Reducing the Competition: A Dual-Purpose Ionic Liquid for the Extraction of Gallium from Iron Chloride Solutions
Abstract
1. Introduction
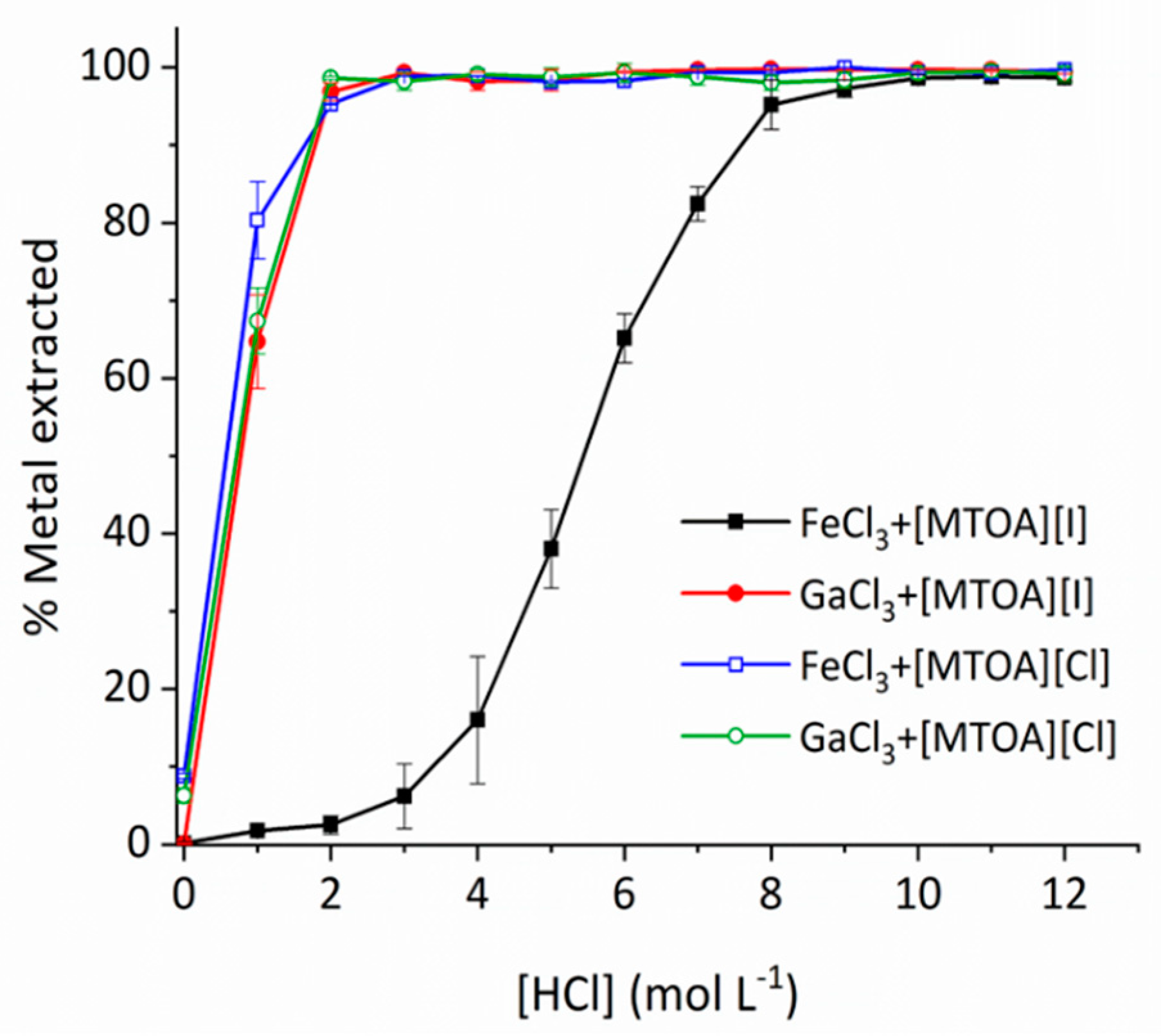
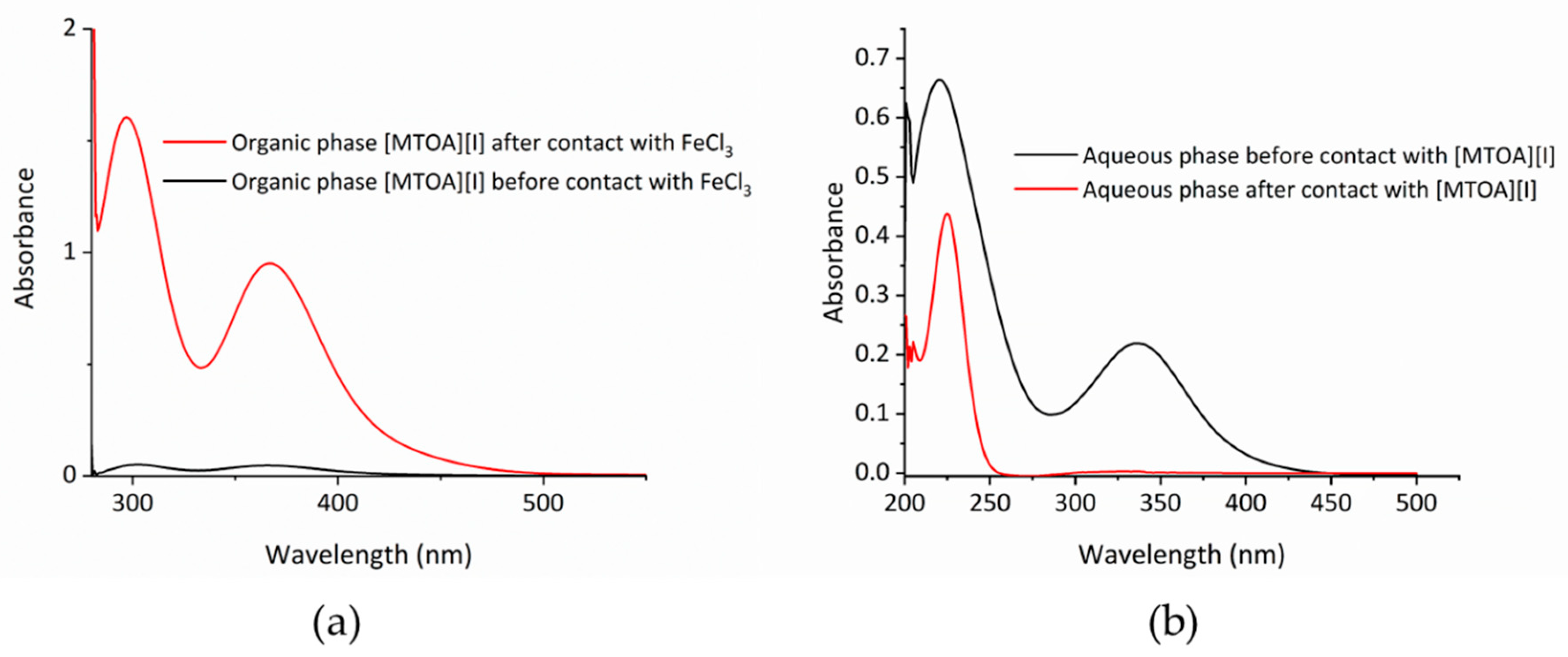
2. Results and Discussion
3. Materials and Methods
3.1. General Solvent Extraction Procedure
3.2. Synthesis of Methyltrioctylammonium Iodide ([MTOA][I])
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wood, S.A.; Samson, I.M. The aqueous geochemistry of gallium, germanium, indium, and scandium. Ore Geol. Rev. 2006, 28, 57–102. [Google Scholar] [CrossRef]
- Liu, W.; Etschmann, B.; Brugger, J.; Spiccia, L.; Foran, G.; McInnes, B. UV–Vis spectrophotometric and XAFS studies of ferric chloride complexes in hyper-saline LiCl solutions at 25–90 °C. Chem. Geol. 2006, 231, 326–349. [Google Scholar] [CrossRef]
- Lu, F.; Xiao, T.; Lin, J.; Ning, Z.; Long, Q.; Xiao, L.; Huang, F.; Wang, W.; Xiao, Q.; Lan, X.; et al. Resources and extraction of gallium: A review. Hydrometallurgy 2017, 174, 105–115. [Google Scholar] [CrossRef]
- Sun, Z.; Cao, H.; Xiao, Y.; Sietsma, J.; Jin, W.; Agterhuis, H.; Yang, Y. Toward Sustainability for Recovery of Critical Metals from Electronic Waste: The Hydrochemistry Processes. ACS Sustain. Chem. Eng. 2017, 5, 21–40. [Google Scholar] [CrossRef]
- European Commission. Critical Raw Materials Factsheets. 2017. Available online: https://ec.europa.eu/growth/sectors/raw-materials/specific-interest/critical_en (accessed on 25 May 2020).
- Nelson, J.J.M.; Schelter, E.J. Sustainable Inorganic Chemistry: Metal Separations for Recycling. Inorg. Chem. 2019, 58, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.M.; Bailey, P.J.; Tasker, P.A.; Turkington, J.R.; Grant, R.A.; Love, J.B. Solvent extraction: The coordination chemistry behind extractive metallurgy. Chem. Soc. Rev. 2014, 43, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.P.; Dolezalova, S.; Ngwenya, B.T.; Morrison, C.A.; Love, J.B. Understanding the Recovery of Rare-Earth Elements by Ammonium Salts. Metals 2018, 8, 465. [Google Scholar] [CrossRef]
- Katsuta, S.; Yoshimoto, Y.; Okai, M.; Takeda, Y.; Bessho, K. Selective Extraction of Palladium and Platinum from Hydrochloric Acid Solutions by Trioctylammonium-Based Mixed Ionic Liquids. Ind. Eng. Chem. Res. 2011, 50, 12735–12740. [Google Scholar] [CrossRef]
- De Los Ríos, A.P.; Hernández-Fernández, F.J.; Lozano, L.J.; Sánchez, S.; Moreno, J.I.; Godínez, C. Removal of metal ions from aqueous solutions by extraction with ionic liquids. J. Chem. Eng. Data 2010, 55, 605–608. [Google Scholar] [CrossRef]
- Nayak, S.; Devi, N. Studies on extraction of gallium(III) from chloride solution using Cyphos IL 104 and its removal from photodiodes and red mud. Hydrometallurgy 2017, 171, 191–197. [Google Scholar] [CrossRef]
- Vereycken, W.; Riaño, S.; Van Gerven, T.; Binnemans, K. Extraction Behavior and Separation of Precious and Base Metals from Chloride, Bromide, and Iodide Media Using Undiluted Halide Ionic Liquids. ACS Sustain. Chem. Eng. 2020, 8, 8223–8234. [Google Scholar] [CrossRef]
- Van den Bossche, A.; Vereycken, W.; Vander Hoogerstraete, T.; Dehaen, W.; Binnemans, K. Recovery of Gallium, Indium, and Arsenic from Semiconductors Using Tribromide Ionic Liquids. ACS Sustain. Chem. Eng. 2019, 7, 14451–14459. [Google Scholar] [CrossRef]
- Macías-Macías, K.Y.; Ceniceros-Gómez, A.E.; Gutiérrez-Ruiz, M.E.; González-Chávez, J.L.; Martínez-Jardines, L.G. Extraction and recovery of the strategic element gallium from an iron mine tailing. J. Environ. Chem. Eng. 2019, 7, 102964. [Google Scholar] [CrossRef]
- Černy, Z.; Macháček, J.; Fusek, J.; Křiž, O.; Čásensky, B.; Tuck, D.G. 71Ga NMR studies of mixtures of gallium trichloride and trimethylgallium. J. Organomet. Chem. 1993, 456, 25–30. [Google Scholar] [CrossRef]
- Awtrey, A.D.; Connick, R.E. The Absorption Spectra of I2, I3−, I−, IO3−, S4O6− and S2O3−. Heat of the Reaction I3− = I2 + I−. J. Am. Chem. Soc. 1951, 73, 1842–1843. [Google Scholar] [CrossRef]
- Gamlen, G.A.; Jordan, D.O. A spectrophotometric study of the iron(III) chloro-complexes. J. Chem. Soc. 1953, 87, 1435–1443. [Google Scholar] [CrossRef]
- Persson, I. Ferric Chloride Complexes in Aqueous Solution: An EXAFS Study. J. Solut. Chem. 2018, 47, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, C.A.; Seward, T.M. A spectrophotometric study of aqueous iron (II) chloride complexing from 25 to 200 °C. Geochim. Cosmochim. Acta 1990, 54, 2207–2221. [Google Scholar] [CrossRef]
- Zhao, R.; Pan, P. A spectrophotometric study of Fe(II)-chloride complexes in aqueous solutions from 10 to 100 °C. Can. J. Chem. 2001, 79, 131–144. [Google Scholar] [CrossRef]
- Ab Rani, M.A.; Borduas, N.; Colquhoun, V.; Hanley, R.; Johnson, H.; Larger, S.; Lickiss, P.D.; Llopis-Mestre, V.; Luu, S.; Mogstad, M.; et al. The potential of methylsiloxanes as solvents for synthetic chemistry applications. Green Chem. 2014, 16, 1282–1296. [Google Scholar] [CrossRef]
- Housecroft, C.E. Inorganic Chemistry, 5th ed.; Pearson Education Ltd.: Harlow, UK, 2018. [Google Scholar]
Sample Availability: Samples of methyltrioctylammonium iodide [MTOA][I] are available from the authors. |


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
M. M. Kinsman, L.; A. Morrison, C.; T. Ngwenya, B.; B. Love, J. Reducing the Competition: A Dual-Purpose Ionic Liquid for the Extraction of Gallium from Iron Chloride Solutions. Molecules 2020, 25, 4047. https://doi.org/10.3390/molecules25184047
M. M. Kinsman L, A. Morrison C, T. Ngwenya B, B. Love J. Reducing the Competition: A Dual-Purpose Ionic Liquid for the Extraction of Gallium from Iron Chloride Solutions. Molecules. 2020; 25(18):4047. https://doi.org/10.3390/molecules25184047
Chicago/Turabian StyleM. M. Kinsman, Luke, Carole A. Morrison, Bryne T. Ngwenya, and Jason B. Love. 2020. "Reducing the Competition: A Dual-Purpose Ionic Liquid for the Extraction of Gallium from Iron Chloride Solutions" Molecules 25, no. 18: 4047. https://doi.org/10.3390/molecules25184047
APA StyleM. M. Kinsman, L., A. Morrison, C., T. Ngwenya, B., & B. Love, J. (2020). Reducing the Competition: A Dual-Purpose Ionic Liquid for the Extraction of Gallium from Iron Chloride Solutions. Molecules, 25(18), 4047. https://doi.org/10.3390/molecules25184047






