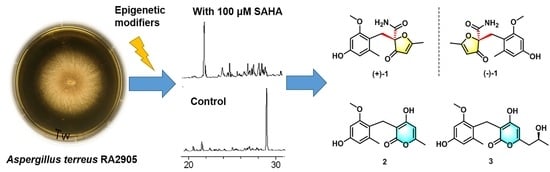
Benzyl Furanones and Pyrones from the Marine-Derived Fungus Aspergillus terreus Induced by Chemical Epigenetic Modification
Abstract
1. Introduction
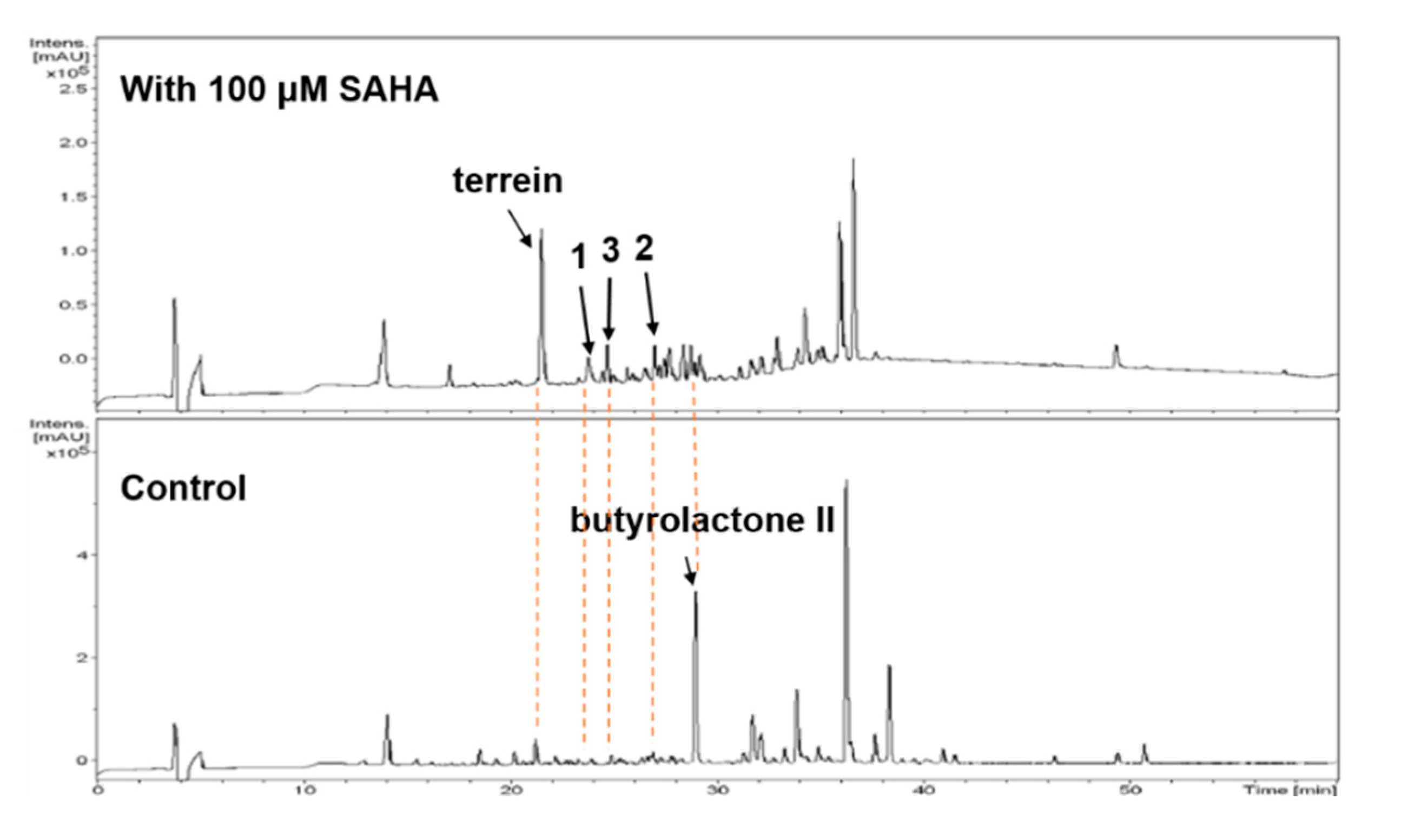
2. Results
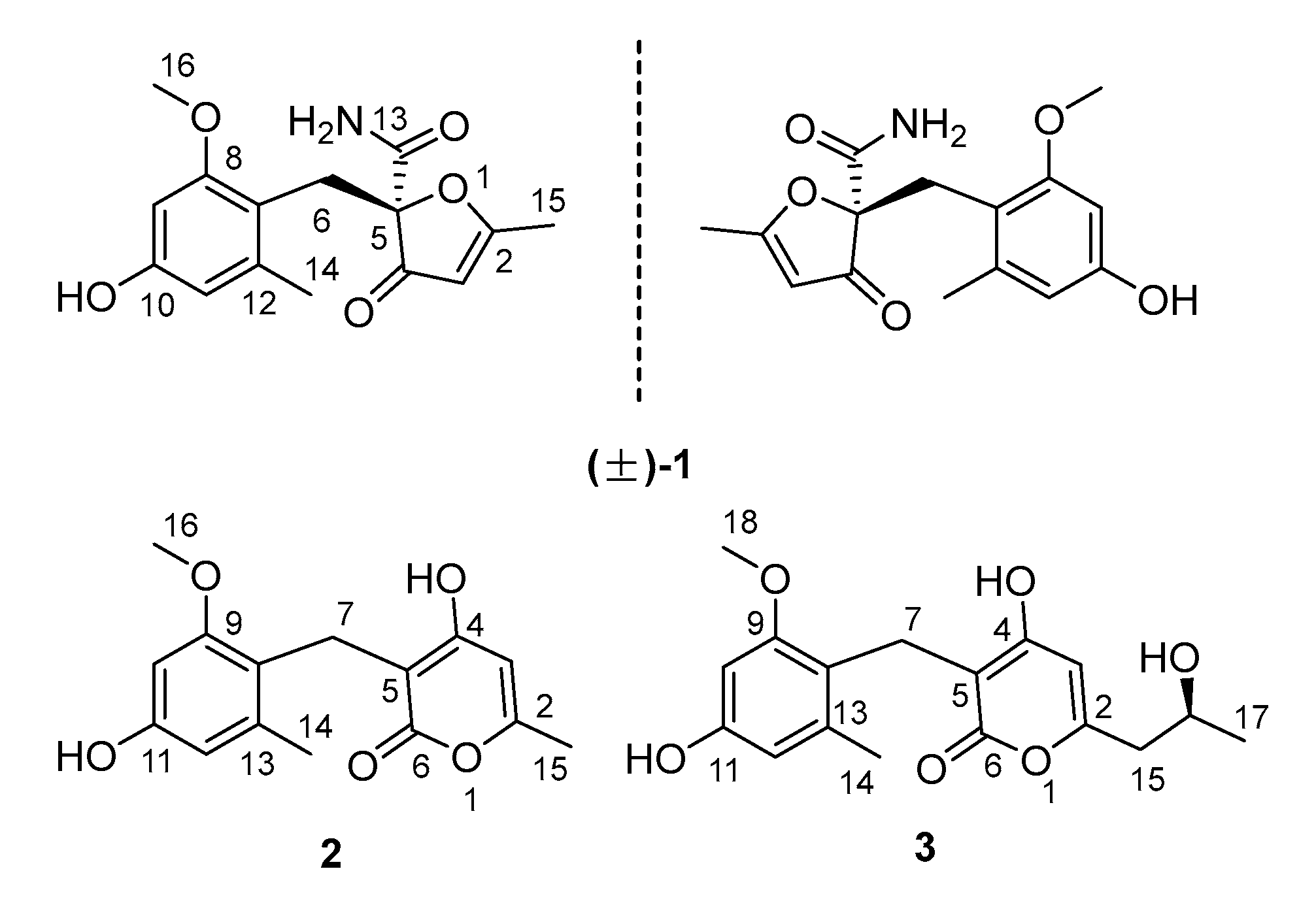
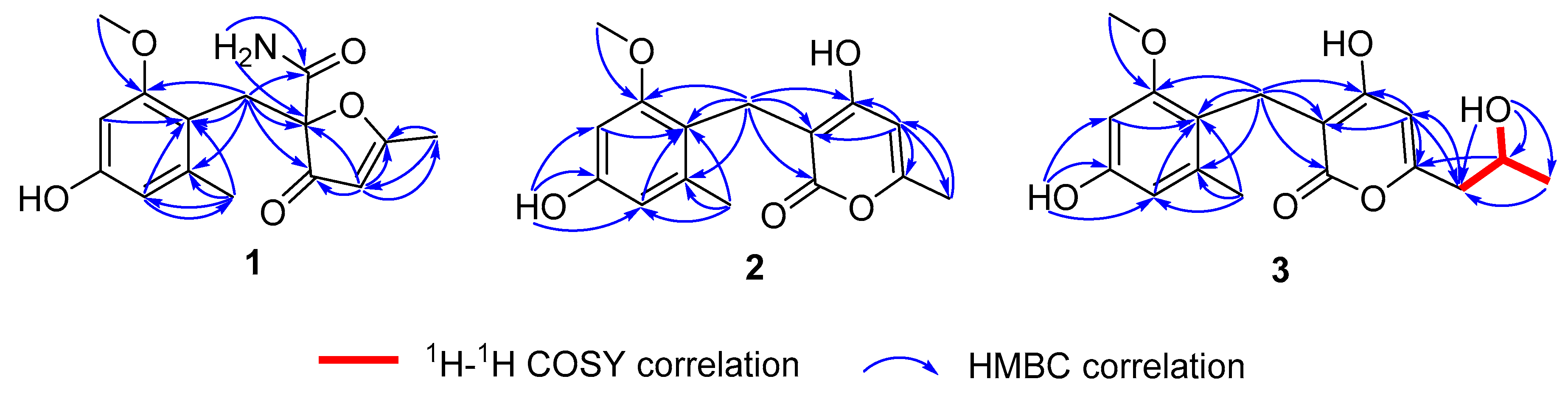
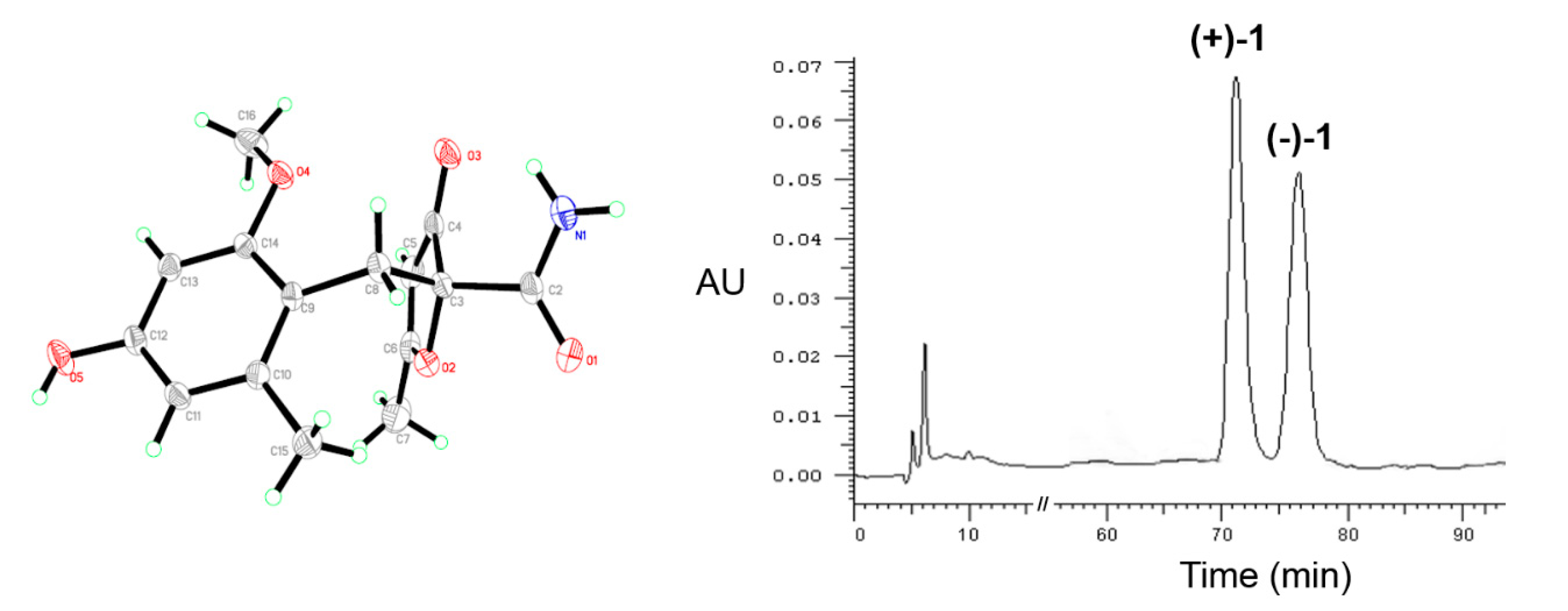
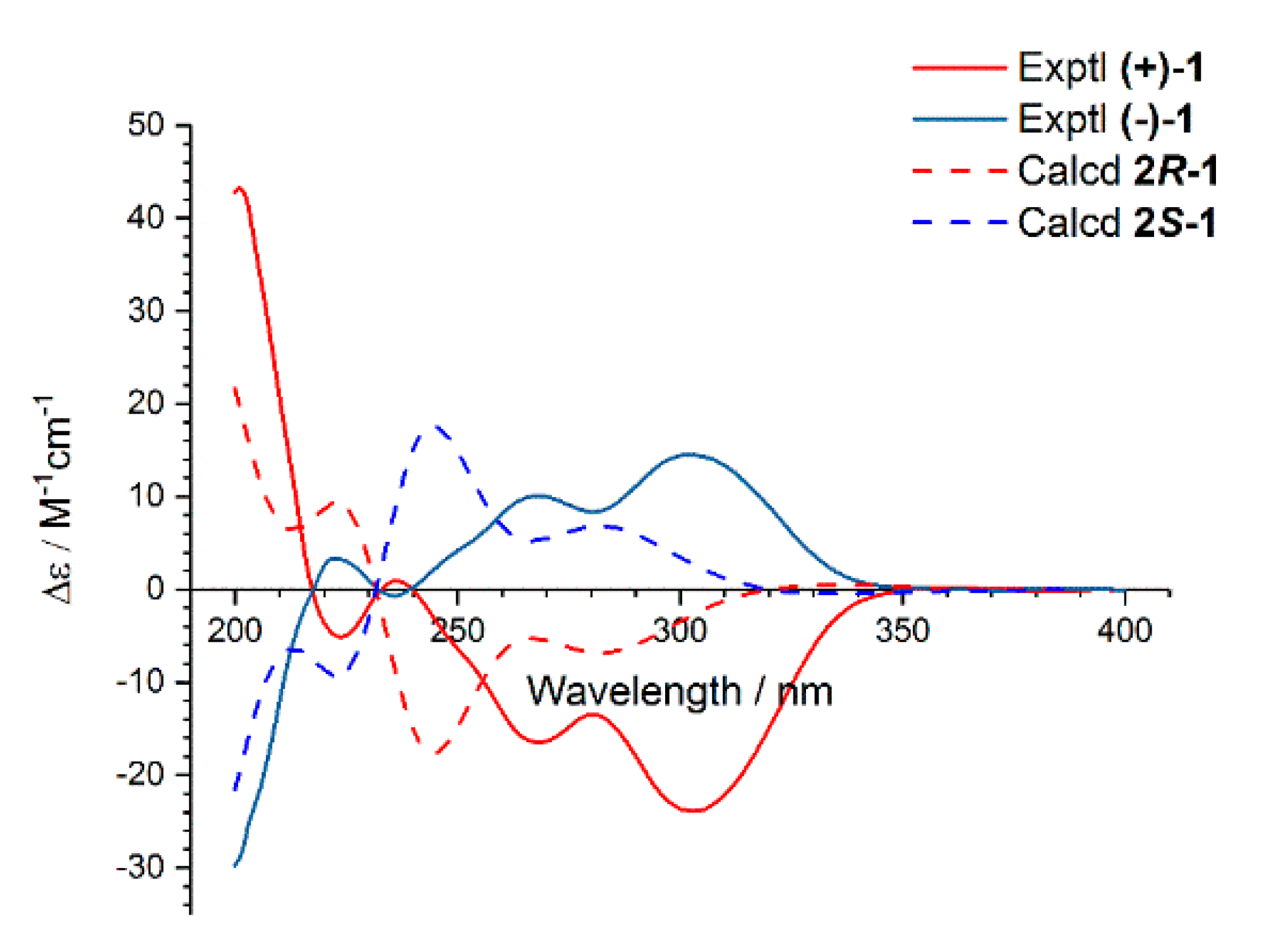
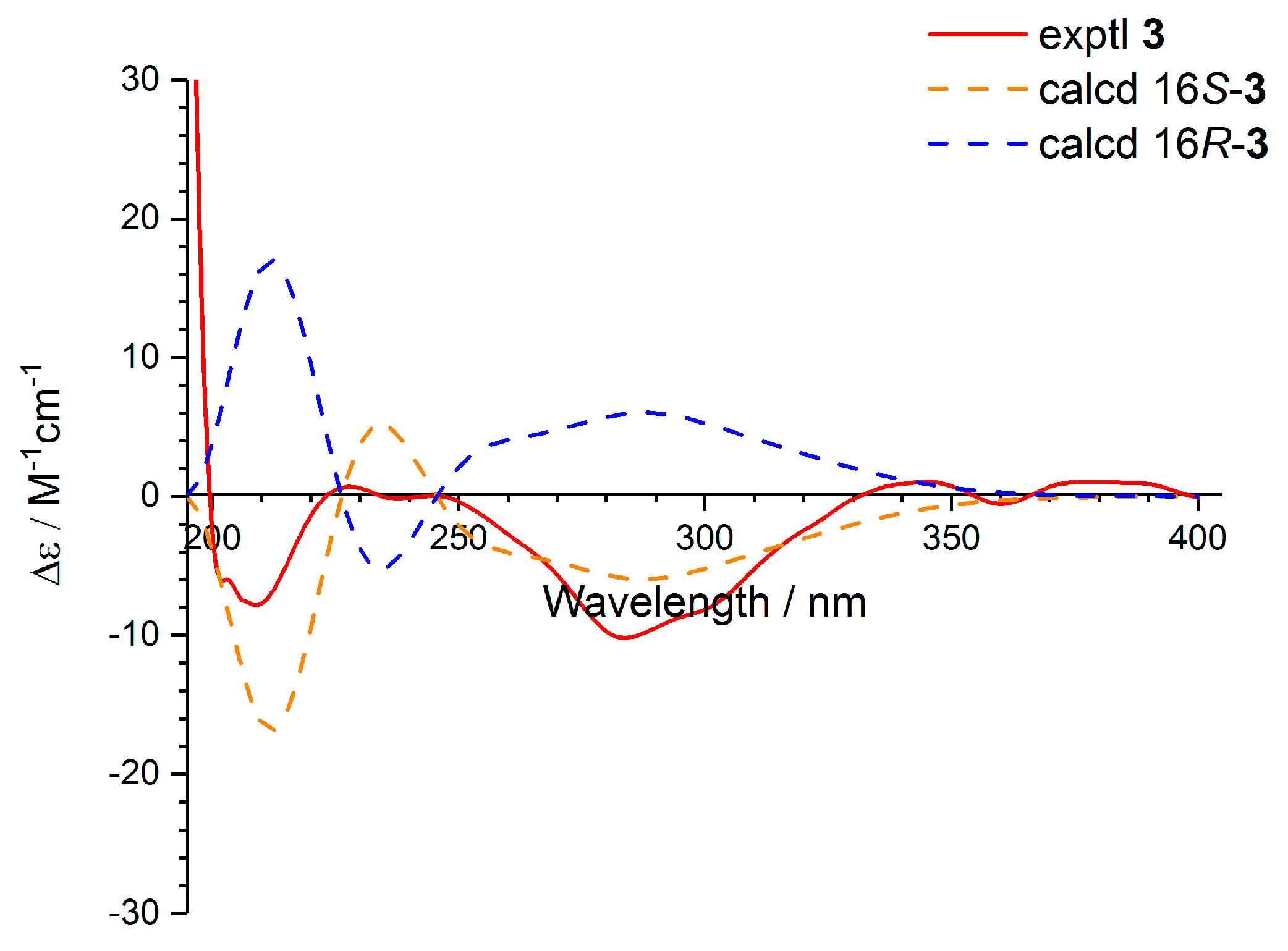
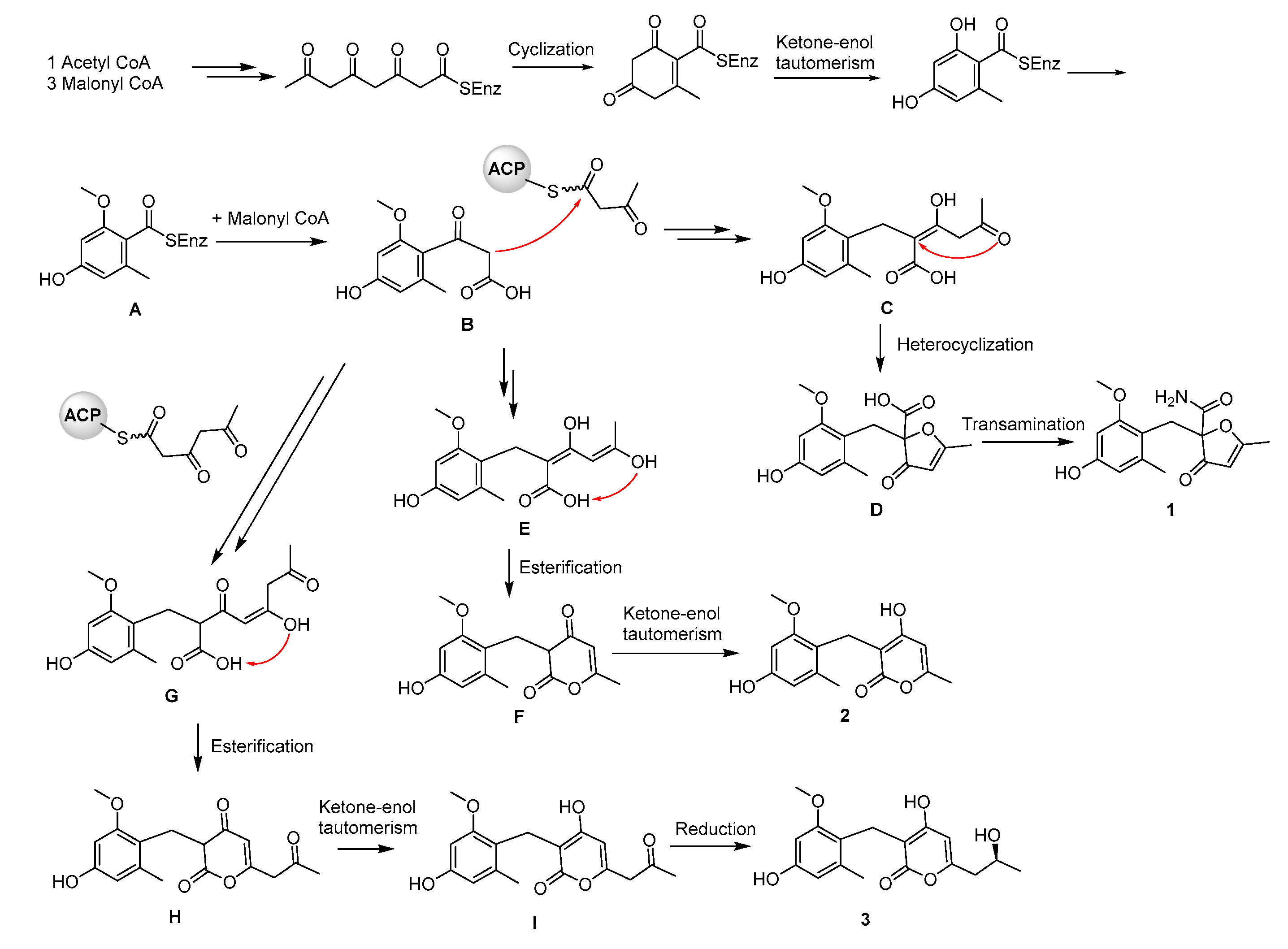
2.1. Structure Elucidation
2.2. Bioassays
3. Materials and Methods
3.1. Instrumentation
3.2. Fungal Material
3.3. Extraction and Isolation
3.4. ECD Calculation for Compounds (+)-1, (−)-1, and 3
3.5. Antimicrobial Assays
3.6. PTP1B Inhibition Assays
3.7. Cytotoxicity Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2020, 37, 175–223. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.W.; Ding, P. New bioactive metabolites from the marine-derived fungi Aspergillus. Mini-Rev. Med. Chem. 2018, 18, 1072–1094. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Kim, M.J.; Li, H.Y.; Zhang, P.; Bao, B.Q.; Lee, K.J.; Jung, J.H. Marine-derived Aspergillus species as a source of bioactive secondary metabolites. Mar. Biotechnol. 2013, 15, 499–519. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, X.M.; Meng, L.; Li, C.S.; Gao, S.S.; Shang, Z.; Proksch, P.; Huang, C.G.; Wang, B.G. Asperolides A–C, tetranorlabdane diterpenoids from the marine alga-derived endophytic fungus Aspergillus wentii EN-48. J. Nat. Prod. 2011, 74, 1787–1791. [Google Scholar] [CrossRef] [PubMed]
- Jillian, R.; Clay, C.C.W. Recent advances in the genome mining of Aspergillus secondary metabolites (covering 2012–2018). Med. Chem. Commun. 2019, 10, 840. [Google Scholar]
- Sun, K.L.; Zhu, G.L.; Hao, J.J.; Wang, Y.; Zhu, W.M. Chemical-epigenetic method to enhance the chemodiversity of the marine algicolous fungus, Aspergillus terreus OUCMDZ-2739. Tetrahedron 2018, 74, 83–87. [Google Scholar] [CrossRef]
- Xiao, L.; Yin, Y.; Sun, W.; Zhang, F.L.; Li, Z.Y. Enhanced production of (+)-terrein by Aspergillus terreus strain PF26 with epigenetic modifier suberoylanilide hydroxamic acid. Process. Biochem. 2013, 48, 1635–1639. [Google Scholar] [CrossRef]
- Chung, Y.M.; Wei, C.K.; Chuang, D.W.; Mohaned, E.S.; Hsieh, C.T.; Asai, T.; Oshima, Y.; Hsieh, T.J.; Hwang, T.L.; Wu, Y.C.; et al. An epigenetic modifier enhances the production of anti-diabetic and anti-inflammatory sesquiterpenoids from Aspergillus sydowii. Bioorg. Med. Chem. 2013, 21, 3866–3872. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zheng, Y.Y.; Shao, C.L.; Wang, C.Y. Metabolites from marine invertebrates and their symbiotic microorganisms: Molecular diversity discovery, mining, and application. Mar. Life Sci. Technol. 2019, 1, 60–94. [Google Scholar] [CrossRef]
- Zhang, W.; Shao, C.L.; Chen, M.; Liu, Q.A.; Wang, C.Y. Brominated resorcylic acid lactones from the marine-derived fungus Cochliobolus lunatus induced by histone deacetylase inhibitors. Tetrahedron Lett. 2014, 55, 4888–4891. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, W.; Shao, C.L.; Chi, Z.M.; Wang, C.Y. DNA methyltransferase inhibitor induced fungal biosynthetic products: Diethylene glycol phthalate ester oligomers from the marine-derived fungus Cochliobolus lunatus. Mar. Biotechnol. 2016, 18, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.S.; Shi, X.H.; Zhang, Y.H.; Yu, J.Y.; Fu, X.M.; Li, X.; Chen, K.X.; Guo, Y.W.; Shao, C.L.; Wang, C.Y. Co-cultivation with 5-azacytidine induced new metabolites from the zoanthid-derived fungus Cochliobolus lunatus. Front. Chem. 2019, 7, 763. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.S.; Yao, G.S.; Shi, X.H.; Rehman, S.U.; Xu, Y.; Fu, X.M.; Zhang, X.L.; Liu, Y.; Wang, C.Y. Epigenetic agents trigger the production of bioactive nucleoside derivatives and bisabolane sesquiterpenes from the marine-derived fungus Aspergillus versicolor. Front. Microbiol. 2020, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Shao, C.L.; Liu, Y.; Zhao, D.L.; Cao, F.; Fu, X.M.; Yu, J.Y.; Wu, J.S.; Zhang, Z.K.; Wang, C.Y. Terpenoids from the coral-derived fungus Trichoderma harzianum (XS-20090075) induced by chemical epigenetic manipulation. Front. Microbiol. 2020, 11, 572. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.S.; Shi, X.H.; Yao, G.S.; Shao, C.L.; Fu, X.M.; Zhang, X.L.; Guan, H.S.; Wang, C.Y. New Thiodiketopiperazine and 3, 4-dihydroisocoumarin derivatives from the marine-derived fungus Aspergillus terreus. Mar. Drugs 2020, 18, 132. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.G.; Wang, H.; Du, L.Q.; Li, M.; Chen, L.; Yu, J.; Cheng, G.G.; Zhan, M.T.; Hu, Q.F.; Zhang, L.H.; et al. Matsuda, Y. Molecular basis for the biosynthesis of an unusual chain-fused polyketide, gregatin A. J. Am. Chem. Soc. 2020, 142, 8464–8472. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement, M100-S22; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Tian, J.L.; Liao, X.J.; Wang, Y.H.; Si, X.; Shu, C.; Gong, E.S.; Xie, X.; Ran, X.L.; Li, B. Identification of cyanidin-3-arabinoside extracted from blueberry as a selective protein tyrosine phosphatase 1B inhibitor. J. Agric. Food Chem. 2019, 67, 13624–13634. [Google Scholar] [CrossRef] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, K.M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
| Position | δC, Type | δH (J in Hz) |
|---|---|---|
| 2 | 189.8, C | |
| 3 | 102.0, CH | 5.32, s |
| 4 | 198.9, C | |
| 5 | 91.4, C | |
| 6 | 30.1, CH2 | 3.25, d (14.2) |
| 3.19, d (14.2) | ||
| 7 | 111.7, C | |
| 8 | 159.2, C | |
| 9 | 96.3, CH | 6.14, d (2.3) |
| 10 | 156.7, C | |
| 11 | 108.8, CH | 6.12, d (2.3) |
| 12 | 139.3, C | |
| 13 | 166.7, C | |
| 14 | 20.0, CH3 | 2.11, s |
| 15 | 16.2, CH3 | 2.02, s |
| 16 | 55.0, CH3 | 3.57, s |
| 10-OH | 9.21, s | |
| 13-NH2 | 7.23, s | |
| 7.31, s |
| Position | 2 | 3 | ||
|---|---|---|---|---|
| δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | |
| 2 | 164.9, C | 160.4, C | ||
| 3 | 100.1, CH | 5.94, s | 100.9, CH | 5.96, s |
| 4 | 159.3, C | 164.4, C | ||
| 5 | 100.5, C | 100.8, C | ||
| 6 | 164.3, C | 164.7, C | ||
| 7 | 19.6, CH2 | 3.46, brs | 19.6, CH2 | 3.46, brs |
| 8 | 117.0, C | 117.0, C | ||
| 9 | 158.6, C | 158.6, C | ||
| 10 | 96.8, CH | 6.15, d (2.3) | 96.8, CH | 6.16, d (2.3) |
| 11 | 155.7, C | 155.7, C | ||
| 12 | 108.9, CH | 6.10, d (2.3) | 108.9, CH | 6.10, d (2.3) |
| 13 | 138.1, C | 138.1, C | ||
| 14 | 19.9, CH3 | 2.17, s | 19.9, CH3 | 2.16, s |
| 15 | 19.2, CH3 | 2.10, s | 42.7, CH2 | 2.39, d (6.4) |
| 16 | 55.3, CH3 | 3.61, s | 64.1, CH | 3.87, m |
| 17 | 23.4, CH3 | 1.07, d (6.1) | ||
| 18 | 55.4, CH3 | 3.61, s | ||
| OH-11 | 9.03, s | 9.03, s | ||
| OH-16 | 4.77, d (5.1) | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.-S.; Shi, X.-H.; Zhang, Y.-H.; Shao, C.-L.; Fu, X.-M.; Li, X.; Yao, G.-S.; Wang, C.-Y. Benzyl Furanones and Pyrones from the Marine-Derived Fungus Aspergillus terreus Induced by Chemical Epigenetic Modification. Molecules 2020, 25, 3927. https://doi.org/10.3390/molecules25173927
Wu J-S, Shi X-H, Zhang Y-H, Shao C-L, Fu X-M, Li X, Yao G-S, Wang C-Y. Benzyl Furanones and Pyrones from the Marine-Derived Fungus Aspergillus terreus Induced by Chemical Epigenetic Modification. Molecules. 2020; 25(17):3927. https://doi.org/10.3390/molecules25173927
Chicago/Turabian StyleWu, Jing-Shuai, Xiao-Hui Shi, Ya-Hui Zhang, Chang-Lun Shao, Xiu-Mei Fu, Xin Li, Guang-Shan Yao, and Chang-Yun Wang. 2020. "Benzyl Furanones and Pyrones from the Marine-Derived Fungus Aspergillus terreus Induced by Chemical Epigenetic Modification" Molecules 25, no. 17: 3927. https://doi.org/10.3390/molecules25173927
APA StyleWu, J.-S., Shi, X.-H., Zhang, Y.-H., Shao, C.-L., Fu, X.-M., Li, X., Yao, G.-S., & Wang, C.-Y. (2020). Benzyl Furanones and Pyrones from the Marine-Derived Fungus Aspergillus terreus Induced by Chemical Epigenetic Modification. Molecules, 25(17), 3927. https://doi.org/10.3390/molecules25173927