1. Introduction
Superparamagnetic iron oxide nanoparticles (SPIONs) are of great importance when grafted with biomarkers for applications in modern biological and biotechnology areas. The surface-modified magnetic nanoparticles (MNPs) can be used both in in-vitro and in-vivo systems effectively. The size of the MNPs needs to be controlled and innovative functionalization techniques need to be utilized for the effective implementation of these modified MNPs in medical applications [
1,
2,
3,
4,
5,
6].
The small size of MNPs (a few nanometers) is essential for them to be able to interact, bind, or penetrate biological entities. This is because in the nano range, their dimensions are comparable to those of proteins, cells, or viruses, and this facilitates their movement through biological structures [
7]. These MNPs are highly attractive for use in biomedical applications such as magnetic resonance imaging (MRI), targeted drug delivery, and treatment of hyperthermia. These are due to certain unique properties that arise due to a combination of their small dimension, enhanced sensing, and nanoscale-dependent magnetism, as well as physiological properties [
8]. MNPs are also very commonly used these days for in-vivo applications as drug carriers in a magnetic ‘tag drag-release’ process, termed as targeted drug delivery. The MNPs are usually loaded with special drug molecules or chemotherapy agents, which are directly vectorized to tumor cells by targeting ligands on their surfaces, or by the application of an external magnetic field. In recent years, substantial interest has been focused on multifunctional MNPs in which diagnostic (MRI) and therapeutic (hyperthermia treatment and drug delivery) capabilities are combined [
9,
10,
11,
12].
The successful design of MNPs for biological applications needs a careful selection of magnetic core and surface coating material, where the former mainly determines the MNPs’ heating and sensing capabilities with regards to application efficiency and the latter specifies the interaction of these MNPs with a physiological environment. To enable the direct use of MNPs in biomedical applications, the MNPs should be further functionalized by conjugating them with functional groups. The surface coating provides a suitable base for the attachment of these functional groups on MNPs. These groups such as antibodies, peptides, polysaccharides, etc., permit specific recognition of cell types and direct the MNPs to a specific tissue or cell type by binding to a cell surface receptor. The silica coating facilitates the functionalization of the surface of the MNPs with either amine groups or carboxylic groups, which help in bonding with biological entities for medical applications. Also, the silica coating does not affect the magnetic property of the Fe
3O
4 core in a substantial way. Among the different types of MNPs, iron oxides (magnetite (Fe
3O
4), maghemite (γ-Fe
2O
3), and hematite (α-Fe
2O
3)) are by far the most commonly employed ones for in-vivo applications since iron is physiologically well tolerated. Silica is inorganic but bio-friendly and it is known for its chemical stability and ease of formation. The biggest advantage of having a surface enriched in silica is the presence of silanol groups, which can easily react with coupling agents, providing strong attachment of surface ligands on MNPs [
13]. There are several successful methods available for the formation of silica coating, amongst which the most commonly used is the Stöber method. Here, a hydrolysis reaction of tetraethyl orthosilicate (TEOS) is governed in alcohol media under the catalytic action of ammonia [
14]. In this paper, a variation of this method is applied to produce more homogeneous coatings of silica.
The coupling strategy used for linking the functionalizing agent to the particle is a pivotal component for success, and will largely depend both on the coating of the MNPs and the available functional groups on the target moiety [
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26]. To improve the sensitivity of sensing devices, signal amplification has been attempted using MNPs. The role of MNPs in our experiment is to pre-concentrate the analyte and, following magnetic separation, remove the undesired effect of the matrix, so that a clean measurable signal can be obtained. In the last decade, MNPs-based EC biosensors [
27,
28,
29,
30,
31,
32,
33,
34,
35,
36,
37], for salivary diagnostics, have received increasing attention because many substances enter saliva from the blood through transcellular or paracellular diffusion [
38]. Consequently, most substances found in blood are also present in saliva. Therefore, saliva is functionally equivalent to serum in reflecting the physiological state of the body but has several advantages over blood [
39], as saliva collection is easy, stress free, requires painless sampling, has a simple matrix (less complex than blood), is non-invasive, and offers the possibility of performing real-time monitoring. Saliva has recently been the prominent body fluid for the study of SARS-CoV-2 during the COVID-19 pandemic. Saliva is not only a reliable tool to detect the virus, but can also help in studying the evolution of the virus [
40,
41,
42]. Hence, there are compelling reasons for exploring saliva as a diagnostic and prognostic fluid in heart failure research [
43,
44] as proposed here. However, saliva is not as constant as blood, as we must keep in mind the change in salivating nature of humans on the sight and smell of food and also depending on the hunger quotient of a particular individual at the specific time. In any case, saliva is there as a potential diagnostic tool due to its ease and non-invasive accessibility along with its abundance of biomarkers, such as genetic material and proteins [
45].
To learn if salivary diagnosis is applicable in the conditions defined earlier on pre-concentration in saliva we tested tumor necrosis factor alpha (TNF alpha), responsible for a diverse range of signaling events within cells, and Interleukin 10 (IL-10) that plays a central role in limiting host immune response to pathogens, thereby preventing damage to the host. To study the feasibility of saliva and, in these preliminary tests, avoid the variability in concentration of the saliva, we considered adequate to test the immunoreagents in artificial saliva and ensure that there is no cross-reactivity between the immunoreagents used. In this research, we have immobilized anti-TNF-α and anti-IL-10 antibodies, which are present in saliva on MNPs as a proof of concept. The 3D nano-collectors generated were evaluated using UV-vis spectrophotometry (JENWAY 7205) for their ability to pre-concentrate the antigens TNF-α and IL-10, respectively, by employing an external magnetic field. Tests were performed both in phosphate buffered saline (PBS) and artificial saliva (AS) as the matrix. The obtained results have proven that our MNPs can represent a promising tool for rapid pre-concentration of heart failure biomarkers (TNF-α/IL-10) in saliva. Further investigation will be carried out to integrate our MNPs with sensors to increase the sensitivity, selectivity, and decrease matrix effect.
On the other hand, the colloidal stability is a big issue with the MNPs or magnetic nanocomposites, since their advent there remains a big issue surrounding the stability of the MNPs in colloids. The more stable they are in a colloidal state, the less easy it is to use them for magnetic separation, while on the other hand, if they are less stable then they are easier to isolate by external magnetic field application. So, for this a compromise has to be made regarding them and based on their application they are synthesized as needed. In order to improve magnetization, larger particles can be synthesized; these show higher magnetization but much less stability in colloidal state as, due to their large sizes, they have a greater tendency to aggregate. MNPs are more prone to aggregation compared to other nanoparticles and agglomeration is even more significant for MNPs as there exists magnetic dipole–dipole attraction among themselves [
46]. In particular, the magnitude of this magnetic dipole–dipole attraction is directly proportional to power 2 of the particle saturation magnetization and power 3 of the particle size. Sedimentation of the agglomerates from its suspension is a challenge [
47] that could limit mobility and applicability of MNPs [
48] especially in biosensing applications.
In order to improve stability and also preserve the magnetic properties, the following few things can be done: Sonication, which is a mechanical way and very effective in disaggregating the particles and dispersing them in colloidal state. One of the other methods is using inorganic shell coating like Silica or carbon coating to improve the stability. Also, surface coating with macromolecules like polymers or surfactants can be used for improving stability. Increasing viscosity of the medium can also prevent aggregation as the particles cannot get dispersed easily and travel through the viscous medium, thus preventing aggregation. This can be done by gum gelation or by emulsion formation.
In this paper, one of the first studies done using saliva as a diagnostic fluid for heart failure research is being reported. There lies a novelty in the synthesis procedure in the use of a non-magnetic mechanical stirrer for the coating of SiO
2 layer on the iron oxide core. This strategy coupled with the concave nature of the reaction flask used results in more homogenized coating of the magnetic core with the Silica layer. The traditional method of using a magnetic bead in this step results in inefficient coating as a major part of the magnetic core becomes attracted to the bead and does not fully participate in the reaction. Using our strategy of a specifically designed mechanical stirrer in the concave flask with Teflon coating on top leads to an increase in the chemical kinetics of the reaction by creating a vortex that leads to homogenized coating with almost negligible reactant losses. Usually, when magnetic nanoparticles tend to aggregate, this causes difficulty in magnetic separation as it is well established that the smaller the size of the particles, the faster is the separation time, while phenomenon like aggregation and flocculation slow down the process [
49].
Furthermore, besides a modification in the synthesis procedure of the MNPs and artificial saliva as the fluid for heart failure detection, colloidal stability of MNPs has been studied here with different surfactants and by increasing the viscosity of the medium by using a surfactant. Relevant issues like colloidal stability of MNPs in long-term use for varied applications and how the medium affects their stability are avoided in most studies dealing with MNPs. In this paper, preliminary results of how the stability varies with the change in viscosity of the medium and how difficult it is to extract with an external magnet is reported. Certain common issues with MNPs, which are not reported when its synthesis and application is stated like its long-term usage, stability, and extraction time using external magnets and stirrer systems, are dealt with here.
2. Results
The syntheses of Fe
3O
4@SiO
2-NH
2 and Fe
3O4@SiO
2-NH
2-COOH were done using co-precipitation method, followed by Stober process and functionalization [
50,
51], though the critical point in the synthesis procedure is to use the mechanical stirrer and not the magnetic bead stirring procedure as is commonly used in other colloidal synthesis routes. This is necessary to avoid the MNPs from sticking to the bead, hence the mechanical stirring is necessary to disperse the MNPs in the solution for the reaction to take place. This is used only in the silica coating procedure; after that, magnetic stirring can be used in these MNPs as, after the silica coating, the direct contact to the magnetite core is prevented by the silica shell. After the synthesis of the magnetite core, it is important to first disperse the MNPs using an ultrasonicator before any further reactions. There is a slight increase in the size of the MNPs after silica coating, which is further increased by functionalizing with the NH
2 and COOH groups. The Fe
3O
4@SiO
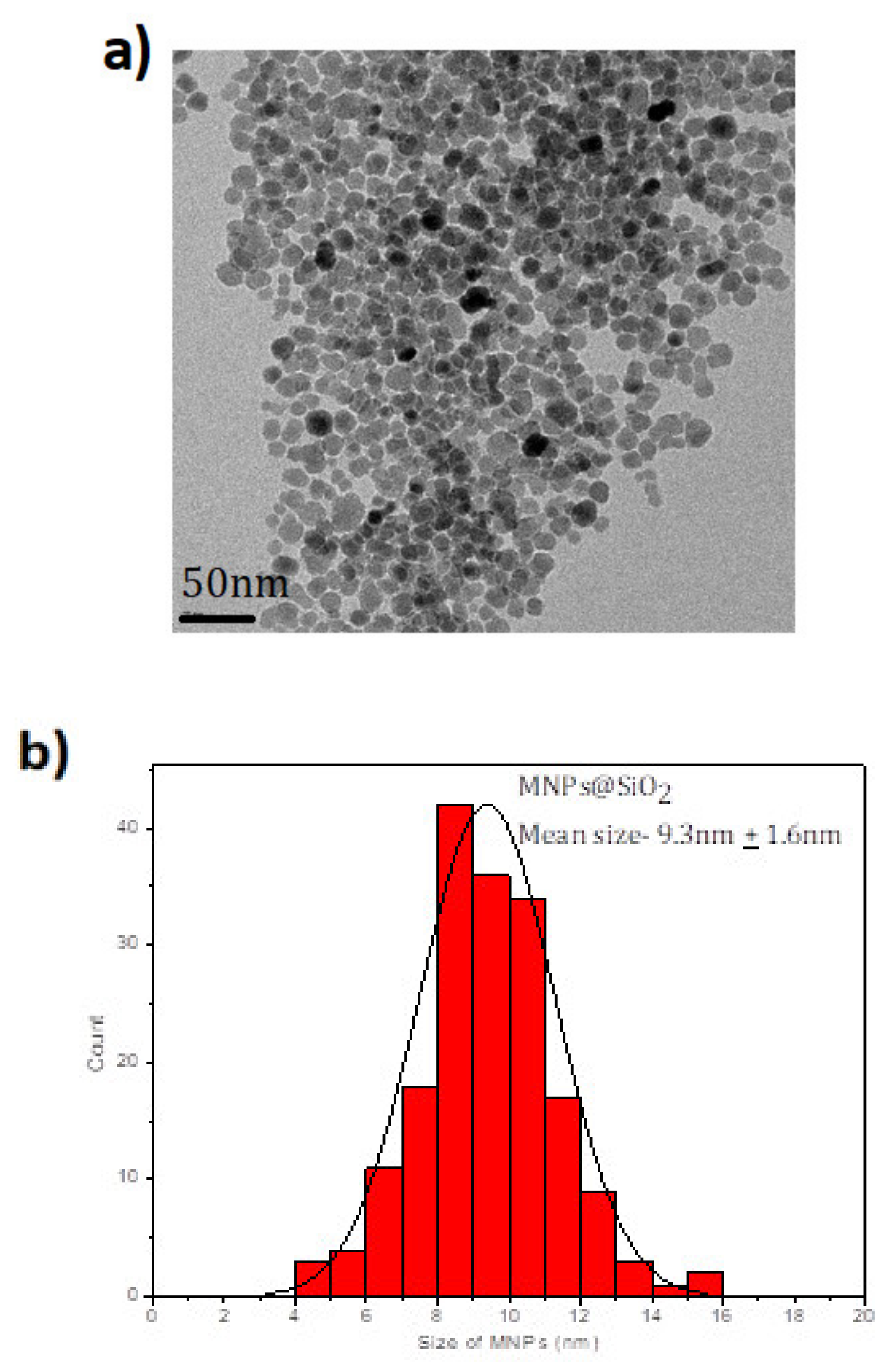
2 MNPs are shown in TEM images (see
Figure 1a) with the characteristic spherical shape of these MNPs with a mean size of 9.3 ± 1.6 nm (see
Figure 1b). When the Fe
3O
4@SiO
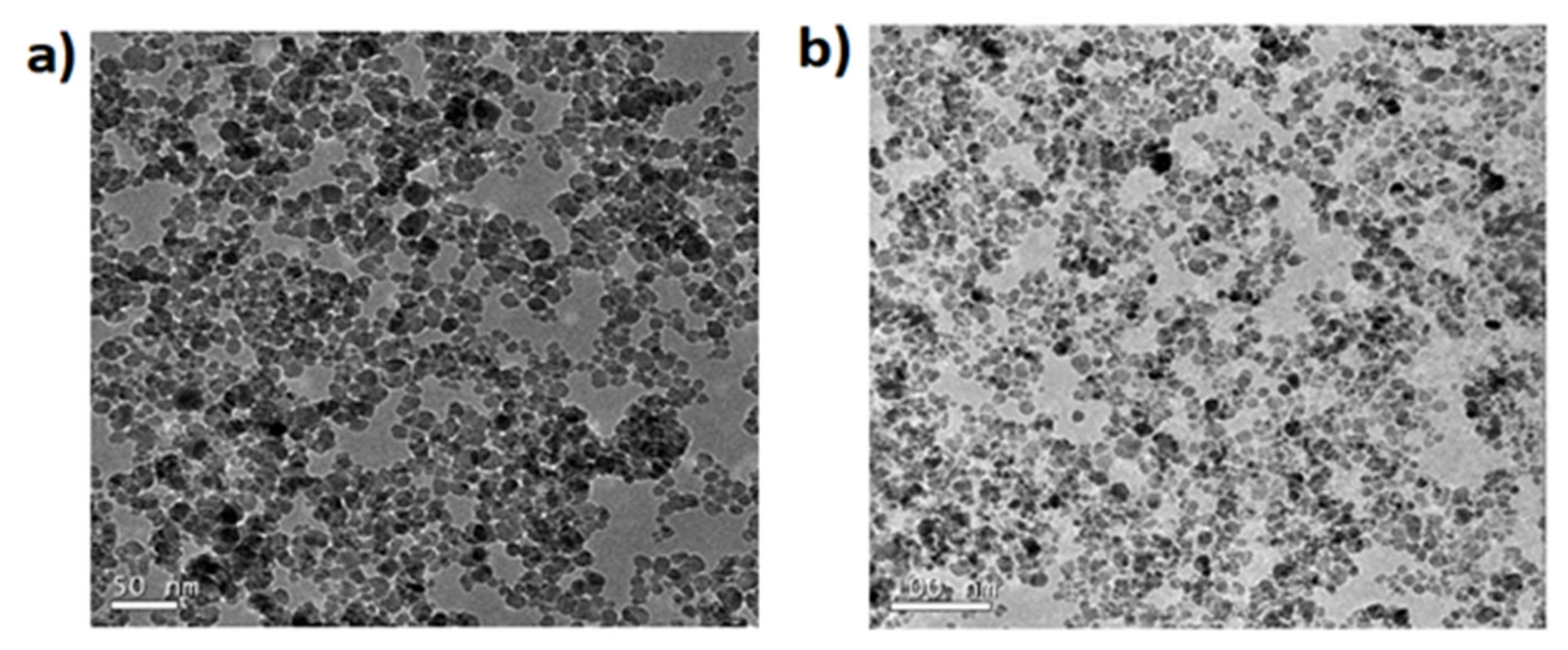
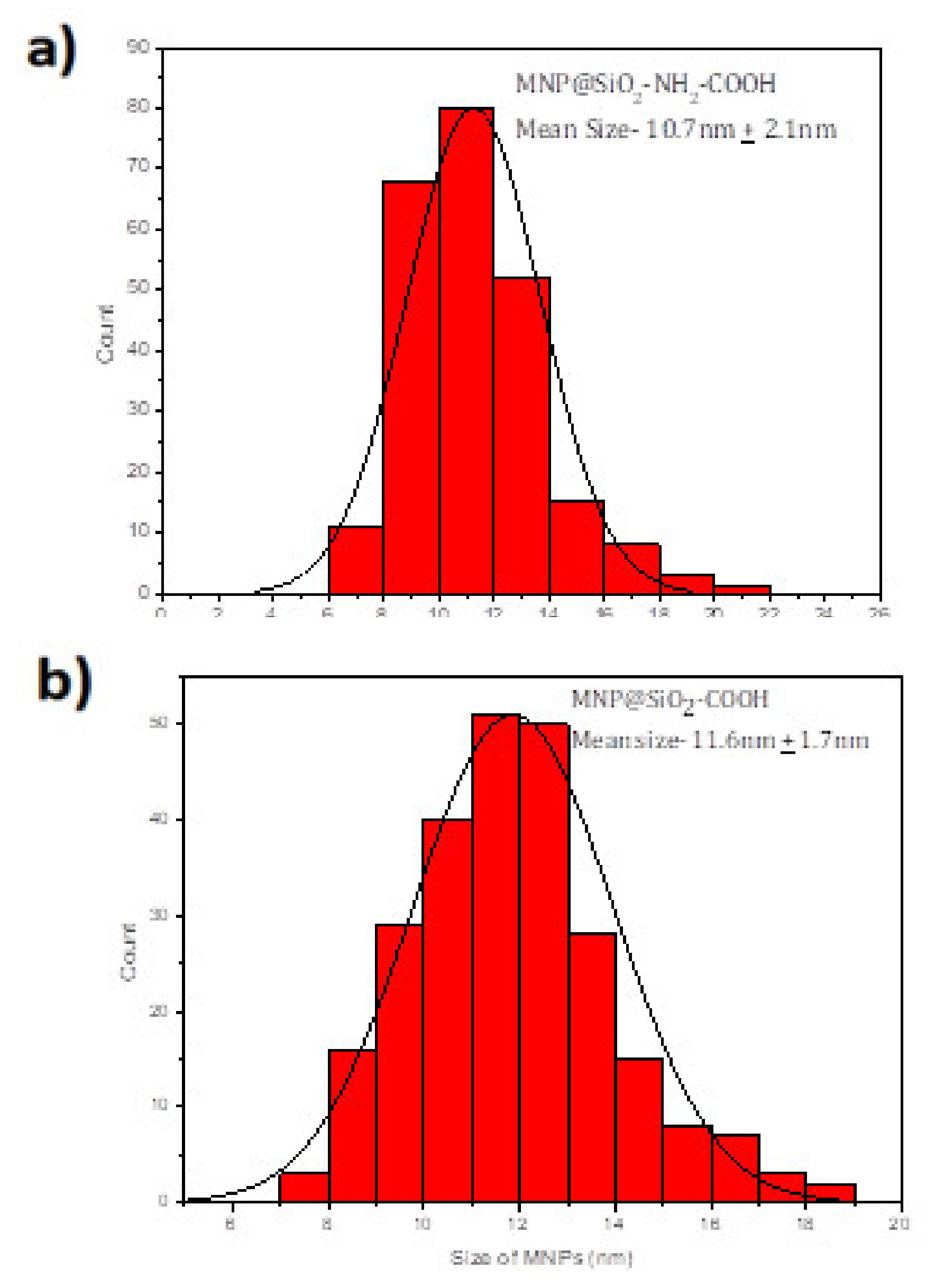
2 MNPs are coated with amine and a carboxylic acid, the spherical shape is maintained as
Figure 2 shows but the average size increases to 10.7 + 2.1 nm and 11.6 ± 1.7 nm (see
Figure 3).
The SEM and EDS techniques provide information about the morphology of the MNPs (
Figure S1a) (indicating that the sample is homogenous) and the composition (
Figure S2), respectively. The sample showed uniform-sized spheres along with a consistent presence of Fe, Si, and O throughout the sample. The composition was studied using EDS and the analysis was done across the whole sample and it was consistent throughout. Using the EDS technique, it is possible to know the percentage coated by SiO
2. In this sample, the coating is 1/3 of Si/Fe that is confirmed with the size we get by TEM (Fe
3O
4 has a size near 7 nm and Fe
3O
4@SiO
2 has 9.3 nm).
Figure S1b shows that the electron diffraction (ED) confirms the cubic spinel structure of the MNPs. This ensures that the magnetization of these MNPs results from the Fe
2+ ions of the MNPs. Also, further chemical composition was analyzed using the IR spectrum. Fe
3O
4@SiO
2 and Fe
3O
4@SiO
2-COOH was measured with IR and the characteristic peaks of Si-O and C=O was observed in the respective samples (
Figure S3) at 1058 cm
−1 and 1739 cm
−1, respectively. Further, these MNPs were also characterized using IR before and after being dispersed in PBS, and the peaks of Si-O and C=O were still visible, thus confirming the presence of the functionalized coating of the MNPs (
Figure S4).
Magnetic characterization of the MNPs was carried out in a superconductive quantum interference device (SQUID) magnetometer (Quantum Design MPMS5XL). Magnetization vs. magnetic field measurements were performed at 300K in a 6T field. The samples were prepared using a polycarbonate capsule each filled with 1 mg of Fe
3O
4@SiO
2-NH
2 or Fe
3O
4@SiO
2-NH
2-COOH and compacted cotton. We already know that the MNPs show the cubic spinel structure by ED. The magnetic property of iron oxide (Fe
3O
4) nanoparticles is dependent on the distribution of Fe ions in octahedral and tetrahedral sites of the spinel structure [
52]. The magnetic spins of the ions in the octahedral sites are ferromagnetically coupled to each other and antiferromagnetically coupled with tetrahedral sites. Since the number of Fe
3+ ions in the octahedral sites and the tetrahedral sites are the same, their magnetic spins cancel out each other. Consequently, the magnetic spins of only Fe
2+ ions in the octahedral sites contribute to the net magnetic moment in a spinel structure.
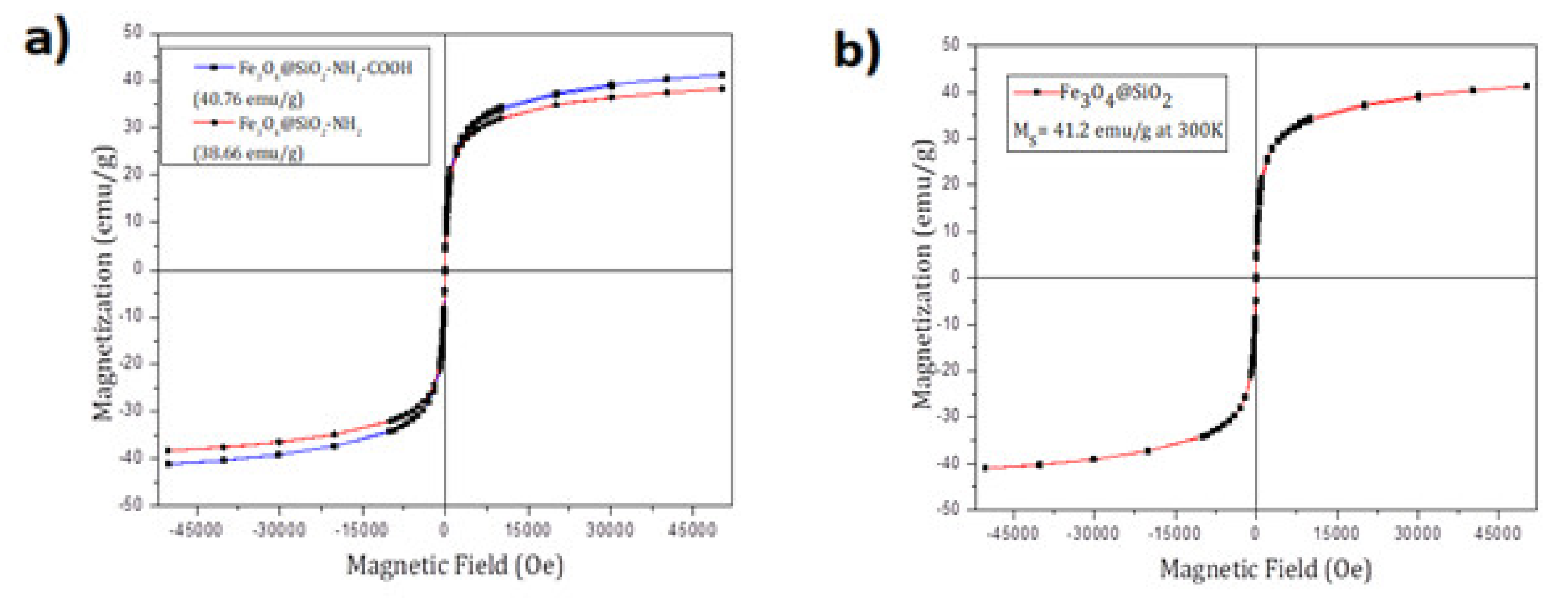
Figure 4 shows a typical magnetization curve at 300K for superparamagnetic nanoparticles in which neither remnant magnetization (magnetization at zero field, M
R) nor coercivity (hysteresis loop, H
c) was observed. The saturation magnetization value of MNPs at 300K was 38.66 emu/g for Fe
3O
4@SiO
2-NH
2 and 40.76 emu/g for Fe
3O
4@SiO
2-NH
2-COOH. Also, the magnetization curve for Fe
3O
4@SiO
2 is shown where the saturated magnetization is 41.2 emu/g. (
Figure 4)
The colloidal stability as mentioned at the start is an issue with the MNPs. The MNPs we synthesized were extremely small in size and hence had a tendency to aggregate due to the magnetic dipole–dipole interaction and precipitate over the course of time. This also aided in easily extracting them with the help of an external magnet. To be able to use these MNPs in biosensors, it was important to test their stability and, if possible, increase it without affecting their facile extraction using an external magnet. Five different surfactants were used for this purpose and they are as follows: Citric Acid, Tricaprylylmethyl ammonium chloride, Cetyl trimethyl ammonium chloride (CTAC), Tetrabutyl ammonium chloride, and Dimethyl di-octadecyl ammonium chloride. Out of these five, the last one was successful in providing long-term stability. This was so because it increased the viscosity of the medium and, hence, stopped the MNPs from aggregating. The surfactant prevented the diffusion of the MNPs through the solvent by increasing the viscosity and hence hindering their aggregation. However, this came with a major drawback, as these MNPs were harder to extract using an external magnet. Different concentrations of the surfactant were studied but the trend observed was the same; the more stable they became, the harder it was to extract them. The zeta-potential values obtained for each surfactant is given in
Table 1 as well as the different zeta-potential values obtained for different concentrations of dimethyl di-n-octadecyl ammonium chloride in
Table 2.
Up until 15 mg of the surfactant, the dispersion was stable, and with time, a small number of MNPs could be extracted by the magnet. However, to obtain them completely dry and free of the surfactant is difficult as it makes the suspension extremely viscous.
Figure S10 shows the different time periods needed to extract the MNPs with increasing stability and viscosity of the medium.
The edges of toroidal external magnets have been used in all the cases to extract the magnets as they comprise of the maximum magnetic fields passing through them. The magnets were used on the sidewalls of the vials or reaction flasks to extract the MNPs.
Figure S11 shows a photo of the external magnet and the vials used for extraction of the MNPs.
To learn about the bio-functionalization efficacy of MNPs with anti-TNF-α antibody, the following experiments were performed: A known amount of MNP@SiO
2-NH
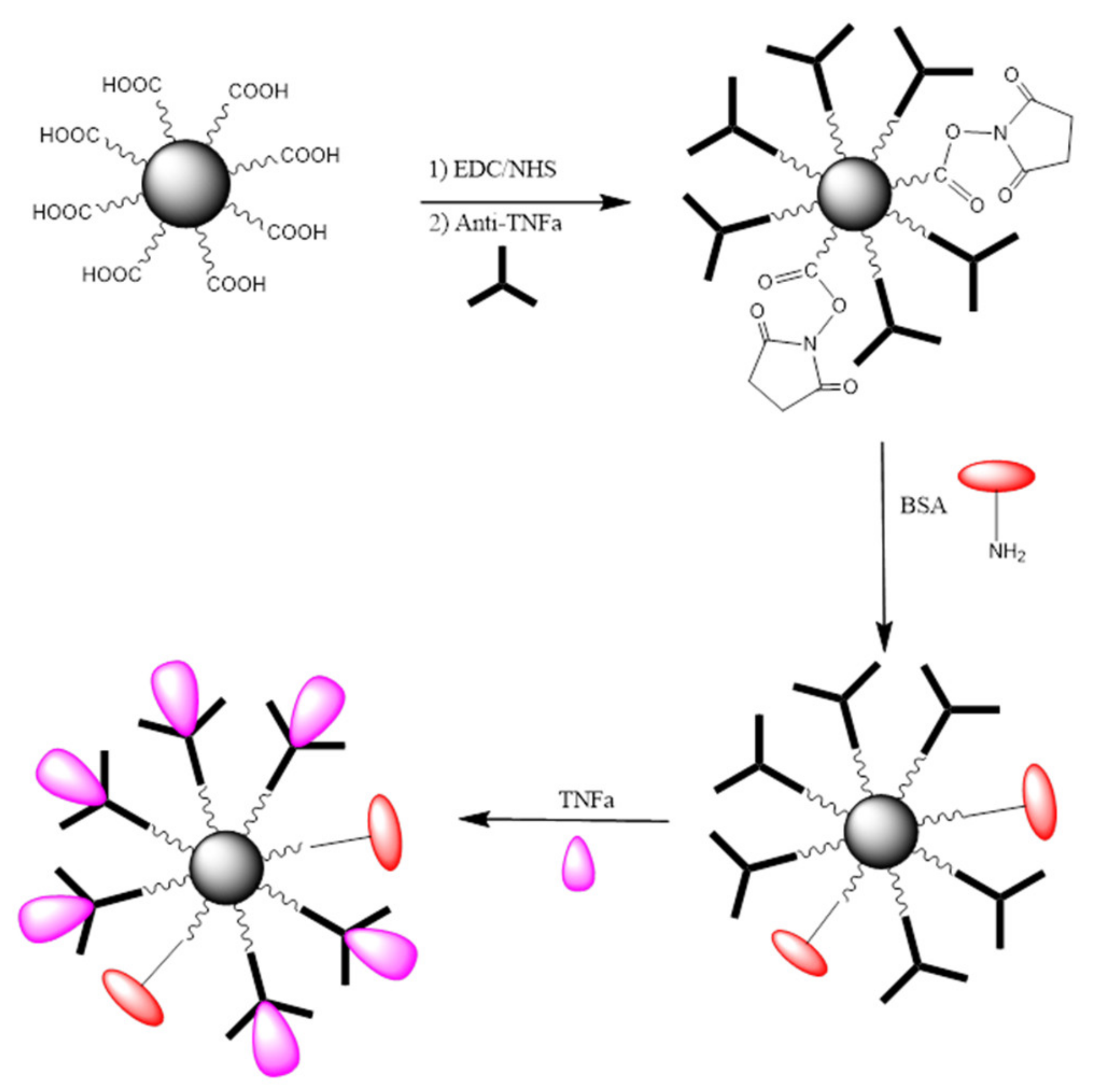
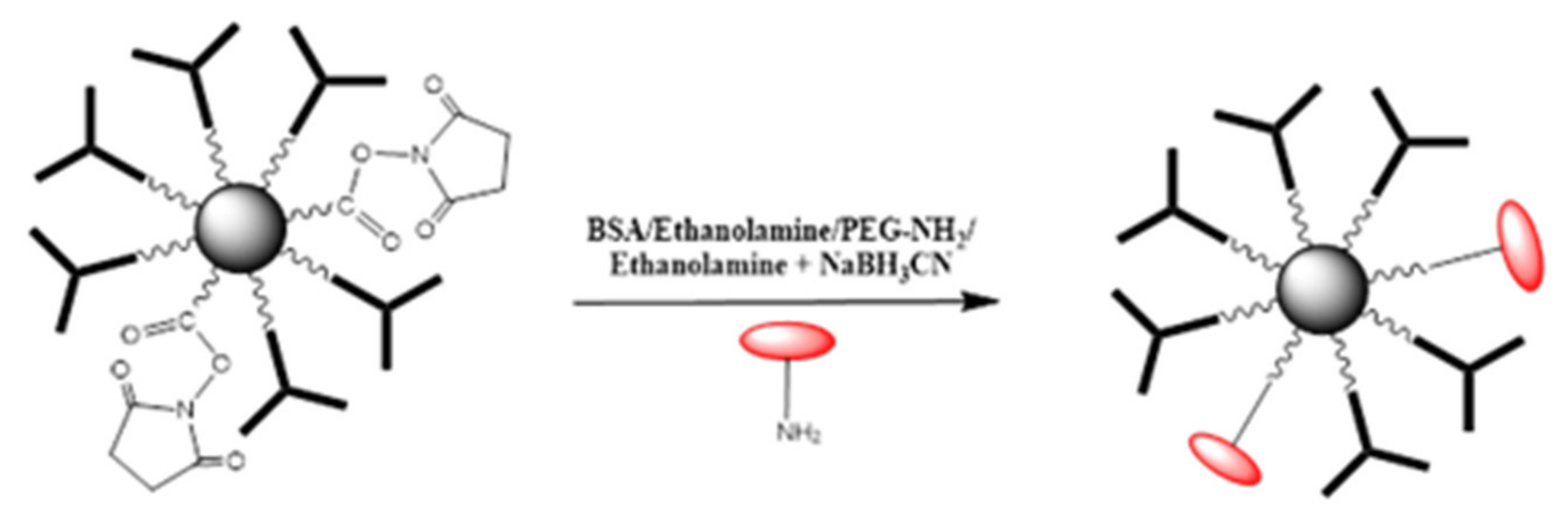
2-COOH activated with EDC/NHS was incubated for a fixed period of time with a known concentration of anti-TNF-α antibody. Following a magnetic separation, the supernatant liquid was analyzed by UV-vis spectrophotometry. The bio-functionalization efficacy was evaluated by comparing the absorbance reading to that obtained for the supernatants of the same concentration of anti-TNF-α antibody after incubation with non-activated MNPs. Consequently, the physisorption of antibodies onto MNPs was taken into account and differences in absorbance can only be attributed to the concentration of unreacted antibody remaining in the supernatants. An illustration of the steps involved in the bio-functionalization of MNPs is presented in
Figure 5. Firstly, the –COOH groups present on the MNPs surface were activated using a mixture of EDC/NHS followed by the incubation of the activated MNPs with a fixed concentration of antibody to create the complex Antibody-MNPs. Afterwards, the unreacted sites R–COO-NHS were deactivated (BSA was used in the illustration). Finally, the 3D nano-collector MNP@SiO
2-NH
2-CO-anti-TNF-α was incubated with the antigen at different concentrations.
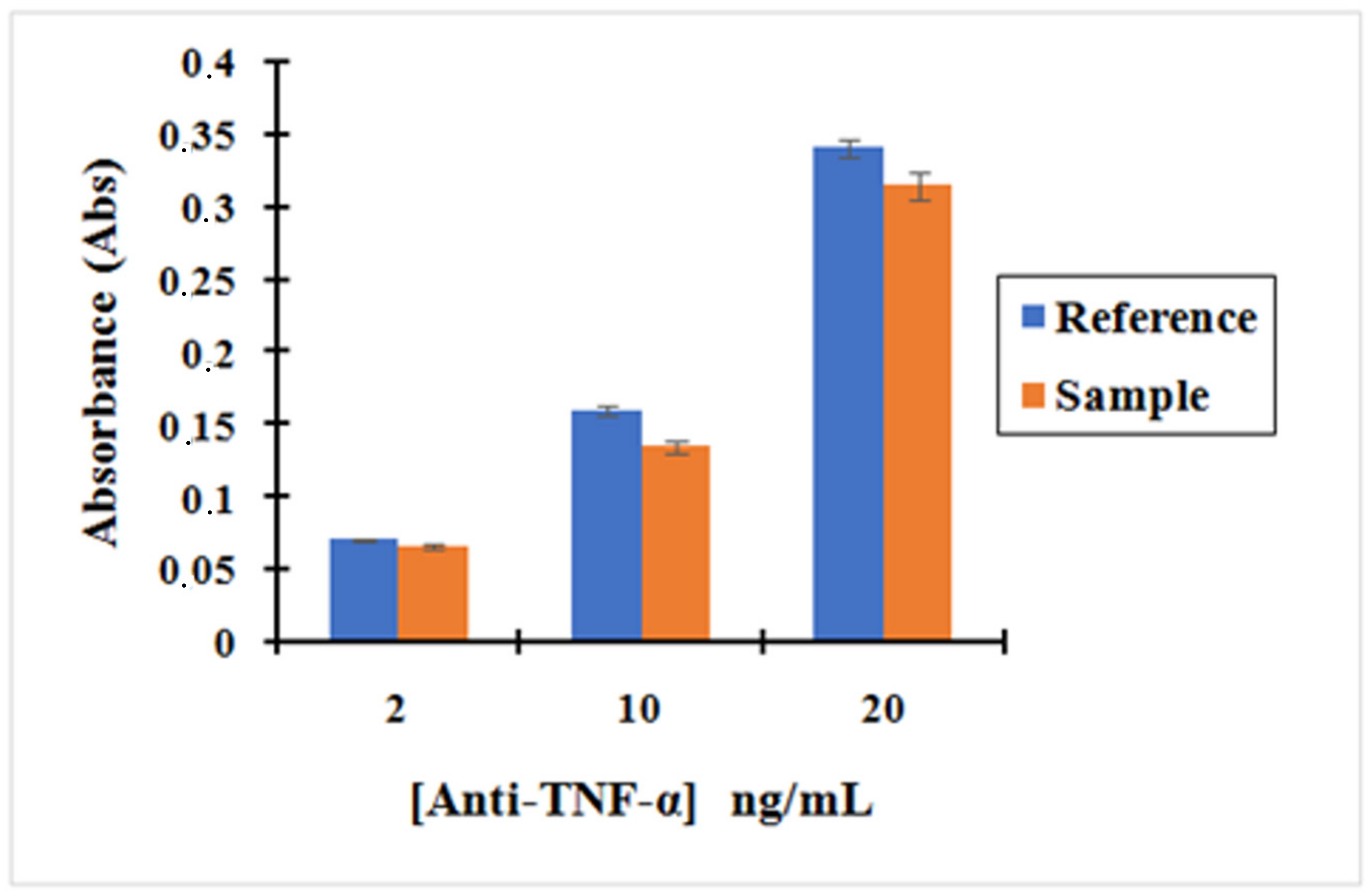
Figure 6 shows that the absorbance of anti-TNF-α antibody solutions after incubating it with activated MNPs is lower than the absorbance of the anti-TNF-α antibody solutions after incubating with non-activated MNPs. The small difference in the absorbance seen between the Activated MNPs and non-activated MNPs confirms that the activated MNPs binds anti-TNF-α antibody on their surface by the reaction between the amine group of antibody TNF-α and R–COO-NHS groups obtained on the MNPs using EDC/NHS.
This was confirmed by the reproducibility of our three replication measurements. In addition, our bio-functionalized MNPs are vortexed before the removal of supernatant so that the weakly bonded by physical interaction are removed and only the strongly bonded by covalent interaction remain, enabling us to evaluate the effectiveness of our chemistry. These results confirm that the bio-functionalization of MNPs with the antibodies was successfully achieved. Moreover, the concentration of anti-TNF-α in the supernatant decreased by 7%, 16%, and 8% for an initial concentration of 2, 10, and 20 ng/mL, respectively, using the "MNP@SiO2-NH2-COOH”. Therefore, based on these results, the concentration of anti-TNF-α antibody to run the incubation was fixed at 10 ng/mL to ensure the successful bio-functionalization of MNPs.
The bio-functionalization efficacy was calculated using Beer–Lambert law. Beer–Lambert Law is difficult for quantitative measurement for wavelengths near 200 nm though it has been used here and has provided satisfactory results.
A is the absorbance, ε is the molar attenuation coefficient, l is the optical path length, and c is the concentration of the attenuation species (Equation (1)).
| Concentrations (ng/mL) | 2 | 10 | 20 |
| Absorbance Sample | 0.066 | 0.135 | 0.315 |
| Absorbance Reference | 0.071 | 0.160 | 0.341 |
The methodology that has been used is the following:
Equation (2)/Equation (3): Asample/Areference = Csample/Creference
Csample = Asample × Creference/Areference
2 ng/mL
Csample = Asample × Creference/Areference = 0.066 × 2/0.071 = 1.85 ng/mL
This is means that the concentration of Antibodies fixed on MNPs is 0.15 ng/mL and the percentage of antibodies fixed in the MNPs is 7%.
10 ng/mL
Csample = Asample × Creference/Areference = 0.135 × 10/0.160 = 8.43 ng/mL
This is means that the concentration of Antibodies fixed on MNPs is 1.57 ng/mL and the percentage of antibodies fixed in the MNPs is 16%.
20 ng/mL
Csample = Asample × Creference/Areference = 0.315 × 20/0.341 = 18.47 ng/mL
This means that the concentration of Antibodies fixed on MNPs is 1.53 ng/mL and the percentage of antibodies fixed in the MNPs is 8%.
The complex MNP@SiO2-NH2-CO-anti-TNF-α described in the previous section was used to pre-concentrate TNF-α in PBS. This section details the experiments performed to incubate TNF-α at different concentrations with the complex MNP@SiO2-NH2-CO-anti-TNF-α and afterwards to measure the supernatants containing the unreacted TNF-α by UV-vis spectrophotometry. Thus, a comparison with the initial concentration of the antigen could be carried out and the percentage of TNF-α, which has been bonded to the complex MNP@SiO2-NH2-CO-anti-TNF-α, could be assessed.
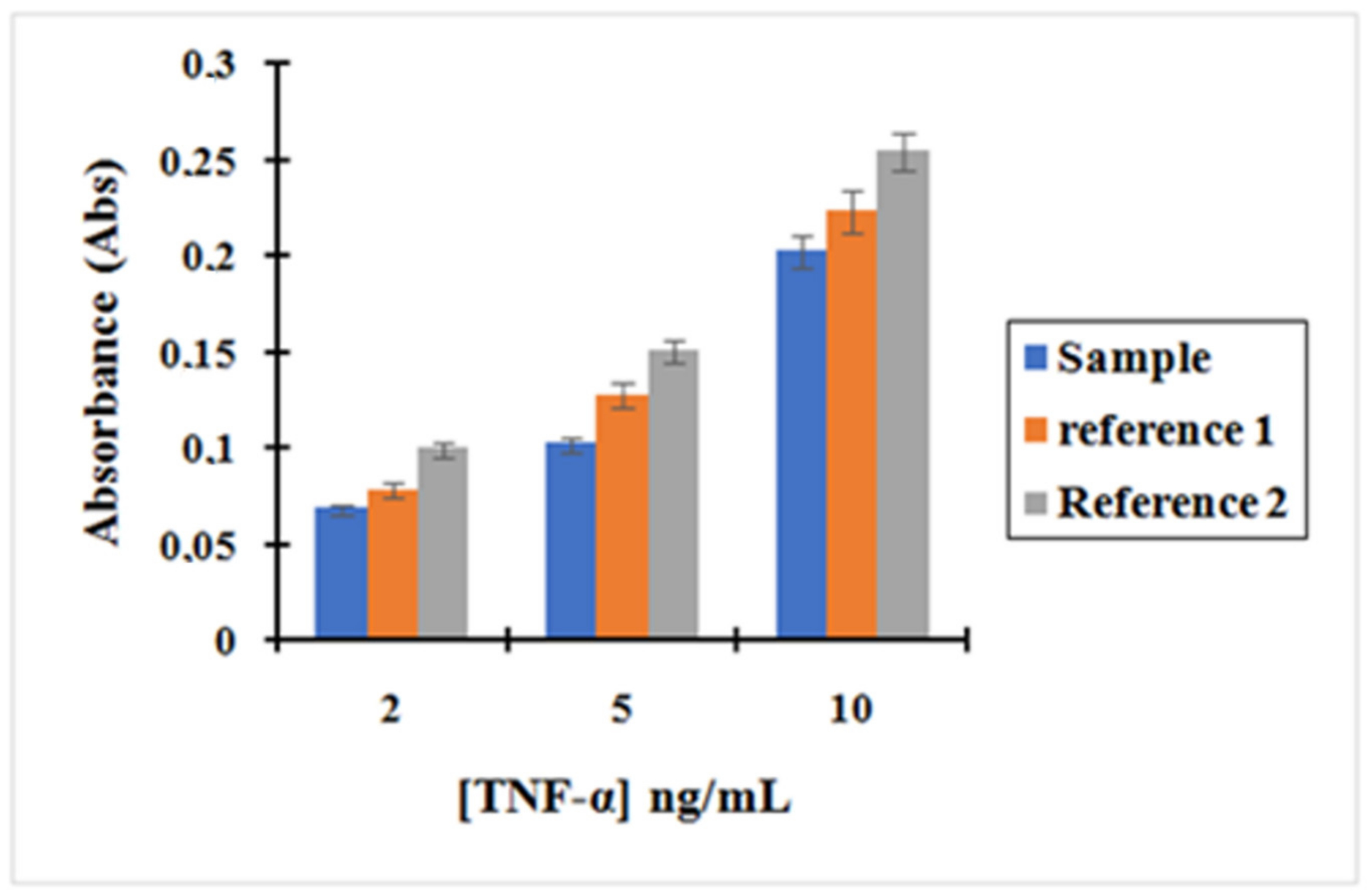
Figure 7 shows the calibration curve obtained for TNF-α after incubation with the complex MNP@SiO
2-NH
2-CO-anti-TNF-α at three different concentrations of TNF-α: 2, 5 and 10 ng/L. The curves, corresponding to reference (reference 1 and 2) to which the former plot was compared, are also shown. Reference (1) depicts supernatants containing unreacted TNF-α after incubation with MNPs that were non-activated with EDC/NHS during the functionalization with anti-TNF-α. Since -COOH groups present on the MNPs were not activated, we did not expect any covalent bonding between anti-TNF-α-MNPs. Therefore, we only expected interaction of TNF-α with those antibodies physisorbed on the MNPs. Reference (2) depicts Supernatants containing unreacted TNF-α after incubation with the initial MNPs that have only been washed four times with 1mL of 10 mM PBS. In this case, the only physisorption of TNF-α on the MNPs was expected.
Based on the results, it can be asserted that the concentration of unreacted TNF-α in the supernatants after incubation with the complex MNP@SiO2-NH2-CO-anti-TNF-α is lower than the concentration of TNF-α after incubation with the other two references.
As shown in
Figure 7, the absorbance decreased following the trend Reference 2 > Reference 1 > Sample. This is consistent with the results expected in Reference 2. The decrease in the amount of TNF-α in the supernatants when compared with its initial concentration can only be attributed to physisorption on the MNPs surfaces. However, in Reference 1, TNF-α can interact with anti-TNF-α antibodies that have been adsorbed to the surface of the MNPs. This effect is shown by a decrease in the absorbance read when compared to Reference 2. Finally, in “Sample”, the complex MNP@SiO
2-NH
2-CO-anti-TNF-α binds the antigen TNF-α more effectively as antibodies are both covalently bonded and physisorbed to the MNPs. Therefore, the pre-concentration effect when incubating TNF-α with “Sample” is much more important.
Thus, we can confirm these two hypotheses:
- (1)
There is an effective coupling between MNPs and anti-TNF-α antibody when the MNPs were activated using EDC/NHS.
- (2)
The complex MNP@SiO2-NH2-CO-Anti-TNF-α can bind the antigen TNF-α and it can be followed by UV-vis spectrophotometry.
The previous experiment as described above was tested using artificial saliva as a matrix. First, a stock solution of artificial saliva (AS) was prepared by dissolving 0.6 g/L Na2HPO4, 0.6 g/L anhydrous CaCl2, 0.4 g/L KCl, 0.4 g/L NaCl, 4 g/L mucin, and 4 g/L urea (purchased from Sigma-Aldrich, France) in deionized water. The pH was adjusted to 7.2 by adding NaOH and it was stored at 4 °C until use.
A dilution profile was performed by UV-vis spectrophotometry to find out the optimal dilution range of artificial saliva at which the matrix effect is removed. For this purpose, AS was diluted using 10 mM PBS to obtain saliva/PBS ratios of 1/1, 1/10, 1/100, 1/500, and 1/1000. The results are shown in
Figure S5. Based on the results obtained, AS diluted with PBS at a ratio 1/500 was chosen as a matrix. Afterwards, a new calibration curve of TNF-α using artificial saliva diluted 1/500 as the matrix was carried out at different concentrations of TNF-α: 2, 5, and 10 ng/L. The results are shown in
Figure S6 and confirmed the dependency of the absorbance with the concentration of TNF-α.
In the first stage, the complex MNP@SiO2-NH2-CO-anti-TNF-α was obtained as described above. Subsequently, TNF-α was prepared in AS diluted in PBS 1/500 at three different concentrations: 2, 5, and 10 ng/mL. The solutions prepared were incubated with the complex MNP@SiO2-NH2-CO-Anti-TNF-α as described above and the supernatants containing the unreacted TNF-α were measured by UV-vis spectrophotometry.
Once again, the solution used for the reference was based on the supernatants containing unreacted TNF-α after incubation with MNPs that were non-activated with EDC/NHS during the functionalization with anti-TNF-α antibody.
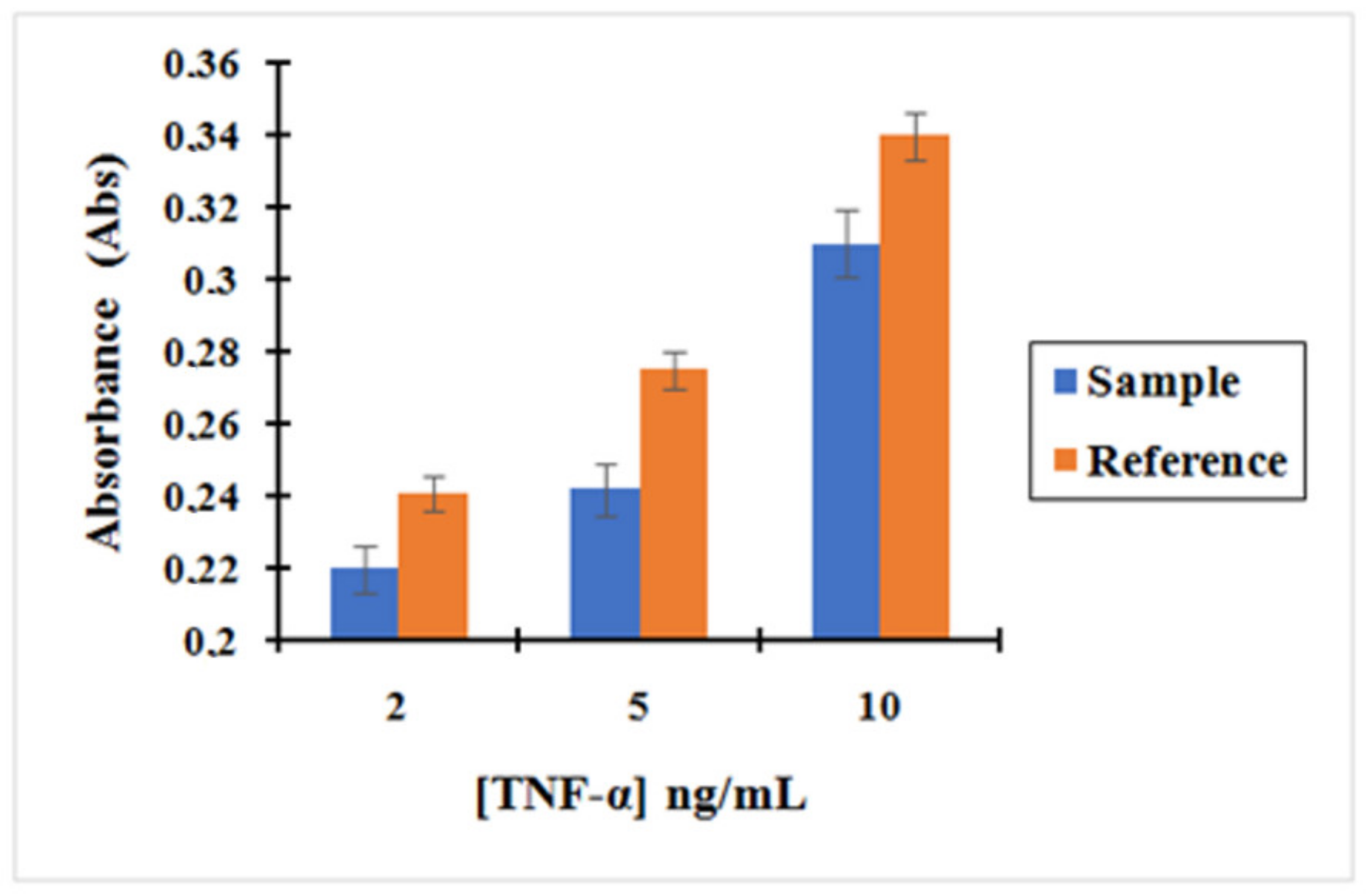
Based on these results shown in
Figure 8, we could confirm that the concentration obtained for the supernatant containing the unreacted amount of TNF-α after incubation with the nano-collector MNP@SiO
2-NH
2-CO-anti-TNF-α was lower than the supernatant of TNF-α after incubation with non-activated MNP (without activation with EDC/NHS) used as a reference. Therefore, it can be concluded that the pre-concentration effect when incubating TNF-α with the complex MNP@SiO
2-NH
2-CO-anti-TNF-α is much more important. The concentration of TNF-α decreased by 9%, 12%, and 9% when compared to the initial concentration of 2, 5, and 10 ng/mL, respectively, using the MNP@SiO
2-NH
2-COOH.
The same experiment described with TNF-α was repeated for the cytokine IL-10 and its corresponding anti-IL-10 antibody. First, the concentration range at which IL-10 can be measurable by UV-vis spectrophotometry in a fixed background of AS diluted 1/500 with PBS was obtained. In the UV region, IL-10 was shown to absorb 10 times less than TNF-α for the same concentration. Consequently, the results showed a working range of IL-10 at a concentration between 10 and 100 ng/mL (
Figure S7).
The concentration of IL-10 decreased by 7%, 6%, and 4% when compared to the initial concentration of 10, 40, and 100 ng/mL, respectively, using MNP@SiO2-NH2-COOH. Once again, the results demonstrated the ability of the complex antibody-MNPs to pre-concentrate the antigen.
A comparative study of different deactivating compounds was carried out. The aim was to assess the adsorption effect of the TNF-α antigen when using different chemicals/proteins for the deactivation step performed after bio-functionalization of MNPs with an antibody. For this purpose, four different deactivation mixtures were used: 0.1% bovine serum albumin (BSA) in 10 mM PBS, ethanolamine 0.1% in 10mM PBS, poly (ethylene glycol) methyl ether amine (PEG-NH2) 0.1% in 10 mM PBS, and ethanolamine cyanoborohydride (NaBH3CN) 0.1% in 10 mM PBS.
The comparative study was performed using activated MNPs with EDC/NHS: MNPs@SiO2-NH2-COOH. They were reacted with anti-TNF-α antibody at a concentration of 10 ng/mL, which was chosen as the antibody for the bio-functionalization of MNPs. The experimental procedure concerning the MNPs bio-functionalization was described above.
The deactivation protocol was the same as described above. The MNPs were activated using a mixture of EDC/NHS. Then, anti-TNF-α antibody was added to the activate MNPs. Afterwards, the deactivating solution was changed consecutively as aforementioned. As an illustration, the deactivation of remaining R-CO-NHS groups using BSA/Ethanolamine/PEG-NH
2/Ethanolamine + NaBH
3CN is presented in
Figure 9.
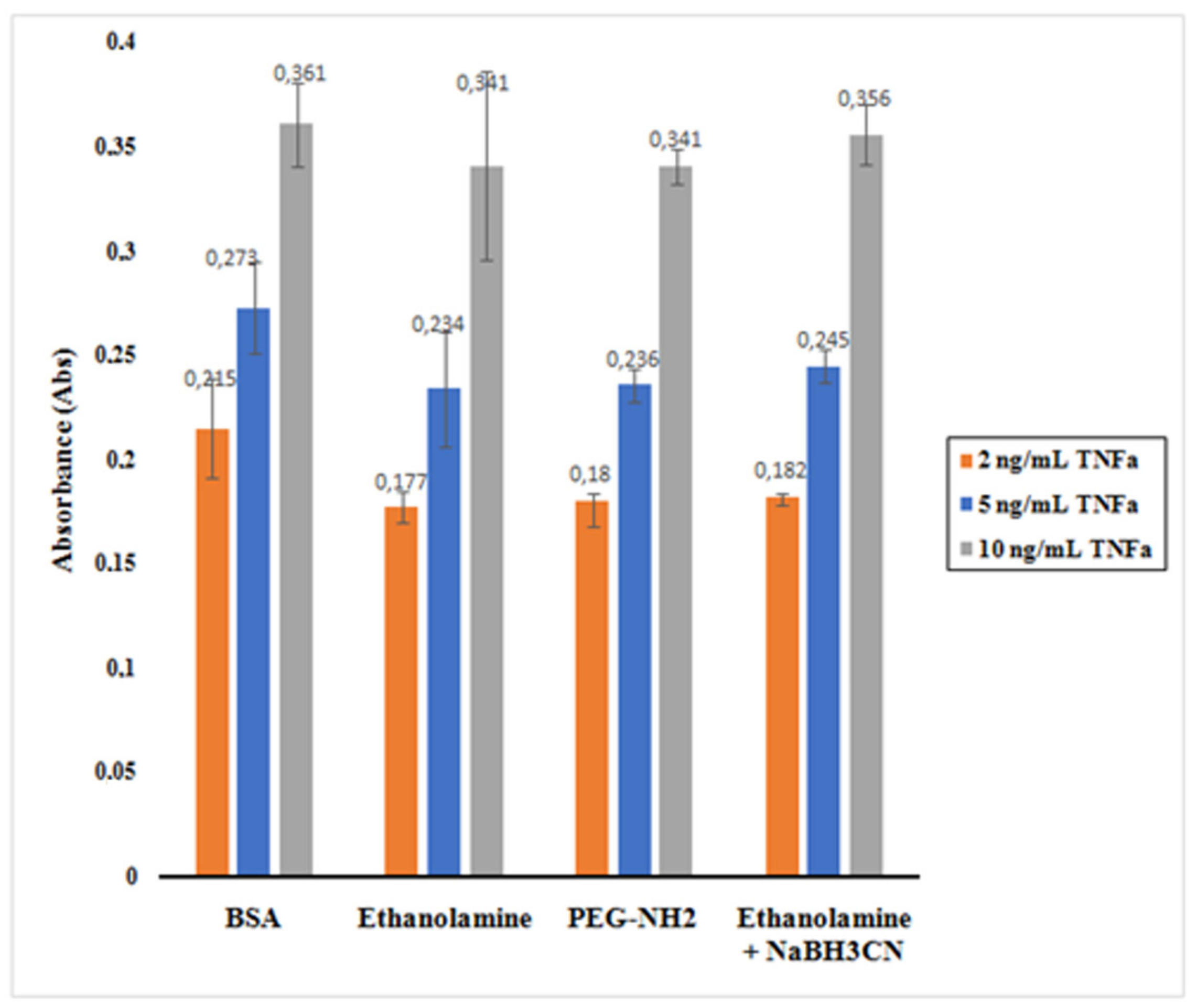
The histogram presented in
Figure 10 shows the absorbance reading for the supernatants containing the unreacted TNF-α after incubation with the nano-collector MNP@SiO
2-NH
2-CO-anti-TNF-α for the different deactivating mixtures.
It is observed that BSA appeared to be the best among the mixtures assessed. When 0.1% of BSA in PBS was used as deactivating mixture, TNF-α was less physisorbed to the nano-collector leading to a higher absorbance as the total amount of unreacted TNF-α remained relatively high. The ethanolamine + NaBH3CN mixture also indicated similar behavior. However, in the case of ethanolamine and PEG-NH2, the absorbance reading was lower as a result of a decreased concentration of unreacted TNF-α.
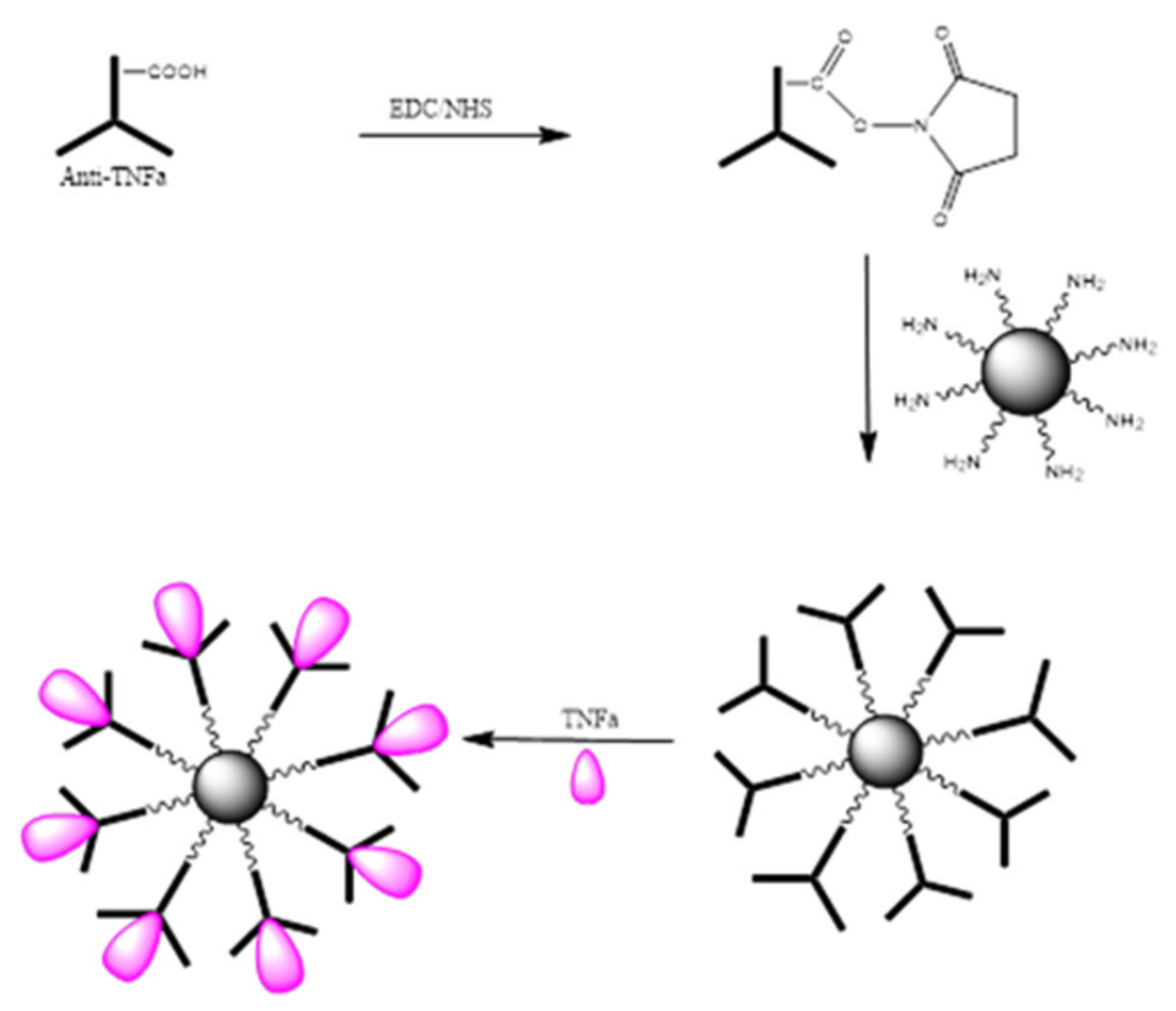
The bio-functionalization of MNPs with anti-TNF-α antibody at different concentrations was carried out. An illustration of the steps involved in the bio-functionalization of MNPs is presented in
Figure 11. Firstly, the –COOH groups present on the anti-TNF-α antibody surface were activated using a mixture of EDC/NHS followed by the incubation of MNPs to the activated anti-TNF-α antibody to create the complex antibody-MNPs. Finally, the 3D nano-collector MNP@SiO
2-NH-anti-TNF-α was incubated with TNF-α.
Based on the results shown in
Figure 12, we can confirm again that the concentration obtained for the supernatant containing the unreacted amount of TNF-α after incubation with the nano-collector MNP@SiO
2-NH-anti-TNF-α is lower than the supernatant of TNF-α after incubation with non-activated anti-TNF-α antibody (without activation with EDC/NHS) used as a reference. Therefore, it can be concluded that the pre-concentration effect when incubating TNF-α with the complex anti-TNF-α-MNPs is much more important. The concentration of TNF-α decreased by 5%, 16%, and 17% when compared to the initial concentration of 2, 5, and 10 ng/mL, respectively.