Encapsulation of Lipid-Soluble Bioactives by Nanoemulsions
Abstract
1. Introduction
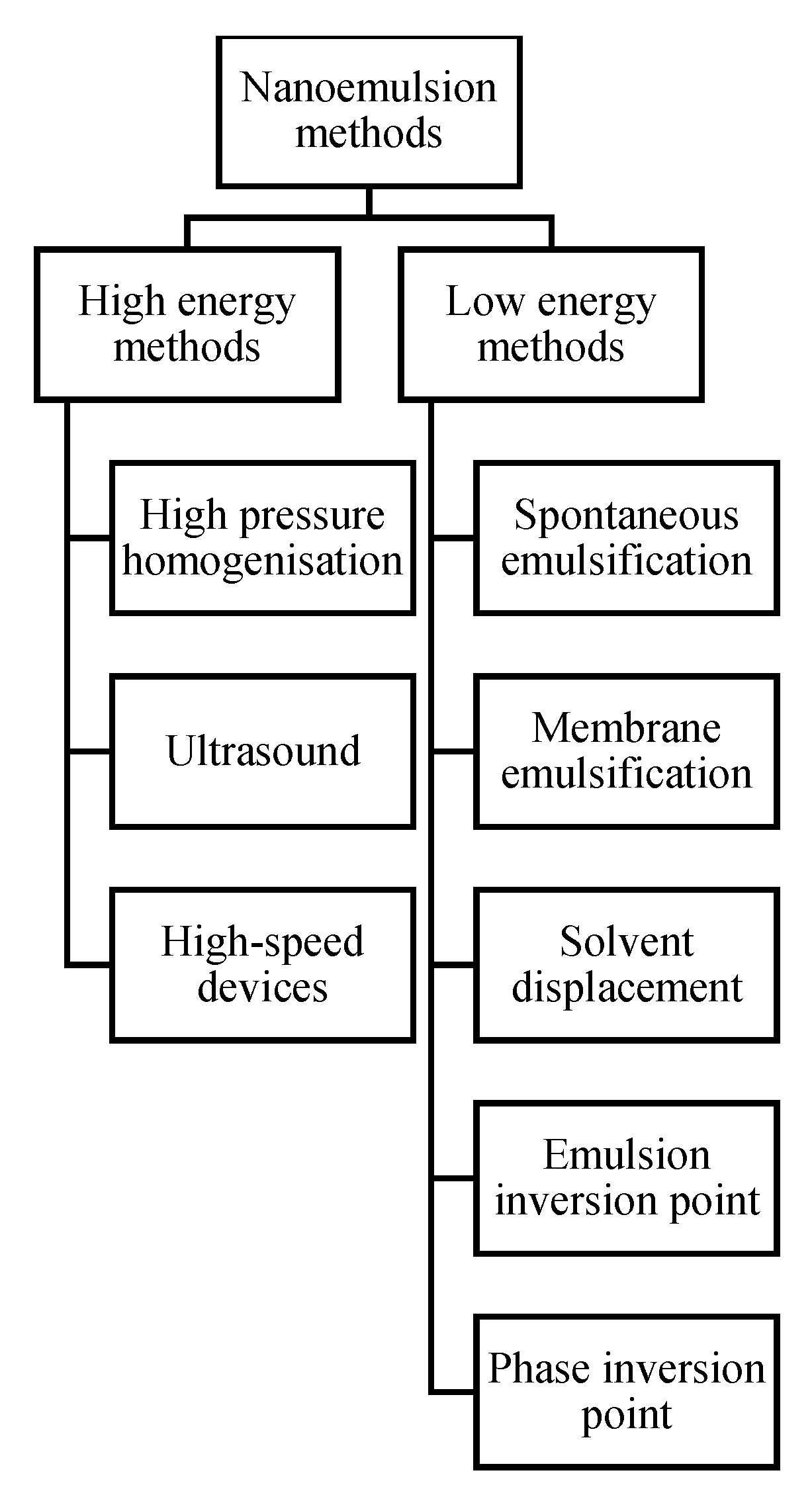
2. Nanoemulsion Technology
2.1. Preparation of Nanoemulsions
2.2. Types of Emulsions
2.3. Ingredients for Nanoemulsion Preparation
2.3.1. Oils
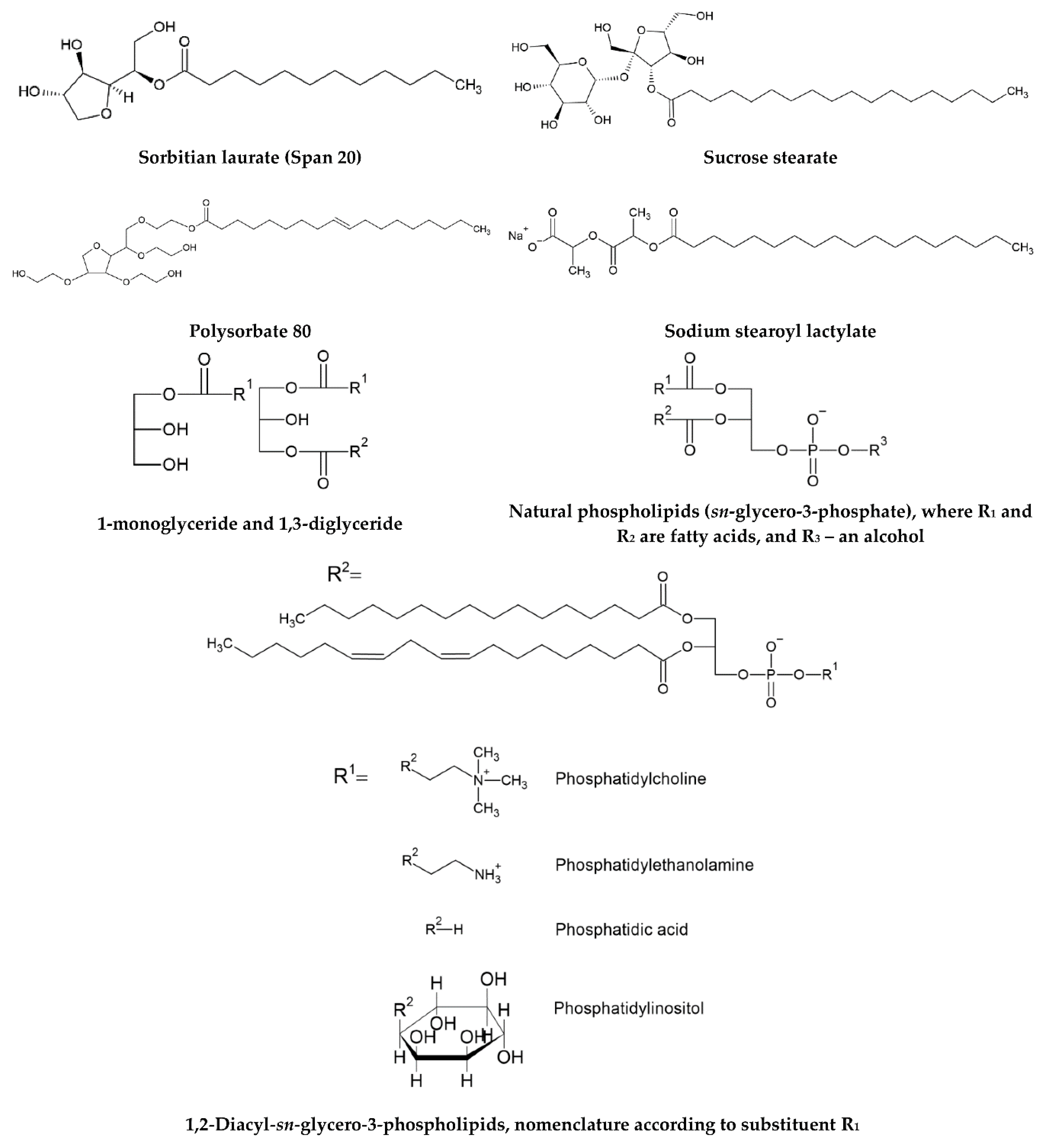
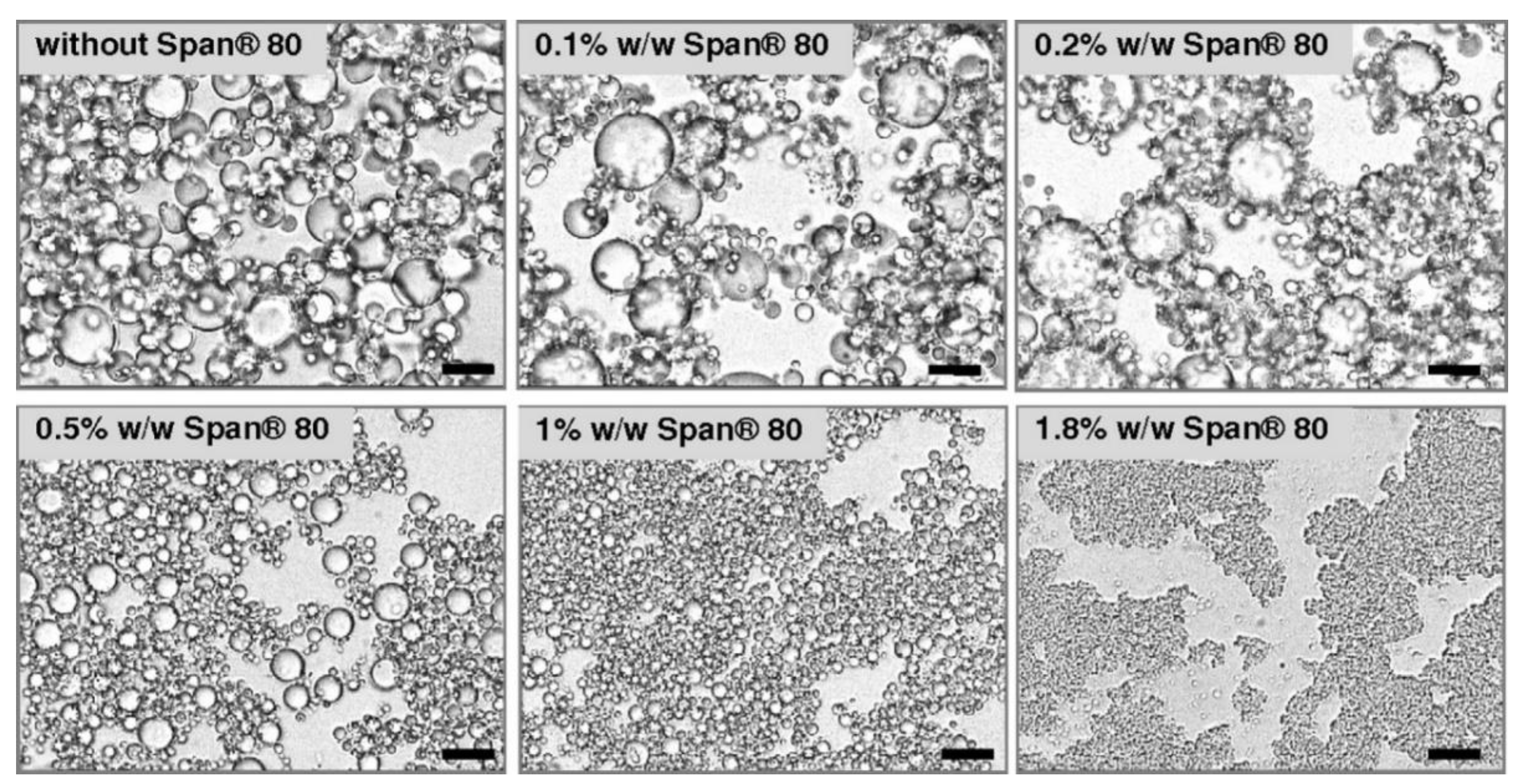
2.3.2. Emulsifiers
2.3.3. Other Ingredients
2.4. Techniques for Measuring Physical and Chemical Stability of Nanoemulsion
2.4.1. Physical Stability
2.4.2. Chemical Stability
3. Encapsulation of Lipid-Soluble Bioactive Compounds by Nanoemulsions
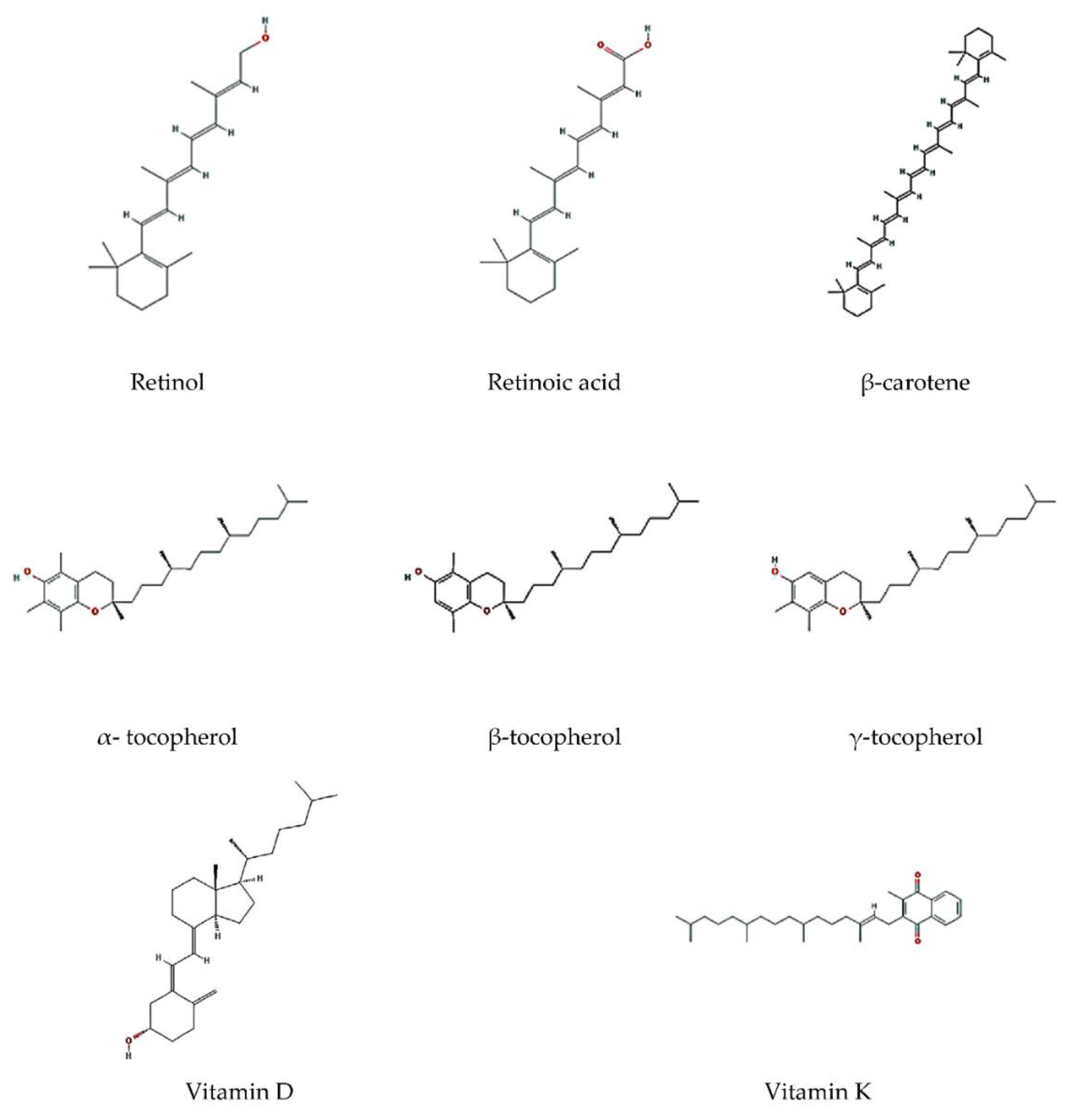
3.1. Vitamin A
3.2. Vitamin E
3.3. Vitamin K
3.4. Vitamin D
3.5. Carotenoids
3.6. β-Carotene
3.7. Polyunsaturated Fatty Acids (PUFA)
3.8. Essential Oils and Flavor Compounds
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Öztürk, B. Nanoemulsions for food fortification with lipophilic vitamins: Production challenges, stability, and bioavailability. Eur. J. Lipid Sci. Technol. 2017, 119, 1500539. [Google Scholar] [CrossRef]
- De Vos, P.; Faas, M.M.; Spasojevic, M.; Sikkema, J. Encapsulation for preservation of functionality and targeted delivery of bioactive food components. Int. Dairy J. 2010, 20, 292–302. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions: Principles, Practices, and Techniques; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- McClements, D.J. Emulsion Design to Improve the Delivery of Functional Lipophilic Components. Annu. Rev. Food Sci. Technol. 2010, 1, 241–269. [Google Scholar] [CrossRef]
- McClements, D.J.; A Decker, E.; Weiss, J. Emulsion-Based Delivery Systems for Lipophilic Bioactive Components. J. Food Sci. 2007, 72, R109–R124. [Google Scholar] [CrossRef]
- McClements, D.J.; A Decker, E. Lipid Oxidation in Oil-in-Water Emulsions: Impact of Molecular Environment on Chemical Reactions in Heterogeneous Food Systems. J. Food Sci. 2000, 65, 1270–1282. [Google Scholar] [CrossRef]
- Chakraborty, S.; Shukla, D.; Mishra, B.; Singh, S. Lipid—An emerging platform for oral delivery of drugs with poor bioavailability. Eur. J. Pharm. Biopharm. 2009, 73, 1–15. [Google Scholar] [CrossRef]
- McClements, D.J.; Li, Y. Structured emulsion-based delivery systems: Controlling the digestion and release of lipophilic food components. Adv. Colloid Interface Sci. 2010, 159, 213–228. [Google Scholar] [CrossRef]
- Sjöström, B.; Bergenståhl, B.; Kronberg, B. A Method for the Preparation of Submicron Particles of Sparingly Water-Soluble Drugs by Precipitation in Oil-in-Water Emulsions. II: Influence of the Emulsifier, the Solvent, and the Drug Substance. J. Pharm. Sci. 1993, 82, 584–589. [Google Scholar] [CrossRef]
- Sjöström, B.; Kronberg, B.; Carlfors, J. A Method for the Preparation of Submicron Particles of Sparingly Water-Soluble Drugs by Precipitation in Oil-in-Water Emulsions. I: Influence of Emulsification and Surfactant Concentration. J. Pharm. Sci. 1993, 82, 579–583. [Google Scholar] [CrossRef]
- GutiérrezJ, M.; González, C.; Maestro, A.; Solè, I.; Pey, C.; Nolla, J. Nano-emulsions: New applications and optimization of their preparation. Curr. Opin. Colloid Interface Sci. 2008, 13, 245–251. [Google Scholar] [CrossRef]
- Leong, T.; Wooster, T.; Kentish, S.E.; AshokKumar, M. Minimising oil droplet size using ultrasonic emulsification. Ultrason. Sonochemistry 2009, 16, 721–727. [Google Scholar] [CrossRef]
- Velikov, K.P.; Pelan, E.G. Colloidal delivery systems for micronutrients and nutraceuticals. Soft Matter 2008, 4, 1964. [Google Scholar] [CrossRef]
- Anton, H.; Benoit, J.-P.; Saulnier, P. Design and production of nanoparticles formulated from nano-emulsion templates—A review. J. Control. Release 2008, 128, 185–199. [Google Scholar] [CrossRef]
- Quintanilla-Carvajal, M.X.; Camacho-Díaz, B.H.; Meraz-Torres, L.S.; Chanona-Pérez, J.; Alamilla-Beltrán, L.; Jiménez-Aparicio, A.; Gutiérrez-López, G.F. Nanoencapsulation: A New Trend in Food Engineering Processing. Food Eng. Rev. 2009, 2, 39–50. [Google Scholar] [CrossRef]
- Sanguansri, P.; Augustin, M.A. Nanoscale materials development—A food industry perspective. Trends Food Sci. Technol. 2006, 17, 547–556. [Google Scholar] [CrossRef]
- Abbas, S.; Hayat, K.; Karangwa, E.; Bashari, M.; Zhang, X. An Overview of Ultrasound-Assisted Food-Grade Nanoemulsions. Food Eng. Rev. 2013, 5, 139–157. [Google Scholar] [CrossRef]
- Boode, K.; Walstra, P. Partial coalescence in oil-in-water emulsions 1. Nature of the aggregation. Colloids Surf. A: Physicochem. Eng. Asp. 1993, 81, 121–137. [Google Scholar] [CrossRef]
- Qian, C.; McClements, D.J. Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: Factors affecting particle size. Food Hydrocoll. 2011, 25, 1000–1008. [Google Scholar] [CrossRef]
- Bouchemal, K.; Briancon, S.; Perrier, E.; Fessi, H. Nano-emulsion formulation using spontaneous emulsification: Solvent, oil and surfactant optimisation. Int. J. Pharm. 2004, 280, 241–251. [Google Scholar] [CrossRef]
- Chu, B.-S.; Ichikawa, S.; Kanafusa, A.S.; Nakajima, M. Preparation and Characterization of β-Carotene Nanodispersions Prepared by Solvent Displacement Technique. J. Agric. Food Chem. 2007, 55, 6754–6760. [Google Scholar] [CrossRef]
- Yin, L.; Chu, B.-S.; Kobayashi, I.; Nakajima, M. Performance of selected emulsifiers and their combinations in the preparation of β-carotene nanodispersions. Food Hydrocoll. 2009, 23, 1617–1622. [Google Scholar] [CrossRef]
- Sadtler, V.; Rondon-Gonzalez, M.; Acrement, A.; Choplin, L.; Marié, E. PEO-Covered Nanoparticles by Emulsion Inversion Point (EIP) Method. Macromol. Rapid Commun. 2010, 31, 998–1002. [Google Scholar] [CrossRef]
- Shinoda, K.; Saito, H. The effect of temperature on the phase equilibria and the types of dispersions of the ternary system composed of water, cyclohexane, and nonionic surfactant. J. Colloid Interface Sci. 1968, 26, 70–74. [Google Scholar] [CrossRef]
- Shinoda, K.; Saito, H. The Stability of O/W type emulsions as functions of temperature and the HLB of emulsifiers: The emulsification by PIT-method. J. Colloid Interface Sci. 1969, 30, 258–263. [Google Scholar] [CrossRef]
- Izquierdo, P.; Feng, J.; Esquena, J.; Tadros, T.F.; Dederen, J.C.; García-Celma, M.; Azemar, N.; Solans, C. The influence of surfactant mixing ratio on nano-emulsion formation by the pit method. J. Colloid Interface Sci. 2005, 285, 388–394. [Google Scholar] [CrossRef]
- Sadurní, N.; Solans, C.; Azemar, N.; García-Celma, M. Studies on the formation of O/W nano-emulsions, by low-energy emulsification methods, suitable for pharmaceutical applications. Eur. J. Pharm. Sci. 2005, 26, 438–445. [Google Scholar] [CrossRef]
- Anton, H.; Gayet, P.; Benoit, J.-P.; Saulnier, P. Nano-emulsions and nanocapsules by the PIT method: An investigation on the role of the temperature cycling on the emulsion phase inversion. Int. J. Pharm. 2007, 344, 44–52. [Google Scholar] [CrossRef]
- Jafari, S.M.; McClements, D.J. Nanoemulsions: Formulation, Applications, and Characterization; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- McClements, D.J. Advances in fabrication of emulsions with enhanced functionality using structural design principles. Curr. Opin. Colloid Interface Sci. 2012, 17, 235–245. [Google Scholar] [CrossRef]
- Delfanian, M.; Razavi, S.M.A.; Khodaparast, M.H.H.; Kenari, R.E.; Golmohammadzadeh, S. Influence of main emulsion components on the physicochemical and functional properties of W/O/W nano-emulsion: Effect of polyphenols, Hi-Cap, basil seed gum, soy and whey protein isolates. Food Res. Int. 2018, 108, 136–143. [Google Scholar] [CrossRef]
- Gunstone, F.D. The Chemical Nature of Lipids. In Oils and Fats in the Food Industry; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 1–10. [Google Scholar]
- Lehtonen, M.; Merinen, M.; Kilpeläinen, P.O.; Xu, C.; Willför, S.M.; Mikkonen, K.S. Phenolic residues in spruce galactoglucomannans improve stabilization of oil-in-water emulsions. J. Colloid Interface Sci. 2018, 512, 536–547. [Google Scholar] [CrossRef]
- Meroni, E.; Raikos, V. Formulating orange oil-in-water beverage emulsions for effective delivery of bioactives: Improvements in chemical stability, antioxidant activity and gastrointestinal fate of lycopene using carrier oils. Food Res. Int. 2018, 106, 439–445. [Google Scholar] [CrossRef]
- Ozturk, B.; McClements, D.J. Progress in natural emulsifiers for utilization in food emulsions. Curr. Opin. Food Sci. 2016, 7, 1–6. [Google Scholar] [CrossRef]
- Choi, S.J.; McClements, D.J. Nanoemulsions as delivery systems for lipophilic nutraceuticals: Strategies for improving their formulation, stability, functionality and bioavailability. Food Sci. Biotechnol. 2020, 29, 149–168. [Google Scholar] [CrossRef]
- Schreiner, T.B.; Santamaria-Echart, A.; Ribeiro, A.; Peres, A.M.; Dias, M.; Pinho, S.P.; Barreiro, M.F. Formulation and Optimization of Nanoemulsions Using the Natural Surfactant Saponin from Quillaja Bark. Molecules 2020, 25, 1538. [Google Scholar] [CrossRef]
- Sharif, H.R.; Goff, H.D.; Majeed, H.; Shamoon, M.; Liu, F.; Nsor-Atindana, J.; Haider, J.; Liang, R.; Zhong, F. Physicochemical properties of β-carotene and eugenol co-encapsulated flax seed oil powders using OSA starches as wall material. Food Hydrocoll. 2017, 73, 274–283. [Google Scholar] [CrossRef]
- Bueschelberger, H.-G. Lecithins. In Emulsifiers in Food Technology; Whitehurst, R.J., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2004; pp. 1–39. [Google Scholar]
- Hasenhuettl, G.L.; Hartel, R.W. Food Emulsifiers and Their Applications; Springer: Berlin, Germany, 2019. [Google Scholar]
- McClements, D.J.; Rao, J. Food-Grade Nanoemulsions: Formulation, Fabrication, Properties, Performance, Biological Fate, and Potential Toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef]
- McClements, D.J.; Gumus, C.E. Natural emulsifiers—Biosurfactants, phospholipids, biopolymers, and colloidal particles: Molecular and physicochemical basis of functional performance. Adv. Colloid Interface Sci. 2016, 234, 3–26. [Google Scholar] [CrossRef]
- Araújo, F.; Kelmann, R.; Araujo, B.; Finatto, R.; Teixeira, H.; Koester, L. Development and characterization of parenteral nanoemulsions containing thalidomide. Eur. J. Pharm. Sci. 2011, 42, 238–245. [Google Scholar] [CrossRef]
- Preetz, C.; Hauser, A.; Hause, G.; Kramer, A.; Mäder, K.; Mäder, K. Application of atomic force microscopy and ultrasonic resonator technology on nanoscale: Distinction of nanoemulsions from nanocapsules. Eur. J. Pharm. Sci. 2010, 39, 141–151. [Google Scholar] [CrossRef]
- Silva, H.D.; Cerqueira, M.Â.P.R.; Cunha-da-Silva, M.; Vicente, A.A. Layer-by-Layer Deposition on-Carotene Oil-in-Water Nanoemulsions: An Edible Multi-Layer System. In Proceedings of the XXII Encontro Nacional SPQ: 100 anos de Química em Portugal, Braga, Portugal, 3–6 July 2011. [Google Scholar]
- Rayner, M.; Dejmek, P. Engineering Aspects of Food Emulsification and Homogenization; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Li, D.; Li, L.; Xiao, N.; Li, M.; Xie, X. Physical properties of oil-in-water nanoemulsions stabilized by OSA-modified starch for the encapsulation of lycopene. Colloids Surf. A: Physicochem. Eng. Asp. 2018, 552, 59–66. [Google Scholar] [CrossRef]
- Thanasukarn, P.; Pongsawatmanit, R.; McClements, D.J. Impact of fat and water crystallization on the stability of hydrogenated palm oil-in-water emulsions stabilized by whey protein isolate. Colloids Surf. A: Physicochem. Eng. Asp. 2004, 246, 49–59. [Google Scholar] [CrossRef]
- Strasdat, B.; Bunjes, H. Incorporation of lipid nanoparticles into calcium alginate beads and characterization of the encapsulated particles by differential scanning calorimetry. Food Hydrocoll. 2013, 30, 567–575. [Google Scholar] [CrossRef]
- Yuan, Y.; Gao, Y.; Zhao, J.; Mao, L. Characterization and stability evaluation of β-carotene nanoemulsions prepared by high pressure homogenization under various emulsifying conditions. Food Res. Int. 2008, 41, 61–68. [Google Scholar] [CrossRef]
- Klang, V.; Matsko, N.; Valenta, C.; Hofer, F. Electron microscopy of nanoemulsions: An essential tool for characterisation and stability assessment. Micron 2012, 43, 85–103. [Google Scholar] [CrossRef]
- Nesterenko, A.; Drelich, A.; Lu, H.; Clausse, D.; Pezron, I. Influence of a mixed particle/surfactant emulsifier system on water-in-oil emulsion stability. Colloids Surf. A: Physicochem. Eng. Asp. 2014, 457, 49–57. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y. Preparation, characterization and evaluation of tea polyphenol–Zn complex loaded β-chitosan nanoparticles. Food Hydrocoll. 2015, 48, 260–273. [Google Scholar] [CrossRef]
- Maurya, V.K.; Aggarwal, M. A phase inversion based nanoemulsion fabrication process to encapsulate vitamin D3 for food applications. J. Steroid Biochem. Mol. Boil. 2019, 190, 88–98. [Google Scholar] [CrossRef]
- Qian, C.; A Decker, E.; Xiao, H.; McClements, D.J. Physical and chemical stability of β-carotene-enriched nanoemulsions: Influence of pH, ionic strength, temperature, and emulsifier type. Food Chem. 2012, 132, 1221–1229. [Google Scholar] [CrossRef]
- Chen, H.; Mao, L.; Hou, Z.; Yuan, F.; Gao, Y. Roles of additional emulsifiers in the structures of emulsion gels and stability of vitamin E. Food Hydrocoll. 2020, 99, 105372. [Google Scholar] [CrossRef]
- Borba, C.M.; Tavares, M.N.; Macedo, L.P.; Araújo, G.S.; Furlong, E.B.; Dora, C.L.; Burkert, J.F. Physical and chemical stability of β-carotene nanoemulsions during storage and thermal process. Food Res. Int. 2019, 121, 229–237. [Google Scholar] [CrossRef]
- Ha, T.V.A.; Kim, S.; Choi, Y.; Kwak, H.-S.; Lee, S.J.; Wen, J.; Oey, I.; Ko, S.-H. Antioxidant activity and bioaccessibility of size-different nanoemulsions for lycopene-enriched tomato extract. Food Chem. 2015, 178, 115–121. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, Y.; Tan, H.; Zhang, R.; McClements, D.J. Vitamin E Encapsulation within Oil-in-Water Emulsions: Impact of Emulsifier Type on Physicochemical Stability and Bioaccessibility. J. Agric. Food Chem. 2019, 67, 1521–1529. [Google Scholar] [CrossRef]
- Yoshida, K.; Sekine, T.; Matsuzaki, F.; Yanaki, T.; Yamaguchi, M. Stability of vitamin A in oil-in-water-in-oil-type multiple emulsions. J. Am. Oil Chem. Soc. 1999, 76, 1–6. [Google Scholar] [CrossRef]
- Campani, V.; Biondi, M.; Mayol, L.; Cilurzo, F.; Pitaro, M.; De Rosa, G. Development of nanoemulsions for topical delivery of vitamin K1. Int. J. Pharm. 2016, 511, 170–177. [Google Scholar] [CrossRef]
- Karthik, P.; Anandharamakrishnan, C. Enhancing omega-3 fatty acids nanoemulsion stability and in-vitro digestibility through emulsifiers. J. Food Eng. 2016, 187, 92–105. [Google Scholar] [CrossRef]
- Dey, T.K.; Koley, H.; Ghosh, M.; Dey, S.; Dhar, P. Effects of nano-sizing on lipid bioaccessibility and ex vivo bioavailability from EPA-DHA rich oil in water nanoemulsion. Food Chem. 2019, 275, 135–142. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, N.K.; Srivastava, A.; Kataria, A.; Dubey, S.; Sharma, S.; Kundu, B. Clove and lemongrass oil based non-ionic nanoemulsion for suppressing the growth of plant pathogenic Fusarium oxysporum f.sp. lycopersici. Ind. Crop. Prod. 2018, 123, 353–362. [Google Scholar] [CrossRef]
- Maia, S.B.; Souza, A.S.R.; Caminha, M.D.F.; Da Silva, S.L.; Cruz, R.D.S.B.L.C.; Santos, C.C.; Filho, M.B. Vitamin A and Pregnancy: A Narrative Review. Nutrients 2019, 11, 681. [Google Scholar] [CrossRef]
- Kulie, T.; Groff, A.; Redmer, J.; Hounshell, J.; Schrager, S.; Marlow, R.A.; Kegowicz, C.L.; Starkey, K.N. Vitamin D: An Evidence-Based Review. J. Am. Board Fam. Med. 2009, 22, 698–706. [Google Scholar] [CrossRef]
- Gröber, U.; Reichrath, J.; Holick, M.; Kisters, K. Vitamin K: An old vitamin in a new perspective. Dermato-Endocrinology 2014, 6, e968490. [Google Scholar] [CrossRef]
- Guttoff, M.; Saberi, A.H.; McClements, D.J. Formation of vitamin D nanoemulsion-based delivery systems by spontaneous emulsification: Factors affecting particle size and stability. Food Chem. 2015, 171, 117–122. [Google Scholar] [CrossRef]
- Stratulat, I.; Britten, M.; Salmieri, S.; Fustier, P.; St-Gelais, D.; Champagne, C.P.; Lacroix, M. Enrichment of cheese with vitamin D3 and vegetable omega-3. J. Funct. Foods 2015, 13, 300–307. [Google Scholar] [CrossRef]
- Ozturk, B.; Argin, S.; Ozilgen, M.; McClements, D.J. Formation and stabilization of nanoemulsion-based vitamin E delivery systems using natural biopolymers: Whey protein isolate and gum arabic. Food Chem. 2015, 188, 256–263. [Google Scholar] [CrossRef]
- Golfomitsou, I.; Mitsou, E.; Xenakis, A.; Papadimitriou, V. Development of food grade O/W nanoemulsions as carriers of vitamin D for the fortification of emulsion based food matrices: A structural and activity study. J. Mol. Liq. 2018, 268, 734–742. [Google Scholar] [CrossRef]
- Meghani, N.; Patel, P.; Kansara, K.; Ranjan, S.; Dasgupta, N.; Ramalingam, C.; Kumar, A.; Rajan, S. Formulation of vitamin D encapsulated cinnamon oil nanoemulsion: Its potential anti-cancerous activity in human alveolar carcinoma cells. Colloids Surf. B: Biointerfaces 2018, 166, 349–357. [Google Scholar] [CrossRef]
- Schoener, A.L.; Zhang, R.; Lv, S.; Weiss, J.; McClements, D.J. Fabrication of plant-based vitamin D3-fortified nanoemulsions: Influence of carrier oil type on vitamin bioaccessibility. Food Funct. 2019, 10, 1826–1835. [Google Scholar] [CrossRef]
- Gulotta, A.; Saberi, A.H.; Nicoli, M.C.; McClements, D.J. Nanoemulsion-Based Delivery Systems for Polyunsaturated (ω-3) Oils: Formation Using a Spontaneous Emulsification Method. J. Agric. Food Chem. 2014, 62, 1720–1725. [Google Scholar] [CrossRef]
- Belhaj, N.; Arab-Tehrany, E.; Linder, M. Oxidative kinetics of salmon oil in bulk and in nanoemulsion stabilized by marine lecithin. Process. Biochem. 2010, 45, 187–195. [Google Scholar] [CrossRef]
- Bush, L.; Stevenson, L.; Lane, K.E. The oxidative stability of omega-3 oil-in-water nanoemulsion systems suitable for functional food enrichment: A systematic review of the literature. Crit. Rev. Food Sci. Nutr. 2017, 59, 1154–1168. [Google Scholar] [CrossRef]
- Ricaurte, L.; Perea-Flores, M.D.J.; Martinez, A.; Quintanilla-Carvajal, M.X. Production of high-oleic palm oil nanoemulsions by high-shear homogenization (microfluidization). Innov. Food Sci. Emerg. Technol. 2016, 35, 75–85. [Google Scholar] [CrossRef]
- Lane, K.E.; Li, W.; Smith, C.J.; Derbyshire, E. The development of vegetarian omega-3 oil in water nanoemulsions suitable for integration into functional food products. J. Funct. Foods 2016, 23, 306–314. [Google Scholar] [CrossRef]
- Sotomayor-Gerding, D.; Oomah, B.D.; Acevedo, F.; Morales, E.; Bustamante, M.; Shene, C.; Rubilar, M. High carotenoid bioaccessibility through linseed oil nanoemulsions with enhanced physical and oxidative stability. Food Chem. 2016, 199, 463–470. [Google Scholar] [CrossRef]
- Raikos, V.; Hayward, N.; Hayes, H.E.; Meroni, E.; Ranawana, V. Optimising the ratio of long- to short-chain triglycerides of the lipid phase to enhance physical stability and bioaccessibility of lycopene-loaded beverage emulsions. Int. J. Food Sci. Technol. 2018, 54, 1355–1362. [Google Scholar] [CrossRef]
- Surh, J.; A Decker, E.; McClements, D.J. Utilisation of spontaneous emulsification to fabricate lutein-loaded nanoemulsion-based delivery systems: Factors influencing particle size and colour. Int. J. Food Sci. Technol. 2017, 5, 1169–1416. [Google Scholar] [CrossRef]
- Qian, C.; A Decker, E.; Xiao, H.; McClements, D.J. Inhibition of β-carotene degradation in oil-in-water nanoemulsions: Influence of oil-soluble and water-soluble antioxidants. Food Chem. 2012, 135, 1036–1043. [Google Scholar] [CrossRef]
- Costa, A.M.; Bueno, K.T.L.; Rosa, A.P.C.; Costa, J.A.V. The antioxidant activity of nanoemulsions based on lipids and peptides from Spirulina sp. LEB18. LWT 2019, 99, 173–178. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, Z.; Lei, F.; Chang, Y.; Gao, Y. Investigation into the bioaccessibility and microstructure changes of β-carotene emulsions during in vitro digestion. Innov. Food Sci. Emerg. Technol. 2012, 15, 86–95. [Google Scholar] [CrossRef]
- Hwang, S.R.; Lim, S.-J.; Park, J.-S.; Kim, C.-K. Phospholipid-based microemulsion formulation of all-trans-retinoic acid for parenteral administration. Int. J. Pharm. 2004, 276, 175–183. [Google Scholar] [CrossRef]
- Jeong, Y. Preparation of poly(?-lactide-co-glycolide) microspheres encapsulating all-trans retinoic acid. Int. J. Pharm. 2003, 259, 79–91. [Google Scholar] [CrossRef]
- Kim, T.-I.; Lim, D.-H.; Kim, S.-B.; Park, S.-M.; Hur, T.-Y.; Ki, K.-S.; Kwon, E.-G.; Vijayakumar, M.; Kim, Y.-J.; Kim, T.G. Preparation of Nanoemulsions of Vitamin A and C by Microfluidization: Efficacy on the Expression Pattern of Milk-Specific Proteins in MAC-T Cells. Molecules 2019, 24, 2566. [Google Scholar] [CrossRef]
- Benzaria, A.; Chevalier, D.; Picart, L.; Hue, P.; López-Pedemonte, T.; Dumay, E. Intracellular fate of retinyl acetate-loaded submicron delivery systems by in vitro intestinal epithelial cells: A comparison between whey protein-stabilised submicron droplets and micelles stabilised with polysorbate 80. Food Res. Int. 2013, 51, 679–692. [Google Scholar] [CrossRef]
- Choudhry, Q.N.; Kim, M.J.; Kim, T.G.; Pan, J.H.; Kim, J.H.; Park, S.J.; Park, J.-W.; Kim, Y.-J. Saponin-Based Nanoemulsification Improves the Antioxidant Properties of Vitamin A and E in AML-12 Cells. Int. J. Mol. Sci. 2016, 17, 1406. [Google Scholar] [CrossRef]
- Tanglao, E.J.; Kumar, A.B.N.; Noriega, R.R.; Punzalan, M.E.; Marcelo, P. Development and physico-chemical characterization of virgin coconut oil-in-water emulsion using polymerized whey protein as emulsifier for Vitamin A delivery. MATEC Web Conf. 2019, 268, 01002. [Google Scholar] [CrossRef]
- Shi, J.; Zhou, S.; Kang, L.; Ling, H.; Chen, J.; Duan, L.; Song, Y.; Deng, Y. Evaluation of the antitumor effects of vitamin K2 (menaquinone-7) nanoemulsions modified with sialic acid-cholesterol conjugate. Drug Deliv. Transl. Res. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Raikos, V. Encapsulation of vitamin E in edible orange oil-in-water emulsion beverages: Influence of heating temperature on physicochemical stability during chilled storage. Food Hydrocoll. 2017, 72, 155–162. [Google Scholar] [CrossRef]
- Saberi, A.H.; Fang, Y.; McClements, D.J. Fabrication of vitamin E-enriched nanoemulsions: Factors affecting particle size using spontaneous emulsification. J. Colloid Interface Sci. 2013, 391, 95–102. [Google Scholar] [CrossRef]
- El Kinawy, O.; Petersen, S.; Ulrich, J. Technological Aspects of Nanoemulsion Formation of Low-Fat Foods Enriched with Vitamin E by High-Pressure Homogenization. Chem. Eng. Technol. 2012, 35, 937–940. [Google Scholar] [CrossRef]
- Xue, J.; Davidson, P.M.; Zhong, Q. Antimicrobial activity of thyme oil co-nanoemulsified with sodium caseinate and lecithin. Int. J. Food Microbiol. 2015, 210, 1–8. [Google Scholar] [CrossRef]
- Ma, Q.; Davidson, P.M.; Zhong, Q. Nanoemulsions of thymol and eugenol co-emulsified by lauric arginate and lecithin. Food Chem. 2016, 206, 167–173. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, C.; Tian, G.; Lu, C.; Li, C.; Bao, Y.; Tang, Z.; McClements, D.J.; Xiao, H.; Zheng, J. Characterization of physical properties and electronic sensory analyses of citrus oil-based nanoemulsions. Food Res. Int. 2018, 109, 149–158. [Google Scholar] [CrossRef]
- Ziani, K.; Fang, Y.; McClements, D.J. Encapsulation of functional lipophilic components in surfactant-based colloidal delivery systems: Vitamin E, vitamin D, and lemon oil. Food Chem. 2012, 134, 1106–1112. [Google Scholar] [CrossRef]
- Guerra-Rosas, M.I.; Morales-Castro, J.; Ochoa-Martínez, L.A.; Salvia-Trujillo, L.; Martín-Belloso, O. Long-term stability of food-grade nanoemulsions from high methoxyl pectin containing essential oils. Food Hydrocoll. 2016, 52, 438–446. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Graü, M.A.; Soliva-Fortuny, R.; Martín-Belloso, O. Effect of processing parameters on physicochemical characteristics of microfluidized lemongrass essential oil-alginate nanoemulsions. Food Hydrocoll. 2013, 30, 401–407. [Google Scholar] [CrossRef]
- Chiu, Y.; Yang, W. Preparation of vitamin E microemulsion possessing high resistance to oxidation in air. Colloids Surfaces 1992, 63, 311–322. [Google Scholar] [CrossRef]
- Dasgupta, N.; Ranjan, S.; Mundra, S.; Ramalingam, C.; Kumar, A. Fabrication of Food Grade Vitamin E Nanoemulsion by Low Energy Approach, Characterization and Its Application. Int. J. Food Prop. 2015, 19, 700–708. [Google Scholar] [CrossRef]
- Yang, Y.; McClements, D.J. Encapsulation of vitamin E in edible emulsions fabricated using a natural surfactant. Food Hydrocoll. 2013, 30, 712–720. [Google Scholar] [CrossRef]
- Van Hasselt, P.; Janssens, G.; Slot, T.K.; Van Der Ham, M.; Minderhoud, T.; Talelli, M.; Akkermans, L.; Rijcken, C.; Van Nostrum, C. The influence of bile acids on the oral bioavailability of vitamin K encapsulated in polymeric micelles. J. Control. Release 2009, 133, 161–168. [Google Scholar] [CrossRef]
- von Lintig, J.; Babino, D. Vitamin A and Other Carotenoids. In Principles of Nutrigenetics and Nutrigenomics; Caterina, R.D.E., Martinez, J.A., Kohlmeier, M., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 237–244. [Google Scholar]
- Grune, T.; Lietz, G.; Palou, A.; Ross, A.C.; Stahl, W.; Tang, G.; Thurnham, D.; Yin, S.-A.; Biesalski, H.K. β-Carotene Is an Important Vitamin A Source for Humans. J. Nutr. 2010, 140, 2268S–2285S. [Google Scholar] [CrossRef]
- Ribeiro, B.D.; Barreto, D.W.; Coelho, M.A.Z. Technological Aspects of β-Carotene Production. Food Bioprocess. Technol. 2011, 4, 693–701. [Google Scholar] [CrossRef]
- Sharif, H.R.; Goff, H.D.; Majeed, H.; Liu, F.; Nsor-Atindana, J.; Haider, J.; Liang, R.; Zhong, F. Physicochemical stability of β-carotene and α-tocopherol enriched nanoemulsions: Influence of carrier oil, emulsifier and antioxidant. Colloids Surf. A: Physicochem. Eng. Asp. 2017, 529, 550–559. [Google Scholar] [CrossRef]
- Tapiero, H.; Ba, G.N.; Couvreur, P.; Tew, K.D. Polyunsaturated fatty acids (PUFA) and eicosanoids in human health and pathologies. Biomed. Pharmacother. 2002, 56, 215–222. [Google Scholar] [CrossRef]
- Lane, K.E.; Li, W.; Smith, C.; Derbyshire, E. The bioavailability of an omega-3-rich algal oil is improved by nanoemulsion technology using yogurt as a food vehicle. Int. J. Food Sci. Technol. 2013, 49, 1264–1271. [Google Scholar] [CrossRef]
- Ferrentino, G.; Morozova, K.; Horn, C.; Scampicchio, M. Extraction of Essential Oils from Medicinal Plants and their Utilization as Food Antioxidants. Curr. Pharm. Des. 2020, 26, 519–541. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, H.; Chen, H.; Lin, J.; Wang, Q. Food-Grade Nanoemulsions: Preparation, Stability and Application in Encapsulation of Bioactive Compounds. Molecules 2019, 24, 4242. [Google Scholar] [CrossRef]
- Ziani, K.; Chang, Y.; McLandsborough, L.; McClements, D.J. Influence of Surfactant Charge on Antimicrobial Efficacy of Surfactant-Stabilized Thyme Oil Nanoemulsions. J. Agric. Food Chem. 2011, 59, 6247–6255. [Google Scholar] [CrossRef]

| Surfactant Type | Surfactant Origin | Examples |
|---|---|---|
| Low-Molecular-Weight Surfactants | Synthetic | esters of sucrose mono- and diglycerides derivatives of monoglycerides polyoxyethylene derivatives (Tween series, Span series, sucrose monopalmitate) derivatives of monoglycerides |
| Natural | phospholipids (phosphotidyletanolamine, phosphatidylcholine, phosphatidic acid, phosphatidylinositol glycolipids (rhamnolipids, sophorolipids, trehalolipids, cellobiose lipids and mannosylerythritol lipids) saponins (Quillaja saponins, tea saponins, ginseng saponins) | |
| High-Molecular-Weight Emulsifiers | Proteins | animal proteins (mainly from milk—whey protein, casein, β-lactoglobulin, sodium caseinate) plant proteins (soy protein isolate, β-conglycinin, glycinin, pea proteins, lentil proteins) mixed proteins (e.g., sodium caseinate + micellar casein) |
| Polysaccharides | gum arabic, corn fiber gum pectin (high methoxylated pectin, ultra-high methoxylated pectin) plant mucilage (from the leaves of pereskia aculeata miller, yellow mustard mucilage) Octenyl succinic anhydride (OSA)-modified polysaccharides (OSA-modified starch, OSA-β-cyclodextrin, OSA-Konjac Glucomannan) |
| Bioactive Compound | Nanoemulsion Production | Type of Emulsion | Surfactant, Emulsifier, Oil Phase | Particle Size | Reference |
|---|---|---|---|---|---|
| Vitamin D | Spontaneous emulsification | Oil-in-water | Tween 20, 40, 60, 80, 85 Oil phase: MCT 1 | <200 nm | [68] |
| Vitamin D | Ultra-Turrax: 17,000 rpm, 2 min. HPH 2: 70 MPa for 2 cycles. 35 MPa for 1 cycle | Oil-in-water | Calcium caseinate (3%, w/v) and soy lecithin 2% | - 8 | [69] |
| Vitamin D3 | High speed blender HPH 2: 83 MPa, for 3 cycles. | Oil-in-water | Active saponins 2% Oil phase: fish oil, corn oil, MCT 1, mineral oil, and orange oil | 0.14–0.19 μm | [70] |
| Vitamin D3 | Magnetic stirrer HPH 2: 10 MPa, 10–15 passages | Oil-in-water | Tween 20 Oil phase: soybean oil, lecithin, cocoa butter | 174 ± 7 nm; 26 ± 3 nm. | [71] |
| Vitamin D | Ultrasonic homogenizer: 20 kHz,10 min, 400 W | Oil-in-water | Tween 80 Oil phase: cinnamon oil Water phase: PBS 3, water, DMEM 4 F12 media | 166.2, 118.0, 170.8 and 40.52 nm | [72] |
| Vitamin D3 | Phase inversion | Water-in-oil | Soybean derived lecithin Oil phase: Monegyl Caprylic-/capric triglyceride | 39.12 ± 0.33–64.11 ± 1.93 nm | [54] |
| Vitamin D3 | High-speed blender HPH 2: 83 MPa, for 5 cycles | Oil-in-water | Pea protein Oil phase: flaxseed oil, corn oil, or fish oil | 0.34 μm | [73] |
| PUFA 5 | Spontaneous emulsification | Oil-in-water | Tween 80 (2.5–10 wt %). Oil phase: MCT 1, lemon oil. | >1000 nm at low surfactant levels, <200 nm at high surfactant levels | [74] |
| Salmon oil | HPH 2: 17 MPa, 5 cycles | Oil-in-water | Marine lecithin (mixture of phospholipids) | 160–207 nm | [75] |
| DHA 6 algae oil | Ultrasound emulsification | Oil-in-water | Soy lecithin | 258 nm | [76] |
| DHA algae oil | Ultra-Turrax: 1000 rpm for 10 min HPH 2: 90 MPa, 5 and 7 cycles; 100 MPa, 5 and 7 cycles | Oil-in-water | Tween 40, Sodium caseinate Soya lecithin | 148 nm; 206 nm; 760 nm | [62] |
| High-oleic palm oil (1–20% w/w) | Ultra-Turrax: 9500 rpm HPH 2: 68–138 MPa, for 1, 2 and 3 cycles | Oil-in-water | Whey (1–20% w/w) | 163.7–2268.0 nm | [77] |
| Flaxseed/high DHA 4 algae oil | Ultra-Turrax: 4000 rpm for 2 min, Ultrasound sonication: 24 kHz | Oil-in-water | Soy lecithin, Tween 40 | 192 nm; 182 nm | [78] |
| Fish oil | HPH 2: 275 MPa, for 10 cycles | Oil-in-water | Tween 20, Span 80, ratio 1:1 (w/w) Oil/surfactant/water ratio 1.5:1:100 (w/w/w) | 89.7 ± 27.7 nm | [63] |
| Lycopene-enriched tomato extract | Ultra-Turrax: 5000 rpm for 5 min HPH 2: at 60, 80, 100, and 140 MPa for 1,2 and 3 cycles | Oil-in-water | Tween 20 | 96 ± 12 nm | [58] |
| Astaxanthin and lycopene | Ultra-Turrax: 5000 rpm for 10 min HPH 2: 5, 10, 15, 30, 70 and 100 MPa for (1–10) cycles. | Oil-in-water | Tween 20, 0.5% w/w Oil phase: linseed oil (1% w/w) | 1, 4 and 10 cycles at 70 MPa (210, 168, 164 nm); 100 MPa: 242, 134, 126 nm. | [79] |
| Lycopene | Ultra-mixer: for 5 min at 1000 rpm, HPH 2: 50 MPa for 2 cycles | Oil-in-water | WPI 7 (3%) Oil phase: short or long chain triglycerides (tributyrin and corn oil). | 2.6 μm; 5.4 μm | [80] |
| Lutein | Spontaneous emulsification | Oil-in-water | Tween 20, 40, 60, 80, 85, sodium benzoate Oil phase: corn oil or MCT | 190–270 nm with MCT 192 nm no lutein, 205 nm with 0.2% lutein, 270 nm with 1.2% lutein | [81] |
| β-carotene | Ultra-Turrax homogenizer HPH 2: 62 MPa, for 3 cycles | Oil-in-water | β-lactoglobulin Oil phase: orange oil | 78 nm | [82] |
| β-carotene | Ultra-Turrax homogenizer | Oil-in-water | Tween 20 (0.5%) | 9.24 ± 0.16–276.77 ± 17.70 nm | [45] |
| Spirulina oil | Ultra Turrax: at 10,000 rpm Ultrasonic bath at 50–60 kHz | Oil-in-water | Spirulina peptides Tween 80 (0, 0.5 and 1% v/v) | 222.9 ± 3.4–466.9 ± 5.3 nm | [83] |
| β-carotene | Ultra Turrax HPH 2: 60 MPa for 3 cycles. | Oil-in-water | Whey protein isolate (4%), soybean soluble polysaccharides (4%), decaglycerol monolaurate (4%) Oil phase: MCT | 579.45–1829.50 nm | [84] |
| β-carotene | Ultra Turrax HPH 2: 62 MPa, for 3 cycles | Oil-in-water | β-lactoglobulin (2%) Tween 20 (1.5%) Oil phase: corn oil | <500 nm | [82] |
| β-carotene | Ultra Turrax: 18,000 rpm, 3 min HPH2: 100 MPa for 5 cycles | Oil-in-water | Tween 80 (2%) OSA-starch (2%) Oil phase: flaxseed oil and MCT | 123.9–185.6 mn | [38] |
| β-carotene | Ultra-Turrax: 14,500 rpm, 2 min, HPH 2: 69 MPa, for 6 cycles | Oil-in-water | Tween 20 (1%) Oil phase: 70% corn oil, 30% Span 80 and β-carotene (0.02%) | 300 nm | [57] |
| Vitamin A | HPH 2: 150 MPa for 8 cycles | Oil-in-water | Egg phosphatidylcholine Oil Phase: corn oil (4.5–15%) | <236.8 ± 26.9 nm | [85] |
| Vitamin A | Emulsification method with two steps | Oil-in-water, Water-in-oil, Oil-in-water-in-oil | 1,3-butanediol Emalex 600 di-IS Oil phase: Liquid paraffin | - | [60] |
| Vitamin A | Ultra-Turrax: 7000 rpm, 10 min | Oil-in-water | Tween 80 Oil Phase: Poly (methyl methacrylate-co-methacrylic acid | - | [86] |
| Vitamin A | Ultra-Turrax: 24,000 rpm, 4 min HPH 2: 7 MPa, for 3 cycles | Oil-in-water | Lecithin (10%) Ethanol (10%) | 475.7 nm | [87] |
| Vitamin A | Ultra-Turrax: 5000 rpm, 10 min HPH 2: 200 Mpa | Oil-in-water | Whey protein isolate (4.3) Oil phase: peanut oil (30%) | <300 nm | [88] |
| Vitamin A in palmitate/peanut oil | HPH 2: 172 MPa, for 7 cycles | Oil-in-water | Lecithin/Saponin (1%) | 115 nm | [89] |
| Vitamin A | Ultra-Turrax: 720, 846.7 and 955.8 rpm. | Oil-in-water | Whey protein isolate Virgin coconut oil (50%) | 5–20 μm | [90] |
| Vitamin K1 | A syringe pump was used to add organic phase to oil phase (flow rate 50 mL/min) | Oil-in-water | Tween 80 Oil phase: 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N- | <253.9 nm | [61] |
| Vitamin K | HPH 2: 14,000 psi, 4 cycles | Oil-in-water | Lipoid E80 (1.5%) Oil Phase: MCT 1 (10%) | <119.3 ± 1.3 nm | [91] |
| Vitamin E | Ultra-Turrax: 2 min HPH2 | Oil-in-water | Whey protein isolate (3.5%) Oil phase: Orange oil (3.5%) | - | [92] |
| Vitamin E | Ultra-Turrax HPH 2: 83 MPa, 3 cycles | Oil-in-water | Gum arabic-Quillaja saponin-whey protein isolate (1.5%) Oil phase: corn oil (80%) | <100 µM | [59] |
| Vitamin E | Ultra-Turrax: 500 rpm, 25 °C | Oil-in-water | Tween 20-40-60-80-85 (10%) | <50 nm | [93] |
| Vitamin E | Ultra-Turrax: 10,000 rpm for 10 min. HPH 2: 20–50 MPa, 3 cycles. | Oil-in-water | Tween 40 Oil phase: Palm Oil (10%) | <1000 nm | [94] |
| Vitamin E | Ultra-Turrax: 2 min at room temperature HPH 2: 83 MPa, 3 cycles | Oil-in-water | Whey protein isolate and gum arabic Oil Phase: Orange Oil (5%) | <0.38 µM | [70] |
| Thyme oil (1% w/v) | Ultra-Turrax: 15,000 rpm for 3 min | Oil-in-water | Sodium caseinate and soy lecithin | 82.5 nm; 125.5 nm | [95] |
| Thymol and eugenol | Ultra Turrax: 15,000 rpm for 6 min | Oil-in-water | Lauric arginate and soy lecithin | 55 (eugenol) and 75 (thymol) nm | [96] |
| Clove and lemongrass oil | Spontaneous emulsification | Oil-in-water | Tween 20, Castor Oil Ethoxylate-40, | 76.73 nm | [64] |
| Bergamot oil and sweet orange oil | Ultra Turrax: 9000 rpm for 3 min HPH 2: 60 MPa for 3 cycles | Oil-in-water | Tween 80, soy lecithin Oil phase: citrus oil mixtures with corn oil and MCT 1 oil | 30 nm (5% oil phase); 190 nm (15% oil phase) | [97] |
| Peppermint oil | Ultra-Turrax: 24,000 rpm for 1 min, HPH 2: 50, 100, and 150 MPa for 1, 3, 5, 7, 10, 15, or 20 cycles. | Oil-in-water | Starch Oil phase: peppermint oil, MCT 1 (pure oils), and mixtures 1:5, 1:1, and 5:1 (v/v) | 146.0 ± 1.5 nm; <200 nm | [97] |
| Thyme oil | Ultra-Turrax: 60 s HPH 2: 0.01 MPa, 5 cycles | Oil-in-water | Oil phase (5% w/w): thyme oil, corn oil (from 0 to 100 wt % corn oil) pH 4.0 Tween 80 (0.5 w/w) | 163 nm | [98] |
| Oregano, thyme, lemongrass and mandarin essential oils (1% w/v) | Ultra Turrax: 9500 rpm for 2 min HPH 2: 150 MPa, 5 cycles. | Oil-in-water | High methoxyl pectin (1% w/v) and Tween 80 (5% w/v) | <50 nm | [99] |
| Lemongrass oil | Ultra-Turrax: 3400 rpm for 2 min. HPH 2: 50, 100 or 150 MPa (1, 2, 3, 4, 5 and 10) cycles | Oil-in-water | Sodium alginate (1% w/v) and Tween 80 (1% v/v) | 53 ± 5 nm, 46 ± 7 nm, 23 ± 2 nm 7.35 ± 1.67 nm | [100] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banasaz, S.; Morozova, K.; Ferrentino, G.; Scampicchio, M. Encapsulation of Lipid-Soluble Bioactives by Nanoemulsions. Molecules 2020, 25, 3966. https://doi.org/10.3390/molecules25173966
Banasaz S, Morozova K, Ferrentino G, Scampicchio M. Encapsulation of Lipid-Soluble Bioactives by Nanoemulsions. Molecules. 2020; 25(17):3966. https://doi.org/10.3390/molecules25173966
Chicago/Turabian StyleBanasaz, Shahin, Ksenia Morozova, Giovanna Ferrentino, and Matteo Scampicchio. 2020. "Encapsulation of Lipid-Soluble Bioactives by Nanoemulsions" Molecules 25, no. 17: 3966. https://doi.org/10.3390/molecules25173966
APA StyleBanasaz, S., Morozova, K., Ferrentino, G., & Scampicchio, M. (2020). Encapsulation of Lipid-Soluble Bioactives by Nanoemulsions. Molecules, 25(17), 3966. https://doi.org/10.3390/molecules25173966









