Evaluation of Antioxidant and Anti-Inflammatory Activity of Anthocyanin-Rich Water-Soluble Aronia Dry Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Total Phenolic Content
2.4. Identification and Quantification of Anthocyanins and Phenolic Acids by HPLC-DAD Method
2.5. Quantification of Anthocyanins by UV-VIS Method
2.6. DPPH Scavenging Activity (Electron Paramagnetic Resonance Test)
2.7. Oxygen Radical Absorbance Capacity (ORAC Assay)
2.8. In Vitro Anti-Inflammatory Activity
2.9. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Aronia Extracts
3.2. Cytotoxicity Assay
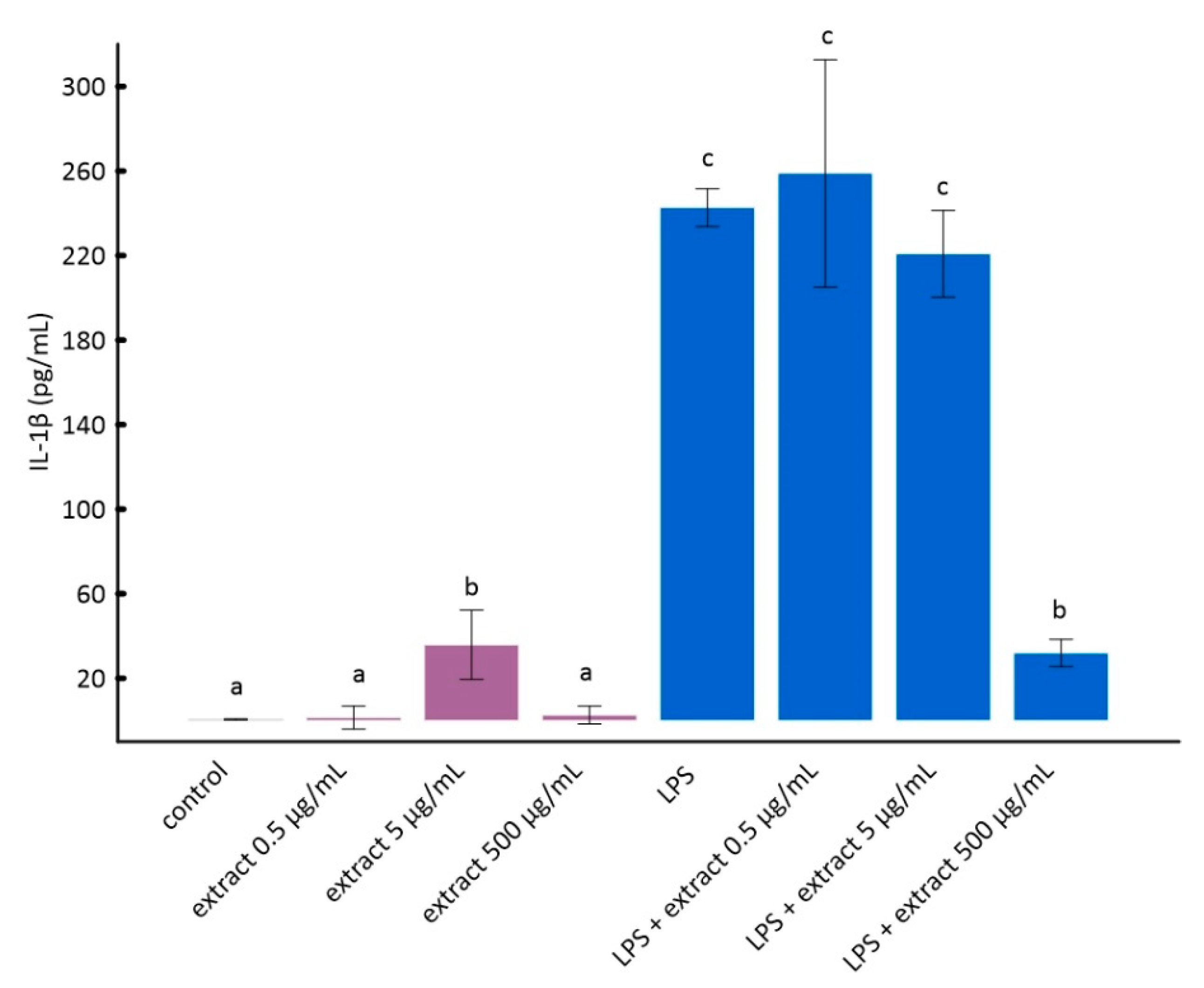
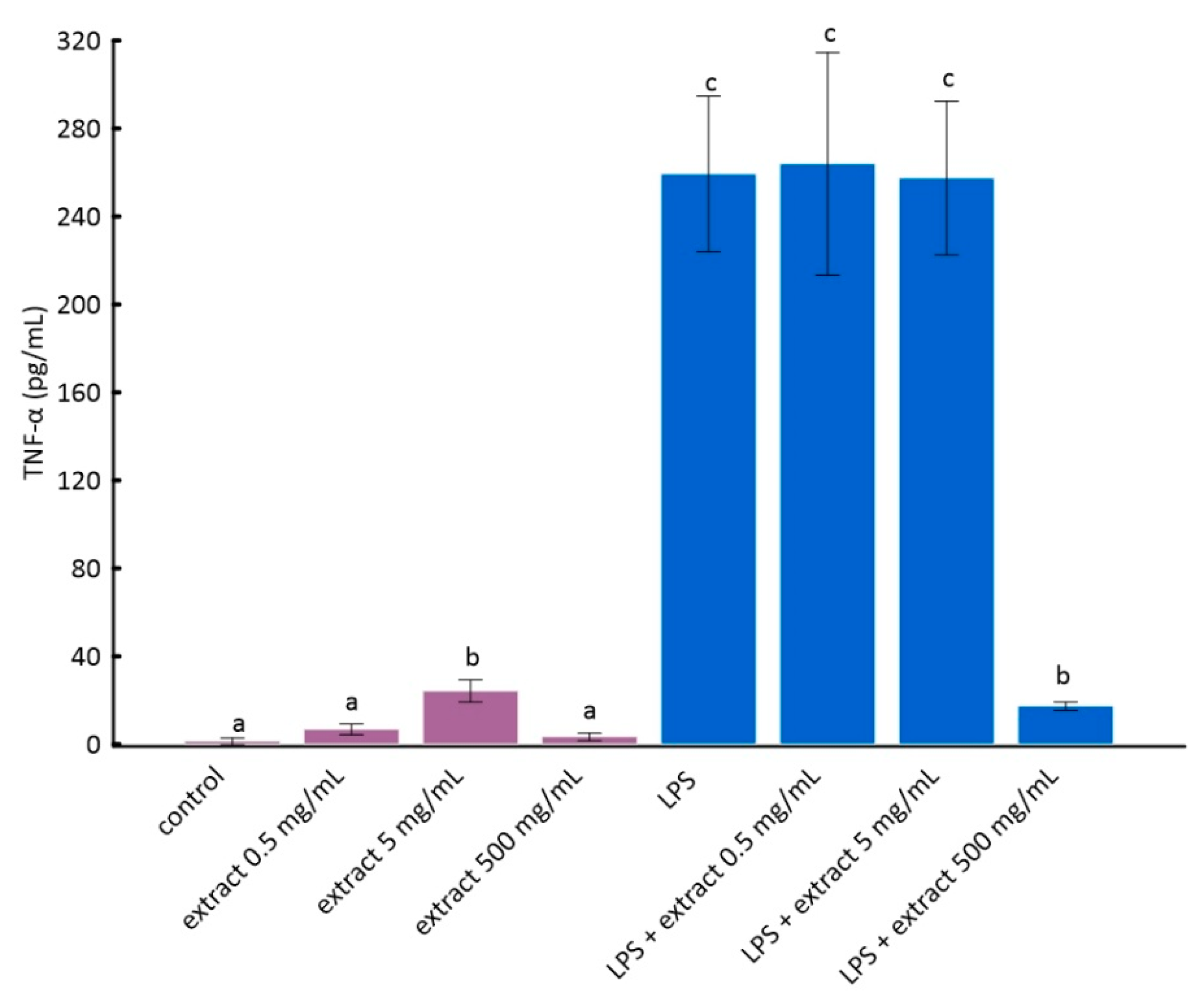
3.3. Effect of Aronia Extract Concentrations on Tumor Necrosis Factor α (TNF-α) and Interleukin 1β (IL-1β) in LPS-Stimulated RAW 264.7 Cells
3.4. Lipid Peroxidation MDA Assay
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Symbols and Abbreviations
| ADE | Aronia dry extract |
| C3G | Cyanidin-3-glucoside chloride |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| EPR | Electron Paramagnetic Resonance |
| FBS | Fetal bovine serum |
| hs-CRP | High-sensitivity C-reactive protein |
| IL-1β | Interleukin-1 beta |
| LPS | Lipopolysaccharide |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| MDA | Malondialdehyde |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| ORAC | Oxygen Radical Absorbance Capacity |
| Ox-LDL | Oxidized low-density lipoprotein |
| PBS | Phosphate-buffered saline |
| PUFAs | Polyunsaturated fatty acids |
| ROS | Reactive oxygen species |
| SPE | Solid Phase Extraction |
| SD | Standard Deviation |
| TNF-α | Tumor necrosis factor α |
References
- Ammendola, M.; Haponska, M.; Balik, K.; Modrakowska, P.; Matulewicz, K.; Kazmierski, L.; Lis, A.; Kozlowska, J.; Garcia-Valls, R.; Giamberini, M.; et al. Stability and anti-proliferative properties of biologically active compounds extracted from Cistus L. after sterilization treatments. Sci. Rep. 2020, 10, 6521. [Google Scholar] [CrossRef] [PubMed]
- Calvani, M.; Pasha, A.; Favre, C. Nutraceutical Boom in Cancer: Inside the Labyrinth of Reactive Oxygen Species. Int. J. Mol. Sci. 2020, 21, 1936. [Google Scholar] [CrossRef] [PubMed]
- Montané, X.; Kowalczyk, O.; Reig-Vano, B.; Bajek, A.; Roszkowski, K.; Tomczyk, R.; Pawliszak, W.; Giamberini, M.; Mocek-Płóciniak, A.; Tylkowski, B. Current Perspectives of the Applications of Polyphenols and Flavonoids in Cancer Therapy. Molecules 2020, 25, 3342. [Google Scholar] [CrossRef] [PubMed]
- Sammar, M.; Abu-Farich, B.; Rayan, I.; Falah, M.; Rayan, A. Correlation between cytotoxicity in cancer cells and free radical-scavenging activity: In vitro evaluation of 57 medicinal and edible plant extracts. Oncol. Lett. 2019, 18, 6563–6571. [Google Scholar] [CrossRef]
- Sawicka, B.; Ziarati, P.; Krochmal-Marczak, B.; Skiba, D. Nutraceuticals in food and pharmacy. A Review. Agron. Sci. 2019, 74, 7–31. [Google Scholar] [CrossRef]
- Kokotkiewicz, A.; Jaremicz, Z.; Luczkiewicz, M. Aronia Plants: A Review of Traditional Use, Biological Activities, and Perspectives for Modern Medicine. J. Med. Food 2010, 13, 255–269. [Google Scholar] [CrossRef]
- Lee, K.P.; Choi, N.H.; Kim, H.S.; Ahn, S.; Park, I.S.; Lee, D.W. Anti-neuroinflammatory effects of ethanolic extract of black chokeberry (Aronia melanocapa L.) in lipopolysaccharide-stimulated BV2 cells and ICR mice. Nutr. Res. Pract. 2018, 12, 13–19. [Google Scholar] [CrossRef]
- Sidor, A.; Drożdżyńska, A.; Gramza-Michałowska, A. Black chokeberry (Aronia melanocarpa) and its products as potential health-promoting factors-An overview. Trends Food Sci. Technol. 2019, 89, 45–60. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.I.; Lee, I.; Lee, S.; Hwang, M.W.; Bae, J.Y.; Heo, J.; Kim, D.; Han, S.Z.; Park, M.S. Aronia melanocarpa and its components demonstrate antiviral activity against influenza viruses. Biochem. Biophys. Res. Commun. 2013, 440, 14–19. [Google Scholar] [CrossRef]
- Walkowiak-Tomczak, D. Changes in antioxidant activity of black chokeberry juice concentrate solutions during storage. Acta Sci. Pol. Technol. Aliment. 2007, 6, 49–54. [Google Scholar]
- Patras, A.; Brunton, N.P.; O’Donnell, C.; Tiwari, B.K. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Gardana, C.; Scialpi, A.; Fachechi, C.; Simonetti, P. Near-infrared spectroscopy and chemometrics for the routine detection of bilberry extract adulteration and quantitative determination of the anthocyanins. J. Spectrosc. 2018, 4751247. [Google Scholar] [CrossRef]
- Davinelli, S.; Bertoglio, J.C.; Zarrelli, A.; Pina, R.; Scapagnini, G. A Randomized Clinical Trial Evaluating the Efficacy of an Anthocyanin–Maqui Berry Extract (Delphinol®) on Oxidative Stress Biomarkers. J. Am. Coll. Nutr. 2015, 34, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Borowska, S.; Tomczyk, M.; Strawa, J.W.; Brzóska, M.M. Estimation of the Chelating Ability of an Extract from Aronia melanocarpa L. Berries and Its Main Polyphenolic Ingredients Towards Ions of Zinc and Copper. Molecules 2020, 25, 1507. [Google Scholar] [CrossRef]
- Scaglione, F.; Pannacci, M.; Petrini, O. The standardised G115® Panax ginseng C.A. Meyer extract: A review of its properties and usage. J. Evid. Based Integr. Med. 2005, 2, 195–206. [Google Scholar] [CrossRef]
- Wu, X.; Gu, L.; Prior, R.L.; McKay, S. Characterization of Anthocyanins and Proanthocyanidins in Some Cultivars of Ribes, Aronia, and Sambucus and Their Antioxidant Capacity. J. Agric. Food Chem. 2007, 52, 7846–7856. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. J. Agric. Food Chem. 2003, 51, 502–509. [Google Scholar] [CrossRef]
- Dąbrowski, A.; Onopiuk, B.M.; Car, H.; Onopiuk, P.; Dąbrowska, Z.N.; Rogalska, J.; Brzóska, M.M.; Dąbrowska, E. Beneficial Impact of an Extract from the Berries of Aronia melanocarpa L. on the Oxidative-Reductive Status of the Submandibular Gland of Rats Exposed to Cadmium. Antioxidants 2020, 9, 185. [Google Scholar] [CrossRef]
- Wangensteen, H.; Bräunlich, M.; Nikolic, V.; Malterud, K.E.; Slimestad, R.; Barsett, H. Anthocyanins, proanthocyanidins, and total phenolics in four cultivars of aronia: Antioxidant and enzyme inhibitory effects. J. Funct. Foods 2014, 7, 746–752. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Sak, A.; Witrowa-Rajchert, D. Influence of the carrier material on the stability of chokeberry juice microcapsules. Int. Agrophys. 2019, 33, 517–525. [Google Scholar] [CrossRef]
- Bednarska, M.A.; Janiszewska-Turak, E. The influence of spray drying parameters and carrier material on the physico-chemical properties and quality of chokeberry juice powder. J. Food Sci. Technol. 2020, 57, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Jurikova, T.; Mlcek, J.; Skrovankova, S.; Sumczynski, D.; Sochor, J.; Hlavacova, I.; Snopek, L.; Orsavova, J. Fruits of black chokeberry Aronia melanocarpa in the prevention of chronic diseases. Molecules 2017, 22, 944. [Google Scholar] [CrossRef] [PubMed]
- Lorenzon dos Santos, J.; Schaan de Quadros, A.; Weschenfelder, C.; Bueno Garofallo, S.; Marcadenti, A. Oxidative Stress Biomarkers, Nut-Related Antioxidants, and Cardiovascular Disease. Nutrients 2020, 12, 682. [Google Scholar] [CrossRef] [PubMed]
- Naruszewicz, M.; Łaniewska, I.; Millo, B.; Dłuzniewski, M. Combination therapy of statin with flavonoids rich extract from chokeberry fruits enhanced reduction in cardiovascular risk markers in patients after myocardial infraction (MI). Atherosclerosis 2007, 194, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.R.; Gochenaur, K. Direct vasoactive and vasoprotective properties of anthocyanin-rich extracts. J. Appl. Physiol. 2006, 100, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Denev, P.N.; Kratchanov, C.G.; Ciz, M.; Lojek, A.; Kratchanova, M.G. Bioavailability and Antioxidant Activity of Black Chokeberry (Aronia melanocarpa) Polyphenols: In vitro and in vivo Evidences and Possible Mechanisms of Action: A Review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 471–489. [Google Scholar] [CrossRef]
- Hwang, S.J.; Kim, Y.W.; Park, Y.; Lee, H.J.; Kim, K.W. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm. Res. 2014, 63, 81–90. [Google Scholar] [CrossRef]
- Ji, H.; Pettit, A.; Ohmura, K.; Ortiz-Lopez, A.; Duchatelle, V.; Degott, C. Critical Roles for Interleukin 1 and Tumor Necrosis Factor α in Antibody-induced Arthritis. J. Exp. Med. 2002, 196, 77–85. [Google Scholar] [CrossRef]
- Feghali, C.A.; Wright, T.M. Cytokines in Acute and chronic inflammation. Front. Biosci. 1997, 2, 12–26. Available online: https://www.bioscience.org/1997/v2/d/feghali1/htmls/feghali.pdf (accessed on 4 September 2020).
- Tolić, M.T.; Krbavčić, I.P.; Vujević, P.; Milinović, B.; Jurčević, I.L.; Vahčić, N. Effects of Weather Conditions on Phenolic Content and Antioxidant Capacity in Juice of Chokeberries (Aronia melanocarpa L.). Pol. J. Food Nutr. Sci. 2017, 67, 67–74. [Google Scholar] [CrossRef]
- Gralec, M.; Wawer, I.; Zawada, K. Aronia melanocarpa berries: Phenolics composition and antioxidant properties changes during fruit development and ripening. Emir. J. Food Agric. 2019, 31, 214–221. [Google Scholar] [CrossRef]
- Kujawski, W.; Sobolewska, A.; Jarzynka, K.; Güell, C.; Ferrando, M.; Warczok, J. Application of osmotic membrane distillation process in red grape juice concentration. J. Food Eng. 2013, 116, 801–808. [Google Scholar] [CrossRef]
- Zainol, M.K.; Abd-Hamid, A.; Yusof, S.; Muse, R. Antioxidative activity and total phenolic compounds of leaf, root and petiole of four accessions of Centella asiatica (L.) Urban. Food Chem. 2003, 81, 575–581. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Sanna, D.; Delogu, G.; Mulas, M.; Schirra, M.; Fadda, A. Determination of Free Radical Scavenging Activity of Plant Extracts Through DPPH Assay: An EPR and UV—Vis Study. Food Anal. Methods 2012, 5, 759–766. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Hambleton, J.; Weinstein, S.L.; Lem, L.; Defranco, A.L. Activation of c-Jun N-terminal kinase in bacterial lipopolysaccharide-stimulated macrophages. Proc. Natl. Acad. Sci. USA 1996, 93, 2774–2778. [Google Scholar] [CrossRef]
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnol. Annu. Rev. 2005, 11, 127–152. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, B.; Ruusunen, V.; Laaksonen, O.; Tahvonen, R.; Hellsten, J.; Kallio, H. Compositional Differences of Phenolic Compounds between Black Currant (Ribes nigrum L.) Cultivars and Their Response to Latitude and Weather Conditions. J. Agric. Food Chem. 2012, 60, 6581–6593. [Google Scholar] [CrossRef]
- Concepción Ramosa, M.; Pérez-Álvarezb, E.P.; Peregrinac, F.; Martínez de Todad, F. Relationships between grape composition of Tempranillo variety and available soil water and water stress under different weather conditions. Sci. Hortic. 2020, 262, 109063. [Google Scholar]
- Zielińska, A.; Siudem, P.; Paradowska, K.; Gralec, M.; Kaźmierski, S.; Wawer, I. Aronia melanocarpa Fruits as a Rich Dietary Source of Chlorogenic Acids and Anthocyanins: 1H-NMR, HPLC-DAD, and Chemometric Studies. Molecules 2020, 25, 3234. [Google Scholar] [CrossRef] [PubMed]
- Wathon, M.H.; Beaumont, N.; Benohoud, M.; Blackburn, R.S.; Rayner, C.M. Extraction of anthocyanins from Aronia melanocarpa skin waste as a sustainable source of natural colorants. Color. Technol. 2019, 135, 5–16. [Google Scholar] [CrossRef]
- Jakobek, L.; Drenjančević, M.; Jukić, V.; Šeruga, M. Phenolic acids, flavonols, anthocyanins and antiradical activity of “Nero”, “Viking”, “Galicianka” and wild chokeberries. Sci. Hortic. 2012, 147, 56–63. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Wang, D.; Huo, Y.; Ji, B. Anthocyanin-rich extracts from blackberry, wild blueberry, strawberry, and chokeberry: Antioxidant activity and inhibitory effect on oleic acid-induced hepatic steatosis in vitro. J. Sci. Food Agric. 2016, 96, 2494–2503. [Google Scholar] [CrossRef] [PubMed]
- Biasutto, L.; Marotta, E.; Bradaschia, A.; Fallica, M.; Mattarei, A.; Garbisa, S.; Zoratti, M.; Paradisi, C. Soluble polyphenols: Synthesis and bioavailability of 3,4′,5-tri(α-d-glucose-3-O-succinyl) resveratrol. Bioorg. Med. Chem. Lett. 2009, 19, 6721–6724. [Google Scholar] [CrossRef]
- Han, S.; Hauzer, A. Sposób otrzymywania barwników antocyjanowych oraz odzyskiwania barwników antocyjanowych z roślinnych odpadów poprodukcyjnych (Method of obtaining anthocyanin dyes and recovering such dyes from vegetal production wastes). Polish Patent PL 192692 B1. 30.11.2006 WUP, 11 June 2000. [Google Scholar]
- García-Gavín, J.; Parente, J.; Goossens, A. Allergic contact dermatitis caused by sodium metabisulfite: A challenging allergen. A case series and literature review. Contact Derm. 2012, 67, 260–269. [Google Scholar] [CrossRef]
- Martin, D.A.; Taheri, R.; Brand, M.H.; Draghi, A.; Sylvester, F.A.; Bolling, B.W. Anti-inflammatory activity of aronia berry extracts in murine splenocytes. J. Funct. Foods 2014, 8, 68–75. [Google Scholar] [CrossRef]
- Zapolska-Downar, D.; Bryk, D.; Małecki, M.; Hajdukiewicz, K.; Sitkiewicz, D. Aronia melanocarpa fruit extract exhibits anti-inflammatory activity in human aortic endothelial cells. Eur. J. Nutr. 2012, 51, 563–572. [Google Scholar] [CrossRef]
- Sun, Y.A.N.; Li, L. Cyanidin-3-glucoside inhibits inflammatory activities in human fibroblast-like synoviocytes and in mice with collagen-induced arthritis. Clin. Exp. Pharmacol. Physiol. 2018, 45, 1038–1045. [Google Scholar] [CrossRef]
- Ferrari, D.; Cimino, F.; Fratantonio, D.; Molonia, M.S.; Bashllari, R.; Busà, R.; Saija, A.; Speciale, A. Cyanidin-3-O-Glucoside Modulates the in Vitro Inflammatory Crosstalk between Intestinal Epithelial and Endothelial Cells. Mediat. Inflamm. 2017, 3454023. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Mojsoska, B. The immunomodulation effect of aronia extract lacks association with its antioxidant anthocyanins. J. Med. Food 2013, 16, 334–342. [Google Scholar] [CrossRef]
- Lee, S.G.; Brownmiller, C.R.; Lee, S.-O.; Kang, H.W. Anti-Inflammatory and Antioxidant Effects of Anthocyanins of Trifolium pratense (Red Clover) in Lipopolysaccharide-Stimulated RAW-267.4 Macrophages. Nutrients 2020, 12, 1089. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Song, L.; Qian, B.; Xu, W.; Ren, J.; Jing, P.; Oey, I. Understanding the effect of anthocyanins extracted from purple sweet potatoes on alcohol-induced liver injury in mice. Food Chem. 2018, 245, 463–470. [Google Scholar] [CrossRef]
- Santos, V.D.S.; Bisen-Hersh, E.; Yu, Y.; Ribeiro Cabral, I.S.; Nardini, V.; Culbreth, M.; Teixeira la Rocha, J.B.; Barbosa, F., Jr.; Aschner, M. Anthocyanin-rich açaí (Euterpe oleracea Mart.) extract attenuates manganese-induced oxidative stress in rat primary astrocyte cultures. J. Toxicol. Environ. Health Part A Curr. Issues 2014, 77, 390–404. [Google Scholar] [CrossRef]
- Kardum, N.; Konić-Ristić, A.; Šavikin, K.; Spasić, S.; Stefanović, A.; Ivanišević, J.; Miljković, M. Effects of Polyphenol-Rich Chokeberry Juice on Antioxidant/Pro-Oxidant Status in Healthy Subjects. J. Med. Food 2014, 17, 869–874. [Google Scholar] [CrossRef]
- Lyall, K.A.; Hurst, S.M.; Cooney, J.; Jensen, D.; Lo, K.; Hurst, R.D.; Stevenson, L.M. Short-term blackcurrant extract consumption modulates exercise-induced oxidative stress and lipopolysaccharide-stimulated inflammatory responses. Am. J. Physiol. Integr. Comp. Physiol. 2009, 297, R70–R81. [Google Scholar] [CrossRef]
- Murkovic, M.; Abuja, P.M.; Bergmann, A.R.; Zirngast, A.; Adam, U.; Winklhofer-Roob, B.M.; Toplak, H. Effects of elderberry juice on fasting and postprandial serum lipids and low-density lipoprotein oxidation in healthy volunteers: A randomized, double-blind, placebo-controlled study. Eur. J. Clin. Nutr. 2004, 58, 244–249. [Google Scholar] [CrossRef]
- Valentová, K.; Ulrichová, J.; Cvak, L.; Šimánek, V. Cytoprotective effect of a bilberry extract against oxidative damage of rat hepatocytes. Food Chem. 2006, 101, 912–917. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
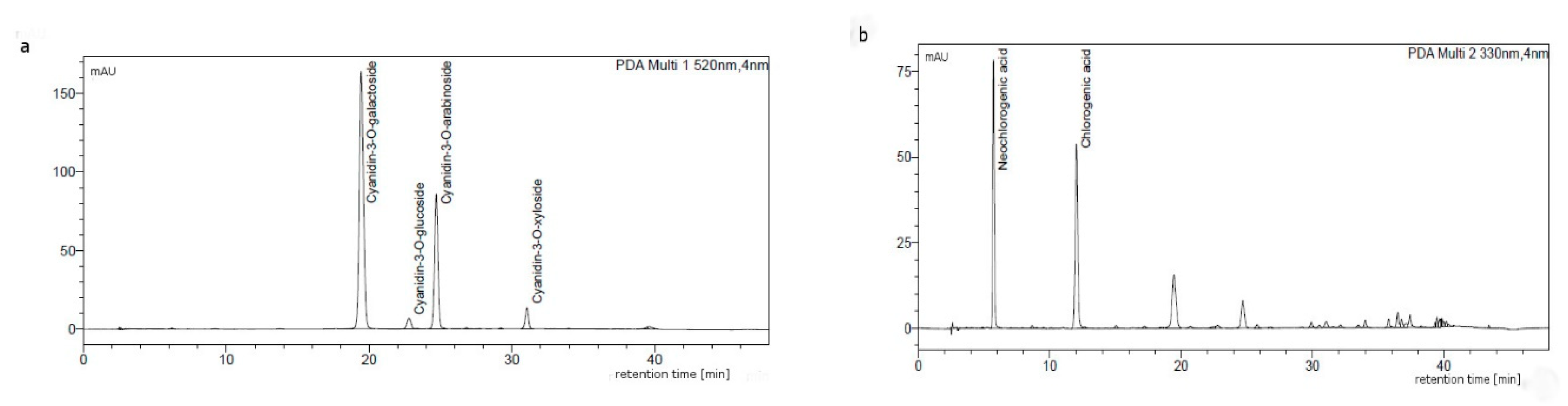

| Batch ID | Total Polyphenols Calculated as Caffeic Acid (UV_VIS) [wt%] | Anthocyanins Calculated as Cy-3-glu Chloride (HPLC) [wt%] | Anthocyanins Calculated as Cy-3-glu (UV-VIS) [wt%] | Phenolic Acids Calculated as Chlorogenic Acid (HPLC) [wt%] | DPPH (Trolox Equivalent) [µmol TE/g] | ORAC (Trolox Equivalent) [µmol TE/g] |
|---|---|---|---|---|---|---|
| B1 | 65.48 ± 1.86 | 33.10 ± 0.28 | 32.54 ± 0.28 | 5.682 ± 0.114 | 6417 ± 93 | 10,519 ± 150 |
| B2 | 57.48 ± 1.55 | 14.47 ± 0.03 | 16.58 ± 0.13 | 6.247 ± 0.171 | 11,127 ± 219 | 15,335 ± 452 |
| Sample | ||||
|---|---|---|---|---|
| Control | Extract 0.5 µg/mL | Extract 5 µg/mL | Extract 500 µg/mL | |
| MTT test results (as A563) | 0.277 ± 0.018 | 0.279 ± 0.017 | 0.279 ± 0.009 | 0.281 ± 0.006 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banach, M.; Wiloch, M.; Zawada, K.; Cyplik, W.; Kujawski, W. Evaluation of Antioxidant and Anti-Inflammatory Activity of Anthocyanin-Rich Water-Soluble Aronia Dry Extracts. Molecules 2020, 25, 4055. https://doi.org/10.3390/molecules25184055
Banach M, Wiloch M, Zawada K, Cyplik W, Kujawski W. Evaluation of Antioxidant and Anti-Inflammatory Activity of Anthocyanin-Rich Water-Soluble Aronia Dry Extracts. Molecules. 2020; 25(18):4055. https://doi.org/10.3390/molecules25184055
Chicago/Turabian StyleBanach, Mariusz, Magdalena Wiloch, Katarzyna Zawada, Wojciech Cyplik, and Wojciech Kujawski. 2020. "Evaluation of Antioxidant and Anti-Inflammatory Activity of Anthocyanin-Rich Water-Soluble Aronia Dry Extracts" Molecules 25, no. 18: 4055. https://doi.org/10.3390/molecules25184055
APA StyleBanach, M., Wiloch, M., Zawada, K., Cyplik, W., & Kujawski, W. (2020). Evaluation of Antioxidant and Anti-Inflammatory Activity of Anthocyanin-Rich Water-Soluble Aronia Dry Extracts. Molecules, 25(18), 4055. https://doi.org/10.3390/molecules25184055







