Bonding Analysis of Compounds with Unusual Coordination of Carbon: Proposed Symmetric Systems with Six-Coordinate Carbon
Abstract
1. Introduction
2. Computational Methods and Software
2.1. Software
2.2. AIM and Bader Terminology
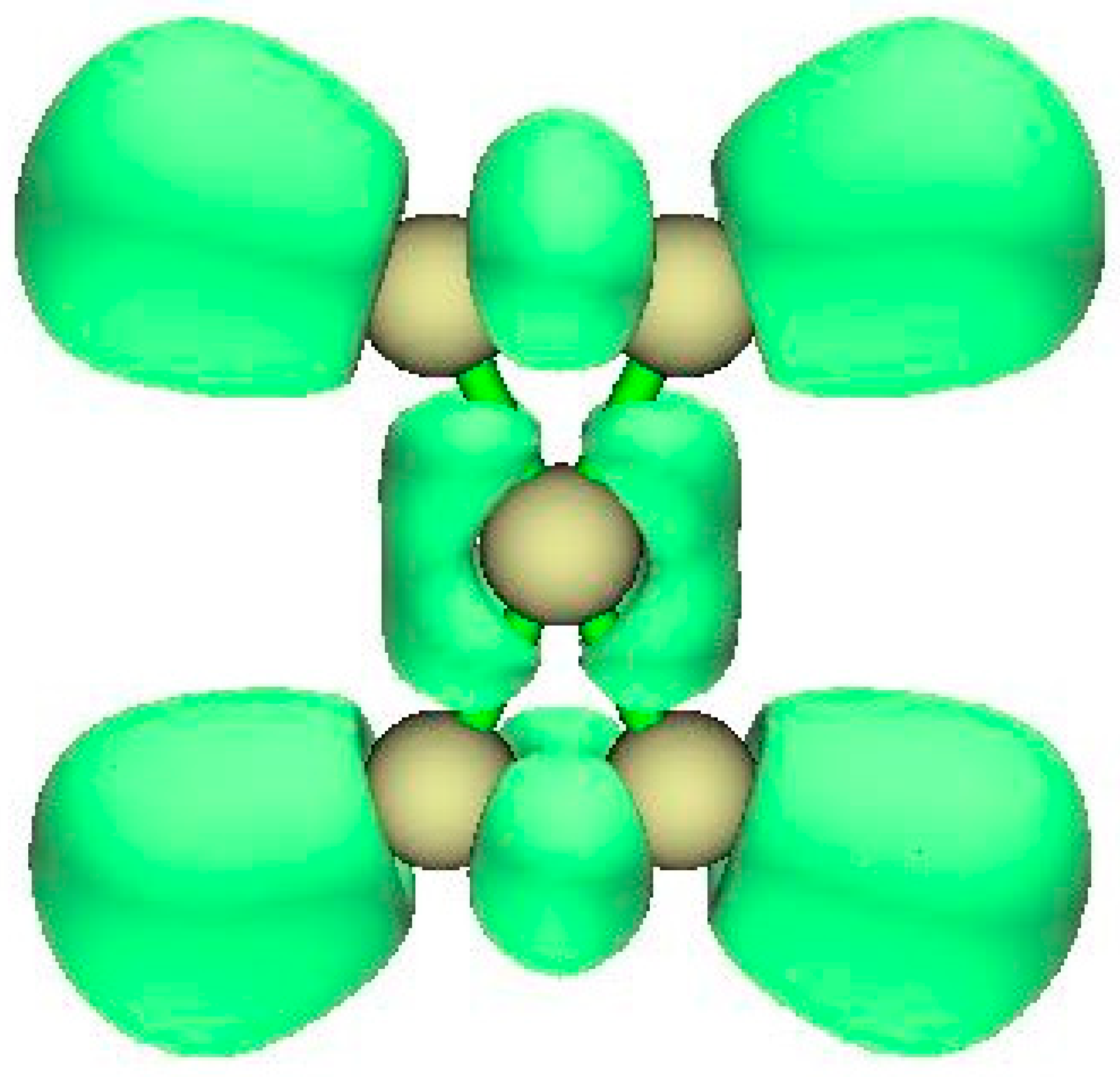
2.3. ELF
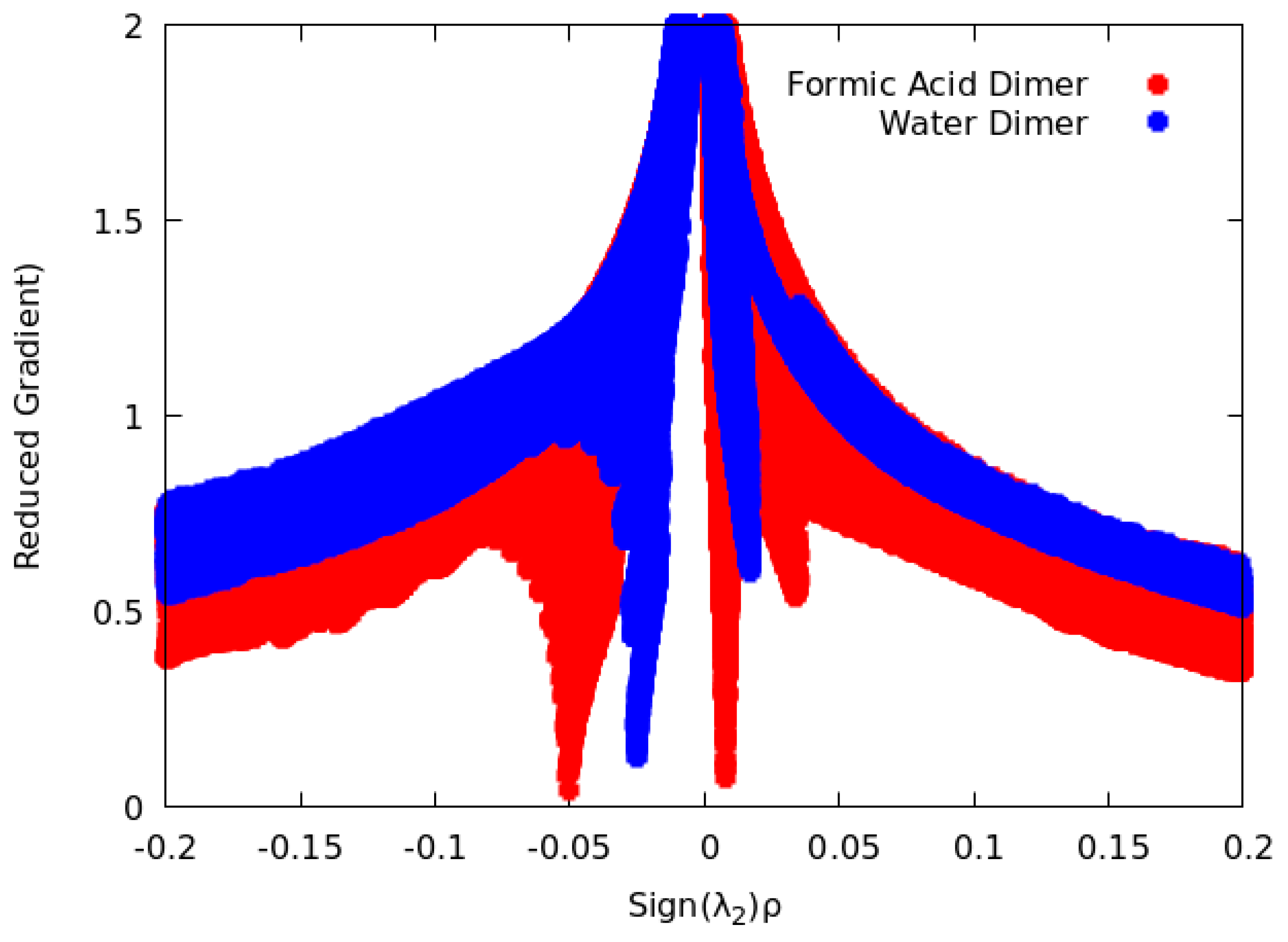
2.4. NCI
3. Density Analysis for Known Examples of Unusual Coordination of Carbon
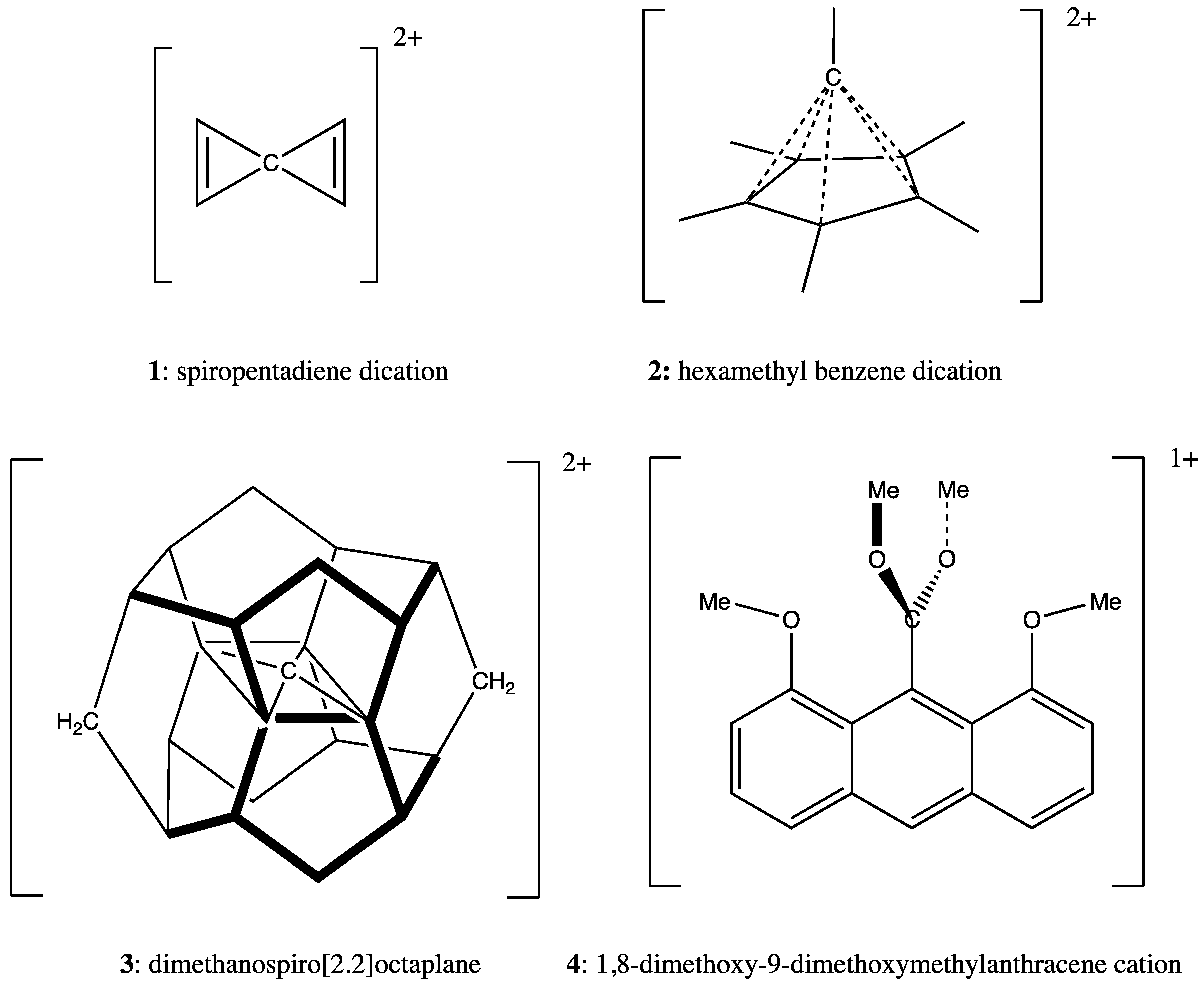
3.1. Example 1: Spiropentadiene Dication
3.1.1. AIM Analysis
3.1.2. ELF Analysis
3.1.3. NCI Analysis
3.2. Example 2: Hexamethylbenzene Dication
3.2.1. AIM Analysis
3.2.2. ELF Analysis
3.2.3. NCI Analysis
3.3. Example 3: Dimethanospiro[2.2]octaplane
3.3.1. AIM Analysis
3.3.2. ELF Analysis
3.3.3. NCI Analysis
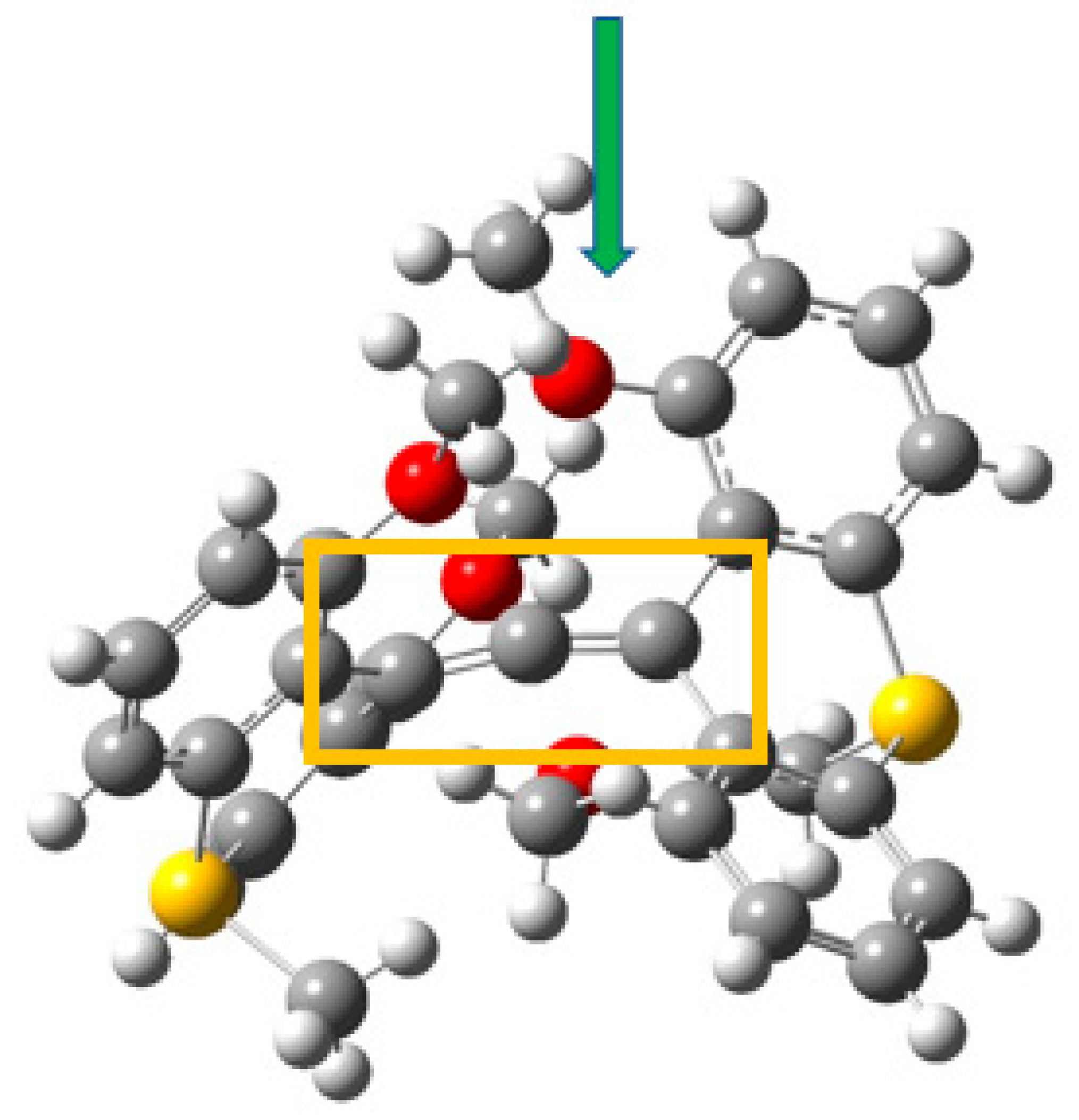
3.4. Example 4: 1,8-Dimethoxy-9-Dimethoxymethylanthracene Cation
3.4.1. AIM Analysis
3.4.2. ELF Analysis
3.4.3. NCI Analysis
4. Proposed Symmetric Variants on a Known Example of Approximately Octahedral Coordination of Carbon
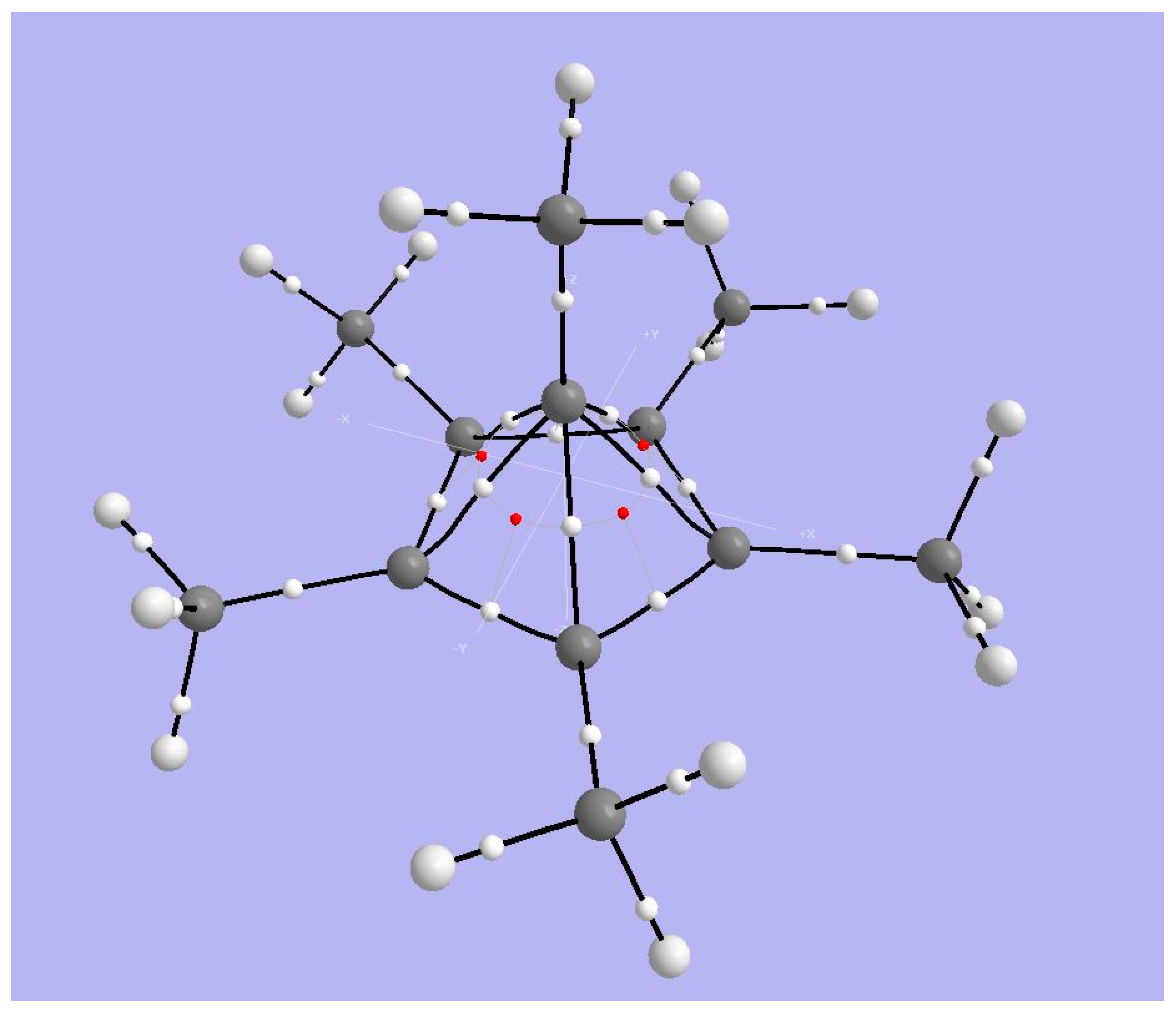
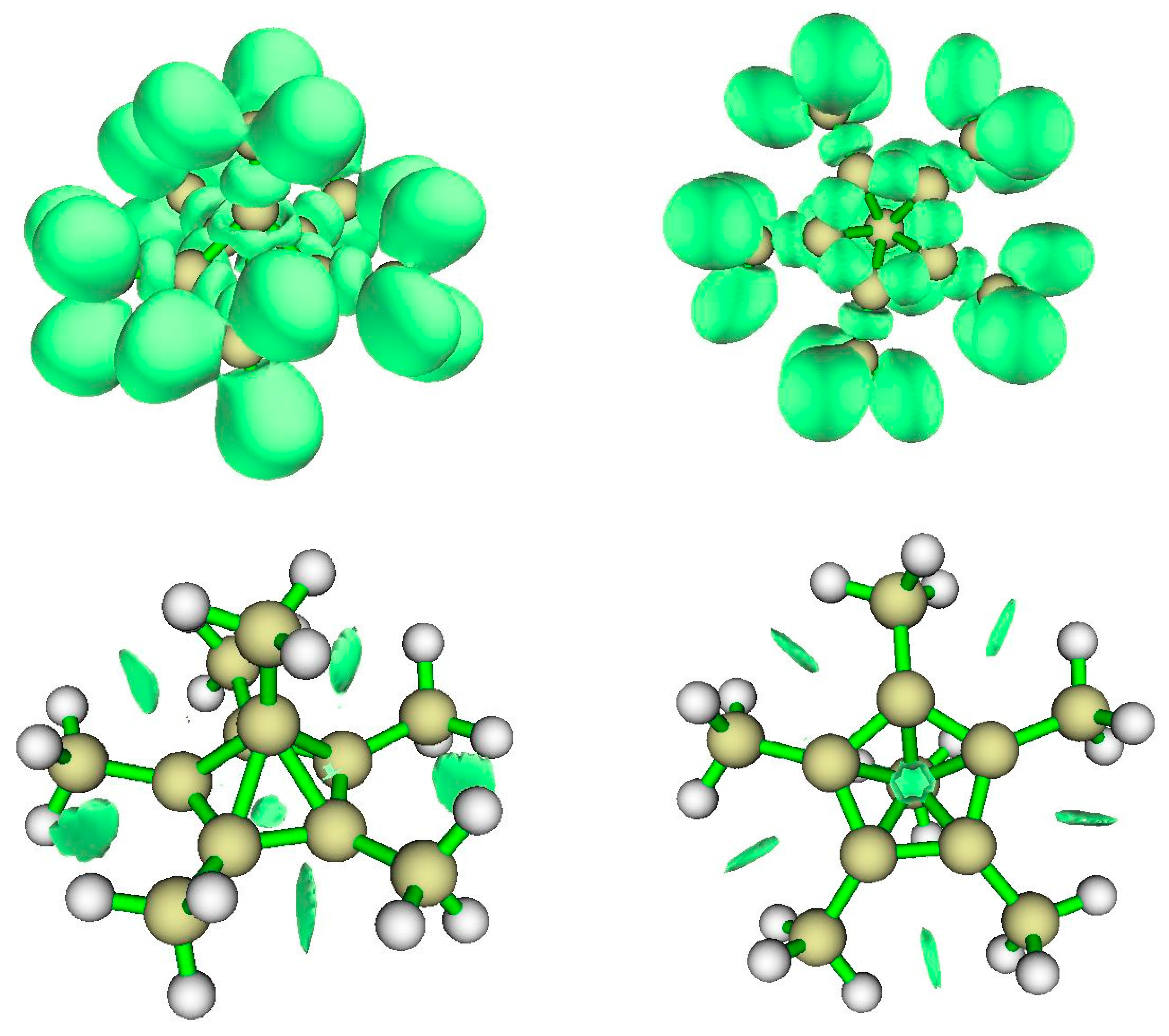
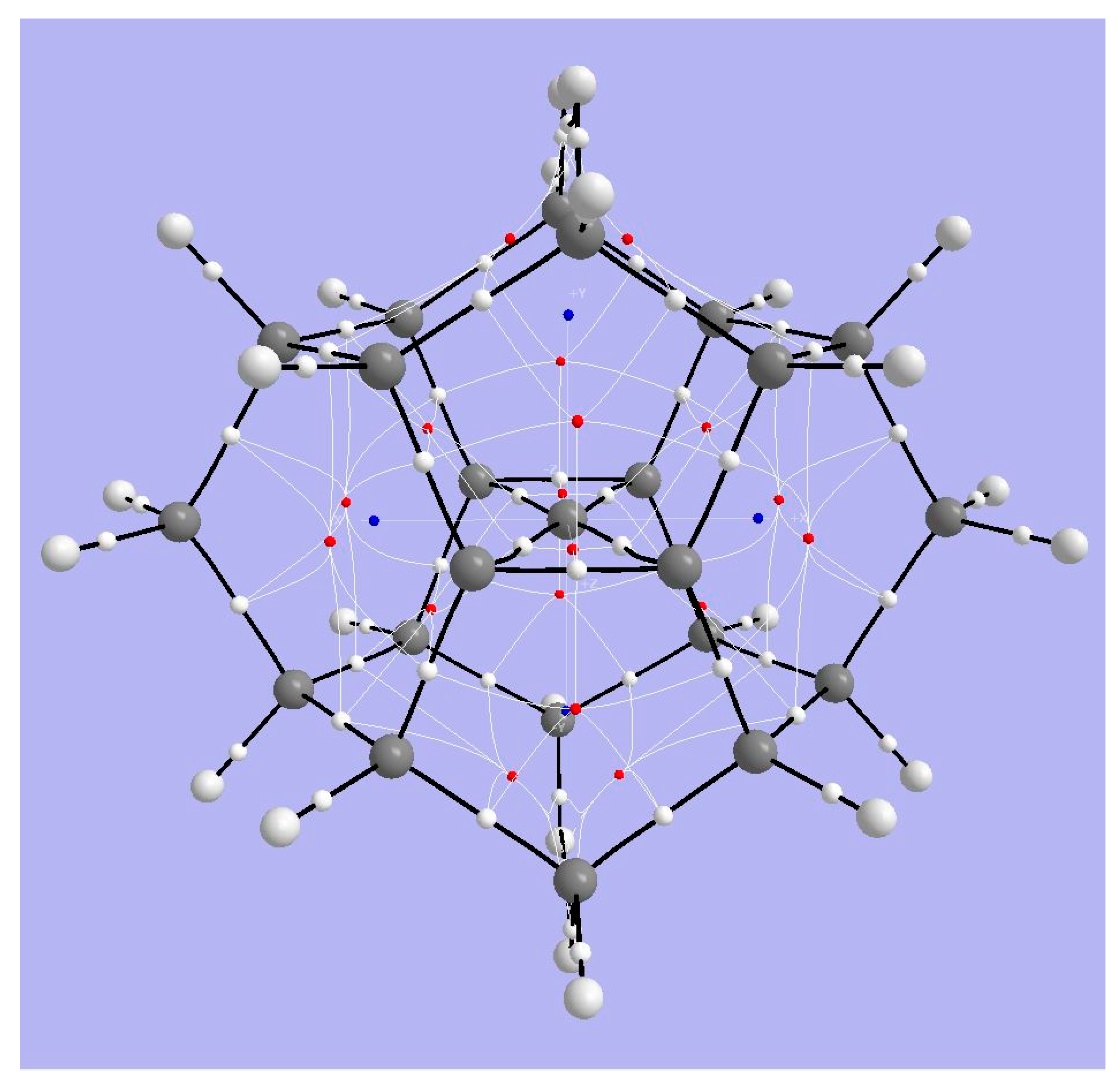
4.1. A Neutral Symmetric System with Six-Coordinate Carbon
4.1.1. AIM Analysis
4.1.2. ELF Analysis
4.1.3. NCI Analysis
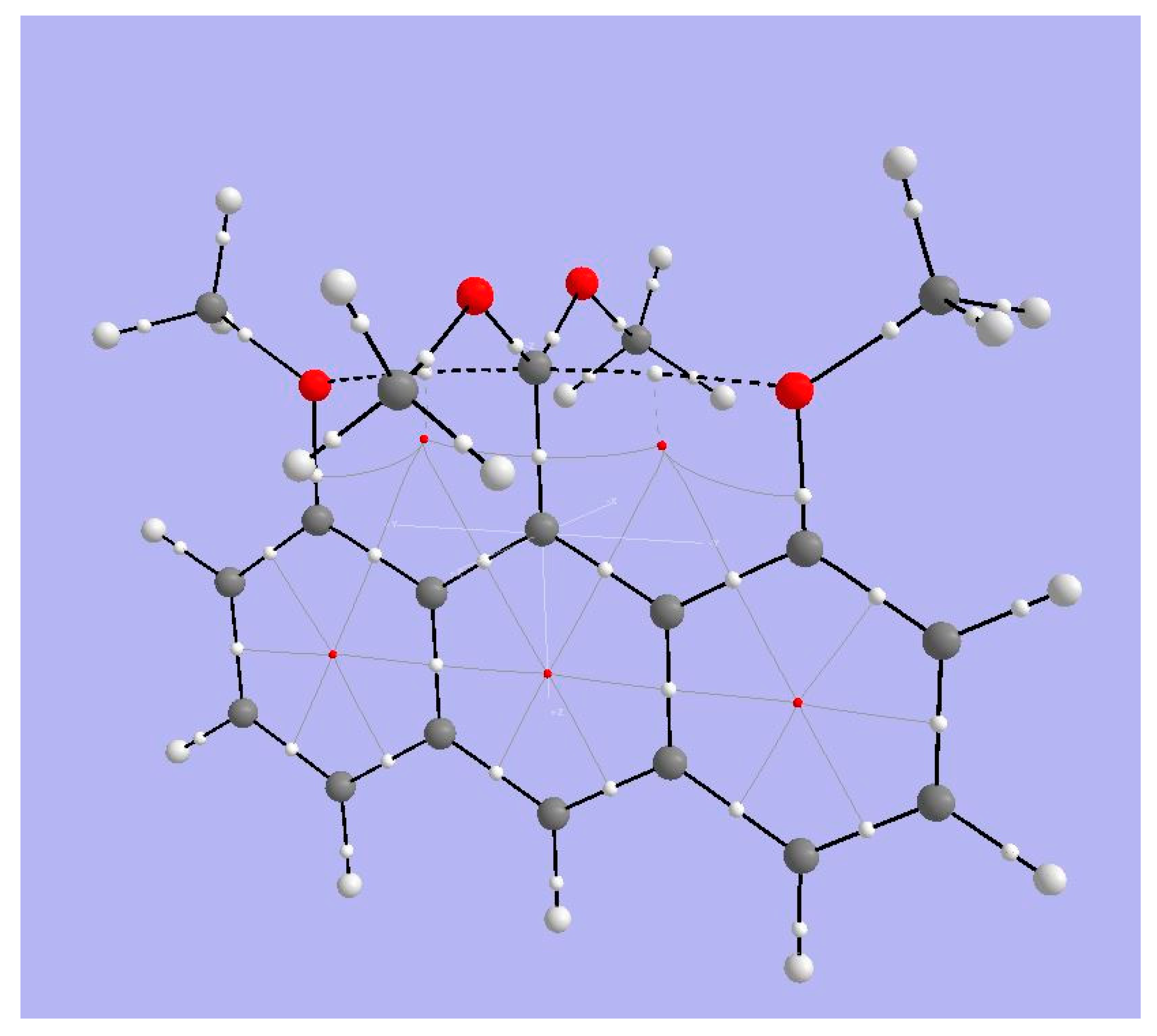
4.2. A Neutral System with Attractive Interactions in the Coordination Sphere
4.2.1. AIM Analysis
4.2.2. ELF Analysis
4.2.3. NCI Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoff, V.J.H. Sur les formules de structure dans l’espace. Arch. Néerl. Sci. Exact. Nat. 1874, 9, 445–454. [Google Scholar]
- Le Bel, J.A. Sur les relations qui existent entre les formules atomiques des corps organiques et le pouvoir rotatoire de leurs dissolutions. Bull. Soc. Chim. Fr. 1874, 22, 337–347. [Google Scholar]
- Leicester, H.M. Le Bel, Joseph.Achille. In Dictionary of Scientific Biography; Gillispie, C.C., Ed.; Charles Scribner’s Sons: New York, NY, USA, 1973; Volume 8, pp. 109–110. [Google Scholar]
- Snelders, H.A.M. van’t Hoff, Jacobus Hendricus. In Dictionary of Scientific Biography; Gillispie, C.C., Ed.; Charles Scribner’s Sons: New York, NY, USA, 1976; Volume 13, pp. 575–581. [Google Scholar]
- Gillespie, R.J.; Nyholm, R.S. Inorganic Stereochemistry. Quart. Rev. Chem. Soc. 1957, 11, 339–380. [Google Scholar] [CrossRef]
- Gillespie, R.J. Fifty years of the VSEPR model. Coord. Chem. Rev. 2008, 252, 1315–1327. [Google Scholar] [CrossRef]
- Hoffmann, R.; Alder, R.W.; Wilcox, C.F., Jr. Planar Tetracoordinate Carbon. J. Am. Chem. Soc. 1970, 92, 4992–4993. [Google Scholar] [CrossRef]
- Hoffmann, R. The Theoretical Design of Novel Stabilized Systems. Pure. Appl. Chem. 1970, 28, 181–194. [Google Scholar] [CrossRef]
- Wong, M.W.; Radom, L. Methane Dication: Planar but Not Square. J. Am. Chem. Soc. 1989, 111, 1155–1156. [Google Scholar] [CrossRef]
- Lammertsma, K.; Schleyer, P.v.R. Structures and energies of isomeric carbodications (C5H42+ and C6H42+). J. Phys. Chem. 1988, 92, 881–886. [Google Scholar] [CrossRef]
- Hogeveen, H.; Kwant, P.W. Direct observation of a remarkably stable dication of unusual structure: (CCH3)62+. Tetrahedron Lett. 1973, 14, 1665–1670. [Google Scholar] [CrossRef]
- Hogeveen, H.; Kwant, P.W.; Postma, J.; van Duynen, P.T. Electronic spectra of pyramidal dications, (CCH3)62+ and (CH)62+. Tetrahedron Lett. 1974, 15, 4351–4354. [Google Scholar] [CrossRef]
- Hogeveen, H.; Kwant, P.W. Chemistry and spectroscopy in strongly acidic solutions XL. (CCH3)6 2+ an unusual dication. J. Am. Chem. Soc. 1974, 96, 2208–2214. [Google Scholar] [CrossRef]
- Radom, L.; Rasmussen, D.R. The planar carbon story. Pure Appl. Chem. 1998, 70, 1977–1984. [Google Scholar] [CrossRef]
- Lyons, J.E.; Rasmussen, D.R.; McGrath, M.P.; Nobes, R.H.; Radom, L. Octaplane: A Saturated Hydrocarbon with a Remarkably Low Ionization Energy Leading to a Cation with a Planar Tetracoordinate Carbon Atom. Angew. Chem. Int. Ed. Engl. 1994, 33, 1667–1668. [Google Scholar] [CrossRef]
- Wang, Z.X.; Von Ragué Schleyer, P. The Theoretical Design of Neutral Planar Tetracoordinate Carbon Molecules with C(C)4 Substructures. J. Am. Chem. Soc. 2002, 124, 11979–11982. [Google Scholar] [CrossRef] [PubMed]
- Von Ragué Schleyer, P.; Boldyrev, A.I. A new, general strategy for achieving planar tetracoordinate geometries for carbon and other second row periodic elements. J. Chem. Soc. Chem. Comm. 1991, 1536–1538. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.S.; Boldyrev, A.I.; Simons, J. Tetracoordinated planar carbon in the Al4C-anion. A combined photoelectron spectroscopy and ab initio study. J. Am. Chem. Soc. 1999, 121, 6033–6038. [Google Scholar] [CrossRef]
- Boldyrev, A.I.; Simons, J. Tetracoordinated Planar Carbon in Pentaatomic Molecules. J. Am. Chem. Soc. 1998, 120, 7967–7972. [Google Scholar] [CrossRef]
- Keese, R. Carbon Flatland: Planar Tetracoordinate Carbon and Fenestranes. Chem. Rev. 2006, 106, 4787–4808. [Google Scholar] [CrossRef]
- Wu, X.-F.; Cheng, Y.-X.; Guo, J.-C. CLi2AlE (E = P, As, Sb, Bi): Planar Tetracoordinate Carbon Clusters with 16 and 14 Valence Electrons. ACS Omega 2019, 4, 21311–21318. [Google Scholar] [CrossRef]
- Exner, K.; Schleyer, P.v.R. Planar Hexacoordinate Carbon: A Viable Possibility. Science 2000, 290, 1937–1940. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory, 1st ed.; Oxford University Press: Oxford, UK, 1994. [Google Scholar]
- Becke, A.D.; Edgecombe, K.E. A simple measure of electron localization in atomic and molecular systems. J. Chem. Phys. 1990, 92, 5397–5403. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing noncovalent interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Dunning, T.H. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Todd, A.; Keith, T.K. AIMAll, Version 17.11.14; Gristmill Software: Overland Park, KS, USA, 2017. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comp. Chem. 2012, 33, 580–592. Available online: http://sobereva.com/multiwfn/ (accessed on 21 August 2020). [CrossRef]
- Contreras-García, J.; Johnson, E.R.; Keinan, S.; Chaudret, R.; Piquemal, J.P.; Beretan, D.; Yang, W. NCIPLOT: A program for plotting noncovalent interaction regions. J. Chem. Theory Comp. 2011, 7, 625–632. [Google Scholar]
- Kumar, P.S.V.; Raghavendra, V.; Subramanian, V.J. Bader’s theory of atoms in molecules (AIM) and its applications to chemical bonding. J. Chem. Sci. 2016, 128, 1527–1536. [Google Scholar] [CrossRef]
- Grabowski, S.J. What is the Covalency of Hydrogen Bonding? Chem. Rev. 2011, 111, 2597–2625. [Google Scholar] [CrossRef]
- Grimme, S.; Mueck-Lichtenfeld, C.; Erker, G.; Kehr, G.; Wang, H.; Beckers, H.; Willner, H. When do interacting atoms form a chemical bond? Spectroscopic measurements and theoretical analyses of dideuteriophenanthrene. Angew. Chem. Int. Ed. 2009, 48, 2592–2595. [Google Scholar] [CrossRef]
- Bader, R.F.W. Bond Paths are Not Chemical Bonds. J. Phys. Chem. A 2009, 113, 10391–10396. [Google Scholar] [CrossRef]
- Cerpa, E.; Andreas Krapp, A.; Flores-Moreno, R. Influence of Endohedral Confinement on the Electronic Interaction between He atoms: A He2@C20H20 Case Study. Chem. Eur. J. 2009, 15, 1985–1990. [Google Scholar] [CrossRef]
- Clark, T.; Murray, J.S.; Politzer, P. A perspective on quantum mechanics and chemical concepts in describing noncovalent interactions. Phys. Chem. Chem. Phys. 2018, 20, 30076–30082. [Google Scholar] [CrossRef]
- Narth, C.; Maroun, Z.; Boto, C.R.; Bonnet, M.-L.; Piquemal, J.-P.; Contreras-Garcia, J. A Complete NCI Perspective from new bonds to reactivity. In Applications of Topological Methods in Molecular Chemistry; Alikhani, E., Chauvin, R., Lepetit, C., Silvi, B., Eds.; Springer: Berlin, Germany, 2016; Volume 22, pp. 491–527. [Google Scholar]
- Esteves, P.M.; Ferreira, N.B.P.; Corrêa, R.J. Neutral Structures with a Planar Tetracoordinated Carbon Based on Spiropentadiene Analogues. J. Am. Chem. Soc. 2005, 127, 8680–8685. [Google Scholar] [CrossRef] [PubMed]
- Firme, C.L.; Barreiro, N.B.P.; Esteves, P.M.; Corrêa, R.J. Understanding the Planar Tetracoordinate Carbon Atom: Spiropentadiene Dication. J. Phys. Chem. A 2008, 112, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Firme, C.L.; Antunes, O.A.C.; Esteves, P.M.; Corrêa, R.J. Derivatives of Spiropentadiene Dication: New Species with Planar Tetracoordinate Carbon (ptC) atom. J. Phys. Chem. A 2009, 113, 3171–3176. [Google Scholar] [CrossRef] [PubMed]
- Malischewski, M.; Seppelt, K. Crystal Structure Determination of the Pentagonal-Pyramidal Hexamethylbenzene Dication (CH3)6C62+. Angew. Chem. Int. Ed. 2017, 56, 368–370. [Google Scholar] [CrossRef] [PubMed]
- Schleyer, P.v.R.; Maerker, C.; Dransfeld, A.; Jiao, H.; van Eikema Hommes, N.J. Nucleus-Independent Chemical Shifts: A Simple and Efficient Aromaticity Probe. J. Am. Chem. Soc. 1996, 118, 6317–6318. [Google Scholar] [CrossRef]
- Klein, J.E.M.N.; Havenith, R.W.A.; Knizia, G. The Pentagonal-Pyramidal Hexamethylbenzene Dication: Many Shades of Coordination Chemistry at Carbon. Chem. Eur. J. 2018, 24, 12340–12345. [Google Scholar] [CrossRef]
- Akiba, K.; Yamashita, M.; Yamamoto, Y.; Nagase, S. Synthesis and Isolation of Stable Hypervalent Carbon Compound (10-C-5) Bearing a 1,8-Dimethoxyanthracene Ligand. J. Am. Chem. Soc. 1999, 121, 10644–10645. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Akiba, K. Synthesis of Hypervalent Pentavalent Carbon and Boron Compounds. J. Synth. Org. Chem. Jpn. 2004, 62, 1128–1137. [Google Scholar] [CrossRef]
- Yamashita, M.; Yamamoto, Y.; Akiba, K.; Hashizume, D.; Iwasaki, F.; Tagaki, N.; Nagase, S. Synthesis and Structures of Hypervalent Carbon and Boron Compounds Bearing an Anthracene Skeleton–Elucidation of Hypervalent Interaction Based on X-ray Analysis and DFT Calculation. J. Am. Chem. Soc. 2005, 127, 4354–4371. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Yamamoto, Y.; Kinoshita, D.; Akiba, K.; Zhang, Y.; Reed, C.A.; Hashizune, D.; Iwasaki, F. Synthesis and Structure of a Hexacoordinate Carbon Compound. J. Amer. Chem. Soc. 2008, 130, 6894–6895. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Yamamoto, Y. Substituent effects on the structure of hexacoordinate carbon bearing two thioxanthene ligands. Pure Appl. Chem. 2013, 85, 671–682. [Google Scholar] [CrossRef]
- Nakai, H.; Okoshi, M.; Atsumi, T.; Kikuchi, T.; Akiba, K. Theoretical Design of Hexacoordinate Hypervalent Carbon Compounds by Analyzing Substituent Effects. Bull. Chem. Soc. Jpn. 2011, 84, 505–510. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Ishii, M.; Akiba, K.; Nakai, H. Discovery of hexacoordinate hypervalent carbon compounds: Density functional study. Chem. Phys. Lett. 2008, 460, 37–41. [Google Scholar] [CrossRef]






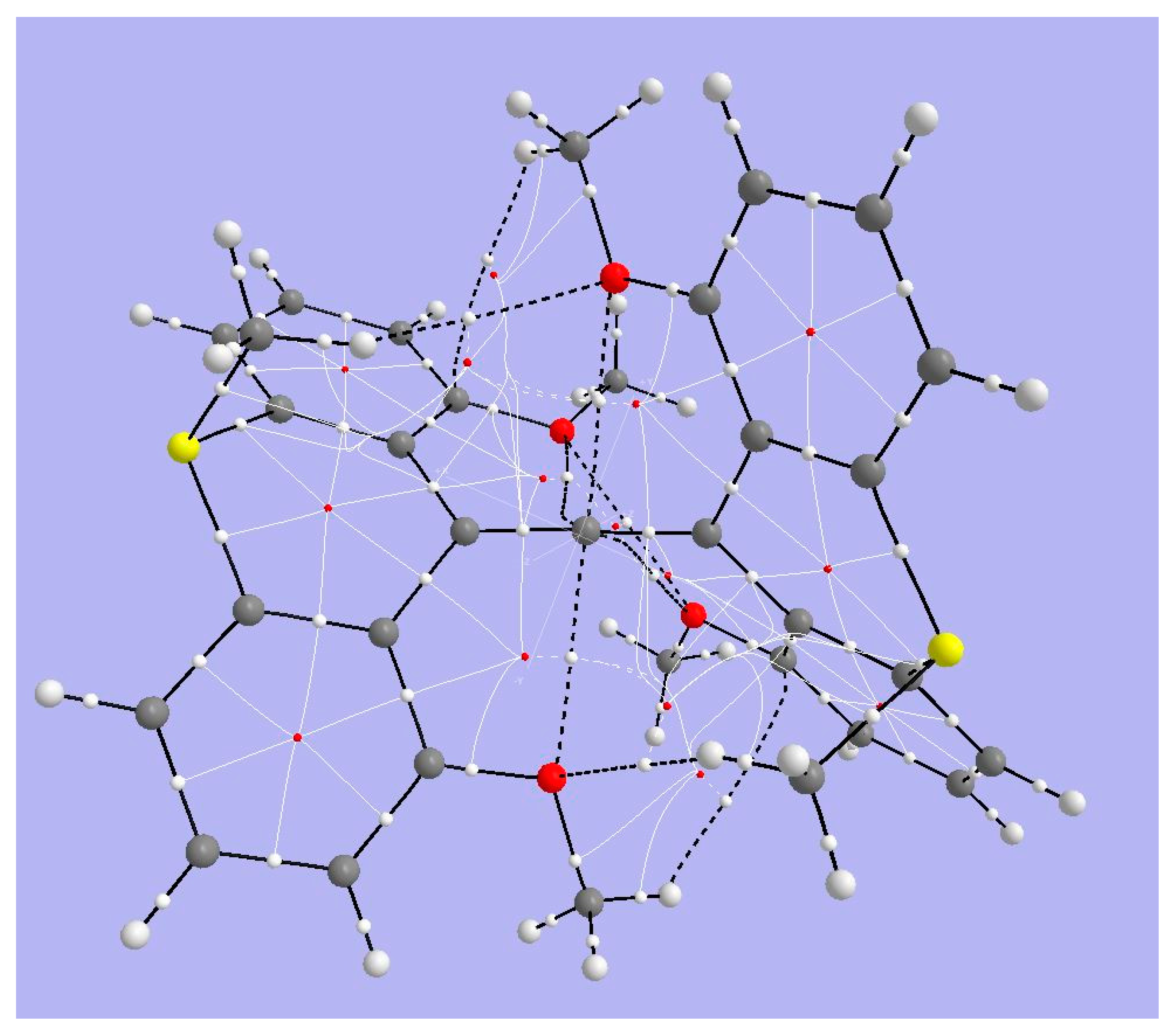
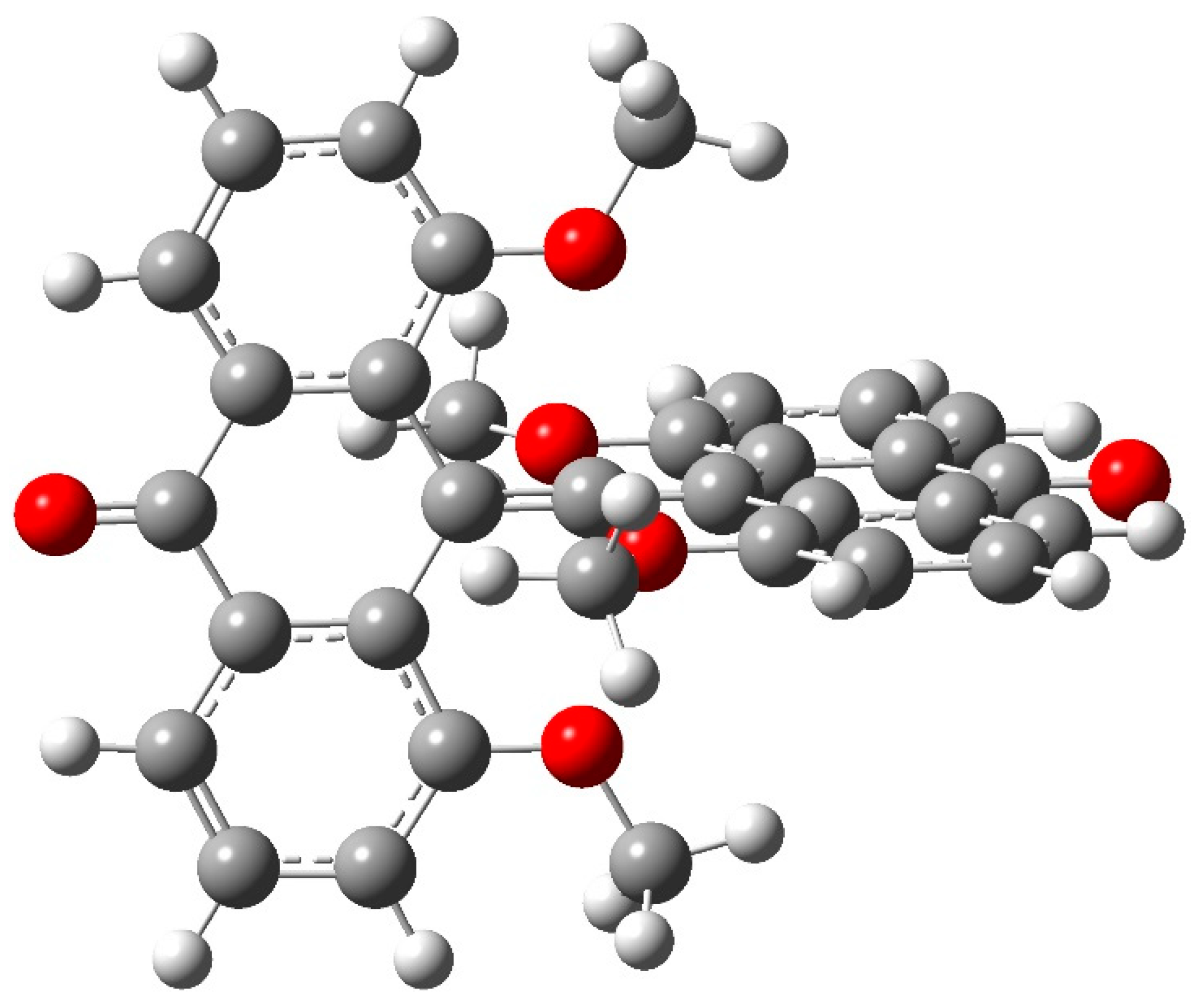
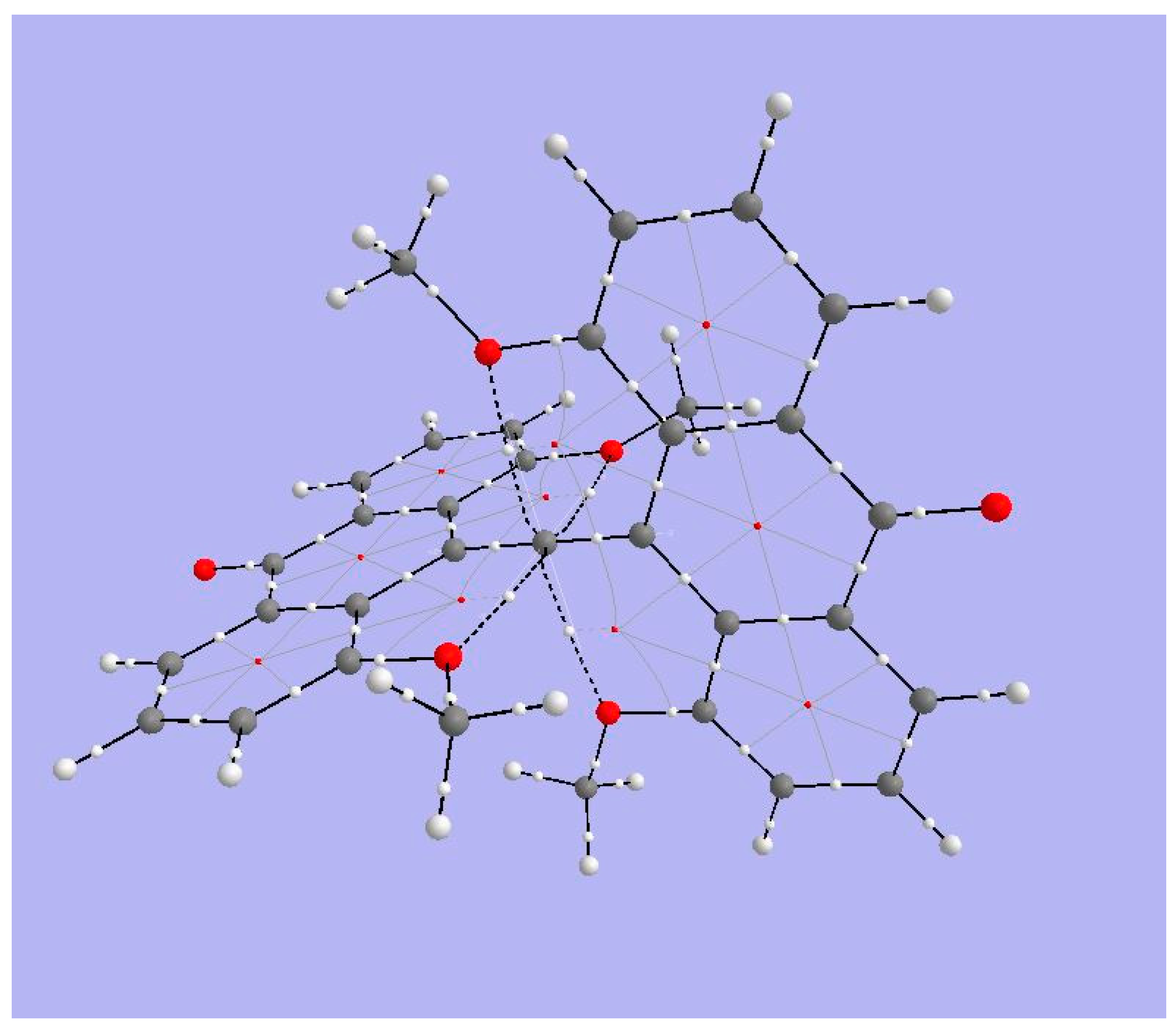

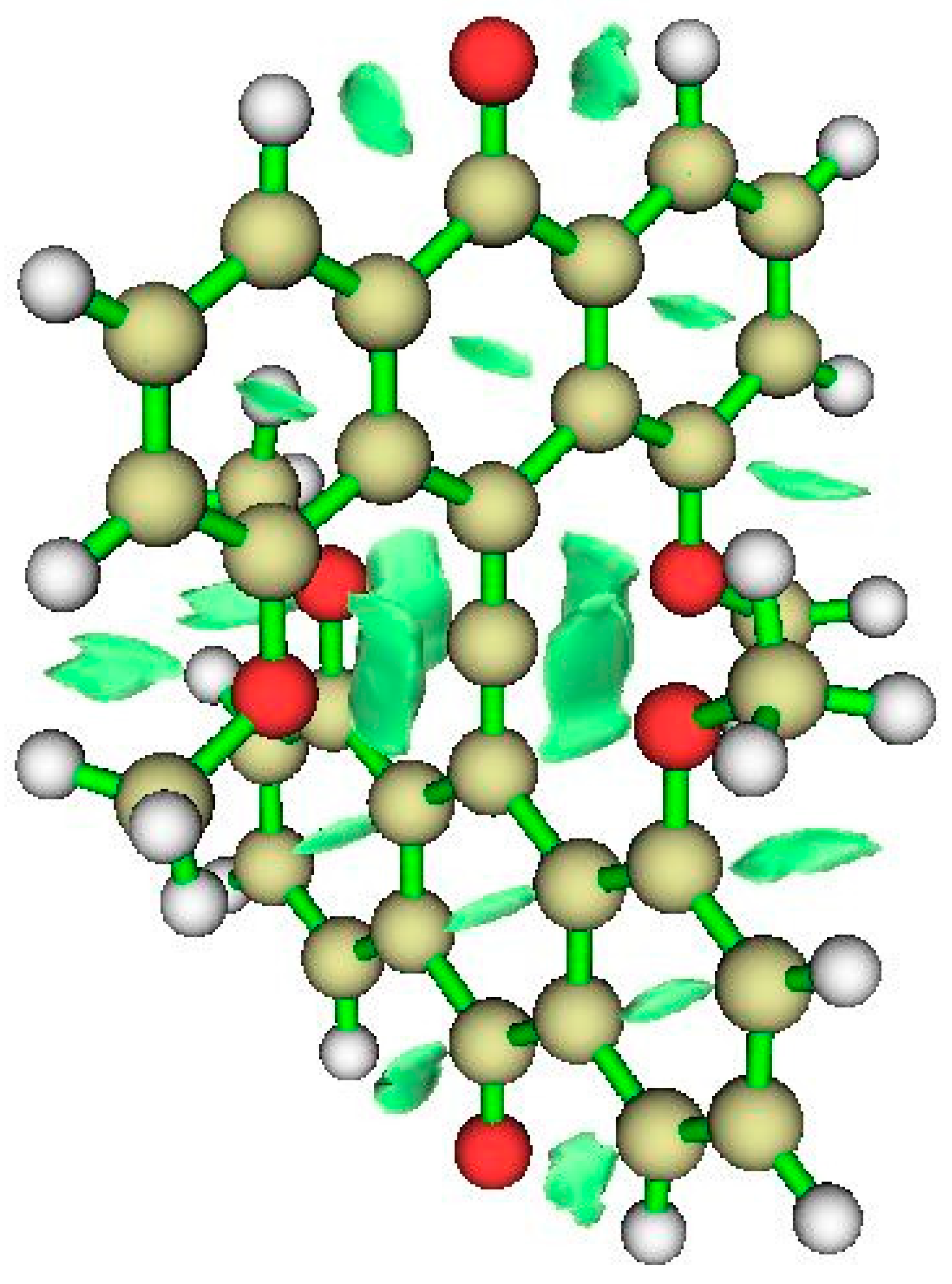
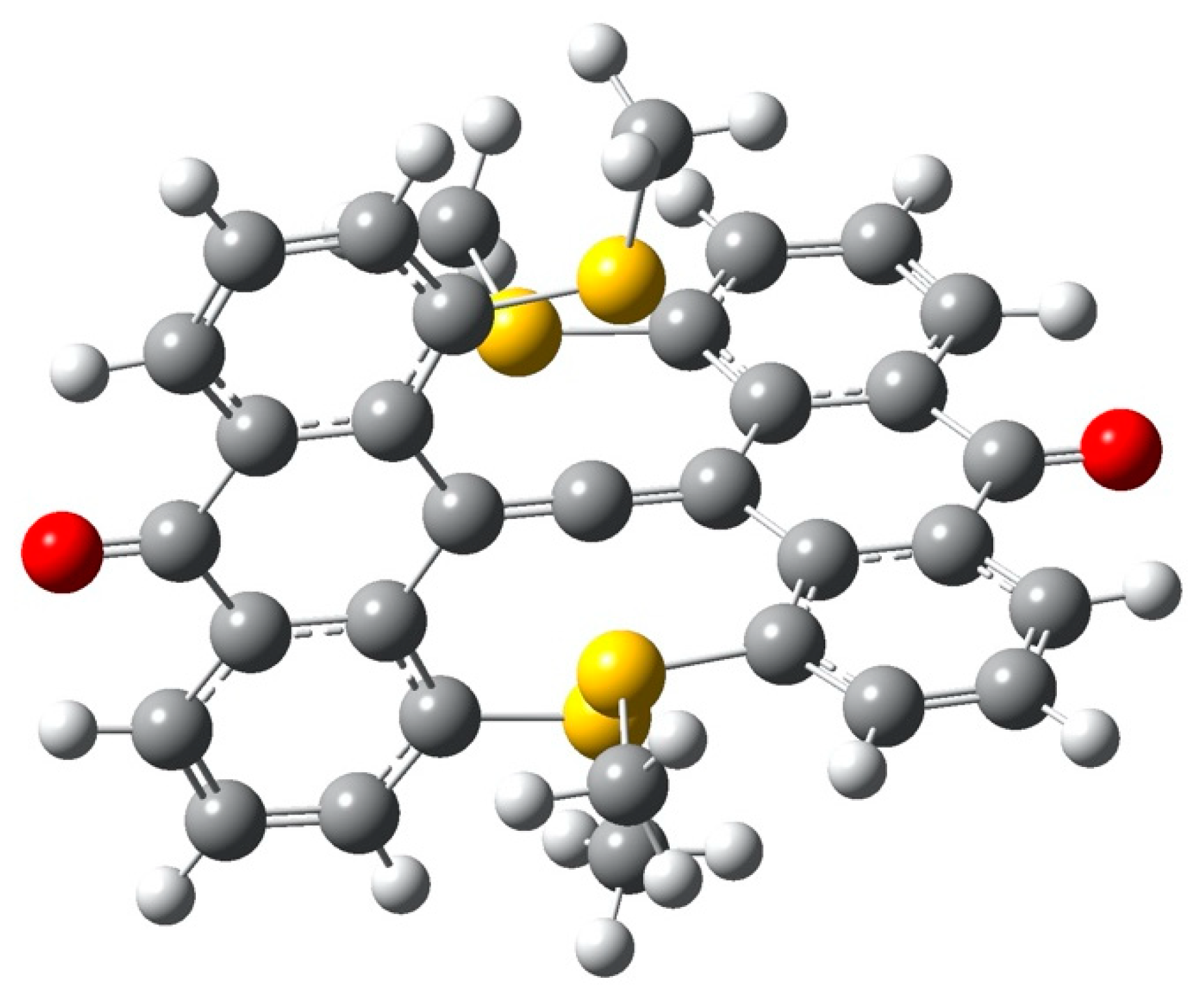


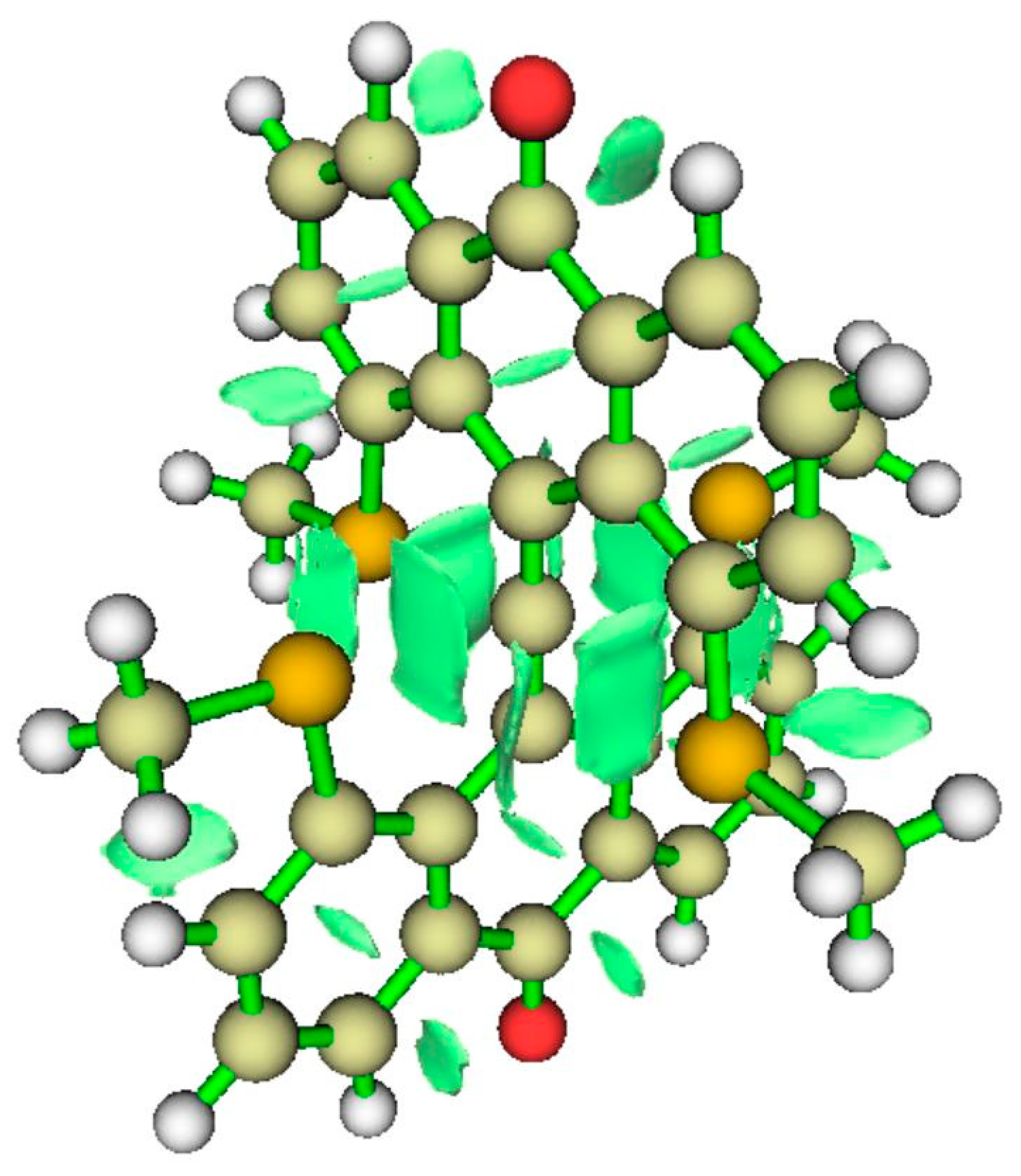
| Critical Point | Density ρ | Laplacian | G | V | |2G/V| |
|---|---|---|---|---|---|
| tpC-Cx: (3, −1) | 0.2323 0.230 | −0.1184 −0.113 | +0.1685 | −0.3506 | 0.961 |
| Cx-Cx (3, −1) | 0.3708 0.369 | −1.1167 −1.109 | +0.1872 | −0.6535 | 0.573 |
| Cx-H (3, −1) | 0.2716 0.275 | −1.0302 −1.050 | +0.0134 | −0.2844 | 0.094 |
| Basin Charges | |||||
| Charge (|e|) | H = +0.3794 | Cx = +0.1691 | tpC = −0.1932 | ||
| Critical Point | Density | Laplacian | G | V | |2G/V| |
|---|---|---|---|---|---|
| C ring-C apex: (3, −1) | 0.1551 | +0.0205 | +0.0913 | −0.1765 | 1.0346 |
| C ring-C ring (3, −1) | 0.2823 | −0.7869 | +0.0894 | −0.3766 | 0.4748 |
| C apex-Me (3, −1) | 0.2705 | −0.5607 | +0.0962 | −0.3254 | 0.5913 |
| C ring-Me (3, −1) | 0.2722 | −0.6231 | +0.0819 | −0.3194 | 0.5128 |
| Basin Charges | |||||
| Charge (|e|) | hcC −0.088 | Ring C −0.019 | Apical methyl C −0.016 | Ring methyl C −0.037 | |
| Critical Point | Density | Laplacian | G | V | |−2G/V| |
|---|---|---|---|---|---|
| rC–tcC: (3, −1) | 0.2286 | −0.2823 | +0.1435 | −0.3576 | 0.8026 |
| rC–rC (3, −1) | 0.2981 | −0.8509 | +0.1084 | −0.4295 | 0.5048 |
| sC–sC (3, −1) | 0.2493 | −0.6234 | +0.0729 | −0.3016 | 0.4834 |
| Critical Point | Density | Laplacian | G | V | |2G/V| |
|---|---|---|---|---|---|
| tbpC-OMe: (3, −1) | 0.3537 | −0.3326 | +0.4764 | −1.0359 | 0.9198 |
| tbpC…OMe (3, −1) | 0.0235 0.022 | +0.0898 +0.078 | +0.0209 | −0.0193 | 2.1658 |
| C-O (3, −1) | 0.2400 | −0.6625 | +0.2650 | −0.5445 | 0.9734 |
| C=C (3, −1) | 0.2618 | −1.0337 | +0.0708 | −0.3073 | 0.4608 |
| Critical Point | Density ρ | Laplacian ∇2ρ | Kinetic Energy Density G | Potential Energy Density V | |2G/V| | ||
|---|---|---|---|---|---|---|---|
| Allenic hcC=Ca | +0.3444 | −1.0187 | +0.1551 | −0.5648 | 0.5492 | ||
| Allenic hcC=Cb | +0.3385 | −0.9761 | +0.1515 | −0.5470 | 0.5539 | ||
| CH3Oa…hcC | +0.0182 | +0.0584 | +0.0128 | −0.0105 | 2.4381 | ||
| CH3Ob…hcC | +0.0192 | +0.0739 | +0.0165 | −0.0146 | 2.2603 | ||
| CH3Oc…hcC | +0.0140 | +0.0593 | +0.0155 | −0.0136 | 2.2794 | ||
| CH3Od…hcC | +0.0133 | +0.0602 | +0.0123 | −0.0099 | 2.4848 | ||
| Basin Charges | |||||||
| Atom | hcC | Allene Ca | Allene Cb | CH3Oa | CH3Ob | CH3Oc | CH3Od |
| AIM Q | −0.1943 | +0.0926 | +0.0898 | −1.0736 | −1.0884 | −1.0753 | −1.0720 |
| Critical Point | ρ | Laplacian | G | V | |2G/V| |
|---|---|---|---|---|---|
| C=O bond (3, −1) | 0.3976 | +0.1874 | +0.7174 | −1.3880 | 1.0337 |
| C=C bond (3, −1) | 0.3455 | −1.0337 | +0.1576 | −0.5736 | 0.5495 |
| H3C-O bond (3, −1) | 0.2523 | −0.2481 | +0.3985 | −0.6692 | 1.1910 |
| MeO … C (3, −1) | 0.0203 | +0.0755 | 0.0171 | −0.0154 | 2.2207 |
| Critical Point | ρ | Laplacian | G | V | |2G/V| |
|---|---|---|---|---|---|
| C=O bond (3, −1) | 0.4045 | −0.0281 | +0.6700 | −1.3470 | 0.9948 |
| C=C bond (3, −1) | 0.3448 | −1.0315 | +0.1624 | −0.5826 | 0.5575 |
| H3C-S bond (3, −1) | 0.1845 | −0.2965 | +0.0497 | −0.1736 | 0.5726 |
| MeS … C (3, −1) | 0.0201 | +0.0619 | +0.0141 | −0.0128 | 2.3125 |
| Basis cc-pVTZ | |||
|---|---|---|---|
| Charges (O) | C(allyl) | hcC | O atom |
| Species 6 | +0.2193 | −0.3279 | −1.1729 |
| Charges (S) | C(allyl) | hcC | S atom |
| Species 7 | +0.1552 | −0.3484 | +0.1130 |
| Basis aug-cc-pVTZ | |||
| Charges (O) | C(allyl) | hcC | O atom |
| Species 6 | +0.2213 | −0.3256 | −1.1815 |
| Charges (S) | C(allyl) | hcC | S atom |
| Species 7 | +0.2077 | −0.4591 | +0.1087 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trindle, C.; Altun, Z.; Bleda, E.A. Bonding Analysis of Compounds with Unusual Coordination of Carbon: Proposed Symmetric Systems with Six-Coordinate Carbon. Molecules 2020, 25, 3937. https://doi.org/10.3390/molecules25173937
Trindle C, Altun Z, Bleda EA. Bonding Analysis of Compounds with Unusual Coordination of Carbon: Proposed Symmetric Systems with Six-Coordinate Carbon. Molecules. 2020; 25(17):3937. https://doi.org/10.3390/molecules25173937
Chicago/Turabian StyleTrindle, Carl, Zikri Altun, and Erdi Ata Bleda. 2020. "Bonding Analysis of Compounds with Unusual Coordination of Carbon: Proposed Symmetric Systems with Six-Coordinate Carbon" Molecules 25, no. 17: 3937. https://doi.org/10.3390/molecules25173937
APA StyleTrindle, C., Altun, Z., & Bleda, E. A. (2020). Bonding Analysis of Compounds with Unusual Coordination of Carbon: Proposed Symmetric Systems with Six-Coordinate Carbon. Molecules, 25(17), 3937. https://doi.org/10.3390/molecules25173937







