Anti-Obesity and Gut Microbiota Modulation Effect of Secoiridoid-Enriched Extract from Fraxinus mandshurica Seeds on High-Fat Diet-Fed Mice
Abstract
1. Introduction
2. Results
2.1. Identification of Secoiridoids in F. mandshurica Seeds
2.2. Quantitation of Secoiridoids in FM by HPLC Analysis
2.3. Inhibitory Effect of Secoiridoids on Pancreatic Lipase
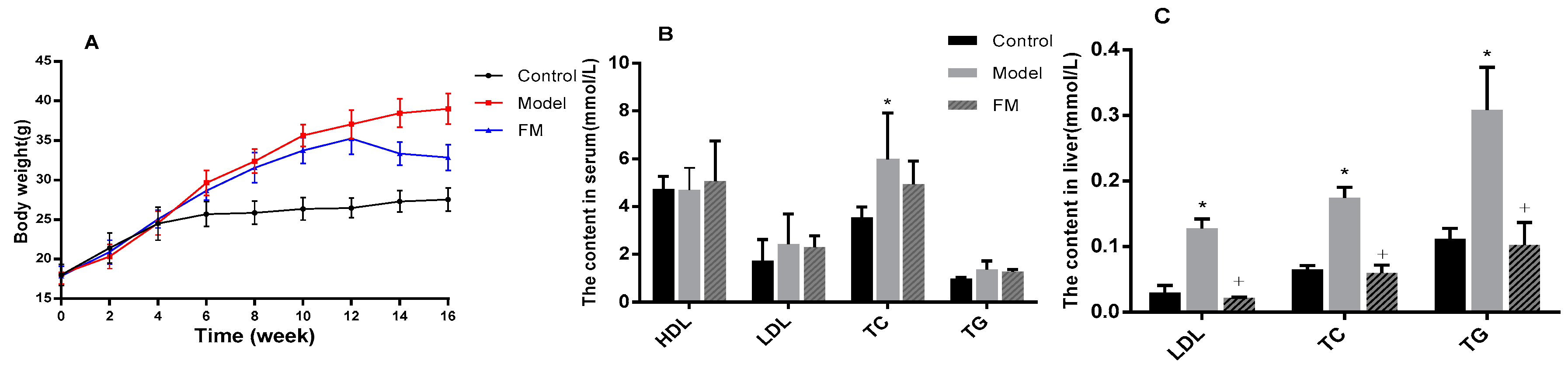
2.4. FM Declined the Body Weight of High-Fat Diet-Induced Obese Mice
2.5. Effects of FM on TC, TG, LDL-C and HDL-C Levels in Serum and Liver
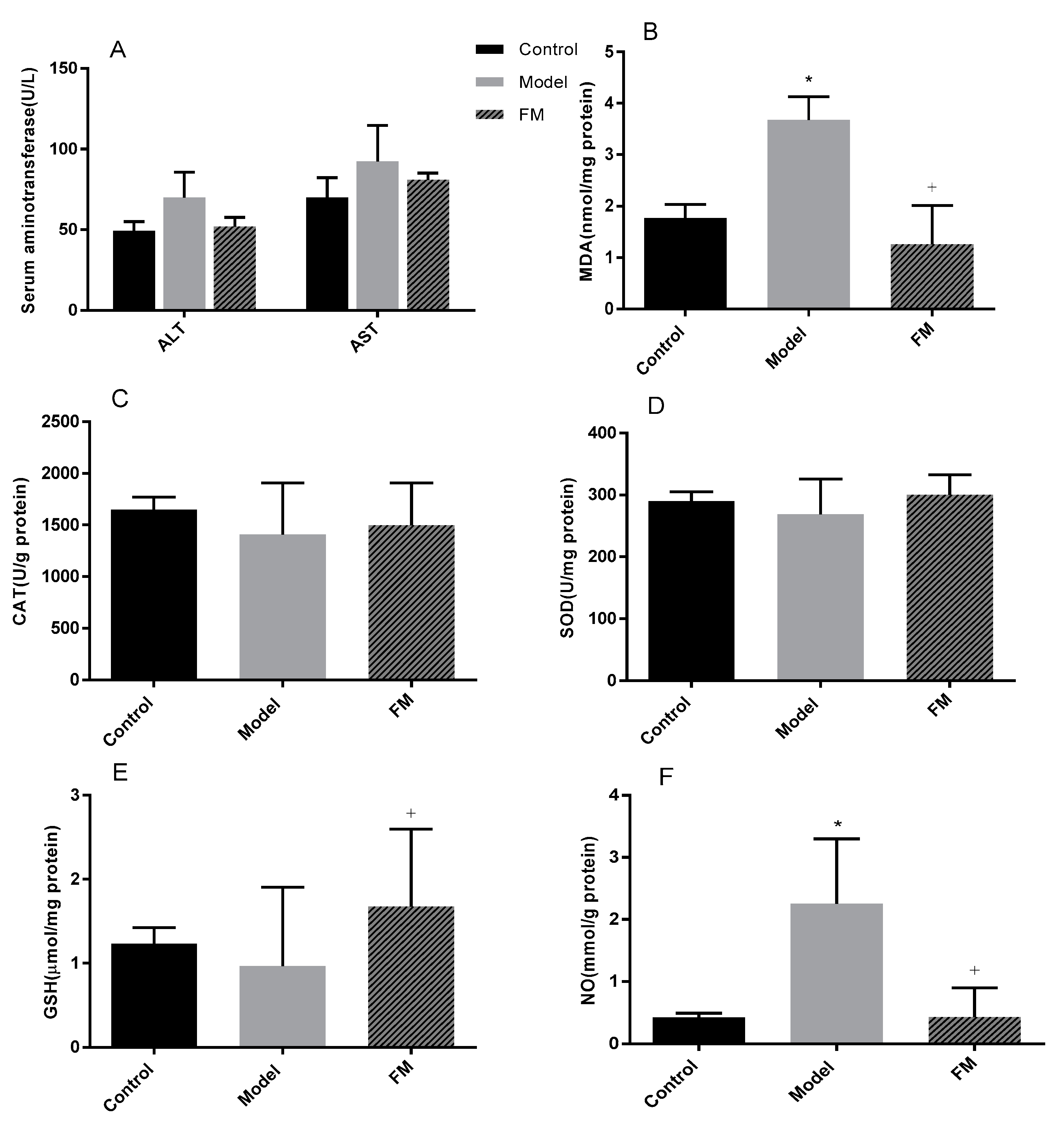
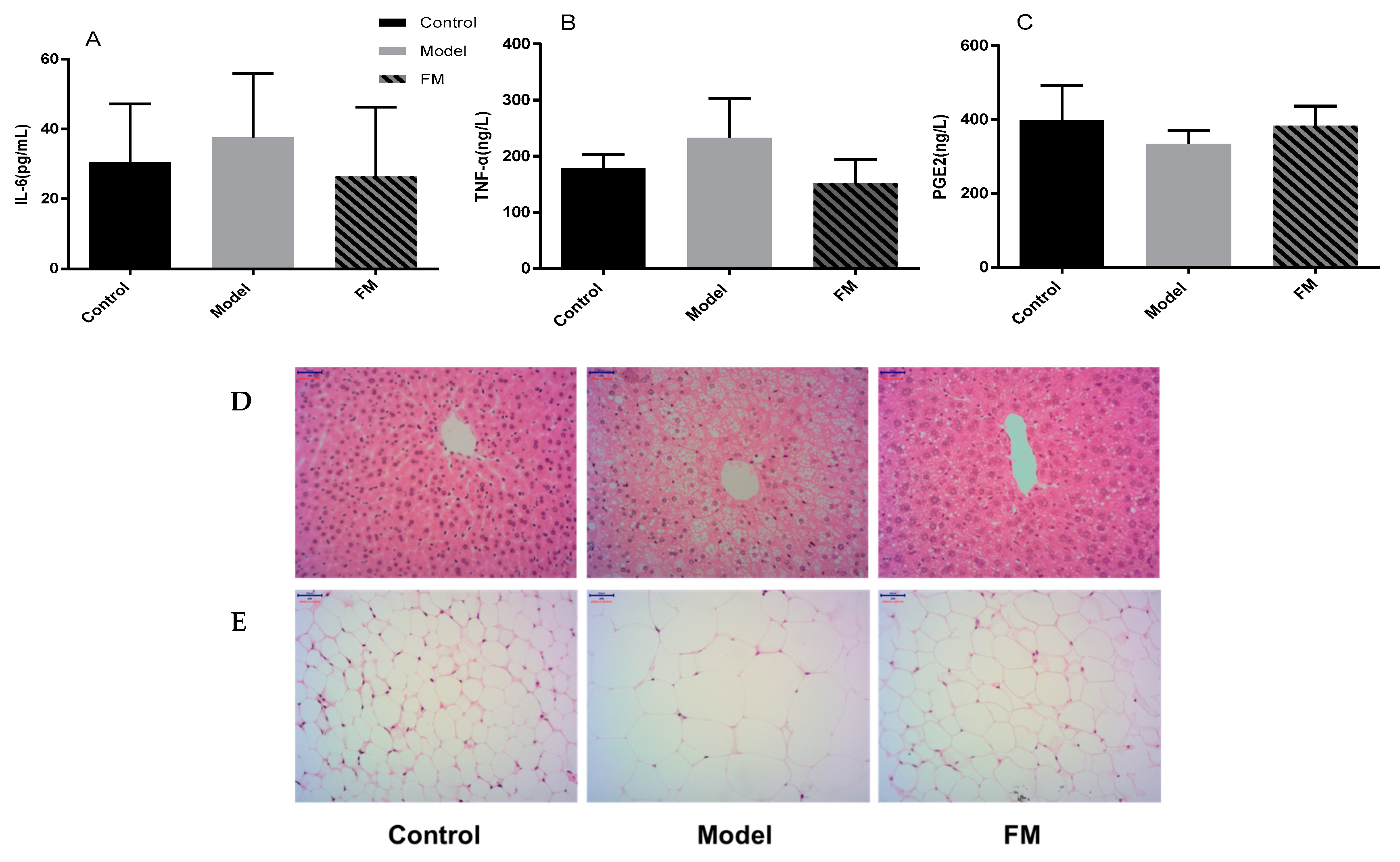
2.6. Effects of FM on Liver Injury, Oxidative Stress and Inflammatory Factors
2.7. Histopathological Examinations of Liver and Epididymal Adipose
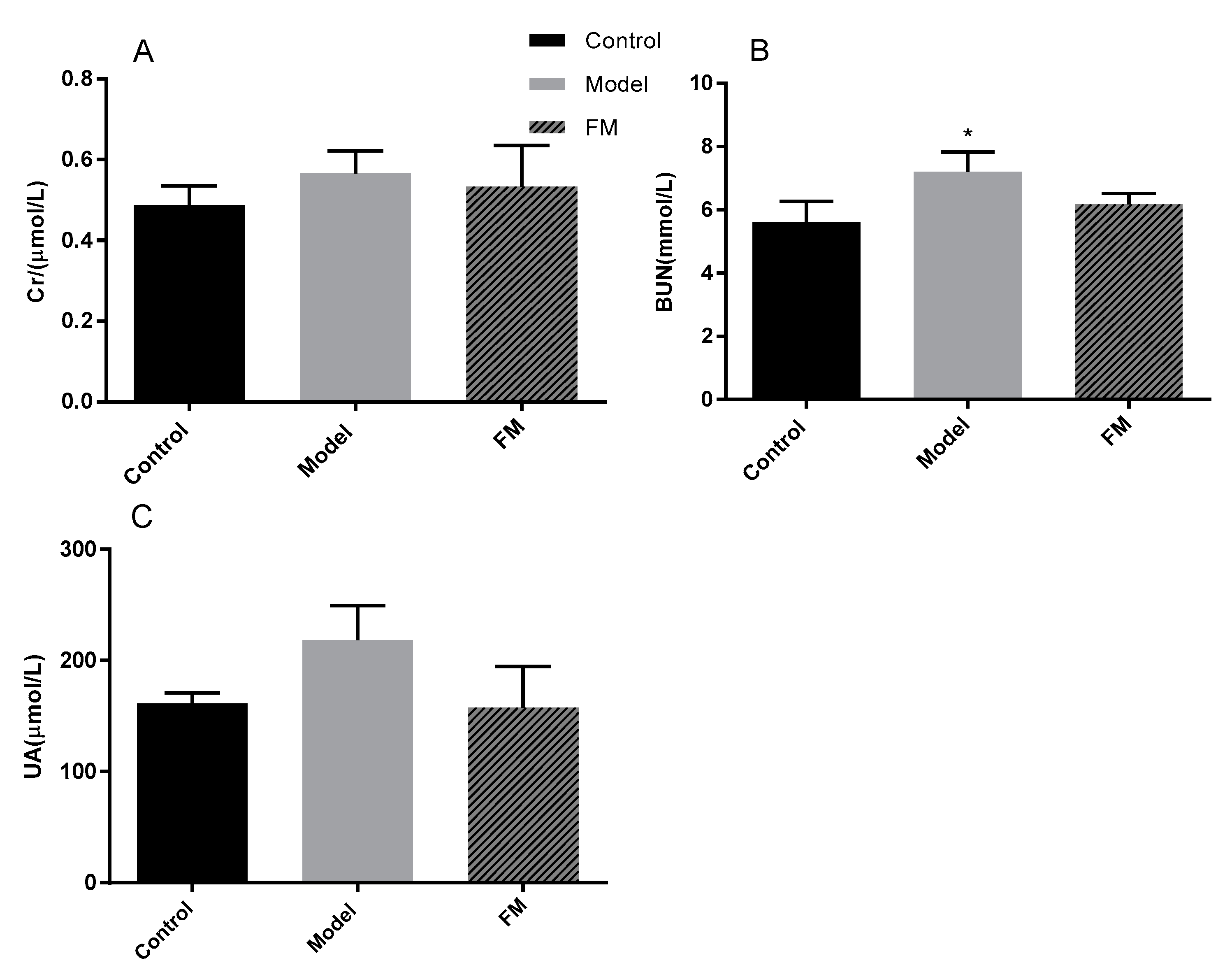
2.8. Effects of FM on Serum Cr, BUN and UA Levels
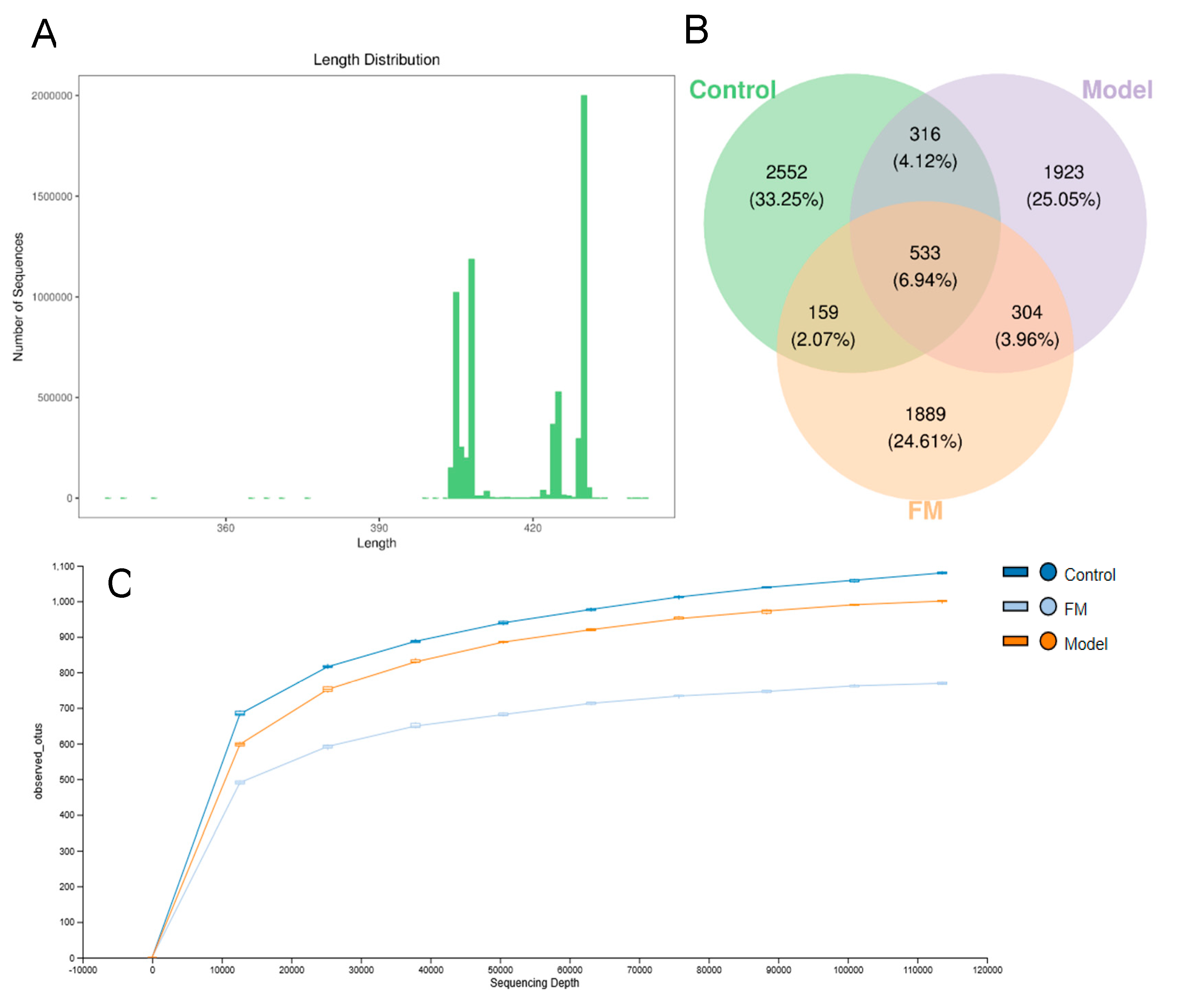
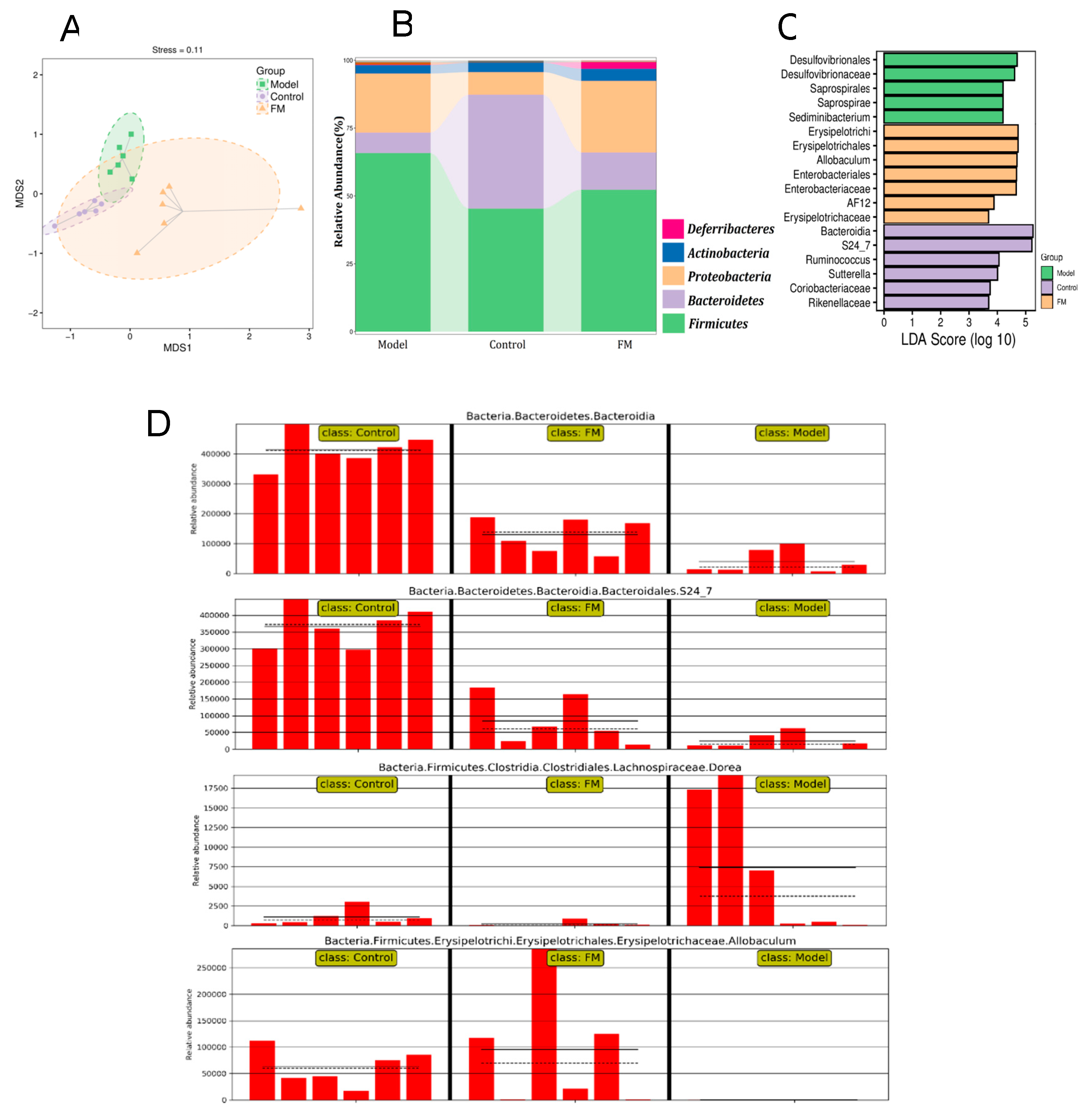
2.9. FM Modulates the Gut Microbiota in High-Fat Diet-Induced Obese Mice
3. Discussion
4. Materials and Methods
4.1. Preparation of F. Mandshurica Seed Extract
4.2. Extraction and Isolation of Secoiridoid Compounds from F. mandshurica Seeds
4.3. Quantification of Secoiridoids in FM by HPLC Analysis
4.4. Pancreatic Lipase Inhibitory Activity of Secoiridoids In Vitro
4.5. Animal Experiments
4.5.1. Animals and Diets
4.5.2. Serum and Hepatic Biochemical Analysis
4.5.3. Histopathological Examinations of Liver and Epididymal Adipose
4.6. Gut Microbiota Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saltiel, A.R.; Kahn, C.R. Insulin signaling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Rahmouni, K.; Correia Correia, L.G.; Haynes, W.G.; Mark, A.L. Obesity-associated hypertension-New insights into mechanisms. Hypertension 2005, 45, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Bastien, M.; Poirier, P.; Lemieux, I.; Despres, J.P. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog. Cardiovasc. Dis. 2014, 56, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Tobias, D.K.; Pan, A.; Jackson, C.L.; O’Reilly, E.J.; Ding, E.L.; Willett, W.C.; Manson, J.E.; Hu, F.B. Body-mass index and mortality among adults with incident type 2 diabetes. N. Engl. J. Med. 2014, 370, 233–244. [Google Scholar] [CrossRef]
- Vucenik, I.; Stains, J.P. Obesity and cancer risk: Evidence, mechanisms, and recommendations. Ann. N. Y. Acad. Sci. 2012, 1271, 37–43. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Liu, W.; Chen, X.; Liu, Z.; Zhou, Z. Improvements in humoral immune function and glucolipid metabolism after laparoscopic sleeve gastrectomy in patients with obesity. Surg. Obes. Relat. Dis. 2019, 15, 1455–1463. [Google Scholar] [CrossRef]
- An, R.; Shen, J.; Yang, Q.; Yang, Y. Impact of built environment on physical activity and obesity among children and adolescents in China: A narrative systematic review. J. Sport Health Sci. 2019, 8, 153–169. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Ma, R.M.; Lao, T.T.; Chen, Z.; Du, M.Y.; Liang, K.; Huang, Y.K.; Zhang, L.; Yang, M.H.; Sun, Y.H.; et al. Maternal gestational diabetes mellitus and overweight and obesity in offspring: A study in Chinese children. J. Dev. Orig. Health Dis. 2015, 6, 479–484. [Google Scholar] [CrossRef]
- Chandrasekaran, C.V.; Vijayalakshmi, M.A.; Prakash, K.; Bansal, V.S.; Meenakshi, J.; Amit, A. Review article: Herbal approach for obesity management. Am. J. Plant Sci. 2012, 3, 1003–1014. [Google Scholar] [CrossRef]
- Apovian, C.M.; Aronne, L.J.; Bessesen, D.H.; McDonnell, M.E.; Murad, M.H.; Pagotto, U.; Ryan, D.H.; Still, C.D. Pharmacological management of obesity: An endocrine society clinical practice guideline. J. Clin. Endocr. Metab. 2015, 100, 342–362. [Google Scholar] [CrossRef]
- Thomas, J.G.; Bond, D.S.; Phelan, S.; Hill, J.O.; Wing, R.R. Weight-loss maintenance for 10 years in the national weight control registry. Am. J. Prev. Med. 2014, 46, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Sjostrom, L.; Rissanen, A.; Andersen, T.; Boldrin, M.; Golay, A.; Koppeschaar, H.P.; Krempf, M. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. Lancet 1998, 352, 167–173. [Google Scholar] [CrossRef]
- Barrett, M.L.; Udani, J.K. A proprietary alpha-amylase inhibitor from white bean (Phaseolus vulgaris): A review of clinical studies on weight loss and glycemic control. Nut. J. 2011, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, R.; Sooriyaarachchi, P.; Ranasinghe, P.; Perera, A.; Hills, A.P. Availability and composition of weight-loss supplements in Sri Lanka. Nutr. Diet. 2020, 77, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Oh, M.R.; Kim, M.G.; Chae, H.J.; Chae, S.W. Anti-obesity effects of Yerba Mate (Ilex paraguariensis): A randomized, double-blind, placebo-controlled clinical trial. BMC Complement. Altern. Med. 2015, 15, 338. [Google Scholar] [CrossRef]
- Chen, Y.J.; Xue, G.; Liu, F.Z.; Gong, X.L. Immunosuppressive effect of extracts from leaves of Fraxinus mandshurica Rupr. Bioengineered 2017, 3, 212–216. [Google Scholar] [CrossRef]
- Chen, Y.J.; Zhang, H.G.; Li, X. Phenylethanoid glycosides from the bark of Fraxinus mandshurica. Chem. Nat. Compd. 2009, 45, 330–332. [Google Scholar] [CrossRef]
- Eyles, A.; Jones, W.; Riedl, K.; Cipollini, D.; Schwartz, S.; Chan, K.; Herms, D.A.; Bonello, P. Comparative phloem chemistry of Manchurian (Fraxinus mandshurica) and two North American ash species (Fraxinus americana and Fraxinus pennsylvanica). J. Chem. Ecol. 2007, 33, 1430–1448. [Google Scholar] [CrossRef]
- Chen, Y.J.; Wang, H.T.; He, L.P.; Zhang, H.G.; Yin, J.L. Study on chemical constituents from bark of Fraxinus mandshurica Rupr. Chin. Pharm. J. 2009, 44, 1133–1136. [Google Scholar]
- Ahn, J.H.; Shin, E.J.; Liu, Q.; Kim, S.B.; Choi, K.M.; Yoo, H.S.; Hwang, B.Y.; Lee, M.K. Secoiridoids from the stem barks of Fraxinus rhynchophylla with pancreatic lipase inhibitory activity. Nat. Prod. Res. 2013, 27, 1132–1135. [Google Scholar] [CrossRef]
- Bai, N.; He, K.; Ibarra, A.; Bily, A.; Roller, M.; Chen, X.; Ruhl, R. Iridoids from Fraxinus excelsior with adipocyte differentiation-inhibitory and PPAR-α activation activity. J. Nat. Prod. 2010, 73, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, A.; Bai, N.; He, K.; Bily, A.; Cases, J.; Roller, M.; Sang, S. Fraxinus excelsior seed extract FraxiPureTM limits weight gains and hyperglycemia in high-fat diet-induced obese mice. Phytomedicine 2011, 18, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Montó, F.; Arce, C.; Noguera, M.A.; Ivorra, M.D.; Flanagan, J.; Roller, M.; Issaly, N.; D’Ocon, P. Action of an extract from the seeds of Fraxinus excelsior L. on metabolic disorders in hypertensive and obese animal models. Food Funct. 2014, 5, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, J.; Meyer, M.; Pasamar, M.A.; Ibarra, A.; Roller, M.; Alvarez, I.; Genoher, N.; Leiva, S.; Gomez-Garc’ıa, F.; Alcaraz, M.; et al. Safety evaluation and nutritional composition of a Fraxinus excelsior seed extract, FraxiPure™. Food Chem. Toxicol. 2013, 53, 10–17. [Google Scholar] [CrossRef]
- Kostova, I.; Iossifova, T. Chemical components of Fraxinus species. Fitoterapia 2007, 78, 85–106. [Google Scholar] [CrossRef]
- LaLonde, R.T.; Wong, C.; Tsai Amy, I.M. Polygluosidic metabolites of Oleaceae. The chain sequence of oleoside aglucon, tyrosol, and glucose units in three metabolites from Fraxinus americana. J. Am. Chem. Soc. 1976, 98, 3007–3013. [Google Scholar] [CrossRef]
- Servili, M.; Baldioli, M.; Selvaggini, R.; Macchioni, A.; Montedoro, G.F. Phenolic compounds of Olive fruit: One-and two-dimensional nuclear magnetic resonance characterization of nuzhenide and its distribution in the constitutive parts of fruit. J. Agric. Food Chem. 1999, 47, 12–18. [Google Scholar] [CrossRef]
- Takenaka, Y.; Tanahashi, T.; Shintaku, M.; Sakai, T.; Nagakura, N. Secoiridoid glucosides from Fraxinus Americana. Phytochemistry 2000, 55, 275–284. [Google Scholar] [CrossRef]
- Zhao, G.Q.; Xia, J.J.; Dong, J.X. Glycosides from flowers of Jasminum officinale L. var. grandiflorum. Acta Pharm. Sin. 2007, 42, 1066–1069. [Google Scholar]
- Wang, X.F.; Li, C.; Shi, Y.P.; Di, D.L. Two new secoiridoid glycosides from the leaves of Olea europaea L. J. Asian Nat. Prod. Res. 2009, 11, 940–944. [Google Scholar] [CrossRef]
- Gao, B.B.; She, G.M.; She, D.M. Chemical constituents and biological activities of plants from the genus Ligustrum. Chem. Biodivers. 2013, 10, 96–128. [Google Scholar] [CrossRef] [PubMed]
- Takao, T.; Hiroko, W.; Atsuko, I. Five secoiridoid glucosides from F. formosana. Phytochemistry 1992, 32, 133–136. [Google Scholar]
- Tanahashi, T.; Takenaka, Y.; Akimoto, M.; Okuda, A. Six secoiridoid glucosides from Jasminum polyanthum. Chem. Pharm. Bull. 1997, 45, 367–372. [Google Scholar] [CrossRef]
- Aoki, S.; Honda, Y.; Kikuchi, T.; Miura, T.; Sugawara, R.; Yaoita, Y.; Kikuchi, M.; Machida, K. Six new secoiridoids from the dried fruits of Ligustrum lucidum. Chem. Pharm. Bull. 2012, 60, 251–256. [Google Scholar] [CrossRef]
- Takenaka, Y.; Okazaki, N.; Tanahashi, T.; Nagakura, N.; Nishi, T. Secoiridoid and iridoid glucosides from Syringa afghanica. Phytochemistry 2002, 59, 779–787. [Google Scholar] [CrossRef]
- Fu, G.M.; Ip, F.C.; Pang, H.H.; Ip, N.Y. New secoiridoid glucosides from Ligustrum lucidum induce ERK and CREB phosphorylation in cultured cortical neurons. Planta Med. 2010, 76, 998–1003. [Google Scholar] [CrossRef]
- He, Z.D.; Dong, H.; Xu, H.X.; Ye, W.C.; Sun, H.D.; HayBut, P.P. Secoiridoid constituents from the fruits of Ligustrum lucidum. Phytochemistry 2001, 56, 327–330. [Google Scholar] [CrossRef]
- Dinda, B.; Debnath, S.; Harigaya, Y. Naturally occurring secoiridoids and bioactivity of naturally occurring iridoids and secoiridoids. A review, part 2. Chem. Pharm. Bull. 2007, 38, 689–728. [Google Scholar] [CrossRef]
- Sugiyama, M.; Machida, K.; Matsuda, N.; Kikuchi, M. A secoiridoid glycoside from Osmanthus asiaticus. Phytochemistry 1993, 34, 1169–1170. [Google Scholar] [CrossRef]
- Tanahashi, T.; Takenaka, Y.; Nagakura, N. Two dimeric secoiridoid glucosides from Jasminum polyanthum. Phytochemistry 1996, 41, 1341–1345. [Google Scholar] [CrossRef]
- Ouyang, X.L.; Wei, L.X.; Wang, H.S.; Pan, Y.M. Antioxidant activity and phytochemical composition of Osmanthus fragrans’ pulps. S. Afr. J. Bot. 2015, 98, 162–166. [Google Scholar] [CrossRef]
- Zheng, D.H.; Ueda, S.; Inoue, K.; Akaji, M.; Fujita, T.; Ren, Y.C. Secoiridoid glucosides from Fraxinus malacophylla. Phytochemistry 1993, 35, 177–181. [Google Scholar] [CrossRef]
- Wu, Z.B.; Liu, Y.; Tian, S.S.; Wen, C. Chemical constituents of the stem bark of Fraxinus rhynchophylla. Chem. Nat. Compd. 2014, 49, 1162–1163. [Google Scholar] [CrossRef]
- Nan-Nong, S.; Tsung-Yen, W.; Chi-Fai, C. Natural dietary and herbal products in anti-obesity treatment. Molecules 2016, 21, 1351. [Google Scholar]
- Cheng, N.; Chen, S.N.; Liu, X.Y.; Zhao, H.A.; Cao, W. Impact of Schisandra Chinensis bee pollen on nonalcoholic fatty liver disease and gut microbiota in high fat diet induced obese mice. Nutrients 2019, 11, 346. [Google Scholar] [CrossRef]
- Giannini, E.G.; Testa, R.; Savarino, V. Liver enzyme alteration: A guide for clinicians. Can. Med. Assoc. J. 2005, 172, 367–379. [Google Scholar] [CrossRef]
- Recknagel, R.O.; Glende, E.A.; Dolak, J.A.; Waller, R.L. Mechanisms of carbon tetrachloride toxicity. Pharmacol. Ther. 1989, 43, 139–154. [Google Scholar] [CrossRef]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, Á.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef]
- Amirkhizi, F.; Siassi, F.; Minaie, S.; Djalali, M.; Rahimi, A.; Chamari, M. Is obesity associated with increased plasma lipid peroxidación and oxidative stress in women? ARYA Atheroscler. 2007, 2, 189–192. [Google Scholar]
- Younis, T.; Khan, M.R.; Sajid, M. Protective effects of Fraxinus xanthoxyloides (Wall.) leaves against CCl4 induced hepatic toxicity in rat. BMC Complement. Altern. Med. 2016, 16, 407. [Google Scholar] [CrossRef]
- Martínez-Morúa, A.; Soto-Urquieta, M.G.; Franco-Robles, E.; Zúñiga-Trujillo, I.; Ramírez-Emiliano, J. Curcumin decreases oxidative stress in mitochondria isolated from liver and kidneys of high-fat diet-induced obese mice. J. Asian Nat. Prod. Res. 2013, 15, 905–915. [Google Scholar]
- Romao, P.R.T.; Tovar, J.; Fonseca, S.G.; Moraes, R.H.; Cruz, A.K.; Hothersall, J.S.; Noronha-Dutra, A.A.; Ferreira, S.H.; Cunha, F.Q. Glutathione and the redox control system trypanothione/trypanothione reductase are involved in the protection of Leishmania spp. against nitrosothiol-induced cytotoxicity. Braz. J. Med. Biol. Res. 2006, 39, 355–363. [Google Scholar] [CrossRef]
- Wu, D.F.; Cederbaum, A.I. Oxidative stress and alcoholic liver disease. Semin. Liver Dis. 2009, 29, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Sikaris, K. The clinical biochemistry of obesity. Clin. Biochem. Rev. 2004, 25, 165–181. [Google Scholar] [PubMed]
- Younis, T.; Khan, M.R.; Sajid, M.; Majid, M.; Zahra, Z.; Shah, N.A. Fraxinus xanthoxyloides leaves reduced the level of inflammatory mediators during in vitro and in vivo studies. BMC Complement. Altern. Med. 2016, 16, 230. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Cao, Y.W.; Tian, Y.; Gu, Y.F.; Lu, H.X.; Zhang, S.Q.; Xu, H.X.; Su, Z.L. PGE2 ameliorated viral myocarditis development and promoted IL-10-producing regulatory B cell expansion via MAPKs/AKT-AP1 axis or AhR signaling. Cell. Immunol. 2020, 347, 104025. [Google Scholar] [CrossRef] [PubMed]
- Edward, N. Obesity and chronic kidney disease. Curr. Opin. Pediatr. 2018, 30, 241–246. [Google Scholar]
- Woting, A.; Blaut, M. The intestinal microbiota in metabolic disease. Nutrients 2016, 8, 202. [Google Scholar] [CrossRef]
- Tremaroli, V.; Backhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef]
- Hildebrandt, M.A.; Hoffmann, C.; Scott, A.; Sherrill–Mix, S.A.; Keilbaugh, S.A.; Hamady, M.; Chen, Y.Y.; Knight, R.; Ahima, R.; Bushman, F.; et al. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 2009, 137, 1716–1724. [Google Scholar] [CrossRef]
- Liu, D.M.; Wen, B.B.; Zhu, K.; Luo, Y.; Li, J.; Li, Y.H.; Lin, H.Y.; Huang, J.A.; Liu, Z.H. Antibiotics-induced perturbations in gut microbial diversity influence metabolic phenotypes in a murine model of high-fat diet-induced obesity. Appl. Microbiol. Biotechnol. 2019, 103, 5269–5283. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Millan, M.; Perez-Matute, P.; Oteo, J. Gut microbiota: A key player in health and disease. A review focused on obesity. J. Physiol. Biochem. 2015, 71, 509–525. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.H.; Ye, Y.; Yan, X.; Chao, X.Y.; Yang, F.; Wang, M.Y.; Zhang, W.C.; Yuan, C.X.; Zeng, Q.M. Effect of banana pulp dietary fibers on metabolic syndrome and gut microbiota diversity in high-fat diet mice. J. Food Biochem. 2020, e13362. [Google Scholar] [CrossRef]
- Shahi, S.K.; Freedman, S.N.; Mangalam, A.K. Gut microbiome in multiple sclerosis: The players involved and the roles they play. Gut Microbes 2017, 8, 607–615. [Google Scholar] [CrossRef]
- Wang, Y.; Ames, N.P.; Tun, H.M.; Tosh, S.M.; Jones, P.J.; Ames, N.P. High molecular weight barley β-glucan alters gut microbiota toward reduced cardiovascular disease risk. Front. Microbiol. 2016, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, P.; Li, D.T.; Hu, X.S.; Chen, F. Beneficial effects of ginger on prevention of obesity through modulation of gut microbiota in mice. Eur. J. Nutr. 2020, 59, 699–718. [Google Scholar] [CrossRef]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.H.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. Dietary fats and cardiovascular disease: A presidential advisory from the American heart association. Circulation 2017, 136, 1–23. [Google Scholar] [CrossRef]
- Jo, Y.H.; Kim, S.B.; Ahn, J.H.; Turk, A.; Kwon, E.B.; Kim, M.O.; Hwang, B.Y.; Lee, M.K. Xanthones from the stems of Cudrania tricuspidata and their inhibitory effects on pancreatic lipase and fat accumulation. Bioorg. Chem. 2019, 92, 103234. [Google Scholar] [CrossRef]
- Qiu, X.L.; Zhang, Q.F. Chemical profile and pancreatic lipase inhibitory activity of Sinobambusa tootsik (Sieb.) Makino leaves. Peer J. 2019, 7, e7765. [Google Scholar] [CrossRef]
- Speakman, J.R. Use of high-fat diets to study rodent obesity as a model of human obesity. Int. J. Obes. 2019, 43, 1491–1492. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
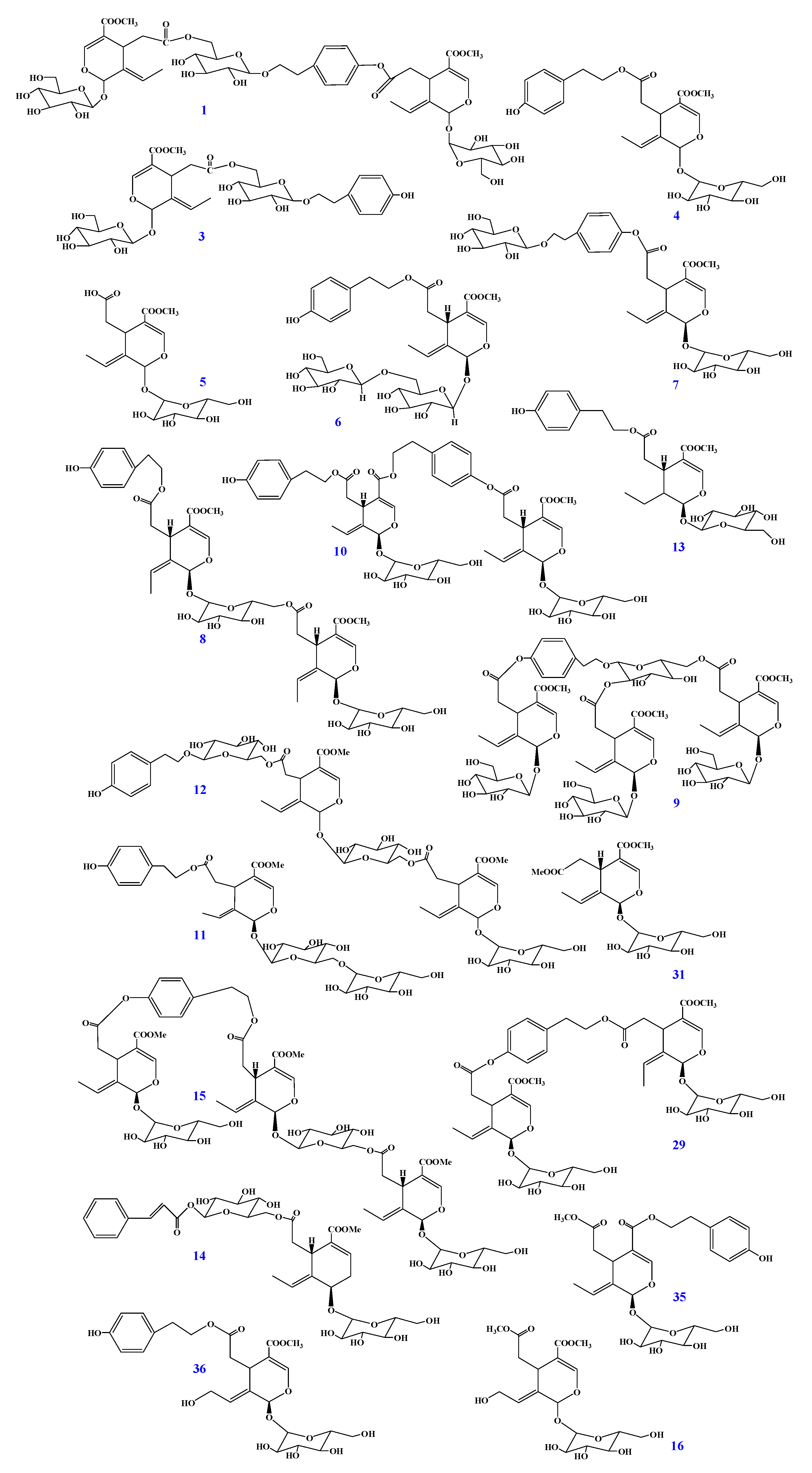
| No. | Name | Calibration Curves a | R2 | Content b (mg/g) | Inhibition% b (100 μM) | IC50 b (μM for Compounds) (mg/L for FM Extract) |
|---|---|---|---|---|---|---|
| 1 | GI3 | Y = 481.08 + 6.30X | 0.9984 | 9.17 ± 0.05 | 53.42 ± 6.33 | 95.36 ± 3.12 |
| 3 | Nuzhenide | Y = 667.40 + 4.41X | 0.9999 | 88.21 ± 0.19 | 43.45 ± 3.28 | 120.97 ± 4.94 |
| 4 | Ligstroside | Y = 40.34 + 13.69X | 0.9986 | 16.88 ± 0.11 | 49.67 ± 4.25 | 100.59 ± 2.74 |
| 5 | Oleoside-11-methyl ester | Y = 153.23 + 3.97X | 0.9991 | 2.90 ± 0.03 | 48.53 ± 5.13 | 102.94 ± 4.13 |
| 6 | Oleuricine A | Y = 32.99 + 2.35X | 0.9997 | 0.42 ± 0.01 | 41.41 ± 1.95 | 126.27 ± 0.87 |
| 7 | Nicotiflorine | Y = −65.95 + 4.79X | 0.9999 | 7.37 ± 0.10 | 37.35 ± 6.34 | 135.84 ± 4.98 |
| 8 | Jaspolyanoside | Y = 108.89 + 0.36X | 0.9995 | 3.19 ± 0.02 | 62.41 ± 3.98 | 66.46 ± 2.20 |
| 9 | Oleopolynuzhenide A | Y = 42.78 + 2.25X | 1.0000 | 0.62 ± 0.03 | 70.25 ± 4.56 | 54.60 ± 2.38 |
| 10 | Safghanoside G | Y = 91.52 + 2.39X | 1.0000 | 5.55 ± 0.09 | 60.16 ± 5.78 | 67.91 ± 3.49 |
| 11 | Excelside B | Y = 169.19 + 2.31X | 0.9999 | 3.22 ± 0.02 | 49.98 ± 4.33 | 100.06 ± 2.26 |
| 12 | Isooleonuezhenide | Y = 81.21 + 3.72X | 0.9999 | 0.89 ± 0.12 | 57.62 ± 4.83 | 90.48 ± 3.67 |
| 13 | Lucidumoside A | Y = 261.83 + 3.66X | 0.9997 | 6.11 ± 0.01 | 47.69 ± 8.36 | 102.37 ± 5.02 |
| 14 | Safghanoside A | Y = 130.90 + 1.87X | 0.9996 | 4.81 ± 0.10 | 33.77 ± 6.14 | 193.81 ± 4.60 |
| 15 | Jaspolyoleoside B | Y = −1.75 + 0.09X | 0.9999 | 1.33 ± 0.05 | 53.32 ± 7.31 | 96.27 ± 4.91 |
| 16 | 10-hydroxoleoside-7,11-dimethyl ester | Y = −1578.72 + 15.65X | 0.9993 | 5.31 ± 0.01 | 43.57 ± 3.09 | 117.42 ± 1.38 |
| 29 | GI5 | Y = −2.04 + 1.72X | 0.9999 | 8.91 ± 0.01 | 58.77 ± 6.01 | 90.51 ± 2.24 |
| 31 | Oleoside-7,11-dimethyl ester | Y = −26.33 + 15.08X | 0.9998 | 10.79 ± 0.02 | 50.11 ± 5.97 | 99.78 ± 3.88 |
| 35 | Isolignstroside | Y = 235.57 + 4.07X | 0.9999 | 0.22 ± 0.03 | 52.07 ± 2.99 | 98.87 ± 2.85 |
| 36 | 10-hydroxyligstroside | Y = 6236.70 + 1.94X | 0.9991 | 5.45 ± 0.01 | 36.35 ± 5.27 | 167.72 ± 3.51 |
| Positive | Orlistat | / | / | / | 73.11 ± 3.23 | 48.92 ± 2.44 |
| Extract | FM | / | / | / | 80.29 ± 1.28 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.; Zhao, H.; Ma, Z.; Zhang, S.; Li, M.; Zheng, Z.; Ren, X.; Ho, C.-T.; Bai, N. Anti-Obesity and Gut Microbiota Modulation Effect of Secoiridoid-Enriched Extract from Fraxinus mandshurica Seeds on High-Fat Diet-Fed Mice. Molecules 2020, 25, 4001. https://doi.org/10.3390/molecules25174001
Guo S, Zhao H, Ma Z, Zhang S, Li M, Zheng Z, Ren X, Ho C-T, Bai N. Anti-Obesity and Gut Microbiota Modulation Effect of Secoiridoid-Enriched Extract from Fraxinus mandshurica Seeds on High-Fat Diet-Fed Mice. Molecules. 2020; 25(17):4001. https://doi.org/10.3390/molecules25174001
Chicago/Turabian StyleGuo, Sen, Haoan Zhao, Zhongxiao Ma, Shanshan Zhang, Mingrou Li, Zhaojing Zheng, Xiameng Ren, Chi-Tang Ho, and Naisheng Bai. 2020. "Anti-Obesity and Gut Microbiota Modulation Effect of Secoiridoid-Enriched Extract from Fraxinus mandshurica Seeds on High-Fat Diet-Fed Mice" Molecules 25, no. 17: 4001. https://doi.org/10.3390/molecules25174001
APA StyleGuo, S., Zhao, H., Ma, Z., Zhang, S., Li, M., Zheng, Z., Ren, X., Ho, C.-T., & Bai, N. (2020). Anti-Obesity and Gut Microbiota Modulation Effect of Secoiridoid-Enriched Extract from Fraxinus mandshurica Seeds on High-Fat Diet-Fed Mice. Molecules, 25(17), 4001. https://doi.org/10.3390/molecules25174001






