossRef]
- Dyan, O.T.; Borodkin, G.I.; Zaikin, P.A. The Diels-Alder reaction for the synthesis of polycyclic aromatic compounds. Eur. J. Org. Chem. 2019, 2019, 7271–7306. [Google Scholar] [CrossRef]
- Clar, E. Über die Konstitution des Perylens; die Synthesen des 2.3,10.11-Dibenz- und des 1.12-Benz-perylens und betrachtungen über die Konstitution des benzanthrons und phenanthrens (Zur kenntnis mehrkerniger aromatischer kohlenwasserstoffe und ihrer abkӧmmlinge, XIV. Mitteil. Ber. Dtsch. Chem. Ges. 1932, 65, 846–859. [Google Scholar]
- Clar, E.; Zander, M.J. Syntheses of coronene and 1: 2–7: 8-dibenzocoronene. Chem. Soc. 1957, 4616–4619. [Google Scholar] [CrossRef]
- Herrero, M.A.; Kremsner, J.M.; Kappe, C.O. Nonthermal microwave effects revisited: on the importance of internal temperature monitoring and agitation in microwave chemistry. J. Org. Chem. 2008, 73, 136–147. [Google Scholar] [CrossRef]
- Manning, S.T.; Bogen, W.; Kelly, L.A. Synthesis, characterization, and photophysical study of fluorescent N-substituted benzo[ghi]perylene “swallow tail” monoimides. J. Org. Chem. 2011, 76, 6007–6013. [Google Scholar] [CrossRef]
- Zhou, C.; Li, W.; Chen, J.; Yang, M.; Li, Y.; Zhu, J.; Yu, C. Real-Time fluorometric turn-on assay for protease activity and inhibitor screening with a benzoperylene probe. Analyst 2014, 139, 1057–1062. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, H.; Li, Y.; Zhang, Q.; Li, J.; Zhang, C.; Zhou, C.; Yu, C. Peroxidase activity of the coronene bisimide supramolecular architecture and its applications in colorimetric sensing of H2O2 and glucose. J. Mater. Chem. C 2017, 32, 6572–6578. [Google Scholar] [CrossRef]
- Alibert-Fouet, S.; Seguy, I.; Bobo, J.-F.; Destruel, P.; Bock, H. Liquid-Crystalline and electron-deficient coronene oligocarboxylic esters and imides by twofold benzogenic Diels-Alder reactions on perylenes. Chem. Eur. J. 2007, 13, 1746–1753. [Google Scholar] [CrossRef]
- Jain, A.; Rao, K.V.; Kulkarni, C.; George, A.; George, S.J. Fluorescent coronene monoimide gels via H-bonding induced frustrated dipolar assembly. Chem. Commun. 2012, 48, 1467–1469. [Google Scholar] [CrossRef]
- Hopff, H.; Schweizer, H.R. Zur kenntnis des coronens. 2. Mitteilung. Dien-anlagerungen in der perylen- und benzperylenreihe. Helv. Chim. Acta 1959, 42, 2315–2333. [Google Scholar] [CrossRef]
- Hirayama, S.; Sakai, H.; Araki, Y.; Tanaka, M.; Imakawa, M.; Wada, T.; Takenobu, T.; Hasobe, T. Systematic control of the excited-state dynamics and carrier-transport properties of functionalized benzo[ghi]perylene and coronene derivatives. Chem. Eur. J. 2014, 20, 9081–9093. [Google Scholar] [CrossRef]
- Tokita, S.; Hiruta, K.; Kitahara, K.; Nishi, H. Diels-Alder reaction of dialkyl diazenedicarboxylates with perylenes; a new synthesis of polycyclic aromatic pyridazines. Synthesis 1982, 3, 229–231. [Google Scholar] [CrossRef]
- Ott, R.; Wiedemann, F.; Zinke, A. Untersuchungen über perylen und seine derivate, 67. Mitt.: Mehrkernige aromaten durch diensynthesen mit perylen. Mon. Chem. 1968, 99, 2032–2047. [Google Scholar] [CrossRef]
- Fort, E.H.; Scott, L.T. One-Step conversion of aromatic hydrocarbon bay regions into unsubstituted benzene rings: A reagent for the low-temperature, metal-free growth of single-chirality carbon nanotubes. Angew. Chem. Int. Ed. 2010, 49, 6626–6628. [Google Scholar] [CrossRef]
- Jackson, E.P.; Sisto, T.J.; Darzi, E.R.; Jasti, R. Probing Diels-Alder reactivity on a model CNT sidewall. Tetrahedron 2016, 72, 3754–3758. [Google Scholar] [CrossRef]
- Fort, E.H.; Donovan, P.M.; Scott, L.T. Diels-Alder reactivity of polycyclic aromatic hydrocarbon bay regions: Implications for metal-free growth of single-chirality carbon nanotubes. J. Am. Chem. Soc. 2009, 131, 16006–16007. [Google Scholar] [CrossRef]
- Domingo, R.E.; Arnó, M.; Contreras, R.; Pérez, P. Density Functional Theory study for the cycloaddition of 1,3-butadienes with dimethyl acetylenedicarboxylate. Polar stepwise vs concerted mechanisms. J. Phys. Chem. A 2002, 106, 952–961. [Google Scholar] [CrossRef]
- Krylov, I.M.; Mailyan, A.K.; Zotova, M.A.; Bruneau, C.; Dixneuf, P.H.; Osipov, S.N. Access to functionalized α-trifluoromethyl-α-aminophosphonates via intermolecular ene-yne metathesis. Synlett 2014, 25, 2624–2628. [Google Scholar] [CrossRef]
- Kurpanik, A.; Matussek, M.; Szafraniec-Gorol, G.; Filapek, M.; Lodowski, P.; Marcol-Szumilas, B.; Ignasiak, W.; Małecki, J.G.; Machura, B.; Małecka, M.; et al. APEX strategy represented by Diels-Alder cycloadditions—New opportunities for the syntheses of functionalised PAHs. Chem. Eur. J. 2020, 26, 12150–12157. [Google Scholar] [CrossRef]
- Pająk, M.; Kurpanik, A.; Zych, D.; Krompiec, S.; Matussek, M.; Marcol, B.; Filapek, M. Method for Obtaining 1′,2′-Bis(Methoxycarbonyl)-1,12-Benzoperylene or 1′,2′-Bis(Ethoxycarbonyl)-1,12-Benzoperylene. PL Patent PL234525B1, 30 January 2018. [Google Scholar]
- Krompiec, S.; Szafraniec-Gorol, G.; Lodowski, P.; Matussek, M.; Marcol-Szumilas, B.; Ignasiak, W. 1,2-Diarylbenzo[ghi]Perylenes and Method of Preparing Them. PL Patent Appl. PL427053A1, 13 September 2018. [Google Scholar]
- Szafraniec-Gorol, G.; Matussek, M.; Krompiec, S.; Jendrzejewska, I.; Marcol-Szumilas, B.; Ignasiak, W.; Gudwański, A. The Method of Preparation of 1,2-Diarylbenzo[ghi]Perylenes. PL Patent Appl. PL431010A1, 30 August 2019. [Google Scholar]
- Zimmermann, G. Cycloaromatization of open and masked 1,3-hexadien-5-ynes—Mechanistic and synthetic aspects. Eur. J. Org. Chem. 2001, 457–471. [Google Scholar] [CrossRef]
- Yang, V.; Bam, R.; Catalano, V.J.; Chalifoux, W.A. Highly regioselective domino benzannulation reaction of buta-1,3-diynes to construct irregular nanographenes. Angew. Chem. Int. Ed. 2018, 57, 14773–14777. [Google Scholar] [CrossRef]
- Chen, T.-A.; Liu, R.-S. Synthesis of polyaromatic hydrocarbons from bis(biaryl)diynes: Large PAHs with low Clar sextets. Chem. Eur. J. 2011, 17, 8023–8027. [Google Scholar] [CrossRef]
- Liu, J.; Li, B.W.; Tan, Y.Z.; Giannakopoulos, A.; Sanchez-Sanchez, C.; Beljonne, D.; Ruffieux, P.; Fasel, R.; Feng, X.; Müllen, K. Toward cove-edged low band gap graphene nanoribbons. J. Am. Chem. Soc. 2015, 137, 6097–6103. [Google Scholar] [CrossRef]
- Lòpez, F.; Mascareñas, J.L. Recent developments in gold-catalyzed cycloaddition reactions. Beilstein J. Org. Chem. 2011, 7, 1075–1094. [Google Scholar] [CrossRef]
- Paternò, G.M.; Chen, Q.; Wang, X.Y.; Liu, J.; Motti, S.G.; Petrozza, A.; Feng, X.; Lanzani, G.; Müllen, K.; Narita, A.; et al. Synthesis of dibenzo[hi,st]ovalene and its amplified spontaneous emission in a polystyrene matrix. Angew. Chem. Int. Ed. 2017, 56, 6753–6757. [Google Scholar] [CrossRef]
- Hitt, D.M.; O’Connor, J.M. Acceleration of conjugated dienyne cycloaromatization. Chem. Rev. 2011, 111, 7904–7922. [Google Scholar] [CrossRef]
- Narita, A.; Wang, X.Y.; Feng, X.; Müllen, K. New advances in nanographene chemistry. Chem. Soc. Rev. 2015, 44, 6616–6643. [Google Scholar] [CrossRef]
- Aguilar, E.; Sanz, R.; Fernández-Rodríguez, M.; García-García, P. 1,3-Dien-5-ynes: Versatile building blocks for the synthesis of carbo- and heterocycles. Chem. Rev. 2016, 116, 8256–8311. [Google Scholar] [CrossRef]
- Szafraniec-Gorol, G.; Krompiec, S.; Ignasiak, W. Pi-Extended Perylene Derivative and the Method of Its Production. PL Patent Appl. PL431011A1, 30 August 2019. [Google Scholar]
- Szafraniec-Gorol, G.; Krompiec, S.; Matussek, M.; Orszulak, L. Pi-Extended Perylene Derivative and the Method of Its Production. PL Patent Appl. PL431015A1, 30 August 2019. [Google Scholar]
- Stork, G.; Matsuda, K. Preparation of Benzocoronene and Intermediates. U.S. Patent US3364275A, 16 January 1968. [Google Scholar]
- Pavlyuk, D.E.; Gundala, S.; Kovalev, I.S.; Kopchuk, D.S.; Krinochkin, A.P.; Budeev, A.V.; Zyryanov, G.V.; Venkatapuram, P.; Rusinov, V.L.; Chupakhin, O.N. Reactions of perylene with aryne intermediates. Russ. J. Org. Chem. 2019, 55, 409–411. [Google Scholar]
- Fort, E.H.; Scott, L.T. Gas-phase Diels-Alder cycloaddition of benzyne to an aromatic hydrocarbon bay region: Groundwork for the selective solvent-free growth of armchair carbon nanotubes. Tetrahedron Lett. 2011, 52, 2051–2053. [Google Scholar] [CrossRef]
- Schuler, B.; Collazos, S.; Gross, L.; Meyer, G.; Pérez, D.; Guitián, E.; Peña, D. From perylene to a 22-ring aromatic hydrocarbon in one-pot. Angew. Chem. Int. Ed. 2014, 53, 9004–9006. [Google Scholar] [CrossRef]
- Rüdiger, E.C.; Porz, M.; Schaffroth, M.; Rominger, F.; Bunz, U.H.F. Synthesis of soluble, alkyne-substituted trideca- and hexadeca-starphenes. Chem. Eur. J. 2014, 20, 12725–12728. [Google Scholar] [CrossRef] [PubMed]
- Pérez, D.; Peña, D.; Guitián, E. Aryne Cycloaddition Reactions in the Synthesis of Large Polycyclic Aromatic Compounds. Eur. J. Org. Chem. 2013, 2013, 5981–6013. [Google Scholar]
- Kumarasinghe, K.G.U.R.; Fronczek, F.R.; Valle, H.U.; Sygulla, A. Bis-corannulenoanthracene: An angularly fused pentacene as a precursor for barrelene-tethered receptors for fullerenes. Org. Lett. 2016, 18, 3054–3057. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, C.; Wen, C.; Zhang, K.; Liu, W.; Chen, Q. Gold-Catalyzed cyclotrimerization of arynes for the synthesis of triphenylenes. Catal. Commun. 2015, 65, 81–84. [Google Scholar] [CrossRef]
- Xu, F.; Xiao, X.; Hoye, T.R. Reactions of HDDA-derived benzynes with perylenes: Rapid construction of polycyclic aromatic compounds. Org. Lett. 2016, 18, 5636–5639. [Google Scholar] [CrossRef]
- Xu, F.; Xiao, X.; Hoye, T.R. Photochemical hexadehydro-Diels-Alder reaction. J. Am. Chem. Soc. 2017, 139, 8400–8403. [Google Scholar] [CrossRef]
- Xiao, X.; Hoye, T.R. The domino hexadehydro-Diels-Alder reaction transforms polyynes to benzynes to naphthynes to anthracynes to tetracynes (and beyond?). Nat. Chem. 2018, 10, 838–844. [Google Scholar] [CrossRef]
- Kurpanik, A.; Krompiec, S.; Marcol-Szumilas, B.; Łucka, J. The Method of Naphtho-, Anthraceno- and Phenanthro[1,2,3,4-ghi]Perylenes Preparation. PL Patent Appl. PL430591A1, 13 July 2019. [Google Scholar]
- Kurpanik, A.; Krompiec, S.; Łucka, J. The Method of Naphtho[1,2,3,4-ghi]Perylene Preparation. PL Patent Appl. PL430590A1, 13 July 2019. [Google Scholar]
- Kurpanik, A.; Krompiec, S.; Marcol-Szumilas, B.; Łucka, J. The Method of Naphtho-, Anthraceno- and Phenanthro[1,2,3,4-ghi]Perylenes Preparation. PL Patent Appl. PL430593A1, 13 July 2019. [Google Scholar]
- Kurpanik, A.; Krompiec, S.; Marcol-Szumilas, B.; Grabowska, A. The Method of Naphtho-, Anthraceno- and Phenanthro[1,2,3,4-ghi]Perylenes Preparation. PL Patent Appl. PL430592A1, 13 July 2019. [Google Scholar]
- Cammidge, A.N.; Gopee, H. Perylenophthalocyanines. Chem. Eur. J. 2006, 12, 8609–8613. [Google Scholar] [CrossRef]
- Gӧltner, C.; Pressner, D.; Müllen, K.; Spiess, H.W. Liquid-Crystalline perylene derivatives as “Discotic pigments”. Angew. Chem. Int. Ed. Engl. 1993, 32, 1660–1662. [Google Scholar] [CrossRef]
- Schlichting, P.; Rohr, U.; Müllen, K. Easy synthesis of liquid crystalline perylene derivatives. J. Mater. Chem. 1998, 8, 2651–2655. [Google Scholar] [CrossRef]
- Jiang, W.; Qian, H.; Li, Y.; Wang, Z. Heteroatom-annulated perylenes: Practical synthesis, photophysical properties, and solid-state packing arrangement. J. Org. Chem. 2008, 73, 7369–7372. [Google Scholar] [CrossRef]
- Kelber, J.; Achard, M.F.; Garreau-de Bonneval, B.; Bock, H. Columnar benzoperylene-hexa- and tetracarboxylic imides and esters: Synthesis, mesophase stabilisation and observation of charge-transfer interactions between electron-donating esters and electron-accepting imides. Chem. Eur. J. 2011, 17, 8145–8155. [Google Scholar]
- Choi, M.; Do, H.Y. Synthesis of perylene dianhydride-incorporated main chain polyimides and sequential structural transformation through a dipolar cycloaddition. React. Func. Polym. 2014, 84, 37–44. [Google Scholar] [CrossRef]
- Langhals, H.; Kirner, S.; Blanke, P.; Speckbacher, M.; Langhals, H.; Kirner, S.; Blanke, P.; Speckbacher, M. Core-Extended Perylene Bisimides. U.S. Patent US6491749B1, 10 December 2002. [Google Scholar]
- Langhals, H.; Kirner, S. Novel fluorescent dyes by the extension of the core of perylenetetracarboxylic bisimides. Eur. J. Org. Chem. 2000, 2000, 365–380. [Google Scholar] [CrossRef]
- Krompiec, S.; Szafraniec-Gorol, G.; Matussek, M.; Ignasiak, W.; Filapek, M. 2,3-Diphenyl-N,N′-bis(2-Ethylhexyl)Benzo[ghi]Perylenediimide and the Method of Its Production. PL Patent Appl. PL433403A1, 31 March 2020. [Google Scholar]
- Krompiec, S.; Szafraniec-Gorol, G.; Matussek, M.; Ignasiak, W.; Lodowski, P. 2,3-bis[7-(9H-Carbazol-9-yl)-9,9-Dibutylfluoren-2-yl]-N,N′-bis(2-Ethylhexyl)Benzo[ghi]Perylenediimide and the Method of Its Production. PL Patent Appl. PL433405A1, 31 March 2020. [Google Scholar]
- Krompiec, S.; Szafraniec-Gorol, G.; Matussek, M.; Ignasiak, W.; Lodowski, P. 2,3-bis[N-(2-Ethylhexyl)Phthalimid-4-yl]-N,N′-bis(2-Ethylhexyl)Benzo[ghi]Perylenediimide and the Method of Its Production. PL Patent Appl. PL433407A1, 31 March 2020. [Google Scholar]
- Krompiec, S.; Szafraniec-Gorol, G.; Matussek, M.; Ignasiak, W.; Filapek, M. 2,3-Diphenyl-N,N′-bis(2,6-Diisopropylphenyl)Benzo[ghi]Perylenediimide and the Method of Its Production. PL Patent Appl. PL433408A1, 31 March 2020. [Google Scholar]
- Kurpanik, A.; Marcol-Szumilas, B.; Krompiec, S.; Gołek, B. Preparation Method of Tetrabenzyl Anthracene[1,2,3,4-ghi]Perylene-7,8,13,14-Tetracarboxylate. PL Patent Appl. PL430585A1, 13 July 2019. [Google Scholar]
- Kurpanik, A.; Marcol-Szumilas, B.; Krompiec, S.; Gołek, B. Preparation Method of Tetrabenzyl Phenanthro[1,2,3,4-ghi]Perylene-6,7,12,13-Tetracarboxylate. PL Patent Appl. PL430587A1, 13 July 2019. [Google Scholar]
- Kurpanik, A.; Marcol-Szumilas, B.; Krompiec, S.; Gołek, B. Preparation Method of Tetrabenzyl Naphtho[1,2,3,4-ghi]Perylene-4,5,10,11-Tetracarboxylate. PL Patent Appl. PL430583A1, 13 July 2019. [Google Scholar]
- Kurpanik, A.; Marcol-Szumilas, B.; Krompiec, S.; Grabowska, A.; Gołek, B. 1,2-Diphenylbenzo[1,2-j]Coronene and the Method of Its Preparation. PL Patent Appl. PL430584A1, 13 July 2019. [Google Scholar]
- Kurpanik, A.; Ignasiak, W.; Krompiec, S.; Matussek, M.; Gołek, B.; Grabowska, A. 1,2-Bis(9,9-Dibutylfluoen-2-yl)Benzo[1,2-j]Coronene and the Method of Its Preparation. PL Patent Appl. PL430588A1, 13 July 2019. [Google Scholar]
- Kurpanik, A.; Szafraniec-Gorol, G.; Krompiec, S.; Matussek, M.; Gołek, B. 1,2-Bis(N-2-Etylhexylphthalimido-4-yl)Benzo[1,2-j]Coronene and the Method of Its Preparation. PL Patent Appl. PL430589A1, 13 July 2019. [Google Scholar]
- Kurpanik, A.; Krompiec, S. 1,2-di(Metoxycarbonyl)Benzo[1,2-j]Coronene and Method of Its Preparation. PL Patent Appl. PL433281A1, 18 March 2020. [Google Scholar]
- Zander, M. Relative reaktionsgeschwindigkeiten der maleinsäureanhydrid-addition an kohlenwasserstoffe der perylen- und 1.12-benzperylen-reihe. Liebigs Ann. Chem. 1969, 723, 27–33. [Google Scholar] [CrossRef]
- Clar, E. Synthesis of ovalene. Nature 1948, 161, 238–239. [Google Scholar] [CrossRef]
- Jinling, L.; Baozhan, X.; Jin, P. Synthesis of bisanthene-based polycyclic aromatic hydrocarbons. Chin. J. Org. Chem. 2015, 35, 1441–1450. [Google Scholar]
- Fort, E.H.; Jeffreys, M.S.; Scott, L.T. Diels-Alder cycloaddition of acetylene gas to a polycyclic aromatic hydrocarbon bay region. Chem. Commun. 2012, 48, 8102–8104. [Google Scholar] [CrossRef]
- Konishi, A.; Hirao, Y.; Matsumoto, K.; Kurata, H.; Kubo, T. Facile synthesis and lateral π-expansion of bisanthenes. Chem. Lett. 2013, 42, 592–594. [Google Scholar] [CrossRef]
- Li, J.; Jiao, C.; Huang, K.W.; Wu, J. Lateral extension of π conjugation along the bay regions of bisanthene through a Diels-Alder cycloaddition reaction. Chem. Eur. J. 2011, 17, 14672–14680. [Google Scholar] [CrossRef]
- Kruse, H.; Goerigk, L.; Grimme, S. Why the standard B3LYP/6-31G* model chemistry should not be used in DFT calculations of molecular thermochemistry: Understanding and correcting the problem. J. Org. Chem. 2012, 77, 10824–10834. [Google Scholar] [CrossRef]
- Cao, Y.; Osuna, S.; Liang, Y.; Haddon, R.C.; Houk, K.N. Diels-Alder reactions of graphene: Computational predictions of products and sites of reaction. J. Am. Chem. Soc. 2013, 135, 17643–17649. [Google Scholar] [CrossRef]
- Lei, H.; Langlois, A.; Fortin, D.; Karsenti, P.L.; Aly, S.M.; Harvey, P.D. Renderingcross-Conjugated azophenine derivatives emissive to probe the silent photophysical properties of emeraldine. Phys. Chem. Chem. Phys. 2017, 19, 21532–21539. [Google Scholar] [CrossRef]
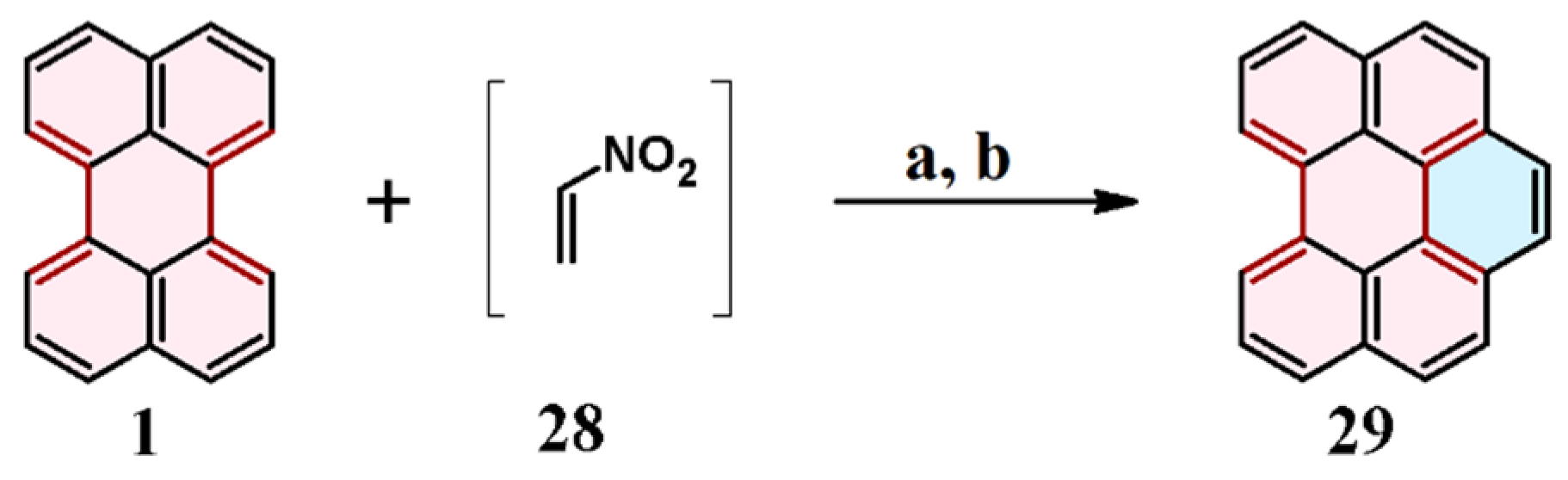
| PAH Structure | 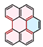 | 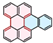 | 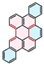 | 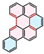 | 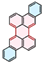 | 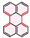 |
|---|---|---|---|---|---|---|
| Relative reaction rate | 1 | 7 | 9 | 38 | 175 | 220 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurpanik, A.; Matussek, M.; Lodowski, P.; Szafraniec-Gorol, G.; Krompiec, M.; Krompiec, S. Diels–Alder Cycloaddition to the Bay Region of Perylene and Its Derivatives as an Attractive Strategy for PAH Core Expansion: Theoretical and Practical Aspects. Molecules 2020, 25, 5373. https://doi.org/10.3390/molecules25225373
Kurpanik A, Matussek M, Lodowski P, Szafraniec-Gorol G, Krompiec M, Krompiec S. Diels–Alder Cycloaddition to the Bay Region of Perylene and Its Derivatives as an Attractive Strategy for PAH Core Expansion: Theoretical and Practical Aspects. Molecules. 2020; 25(22):5373. https://doi.org/10.3390/molecules25225373
Chicago/Turabian StyleKurpanik, Aneta, Marek Matussek, Piotr Lodowski, Grażyna Szafraniec-Gorol, Michał Krompiec, and Stanisław Krompiec. 2020. "Diels–Alder Cycloaddition to the Bay Region of Perylene and Its Derivatives as an Attractive Strategy for PAH Core Expansion: Theoretical and Practical Aspects" Molecules 25, no. 22: 5373. https://doi.org/10.3390/molecules25225373
APA StyleKurpanik, A., Matussek, M., Lodowski, P., Szafraniec-Gorol, G., Krompiec, M., & Krompiec, S. (2020). Diels–Alder Cycloaddition to the Bay Region of Perylene and Its Derivatives as an Attractive Strategy for PAH Core Expansion: Theoretical and Practical Aspects. Molecules, 25(22), 5373. https://doi.org/10.3390/molecules25225373